Abstract
Objectives
This study evaluated the effect of serum calcium, parathyroid hormone (PTH), vitamin D, and uric acid levels on pulp stone formation.
Materials and Methods
Patients who were admitted to the Muğla Sıtkı Koçman University, Faculty of Dentistry, Department of Oral and Maxillofacial Radiology for dental complaints were registered. Among these patients, individuals who had routine biochemical tests at the same period in the Outpatient Clinics of Muğla Sıtkı Koçman University Training and Research Hospital were included in the study. The patients with at least 1 pulp stone on panoramic radiographs recorded as the “pulp stone group” while patients without any pulp stones were the “control group”. Demographic data and serum levels of calcium, PTH, vitamin D, and uric acid were retrospectively evaluated in both groups. Student t-test or Mann-Whitney U test was used to evaluate the differences between the groups.
Results
Among 151 patients, dental pulp stone was detected in 53.6% of patients, and 82.7% of these patients were female. Female sex and pulp stone formation were significantly associated (p = 0.001). The mean age of the pulp stone group was 43.9, while it was 39.9 in the control group, without any significant correlation between age and pulp stone (p > 0.05). Similarly, there were no significant differences in serum levels of PTH, vitamin D, uric acid and calcium between groups (p > 0.05).
Conclusions
According to the present study, the effect of dental factors rather than systemic factors should be considered primarily in pulp stone formation.
Keywords: Calcium, Parathyroid hormone, Pulp stone, Uric acid, Vitamin D
INTRODUCTION
Pulp stones are calcifications that consist the calcium phosphate, calcium carbonate, and magnesium phosphate, and can be observed in both the coronal and the root pulp of the tooth [1]. Having a prevalence of up to 50%, pulp stones are considered one of the most common dental anomalies, with varying sizes (large or microscopic size) and numbers (single or multiple) [2,3]. Although these calcifications are mostly perceived in permanent molars, they may be present in anterior teeth, primary teeth or unerupted teeth [4].
Although the exact etiology of pulp stones is still unclear, numerous studies have reported various potential factors for pulpal calcifications. The natural aging process, traumatic occlusion, orthodontic treatments, periodontal pathologies and caries lesions have been reported as causative factors in the literature [5]. Among these, the anomaly is primarily associated with caries and orthodontic treatment. It has been reported that the incidence of pulp stones in irritated pulp affected by long-standing irritants such as caries, and deep fillings is significantly high due to the self-repair mechanism of the pulp tissue [5,6]. Another principal theory regarding the etiology of the pulp stones suggests that the calcifications are caused by the changes in pulp blood flow due to orthodontic forces [7]. Because of their asymptomatic nature, the pulp stones are usually detected on the panoramic, bitewing, and periapical radiographs during routine radiographic examination. Pulp stones within the pulp chamber may be free, attached to the chamber wall or embedded into the dentine tissue, and have radiological appearances as round/ovoid, radiopaque, and small calcified masses [8].
The large pulp stones or the ones attached/embedded into the pulpal chamber cause problems in clinical practice, especially during endodontic treatment, because these calcified structures block access to canal orifices and prevent performing adequate root canal preparation. Consequently, many endodontic complications such as perforations, loss of tooth hard tissues, and loss of root canal working length that may lead to endodontic treatment failure may be observed [6].
Besides the endodontic importance of pulp stones, many current studies have focused on whether pulp stones may be a diagnostic marker for systemic diseases such as vascular calcifications, endocrine disorders, and renal diseases [5,9,10,11]. Although oral signs and symptoms of various systemic diseases have been already reported, the relationship between pulp stones and systemic factors is still controversial in literature [10]. Therefore, the aim of the present study is to investigate the relationship between the presence of the pulp stones and the biochemical markers that are related to mineralization.
MATERIALS AND METHODS
Ethical approval
This retrospective study was approved by the Ethics Committee of the Muğla Sıtkı Koçman University (approval no/date: 230009-21/5.03.2023), and was performed according to the Ethical Principles Declaration of Helsinki.
Study design and patient selection
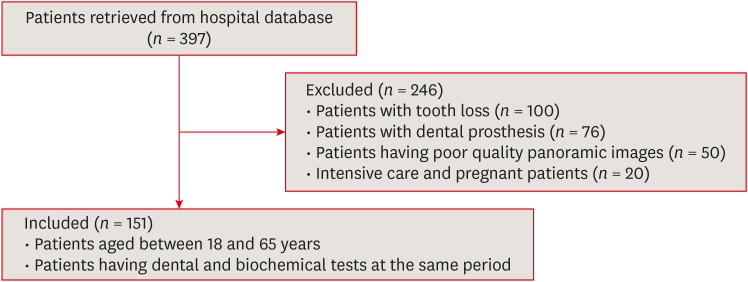
Patients who were radiologically examined in the Muğla Sıtkı Koçman University, Faculty of Dentistry, Department of Oral and Maxillofacial Radiology for any dental complaint between January 2022 and December 2022 were registered. Among these patients, individuals who had vitamin D, parathyroid hormone (PTH), calcium, and uric acid tests at the same period in the Outpatient Clinics of the Muğla Sıtkı Koçman University Training and Research Hospital were included in the study. The maximum time allowed between dental examination and biochemical tests of patients was determined as 3 months. In our hospital, the levels of vitamin D (ng/mL) and PTH (pg/mL) were determined by the electrochemiluminescent method, calcium (mg/dL) and uric acid (mg/dL) levels were determined by enzymatic methods on a COBAS 8000 (c702) biochemical analyzer (Roche Diagnostics GmbH, Mannheim, Germany). After 397 patients were evaluated according to the workflow diagram (Figure 1), 151 patients were included in the study. Patients with tooth loss or with poor-quality panoramic images were excluded from the study. Because the presence of fixed prosthesis prevents the radiological examination of the pulp chamber, patients with prosthetic rehabilitations were excluded from the study. Additionally, pregnant patients were not included in the study because hormonal and metabolic changes during pregnancy affect the biochemical profiles of patients and unstable blood values depending on the pregnancy period may change our findings. Similarly, intensive care unit patients were not included in the study because it could not be estimated how acute deteriorations in blood biochemical parameters related to progressive diseases might affect our findings.
Figure 1. Flowchart diagram showing patient selection criteria.
Radiological examination
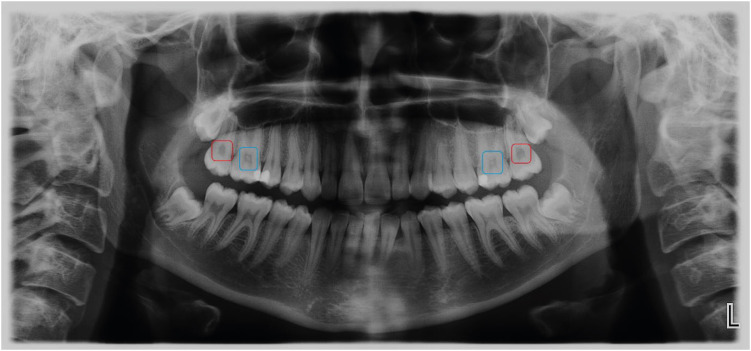
The panoramic images requested for dental diagnostic purposes were obtained by using the Planmeca ProOne Digital Panoramic X-Ray Machine with 60–70 kVp, 2–7 mA and 2–10 seconds exposure time (Planmeca Group, Helsinki, Finland). The radiographic images were evaluated under standard radiological examination conditions according to the presence or absence of pulp stones (Figure 2). On the panoramic images, the patients having a pulp stone in at least 1 tooth were recorded as the “pulp stone group” while patients who had no pulp stone in any teeth were the “control group”. Considering that the presence of pulp stones within the pulps of the extracted teeth may influence the findings of the present study, the patients with tooth loss (except third molars) were excluded from the study.
Figure 2. Blue and red boxes on the panoramic image show teeth with or without pulp stone, respectively.
All evaluations were performed by single oral and maxillofacial radiologist with 10 years of experience. In order to test the intraobserver agreement, randomly selected images were evaluated twice with an interval of 1 month.
Statistical analysis
Demographic data of all patients were collected from the hospital information system. Serum levels of calcium, PTH, vitamin D, and uric acid were retrospectively evaluated. Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Demographic data were analyzed using descriptive analysis. Data were given as percentage, rate, mean, and standard deviation. According to the conformity of the data to the normal distribution, Student t-test or Mann-Whitney U test was used to evaluate the differences between the pulp stone group and the control group. The chi-square test was used to determine the association between categorical variables. Cohen's kappa coefficient (k) was used to measure intraobserver agreement. The sample size and power calculations were carried out using the G*Power software version 3.1 (Düsseldorf University, Düsseldorf, Germany). The effect size was determined as 0.85 and the sample size was 140. The level of significance was set at p = 0.05.
RESULTS
Among 151 patients who were enrolled in the study, dental pulp stone was detected in 81 patients (53.6%), while no pulp stone was observed in 70 patients (46.4%). Of the patients who had pulp stones, 83% were female and 17% were male. When the relationship between sex and the pulp stone formation was statistically evaluated, a significant association between female sex and the presence of pulp stone was observed (p = 0.001). The mean age of the pulp stone group was 44 ± 11 years, while it was 41 ± 11 years in the control group. There was no significant correlation between the age and the pulp stone formation (p > 0.05). In this study, serum levels of PTH, vitamin D, uric acid, and calcium in both groups were comparatively evaluated. There was no significant difference between the biochemical parameters of mineralization and pulp stone formation (p > 0.05, Table 1). According to the Kappa analysis and Landis-Koch scale, intraobserver agreement was determined as 0.98 and almost perfect, respectively.
Table 1. Comparison of calcium, PTH, vitamin D, and uric acid levels between groups.
| Variables | Pulp stone group (n = 81) | Control group (n = 70) | p value | |
|---|---|---|---|---|
| Sex | 0.001* | |||
| Male | 14 (17) | 30 (43) | ||
| Female | 67 (83) | 40 (57) | ||
| Age (yr) | 44 ± 11 | 41 ± 11 | 0.063† | |
| Calcium (mg/dL) | 9.50 ± 0.49 | 9.54 ± 0.49 | 0.551† | |
| PTH (pg/mL) | 39.8 ± 20.6 | 38.3 ± 20.9 | 0.468‡ | |
| Vitamin D (ng/mL) | 24.5 ± 12.5 | 24.7 ± 14.0 | 0.770‡ | |
| Uric acid (mg/dL) | 4.6 ± 2.1 | 4.6 ± 2.2 | 0.786‡ | |
Data are presented as mean ± standard deviation or number (%).
Bold p values indicate statistical differences between groups.
PTH, parathyroid hormone.
*Chi-square test; †Student t-test; ‡Mann-Whitney U test.
DISCUSSION
The general opinion in the literature is that the incidence of pulp stones usually increases in elderly persons due to the rise in predisposing factors for pulpal calcifications [1,3,12,13,14]. However, the findings of our study did not show any correlation between age and pulp stone formation, and this result was similar to those of the studies made by Kannan et al. [2], Ashutosh et al. [3], Turkal et al. [12], and Sisman et al. [15]. Akin to the relationship between age and pulp stone formation, there is no consensus in dental literature about the effect of sex on the frequency of pulp stone occurrence [12]. Some studies have reported higher pulp stone incidence in females than in males whereas others have failed to demonstrate the same result [2,14,15,16,17,18,19,20,21]. The results of the present study revealed that pulp stone incidence in the female group is significantly higher compared to the male group (p = 0.001).
In literature, there are a small number of studies investigating whether pulp stones occur as a consequence of biochemical changes. Up to date, any local or systemic mechanism related to pulp stone formation has not been still established in the literature [3]. Except for local dental mechanisms such as caries, orthodontic tooth movement, and occlusal trauma; there are various studies researching the role of systemic factors on pulp stone etiology [3,5,8,9,11,22,23,24,25,26,27,28,29]. In these studies, the relationship between pulp stones and systemic factors has been investigated using 2 different methods: some studies have comparatively evaluated the incidence of pulp stones in systemic disease and healthy groups [5,8,9,22,23,24,25,26,27]. On the other hand, various studies have examined whether biochemical alterations in the body have affected the pulp stone occurrence [3,5,11,29]. Some authors reported that pulp stones may be incidental findings of renal or ischemic heart diseases [2,4,5,9,23,25,26,29,30]. However, some others stated a positive correlation between systemic disease and pulp stone formation, and even announced that pulp stones may be used as a diagnostic marker of renal and arterial calcifications [8,10,22,27].
The comparison of serum/salivary biochemical parameters of people with and without pulp stones mostly focused on the calcium values due to the presence of hypercalcemia in renal stones [3,5,11,29]. As observed in the present study, most of these studies have not confirmed a statistical correlation between serum/saliva calcium levels and pulp stone formation [3,11,29]. Only 1 study performed in hemodialysis patients showed that serum calcium levels of patients with pulp stones were statistically higher than those of the control group [5]. Excluding this study, the results of the present study were consistent with the literature.
The correlation between PTH which is the main cause of hypercalcemia and pulp stone formation was evaluated in the present study [31]. When PTH levels in patients with and without pulp stones were compared, no significant correlation was observed between these groups. In the literature, only 1 study investigated the effect of PTH levels on pulp stone formation in chronic hemodialysis patients [5]. The authors have disclosed higher serum PTH levels in patients with pulp stones, however, the results of the present study did not support those findings [5].
Recent papers reported the escalation of dental problems such as periodontitis and caries with Vitamin D deficiency [32,33]. Although no study has investigated the incidence of pulpal calcification in hypervitaminosis D, only 1 case report mentioned focal pulp calcifications in maxillary permanent central incisors in hypercalcemia, which was due to secondary to excess vitamin D because of excessive milk consumption [34]. The results of the current study have not verified those of the case report, and large-scale clinical studies are required to clarify the effect of vitamin D on pulp stone etiology.
There were few studies evaluating uric acid levels in the literature [5,11]. One of these studies compared the serum uric acid level in hemodialysis patients with and without pulp stones, while the other analyzed the salivary uric acid level in patients requiring dental treatment [5,11]. The findings of the present study endorsed the results of Gunen Yılmaz et al. [5], and granted no significant differences between uric acid levels in patients with and without pulp stones.
The single-center design of the study and retrospective design may be regarded as limitations of this study. Therefore, the results of our study might not be applicable to other populations. Further multicenter prospective studies including many populations may provide more accurate results reflecting the relationship between biochemical parameters and pulp stone formation.
CONCLUSIONS
Although the etiology of pulp stones is not clearly known, the findings of our study showed that there was a significant relation between sex and pulp stone formation in favor of the female sex. However, the levels of calcium, PTH, vitamin D, and uric acid were not significantly different between the patients with and without pulp stones in this study. These findings indicated the need to investigate the effect of local dental factors such as caries, occlusal trauma or orthodontic forces rather than the systemic factors on pulp stone formation.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Pelin Güneri for her language support and valuable advice.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Gürhan C.
- Data curation: Gürhan C, Saruhan E.
- Formal analysis: Saruhan E.
- Investigation: Gürhan C, Saruhan E.
- Methodology: Gürhan C, Saruhan E.
- Project administration: Gürhan C, Saruhan E.
- Writing - original draft: Gürhan C.
- Writing - review & editing: Gürhan C, Saruhan E.
References
- 1.Jain P, Patni P, Hiremath H, Jain N. Successful removal of a 16 mm long pulp stone using ultrasonic tips from maxillary left first molar and its endodontic management. J Conserv Dent. 2014;17:92–95. doi: 10.4103/0972-0707.124170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan S, Kannepady SK, Muthu K, Jeevan MB, Thapasum A. Radiographic assessment of the prevalence of pulp stones in Malaysians. J Endod. 2015;41:333–337. doi: 10.1016/j.joen.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Ashutosh H, Pragya H, Divya KJ, Ramniwas K, Sharad P, Mansoor S. Correlation between pulp stones and serum calcium levels: a preliminary study. Int J Health Clin Res. 2021;4:145–147. [Google Scholar]
- 4.Patil S, Sinha N. Pulp stone, haemodialysis, end-stage renal disease, carotid atherosclerosis. J Clin Diagn Res. 2013;7:1228–1231. doi: 10.7860/JCDR/2013/5087.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunen Yilmaz S, Yilmaz F, Bayrakdar IS, Harorli A. The relationship between carotid artery calcification and pulp stone among hemodialysis patients: a retrospective study. Saudi J Kidney Dis Transpl. 2019;30:755–763. doi: 10.4103/1319-2442.265449. [DOI] [PubMed] [Google Scholar]
- 6.Goga R, Chandler NP, Oginni AO. Pulp stones: a review. Int Endod J. 2008;41:457–468. doi: 10.1111/j.1365-2591.2008.01374.x. [DOI] [PubMed] [Google Scholar]
- 7.Ertas ET, Veli I, Akin M, Ertas H, Atici MY. Dental pulp stone formation during orthodontic treatment: a retrospective clinical follow-up study. Niger J Clin Pract. 2017;20:37–42. doi: 10.4103/1119-3077.164357. [DOI] [PubMed] [Google Scholar]
- 8.S N, Chandran A, B S, S G, A M, Muddebihal F, et al. Pulp stones: diagnostic significance in early diagnosis and radiographic correlation with ischemic heart diseases. Indian J Radiol Imaging. 2021;31:277–283. doi: 10.1055/s-0041-1731829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil SR. Prevalence of and relationship between pulp and renal stones: a radiographic study. J Oral Biol Craniofac Res. 2015;5:189–192. doi: 10.1016/j.jobcr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabardo MCL, Wambier LM, Rocha JS, Küchler EC, de Lara RM, Leonardi DP, et al. Association between pulp stones and kidney stones: a systematic review and meta-analysis. J Endod. 2019;45:1099–1105.e2. doi: 10.1016/j.joen.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Gabardo MCL, Kublitski PMO, Sette IR, Lauschner T, Juglair MM, Baratto-Filho F, et al. Sialometric and sialochemical analysis in individuals with pulp stones. Front Cell Dev Biol. 2020;8:403. doi: 10.3389/fcell.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turkal M, Tan E, Uzgur R, Hamidi M, Colak H, Uzgur Z. Incidence and distribution of pulp stones found in radiographic dental examination of adult Turkish dental patients. Ann Med Health Sci Res. 2013;3:572–576. doi: 10.4103/2141-9248.122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanauskaitė D, Kubiliūtė D, Janavičienė D, Brukienė V. Prevalence of pulp stones in molars based on bitewing and periapical radiographs. Stomatologija. 2021;23:9–15. [PubMed] [Google Scholar]
- 14.Gulsahi A, Cebeci AI, Ozden S. A radiographic assessment of the prevalence of pulp stones in a group of Turkish dental patients. Int Endod J. 2009;42:735–739. doi: 10.1111/j.1365-2591.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- 15.Sisman Y, Aktan AM, Tarim-Ertas E, Ciftçi ME, Sekerci AE. The prevalence of pulp stones in a Turkish population. A radiographic survey. Med Oral Patol Oral Cir Bucal. 2012;17:e212–e217. doi: 10.4317/medoral.17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravanshad S, Khayat S, Freidonpour N. The prevalence of pulp stones in adult patients of Shiraz dental school, a radiographic assessment. J Dent (Shiraz) 2015;16:356–361. [PMC free article] [PubMed] [Google Scholar]
- 17.Jannati R, Afshari M, Moosazadeh M, Allahgholipour SZ, Eidy M, Hajihoseini M. Prevalence of pulp stones: a systematic review and meta-analysis. J Evid Based Med. 2019;12:133–139. doi: 10.1111/jebm.12331. [DOI] [PubMed] [Google Scholar]
- 18.Ranjitkar S, Taylor JA, Townsend GC. A radiographic assessment of the prevalence of pulp stones in Australians. Aust Dent J. 2002;47:36–40. doi: 10.1111/j.1834-7819.2002.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 19.Ravichandran S, Vadivel JK. Prevalence of pulp stones in IOPA radiographs. J Adv Pharm Technol Res. 2022;13:S63–S66. doi: 10.4103/japtr.japtr_126_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CY, Wu YC, Su CC, Chung MP, Huang RY, Ting PY, et al. The prevalence and distribution of radiopaque, calcified pulp stones: a cone-beam computed tomography study in a northern Taiwanese population. J Dent Sci. 2018;13:138–144. doi: 10.1016/j.jds.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu SJ, Swarnalatha C, Rao AP, Kumar BB, Tilak BP, Naidu RB, et al. Pulp stones as risk predictors for coronary artery disease. Int J Prev Med. 2020;11:7. doi: 10.4103/ijpvm.IJPVM_68_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleksova P, Serafimoski V, Popovska M, Ristovski M. Pulp stones can help in detection of calculus in the kidneys and/or in the bile--fact or fiction? Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2013;34:159–167. [PubMed] [Google Scholar]
- 23.Tarim Ertas E, Inci M, Demirtas A, Ertas H, Yengil E, Sisman Y, et al. A radiographic correlation between renal and pulp stones. West Indian Med J. 2014;63:620–625. doi: 10.7727/wimj.2013.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galav A, Vyas T, Kaur M, Chauhan M, Satija N, et al. Association of pulp stones & renal stones- a clinical study. Int J Res Health Allied Sci. 2018;4:82–84. [Google Scholar]
- 25.Kumar T, Puri G, Aravinda K, Laller S, Jatti D, Gupta R. Correlation between prevalence of pulp stones and renal stones in Panchkula region of India. SRM J Res Dent Sci. 2015;6:150–154. [Google Scholar]
- 26.Movahhedian N, Haghnegahdar A, Owji F. How the prevalence of pulp stone in a population predicts the risk for kidney stone. Iran Endod J. 2018;13:246–250. doi: 10.22037/iej.v13i2.18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moudi E, Kazemi A, Madani Z, Haghanifar S, Moudi E. A radiographic correlation between the presence of pulp stones and kidney stones. Casp J Appl Sci Res. 2015;4:1–7. [Google Scholar]
- 28.Jawahar G, Rao GN, Vennila AA, Fathima SD, Lawanya MKK, Doss DM, et al. Clinicopathological correlation of pulp stones and its association with hypertension and hyperlipidemia: an hospital-based prevalence study. J Pharm Bioallied Sci. 2021;13:S1268–S1274. doi: 10.4103/jpbs.jpbs_475_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeluri G, Kumar CA, Raghav N. Correlation of dental pulp stones, carotid artery and renal calcifications using digital panoramic radiography and ultrasonography. Contemp Clin Dent. 2015;6:S147–S151. doi: 10.4103/0976-237X.166837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kansu O, Ozbek M, Avcu N, Aslan U, Kansu H, Gençtoy G. Can dental pulp calcification serve as a diagnostic marker for carotid artery calcification in patients with renal diseases? Dentomaxillofac Radiol. 2009;38:542–545. doi: 10.1259/dmfr/13231798. [DOI] [PubMed] [Google Scholar]
- 31.Auron A, Alon US. Hypercalcemia: a consultant’s approach. Pediatr Nephrol. 2018;33:1475–1488. doi: 10.1007/s00467-017-3788-z. [DOI] [PubMed] [Google Scholar]
- 32.Botelho J, Machado V, Proença L, Delgado AS, Mendes JJ. Vitamin D deficiency and oral health: a comprehensive review. Nutrients. 2020;12:1471. doi: 10.3390/nu12051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uwitonze AM, Rahman S, Ojeh N, Grant WB, Kaur H, Haq A, et al. Oral manifestations of magnesium and vitamin D inadequacy. J Steroid Biochem Mol Biol. 2020;200:105636. doi: 10.1016/j.jsbmb.2020.105636. [DOI] [PubMed] [Google Scholar]
- 34.Giunta JL. Dental changes in hypervitaminosis D. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:410–413. doi: 10.1016/s1079-2104(98)90066-x. [DOI] [PubMed] [Google Scholar]