Abstract
Atrial fibrillation/flutter (AF) is a major public health problem and is associated with stroke, heart failure, dementia, and death. It is estimated that 20%–30% of Americans will develop AF at some point in their life. Current medications to prevent AF have limited efficacy and significant adverse effects. Newer and safer therapies to prevent AF are needed. Ventricular arrhythmias are less prevalent than AF but may have significant consequences including sudden cardiac death. Metformin is the most prescribed, first-line medication for treatment of diabetes mellitus (DM). It decreases hepatic glucose production but also reduces inflammation and oxidative stress. Experimental studies have shown that metformin improves metabolic, electrical, and histologic risk factors associated with AF and ventricular arrhythmias. Furthermore, in large clinical observational studies, metformin has been associated with a reduced risk of AF in people with DM. These data suggest that metformin may have antiarrhythmic properties and may be a candidate to be repurposed as a medication to prevent cardiac arrhythmias. In this article, we review the clinical observational and experimental evidence for the association between metformin and cardiac arrhythmias. We also discuss the potential antiarrhythmic mechanisms underlying this association. Repurposing a well-tolerated, safe, and inexpensive medication to prevent cardiac arrhythmias has significant positive public health implications.
Keywords: Arrhythmia, Atrial fibrillation, Metformin, Prediabetes, Ventricular fibrillation, Ventricular tachycardia
Key Findings.
-
▪
A growing body of experimental and observational clinical evidence suggests that metformin conveys antiarrhythmic effects, potentially protecting against atrial fibrillation/flutter and ventricular arrhythmias.
-
▪
Underlying such favorable actions may be metformin’s effects on activating adenosine monophosphate-activated protein kinase and attenuating inflammation, oxidative stress, dysregulated calcium handling, and pathologic structural remodeling of myocardium, while promoting myocardial oxidative metabolism and energetic integrity.
-
▪
However, starting metformin in patients without diabetes for the sole purpose of preventing arrhythmias would not be justified because the clinical evidence supporting its antiarrhythmic activity originates from observational studies, which may be affected by confounding.
-
▪
The ongoing VA-IMPACT (Veterans Affairs–Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes) trial (ClinicalTrials.gov Identifier: NCT02915198) provides a unique opportunity to test metformin’s antiarrhythmic effects.
Atrial fibrillation and flutter (AF) is a public health issue that is highly prevalent on a global scale.1, 2, 3 Adverse outcomes related to AF include stroke, heart failure, dementia, and death.4, 5, 6 In the United States approximately 20%–30% of adults will develop AF at some point in their life.7 The annual economic burden of AF treatment exceeds $26 billion.8
Although less prevalent than AF, ventricular arrhythmias, such as ventricular fibrillation (VF) and ventricular tachycardia (VT), are frequently the final arrhythmias before sudden cardiac death.9, 10, 11, 12 Prevention of AF and VT/VF in high-risk populations is of utmost importance.13, 14, 15, 16 However, current antiarrhythmic medications have limited efficacy and significant adverse effects, thus limiting their use.17 Determining alternative medications with favorable risk profiles is essential to reduce the societal burden of these highly prevalent and impactful arrhythmias.
Diabetes mellitus (DM) and prediabetes are prevalent risk factors for AF. The risk of AF is 34% higher in patients with DM and 20% higher in those with prediabetes compared to individuals with normal glucose tolerance.18 Metformin, which has been in clinical use for 6 decades, is a first-line therapy for DM. It is inexpensive and has a favorable safety profile. Metformin is an activator of adenosine monophosphate-activated protein kinase (AMPK). This mechanism is primarily responsible for metformin’s effect in decreasing hepatic gluconeogenesis and may also contribute to reduced inflammation and oxidative stress.19 Clinical trials and observational data indicate that metformin may offer cardioprotective effects, including reduced risk of stroke, heart failure, myocardial infarction, and all-cause mortality.19,20 However, less is known about potential antiarrhythmic effects of metformin. Experimental studies have shown that metformin may ameliorate the metabolic, electrical, and histologic conditions that lead to AF or ventricular arrhythmias.19,21 Furthermore, in observational studies using administrative data, metformin use was associated with a lower risk of AF in people with DM.19,22,23 The objective of this article is to review the current clinical observational and experimental evidence for the association between metformin and cardiac arrhythmias and to discuss the potential antiarrhythmic mechanisms underlying this association (Figure 1). Compared to a previous review on metformin and arrhythmias published in 2020, the current article is more comprehensive, including important new data that have been published since then.
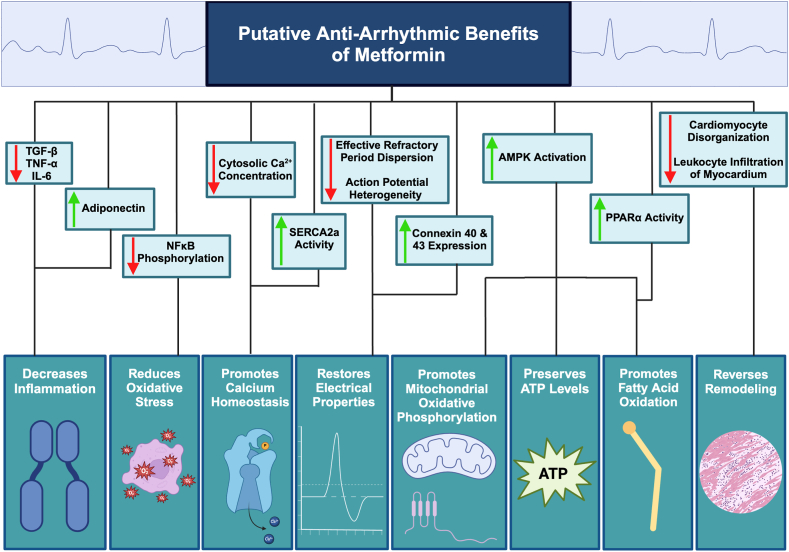
Figure 1.
Putative antiarrhythmic benefits of metformin. AMPK = adenosine monophosphate-activated protein kinase; IL-6 = interleukin 6; NFκB = nuclear factor kappa-B; PPARγ = peroxisome proliferator-activated receptor-gamma; SERCA2a = sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase; TGF-β = transforming growth factor-beta; TNF-α = tumor necrosis factor-alpha. Created with BioRender.com.
Clinical evidence for antiarrhythmic effects of metformin
Atrial arrhythmias
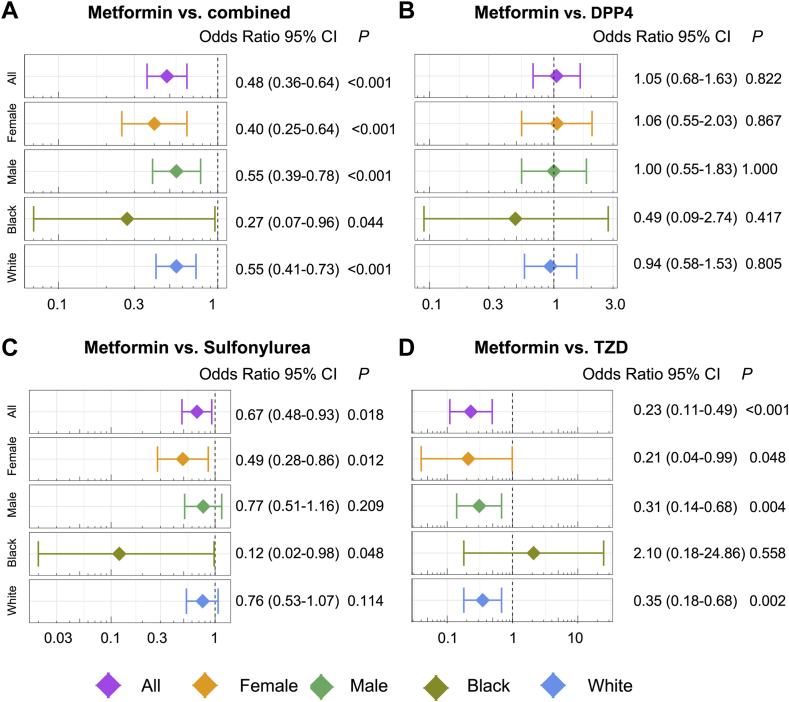
Recent observational cohort studies indicate that metformin is associated with reduced incidence of atrial arrhythmias (Table 1). Among 645,710 patients with DM, metformin users had a lower risk of AF than nonusers after adjusting for age, sex, and traditional cardiovascular risk factors (hazard ratio [HR] 0.81; 95% confidence interval [CI] 0.76–0.86).24 In other studies, patients using metformin had lower rates of hospitalization for AF compared to nonusers.23,25 Furthermore, in a network meta-analysis prioritizing drug repurposing for AF, Lal et al26 found that metformin use is associated with a lower risk of AF (OR 0.48; 95% CI 0.36–0.64) compared with other diabetes treatments (Figure 2).
Table 1.
Clinical studies of metformin and arrhythmia risk
| Study | Objective | Design | Sample (N) | Follow-up | Conclusion |
|---|---|---|---|---|---|
| Chang et al24 (2014) | Compare the incidence of AF in patients with DM on MET to those with DM not on MET | Retrospective cohort | 645,710 | 13 years | After adjusting for comorbidities and meds, MET reduced new-onset AF. |
| Ostropolets et al22 (2021) | Determine the risk of arrhythmias in patients on single oral hypoglycemic medications | Retrospective cohort | 410,000 | 8 years | MET reduced the risk of AF compared to sulfonylureas, DPP4s, and TZDs. MET also reduced the risk of VT/VF compared to sulfonylureas. |
| Tseng et al25 (2021) | Compare the incidence of hospitalization for AF in patients who had ever used MET to those who never used MET | Retrospective cohort with propensity matching | 200,000 | 6 years | Incidence rate of AF-related hospitalization was 56.9 vs 92.5 per 100,000 person-years for ever-users vs never-users of MET, respectively. |
| Deshmukh et al32 (2021) | Determine the risk of AF recurrence after catheter ablation in patients with DM on MET vs not on MET | Retrospective cohort | 271 | 13 months | Sinus rhythm was maintained in 55% of patients treated with MET vs 40% of patients not on MET |
| Basnet et al36 (2017) | Determine the risk of postoperative AF after cardiac surgery in patients with DM on MET vs not on MET | Matched retrospective cohort | 1283 | No difference in the rate of postoperative AF after cardiac surgery in patients with DM on MET vs not on MET | |
| Liou et al23 (2018) | Compare the risk of AF between different classes of hypoglycemic medications | Nested case control | 14,410 | MET users had reduced rate of AF compared to nonusers. | |
| Davis et al104 (1998) | Determine the risk of post-MI arrhythmias with respect to hypoglycemic medications | Cohort | 745 | 28 days | MET in combination with other hypoglycemic medications, but not alone, reduced the risk of post-MI AF. No difference in VT/VF. |
| Chen et al28 (2017) | Compare the risk of AF between different classes of hypoglycemic medications | Nested case control | 9790 | No significant association of MET with AF incidence. | |
| El Messaoudi et al37 (2015) | Determine if MET pretreatment before CABG reduced adverse outcomes in patients without DM | Randomized controlled Trial | 100 | 24 hours | MET administered 3 days before CABG did not reduce arrhythmias within postoperative 24 hours |
| Lal et al26 (2022) | Compare the risk of AF with MET vs other hypoglycemic medications | Network meta-analysis | 35,824 | MET use was associated with 52% reduction in risk of AF vs those taking sulfonylureas, TZDs, and a combination regimen of hypoglycemic medications (TZD, DPP4, GLP1RA, and sulfonylureas). | |
| Iqbal et al29 (2022) | Determine AF incidence in patients with DM initially treated with MET vs other hypoglycemic medications | Retrospective cohort | 5664 | 10 years | Ten-year cumulative incidence of AF in patients initially treated with MET was 5.2% vs 8.1% with other hypoglycemic medications, which was not statistically different (P = .55). |
| Zhou et al27 (2022) | Compare AF incidence in patients with DM on MET vs sulfonylureas | Retrospective cohort with propensity matching | 108,000 | AF risk was higher in patients with DM on sulfonylurea monotherapy vs MET monotherapy. | |
| Lee et al38 (2022) | Compare risk of VT/VF or SCD in patients with DM on MET vs sulfonylurea | Retrospective cohort with propensity matching | 33,192 | 4.9 years | Sulfonylurea use was associated with a higher risk of VT/VF or SCD than MET. |
| Islam et al39 (2023) | Compare risk of VT/VF or SCD in patients with DM on MET vs sulfonylurea | Retrospective cohort with propensity matching | 599,520 | Sulfonylurea use was associated with a higher risk of VT/VF or SCD than MET. |
AF = atrial fibrillation; CABG = coronary artery bypass graft; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4 inhibitor; GLP1RA = glucagon-like peptide 1 receptor agonist; MET = metformin; MI = myocardial infarction; SCD = sudden cardiac death; TZD = thiazolidinedione; VF = ventricular fibrillation; VT = ventricular tachycardia.
Figure 2.
Pharmacoepidemiologic validation of metformin in reducing atrial fibrillation (AF) occurrence odds ratio (OR) and 95% confidence interval (CI) for metformin vs combination of the 4 drug groups (all: dipeptidyl peptidase-4 sulfonylurea [DPP4], thiazolidinedione [TZD], sulfonylurea, and glucagon-like peptide 1 receptor agonist [GLP1RA]) (n = 3578) (A), DPP4 (n = 1244) (B), sulfonylurea (n = 2352) (C), and TZD (n = 288) (D). For each of the 4 comparisons, the results for comparisons between subgroups (including female, male, Black, and White) are also shown. Patient groups were matched using propensity score matching with the variables age, gender, race, and comorbidities for the overall group comparisons. For the subgroup of male and female, the matching variables excluded gender, and for the subgroup Black and White, the matching variables excluded race. Logistic regression models were used for statistical inference of the AF ORs. Subgroup analyses were performed in females (orange), males (green), Black Americans (dark green), and White Americans (blue).P <.05. (Lal et al.26 Reprinted with permission.)
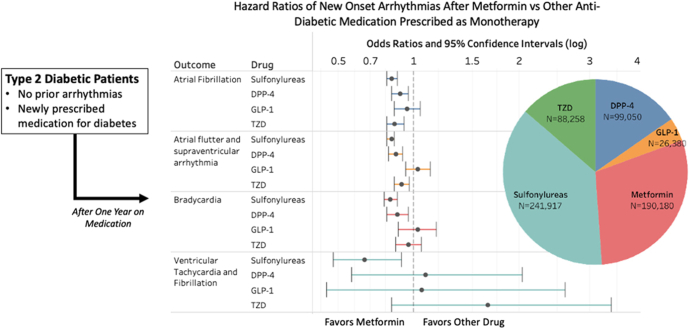
Metformin also performed better than other diabetes medications. Ostropolets et al22 found that compared to sulfonylureas, dipeptidyl peptidase-4 inhibitors (DPP4s), and thiazolidinediones, metformin monotherapy was associated with a lower risk of AF (Figure 3). Furthermore, in addition to a lower risk of incident AF, Zhou et al27 observed that metformin users had a lower risk of stroke, cardiovascular mortality, and all-cause mortality when compared to users of sulfonylurea. In a case-matched study of patients with DM, users of metformin and thiazolidinediones had lower risk of AF compared to nonusers (OR 0.81, 95% CI 0.71–0.95; OR 0.72, 95% CI 0.63–0.83, respectively), whereas insulin users had a higher risk of AF (OR 1.19; 95% CI 1.06–1.35).23
Figure 3.
Association of metformin monotherapy with arrhythmias compared to other medications for treatment of diabetes mellitus. Abbreviations as in Figure 2. (Ostropolets et al.22 Reprinted with permission.)
Although these studies suggest a potential association of metformin with lower risk of AF, other studies are nonconcordant. For example, Chen et al28 found no significant difference in the risk of AF between metformin users and nonusers (OR 1.01; 95% CI 0.88–1.15) while corroborating that insulin was associated with a higher risk of AF (OR 1.58; 95% CI 1.37–1.82). Similarly, a retrospective cohort study demonstrated similar rates of AF with metformin vs other noninsulin hypoglycemic medications.29
Postablation AF recurrence
Recurrent AF occurs in 30%–40% of patients after catheter ablations.30,31 Among 271 patients with DM undergoing AF ablation, Deshmukh et al32 observed that those using metformin were more likely to maintain sinus rhythm by 13 months compared to those not using metformin (adjusted HR 0.63; 95% CI 0.42–0.96).32 This effect was independent of blood glucose level.
Postoperative AF
Postoperative AF complicates 30%–40% of cardiac surgical procedures and has been linked to an increased risk of stroke and mortality.33, 34, 35 Among 1283 patients with DM who underwent cardiac surgery, Basnet et al36 found that preoperative treatment with metformin did not reduce the risk of postoperative AF. Furthermore, a randomized controlled trial demonstrated that metformin treatment 3 days before cardiac surgery did not alter the incidence of postoperative AF.37
Ventricular arrhythmias
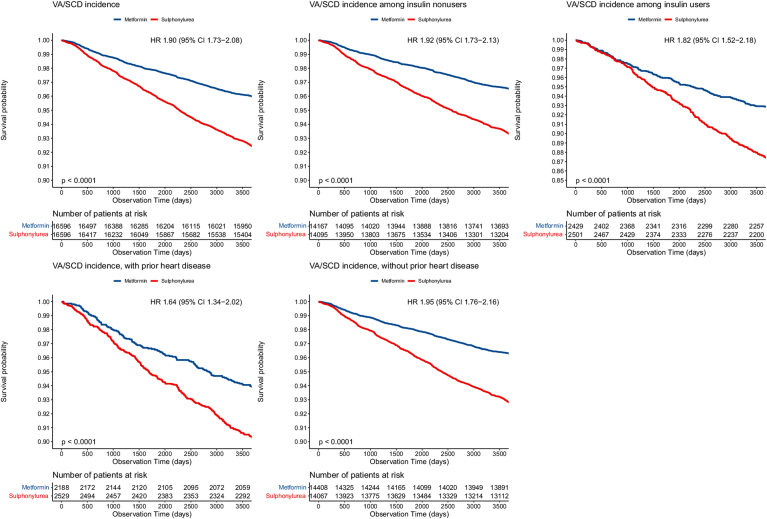
Compared to AF, fewer data are available on the association between metformin use and ventricular arrhythmias. Ostropolets et al22 observed a lower risk of VT/VF in metformin users compared to sulfonylurea (HR 0.66; 95% CI 0.47–0.91). In a matched cohort study, participants with DM receiving sulfonylurea had a 2× times higher risk of ventricular arrhythmias and sudden cardiac death compared to those receiving metformin (Figure 4).38 Islam et al39 found similar results in a population-based cohort study, with sulfonylurea users experiencing higher incidence of ventricular arrhythmias compared to metformin users (HR 1.42; 95% CI 1.18–1.69). Whether these data indicate a deleterious effect of sulfonylureas and/or a favorable effect of metformin is uncertain.
Figure 4.
Kaplan-Meier survival curves of ventricular arrhythmias and sudden cardiac death associated with metformin vs sulfonylurea. CI = confidence interval; HR = hazard ratio; SCD = sudden cardiac death; VA = ventricular arrhythmia. (Lee et al.38 Reprinted with permission.)
Independence from blood glucose control
The association of metformin with decreased risk of cardiac arrhythmias does not seem to be a consequence of better diabetes control but an independent antiarrhythmic effect. In observational clinical studies, metformin had a similar effect on blood glucose as other diabetes medications in patients with diabetes and had minimal effect on blood glucose in individuals without diabetes, while showing antiarrhythmic effects in both groups.40,41 Furthermore, there is clinical and experimental evidence that AF risk is not modified by blood glucose level. Post hoc analysis of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, which randomized patients with diabetes to an intensive vs standard glucose control strategy, found no difference in incident AF between the treatment groups.42 In experimental animal models of myocardial infarction, the antiarrhythmic effects of metformin on VT/VF were also independent of blood glucose concentrations.43,44
Mechanisms of action
Mechanisms for a putative antiarrhythmic effect of metformin are summarized in Figure 1.
Moderation of inflammation and cytokine profile
There is increasing evidence that inflammation is associated with arrhythmia development, severity, persistence, and recurrence.45, 46, 47, 48 Therefore, therapies that attenuate inflammation may also reduce AF incidence. In vitro, metformin treatment in human induced pluripotent stem cell–derived atrial-like cardiomyocytes downregulated expression of proinflammatory cytokines, such as transforming growth factor-beta1 (TGF-β1).26 In a canine AF model of rapid atrial pacing (RAP), Li et al49 found that metformin treatment attenuated the RAP-induced increase in proinflammatory cytokines (interleukin-6 [IL-6], TGF-β1, and tumor necrosis factor-alpha [TNF-α]). Metformin treatment also attenuated the RAP-induced reduction in adiponectin, an anti-inflammatory adipokine. In addition, the concentrations of peroxisome proliferator-activated receptor-gamma (PPAR ), a key transcription factor controlling the expression of adiponectin, was increased.49 With its antioxidant and anti-inflammatory properties, adiponectin may have effects on counteracting atrial fibrosis and on the development of AF.50 Decreased levels of adiponectin and increased activity of TGF-β1, IL-6, and TNF-α have been noted in AF and may serve as therapeutic targets of metformin therapy.51, 52, 53, 54, 55, 56
Reduction of oxidative stress
Recent evidence suggests a link between oxidative stress and AF.57,58 In the setting of tachyarrhythmias, oxidative damage and atrial cellular remodeling induce AF.24,59 The accumulation of reactive oxygen species (ROS) and activation of various signaling pathways augments this process. Patients with DM are thought to be more prone to developing AF in part due to increased oxidative stress and nuclear factor kappa-B (NF- B) signaling.60, 61, 62
In a canine model, RAP led to increased phosphorylation of NF- B and higher contents of ROS in the left atrium and epicardial adipose tissue compared to controls. Metformin therapy reduced oxidative stress that occurred in this model through decreased phosphorylation of NF- B.24,49
Promotion of calcium homeostasis
Calcium (Ca2+) homeostasis is critical for optimal cardiomyocyte function. Intracellular Ca2+ handling is significantly altered in atrial myocytes in AF.63 During the cardiac action potential, Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR) allows Ca2+ to bind to myofilaments and initiate myocyte contraction. Atrial relaxation occurs partly through the retreat of Ca2+ back into the SR through a protein known as sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase (SERCA2a). In the atria, phospholamban regulates SERCA2a activity and cytosolic Ca2+ concentrations. Dysregulation of this process is thought to increase the risk of ectopic activity and the likelihood of reentry.64, 65, 66 There is evidence that AF is triggered by delayed afterdepolarizations and spontaneous depolarizations that are propagated by alterations in calcium handling and increased cytosolic Ca2+.67,68 In vitro, metformin has been shown to attenuate inflammation-induced decreases in SERCA2a expression and increases in cytosolic Ca2+.49 This may occur through the upregulation of adiponectin, which increases SERCA2a expression in cardiomyocytes.69
Preservation of ATP levels in ischemic myocardium
Ischemic or infarcted myocardium serves as a substrate for VT/VF.10,70, 71, 72, 73, 74, 75 Myocardial ischemia causes a rundown in myocardial ATP levels. Reduced ATP concentration leads to opening of ATP-sensitive potassium channels with consequent shortening of the action potential in ischemic myocardium.70 Heterogeneity of action potential duration and refractoriness in ischemic vs healthy myocardial regions may establish conditions conducive to VT/VF.71,76
AMPK is activated, as a compensatory mechanism, to preserve cellular energy metabolism in ischemia.77,78 Amplification of AMPK by metformin may serve as a safeguard against the deleterious effects of myocardial ischemia. Kawabata et al79 showed that metformin attenuated the rundown of ATP levels in ischemic isolated rabbit hearts. Lu et al44 demonstrated that domestic pigs treated with metformin had preserved myocardial ATP concentration, reduced heterogeneity of myocardial action potential duration, and a lower incidence of VF during ischemia compared to untreated controls. This antiarrhythmic effect was associated with amplified AMPK activity and preserved ATP concentration in ischemic porcine myocardium but was independent of blood glucose level.
Promotion of mitochondrial oxidative phosphorylation
A potential mechanism for metformin’s benefit on metabolism is through AMPK-mediated reversal of the Warburg effect, a metabolic shift from mitochondrial oxidative phosphorylation to glycolysis even when oxygen supply is sufficient (so-termed “aerobic glycolysis”).80,81 Decreased activity of AMPK is associated with aerobic glycolysis and the development and persistence of AF. By activating AMPK, metformin may diminish aerobic glycolysis, enhance oxidative metabolism, and reduce the incidence of AF.82
Promotion of myocardial fatty acid oxidation
The antiarrhythmic effect of metformin in experimental models may be related to enhancement of fatty acid oxidation. Dysregulated cardiac fatty acid metabolism with accumulation of intracellular lipids is known to contribute to AF progression.83 Compared to patients in sinus rhythm, those with AF have been noted to have higher plasma levels of saturated fatty acids, lower plasma levels of unsaturated fatty acids, and increased atrial expression of fatty acid binding protein 3, which is involved in fatty acid uptake and intracellular transport.83,84 Furthermore, cardiac adiposity has been associated with an increased risk and severity of AF.85 Promotion of fatty acid oxidation and autophagy by metformin may protect against development of AF by attenuating lipotoxicity.83,86,87 Liu et al21 demonstrated that RAP increased the expression of factors that augment aerobic glycolysis, such as hypoxia inducible factor-1a (HIF-1a). RAP also downregulated AMPK and peroxisome proliferator-activated receptor coactivator 1ɑ (PPAR-ɑ) activity, reducing fatty acid oxidation and leading to intramyocardial lipid accumulation. Treatment with metformin blunted these effects by increasing AMPK and PPAR-ɑ activity.21 Bai et al88 demonstrated that while RAP decreased transcription and expression of PPAR-ɑ, leading to intracellular lipid accumulation in atrial myocytes, those effects were attenuated by metformin treatment.
Reversal of remodeling
Atrial fibrosis creates conduction disturbances that facilitate reentry and AF.89 Once established, AF worsens the progression of atrial fibrosis.90 Therefore, mitigating fibrosis may be a potential therapeutic target to prevent AF.
Metformin treatment has been shown to attenuate or reverse atrial fibrosis in vivo.21,49 In diabetic and arrhythmogenic rodents, metformin limits cardiomyocyte disorganization and leukocyte infiltration in atrial tissue.82,91 This is thought to occur through activation of AMPK, which protects against the dysregulation of transcription factors needed to maintain expression of ion channels and gap junction proteins. Decreased activity of AMPK in the atria promotes electrophysiological changes and ectopic activity that may lead to AF.92,93 The relationship between metformin and AMPK has been observed on a genetic level. Approximately 100 AF susceptibility loci have been identified through genomewide association studies. In an AF network module consisting of differentially expressed genes in atrial tissue, metformin was shown to target genes encoding subunits of AMPK, such as PRKAB1.26 Thus, experimental data indicate that metformin, through activation of AMPK, may prevent deleterious fibrotic changes that are both a cause and a consequence of AF.
Restoration of electrical properties
Shortening and increased dispersion of the atrial effective refractory period (ERP) increases vulnerability to AF.49,94,95 Li et al49 demonstrated that RAP decreased atrial ERP in dogs. However, treatment with metformin attenuated this effect. RAP also increased ERP dispersion compared to controls; however, metformin treatment fully reversed this effect.49 In another AF canine model, Liu et al21 observed that metformin treatment restored ERP values and significantly reduced ERP dispersion, AF inducibility, and window of vulnerability.
Gap junctions, such as connexin43, are critical for cell-to-cell electrical conduction and are downregulated in AF. Whereas RAP downregulated connexin43 in canines, treatment with metformin minimized this effect.96 Furthermore, Ozcan et al82 noted restoration of connexin40 and connexin43 concentrations in an AF mouse model after treatment with metformin.
Small conductance calcium-activated potassium channels (SK channels) also play a critical role in the action potential repolarization of cardiomyocytes, although their role in the pathophysiology of AF is less clear. The genetic knockout of SK2 channels and pharmacologic inhibition of SK channels in atrial myocytes have been shown to induce early afterdepolarizations that create arrhythmogenic substrates.97,98 In contrast, the knockdown of SK3 channels has been shown to reduce the number of AF episodes and AF duration in rats.99 In concordance with these studies, Fu et al91 demonstrated in diabetic rats that metformin treatment upregulates SK2 and downregulates SK3 channels. These findings suggest that SK channels could play a role in AF and may partially explain the antiarrhythmic benefits of metformin.
Under conditions of ischemia, susceptibility to ventricular arrhythmias such as VT and VF increases.100 One reason for this is shortening of action potentials.101 In metabolically normal pigs that underwent experimental myocardial ischemia, those treated with metformin demonstrated less heterogeneity of action potential duration between ischemic and healthy regions and a lower incidence of ischemic VF compared with untreated pigs.44 Regional heterogeneity in the action potential duration (dispersion) between ischemic and nonischemic regions is also known to precipitate VF.100,102,103
Conclusion
A considerable and growing body of experimental and clinical evidence suggests that metformin conveys antiarrhythmic effects, potentially protecting against both atrial and ventricular arrhythmia. Underlying such favorable actions may be metformin’s effects in activating AMPK and attenuating inflammation, oxidative stress, dysregulated calcium handling, and pathologic structural remodeling of myocardium while promoting myocardial oxidative metabolism and energetic integrity (Figure 1). Each of these effects could stabilize or ameliorate the electrophysiological conditions leading to arrhythmias.
However, starting metformin in patients without diabetes for the sole purpose of preventing arrhythmias would not be justified because the clinical evidence supporting the antiarrhythmic activity of metformin originates from observational studies, which may be affected by confounding (Table 1). Randomized controlled trials are needed to establish metformin as an effective antiarrhythmic medication. The TRIM-AF (Targeting Risk Interventions and Metformin for Atrial Fibrillation) trial (ClinicalTrials.gov Identifier: NCT03603912) is testing the effects of lifestyle risk modification and metformin on AF burden detected on cardiac implantable devices. The VA-IMPACT (Veterans Affairs–Investigation of Metformin in Pre-diabetes on Atherosclerotic Cardiovascular OuTcomes) trial (ClinicalTrials.gov Identifier: NCT02915198) is randomizing patients with prediabetes and cardiovascular disease to metformin or placebo to test the hypothesis that metformin reduces major adverse cardiovascular outcomes. In the trial, all new clinical AF events will be collected as adverse events, and patients with cardiac implantable devices are being followed centrally for arrhythmias. In this regard, the VA-IMPACT trial provides a unique opportunity to test metformin’s antiarrhythmic effects.
Acknowledgments
This manuscript is partially the result of work supported with resources and use of facilities of the Minneapolis Veterans Affairs Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Funding Sources
The Veterans Affairs–Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular Outcomes (VA-IMPACT; ClinicalTrials.gov Identifier: NCT02915198) is funded by the U.S. Department of Veterans Affairs Cooperative Studies Program.
Disclosures
The authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2024.04.003.
References
- 1.Brundel B.J.J.M., Ai X., Hills M.T., Kuipers M.F., Lip G.Y.H., de Groot N.M.S. Atrial fibrillation. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baman J.R., Passman R.S. Atrial fibrillation. JAMA. 2021;325:2218. doi: 10.1001/jama.2020.23700. [DOI] [PubMed] [Google Scholar]
- 3.Kornej J., Börschel C.S., Benjamin E.J., Schnabel R.B. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-López J.A., Sterne J.A.C., Thom H.H.Z., et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359 doi: 10.1136/bmj.j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlisle M.A., Fudim M., DeVore A.D., Piccini J.P. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019;7:447–456. doi: 10.1016/j.jchf.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Ruddox V., Sandven I., Munkhaugen J., Skattebu J., Edvardsen T., Otterstad J.E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1555–1566. doi: 10.1177/2047487317715769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mou L., Norby F.L., Chen L.Y., et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC Study (Atherosclerosis Risk in Communities) Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 9.Adabag A.S., Luepker R.V., Roger V.L., Gersh B.J. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adabag A.S., Therneau T.M., Gersh B.J., Weston S.A., Roger V.L. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adabag A.S., Peterson G., Apple F.S., Titus J., King R., Luepker R.V. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooks M., Downey M.C., Joppa S., et al. Arrhythmic causes of in-hospital cardiac arrest among patients with heart failure with preserved ejection fraction. Heart Rhythm. O2 2021;2:665–667. doi: 10.1016/j.hroo.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adabag S., Langsetmo L. Sudden cardiac death risk prediction in heart failure with preserved ejection fraction. Heart Rhythm. 2020;17:358–364. doi: 10.1016/j.hrthm.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Anantha Narayanan M., Vakil K., et al. Efficacy of implantable cardioverter-defibrillator therapy in patients with nonischemic cardiomyopathy: a systematic review and meta-analysis of randomized controlled trials. JACC Clin Electrophysiol. 2017;3:962–970. doi: 10.1016/j.jacep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Adabag A.S., Maron B.J. Implications of arrhythmias and prevention of sudden death in hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2007;12:171–180. doi: 10.1111/j.1542-474X.2007.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adabag S., Hodgson L., Garcia S., et al. Outcomes of sudden cardiac arrest in a state-wide integrated resuscitation program: results from the Minnesota Resuscitation Consortium. Resuscitation. 2017;110:95–100. doi: 10.1016/j.resuscitation.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Agdamag A.C., Westanmo A., Gravely A., Angsubhakorn N., Chen L.Y., Adabag S. Mortality associated with antiarrhythmic medication for atrial fibrillation among patients with left ventricular hypertrophy. Pacing Clin Electrophysiol. 2023;46:738–744. doi: 10.1111/pace.14711. [DOI] [PubMed] [Google Scholar]
- 18.Aune D., Feng T., Schlesinger S., Janszky I., Norat T., Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complications. 2018;32:501–511. doi: 10.1016/j.jdiacomp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Nantsupawat T., Wongcharoen W., Chattipakorn S.C., Chattipakorn N. Effects of metformin on atrial and ventricular arrhythmias: evidence from cell to patient. Cardiovasc Diabetol. 2020;19:198. doi: 10.1186/s12933-020-01176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. Erratum in: Lancet 1998;352:1558. [PubMed] [Google Scholar]
- 21.Liu Y., Bai F., Liu N., et al. Metformin improves lipid metabolism and reverses the Warburg effect in a canine model of chronic atrial fibrillation. BMC Cardiovasc Disord. 2020;20:50. doi: 10.1186/s12872-020-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostropolets A., Elias P.A., Reyes M.V., et al. Metformin is associated with a lower risk of atrial fibrillation and ventricular arrhythmias compared with sulfonylureas: an observational study. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liou Y.S., Yang F.Y., Chen H.Y., Jong G.P. Antihyperglycemic drugs use and new-onset atrial fibrillation: a population-based nested case control study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S.H., Wu L.S., Chiou M.J., et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. doi: 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng C.H. Metformin use is associated with a lower incidence of hospitalization for atrial fibrillation in patients with type 2 diabetes mellitus. Front Med (Lausanne) 2021;7 doi: 10.3389/fmed.2020.592901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal J.C., Mao C., Zhou Y., et al. Transcriptomics-based network medicine approach identifies metformin as a repurposable drug for atrial fibrillation. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J., Zhang G., Chang C., et al. Metformin versus sulphonylureas for new onset atrial fibrillation and stroke in type 2 diabetes mellitus: a population-based study. Acta Diabetol. 2022;59:697–709. doi: 10.1007/s00592-021-01841-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen H.Y., Yang F.Y., Jong G.P., Liou Y.S. Antihyperglycemic drugs use and new-onset atrial fibrillation in elderly patients. Eur J Clin Invest. 2017;47:388–393. doi: 10.1111/eci.12754. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal A., Tekin Z., Kattan M.W., et al. Association between first-line monotherapy with metformin and the risk of atrial fibrillation (AMRAF) in patients with type 2 diabetes. J Diabetes Complications. 2022;36 doi: 10.1016/j.jdiacomp.2022.108315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darby A.E. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9:1427. doi: 10.4022/jafib.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voight J., Akkaya M., Somasundaram P., et al. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11:1884–1889. doi: 10.1016/j.hrthm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh A., Ghannam M., Liang J., et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:1232–1239. doi: 10.1111/jce.14954. [DOI] [PubMed] [Google Scholar]
- 33.Dobrev D., Aguilar M., Heijman J., Guichard J.B., Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- 34.Lin M.H., Kamel H., Singer D.E., Wu Y.L., Lee M., Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50:1364–1371. doi: 10.1161/STROKEAHA.118.023921. [DOI] [PubMed] [Google Scholar]
- 35.Mithani S., Akbar M.S., Johnson D.J., et al. Dose dependent effect of statins on postoperative atrial fibrillation after cardiac surgery among patients treated with beta blockers. J Cardiothorac Surg. 2009;4:61. doi: 10.1186/1749-8090-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basnet S., Kozikowski A., Sun H., Troup M., Urrutia L.E., Pekmezaris R. Metformin therapy and postoperative atrial fibrillation in diabetic patients after cardiac surgery. J Intensive Care. 2017;5:60. doi: 10.1186/s40560-017-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Messaoudi S., Nederlof R., Zuurbier C.J., et al. Effect of metformin pretreatment on myocardial injury during coronary artery bypass surgery in patients without diabetes (MetCAB): a double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:615–623. doi: 10.1016/S2213-8587(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 38.Lee T.T.L., Hui J.M.H., Lee Y.H.A., et al. Sulfonylurea is associated with higher risks of ventricular arrhythmia or sudden cardiac death compared with metformin: a population-based cohort study. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.026289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam N., Reynier P., Douros A., Yu O.H.Y., Filion K.B. Sulphonylureas versus metformin and the risk of ventricular arrhythmias among people with type 2 diabetes: a population-based cohort study. Diabetes Obes Metab. 2023;25:1523–1533. doi: 10.1111/dom.15000. [DOI] [PubMed] [Google Scholar]
- 40.Xu C., He L., Zhang J., Xu L., Dong J., Liao L. The cardiovascular benefits and infections risk of sglt2i versus metformin in type 2 diabetes: a systemic review and meta-analysis. Metabolites. 2022;12:979. doi: 10.3390/metabo12100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fatemi O., Yuriditsky E., Tsioufis C., et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study) Am J Cardiol. 2014;114:1217–1222. doi: 10.1016/j.amjcard.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apaijai N., Chinda K., Palee S., Chattipakorn S., Chattipakorn N. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu L., Ye S., Scalzo R.L., Reusch J.E.B., Greyson C.R., Schwartz G.G. Metformin prevents ischaemic ventricular fibrillation in metabolically normal pigs. Diabetologia. 2017;60:1550–1558. doi: 10.1007/s00125-017-4287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez A., Van Wagoner D.R. Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: an update. J Cardiovasc Pharmacol. 2015;66:523–529. doi: 10.1097/FJC.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y.F., Chen Y.J., Lin Y.J., Chen S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 47.Korantzopoulos P., Kalantzi K., Siogas K., Goudevenos J.A. Long-term prognostic value of baseline C-reactive protein in predicting recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin Electrophysiol. 2008;31:1272–1276. doi: 10.1111/j.1540-8159.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 48.Adabag S., Zimmerman P., Black A., Madjid M., Safavi-Naeini P., Cheng A. Implantable cardioverter-defibrillator shocks during COVID-19 outbreak. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B., Po S.S., Zhang B., et al. Metformin regulates adiponectin signalling in epicardial adipose tissue and reduces atrial fibrillation vulnerability. J Cell Mol Med. 2020;24:7751–7766. doi: 10.1111/jcmm.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafaqat S., Rafaqat S., Rafaqat S. Pathophysiological role of major adipokines in atrial fibrillation. Int J Arrhythm. 2021;22:18. [Google Scholar]
- 51.Marcus G.M., Whooley M.A., Glidden D.V., Pawlikowska L., Zaroff J.G., Olgin J.E. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren M., Li X., Hao L., Zhong J. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: a novel potential therapeutic target? Ann Med. 2015;47:316–324. doi: 10.3109/07853890.2015.1042030. [DOI] [PubMed] [Google Scholar]
- 53.Gramley F., Lorenzen J., Koellensperger E., Kettering K., Weiss C., Munzel T. Atrial fibrosis and atrial fibrillation: the role of the TGF-β1 signaling pathway. Int J Cardiol. 2010;143:405–413. doi: 10.1016/j.ijcard.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Yang Y., Ng C.Y., Zhang Z., Liu T., Li G. Association of plasma transforming growth factor-β1 levels and the risk of atrial fibrillation: a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kourliouros A., Karastergiou K., Nowell J., et al. Protective effect of epicardial adiponectin on atrial fibrillation following cardiac surgery. Eur J Cardiothorac Surg. 2011;39:228–232. doi: 10.1016/j.ejcts.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Assar O. Low adiponectin level may contribute to higher incidence of postcardiac surgery atrial fibrillation in obese patients. Ann Thorac Surg. 2012;93:1762–1763. doi: 10.1016/j.athoracsur.2011.09.025. author reply 1763. [DOI] [PubMed] [Google Scholar]
- 57.Hadi H.A., Alsheikh-Ali A.A., Mahmeed W.A., Suwaidi J.M. Inflammatory cytokines and atrial fibrillation: current and prospective views. J Inflamm Res. 2010;3:75–97. doi: 10.2147/JIR.S10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 59.Yeh Y.H., Kuo C.T., Lee Y.S., et al. Region-specific gene expression profiles in the left atria of patients with valvular atrial fibrillation. Heart Rhythm. 2013;10:383–391. doi: 10.1016/j.hrthm.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Lorenzo O., Picatoste B., Ares-Carrasco S., Ramírez E., Egido J., Tuñón J. Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediators Inflamm. 2011;2011 doi: 10.1155/2011/652097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiordaliso F., Cuccovillo I., Bianchi R., et al. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79:121–129. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 62.Ziolo M.T., Mohler P.J. Defining the role of oxidative stress in atrial fibrillation and diabetes. J Cardiovasc Electrophysiol. 2015;26:223–225. doi: 10.1111/jce.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan A.D., Joca H.C., Boyman L., Greiser M. Calcium signaling silencing in atrial fibrillation: implications for atrial sodium homeostasis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heijman J., Voigt N., Nattel S., Dobrev D. Calcium handling and atrial fibrillation. Wien Med Wochenschr. 2012;162:287–291. doi: 10.1007/s10354-012-0109-9. [DOI] [PubMed] [Google Scholar]
- 65.Dobrev D. Atrial Ca2+ signaling in atrial fibrillation as an antiarrhythmic drug target. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:195–206. doi: 10.1007/s00210-009-0457-1. [DOI] [PubMed] [Google Scholar]
- 66.Dobrev D., Voigt N., Wehrens X.H. The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89:734–743. doi: 10.1093/cvr/cvq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Z., Ko C.Y., Nivala M., Weiss J.N., Qu Z. Calcium-voltage coupling in the genesis of early and delayed afterdepolarizations in cardiac myocytes. Biophys J. 2015;108:1908–1921. doi: 10.1016/j.bpj.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sardu C., Santulli G., Guerra G., et al. Modulation of SERCA in patients with persistent atrial fibrillation treated by epicardial thoracoscopic ablation: the CAMAF study. J Clin Med. 2020;9:544. doi: 10.3390/jcm9020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boddu N.J., Theus S., Luo S., Wei J.Y., Ranganathan G. Is the lack of adiponectin associated with increased ER/SR stress and inflammation in the heart? Adipocyte. 2014;3:10–18. doi: 10.4161/adip.26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luqman N., Sung R.J., Wang C.L., Kuo C.T. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119:283–290. doi: 10.1016/j.ijcard.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 72.Vakil K., Florea V., Koene R., Kealhofer J.V., Anand I., Adabag S. Effect of coronary artery bypass grafting on left ventricular ejection fraction in men eligible for implantable cardioverter-defibrillator. Am J Cardiol. 2016;117:957–960. doi: 10.1016/j.amjcard.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 73.Adabag S., Zimmerman P., Lexcen D., Cheng A. Predictors of sudden cardiac arrest among patients with post-myocardial infarction ejection fraction greater than 35. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adabag S., Carlson S., Gravely A., Buelt-Gebhardt M., Madjid M., Naksuk N. Improvement of left ventricular function with surgical revascularization in patients eligible for implantable cardioverter-defibrillator. J Cardiovasc Electrophysiol. 2022;33:244–251. doi: 10.1111/jce.15315. [DOI] [PubMed] [Google Scholar]
- 75.Konety S.H., Koene R.J., Norby F.L., et al. Echocardiographic predictors of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ Cardiovasc Imaging. 2016;9:e004431. doi: 10.1161/CIRCIMAGING.115.004431. 10.1161/CIRCIMAGING.115.004431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai C.F., Ueng K.C., Wu D.J., Tsai T.P., Lin C.S. Remodeled left ventricular myocardium remote to infarction sites is the arrhythmogenic substrate for sudden cardiac death. Med Hypotheses. 2010;75:368–371. doi: 10.1016/j.mehy.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 77.Bairwa S.C., Parajuli N., Dyck J.R. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016;1862:2199–2210. doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Omar M.A., Fraser H., Clanachan A.S. Ischemia-induced activation of AMPK does not increase glucose uptake in glycogen-replete isolated working rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H1266–H1273. doi: 10.1152/ajpheart.01087.2007. [DOI] [PubMed] [Google Scholar]
- 79.Kawabata H., Ishikawa K. Cardioprotection by metformin is abolished by a nitric oxide synthase inhibitor in ischemic rabbit hearts. Hypertens Res. 2003;26:107–110. doi: 10.1291/hypres.26.107. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y., Bai F., Liu N., Ouyang F., Liu Q. The Warburg effect: a new insight into atrial fibrillation. Clin Chim Acta. 2019;499:4–12. doi: 10.1016/j.cca.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 81.Liberti M.V., Locasale J.W. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozcan C., Dixit G., Li Z. Activation of AMP-activated protein kinases prevents atrial fibrillation. J Cardiovasc Transl Res. 2021;14:492–502. doi: 10.1007/s12265-020-10069-6. [DOI] [PubMed] [Google Scholar]
- 83.Shingu Y., Takada S., Yokota T., et al. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS One. 2020;15 doi: 10.1371/journal.pone.0224713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung Y., Cho Y., Kim N., et al. Lipidomic profiling reveals free fatty acid alterations in plasma from patients with atrial fibrillation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatem S.N., Redheuil A., Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc Res. 2016;109:502–509. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Fu Y., Jiang T., et al. Enhancing fatty acids oxidation via L-carnitine attenuates obesity-related atrial fibrillation and structural remodeling by activating AMPK signaling and alleviating cardiac lipotoxicity. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.771940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenski M., Schleider G., Kohlhaas M., et al. Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res Cardiol. 2015;110:40. doi: 10.1007/s00395-015-0497-2. [DOI] [PubMed] [Google Scholar]
- 88.Bai F., Liu Y., Tu T., et al. Metformin regulates lipid metabolism in a canine model of atrial fibrillation through AMPK/PPAR-α/VLCAD pathway. Lipids Health Dis. 2019;18:109. doi: 10.1186/s12944-019-1059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xintarakou A., Tzeis S., Psarras S., Asvestas D., Vardas P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. Europace. 2020;22:342–351. doi: 10.1093/europace/euaa009. [DOI] [PubMed] [Google Scholar]
- 90.Platonov P.G. Atrial fibrosis: an obligatory component of arrhythmia mechanisms in atrial fibrillation? J Geriatr Cardiol. 2017;14:233–237. doi: 10.11909/j.issn.1671-5411.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu X., Pan Y., Cao Q., et al. Metformin restores electrophysiology of small conductance calcium-activated potassium channels in the atrium of GK diabetic rats. BMC Cardiovasc Disord. 2018;18:63. doi: 10.1186/s12872-018-0805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su K.N., Ma Y., Cacheux M., et al. Atrial AMP-activated protein kinase is critical for prevention of dysregulation of electrical excitability and atrial fibrillation. JCI Insight. 2022;7 doi: 10.1172/jci.insight.141213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harada M., Tadevosyan A., Qi X., et al. Atrial fibrillation activates AMP-dependent protein kinase and its regulation of cellular calcium handling: potential role in metabolic adaptation and prevention of progression. J Am Coll Cardiol. 2015;66:47–58. doi: 10.1016/j.jacc.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 94.Yu W.C., Chen S.A., Lee S.H., et al. Tachycardia-induced change of atrial refractory period in humans: rate dependency and effects of antiarrhythmic drugs. Circulation. 1998;97:2331–2337. doi: 10.1161/01.cir.97.23.2331. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira M., da Silva M.N., Timoteo A.T., et al. Inducibility of atrial fibrillation during electrophysiologic evaluation is associated with increased dispersion of atrial refractoriness. Int J Cardiol. 2009;136:130–135. doi: 10.1016/j.ijcard.2008.04.097. [DOI] [PubMed] [Google Scholar]
- 96.Li J., Li B., Bai F., et al. Metformin therapy confers cardioprotection against the remodeling of gap junction in tachycardia-induced atrial fibrillation dog model. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117759. [DOI] [PubMed] [Google Scholar]
- 97.Li N., Timofeyev V., Tuteja D., et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587(Pt 5):1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsueh C.H., Chang P.C., Hsieh Y.C., Reher T., Chen P.S., Lin S.F. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm. 2013;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saljic A., Soattin L., Trachsel D.S., Boddum K., Jespersen T. In vivo knockdown of SK3 channels using antisense oligonucleotides protects against atrial fibrillation in rats. J Mol Cell Cardiol. 2020;147:18–26. doi: 10.1016/j.yjmcc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 100.Koplan B.A., Stevenson W.G. Ventricular tachycardia and sudden cardiac death. Mayo Clin Proc. 2009;84:289–297. doi: 10.4065/84.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Diego J.M., Antzelevitch C. Ischemic ventricular arrhythmias: experimental models and their clinical relevance. Heart Rhythm. 2011;8:1963–1968. doi: 10.1016/j.hrthm.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winter J., Brack K.E., Coote J.H., Ng G.A. Cardiac contractility modulation increases action potential duration dispersion and decreases ventricular fibrillation threshold via β1-adrenoceptor activation in the crystalloid perfused normal rabbit heart. Int J Cardiol. 2014;172:144–154. doi: 10.1016/j.ijcard.2013.12.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maheshwari A., Norby F.L., Somilan E.Z., et al. Relation of prolonged P-wave duration to risk of sudden cardiac death in the general population (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2017;119:1302–1306. doi: 10.1016/j.amjcard.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis T.M., Parsons R.W., Broadhurst R.J., Hobbs M.S., Jamrozik K. Arrhythmias and mortality after myocardial infarction in diabetic patients. Relationship to diabetes treatment. Diabetes Care. 1998;21:637–640. doi: 10.2337/diacare.21.4.637. [DOI] [PubMed] [Google Scholar]