Abstract
Objective:
The meta-analysis was conducted to test the link between pancreatic cancer (PC) risk and dietary inflammatory index (DII®) score.
Design:
Systematic review and meta-analysis.
Setting:
We searched PubMed, Embase, Web of Science and the Cochrane Library up to 22 November 2020 to identify the relevant studies. Studies that reported the risk estimates and the corresponding 95 % CI for the DII category and PC risk were included. The effect sizes were pooled using the random-effects model. Dose–response analysis was conducted where possible.
Participants:
Two prospective cohort studies of 634 705 participants (3152 incident cases), and four case–control studies of 2737 cases and 4861 controls.
Results:
Overall, the pooled risk ratio (RR) indicated that individuals in the highest category compared with the lowest category had an increased PC risk (RR = 1·45; 95 % CI 1·11, 1·90; P = 0·006). Meanwhile, significant heterogeneity was also revealed. The dose–response meta-analysis indicated that a 1-unit increase in the DII score was associated with the PC risk (RR = 1·08; 95 % CI 1·002, 1·166; P = 0·045; I2 = 94·1 %, P < 0·001). Nonlinear result showed an increased risk of moving from fewer to more inflammatory borders with increasing DII score (Pnonlinearity = 0·003; I2 = 76·5 %, P < 0·001). Subgroup analyses found that significant positive association between PC risk and DII score appeared to be in case–control studies (RR = 1·70; 95 % CI 1·16, 2·50; P = 0·007) and studies with ≤ 31 DII components (RR = 1·76; 95 % CI 1·14, 2·72; P = 0·011).
Conclusion:
These findings suggested dietary habits with high inflammatory features (high DII score) might increase PC risk.
Keywords: Dietary inflammatory index, Pancreatic cancer, Anti-inflammatory diet, Pro-inflammatory diet, Meta-analysis
Pancreatic cancer (PC) is a highly fatal disease with a 5-year overall survival of approximately 10 % in the USA(1). PC risk factors include family history, chronic pancreatitis, type 2 diabetes, obesity and heavy tobacco usage(2–4). Chronic inflammation is implicated in PC and supports cancer cells to evade immune elimination and accelerates malignant progression and metastasis to distant organs(5,6). C-reactive protein, TNF-α, tumour growth factor-β, IL-1β, IL-6, IL-10 and IL-17 have been suggested as important roles in PC(7–11).
Scientific evidence shows the consumption of energetically rich diets evokes a state of chronic metabolic inflammation(12). Exploring the association between higher inflammation in the diet and cancer risk is of great significance. A recent randomised cross-over trial reported whole grains diet reduced weight and systemic hypo-inflammation(13). Another randomised controlled trial found low-fat dietary intervention was associated with reduced PC incidence(14).
The dietary inflammatory index (DII®) is based on the published review of the articles evaluating the effects of the specific foods or food components on six biomarkers of inflammation (C-reactive protein, TNF-α, IL-1β, IL-4, IL-6 and IL-10)(15). Recent systematic reviews showed that the higher the DII score, the higher the risks of gynaecological cancer, urologic cancer, breast cancer and colorectal cancer(16–20). Some studies have described the link between DII score and PC risk, but the results are inconsistent. Up to now, we have not been able to identify any systematic review or meta-analysis assessing the relationship between DII score and PC risk. We conducted this meta-analysis to summarise the evidence on the association between DII score and PC risk.
Methods
Search strategy
Four electronic databases (PubMed, Embase, Web of Science and the Cochrane Library) were searched up to 22 November 2020, using the following keywords: (dietary inflammatory index OR inflammatory diet OR anti-inflammatory diet OR pro-inflammatory diet OR inflammatory potential of diet OR dietary score) AND (pancreatic cancer OR pancreatic carcinoma OR pancreatic neoplasm OR pancreatic adenoma OR pancreatic ductal adenocarcinoma). Search only for articles published in English. Search history is shown in online supplementary material, Supplemental Table 1. References of all relative articles were also manually checked to identify any potential additional studies. Two independent authors (Z.G., Y.H.) reviewed the titles, abstracts and full text of all the articles we identified. In case of any disagreements, a third investigator (Y.C.) was sought.
Eligibility
The inclusion criteria included: (1) studies conducted on human beings aged ≥ 18 years old who had completed all questionnaires; (2) studies that reported DII score category as exposure; (3) studies that reported the incidence of PC as the outcome measure; (4) studies that provided multi-covariate adjusted OR or hazard ratios and 95 % CI of PC risk associated with the DII score (the highest category v. the lowest category) and (5) randomised controlled trials, observational studies or case–control. The exclusion criteria included: (1) studies without reporting multi-covariate adjusted risk estimates of PC risk; (2) DII score measured as a continuous variable (see online supplementary material, Supplemental Table 2). The meta-analysis conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist(21). The protocol for this review was registered with PROSPERO (CRD42021237985).
Data extraction and quality assessment
Two researchers (Z.G., Y.H.) independently collected the information from each eligible studies as follows: surname of first researcher, time of publication, countries and regions, study design, sample size, gender proportion, mean age or age range (years), method of dietary assessment, number of DII components, risk estimates and their 95 % CI for DII score category and PC risk, the DII stratification interval, DII score comparison, covariates adjusted in the multivariate model and follow-up time for cohort studies (years). The quality assessment of incorporated studies was conducted by the same two authors using the Newcastle–Ottawa Scale(22) (studies ≤ 3 scores are classified as low quality, 4–6 scores as moderate quality and ≥ 7 scores as high quality). The Kappa statistic for the agreement of the two investigators for quality assessment as well as data collection was computed. The disagreement was resolved through discussion or, if required, consulting a third researcher (Y.C.) (see online supplementary material, Supplemental Table 3).
Statistical analysis
The pooled risk ratio (RR) and 95 % CI were calculated for the highest DII score category v. the lowest DII score category. The reported OR and hazard ratios were considered as equivalent to RR(23). Cochrane’s Q and I2 statistics were used to assess the heterogeneity across studies(24). The Cochrane’s Q test P-value < 0·10 or I2 > 50 % was considered to be significantly heterogeneous(24). In the presence of significant heterogeneity, the random-effects model was chosen to combine the results; otherwise, the fixed-effects model was selected.
We used the generalised least-squares trend estimation, the methods developed by Orsini et al.(25), to measure the linear dose–response relation of the DII levels and PC risk. The results were combined using a random-effects model. The median in each category of the DII was assigned. If medians were not reported, the midpoint of lower and upper limits was designated as the assigned dose. If highest category or the lowest category was open-end, we used the reported maximum and minimum range of the DII, respectively. If the maximum and minimum range had not been reported, we estimated it from the reported mean and sd values (mean ± 3 sd) in the study. The potential nonlinear dose–response relationship was examined through restricted cubic splines with 4 knots at fixed percentiles (5, 35, 65 and 95 %) of the distribution(26). Then the estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis(27). A P nonlinearity of the meta-analysis was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero(28).
Stratification analyses were conducted to explore the potential sources of heterogeneity whenever possible, by stratifying study designs, regions, the number of the DII components and adjustment factors. Sensitivity analyses were conducted to test the potential effect of each study on pooled effect size, by excluding each single study in each turn. We did not test publication bias with the formal statistical tests, because they have limited power when there are < 10 studies. The quality of evidence for result was assessed using the Grading of Recommendations Assessment, Development, and Evaluation and ranked as high, moderate, low or very low. All calculations were implemented using Stata/se version 15.0 (Stata Corp).
Results
Study characteristics
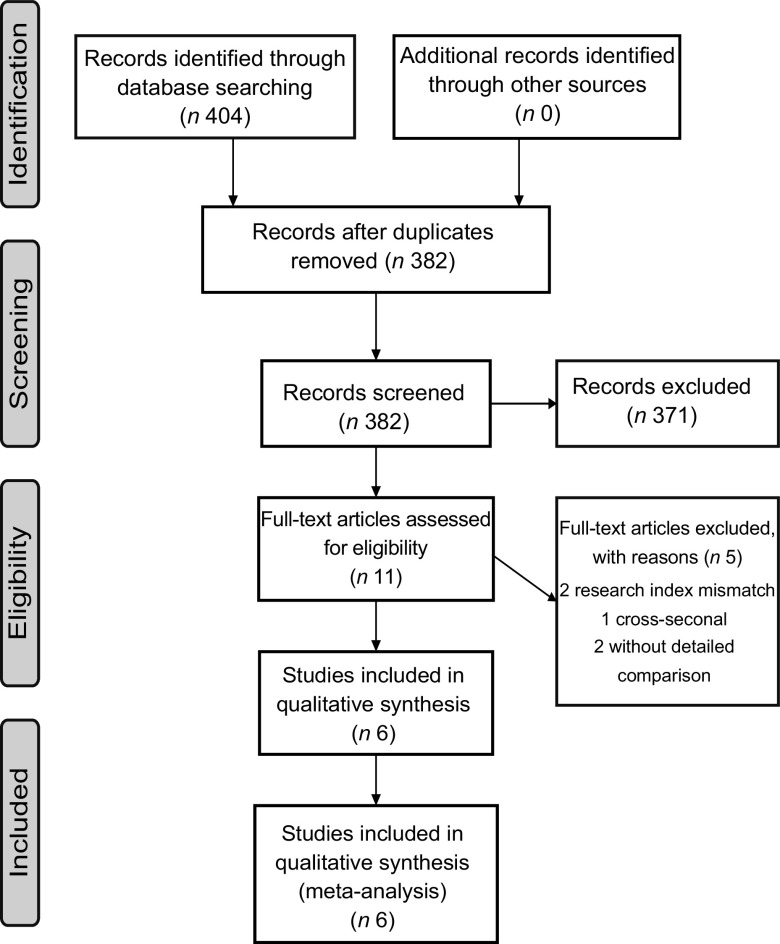
Figure 1 shows the detailed retrieval and screening process of literature. A total of 404 studies were identified. Eleven full-text articles were evaluated for eligibility. Finally, six studies(29–34) met the inclusion criteria for meta-analysis, including two prospective cohort studies(33,34) with a total of 634 705 individuals (3152 incident cases) and four case–control design studies(29–32) with 2737 cases and 4861 controls. Three studies were performed in the USA(30,33,34), two in Italy(29,32) and one in the USA, Italy and Asia(31). The FFQ were used in all studies to estimate dietary intakes and the DII was calculated by the method developed by Shivappa et al.(15). All studies reported the adjustment for smoking as the exposure. Four studies(29,32–34) reported the adjustment for alcohol consumption and one study(31) reported adjustment for history of PC as the exposure. The Kappa statistic for the agreement of the two investigators for data collection was 0·9511. The Newcastle–Ottawa Scale scores of all included studies reached with 7–8 stars and the Kappa statistic for the agreement of the two investigators for quality assessment was 0·6667. More characteristics of the included studies are shown in Table 1.
Fig. 1.
Literature search and study selection process for inclusion in meta-analysis of dietary inflammatory index and pancreatic cancer risk
Table 1.
General characteristics of included studies in the meta-analysis of DII score and pancreatic cancer risk
| Studies | Regions | Study design | Sample size | Gender (male: female) | Age, mean/range (years) | No. of DII components, dietary assessment tool | DII score comparison | Risk estimates | 95 % CI | Adjustment for covariate | Follow-up (years) | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accardi et al.
(2019)(29) |
Italy | Case–control | Case: 326, Con: 652 | 522: 456 | Median: 63, Range: NR |
31, FFQ | Quintile 5 v. 1 (NR) |
1·24 | 1·11, 1·38 | Smoking, alcohol drinking, BMI, diabetes | NR | 7 |
| Antwi (a*) et al.
(2016)(30) |
USA | Case–control | Case: 817, Con: 1756 | 1416: 1157 | Case: 66·7, Con: 65·4 | 28, FFQ | Quintile 5 v. 1 (> –0·03 v. < –3·07) |
2·54 | 1·87, 3·46 | Age, sex, race, diabetes, BMI, smoking, education | NR | 7 |
| Antwi (b*) et al.
(2018)(31) |
USA, Italy, and Asia | Case–control | Case: 1268, Con: 4215 | 4615: 868 | Case: 67·2, Con: 62·7 | 45, FFQ | Quintile 5 v. 1 (NR) |
1·23 | 0·92, 1·66 | Age, sex, race, diabetes, family history of pancreatic cancer, BMI, smoking, study site | NR | 7 |
| Shivappa et al.
(2015)(32) |
Italy | Case–control | Case: 326, Con: 652 | 522: 456 | Case: Median: 63, range: NR Con: Median: 64, Range: NR |
31, FFQ | Quintile 5 v. 1 (> +1·27 v. < –1·28) |
2·48 | 1·50, 4·10 | Age, sex, study centre, year of interview, education, BMI, smoking, alcohol drinking, history of diabetes | NR | 7 |
| Zheng (a*) et al.
(2019)(33) |
USA | Cohort | Participant: 533 256; Incident case: 2824 |
314 139: 219 117 |
Range: 50–71 |
33, FFQ | Quintile 5 v. 1 (> –1·50 v. < –5·61) |
0·96 | 0·85, 1·08 | Age, sex, BMI, smoking, total energy (kcal/d), alcohol drinks per day, diabetes history, education level |
13·4 | 8 |
| Zheng (b*) et al.
(2018)(34) |
USA | Cohort | Participant: 101 449; incident case: 328 | 49 347: 52 102 | Range: 52–78 |
31, FFQ | Quintile 5 v. 1 (> –1·21 v. < –5·32) |
1·31 | 0·83, 2·08 | Age, sex, BMI, history of diabetes, smoking, total energy intake (kcal/d), alcohol drinking, educational level | 8·5 | 8 |
DII, dietary inflammatory index; NOS, Newcastle–Ottawa Scale; NR, not reported.
a and b represent different studies with the same author name.
Meta-analysis of dietary inflammatory index score and pancreatic cancer risk
Overall, the pooled RR indicated that individuals in the highest category compared with the lowest category had a 45 % increased PC risk (RR = 1·45; 95 % CI 1·11, 1·90; P = 0·006) when using a random-effects model (see Fig. 2). However, significant heterogeneity among the incorporated studies was also found (I2 = 88·8 %, P < 0·001).
Fig. 2.
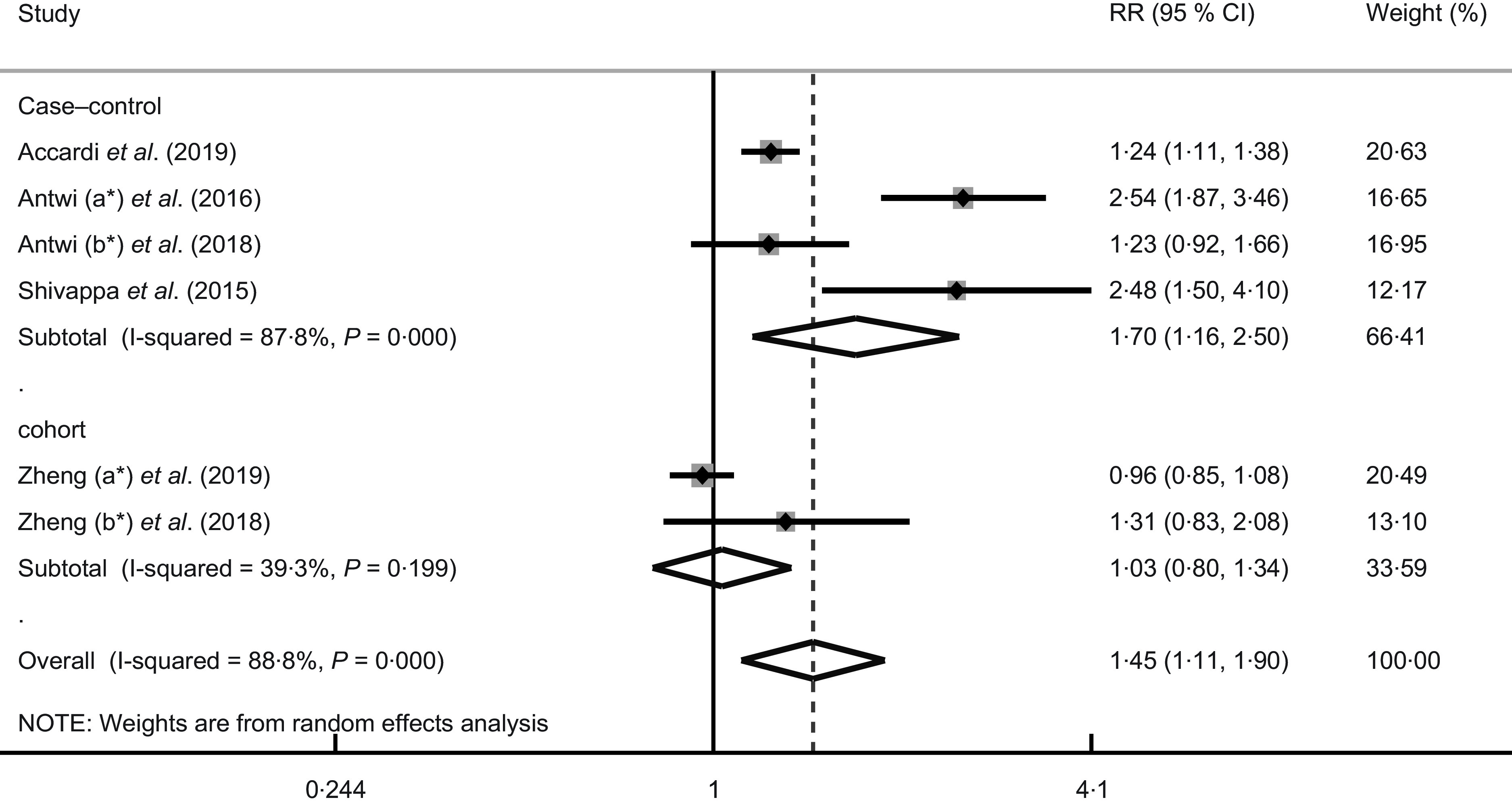
Forest plots for risk ratios (RR) of the highest compared with the lowest category of DII score and pancreatic cancer. *a and b represent different studies with the same author name
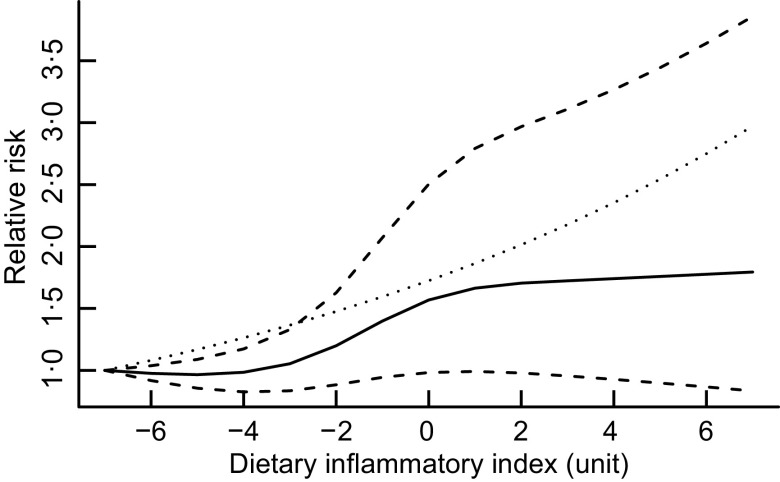
Two studies(29,31) did not present cut-off points for DII. The dose–response meta-analysis of four studies(30,32–34) indicated that a 1-unit increase in the DII score was associated with the PC risk (RR = 1·08; 95 % CI 1·002, 1·166; P = 0·045; I2 = 94·1 %, P < 0·001). Nonlinear dose–response meta-analysis showed an increased risk of moving from fewer to more inflammatory borders with increasing DII (P nonlinearity = 0·003; I2 = 76·5 %, P < 0·001) (Fig. 3). The Wald test indicated dose–response relation was consistent with a nonlinear model (χ2 = 9·8, P = 0·0074).
Fig. 3.
Dose–response associations between the dietary inflammatory index and pancreatic cancer risk.  , spline model;
, spline model;  , linear model;
, linear model;  , 95 % CI
, 95 % CI
Subgroup analyses
Due to the high heterogeneity among the incorporated studies, subgroup analyses were performed by stratifying study designs, regions, the number of the DII components and adjustment factors. The results are shown in Table 2. The summary RR indicated a significant positive correlation between high DII score and PC risk was found in the case–control subgroup (RR = 1·70; 95 % CI 1·16, 2·50; P = 0·007), with high heterogeneity (I2 = 87·8 %, P < 0·001). On the contrary, the cohort subgroup had lower heterogeneity (I2 = 39·3 %, P = 0·20) but no significant increase in the risk of PC (RR = 1·03; 95 % CI 0·80, 1·34; P = 0·81) (see Fig. 2). In subgroup analysis on countries and regions, a nonsignificant association was found. A significant positive association appeared to be in studies with ≤ 31 DII components (RR = 1·76; 95 % CI 1·14, 2·72; P = 0·011) but not in studies with >31 components (RR = 1·05; 95 % CI 0·83, 1·32; P = 0·70), and both groups had medium–high heterogeneity (≤31 DII components, I2 = 87·5 %, P < 0·001; > 31 DII components, I2 = 57·0 %, P = 0·127). Positive correlations were not observed in studies stratified to adjust for alcohol consumption (RR = 1·28; 95 % CI 0·98, 1·66; P = 0·07; I2 = 84·8 %, P < 0·001), PC family history (RR = 1·23, 95 % CI 0·92, 1·65; P = 0·17), race (RR = 1·77; 95 % CI 0·87, 3·59; P = 0·12; I2 = 91·0 %, P = 0·001) and total energy intake (RR = 1·03; 95 % CI 0·80, 1·34; P = 0·81; I 2 = 39·3 %, P = 0·20). Subgroup analyses indicated study design and adjustment for total energy intake appeared to be the potential sources of the heterogeneity.
Table 2.
Subgroups analyses of studies reporting the pancreatic cancer risk for the highest v. the lowest category of DII score
| Subgroups | No. of studies | RR | 95 % CI | Heterogeneity | Z value | P | |
|---|---|---|---|---|---|---|---|
| I 2 (%) | P | ||||||
| Overall | 6 | 1·45 | 1·11, 1·90 | 88·8 | < 0·001 | 2·73 | 0·006 |
| Study design | < 0·001 (between-group) | 0·034 | |||||
| Cohort | 2 | 1·03 | 0·80, 1·34 | 39·3 | 0·199 | 0·24 | 0·81 |
| Case–control | 4 | 1·70 | 1·16, 2·50 | 87·8 | < 0·001 | 2·70 | 0·007 |
| Countries and regions | 0·155 (between-group) | 0·27 | |||||
| USA | 3 | 1·46 | 0·75, 2·84 | 94·1 | < 0·001 | 1·13 | 0·26 |
| Italy | 2 | 1·68 | 0·85, 3·29 | 85·7 | 0·008 | 1·50 | 0·13 |
| USA, Italy and Asia | 1 | 1·23 | 0·92, 1·65 | Ns | Ns | 1·37 | 0·17 |
| Number of the DII components | < 0·001 (between-group) | 0·040 | |||||
| 31 | 4 | 1·76 | 1·14, 2·72 | 87·5 | < 0·001 | 2·54 | 0·01 |
| > 31 | 2 | 1·05 | 0·83, 1·32 | 57·0 | 0·127 | 0·38 | 0·70 |
| Adjustment for family history of pancreatic cancer | 0·828 (between-group) | 0·35 | |||||
| Yes | 1 | 1·23 | 0·92, 1·65 | Ns | Ns | 1·37 | 0·17 |
| No | 5 | 1·51 | 1·10, 2·07 | 91 | < 0·001 | 2·58 | 0·01 |
| Adjustment for alcohol drinking | < 0·001 (between-group) | 0·40 | |||||
| Yes | 4 | 1·28 | 0·98, 1·66 | 84·8 | < 0·001 | 1·85 | 0·07 |
| No | 2 | 1·77 | 0·87, 3·59 | 91·0 | 0·001 | 1·57 | 0·12 |
| Adjustment for race | < 0·001 (between-group) | 0·40 | |||||
| Yes | 2 | 1·77 | 0·87, 3·59 | 91·0 | 0·001 | 1·57 | 0·12 |
| No | 4 | 1·28 | 0·98, 1·66 | 84·8 | < 0·001 | 1·85 | 0·07 |
| Adjustment for total energy intake | < 0·001 (between-group) | 0·034 | |||||
| Yes | 2 | 1·03 | 0·80, 1·34 | 39·3 | 0·199 | 0·24 | 0·81 |
| No | 4 | 1·70 | 1·16, 2·50 | 87·8 | < 0·001 | 2·70 | 0·007 |
DII, dietary inflammatory index; RR, risk ratio.
Sensitivity analyses
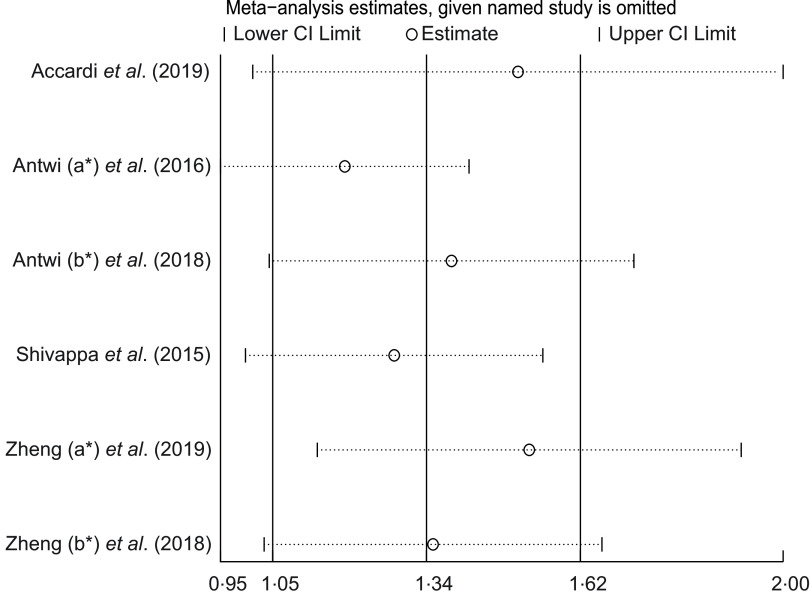
Sensitivity analysis (Fig. 4), after excluding the study of Antwi (a) et al.(30), also obtained positive affected results (RR = 1·26; 95 % CI 1·01, 1·58; P = 0·038), and heterogeneity slightly decreased (I2 = 80·0 %, P = 0·001). Meanwhile, the exclusion of another individual study also did not have a significant impact on the results of the meta-analysis, indicating the stability of the results.
Fig. 4.
Sensitivity analysis was conducted by removing each study in turn and recalculating the pooled risk ratio (RR) estimates. *a and b represent different studies with the same author name
Grading of Recommendations Assessment, Development, and Evaluation evidence
The Grading of Recommendations Assessment, Development, and Evaluation evidence for meta-analysis of DII score and PC risk is shown in online supplementary material, Supplemental Table 4. The quality of evidence for the result of the meta-analysis of DII score and PC risk was moderate.
Discussion
This meta-analysis summarised the evidence on the relationship between DII score and the incidence of PC. The current study included two prospective cohort studies and four case–control studies, with a total sample size of 642 303 participants. Meta-analysis findings indicated that the highest DII score might increase PC risk up to 45 % compared with the lowest DII score. The dose–response meta-analysis indicated that a 1-unit increase in the DII score was associated with an 8 % increase in PC risk. Nonlinear dose–response meta-analysis showed an increased risk of moving from fewer to more inflammatory borders with increasing DII score. Previous studies suggested better diet quality was associated with a reduced PC risk. Meanwhile, a recent systematic review including eight cohort studies and eight case–control studies indicated that PC risk and the animal products, starch-rich and western dietary patterns had significant positive associations. Significant inverse correlations were observed between the risk of PC and the specified dietary pattern of vegetables and fruits, vitamins and fibre(35). A review from the International Agency for Research on Cancer and another 2017 review both reported being obese or overweight increases the risk of developing PC, probably because obesity produces an inflammatory state, increasing IL-6 in pancreatic tissue(36,37). This review further indicated that a higher DII score (means a more pro-inflammatory and less anti-inflammatory diet) may increase the risk of PC(36).
Subgroup analyses showed case–control subgroup had a more significant positive correlation between the DII score and PC risk compared with the cohort subgroup. However, case–control studies are more prone to bias in selection, recall and reverse causality. This difference may be due to the fact that there are only two articles included in the cohort subgroup, and both articles are from the same author(33,34). In the subgroup analysis, the number of components used to calculate DII scores decreased and the associations increased instead, suggesting that the reduction in the number of components strengthened the link between DII scores and PC risk. However, owing to the small number of studies, we were unable to explore how this relationship changed with changes in DII components. Therefore, this result had some limitations and needed to be interpreted carefully. The eating habits of Western and Asia are different. However, in subgroup analysis based on countries and regions, three subgroups have similar results on the link between DII score and PC risk. The reason may be that only one study involved the Asian race(31). Meanwhile, the current study did not perform subgroup analysis based on region, only based on the whites and other races. Moreover, the results were adjusted for race. We further explored the risk factors adjustment, including a family history of PC, race, alcohol consumption and total energy intake, using subgroup analyses, but the positive association was not found.
Several potential mechanisms have been proposed to explain the possible association between pro-inflammatory diet (means higher DII score) and PC risk. In PC, down-regulated tumour suppressor miRNA and up-regulated oncogenic miRNA are considered to be associated with tumour growth and metastases(38). Growth factors and cytokines might enhance or inhibit the expression levels of miRNA. Also, the expression of growth factors, cytokines and their receptors might be regulated by miRNA(39). Meanwhile, anti-TNF-α can up-regulate some miRNA (i.e., hsa-miR-23a, hsa-miR-197 and hsa-miR-221, etc.) which are considered to be associated with PC(39), which means cancer-associated inflammatory responses might be influenced by the balance between cancer-derived and inflammatory cell-derived cytokines and chemokines. In this case, TNF-α, produced by tumour cells themselves and tumour-infiltrating inflammatory cells, is involved in tumourigenesis, growth, metastasis and anti-cancer immune regulation(39). In a systematic review, the authors summarised that levels of IL-2, IL-6, IL-8, IL-10, macrophage colony-stimulating factor, macrophage inhibitory cytokine-1 and vascular endothelial growth factor were higher in patients with PC than in those without PC(40). And the DII scoring system exactly focuses on the dietary inflammatory potential.
Another potential mechanism between inflammatory and PC was the activation of transcription factors NF-κB. A recent study reported that the activation of NF-κB promotes the production of growth and differentiation factor 15, a member of the tumour growth factor-β superfamily, in pancreatic cells. Growth and differentiation factor-15 acts on tumour-related macrophages by inhibiting NF-κB signalling and reduces the synthesis of TNF-α, thereby reducing TNF-α-dependent tumour cell apoptosis and enhancing tumour growth(41).
Some limitations should be considered in the present meta-analysis. First, owing to the small number of studies and no randomised controlled trials included, meta-analyses results may be affected, but the included studies all have good quality. Second, high heterogeneity was observed in the meta-analysis results. Further subgroup analyses and sensitivity analyses were conducted, and it was found that the heterogeneity might be due to the different designs of the included studies. Third, we did not assess the publication bias using the formal statistical tests as they have insufficient power when there are limited studies (n < 10). Besides, the research population is mainly limited to Europe and the USA, which may lead to the results cannot be promoted globally.
The present meta-analysis also has some strengths. First, the DII score used in each study is calculated based on the same dietary assessment tool (FFQ), which increases the comparability of the study. Second, the exclusion of another individual study also did not have a significant impact on the results of the meta-analysis, indicating the stability of the results.
In conclusion, the analysis of the evidence from included studies suggested dietary habits with high inflammatory features (high DII score) might increase PC risk. However, these findings should be interpreted with caution due to the limited number of studies and potential bias, and the need for further validation. Future studies would benefit from improved designs, larger sample sizes and better confounding controls and should emphasise the potential dose–response effect on DII score and PC risk.
Acknowledgements
Acknowledgments: We would like to thank Ph.D. Min Zhao for providing language help and writing assistance. Financial support: The current study was supported by the National Natural Science Foundation of China (grant no. 81701950), Medical Research Projects of Chongqing (grant no. 2018MSXM132) and the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (grant no. KY2019Y002). Conflict of interest: There are no conflicts of interest. Authorship: Z.G. and Y.C. designed the research; Z.G., Y.H. and Y.C. conducted the research; Z.G. analysed the data; Z.G. wrote the paper. Y.C. had primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: not applicable. Because analysis is only based on published article data.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021001579.
click here to view supplementary material
References
- 1. Siegel RL, Miller KD & Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Mizrahi JD, Surana R, Valle JW et al. (2020) Pancreatic cancer. Lancet 395, 2008–2020. [DOI] [PubMed] [Google Scholar]
- 3. Kirkegård J, Mortensen FV & Cronin-Fenton D (2017) Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol 112, 1366–1372. [DOI] [PubMed] [Google Scholar]
- 4. Koyanagi YN, Matsuo K, Ito H et al. (2018) Body-mass index, pancreatic cancer incidence: a pooled analysis of nine population-based cohort studies with more than 340 000 Japanese subjects. J Epidemiol 28, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aziz MH, Sideras K, Aziz NA et al. (2019) The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on Bilirubin levels: a retrospective multicenter cohort study. Ann Surg 270, 139–146. [DOI] [PubMed] [Google Scholar]
- 6. Stone ML & Beatty GL (2019) Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther 201, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng R, Morine Y, Ikemoto T et al. (2018) Nab-paclitaxel interrupts cancer-stromal interaction through C-X-C motif chemokine 10-mediated interleukin-6 downregulation in vitro. Cancer Sci 109, 2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karakhanova S, Link J, Heinrich M et al. (2015) Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology 4, e998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kratochvill F, Neale G, Haverkamp JM et al. (2015) TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep 12, 1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomura A, Gupta VK, Dauer P et al. (2018) NFκB-mediated invasiveness in CD133+ pancreatic TICs is regulated by autocrine and paracrine activation of IL1 signaling. Mol Cancer Res 16, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Zoltan M, Riquelme E et al. (2018) Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology 155, 210–223.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christ A, Lauterbach M & Latz E (2019) Western diet and the immune system: an inflammatory connection. Immunity 51, 794–811. [DOI] [PubMed] [Google Scholar]
- 13. Munch Roager H, Vogt JK, Kristensen M et al. (2019) Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut 68, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiao L, Chen L, White DL et al. (2018) Low-Fat dietary pattern, pancreatic cancer risk in the women’s health initiative dietary modification randomized controlled trial. J Natl Cancer Inst 110, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shivappa N, Steck SE, Hurley TG et al. (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17, 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu DL, Ren ZJ, Zhang Q et al. (2018) Meta-analysis of the association between the inflammatory potential of diet, urologic cancer risk. PLoS One 13, e0204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayedi A, Emadi A & Shab-Bidar S (2018) Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Adv Nutr 9, 388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu ZY, Gao XP, Zhu S et al. (2019) Dietary inflammatory index and risk of gynecological cancers: a systematic review and meta-analysis of observational studies. J Gynecol Oncol 30, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Liu C, Zhou C et al. (2019) Meta-analysis of the association between the dietary inflammatory index (DII), breast cancer risk. Eur J Clin Nutr 73, 509–517. [DOI] [PubMed] [Google Scholar]
- 20. Shivappa N, Godos J, Hébert JR et al. (2017) Dietary inflammatory index and colorectal cancer risk: a meta-analysis. Nutrients 9, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, 332–336. [PMC free article] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O’connell D et al. (2014) The Newcastle-Ottawa Scale NOS for assessing the quality of nonrandomized studies in meta-analysis. Appl Eng Agric 18, 727–734. [Google Scholar]
- 23. Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9, 1–30. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. Br Med J 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orsini N, Bellocco R & Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6, 40–57. [Google Scholar]
- 26. Orsini N, Li R, Wolk A et al. (2012) Meta-analysis for linear, nonlinear dose-response relations: examples, an evaluation of approximations, software. Am J Epidemiol 175, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson D, White IR & Thompson SG (2010) Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 29, 1282–1297. [DOI] [PubMed] [Google Scholar]
- 28. Desquilbet L & Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29, 1037–1057. [DOI] [PubMed] [Google Scholar]
- 29. Accardi G, Shivappa N, Di Maso M et al. (2019) Dietary inflammatory index and cancer risk in the elderly: a pooled-analysis of Italian case-control studies. Nutrition 63, 205–210. [DOI] [PubMed] [Google Scholar]
- 30. Antwi SO, Oberg AL, Shivappa N et al. (2016) Pancreatic cancer: Associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinog 37, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antwi SO, Bamlet WR, Pedersen KS et al. (2018) Pancreatic cancer risk is modulated by inflammatory potential of diet and ABO genotype: a consortia-based evaluation and replication study. Carcinog 39, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shivappa N, Bosetti C, Zucchetto A et al. (2015) Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr 113, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng J, Wirth MD, Merchant AT et al. (2019) Inflammatory potential of diet, inflammation-related lifestyle factors, and risk of pancreatic cancer: results from the NIH-AARP Diet and health study. Cancer Epidemiol Biomarkers Prev 28, 1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng J, Merchant AT, Wirth MD et al. (2018) Inflammatory potential of diet and risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Int J Cancer 142, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng J, Guinter MA, Merchant AT et al. (2017) Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev 75, 883–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lauby-Secretan B, Scoccianti C, Loomis D et al. (2016) Body fatness and cancer: viewpoint of the IARC working group. N Engl J Med 375, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kerr J, Anderson C & Lippman SM (2017) Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol 18, e457–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hawa Z, Haque I, Ghosh A et al. (2016) The miracle in pancreatic cancer by miRNAs: tiny angels or devils in disease progression. Int J Mol Sci 17, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Padoan A, Plebani M & Basso D (2019) Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci 20, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yako YY, Kruger D, Smith M et al. (2016) Cytokines as biomarkers of pancreatic ductal adenocarcinoma: a systematic review. PLoS One 11, e0154016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ratnam NM, Peterson JM, Talbert EE et al. (2017) NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J Clin Invest 127, 3796–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021001579.
click here to view supplementary material