Abstract
It is well known that hepatitis B virus infections can be transient or chronic, but the basis for this dichotomy is not known. To gain insight into the mechanism responsible for the clearance of hepadnavirus infections, we have performed a molecular and histologic analysis of liver tissues obtained from transiently infected woodchucks during the critical phase of the recovery period. We found as expected that clearance from transient infections occurred subsequent to the appearance of CD4+ and CD8+ T cells and the production of interferon gamma and tumor necrosis factor alpha in the infected liver. These events were accompanied by a significant increase in apoptosis and regeneration of hepatocytes. Surprisingly, however, accumulation of virus-free hepatocytes was delayed for several weeks following this initial influx of lymphocytes. In addition, we observed that chronically infected animals can exhibit levels of T-cell accumulation, cytokine expression, and apoptosis that are comparable with those observed during the initial phase of transient infections. Our results are most consistent with a model for recovery predicting replacement of infected hepatocytes with regenerated cells, which by unknown mechanisms remain protected from reinfection in animals that can be cured.
Human hepatitis B virus (HBV), as well as the related woodchuck hepatitis virus (WHV), can cause transient or chronic infections in its native host (11, 24, 27). The molecular basis for the dichotomy of disease outcomes is not known. As in humans, in woodchucks chronic, lifelong WHV infections generally occur when virus is transmitted during or soon after birth. Infection of adults leads to transient infections in over 90% of cases. Experiments with woodchucks have shown that clearance of infections can occur within a few weeks even when nearly all hepatocytes in the liver have been infected (14, 20). Thus, a major question concerns the molecular mechanism responsible for the regulation of clearance of virus from infected hepatocytes.
Clearance from infections with noncytopathic viruses, such as hepadnaviruses, requires the elimination of infected cells by cytotoxic T lymphocytes (CTLs) and the production of neutralizing antibodies directed against one or several viral proteins (13). A role for T cells in the recovery from natural hepadnavirus infections has been demonstrated through treatment with cyclosporin A, a known suppressor of T-cell function, which prevents recovery from otherwise transient WHV infections in adult woodchucks (4). It also appears that the number of CTLs present in the peripheral blood of chronically infected patients is approximately 10 to 100 times lower than that in the blood of patients with transient infections (23), suggesting that a critical number of reactive CTLs are required for recovery. In this scenario all infected hepatocytes would have to be killed by CTLs and replaced by uninfected cells. In order to sustain sufficient liver function, the rate of cell death should not exceed the rate of cell replacement over a prolonged time period. Moreover, replaced hepatocytes have to be protected from virus produced by cells that are still infected.
Observations made for chronic HBV carriers that presented with hepatitis A virus or hepatitis D virus superinfections revealed that HBV titers can decline during the recovery phase of the superinfection, suggesting that certain nonspecific mediators of the immune response, such as cytokines, can suppress HBV replication and may protect hepatocytes from de novo infection or reinfection (5, 15, 26). This view has been supported by experiments with transgenic mice expressing HBV that demonstrated that alpha interferon (IFN-α) and IFN-γ, as well as tumor necrosis factor alpha (TNF-α), can suppress, at least temporarily, HBV titers by at least one order of magnitude (6, 7). Thus, it is conceivable that CTL-mediated killing combined with a concomitant inhibition of replication by cytokines is critical for recovery from transient infections. It is also probable that virus-neutralizing antibodies, which usually but not always arise late in infection, are key mediators of recovery (12).
To gain insight into the mechanism responsible for the clearance of natural hepadnavirus infections, we have performed a molecular and histologic analysis of liver tissues obtained from transiently and chronically infected woodchucks. The purpose of this study was threefold. First, to document the kinetic profile of selected immunological markers during the course of natural, transient hepadnavirus infections; second, to investigate the fate of hepatocytes during the recovery phase, i.e., to investigate whether infected hepatocytes are removed from the liver or cured of replicating virus; and third, to determine whether transient and chronic infections exhibit obvious differences that could help in defining the molecular parameters controlling the outcome of an infection.
MATERIALS AND METHODS
Infection of woodchucks with WHV.
Experiments with woodchucks were reviewed and approved by the Institutional Animal Care and Use Committee of the Fox Chase Cancer Center. Woodchucks negative for serological markers of WHV infection were purchased from North-Eastern Wildlife (South Plymouth, N.Y.). Woodchucks were infected intravenously with 7 ml of WHV-positive serum containing 1010 virions/ml, obtained from a chronically infected animal.
Woodchucks were bled and subjected to liver biopsies several weeks before WHV inoculation and at various intervals postinfection (p.i.). Blood collections and liver biopsies (preinfection and 4 weeks p.i.) were performed as described by Kajino et al. (14). Additional liver needle biopsies were collected at the indicated time points. Sera were stored at −80°C. Liver biopsy specimens were divided into two parts. One aliquot was frozen in liquid nitrogen and stored at −80°C for the extraction of nucleic acids; another aliquot was fixed in acetic acid-ethanol (1:3), as previously described (14). The procedures for fixation, paraffin embedding, and subsequent processing of tissue sections have been described previously (12).
Tissue samples from four chronically infected woodchucks (4705, 4707, 4714, and 4723) and two uninfected control animals (2598 and 2732) were a kind gift from Bud C. Tennant (Cornell University, Ithaca, N.Y.).
Analysis of WHV DNA in serum and liver tissues.
To determine the virus titers in sera of WHV-infected woodchucks, 5 μl of serum was spotted onto a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.). The membrane was dried and then soaked in a solution of 0.5 N NaOH and 1.5 M NaCl for 15 min and neutralized in a solution of 1 M Tris-HCl (pH 7.0) and 1.5 M NaCl for 15 min. Viral DNA was immobilized by baking the membrane at 80°C for 2 h. To detect viral DNA, the membrane was incubated overnight at 42°C in a hybridization solution containing 50% formamide, 5× SSC (0.75 M sodium chloride, 0.075 M sodium citrate [pH 7.0]), 1× Denhardt's solution (0.2 mg of Ficoll per ml, polyvinylpyrrolidone, bovine serum albumin), 20 mM sodium phosphate (pH 6.8), 0.2% sodium dodecyl sulfate (SDS), 10 μg of yeast RNA per ml, 5 mM EDTA, 50 μg of salmon sperm DNA per ml, and 32P-labeled WHV DNA and then washed twice in 0.1× SSC at 65°C for 20 min. The hybridization signals were quantified with a Fuji BAS 1000 Bioimaging system, and the amount of viral DNA was determined with the help of plasmid DNA standards.
Isolation and culture of PBMC.
Six milliliters of blood drawn from a WHV-negative woodchuck was diluted with an equal volume of phosphate-buffered saline and layered on 12 ml of a Ficoll-Hypaque solution (Pharmacia Biotech, Piscataway, N.J.). The sample was centrifuged at 400 × g for 30 min at room temperature. The platelets and lymphocytes were transferred to a new centrifuge tube, and the cells were washed twice with Hank's balanced salt solution. Approximately 106 peripheral blood mononuclear cells (PBMC) were cultured in 2 ml of complete RPMI 1640 medium containing concanavalin A (5 U/ml) for 12 to 24 h at 37°C in a humidified incubator containing 5% CO2.
Isolation of RNA.
The cultured PBMC and liver specimens were lysed or homogenized with 1 ml of Tri-Reagent solution (Molecular Research Center, Inc., Cincinnati, Ohio). After the addition of 0.2 ml of chloroform, the mixture was centrifuged at 12,000 × g for 10 min at 4°C in an Eppendorf centrifuge. Following centrifugation, the aqueous phase containing the RNA was transferred to a new tube and the RNA was precipitated with 0.7 volumes of isopropanol. The RNA pellets were resuspended in diethylpyrocarbonate-treated water. The concentration of the RNA was determined with a UV spectrophotometer.
Cloning of woodchuck T-cell markers and cytokines.
cDNA was synthesized from 1 μg of total RNA isolated from PBMC and 10 μg of total RNA isolated from liver with Superscript II RNase H− reverse transcriptase (GIBCO-BRL, Grand Island, N.Y.) in a 20-μl volume, with the reaction carried out at 42°C for 1 h. cDNAs were stored at −20°C. For the amplification of T-cell markers and cytokines 1/20 of the cDNA solution was added to the PCR mixture. The PCRs were carried out in a 25-μl reaction mixture with the Advantage cDNA PCR kit (Clontech, Palo Alto, Calif.) in a Gene-Amp 2400 thermal cycler (Perkin-Elmer, Norwalk, Conn.). The PCR annealing temperatures selected varied depending on the primers selected for amplification. The primers for T-cell markers and cytokines were designed based on available nucleotide sequences from human and mouse homologues. Amplification for 30 cycles generally yielded the expected DNA products. Amplified DNA fragments were isolated from agarose gels by using the QIAEX II gel extraction kit (Qiagen Inc., Valencia, Calif.) and cloned into the pGEM-TEasy vector (Promega, Madison, Wis.). The cloned DNA fragments were sequenced with an automatic DNA sequencer. The nucleotide sequences of the cloned woodchuck cDNAs were submitted to GenBank.
Reverse transcription-PCR (RT-PCR) assay.
The linear range of the DNA amplification reaction was determined with serial dilutions of the cDNA reaction mixtures and through variations in the number of DNA amplification cycles. To quantitate mRNAs for the T-cell markers CD3, CD4, and CD8 and the cytokines IFN-γ and TNF-α, 0.5 μl of each cDNA reaction mixture was amplified for 25 cycles. For the amplification of actin mRNA, the cDNA reaction was diluted 2,500-fold and 10 μl of the diluted sample was amplified with 25 cycles.
To quantitate the amplified DNA products, a 5-μl aliquot of each reaction mixture was electrophoresed through 1.5% agarose gels and transferred to nylon membranes (Amersham) in a solution of 20× SSC. The blots were hybridized with 5′ end-labeled oligonucleotides at 42°C overnight in a buffer containing 50% formamide, 5× SSC, 1× Denhardt's solution, 20 mM sodium phosphate (pH 6.8), 0.2% SDS, 5 mM EDTA, 10 μg of yeast RNA per ml, and 50 μg of salmon sperm DNA per ml. The oligonucleotides were labeled at their 5′ ends with [γ-32P]ATP (3,000 Ci/mmol; NEN, Boston, Mass.) and with T4 polynucleotide kinase (GIBCO-BRL). Following the hybridization reaction, the blots were washed twice in 2× SSC at 60°C. The hybridization signals were quantified with a Fuji BAS 1000 Bioimaging system.
RNase protection assay.
Total RNA was extracted from frozen liver biopsy samples with Tri-Reagent (Molecular Research Center, Inc.), following the manufacturer's directions. RNase protection assays for the analysis of cytokine and T-lymphocyte transcripts was carried out essentially as described by Hobbs et al. (10). Briefly, the antisense RNA probes for TNF-α, IFN-γ, CD4, CD8, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were synthesized by using NcoI or AvaII linearized templates, SP6 RNA polymerase, and the Riboprobe Gemini II system (Promega) as recommended by the manufacturer. A 20-μl in vitro transcription reaction mixture contained 60 μCi [α-32P]UTP, 12 μM UTP, 500 μM (each) GTP, ATP, and CTP, 10 mM dithiothreitol, 1× transcription optimized buffer, 24 U of RNAsin, 20 U of SP6 RNA polymerase, and 1 μg of linearized template DNA. After 1 h of incubation at 39°C, the mixture was treated with 2 U of RNase-free DNase I (RQ1; Promega) at 37°C for 15 min. The reaction mixture was extracted with phenol:chloroform, and RNA was precipitated with ethanol. The RNA pellet was dissolved in 30 μl of gel loading buffer (90% formamide and dye), heated for 3 min at 95°C, and loaded onto a 0.75-mm-thick 8 M urea–5% acrylamide gel. After electrophoresis, the gel was covered with plastic wrap and exposed to X-ray film for 2 min, and then the full-length probe was cut out and eluted from the gel with 400 μl of elution buffer (500 mM ammonium acetate, 1 mM EDTA, 0.2% SDS) at 37°C for 3 to 5 h. For hybridization, an aliquot containing 2 × 105 cpm of each probe was mixed with 10 μg of total liver RNA or yeast RNA and ammonium acetate was added to a final concentration of 0.5 M. The RNA was precipitated with 2.5 volumes of ethanol at −20°C for 30 min. The precipitated RNA was recovered by centrifugation for 15 min in a microcentrifuge at 4°C. After the ethanol was carefully removed, the pellets were allowed to dry for 5 min. The dried RNA pellets were resuspended in 10 μl of hybridization buffer (80% formamide, 100 mM sodium citrate [pH 6.4], 300 mM sodium acetate [pH 6.4], 1 mM EDTA), heated to 95°C for 5 min, and hybridized at 45°C for 12 h. Digestion of single-stranded RNA was done as described by Hobbs et al. (10). The protected RNA bands were quantitated with the help of a Fuji BAS 1000 imager.
Histopathology.
To detect CD3+ T cells, sections of acetic acid-ethanol-fixed liver tissue were first incubated with a rabbit polyclonal antibody raised against a peptide (amino acids 156 to 168) of the human CD3 epsilon chain (DAKO, Inc.) Core antigen-positive hepatocytes were detected with a rabbit serum raised against recombinant WHV core protein produced in Escherichia coli. Proliferating cell nuclear antigen (PCNA) in sections of acid-ethanol-fixed liver tissue was detected with a monoclonal antibody against PCNA (DAKO, Inc.). Biotinylated antibodies against rabbit and mouse immunoglobulin G (DAKO, Inc.) were used as secondary antibodies. Tissue sections were incubated with peroxidase-labeled streptavidin and developed with 0.5 mg of diaminobenzidine per ml in 0.03% hydrogen peroxide–phosphate-buffered saline. Sections were counterstained with hematoxylin, dehydrated in ethanol, and mounted with Permount.
Nucleotide sequence accession numbers.
The nucleotide sequence accession numbers for sequences submitted to GenBank were as follows: for CD3-γ, AF082493; for CD4, AF082497; for CD8, AF082499; for IFN-γ, AF081502; for TNF-α, AF082491; and for 2′-5′ oligoadenylate synthetase (2′-5′ OAS), AF082498.
RESULTS
Infection of woodchucks with WHV.
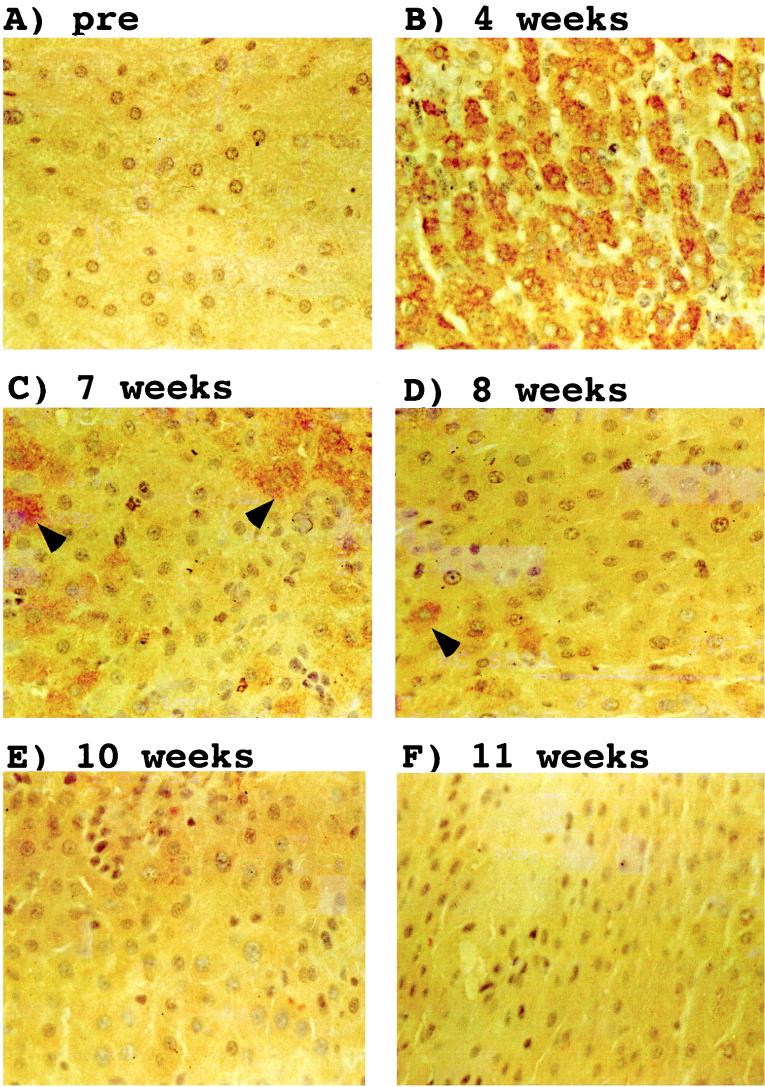
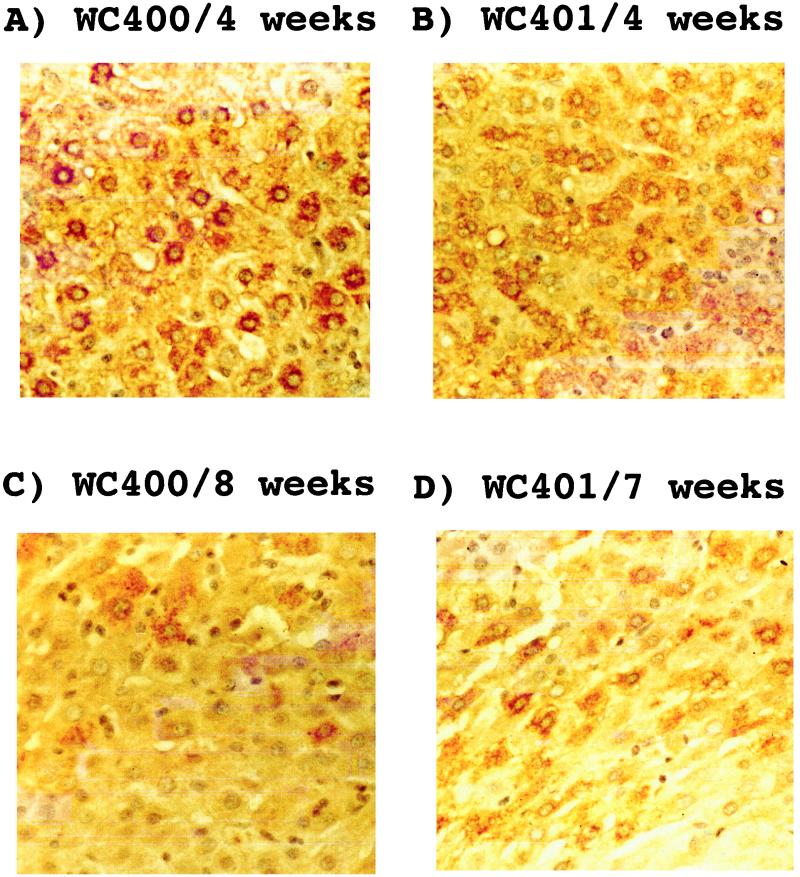
For this study we have used liver biopsy samples obtained from nine transiently WHV-infected woodchucks and one uninfected control animal. The course of the viremia observed with woodchucks 22, 35, 36, and 38, collectively referred to as cohort I, has been described previously (14). Infections of all four animals with approximately 1011 WHV particles led to a transient viremia after an incubation period of 1 to 2 weeks (Table 1). Liver biopsies were performed 4 weeks prior to infection and subsequent to infection as indicated in Table 1. Immunohistochemical analyses of liver samples suggested that in these animals every hepatocyte was infected during the peak of the viremic phase (14). Since the number of biopsy specimens available during the recovery phase was limited, a second experiment with five transiently infected woodchucks and an uninfected control animal was performed. These animals, designated cohort II, were infected, like cohort I, with approximately 1011 WHV particles obtained from a chronically infected, viremic woodchuck. The viremic phase in animals belonging to the second cohort was much shorter than that observed with the woodchucks in cohort I (Table 1). It lasted approximately 2 to 3 weeks in animals 400, 401, 402, and 403 and 5 weeks in woodchuck 405. Liver biopsies were obtained 4 weeks p.i., and then at the time points indicated in Table 1. Immunohistochemical analysis of liver specimens obtained 4 weeks p.i. with an antibody directed against the WHV core antigen showed that more than 95% of hepatocytes were infected in all five animals examined, similar to the observations made previously with cohort I animals (Fig. 1). As was observed for cohort I, core antigen persisted to at least 7 weeks p.i. and, except in the case of woodchuck 405, had disappeared by 10 weeks. Moreover, the availability of additional liver biopsies from cohort II during the recovery phase allowed us to confirm that core antigen-positive hepatocytes can disappear in a piecemeal rather than a global fashion (Fig. 2), as previously suggested (12, 14, 20).
TABLE 1.
Viremia in woodchucks infected with WHVa
| No. of weeks p.i. | Virus titer (108 virions/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort I
|
Cohort II
|
|||||||||
| 22 | 35 | 36 | 38 | 400 | 401 | 402 | 403 | 405 | 406 | |
| 0 | — (B) | — (B) | — (B) | — (B) | — (B) | — (B) | — (B) | — (B) | — (B) | — (B) |
| 1 | 2 | 1.4 | — | — | — | 2.1 | 5 | — | 1.8 | — |
| 2 | 13 (B) | 24 | 2 | 0.3 | 1.7 | 20 | 115 | 4 | 17 | — |
| 3 | 28 | 38 (B) | 27 (B) | 5.2 (B) | 30 | 1.7 | — | 76 | 850 | — |
| 4 | 37 | 16 | 14 | — (B) | — (B) | — (B) | 4.5 (B) | 40 (B) | — (B) | |
| 5 | 29 | 29 | 12 | 4.5 | — | — | — | — | 5 | — |
| 6 | 33 (B) | 10 | 13 | 4.0 | — | — | — | — | — | — |
| 7 | 9 | 3 (B) | 6 (B) | 4.8 (B) | B | B | B | |||
| 8 | 9 | 0.6 | 2 | 5.9 | B | B | ||||
| 9 | 2 | 0.1 | — | 5.6 | ||||||
| 10 | B | — | — | 3.9 | B | B | B | B | B | B |
| 11 | — | — (B) | — (B) | 4.2 (B) | B | B | ||||
| 12 | — | |||||||||
| 13 | 1.5 | |||||||||
| 14 | — | |||||||||
| 15 | 0.7 | |||||||||
| 16 | 0.03 | |||||||||
| 17 | B | — (B) | ||||||||
| 18 | — | |||||||||
| 19 | B | |||||||||
| 21 | — (B) | |||||||||
| 22 | B | B | ||||||||
| 29 | B | |||||||||
Virus titers of cohort I animals were determined by Southern blot analysis of virion DNA extracted from virus particles that were pelleted from serum samples by ultracentrifugation (14). Virus titers of cohort II animals were determined by dot blot hybridization analysis of 5 μl of serum. —, below the level of detection; B, liver biopsy was performed at time point indicated.
FIG. 1.
Resolution of the transient WHV infection from the liver of woodchuck 403. Sections from ethanol-acetic acid-fixed liver tissues obtained at the indicated time points were reacted with a rabbit WHV core antigen antibody. The antibody was detected by staining with immunoperoxidase. Hepatocytes that express core antigen are marked with arrows. Magnification, ×388. pre, preinfection.
FIG. 2.
Piecemeal recovery from transient WHV infections in woodchucks 400 and 401. Liver sections were treated and processed as described in the legend to Fig. 1. Magnification, ×400.
Immune response during transient WHV infections.
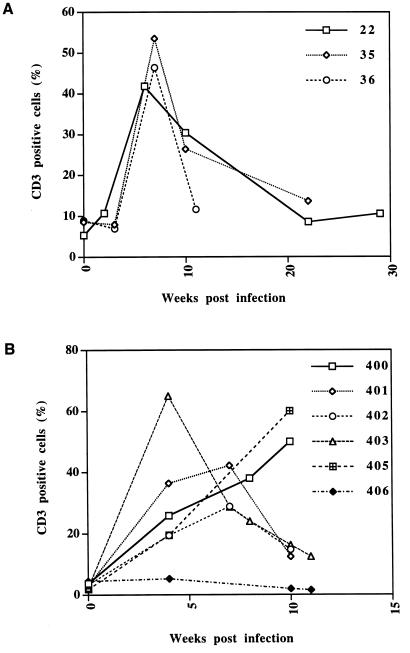
To obtain a first estimate of the vigor of the immune response in transient infections, we performed an immunohistologic analysis with a rabbit antibody raised against a peptide of human CD3ɛ, which also recognizes the woodchuck homologue (17). This analysis showed that viremia in cohort I animals was accompanied by an influx of CD3+ lymphocytes into the liver that began less than 2 weeks p.i. and reached higher levels prior to and during the recovery phase (Fig. 3A and Table 1). In the biopsies taken closest to the presumed peak of the infection, approximately one CD3+ lymphocyte for every two to three hepatocytes within the lobule, not including lymphocytes in the portal tracts, was observed. Similar results were obtained with cohort II (Fig. 3B). Thus, a major burst in the immune response to the hepatic infection apparently occurs under the selected infection conditions between 2 and 4 weeks p.i.
FIG. 3.
Infiltration of CD3+ T cells into the livers of WHV-infected woodchucks. The graphs indicate the number of CD3+ cells, not including those in the portal tract, per hepatocyte during the transient infections in woodchucks belonging to cohort I (A) and cohort II (B).
Since immunological reagents to characterize T-cell subsets in woodchucks were not available, we isolated relevant woodchuck-specific cDNA clones for RNase protection and RT-PCR assays (see Materials and Methods). These reagents were then used to monitor the presence of the T-cell subsets CD4+ and CD8+ and the expression of IFN-γ and TNF-α, both believed to play a major role in T-cell immunity and antiviral response (22). IFN-γ is expressed in response to antigen stimulus primarily by CD8+ T cells but also by CD4+ T cells of the Th1 subtype. TNF-α is mainly produced in macrophages or the related Kupffer cells in the liver in response to cytokines such as IFN-γ.
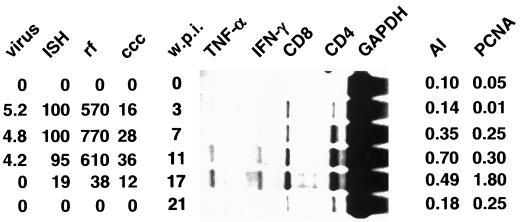
RNase protection analysis with RNA isolated from liver biopsy samples obtained from woodchuck 38 revealed an influx of CD4+ and CD8+ T cells within the first 3 weeks of infection (Fig. 4). The highest levels of both markers coincided with the time of recovery, which began between 11 and 17 weeks p.i. (Table 1). Expression of the cytokines IFN-γ and TNF-α coincided with the observed increase in the levels of the two T-cell subsets and reached a peak at 17 weeks p.i. At week 21, when the animal had recovered from the infection, all four markers declined to nearly normal levels, with the levels observed prior to the infection being considered normal.
FIG. 4.
Transient WHV infection in woodchuck 38. The left side of the figure shows the results from the analysis of WHV DNA markers during transient WHV infection as determined previously by Kajino et al. (14). The types of values shown in the four leftmost columns are as follows: virus, the number of virions per milliliter of serum (multiplied by 10−9); ISH, the fraction (percentage) of hepatocytes that showed a positive signal in an in situ hybridization (ISH) assay with a WHV probe; and rf and ccc, the copy numbers of replicative form (rf) and cccDNA, respectively, relative to the total number of hepatocytes. The central panel shows the results from the RNase protection analysis with RNA obtained at the indicated number of weeks p.i. (w.p.i.). The right side shows the results from the histological examination of liver sections for apoptotic hepatocytes and for hepatocytes with nuclear PCNA staining. The columns contain the following types of values: the AI, the fraction of hepatocytes that showed morphological signs of apoptosis, and the fraction of hepatocytes with nuclei that were positive for PCNA, respectively.
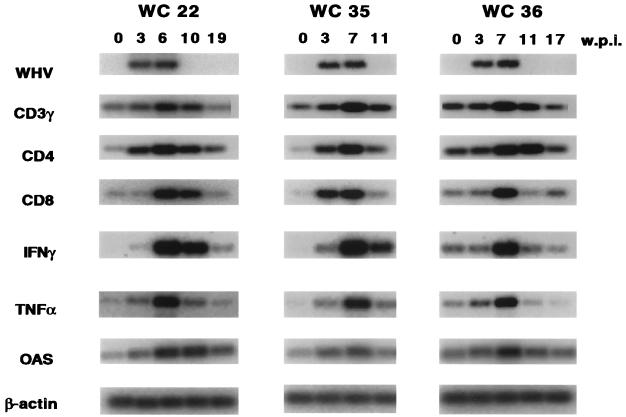
The results obtained with woodchuck 38 were confirmed by RT-PCR analysis of three additional animals in cohort I (Fig. 5). In addition to the four genes monitored with the RNase protection assay, we determined the expression levels of CD3-γ and 2′-5′ OAS, which is induced by IFN-α. RT-PCR analysis showed that the increase of CD3 mRNA from preinfection to the peak of the infection ranged from approximately sixfold in animal 22 to ninefold in animal 35. Animal 36 exhibited only a three- to fourfold increase during the peak of the infection but had a relatively high CD3 mRNA level prior to the WHV infection. This woodchuck most likely had an unrelated infection at the time it was inoculated with WHV. The presence of CD4+ T cells correlated well with the presence of CD3+ T cells. Animals 22 and 36 exhibited approximately 9- and 20-fold increases of CD4 mRNA, respectively, whereas animal 36 showed only a 3- to 4-fold increase. Accumulation of CD8+ T cells occurred in two woodchucks, 22 and 36, after the accumulation of CD4 cells, whereas in woodchuck 35 both T-cell subsets appeared at the same time. The increase of CD8+ cells was the most pronounced among the three markers, reaching levels greater than preinfection levels by approximately 13- and 17-fold for woodchucks 22 and 35, respectively, and by approximately fivefold for woodchuck 36. Of note is that in all three animals, the highest levels of CD8 mRNAs were observed at time points when all hepatocytes were still infected and viral DNA was still present in the livers and sera of these animals (Table 1 and Fig. 5) (14).
FIG. 5.
Analysis of WHV and cellular mRNAs in transiently infected woodchucks by RT-PCR. The results of the RT-PCR analysis to detect mRNAs corresponding to WHV, CD3-γ, CD4, and CD8-α and the cytokines IFN-γ and TNF-α and for 2′-5′ OAS and β-actin (details of the procedures are described in Materials and Methods) are shown. w.p.i., weeks p.i.; WC, woodchuck.
We observed a good correlation between the patterns of IFN-γ and TNF-α and the presence of CD8+ T cells (Fig. 5). The relative increases measured in woodchucks 22 and 35 were more than 100-fold for IFN-γ and 23- to 25-fold for TNF-α. In woodchuck 36 the relative increases were less pronounced due to the elevated mRNA levels observed prior to the WHV infection. Thus, as observed with woodchuck 38 (Fig. 4) the apparent peak mRNA levels of both cytokines coincided with the highest levels of CD4 and CD8 mRNAs in the infected livers. All three animals examined also exhibited elevated mRNA levels specific for 5′-3′ OAS, which is induced in response to IFN-α, during the peak of the infection. 2′-5′ OAS levels were greater than preinfection levels in animals 22, 35, and 36 by eight-, five-, and threefold, respectively.
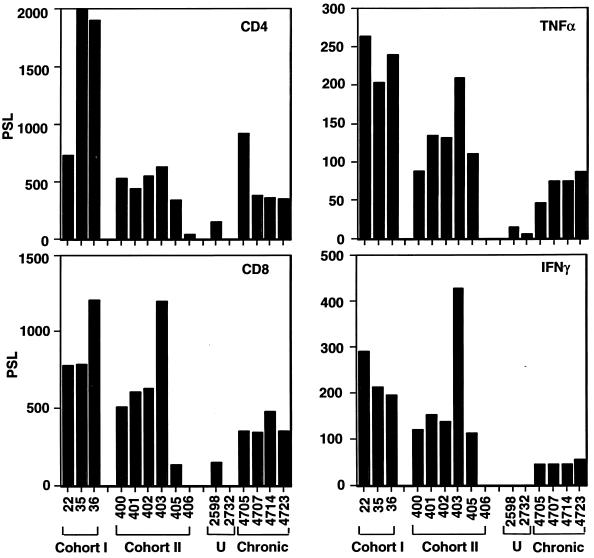
As was observed for cohort I, infections of all animals in cohort II, save for the uninfected control animal 406, were associated with elevations in both CD4 and CD8 mRNAs, as determined by RNase protection analysis (Fig. 6). The peak levels of cohort I animals, which were identified by RT-PCR (Fig. 5), were measured again by RNase protection assays (Fig. 6) to permit a direct comparison with those of cohort II animals. The CD4 and CD8 mRNA levels observed in cohort II animals at 4 weeks p.i. were still below the peak levels observed in cohort I animals at a later time point. An exception was animal 403, which exhibited CD8 mRNA expression levels very similar to those observed with woodchuck 36. TNF-α and IFN-γ levels were also elevated in woodchucks of cohort II 4 weeks p.i. With the exception of those in woodchuck 403, the levels of both cytokines were approximately 50% below the levels observed with cohort I animals at later times p.i. Animal 403 exhibited the highest levels of IFN-γ measured among all animals examined.
FIG. 6.
Analysis of cellular mRNAs in transiently and chronically infected woodchucks by RNase protection. The histograms summarize the results obtained from RNase protection experiments with woodchucks in cohorts I and II as well as two uninfected (U) woodchucks (2598 and 2732) and four chronically infected animals (4705, 4707, 4714, and 4723) (the probes used are indicated in the upper right corner of each histogram). Quantitation of the RNase protection analysis was performed with a phosphorimager. The values were expressed in arbitrary units (PSL).
In summary, these results demonstrated that during transient infections T cells can accumulate in the liver to varying levels, reaching up to two-thirds of the total number of hepatocytes. It appears that accumulation of T cells increases with the duration of the infection. Furthermore, the results showed that the presence of infiltrating lymphocytes in the livers of transiently and chronically infected animals is accompanied by elevated levels of TNF-α, IFN-γ, and, by inference, IFN-α.
Immune response in chronic carriers of WHV.
Examination of tissue sections from three chronically infected woodchucks revealed CD3+ T-cell levels ranging from 8 to 14% of the total hepatocyte population depending on the animal examined, which was below the levels observed with the cohort II animals at 4 weeks p.i. (Fig. 3B). However, RNase protection analysis revealed that three of the four chronically infected woodchucks exhibited CD4 mRNA levels very similar to those of cohort II animals at 4 weeks (Fig. 6). Woodchuck 4705 showed an approximately twofold increase compared to the other three animals. The CD8 mRNA levels of the chronically infected animals were approximately 25% below the levels observed in woodchucks 400, 401, and 402 (Fig. 6). The levels of both mRNAs were significantly higher than those observed with woodchuck 405, which showed delayed core antigen clearance, and with the uninfected control animals 406, 2598, and 2732. All four of the chronically infected woodchucks showed increased levels of the two cytokines compared to the three uninfected controls. While expression levels of TNF-α were in the same range as those observed with cohort II animals (4 weeks p.i.), save for animal 403, expression of IFN-γ was 50 to 75% reduced.
In summary, the results showed that chronically infected animals also accumulate CD4+ and CD8+ T cells in their livers, but that the levels of the CD8+ T-cell subsets appear to be generally lower than the peak levels reached in transiently infected animals. Furthermore, all chronically infected woodchucks displayed lower levels of TNF-α and especially IFN-γ than cohort I or II animals.
Increased apoptosis of hepatocytes during recovery from transient and chronic infections.
CD8+ T cells are known to exhibit a cytotoxic activity that causes apoptosis of infected target cells. To determine whether WHV-infected hepatocytes undergo programmed cell death, we conducted histopathologic examinations of tissue sections obtained from liver biopsies of woodchucks 38, 22, and 36 in cohort I, all six woodchucks in cohort II, the four chronically infected woodchucks, and two negative controls. Moreover, to assess whether the increased level of hepatocyte apoptosis observed in WHV-infected woodchucks is accompanied by hepatocyte regeneration, we performed an immunohistochemical analysis of liver sections with an antibody to the PCNA, an indicator of DNA synthesis.
All of the transiently infected woodchucks examined showed distortions of the lobular structures, mononuclear cell infiltrations in the portal areas and lobules, and the presence of scattered apoptotic hepatocytes. Sections from the chronically infected woodchucks showed inflammatory changes and, as was observed with acute infections, apoptotic hepatocytes. In contrast to transiently infected animals, where inflammation appeared to be more widespread (panlobular activity), inflammation in chronic infections was more concentrated around the portal areas. Apoptotic cells were particularly obvious in hepatocytes around the limiting plate at the portal area, which is consistent with piecemeal necrosis observed for chronic HBV infection.
To determine the fraction of apoptotic hepatocytes in livers of WHV-infected woodchucks, between 2,000 and 7,000 hepatocytes were scored on liver biopsy slides. Criteria for the identification of apoptotic hepatocytes included the detachment of hepatocytes from the liver plate, reduced size of hepatocytes, eosinophilic staining, cells with pyknotic, fragmented nuclei, and peripheral aggregation of chromatin. The fraction of PCNA-positive hepatocytes was determined from a microscopic analysis of 7,000 to 24,000 cells per slide, depending on the fraction of positive cells.
In woodchuck 38 the apoptotic index (AI), the percentage of the total number of hepatocytes that were apoptotic, increased in parallel with the increase of CD4+ and CD8+ T cells and reached peak levels at 11 weeks p.i. (Fig. 4). This increase in apoptosis of hepatocytes coincided with an increase in the number of PCNA-positive hepatocytes. The peak levels of PCNA-positive cells was observed 17 weeks p.i. and thus lagged somewhat behind the observed peak of apoptosis.
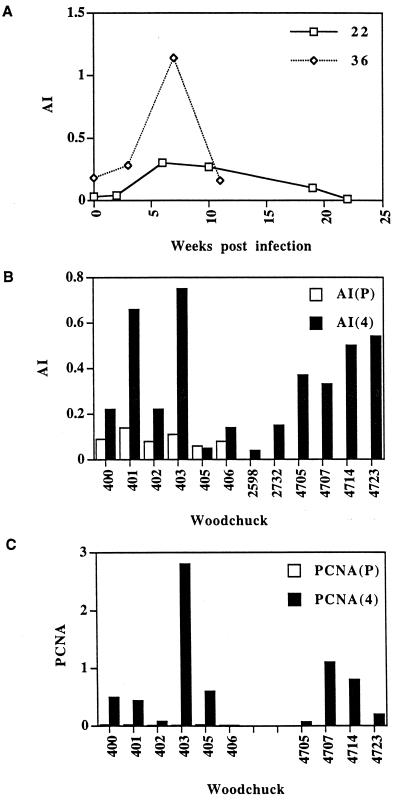
Similar observations were made with woodchuck 22, for which the AI increased 7- to 10-fold compared to levels measured before infection or at 2 weeks p.i., to 0.3% at 6 and 10 weeks p.i. (Fig. 7A). The index declined progressively to preinfection levels following recovery from the WHV infection. In woodchuck 36 we observed a sixfold increase in the AI (1.1%) 7 weeks p.i. Interestingly, this woodchuck exhibited a significantly higher index prior to infection, correlating with elevated levels of CD4 mRNA (Fig. 5).
FIG. 7.
Determination of the AI and expression of PCNA in transiently and chronically infected woodchucks. (A) The results of determinations of apoptotic cells counts from slides of tissue from woodchucks 22 and 36 at the indicated time points. (B) The results of determinations of apoptotic cell counts of cohort II animals prior to infection (P) and 4 weeks p.i. (4). Panel B also shows the results obtained with two uninfected control animals, 2598 and 2732, and four chronically infected woodchucks, 4705, 4707, 4714, and 4723. The AI is the fraction (percentage) of apoptotic hepatocytes per normal hepatocyte. (C) The results of the analysis of PCNA-positive hepatocytes of sections from cohort II animals and the four chronically infected woodchucks.
In cohort II, woodchuck 405 and the uninfected control animal 406 did not show any significant increase in apoptosis 4 weeks p.i. (Fig. 7B). Their levels were also comparable to the levels observed with two additional uninfected woodchucks, 2598 and 2732. In contrast, the other four woodchucks in this group exhibited two- to sevenfold elevations in the AI compared to the levels observed prior to the infection. The highest increase, reaching an AI of 0.75%, was observed in woodchuck 403, which also showed the highest levels of CD8+ T cells, IFN-γ, and TNF-α among cohort II animals (Fig. 6).
The AIs of the four chronically infected woodchucks were all increased compared to those of uninfected control animals or even woodchuck 405, which had the most delayed clearance of virus antigen from the liver. AIs measured for the chronically infected animals ranged from 0.3 to 0.5% and were higher than the values observed with some of the transiently infected animals, including woodchucks 400 and 402 (Fig. 7B).
Among cohort II animals, woodchuck 403 exhibited the largest fraction of PCNA-positive hepatocytes (Fig. 7C). Nearly 3% of the hepatocyte population expressed PCNA at 4 weeks p.i., which represented an approximately 30-fold increase compared to levels measured for the uninfected control animal 406 or samples taken prior to infection of the other five woodchucks in cohort II. Three of the four remaining animals in cohort II exhibited approximately 0.5% PCNA-positive hepatocytes. The number of PCNA-positive hepatocytes in chronically infected woodchucks varied from less than 0.1% to approximately 1.2%. Thus, with the exception of the negative control animal 406, all infected woodchucks showed evidence of increased liver cell regeneration, necessary, most likely, to compensate for the loss of hepatocytes due to apoptosis.
DISCUSSION
This study was meant to provide a molecular and histologic description of events as they occur in livers during natural transient and chronic hepadnavirus infections in an outbred population of animals. Naturally, such an analysis cannot be performed with HBV, since multiple liver biopsy samples are not available from patients. It has become evident from this study that the time point for the liver biopsy during the recovery period is critical. Our results showed that recovery from transient WHV infections is preceded by a substantial influx of CD3+ T cells into the liver that can reach up to 65% of the number of hepatocytes (Fig. 3). The presence of T cells is accompanied by the expression of IFN-γ and TNF-α. While we found a good correlation between eventual recovery and the expression of the two cytokines, we also noted that in their presence the accumulation of viral DNA can still occur (14) (Table 1 and Fig. 5), suggesting that these cytokines per se may not inhibit viral replication. However, we cannot exclude the possibility that the biopsies predated the maximal cytokine expression levels. Although expressed at lower levels, TNF-α and IFN-γ were also detected in all four chronically infected animals. Both cytokines are known to exert antiviral effects and can even trigger the disappearance of viral DNA markers in HBV transgenic mice (7). What is not known is whether the expression of these two cytokines in a natural infection induces changes in gene expression in infected hepatocytes or whether they primarily act to sustain the activity of T cells and thus the immune response. Future studies will have to be directed toward this important issue.
Our results clearly showed that hepatocytes of transiently and chronically infected woodchucks undergo programmed cell death at an increased rate compared to uninfected control animals (Fig. 7A and B). We arrived at this conclusion through the direct counting of apoptotic hepatocytes. To interpret our results we considered a study by Bursch et al. (1), who found that the detection time of apoptotic bodies of hepatocytes in rat livers is approximately 3 h. Under the assumption that this number can be applied to woodchucks and that the rate of apoptosis remains constant over time, our results indicated that uninfected control animals with an AI of 0.1% replace an entire liver within approximately 125 days. This observation is in good agreement with estimates of Columbano et al. (3), who found an AI of 0.05% for hepatocytes in healthy Wistar rats. Based on our results, cells in the liver of transiently infected woodchuck 22 could have undergone turnover within 41 days provided that the AI remained constant between weeks 6 and 10 (Fig. 7A). In fact, this may be an underestimate, since higher rates of cell death may have occurred between 6 and 10 weeks.
We noted substantial differences in the AI as well as in the amount of PCNA staining among different woodchucks. In part, these differences can be explained by the selection of the time points for liver biopsies, which varied in relation to the course of the recovery process for each individual animal. However, other factors, including genetic variation of an outbred population as well as variations due to differences in circadian rhythms should also be taken into consideration (1).
Our results seem to differ with a recent study by Guidotti et al. (8), who found that the recovery from transient HBV infections in two chimpanzees was accompanied by a form of innate immunity and not, as expected and demonstrated in this study, by a CTL response. This difference may be explained by the fact that the two animal hosts as well as the two viruses are different from each other. The differences between the two studies could also be due to the differences in viral replication observed with the two animal models. Woodchucks, like humans infected with their indigenous virus, generally exhibit an approximately 10-fold excess of cytoplasmic viral DNA intermediates over nuclear covalently closed circular DNA (cccDNA), whereas in this particular chimpanzee experiment this ratio was significantly lower, perhaps due to the nature of the HBV isolate used to infect the primates.
An interpretation of our results is that killing of hepatocytes occurs over a prolonged period of time. In fact, the relatively slow dynamics of this process may guarantee the survival of the host, since more rapid killing could impair liver function and cause terminal liver failure. Based on limiting dilution assays Rehermann and colleagues estimated that the precursor frequency of HBV-specific CTLs is approximately 10−4 (23), which would amount to a CTL-to-hepatocyte ratio of 1:1,000 (assuming that there are 1011 hepatocytes in the liver and 1012 lymphocytes in the body of a human), perhaps too low to account for the removal of every hepatocyte. Recently, however, Murali-Krishna et al. (18, 19) showed by direct counting of antigen-specific CTLs that results from limiting dilution assays can cause the precursor frequency to be underestimated by a factor of 20 to 200 and that up to 50 to 70% of activated CD8+ cells can be virus specific. Applying these recent results to hepadnavirus infections would increase the potential CTL-to-hepatocyte ratio to 1:5. Thus, even when CTLs could kill only once, an unproven assumption, the continuous expansion of CD8+ T cells could account for the replacement of an entire liver over a relatively short period. The potential of CTLs to induce the turnover of a whole liver in a short time span has recently been demonstrated with adenovirus infections in mice (2). In this system, clearance of an infection involving nearly every hepatocyte occurs by CTLs in a Fas-dependent pathway.
According to our results, liver turnover is accelerated three to five times in chronically infected woodchucks compared to that in uninfected controls. One interpretation of our observations is that CTLs present in chronically infected livers are as effective in inducing apoptosis as their counterparts in transiently infected livers. Therefore, hepatocyte killing per se can only account for the cure of an infected liver provided that newly regenerated cells both lose existing virus and remain protected from reinfection.
To explain why regenerated hepatocytes remain virus free, we favor two models. The first model would predict that virus-specific antibodies are present during the early phase of recovery. This might explain the loss of detectable viremia in cohort II weeks prior to the loss of virus from the liver. Although our previous study (14) indicated that virus-neutralizing antibodies sometimes appear after recovery, nonneutralizing antibodies to the virus envelope would also suffice to deplete the intrahepatic virus pool in the presence of activated complement. The second model would predict that during the recovery phase hepatocytes are not permissive for infection. For example, the presence of certain cytokines or chemokines could induce changes in gene expression of hepatocytes that would produce cellular immunity to reinfection, similar to IFN-α-induced expression of Mx protein during influenza virus infection (25). Indeed, early steps of the viral replication cycle are known to be very sensitive to environmental changes of hepatocytes. For example, Pugh and colleagues (21) demonstrated that primary hepatocyte cultures lose susceptibility to infection after a few days in culture due to the failure of the virus to attach to cells. In addition, Hild et al. (9) showed that addition of glucagon to the culture medium of primary hepatocyte cultures induces immunity to infection due to increases in intracellular cyclic AMP levels.
Both models require that the nuclear cccDNA, the template for viral RNA synthesis, be lost from infected hepatocytes as they divide and replace cells killed by CTLs. So far, little is known about the fate of this DNA species during regeneration of hepatocytes in the liver. However, it seems unlikely that a DNA species without a centromer can be maintained during mitosis, since, at least in yeast, plasmids lacking a functional centromer cannot be propagated in a stable fashion. Moreover, recent data indicated that the maintenance of the Epstein-Barr virus plasmid, also lacking a centromere, in latently infected cells requires the EBNA1 protein; in the absence of EBNA1, replicated DNA is lost from proliferating cultures (16).
In summary, our study revealed that recovery from WHV infections is a dynamic process associated with a dramatic influx of T cells into the parenchyma of the liver. This event, in turn, is associated with the expression of cytokines and the killing of infected hepatocytes by apoptosis. A major issue that remains unresolved is how regenerated hepatocytes lose existing intracellular viral particles and, in particular, the nuclear cccDNA. In addition, it will be important to investigate how regenerated hepatocytes are protected from reinfection. Answers to these questions are paramount, since they will reveal the mechanism for the persistence of virus in chronic infections and thus provide a basis for the development of effective antiviral therapies.
ACKNOWLEDGMENTS
J.-T.G. and H.Z. contributed equally to this work.
We thank Kerry Campbell and Glenn Rall for their helpful suggestions and comments on the manuscript. We thank Bud Tennant (Cornell University) for woodchuck liver tissues that were essential for the conduct of this study. We acknowledge the assistance received from the following facilities at the Fox Chase Cancer Center: nucleotide sequencing facility, histopathology facility, imaging facility, and animal care facility.
This work was supported by grants from the National Institutes of Health and by an appropriation from the Commonwealth of Pennsylvania.
REFERENCES
- 1.Bursch W, Paffe S, Putz B, Barthel G, Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990;11:847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- 2.Chirmule N, Moscioni A D, Qian Y, Qian R, Chen Y, Wilson J M. Fas-Fas ligand interactions play a major role in effector functions of cytotoxic T lymphocytes after adenovirus vector-mediated gene transfer. Hum Gene Ther. 1999;10:259–269. doi: 10.1089/10430349950019048. [DOI] [PubMed] [Google Scholar]
- 3.Columbano A, Ledda-Columbano G M, Coni P P, Faa G, Liguori C, Santa Cruz G, Pani P. Occurrence of cell death (apoptosis) during the involution of liver hyperplasia. Lab Investig. 1985;52:670–675. [PubMed] [Google Scholar]
- 4.Cote P J, Korba B E, Steinberg H, Ramirez M C, Baldwin B, Hornbuckle W E, Tennant B C, Gerin J L. Cyclosporin A modulates the course of woodchuck hepatitis virus infection and induces chronicity. J Immunol. 1991;146:3138–3144. [PubMed] [Google Scholar]
- 5.Dienes H P, Purcell R H, Popper H, Ponzetto A. The significance of infections with two types of viral hepatitis demonstrated by histologic features in chimpanzees. J Hepatol. 1990;10:77–84. doi: 10.1016/0168-8278(90)90076-4. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti L G, Guilhot S, Chisari F V. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1993;68:1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 9.Hild M, Weber O, Schaller H. Glucagon treatment interferes with an early step of duck hepatitis B virus infection. J Virol. 1998;72:2600–2606. doi: 10.1128/jvi.72.4.2600-2606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs M V, Weigle W O, Noonan D J, Torbett B E, McEvilly R J, Koch R J, Cardenas G J, Ernst D N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 11.Hollinger F B. Hepatitis B virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2738–2807. [Google Scholar]
- 12.Jilbert A R, Wu T-T, England J M, Hall P de la M, Carp N Z, O'Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 14.Kajino K, Jilbert A R, Saputelli J, Aldrich C E, Cullen J, Mason W S. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeffe E B. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 16.Mackey D, Sugden B. The linking regions of EBNA1 are essential for its support of replication and transcription. Mol Cell Biol. 1999;19:3349–3359. doi: 10.1128/mcb.19.5.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menne S, Maschke J, Lu M, Grosse-Wilde H, Roggendorf M. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J Virol. 1998;72:6083–6091. doi: 10.1128/jvi.72.7.6083-6091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Altman J D, Suresh M, Sourdive D, Zajac A, Ahmed R. In vivo dynamics of anti-viral CD8 T cell responses to different epitopes. An evaluation of bystander activation in primary and secondary responses to viral infection. Adv Exp Med Biol. 1998;452:123–142. doi: 10.1007/978-1-4615-5355-7_14. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 20.Ponzetto A, Cote P J, Ford E C, Purcell R H, Gerin J L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984;52:70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugh J C, Di Q, Mason W S, Simmons H. Susceptibility to duck hepatitis B virus infection is associated with the presence of cell surface receptor sites that efficiently bind viral particles. J Virol. 1995;69:4814–4822. doi: 10.1128/jvi.69.8.4814-4822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 23.Rehermann B, Lau D, Hoofnagle J H, Chisari F V. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Investig. 1996;97:1655–1665. doi: 10.1172/JCI118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeger C, Mason W S. Woodchuck and duck hepatis B viruses. In: Ahmed R, Chen I, editors. Persistent viral infections. J. New York, N.Y: Wiley & Sons, Ltd.; 1999. pp. 607–622. [Google Scholar]
- 25.Staeheli P, Horisberger M A, Haller O. Mx-dependent resistance to influenza viruses is induced by mouse interferons alpha and beta but not gamma. Virology. 1984;132:456–461. doi: 10.1016/0042-6822(84)90050-3. [DOI] [PubMed] [Google Scholar]
- 26.Tassopoulos N, Papaevangelou G, Roumeliotou-Karayannis A, Kalafatas P, Engle R, Gerin J, Purcell R H. Double infections with hepatitis A and B viruses. Liver. 1985;5:348–353. doi: 10.1111/j.1600-0676.1985.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 27.Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]