Abstract
Interferon (IFN) mediates its antiviral effects by inducing a number of responsive genes, including the double-stranded RNA (dsRNA)-dependent protein kinase, PKR. Here we report that inducible overexpression of functional PKR in murine fibroblasts sensitized cells to apoptosis induced by influenza virus, while in contrast, cells expressing a dominant-negative variant of PKR were completely resistant. We determined that the mechanism of influenza virus-induced apoptosis involved death signaling through FADD/caspase-8 activation, while other viruses such as vesicular stomatitis virus (VSV) and Sindbis virus (SNV) did not significantly provoke PKR-mediated apoptosis but did induce cytolysis of fibroblasts via activation of caspase-9. Significantly, treatment with IFN-α/β greatly sensitized the fibroblasts to FADD-dependent apoptosis in response to dsRNA treatment or influenza virus infection but completely protected the cells against VSV and SNV replication in the absence of any cellular destruction. The mechanism by which IFN increases the cells' susceptibility to lysis by dsRNA or certain virus infection is by priming cells to FADD-dependent apoptosis, possibly by regulating the activity of the death-induced signaling complex (DISC). Conversely, IFN is also able to prevent the replication of viruses such as VSV that avoid triggering FADD-mediated DISC activity, by noncytopathic mechanisms, thus preventing destruction of the cell.
The interferons (IFNs) are cytokines with potent antiviral, antiproliferative, and immunomodulatory activity that are categorized into two major subtypes: alpha/beta (IFN-α/β) and gamma (IFN-γ). IFN-α and -β are synthesized by most cell types and share a common receptor, while IFN-γ is predominantly produced from T lymphocytes (37). Although considerable evidence indicates that the IFNs constitute a vital component of host antiviral and antitumor defense, the detailed mechanisms of their effects against viruses and cancer as well as their role in apoptosis remain elusive. However, it is known that the IFNs exert their multiple properties by inducing a number of cellular genes, one of the best characterized being the double-stranded RNA (dsRNA)-dependent, serine/threonine protein kinase, PKR (28). Interaction with dsRNA causes PKR to autophosphorylate and to catalyze the phosphorylation of substrate targets, the best described being the alpha subunit of eukaryotic initiation factor 2 (eIF2α), which causes a reduction in protein synthesis rates in the cell (24, 28). PKR has also been proposed to function in signaling cascades regulated by dsRNA and to influence the control of selected pathways such as those involving NF-κB, platelet-derived growth factor, and IRF-1 (21, 29, 43). More recently, it has similarly been revealed that PKR plays a critical role in mediating dsRNA-induced apoptosis in the cell (2, 8, 23, 36, 38, 45).
The process of apoptosis, or programmed cell death, is an important mechanism used by the host to limit virus production following infection of the cell. One mechanism of apoptosis can occur through ligation of cell surface death receptors such as Fas/CD95, a member of the tumor necrosis factor receptor (TNFR) family (13). This event leads to the recruitment and activation of an adapter protein, FADD (Fas-associated death domain-containing protein), and to the subsequent activation of caspases, a family of cysteine proteases that exist as inactive zymogens in normal cells (5, 34). The apical target of FADD is caspase-8/FLICE (30). Activated caspase-8 is able to cleave additional downstream caspases, which include caspase-3, to ultimately elicit the morphological hallmarks of apoptosis, including DNA fragmentation and cell shrinkage. In addition to this mechanism of death signaling, it is apparent that the mitochondrion similarly plays a key role in the regulation of apoptosis. For example, active caspase-8 is also known to activate Bid, a proapoptotic member of the Bcl-2 family of proteins that can trigger a loss in mitochondrial transmembrane potential and cause an efflux of cytochrome c into the cytoplasm (26). Cytochrome c then complexes with Apaf-1 to activate procaspase-9, which in turn targets and activates caspase-3 (22, 42). Other members of the Bcl-2 family, including Bcl-2 itself (antiapoptotic) and Bax (proapoptotic), can also influence the cell death process, possibly by acting as outer mitochondrial membrane channel proteins that regulate cytochrome release into the cytosol (20).
Activation of apoptosis by the host cell in response to virus infection is highly detrimental to the virus, at least until progeny viruses have been safely replicated. Thus, numerous viruses have evolved a variety of mechanisms to neutralize not only the antiviral effects of IFN and their inducible genes but also host-mediated programmed cell death (15, 27, 40). For example, cowpox virus CrmA and baculovirus p35 have been shown to inhibit caspase activation, while adenovirus encodes E1B 19K, a Bcl-2 homologue that prevents mitochondrial, and in certain cases caspase-8, input into Apaf-1-mediated activation of the executioner caspase-3 (40). In addition, it is well documented that viruses such as vaccinia virus (E3L and K3L), adenovirus (VAI RNA), and hepatitis C virus (NS5a and E2) encode products to inhibit IFN signaling pathways and IFN-inducible gene products such as the key antiviral protein, PKR (7, 10, 15, 27, 39).
Efforts to analyze the antiviral function of IFN have included attempting to examine the cellular expression of individual IFN-induced genes, such as the PKR gene, in the absence of other IFN or virus-induced products. In the case of PKR, this has proven difficult since previous efforts to establish cell lines that overexpress the kinase failed due to PKR's toxic phenotype (4, 18, 19). To overcome this obstacle, we established tetracycline-regulated 3T3 L1 cell lines that inducibly express wild-type PKR or a catalytically inactive dominant-negative PKR variant referred to as PKRΔ6 (2). PKR-overexpressing cells were greatly sensitized to the effects of dsRNA, and treatment with synthetic dsRNA poly(I-C) caused autophosphorylation of the heterologous PKR and the induction of Fas and TNFR-1 and initiated cellular apoptosis via activation of the FADD death signaling pathway (2). Conversely, fibroblasts expressing the dominant-negative variant PKRΔ6 lacked a number of key proapoptotic signaling components such as FADD and Bax and were resistant to dsRNA-, Fas-, and TNF-α-induced cell death (2).
To extend our studies on the antiviral and proapoptotic properties of IFN and PKR, we examined the effects of infection of murine cells overexpressing functional PKR or the dominant-negative variant PKRΔ6 with selected RNA viruses. We determined that cells overexpressing PKR were significantly more sensitive to apoptosis induced by influenza A/WSN virus (WSN), but not by vesicular stomatitis virus (VSV) or Sindbis virus (SNV), compared to control cells carrying the vector alone. Significantly, treating control fibroblasts or PKR-overexpressing cells with IFN-α/β greatly enhanced WSN-induced apoptosis and reduced viral replication. The mechanism of WSN induced apoptosis was demonstrated to be via activation of the FADD/caspase-8 pathway. Conversely, VSV and SNV viral replication principally triggered apoptosis through Apaf-1-mediated activation of caspase-9. Notably, IFN-α/β treatment completely blocked the replication of VSV and SNV without the induction of apoptosis and any damage to the cell. Thus, IFN-α/β can concomitantly invoke disparate mechanisms to prevent virus replication. One antiviral pathway involves sensitization of cells to apoptosis through death-induced signaling complexes (DISC), dependent on FADD, while a second involves the inhibition of viral replication in the absence of any damage to the host. The physiological and clinical significance of these observations is discussed.
MATERIALS AND METHODS
Cell lines.
The establishment and maintenance of cell lines inducibly expressing functional PKR and PKRΔ6 in response to tetracycline and doxycycline (Dox) have been previously reported (2). Cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Gibco-BRL, Gaithersburg, Md.) in the presence or absence of Dox (5 μg/ml) for 2 days before experiments were performed. FADD- and Apaf-1-deficient fibroblasts were generated as described previously (44, 46). Murine fibroblast IFN (IFN-α/β) used in these studies was obtained from Sigma (St. Louis, Mo.) and used to treat cells at 1,000 U/ml for 18 h prior to infection.
Virus growth and infection studies.
Cells were infected with virus at a multiplicity of infection (MOI) of 10 (32). Titers of released virus were determined by standard plaque assay of serially diluted virus suspensions either in MDCK cells in the presence of trypsin for WSN or in BHK-21 cells for VSV (Indiana strain) (3) and SNV (6). Virus yields were expressed as PFU of released virus per cell.
Immunoblot analysis.
Western blot analyses were carried out following disruption of cells in lysis buffer as described previously (2). Proteins were electrophoresed on sodium dodecyl sulfate–10% polyacrylamide gels and transferred to nitrocellulose. After blocking in milk extract, blots were incubated with antibodies as described previously (2). Antibodies to murine Fas and PKR were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.); antibody directed to phosphorylated and total eIF2α has been reported previously (2). Anti-FADD and human PKR antibodies were gifts from L. Boise and A. Hovanessian, respectively.
Apoptosis and cell viability analysis.
After 12 to 48 h, virus-infected cells were washed in cold phosphate-buffered saline and incubated for 15 min with fluorescein-conjugated annexin V- and propidium iodide (R&D Systems, Minneapolis, Minn.). Flow cytometry of annexin V- and propidium iodide-stained cells was performed on a Becton Dickinson FACScan machine and analyzed with CellQuest software. Cell viability was determined by trypan blue exclusion analysis. For TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-FITC nick end labeling) assays, cells were fixed with paraformaldehyde (1%) at room temperature and labeled as described by the manufacturer (In Situ Cell Death Detection kit; Boehringer Mannheim, Indianapolis, Ind.). Caspase activity was measured with an Apoalert kit (Clontech, Palo Alto, Calif.).
RESULTS
Cells overexpressing PKR are sensitized to WSN-induced apoptosis.
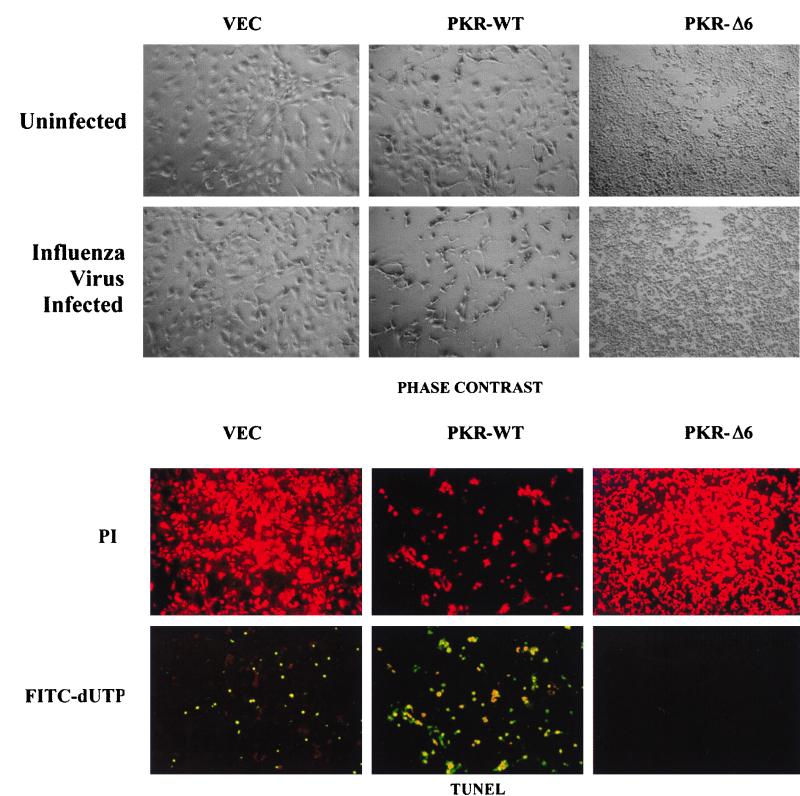
To analyze the antiviral role of PKR in the absence of other IFN-induced proteins, we infected murine 3T3 L1 cell lines that overexpress wild-type PKR or a catalytically inactive PKR variant, PKRΔ6, with WSN, a negative-stranded RNA virus with a segmented genome (32). We initially chose WSN since previous studies had demonstrated the induction of Fas/CD95 and subsequent apoptosis in cells infected with this member of the orthomyxovirus family (38). Importantly, studies by our own group had demonstrated that activation of PKR by dsRNA induces apoptosis by enhancing Fas expression and by triggering FADD-dependent death signaling (2). Taken together, the data suggested the possibility that PKR played a role in influenza virus-induced apoptosis. For this analysis we used PKR-inducible murine fibroblasts, which, although not fully permissive to WSN replication, permit viral protein synthesis and produce low levels of progeny virus (9). PKR-expressing cells were thus infected with WSN for up to 48 h. After 24 h of infection, WSN-infected control and wild-type PKR-overexpressing cells started to morphologically exhibit signs of apoptosis, including blebbing of the plasma membrane and cell shrinkage (Fig. 1, top). In contrast, cells expressing PKRΔ6, which morphologically appear smaller as a result of being malignantly transformed, did not show any evidence of apoptosis or loss of cell viability even 72 h postinfection. To further examine if apoptosis was in fact occurring in cells infected with WSN, cells were fixed and examined by TUNEL, an assay that detects DNA strand breakage, a hallmark of apoptosis. As can be seen in the bottom part of Fig. 1, a small proportion (<10%) of the WSN-infected control cells expressing vector alone showed signs of DNA fragmentation. However, nearly all of the WSN-infected PKR-overexpressing cells exhibited TUNEL staining as determined by fluorescence microscopy. In contrast, cells expressing PKRΔ6 showed no evidence of TUNEL staining or cell death, indicating resistance to apoptosis. To complement our analysis, WSN-infected cells overexpressing PKR or PKRΔ6 were analyzed by annexin V staining, which measures the presence of phosphatidylserine on the outer membrane of apoptotic cells. Approximately 56% of the PKR-overexpressing, virus-infected cells exhibited loss of phosphatidylserine to the outer membrane, compared to less than 17% of the control cells (data not shown). Again, cells expressing PKRΔ6 showed no evidence of apoptosis, as determined by annexin V staining (data not shown) or trypan blue exclusion analysis (see Fig. 3a). Collectively, these data indicate that cells overexpressing PKR are sensitized not only to dsRNA-induced apoptosis but also to apoptosis induced by WSN. Comparable results were obtained with parainfluenza virus type III (data not shown). In contrast, cells expressing the dominant-negative PKRΔ6 are resistant to WSN-induced apoptosis, as well as to apoptosis mediated by dsRNA, Fas ligand, and TNF-α, as we have previously demonstrated (2).
FIG. 1.
Cells inducibly expressing PKR are sensitive to WSN-induced apoptosis. Murine 3T3 L1 cells (VEC, control cells carrying vector alone; PKR-WT, cells overexpressing PKR under control of a tetracycline/doxycycline-inducible promoter; PKR-Δ6, cells overexpressing a dominant-negative variant of PKR under the same inducible promoter) were infected with WSN at an MOI of 10. (Top) Cells mock infected or infected with WSN were photographed 24 h postinfection (magnification, ×100). (Bottom) WSN-infected cells were fixed and incubated with the TUNEL reaction mixture (see Materials and Methods) to detect apoptotic cells and subsequently stained with propidium iodide to detect all cells (magnification, ×100).
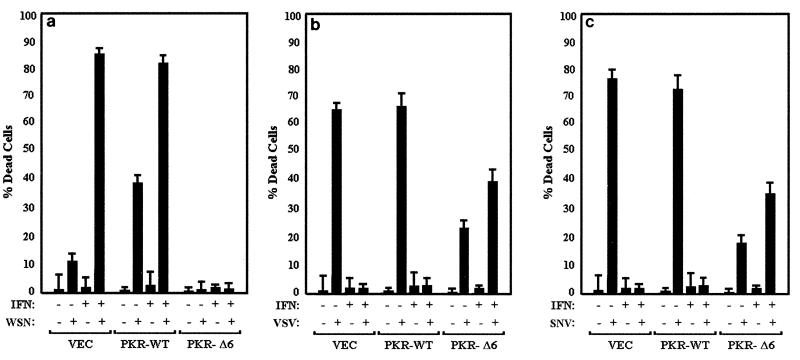
FIG. 3.
IFN sensitizes infected cells to or protects them from apoptosis. IFN-treated or untreated 3T3 L1 control cells (VEC) or cells inducibly overexpressing wild-type PKR (PKR-WT) or a dominant-negative variant (PKR-Δ6) were mock infected or infected with WSN (a), VSV (b), or SNV (c) at an MOI of 10 in the presence or absence of IFN pretreatment; 24 (WSN) or 48 (VSV and SNV) h later, trypan blue exclusion analysis was performed. Data represent the mean from three experiments ± standard deviation.
Role of IFN in virus-induced apoptosis.
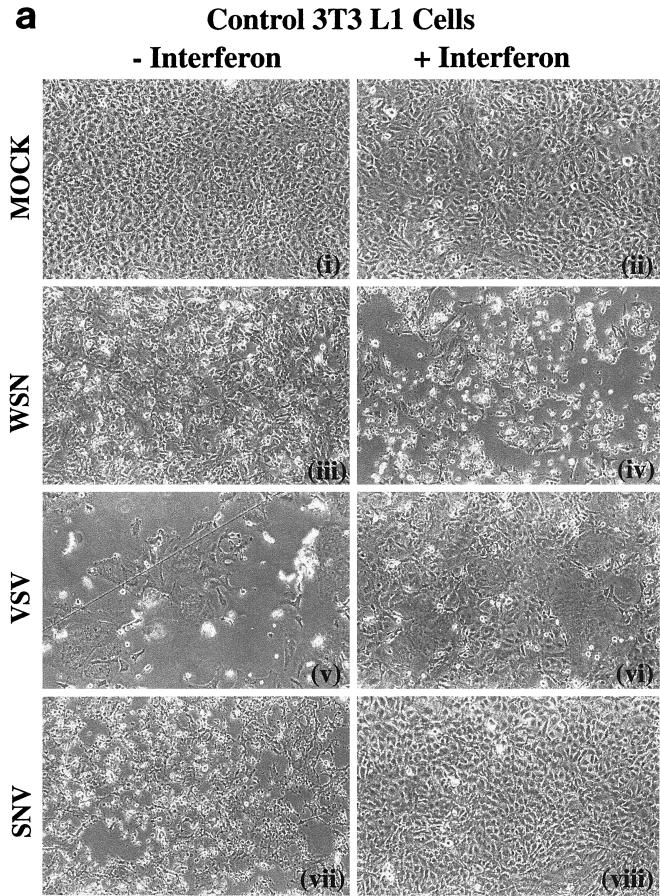
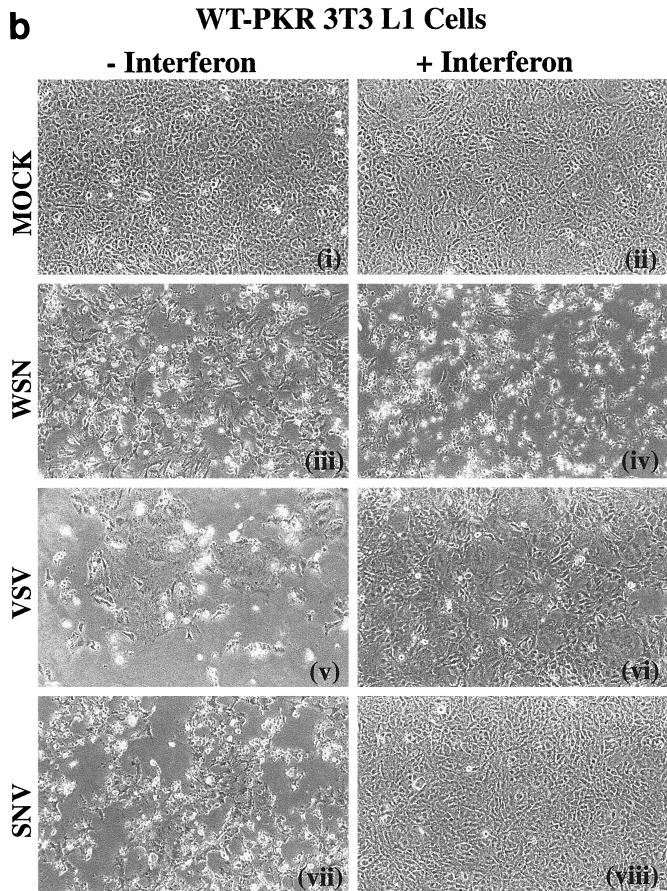
To further examine the role of PKR in IFN-mediated antiviral host defense, we infected control cells or cells overexpressing wild-type PKR or variant PKRΔ6 with the rhabodovirus VSV or the alphavirus SNV. Both viruses are known to be sensitive to the antiviral effects of IFN (37). As part of these studies, we also examined the effects of IFN on the cell's response to viral infection. Figure 2 shows the morphologies of cells expressing vector or wild-type PKR treated with or without IFN and of cells mock infected or infected with either WSN, VSV, or SNV. As described earlier, WSN infection resulted in the induction of apoptosis in the control cells, with an enhanced effect in cells overexpressing PKR [Fig. 2a (iii) and 2b (iii)]. However, pretreatment with IFN augmented the virus-induced apoptotic effect in both cell types [Fig. 2a (iv) and 2b (iv)]. To complement our morphological assessment, we also tested the infected cells by trypan blue exclusion analysis. Over 80% of IFN-treated, WSN-infected control cells or cells overexpressing PKR were unable to exclude trypan blue, indicating loss of viability and compromised plasma membrane (Fig. 3a). That this effect was apoptotic was confirmed, as in Fig. 1, by both the TUNEL assay and annexin V staining. Trypan blue exclusion analysis revealed that cells expressing PKRΔ6 remained viable after WSN infection, whether IFN pretreated or not, presumably because these cells lack key components of the apoptotic signaling pathway (2) (Fig. 3a).
FIG. 2.
Micrographs of WSN-, VSV-, and SNV-infected cells inducibly overexpressing PKR and treated with IFN. 3T3 L1 cells carrying vector alone (a) or PKR-overexpressing 3T3 L1 cells (b) were mock infected or infected with WSN, VSV, or SNV at an MOI of 10 in the presence (+) or absence (−) of murine fibroblast IFN-α/β (1,000 U/ml) pretreatment. Photomicrographs were taken 24 (WSN) or 48 (VSV and SNV) h postinfection at a magnification of ×100. FIG. 2—Continued.
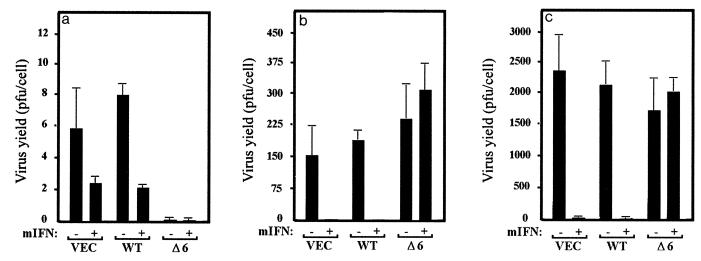
To extend our analysis, viral yields were measured in the IFN-treated, WSN-infected cells. Figure 4a shows that WSN infection is essentially nonproductive in 3T3 L1 cells, with PFU-per-cell yields being less than 10 in the control and PKR-expressing cells. However, IFN pretreatment reduced viral production two- to fourfold in both control and PKR-overexpressing cell types to barely detectable levels. Cells expressing PKRΔ6 did not appear to produce any WSN whether they were treated with IFN or not, for reasons that remain undetermined. Given the low virus yields, it remained conceivable that WSN was not efficiently infecting any of the cell lines. However, immunofluorescent staining of WSN-infected cells overexpressing the wild-type PKR or PKRΔ6, or control cells with anti-WSN antiserum (as well as immunoprecipitating WSN nucleoprotein), confirmed that the virus was indeed infecting the cells and that synthesis of viral proteins was occurring (data not shown). Taken together, our data indicate that PKR can play a role in IFN-mediated host defense against WSN infection by sensitizing the cells to virus-induced apoptosis. This effect was significantly enhanced by pretreatment with IFN but not in cells expressing PKRΔ6.
FIG. 4.
Effects of PKR and IFN on viral production. IFN-treated or untreated 3T3 L1 cells (VEC) inducibly expressing wild-type PKR (WT) or dominant-negative variant PKR-Δ6 (Δ6) were infected with WSN (a), VSV (b), or SNV (c) at an MOI of 10; 24 (WSN) or 48 (VSV and SNV) h later, samples of medium from the infected cells were retrieved and viral production was determined by plaque assay. Data represent means of three experiments ± standard deviation.
Interestingly, a different pattern of response can be seen in PKR-overexpressing cells infected with VSV or SNV. Infection of either control or wild-type PKR-overexpressing cells with VSV caused extensive cell death after 48 h, as determined morphologically and by trypan blue exclusion analysis [Fig. 2a (v), 2b (v), and 3b]. Also, in contrast to WSN, VSV-mediated apoptosis was completely inhibited in the control and PKR-overexpressing cells following treatment with IFN [Fig. 2a (vi) and 2b (vi)]. Pretreatment of the cells with IFN not only protected the cells against cell death but also greatly limited the production of progeny viruses (Fig. 3b and 4b). Interestingly, cell death was also observed in VSV-infected cells expressing PKRΔ6, probably as a direct result of virus replication and damage to the cell. IFN did not inhibit VSV production or apoptosis in PKRΔ6-expressing cells, indicating that the mechanism of the IFN antiviral response to VSV infection had been compromised.
Infection of 3T3 L1 cells with SNV showed a pattern of response similar to those observed in VSV-infected cells [Fig. 2a (vii) and 2b (vii)]. For example, IFN also protected against SNV-induced apoptosis and viral replication in the control or PKR-overexpressing cells but not in cells expressing PKRΔ6 [Fig. 2 (viii), 3c, and 4c].
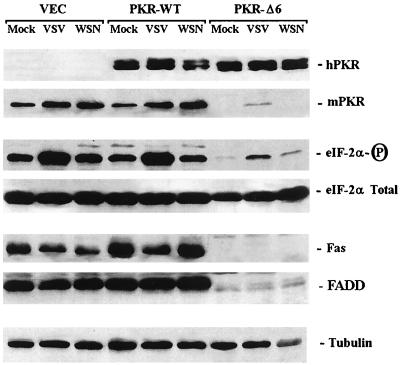
To complement the above study, we analyzed the levels of PKR and eIF2α in WSN- and VSV-infected cells. Immunoblot analysis revealed a slightly higher level of the endogenous murine PKR following infection with WSN and VSV (Fig. 5). For reasons that remain to be clarified, endogenous PKR is ablated in cells expressing PKRΔ6; consequently, phosphorylated eIF2α levels are very low, as we have previously reported (2). Interestingly, levels of eIF2α phosphorylation greatly increased in response to VSV infection but not significantly in cells infected with WSN. These data were complemented by analyzing PKR activity during viral infection, and phosphorylation of PKR was seen to increase slightly in response to VSV but not following infection by WSN as has been described by other groups (16) (data not shown).
FIG. 5.
Protein analysis of PKR, eIF2α, and Fas. Murine 3T3 L1 cells (VEC) inducibly expressing wild-type PKR (PKR-WT) or a dominant-negative variant (PKR-Δ6) were infected with VSV or WSN; 24 h postinfection, equal amounts of cell lysates were examined by Western blotting using antibodies to human PKR (hPKR), the endogenous murine PKR (mPKR), phosphorylated eIF2α (eIF-2α-P) total eIF2α, Fas, and FADD. Similar levels of tubulin in each lane confirm that equivalent amounts of protein were loaded.
Differential activation of caspases in virus-infected 3T3 L1 cells.
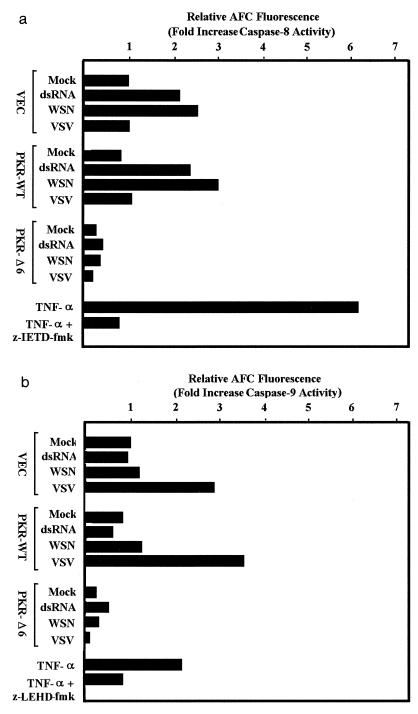
To start to dissect the mechanisms of virus-induced apoptosis, we analyzed cell extracts previously infected with WSN and VSV for activation of known caspases (34). Binding of ligand to death receptors such as Fas or TNFR-1 leads to recruitment and activation of FADD, which in turn leads to cleavage of caspase-8 (5, 30). This event causes activation of the executioner caspases, such as caspase-3, leading directly to the apoptotic disassembly of the cell. Cell death signals can also be initiated through the release of cytochrome c resulting from mitochondrial damage. This pathway requires the function of Apaf-1, which is responsible for the recruitment and subsequent activation of caspase-9 (22, 42). Cross talk between the two (i.e., the cell surface and mitochondria) pathways can also occur through caspase-8-mediated cleavage of Bid, a member of the Bcl-2 family, an effect which induces the release of cytochrome c (26). To measure caspase-8 protease activity in infected cells, we used the substrate 7-amino-4-trifluoromethylcoumarin (AFC) conjugated to the tetrapeptide Ile-Glu-Thr-Asp (IETD). Cleavage of this substrate by caspase-8 releases AFC, which can be measured fluorometrically. Our results indicated that extracts from dsRNA- and WSN-treated cells contained two- to threefold more caspase-8 activity than extracts from untreated cells (Fig. 6a). However, little activation of caspase-8 occurred in VSV- or SNV-infected cells, even though the cells were clearly undergoing apoptosis as determined morphologically by annexin V staining and by TUNEL assay (Fig. 2 and 3 and data not shown). Similarly treated PKRΔ6-expressing cells demonstrated very little IETD-AFC cleavage because of the absence of caspase-8 in these cells (2). These data indicate that following VSV and SNV infection, apoptosis was being induced through a pathway independent of caspase-8 activation. To investigate this further, we used AFC-conjugated Leu-Glu-His-Asp (LEHD), a fluorogenic substrate for caspase-9. Our data indicated that caspase-9 activity was increased threefold in VSV-infected control and PKR-overexpressing cells, indicating the involvement of the mitochondria in mediating cell death. Very little caspase-9 activation was observed in PKRΔ6-expressing cells, perhaps due to low levels of caspase-9 (data not shown).
FIG. 6.
Caspase activity in cells infected with WSN or VSV. Control 3T3 L1 cells (VEC) or 3T3 L1 cells overexpressing wild-type PKR (PKR-WT) or a dominant-negative variant (PKRΔ6) were transfected with poly(I-C) by using Lipofectamine (Gibco-BRL) or infected with WSN or VSV at an MOI of 10. After 24 h, cell lysates were prepared and incubated with either z-IETD-AFC, the caspase-8 fluorogenic substrate (a), or z-LEHD-AFC, the caspase-9 fluorogenic substrate (Apoalert; Clontech) (b). Samples were excited at 400 nm, and fluorescent emissions were read at 505 nm on a fluorometer. As controls, cell lysates prepared from 3T3 L1 cells treated for 18 h with 50 ng of murine TNF-α (R&D Systems) per ml were preincubated with or without the caspase-8-specific inhibitor z-LEHD-fmk or the caspase-9 specific blocker z-LEHD-fmk prior to incubation with the fluorogenic substrates as described above. Data represent means of two experiments.
We have previously shown that PKR-mediated, dsRNA-induced apoptosis also coincides with the upregulation of Fas (2). To extend these analyses, we measured, by immunoblotting, Fas and FADD levels in virus-infected, PKR-overexpressing cells. Our data indicated that cells overexpressing PKR contained somewhat more Fas compared to controls, (Fig. 5), as we have previously shown (2). Fas levels decreased in VSV-infected cells and did not markedly rise in cells infected with WSN. Similarly, FADD levels also appeared to be moderately higher in cells overexpressing PKR and did not appear to fluctuate significantly during viral infection.
IFN-induced apoptosis requires FADD.
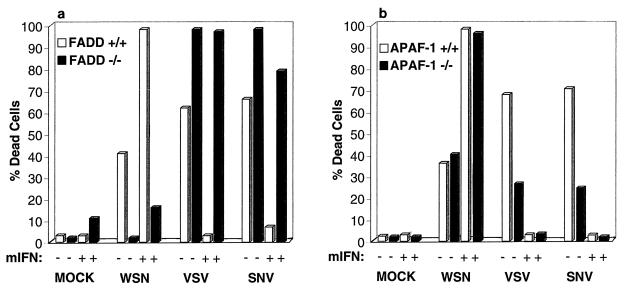
To further clarify the mechanisms of IFN-stimulated apoptosis in the virus-infected cells, we used embryonic fibroblasts that lack the key death receptor signal transducer FADD (44). Although mice lacking FADD die in utero, FADD-deficient embryonic fibroblasts can be generated and were infected with WSN as well as VSV and SNV. Morphological examination, annexin V staining, and trypan blue exclusion analysis revealed that murine embryonic fibroblasts lacking FADD had reduced sensitivity to WSN-induced apoptosis compared to control fibroblasts (Fig. 7a and data not shown). Strikingly, WSN viral yields were dramatically increased in FADD−/− cells (Table 1). Similar to the effects of dsRNA, WSN-induced apoptosis was enhanced by IFN in the control embryonic fibroblasts but not in cells lacking FADD, signifying that IFN mediates apoptosis through activation of caspase-8 and perhaps other caspases that associate with FADD and the DISC. Interestingly, IFN treatment reduced WSN titers in the absence of FADD, indicating that IFN may stimulate other antiviral mechanisms in addition to apoptosis. However, VSV and SNV induced apoptosis equally well whether the cells contained FADD or not, indicating that the FADD death signaling pathway is not the predominant pathway activated by these viruses in these cells. In fact, lack of FADD increased the degree of apoptosis induced by VSV and SNV by mechanisms that remain to be clarified.
FIG. 7.
Murine fibroblasts lacking FADD but not Apaf-1 show resistance to WSN-induced apoptosis. Fibroblasts lacking FADD (a) or Apaf-1 (b) were infected with WSN, VSV, or SNV at an MOI of 10; 24 (WSN) or 48 (VSV and SNV) h postinfection, cell viability was determined by trypan blue exclusion analysis. Data represent means of two experiments.
TABLE 1.
Viral titers in cells lacking FADD and Apaf-1a
| Cell type | IFN | Viral titer
|
||
|---|---|---|---|---|
| WSN | VSV | SNV | ||
| Control | − | <1 | <1 | 36 |
| + | <1 | <1 | 5 | |
| FADD−/− | − | 1,975 | 1,253 | 640 |
| + | 89 | 100 | 333 | |
| Apaf-1−/− | − | 25 | 55 | 323 |
| + | 1 | <1 | <1 | |
Control, FADD-deficient, or Apaf-1-deficient cells were infected with WSN, VSV, or SNV at an MOI of 10, and virus progeny yield was measured 24 (WSN) or 48 (VSV and SNV) h postinfection as described in Materials and Methods.
To further evaluate the mechanisms of WSN-, VSV-, and SNV-induced apoptosis, we examined the response of murine embryonic fibroblasts lacking Apaf-1 to infection (46). Figure 7b indicates that WSN induced cell death equally well in cells containing or lacking Apaf-1 and that IFN enhanced this apoptotic effect in both cell types. However, both VSV and SNV induced markedly less apoptosis in cells devoid of Apaf-1. VSV and SNV titers were also higher in the Apaf-1-lacking cells than in control cells, indicating that reduction in apoptosis may facilitate the replication of these viruses (Table 1). However, IFN treatment also prevented VSV-induced apoptosis as well as viral progeny replication (37). It is therefore plausible that VSV inadvertently triggers host-mediated cell death following infection of the cell. Thus, aside from confirming that IFN sensitizes cells to WSN-induced apoptosis largely independently of Apaf-1 and principally through activation of FADD, these data suggest that VSV and SNV trigger apoptosis in fibroblasts predominantly through activation of the mitochondrion-related Apaf-1/caspase-9 pathway.
DISCUSSION
The IFNs, important components of host defense, are known to manifest their multiple properties by inducing a number of cellular genes. IFN-induced genes can initiate direct cytotoxic effects on the cell or can contribute to indirect cytotoxic effects by modulating the host's immune response (37). In the latter scenario, the IFNs have been shown to augment major histocompatibility complex class I and TNF-related apoptosis-inducing ligand (TRAIL) expression, which can lead to the enhancement of cytotoxic T-cell activity (17). To further examine the mechanisms of IFN action, we have for the first time used cells that inducibly overexpress the key IFN-regulated, dsRNA-activated protein kinase, PKR, and analyzed their response to infection with different RNA viruses in the absence of other IFN-induced genes. We discovered that overexpression of PKR sensitizes cells to apoptosis following infection with WSN but not to other RNA viruses, such as VSV and SNV.
Interestingly, PKR did not appear to be significantly activated in vivo following infection with WSN, possibly because the viral RNAs are poor activators of the kinase or because WSN can suppress PKR function (11, 16, 18). It is therefore conceivable that small amounts of activated PKR are sufficient to induce or sensitize cells to apoptosis. Whether this event occurs through phosphorylation of eIF2α or another, as yet uncharacterized PKR target is unclear since very little increase in phosphorylation of eIF2α was observed in WSN-infected cells. Our studies are in agreement with others who have also implicated PKR in mediating influenza virus-induced apoptosis. For example, PKR was reported to be potentially involved in Fas expression following the infection of HeLa cells with influenza virus strain A/Udorn (38). Although we detected significant levels of caspase-8 activity in WSN-infected, PKR-overexpressing 3T3 L1 cells, immunoblot analysis did not reveal significantly higher levels of Fas or FADD, possibly due to WSN mediating host protein synthesis shutoff, which we established to occur by 9 h postinfection (data not shown). However, an increase in DISC activity does not necessarily correlate with an increase in the expression of molecules associated with the DISC, such as FADD and caspase-8 (35). Collectively, the data show that the FADD/caspase-8 pathway plays a significant role in WSN-induced apoptosis in murine fibroblasts. This is underscored by analyzing the replication of viruses in FADD-deficient fibroblasts, for the first time, and verifying that such cells are significantly resistant to WSN-mediated apoptosis compared to their FADD-containing counterparts. Furthermore, although caspase-8 inhibitors such as z-IETD-fluoromethylketone (fmk) were found to be toxic to the primary and immortalized fibroblasts and could not be used, we have also observed that HeLa cells pretreated with z-IETD-fmk or expressing a dominant-negative variant of FADD are resistant to WSN-induced apoptosis (data not shown). These data were also complemented by analyzing the effects of virus infection on Apaf-1-deficient fibroblasts. For example, we have demonstrated that WSN triggered apoptosis to a similar degree in Apaf-1-deficient cells compared to control cells. It is also noteworthy that although 3T3 L1 or HeLa cells do not produce significant levels of infectious WSN, it has similarly been shown that influenza virus induces apoptosis in MDCK cells, which are permissive for these viruses (12). Similar studies in our laboratory have demonstrated that WSN-mediated apoptosis of MDCK cells is also caspase-8 dependent (data not shown).
Our studies indicate that treatment of control or PKR-overexpressing 3T3 L1 cells with IFN greatly sensitized the cells to apoptosis following infection with WSN or treatment with dsRNA in a FADD-dependent manner. Although the cytotoxicity of dsRNA in combination with IFN has been documented previously, the mechanisms of apoptotic priming by IFN remains unclear (37a, 38a). Possibly, IFN can augment the levels of endogenous PKR in both the control and PKR-overexpressing cells, or possibly another IFN-induced gene(s) regulates the activity of the DISC (2). Interestingly, a number of IFN-induced genes, including those encoding 2′,5′-oligoadenylate synthetase/RNase L, the promyelocytic leukemia protein PML, and the cytotoxic ligand TRAIL, have been shown to be mediators of apoptosis and to have antiviral activity (17, 41, 47). It is possible that the Mx genes, which are also IFN inducible, are involved in preventing orthomyxovirus replication. However, the Mx family has not been reported to be involved in apoptosis. Further, the cell lines used for these studies were derived from animals defective in known Mx function (14). Given that IFN induces a large number of genes, it is plausible that in addition to PKR, as yet uncharacterized antiviral proteins play a role in IFN-mediated host defense. In agreement with this, preliminary studies with mice lacking PKR indicate that such animals retain a substantial antiviral response compared to control mice (1, 48).
While overexpression of PKR sensitized cells to apoptosis in response to some viruses, such as WSN, excess kinase did not appear to significantly influence apoptosis in response to infection by other viruses such as VSV or SNV. Despite this, the levels of phosphorylated eIF2α were somewhat higher in VSV-infected cells than in WSN-infected cells, suggesting that eIF2α may not play a significant role in PKR-mediated apoptosis and that possibly other undefined substrates of the kinase are involved in the regulation of cell death. VSV and SNV were also found to induce apoptosis predominantly through activation of caspase-9 and not through the PKR-influenced caspase-8 pathway. The requirement for caspase-9 and not caspase-8 activation was underscored by demonstrating that VSV and SNV induced significantly less apoptosis in cells lacking Apaf-1 than control cells and cells lacking FADD. Although it cannot be entirely ruled out that VSV can invoke an as yet uncharacterized inhibitor(s) of FADD- or DISC-associated molecule, previous reports show that the mitochondrion-associated bcl-2 can similarly reduce SNV-mediated cell death (25), supporting the findings reported here. The viral antiapoptotic gene encoding CrmA, a putative inhibitor of the Fas/FADD/caspase-8 pathway, has also been reported to inhibit SNV-mediated apoptosis (31). However, the effects of CrmA may depend on the type of cell line used for the study. For example, in certain cells (referred to as type II), it has been reported that caspase-8 and caspase-3 are activated downstream of the mitochondria (35). Thus, CrmA may suppress SNV-mediated apoptosis and block caspase-8 activity, indirectly, by preventing mitochondrial input into the cell death process. In contrast, the murine fibroblasts used in this study may be of the type I variety where caspase-8 is rapidly activated at the DISC followed by activation of caspase-3.
Depending on the route of administration, IFN has been shown to influence influenza virus infection in vivo (33). Indeed, since influenza viruses are capable inducers of IFN, it is plausible that apoptosis via FADD contributes to the acute respiratory disease that epitomizes this particular orthomyxvirus infection (11a). Regarding other viruses, it is well documented that IFN exerts a potent inhibitory effect on VSV replication and progeny virus production both in vitro and in vivo (3). In this circumstance, both primary transcription of VSV mRNAs and their translation may be impaired in cells treated with IFN (3). It is possible that IFN prevents VSV-induced apoptosis by blocking the early stages of replication of the virus (3). Interestingly, IFN exacerbated the apoptotic effect mediated by VSV and SNV in FADD-deficient fibroblasts but not in cells lacking Apaf-1, insinuating that in certain circumstances, FADD may have an antiapoptotic role. Mice lacking FADD die before birth, underlining the critical and likely complex biological role of this molecule (44). Although FADD appears to play a central role in governing IFN-mediated, virus-induced apoptosis, it remains to be fully clarified why IFN invokes cell destruction in response to certain viral infection such as WSN and not others. Evidence indicates that numerous antiviral pathways are concomitantly established by IFN. It is possible that viruses escaping IFN's early inhibitory effects subsequently face alternate host defense mechanisms that involve the triggering of apoptosis either directly or indirectly via cytotoxic T-lymphocyte activity. Certainly in the case of WSN, virus yields were increased in FADD-deficient fibroblasts, indicating that apoptosis triggered by this virus, and augmented by IFN, is detrimental for its survival. However, it is noteworthy that in many cases the induction of apoptosis does not appear to dramatically effect virus yields (31, 40). Thus, apoptosis may also serve to prevent the establishment of latent viral infection. We speculate that blocking mechanisms of death signaling may play a part in enabling viruses to establish persistent infections of the cell.
Collectively, our data indicate that IFN can establish an antiviral state that, depending on the type of viral infection, can result in disparate effects to the host. One possible outcome results in sensitization of cells to rapid FADD-dependent apoptosis, as indicated by dsRNA treatment and WSN infection. Presumably this would limit the possibilities of latent infection from occurring, ensure a minimal host-mediated inflammatory response, and prevent viral dissemination. Alternate IFN-regulated, antiviral mechanisms involve the early inhibition of viral replication, prior to any triggering of apoptosis, which presumably contributes to noncytopathic viral clearance and thus minimizes injury to the host.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Tak W. Mak, University of Toronto, for FADD and Apaf-1-deficient and control fibroblasts and Larry Boise for critical review of the manuscript.
This work was supported by Public Health Service grants R01-CA84247-01 and R29-CA72648-01 (G.N.B.) from the National Institutes of Health.
REFERENCES
- 1.Abraham N, Stojl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with a targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;247:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Kim C N, Yeh W-C, Mak T W, Bhalla K N, Barber G N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987;51:66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber G N, Wambach M, Wong M L, Dever T E, Hinnebusch A G, Katze M G. Translational regulation by the interferon-induced double-stranded RNA-activated 68-kDa protein kinase. Proc Natl Acad Sci USA. 1993;90:4621–4625. doi: 10.1073/pnas.90.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, O'Rouke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 6.Compans R W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971;229:114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- 7.Davies M V, Chang H W, Jacobs B L, Kaufman R J. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase PKR through different mechanisms. J Virol. 1993;67:1688–1692. doi: 10.1128/jvi.67.3.1688-1692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Der S D, Yang Y L, Weissmann C, Williams B R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin R M, Breitenfeld P M. The abortive infection of Earle's L cells by fowl plague virus. Virology. 1959;8:293–299. doi: 10.1016/0042-6822(59)90031-5. [DOI] [PubMed] [Google Scholar]
- 10.Gale M J, Jr, Korth M J, Tang N M, Tan S L, Hopkins D A, Dever T E, Polyak S J, Gretch D R, Katze M G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 11.Hatada E, Saito S, Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Hill D A, Baron S, Perkins J C, Worthington M, Van Kirk J E, Mills J, Kapikian A Z, Chanock R M. Evaluation of an interferon inducer in viral respiratory disease. JAMA. 1972;219:1179–1184. [PubMed] [Google Scholar]
- 12.Hinshaw V S, Olsen C W, Sissoko-Dybdahl N, Evans D. Apoptosis: a mechanism of killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 14.Jin H K, Yamashita T, Ochiai K, Haller O, Watanabe T. Characterization and expression of the Mx1 gene in wild mouse species. Biochem Genet. 1998;36:311–322. doi: 10.1023/a:1018741312058. [DOI] [PubMed] [Google Scholar]
- 15.Katze M G. Games viruses play: a strategic initiative against the IFN-induced, dsRNA-activated protein kinase. Semin Virol. 1993;4:259–268. [Google Scholar]
- 16.Katze M G, Tomita J, Black T, Krug R M, Safer B, Hovanessian A G. Influenza virus regulates protein synthesis during infection by repressing autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J Virol. 1988;62:3710–3717. doi: 10.1128/jvi.62.10.3710-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayagaki N, Yamaguchi N, Makayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman R J, Davies M V, Pathak V K, Hershey J W. The phosphorylation state of eukaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989;9:946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of a caspase-9/apaf-1 complex initiates an apoptotic cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 24.Levin D, London I M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylayes eukaryotic initiation factor 2. Proc Natl Acad Sci USA. 1978;75:1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 27.Mathews M B. Viral evasion of cellular defence mechanisms: regulation of the protein kinase DAI by RNA effectors. Semin Virol. 1993;4:507–512. [Google Scholar]
- 28.Meurs E F, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 29.Mundschau L J, Faller D V. Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. J Biol Chem. 1995;270:3100–3106. doi: 10.1074/jbc.270.7.3100. [DOI] [PubMed] [Google Scholar]
- 30.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rouke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 31.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts P C, Lamb R A, Compans R W. The M1 and M2 proteins of influenza A are important determinants in filamentous particle formation. Virology. 1998;240:127–137. doi: 10.1006/viro.1997.8916. [DOI] [PubMed] [Google Scholar]
- 33.Saito N, Suzuki F, Ishida N. Antiviral effect of interferon on influenza virus infection in mice. Tohoku J Exp Med. 1983;139:355–363. doi: 10.1620/tjem.139.355. [DOI] [PubMed] [Google Scholar]
- 34.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 35.Scaffidi C, Fluda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (Apo-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 37.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 37a.Stewert W E, DeClerq E, Billiau A, Desmyter J, Desomer P. Increased susceptibility of cells treated with interferon to the toxicity of polyriboinosinic-polyribocytidylic acid. Proc Natl Acad USA. 1972;69:1851–1854. doi: 10.1073/pnas.69.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Tanaka N, Sato M, Lamphier M S, Nozawa H, Oda E, Noguchi S, Schreiber R D, Tsujimoto Y, Taniguchi T. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998;3:29–37. doi: 10.1046/j.1365-2443.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 39.Taylor D R, Shi S T, Romano P R, Barber G N, Lai M C. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 40.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z G, Ruggero D, Ronchetti S, Zhong S, Gaboli M, Rivi R, Pandolfi P P. PML is essential for multiple apoptotic pathways. Nat Genet. 1998;20:266–272. doi: 10.1038/3073. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh W-C, Pompa J L, McCurrach M E, Shu H-B, Elia A J, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, el-Deiry W S, Lowe S W, Goeddel D V, Mak T W. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 45.Yeung M C, Liu J, Lau A S. An essential role for the interferon-inducible protein kinase PKR in tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida H, Kong Y-Y, Yoshida R, Elia A J, Hakem A, Hakem R, Penninger J F, Mak T W. Apaf-1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou A, Paranjpe J, Brown T L, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman R H. Interferon action and apoptosis defective in mice devoid of 2′,5′-oligoadelyate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou A, Paranjape J M, Der S D, Williams B R G, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]