This cross-sectional study examines data for patients with acute ischemic stroke to determine whether dynamic changes in high-sensitivity cardiac troponin values can indicate myocardial infarction.
Key Points
Question
Does a “rise and/or fall” pattern (dynamic change) of troponin values in patients with acute ischemic stroke and elevated troponin indicate myocardial infarction?
Findings
This cross-sectional study found that a dynamic change in troponin was not associated with myocardial infarction in patients with ischemic stroke whereas baseline absolute troponin value was independently associated with type 1 myocardial infarction. The best cutoffs for predicting type 1 myocardial infarction were 5 to 10 times the upper limit of normal.
Meaning
In acute ischemic stroke, a dynamic change in troponin values is not helpful in detecting myocardial infarction, emphasizing that dynamic changes do not reveal the underlying pathophysiological mechanism of myocardial injury.
Abstract
Importance
Elevated values of high-sensitivity cardiac troponin (hs-cTn) are common in patients with acute ischemic stroke and are associated with poor prognosis. However, diagnostic and therapeutic implications in patients with ischemic stroke remain unclear.
Objective
To identify factors indicative of myocardial infarction (MI) in patients with acute ischemic stroke and hs-cTn elevation. The primary hypothesis was that a dynamic change of hs-cTn values (>50% change) in patients with acute ischemic stroke indicates MI.
Design, Setting, and Participants
This cross-sectional study was a prospective, observational study with blinded end-point assessment conducted across 26 sites in Germany. Patients were included if they had acute ischemic stroke within 72 hours and either (1) highly elevated hs-cTn values on admission (>52 ng/L) or (2) hs-cTn levels above the upper limit of normal and a greater than 20% change at repeated measurements. Patients were enrolled between August 2018 and October 2020 and had 1 year of follow-up. Statistical analysis was performed between April 2022 and August 2023.
Exposure
Standardized electrocardiography, echocardiography, and coronary angiography.
Main Outcome and Measures
Diagnosis of MI as adjudicated by an independent end-point committee based on the findings of electrocardiography, echocardiography, and coronary angiography.
Results
In total, 254 patients were included. End points were adjudicated in 247 patients (median [IQR] age, 75 [66-82] years; 117 were female [47%] and 130 male [53%]). MI was present in 126 of 247 patients (51%) and classified as type 1 MI in 50 patients (20%). Dynamic change in hs-cTn value was not associated with MI in univariable (32% vs 38%; χ2 P = .30) or adjusted comparison (odds ratio, 1.05; 95% CI, 0.31-3.33). The baseline absolute hs-cTn value was independently associated with type 1 MI. The best cutoffs for predicting type 1 MI were at hs-cTn values 5 to 10 times the upper limit normal.
Conclusions and Relevance
This study found that in patients with acute ischemic stroke, a dynamic change in hs-cTn values did not identify MI, underscoring that dynamic changes do not identify the underlying pathophysiological mechanism. In exploratory analyses, very high absolute hs-cTn values were associated with a diagnosis of type 1 MI. Further studies are needed how to best identify patients with stroke who should undergo coronary angiography.
Introduction
Elevated values of cardiac troponin (cTn) in patients with acute ischemic stroke are a diagnostic and therapeutic challenge in daily clinical practice. Current guidelines recommend routine measurement of cTn for the early management of patients with acute ischemic stroke.1 Elevations of cTn above the upper limit of normal (above the 99% percentile) indicate myocardial damage and are detected in approximately 1 in 2 patients with acute ischemic stroke when measured with high-sensitivity assays (hs-cTn).2 Levels of cTn that meet the European Society of Cardiology (ESC) diagnostic criteria for suspected non–ST-elevation myocardial infarction (MI) are present in about 1 in 7 patients with acute ischemic stroke.3,4 Elevated values of cTn are strongly associated with poorer outcomes in patients with stroke.2,5,6 One explanation for the poor outcome could be MI, because cTn elevation in acute ischemic stroke is strongly associated with a higher risk for coronary events and vascular death.7,8 However, distinguishing MI, in particular type 1 MI, from other pathophysiologies of myocardial injury, such as neurogenic or stress-induced myocardial injury, remains difficult.2,6,9
Currently, there is limited evidence on how best to diagnose MI in the setting of acute ischemic stroke and, in particular, which patients should undergo coronary angiography.10 Current guidelines for the early management of patients with acute ischemic stroke do not provide clear recommendations except for the measurement of hs-cTn.1
The aim of this study was therefore to clarify the significance of hs-cTn elevation and its time course for the diagnosis of MI and type 1 MI in acute ischemic stroke. The prespecified primary hypothesis of this study was that a dynamic change of hs-cTn values (ie, >50% change at repeated measurements) in patients with an acute ischemic stroke is indicative of MI.
Methods
Study Design
The Prediction of Acute Coronary Syndrome in Acute Ischemic Stroke (PRAISE) study was a prospective, multicenter, observational clinical study with external monitoring; central reading by core laboratories; blinded end-point assessment by an end-point adjudication committee (EAC), data and safety monitoring board, and critical events committee; predefined end points; and sample size calculation. The study protocol was approved by the ethics committee at each participating center and the ethics committee of the lead center, Charité, Berlin (No. EA1/057/18). The protocol was published before the final analysis.11 A detailed statistical analysis plan was finalized before the statistical analyses were performed.
Eligible patients had to provide written informed consent for study enrollment. The ethics committee approved the method of consent. The study conforms to the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guidelines for diagnostic accuracy studies.12
Setting and Participants
The study was conducted at 26 study sites in Germany. In accordance with current guidelines, the study sites performed hs-cTn testing as part of routine clinical practice in all patients with stroke at the time of admission and after 3 hours.1
Patients were included if they were 18 years or older and had a clinical diagnosis of acute ischemic stroke (including all subtypes) based on neurological assessment and brain imaging or high-risk transient ischemic attack (TIA, defined as an ABCD2 score ≥4). Time from symptom onset to hospital admission had to be less than 72 hours for study inclusion. Patients were eligible for inclusion if their hs-cTn levels were above the cutoffs recommended by the ESC guidelines for non–ST-elevation (NSTE) acute coronary syndrome to prompt early coronary angiography,4,13 ie, 1 “highly abnormal” hs-cTn value (ie, >52 ng/L if hs-cTnT, Roche Elecsys assay, or >52 ng/L, if hs-cTnI, Abbott Architect assay, or >107 ng/L, if hs-cTnI, Dimension Vista assay) or, alternatively, change greater than 20% at serial measurements and 1 hs-cTn value higher than the upper limit of normal (ULN) depending on assay.
Exclusion criteria were severe renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2), uncontrolled thyroid dysfunction, large ischemic brain lesions, pregnancy or breastfeeding, limited life expectancy (<1 year), and a high premorbid degree of disability (defined as a modified Rankin Scale score >3).14 Details were published previously.11
Study Procedures
Patients received repeated 12-lead electrocardiograms (ECGs), transthoracic echocardiography, and coronary angiography following specific standard procedures of the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung).15 All images were analyzed in separate central academic core laboratories as described earlier.11 Core laboratories were blinded for clinical information.11 Blood samples for hs-cTn measurement were deep frozen, stored, and reanalyzed in an independent core laboratory at the University Heart Center in Hamburg using the Roche Elecsys assay and the Abbott Architect assay.
The end point and adjudication process was performed by an independent EAC based on clinical data (including patient symptoms and vital signs) and the reports from the central academic core laboratories. The primary outcome was the presence of MI, which was adjudicated subject to an EAC charter (eMethods in Supplement 1) based on the then-valid third universal definition of MI.16 MI was considered present if the following criteria were met: the presence of coronary artery disease with stenosis on coronary angiogram and at least 1 of ischemic ECG changes, regional wall motion abnormalities on echocardiography, and/or culprit lesions on coronary angiogram, as reported by the respective central core laboratory. The EAC further differentiated MI into type 1 and type 2 MI based on the coronary angiogram.16 Type 1 MI was adjudicated when the coronary angiogram revealed a culprit lesion and the clinical presentation concurred. A culprit lesion was defined as a lesion with irregular borders, ulceration, filling defects, or the presence of intraluminal thrombus.16,17 Type 2 MI was considered if there were no such angiographic findings, but the patient had wall motion abnormalities on echocardiography and/or ischemic ECG changes. If type 1 and 2 MI were not diagnosed and thus MI was excluded, nonischemic myocardial injury was diagnosed.
Secondary end points were major adverse cardiovascular events (MACE) defined as (recurrent) stroke, TIA, MI, and all-cause death. Safety end points included predefined severe adverse events of special interest (SAESI), ie, MI, repeated coronary intervention, ischemic stroke or TIA, peripheral embolic artery occlusion, intracranial hemorrhage, major bleeding (type 3-5 according to the Bleeding Academic Research Consortium [BARC] classification), and death.18 MACE and SAESI were recorded during the in-hospital stay and at 3 and 12 months. All SAESI were evaluated by the critical events committee regarding a possible causal relationship with coronary angiography. Reports were made to the data and safety monitoring board after the inclusion of 100 and 200 patients as well as after the end of the study. The critical events committee and data and safety monitoring board agreed on a charter defining appropriate conduct and obligation before the beginning of the study (eMethods in Supplement 1).
All data were recorded in an electronic case report form. Remote monitoring was conducted at all sites to ensure data completeness and correctness.
Statistical Analysis
Sample size calculation and choice of threshold for the definition of dynamic hs-cTn change (>50%) was based on the results of the TRELAS study.17 The study protocol has already been published.11 Differences between the 2 diagnostic groups (presence/absence of type 1 MI) were described using the χ2 test for dichotomous variables and the Wilcoxon rank sum test for continuous variables.
The primary hypothesis (ie, presence of any MI in relation to a dynamic hs-cTn change >50%) was analyzed by calculation of the diagnostic odds ratio (OR). In an explorative approach, multivariable (adjusted) backwards-stepwise logistic regression was used to identify factors associated with MI overall (primary outcome) and type 1 MI in particular.
In sensitivity analysis, the main analysis was repeated after excluding patients with TIA. In a second sensitivity analyses, the predictive accuracy for different thresholds of relative hs-cTn changes (any cutoff between relative change of 0% and 100%) for the presence of any MI and type 1 MI was evaluated by calculating the area under the receiver operating characteristic curve (ROC-AUC). The best cutoff was determined by the maximum AUC.
This approach was also applied for absolute baseline hs-cTn values using standardized log-transformed hs-cTn values (expressed as multiples of the ULN). Moreover, sensitivity analyses for best cutoff of absolute hs-cTn on admission to predict type 1 MI was performed in the subgroups of patients with hs-cTnT values measured by the Roche Elecsys assay and hs-cTnI values measured by the Abbott Architect assay. Cox regression analysis was used to estimate hazard ratios (HRs) for MACE and mortality at 12 months for patients with stroke with and without a dynamic change in hs-cTn value. All statistical tests were 2-sided and performed using a 5% significance level. All analyses, except for the primary analysis, were conducted in an exploratory manner without adjustment for multiple testing. Statistical analyses were performed using R version 4.0.5 (R Foundation).
Results
Between August 2018 and October 2020, 254 patients were prospectively enrolled. Of these, 247 patients (97%) underwent coronary angiography (median [IQR] age, 75 [66-82] years; 117 were female [47%] and 130 male [53%]) allowing adjudication of the primary end point (Figure 1). The EAC diagnosed any MI in 126 of 247 patients (51%) and type 1 MI in 50 of 247 patients (20%).
Figure 1. Participant Flow in the PRAISE Study.
TIA indicates transient ischemic attack.
Baseline characteristics of patients with and without MI are shown in Table 1. In unadjusted comparisons, patients with MI were less often female, had shorter times from symptom onset to angiography, were more likely to have dyslipidemia, were more likely to have known coronary artery disease (CAD), and had higher heart rate on admission. In the majority of patients diagnosed with MI, CAD was unknown before the coronary angiography (82/126 [65%]). Patients with type 1 MI had shorter symptom-to-angiography times and higher creatinine values (Table 2). The predefined dynamic change of hs-cTn was detected in 85 of 247 patients (34%). The number of patients with any MI and type 1 MI per study site is shown in eTable 1 in the Supplement 1.
Table 1. Baseline Characteristics of Patients With and Without Myocardial Infarction (N = 247).
| Characteristic | No. of patients | No./total No. of patients (%) | P values of univariable comparisons | From multivariable model | ||
|---|---|---|---|---|---|---|
| MI (n = 126) | No MI (n = 121) | OR (95% CI) | P value | |||
| Age, median (IQR), y | 247 | 74 (64-82) | 76 (67-82) | .54a | NA | NA |
| ≥75 y | 62/126 (49) | 67/121 (55) | .33b | NA | NA | |
| Sex | 247 | .03b | NA | NA | ||
| Female | 51/126 (40) | 66/121 (55) | ||||
| Male | 75/126 (60) | 55/121 (45) | ||||
| Time from symptom onset to angiography, median (IQR), d | 247 | 2 (1-4) | 3 (2-4) | .04a | NA | NA |
| Diabetes | 247 | 42/126 (33) | 28/121 (23) | .08b | NA | NA |
| Hypertension | 247 | 102/126 (81) | 91/121 (75) | .28b | NA | NA |
| Dyslipidemia | 247 | 61/125 (49) | 43/121 (36) | .04b | NA | NA |
| Known coronary artery disease | 247 | 44/126 (35) | 23/121 (19) | <.005b | 4.74 (1.63-15.3) | .006 |
| Atrial fibrillation | 246 | 33/126 (26) | 36/120 (30) | .51b | NA | NA |
| Heart failure | 246 | 13/126 (10) | 14/120 (12) | .74b | 0.10 (0.01-0.54) | .02 |
| Previous stroke | 247 | 34/126 (27) | 25/121 (21) | .24b | NA | NA |
| Current smoker | 247 | 28/122 (23) | 31/120 (26) | .87b | NA | NA |
| Ex-smoker | 242 | 27/122 (22) | 25/120 (21) | |||
| IV thrombolysis (rTPA) | 246 | 49/126 (39) | 44/120 (37) | .72b | NA | NA |
| Heart rate, median (IQR), /min | 247 | 78 (66-88) | 72 (64-84) | .04a | 0.22 (0.04-1.03) | .06c |
| Systolic BP, median (IQR), mm Hg | 247 | 138 (125-150) | 135 (120-148) | .39a | NA | NA |
| Diastolic BP, median (IQR), mm Hg | 247 | 80 (68-89) | 76 (68-85) | .40a | NA | NA |
| β-Blocker use | 245 | 62/124 (50) | 62/121 (51) | .85b | NA | NA |
| Statin use | 245 | 93/124 (75) | 87/121 (72) | .58b | NA | NA |
| NIHSS score on visit 1, median (IQR) | 246 | 2 (0-5) | 2 (0-3) | .11a | 1.07 (0.94-1.22) | .30 |
| GRACE score, median (IQR) | 247 | 139.5 (119-158) | 137 (118-151) | .35a | NA | NA |
| Creatinine, median (IQR), mg/dL | 247 | 1.00 (0.81-1.20) | 0.95 (0.80-1.14) | .20a | NA | NA |
| Hemoglobin, median (IQR), g/dL | 247 | 13.4 (12.0-14.7) | 13.4 (12.0-14.8) | .67a | 2.73 (0.98-8.50) | .07c |
| Variables assessed by the different core laboratories (known to the EAC) | ||||||
| Culprit lesion present | 247 | 45/126 (36) | 2/121 (2) | <.001b | NA | NA |
| T inversion on any ECG | 243 | 45/124 (36) | 45/119 (38) | .81b | NA | NA |
| ST depression in any ECG | 243 | 12/124 (10) | 1/119 (1) | <.001b | NA | NA |
| ACS-typical ECG changes | 190 | 43/95 (45) | 30/95 (32) | .052b | 2.37 (0.79-7.55) | .13 |
| Left ventricular ejection fraction, median (IQR), % | 181 | 55 (50-60) | 59 (54-64) | <.001a | NA | NA |
| Left ventricular ejection fraction ≥50% | 181 | 76/99 (77) | 73/82 (89) | .03b | NA | NA |
| Wall motion abnormality (data from core lab) | 163 | 29/88 (33) | 3/75 (4) | <.001b | 30.89 (6.35-279.5) | <.001 |
| Wall motion abnormality (data from centers) | 195 | 34/99 (34) | 9/96 (9) | <.001b | NA | NA |
| Variables of interest (not known to the EAC) | ||||||
| Dynamic hs-cTn change >50% | 244 | 40/126 (32) | 45/118 (38) | .30b | 0.72 (0.31-1.61) | .42d |
| Dynamic hs-cTn change >20% | 244 | 67/126 (53) | 70/118 (59) | .33b | NA | NA |
| Baseline hs-cTn (multiples of ULN), median (IQR) | 243 | 5.98 (3.00-14.86) | 5.11 (3-00-11.46) | .28a | NA | NA |
| Baseline hs-cTnT, median (IQR), ng/Le | 202 | 76 (38-180) | 70 (43-146) | .71a | NA | NA |
| Baseline hs-cTnI, median (IQR), ng/Lf | 159 | 234 (46-815) | 116 (53-614) | .46a | NA | NA |
| Other variables not known to the EAC | ||||||
| Percutaneous transluminal coronary angioplasty stent | 247 | 62/126 (49) | 8/121 (7) | <.001b | NA | NA |
| Time from symptom onset to first troponin measurement, median (IQR), h | 247 | 4 (2-9) | 4 (2-10) | .97a | NA | NA |
Abbreviations: ACS, acute coronary syndrome; EAC, end-point adjudication committee; GRACE, Global Registry of Acute Coronary Events; hs-cTn, high-sensitivity cardiac troponin; MI, myocardial infarction; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; rTPA, recombinant tissue-type plasminogen activator; ULN, upper limit of normal.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Wilcoxon rank sum test.
Likelihood ratio χ2 test. ORs were derived from the fully adjusted logistic regression model after backward selection and inclusion of hs-cTn.
Heart rate (>100/min) and hemoglobin (≥12 g/dL in women and ≥13 g/dL in men) were included as binary variables in the regression model.
Dynamic hs-cTn change was included as a continuous variable in the regression model.
Roche Elecsys subgroup.
Abbott Architect subgroup.
Table 2. Baseline Characteristics of Patients With and Without Type 1 Myocardial Infarction (N = 247).
| Characteristic | No. of patients | No./total No. of patients (%) | P values of univariable comparisons | From multivariable model | ||
|---|---|---|---|---|---|---|
| Type 1 MI (n = 50) | No type 1 MI (n = 197) | OR (95% CI) | P value | |||
| Age, median (IQR), y | 247 | 77 (66-82) | 75 (65-82) | .50a | NA | NA |
| ≥75 y | 27/50 (54) | 102/197 (52) | .78b | NA | NA | |
| Sex | 247 | .59b | NA | NA | ||
| Female | 22/50 (44) | 95/197 (48) | ||||
| Male | 28/50 (56) | 102/197 (52) | ||||
| Time from symptom onset to angiography, median (IQR), d | 247 | 2 (0-4) | 2 (1-4) | .02a | NA | NA |
| Diabetes | 247 | 17/50 (32) | 53/197 (27) | .33b | NA | NA |
| Hypertension | 247 | 41/50 (82) | 152/197 (77) | .45b | NA | NA |
| Dyslipidemia | 247 | 24/49 (49) | 80/197 (41) | .29b | NA | NA |
| Known coronary artery disease | 247 | 13/50 (26) | 54/197 (27) | .84b | NA | NA |
| Atrial fibrillation | 246 | 12/50 (24) | 57/196 (29) | .47b | NA | NA |
| Heart failure | 246 | 5/50 (10) | 22/196 (11) | .80b | NA | NA |
| Previous stroke | 247 | 16/50 (32) | 43/197 (22) | .14b | NA | NA |
| Current smoker | 247 | 12/49 (25) | 47/193 (24) | .98b | NA | NA |
| Ex-smoker | 242 | 11/49 (22) | 41/193 (21) | |||
| IV thrombolysis (rTPA) | 246 | 21/50 (42) | 72/196 (37) | .50b | NA | NA |
| Heart rate, median (IQR), /min | 247 | 80 (68-85) | 74 (64-86) | .31a | NA | NA |
| Systolic BP, median (IQR), mm Hg | 240 | 137 (126-145) | 136 (122-150) | .75a | NA | NA |
| Diastolic BP, median (IQR), mm Hg | 240 | 77 (70-85) | 78 (68-86) | .97a | NA | NA |
| β-Blocker use | 245 | 23/49 (47) | 101/196 (52) | .57b | NA | NA |
| Statin use | 245 | 34/49 (69) | 146/196 (75) | .47b | NA | NA |
| NIHSS on visit 1, median (IQR) | 246 | 2 (1-6) | 2 (0-4) | .24a | NA | NA |
| GRACE score, median (IQR) | 247 | 146 (122-164) | 136 (118-151) | .05a | NA | NA |
| Creatinine, median (IQR), mg/dL | 247 | 1.10 (1.00-1.27) | 0.95 (1.0-1.13) | .01a | NA | NA |
| Hemoglobin, median (IQR), g/dL | 247 | 13.6 (12.0-14.9) | 13.4 (12.0-14.7) | .58a | NA | NA |
| Variables assessed by the different core laboratories (known to the EAC) | ||||||
| Culprit lesion present | 247 | 42/50 (84) | 5/197 (3) | <.001b | NA | NA |
| T inversion on any ECG | 243 | 15/50 (30) | 75/193 (39) | .24b | NA | NA |
| ST depression in any ECG | 243 | 4/50 (8) | 9/193 (5) | .37b | NA | NA |
| ACS-typical changes in any ECG | 190 | 13/34 (38) | 60/156 (38) | .98b | NA | NA |
| Left ventricular ejection fraction, median (IQR), % | 181 | 56 (501-61) | 58 (52-62) | .29a | NA | NA |
| Left ventricular ejection fraction ≥50% | 181 | 35/41 (85) | 114/140 (81) | .55b | NA | NA |
| Wall motion abnormality (data from core lab) | 163 | 10/38 (26) | 22/125 (18) | .25b | NA | NA |
| Wall motion abnormality (data from centers) | 195 | 12/41 (29) | 31/154 (20) | .22b | NA | NA |
| Variables of interest (not known to the EAC) | ||||||
| Dynamic hs-cTn change >50% | 244 | 18/50 (36) | 67/194 (35) | .85b | NA | NA |
| Dynamic hs-cTn change >20% | 244 | 23/50 (46) | 114/194 (59) | .11b | NA | NA |
| Baseline hs-cTn, median (IQR)c | 243 | 9.56 (4.00-23.26) | 5.19 (3.00-12.00) | .01a | 1.35 (1.07-1.70) | .01c |
| Baseline hs-cTnT, median (IQR), ng/Ld | 202 | 117 (52-253) | 66 (40-143) | .007a | 3.61 (1.49-9.51) | .006d |
| Baseline hs-cTnI, median (IQR), ng/Le | 159 | 326 (64-800) | 138 (46-717) | .28a | 1.10 (0.89-1.37) | .38e |
| Other variables not known to the EAC | ||||||
| Percutaneous transluminal coronary angioplasty stent | 247 | 39/50 (78) | 31/197 (16) | <.001b | NA | NA |
| Time from symptom onset to first troponin measurement, median (IQR), h | 247 | 3 (2-8) | 4 (2-10) | .47 | NA | NA |
Abbreviations: ACS, acute coronary syndrome; EAC, end-point adjudication committee; GRACE, Global Registry of Acute Coronary Events; hs-cTn, high-sensitivity cardiac troponin; MI, myocardial infarction; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; rTPA, recombinant tissue-type plasminogen activator; ULN, upper limit of normal.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Wilcoxon rank sum test.
Likelihood ratio χ2 test. ORs were derived from the fully adjusted logistic regression model after backward selection and inclusion of hs-cTn.
Entered as multiples of ULN (thereby all assays could be used) and log-transformed to a continuous variable in the model.
Entered as an absolute value (Roche Elecsys assay) and log-transformed to a continuous variable in the model.
Entered as an absolute value (Abbott Architect assay) and log-transformed to a continuous variable in the model.
A dynamic hs-cTn change greater than 50% was not associated with the diagnosis of MI (diagnostic OR, 0.76; 95% CI, 0.45-1.28; primary hypothesis). Moreover, sensitivity analyses showed that dynamic hs-cTn changes with a threshold greater than 20% (OR, 0.78; 95% CI, 0.47-1.29) or any cutoff between 0% and 100% for relative hs-cTn changes did not improve the association with MI (Table 1, Figure 2, and Figure 3). ST depression on ECG, regional wall motion abnormalities on echocardiography (both assessed by core laboratories), a history of CAD, and absence of heart failure were independently associated with the diagnosis of MI (Table 1). There was no interaction between treatment with intravenous thrombolysis and a dynamic change in hs-cTn. The sensitivity analysis after the exclusion of 19 patients with TIA confirmed the results of the main analysis: there was still no association between dynamic change in hs-cTn and diagnosis of any MI (eTable 2 in Supplement 1). Moreover, regional wall motion abnormalities, acute coronary syndrome–typical ECG changes, history of CAD, and absence of heart failure remained associated with a diagnosis of any MI.
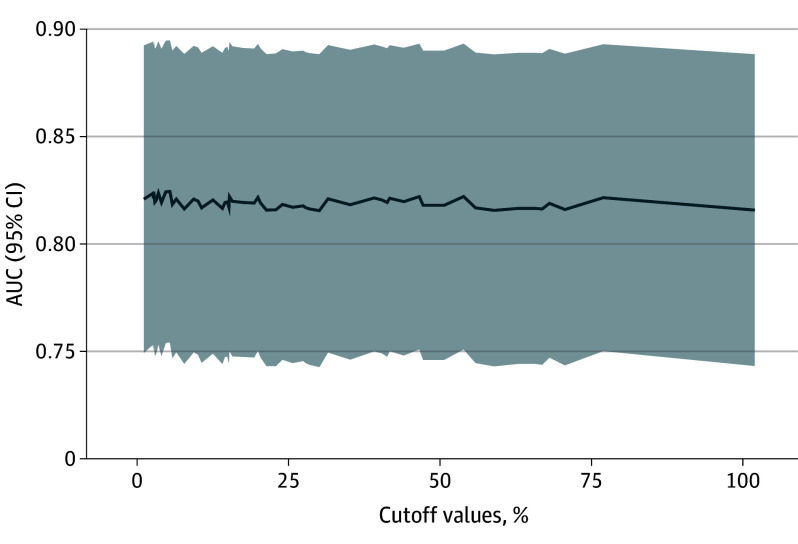
Figure 2. Diagnostic Accuracy of Adding High-Sensitivity Cardiac Troponin (hs-cTn) Changes to a Predefined Regression Model for Myocardial Infarction (MI).
Predictive accuracy for different thresholds (x-axis) of relative hs-cTn changes (any cutoff between relative change of 0% and 100%) for the presence of MI as given by area under the curve (AUC). The higher the AUC, the better the predictive accuracy of the respective cutoff.
Figure 3. Diagnostic Accuracy of Adding Baseline Absolute High-Sensitivity Cardiac Troponin (hs-cTn) Values to a Predefined Regression Model for Type 1 Myocardial Infarction (MI).
Predictive accuracy for different thresholds (x-axis) of hs-cTn on admission (as a multiple of upper limits of normal [ULN]) for the presence of MI as given by the area under the curve (AUC). The higher the AUC, the better the predictive accuracy of the cutoff. The optimal cutoff hs-cTn to predict type 1 MI is at 5.39 times ULN with AUC = 0.608 (95% CI, 0.535-0.681) and at 10.42 times ULN with AUC = 0.608 (95% CI, 0.531-0.685).
Using multiples of the ULN, log-transformed hs-cTn on admission was associated with type 1 MI (OR, 1.35; 95% CI, 1.07-1.70; P = .01). Backward selection of clinical predictors led to an empty model; that is, no additional predictors of type 1 MI were identified. According to the ROC analysis, the best hs-cTn cutoffs to identify type 1 MI were at 5.4 times ULN and 10.4 times ULN with an ROC-AUC of 0.608 (95% CI, 0.535-0.681) and 0.608 (95% CI, 0.531-0.685), respectively. After exclusion of patients with TIA, the absolute initial hs-cTn value was still the variable with the strongest association with type 1 MI. Similarly to the original analysis, the best cutoff of hs-cTn to predict type 1 MI according to calculation of the ROC-AUC was at 10 times the upper reference limit (eTable 3 and eFigure in Supplement 1).
Sensitivity analyses were also performed using absolute hs-cTn values, which were available in 202 of 247 patients (82%) using the Roche Elecsys fifth-generation assay for hs-cTnT and in 159 of 247 patients (64%) patients using the Abbot Architect assays for hs-cTnI. Patients diagnosed with type 1 MI had higher absolute log-transformed hs-cTnT values on admission (median [IQR] hs-cTnT on admission, 117 [52-253] vs 66 [40-143] ng/L; P = .007; Roche Elecsys assay). Values for hs-cTnI were numerically higher but did not differ significantly (median [IQR], 326 [64-800] vs 138 [46-717] ng/L; P = .27; Abbott Architect assay). Log-transformed hs-cTnT (Roche Elecsys assay) on admission remained independently associated with type 1 MI in adjusted regression analysis (adjusted OR, 3.61; 95% CI, 1.49-9.51; P = .006). According to ROC analysis, the best hs-cTnT cutoff to identify type 1 MI was 76 ng/L for the Roche Elecsys assay (ie, 5.4 times the ULN) with an AUC of 0.625 (95% CI, 0.545-0.705). Overall, 62 of 126 patients (49%) with MI and 39 of 50 (78%) with type 1 MI received immediate percutaneous transluminal coronary angioplasty.
Concerning safety, 7 SAESI in 6 patients were classified as “potentially attributable to coronary intervention” (ie, 1 MI, 1 death, 1 ischemic stroke, 1 TIA, 1 peripheral artery occlusion, and 2 major bleedings [BARC class 3, both of which were groin hematomas], but no intracranial bleeding). No safety concerns were raised by the data and safety monitoring board.
Follow-up at 12 months was available in 229 of 247 patients (91%) with respect to MACE and in 247 of 247 (100%) with respect to vital status. MACE occurred in 60 of 229 patients (26%), and 39 of 247 patients (16%) died. A dynamic change of hs-cTn was not associated with MACE (HR, 0.76; 95% CI, 0.43-1.33; secondary hypothesis). On the contrary, a dynamic change of hs-cTn was associated with reduced mortality at 12 months in unadjusted analyses (HR, 0.46; 95% CI, 0.21-0.99; P = .05).
Discussion
The prospective, multicenter PRAISE study included patients with acute ischemic stroke and elevated hs-cTn values above rule-in cutoffs to suspect NSTE-MI and tested the hypothesis that a dynamic hs-cTn change is indicative of MI (including type 1 MI). Elevated cTn values that meet the ESC diagnostic criteria for suspected NSTE-MI are present in about 1 in 7 patients with acute ischemic stroke.3
The study has the following main findings, First, in this selected population of patients with acute ischemic stroke and elevated hs-cTn, MI (including type 1 MI) is relatively common as assessed by an independent EAC. Second, patients with stroke and elevated hs-cTn values have a high risk of MACE and death over 12 months. Third, coronary angiography carries a decent risk of serious adverse events of 2.4%. Fourth, dynamic hs-cTn changes (ie, a rise and/or fall pattern) alone are not helpful to distinguish MI from other causes of acute myocardial injury and to predict MACE at 12 months. In contrast, the diagnosis of MI was associated with ECG and echocardiography, whereas patients with type 1 MI had higher baseline absolute hs-cTn values.
Based on data from our earlier, much smaller TRELAS study, our prespecified primary hypothesis was that a dynamic change in hs-cTn values would help identify patients with MI. However, we found that neither a prespecified delta of 50% in hs-cTn values nor any dynamic changes were associated with a final diagnosis of any MI or type 1 MI.
In acute ischemic stroke, dynamic hs-cTn changes indicative of acute myocardial injury are therefore not suitable for detecting myocardial infarction, and they do not distinguish between different mechanisms of acute myocardial injury. Alternatively, in patients with acute ischemic stroke, acute myocardial injury may be caused by neurocardiogenic dysregulation rather than acute coronary syndrome.2,6 This phenomenon has recently obtained much attention, giving birth to the term stroke-heart syndrome. For example, ischemic brain lesions in the right anterior dorsal insula (representing the central autonomic network) are associated with dynamic hs-cTn elevation.19,20
On the other hand, 1 in 2 patients with stroke and elevated hs-cTn (ie, myocardial injury) was diagnosed with MI and 1 in 5 with type 1 MI, which is in good agreement with the previous TRELAS study.17 Thus, our study shows that type 1 MI is a common mechanism of myocardial injury in stroke. Of note, the majority of patients did not have known CAD, suggesting a high prevalence of silent CAD in patients with stroke. This apparent underdiagnosis is particularly important for clinical practice because patients with stroke in general (and those with elevated troponin values in particular) are at increased risk for recurrent vascular events and death.7,8 Our data suggest that more patients with acute stroke should be evaluated accordingly. Timely evaluation for and treatment of concomitant CAD and MI may help reduce the risk of future cardiovascular events in these patients.
Although stroke severity was not particularly high in patients included in the PRAISE study, 1 in 4 patients had a recurrent event, and 1 in 6 died within 1 year after stroke. In PRAISE, MACE at 12 months occurred in 26% of patients, which is about 3 times as high as in a large Canadian cohort of patients with first ever ischemic stroke and unknown hs-cTn values (9%).21 Our study suggests that ECG and echocardiography may be helpful to select patients who likely have ischemic myocardial injury (ie, MI). An interesting finding of the PRAISE study is that the baseline value of hs-cTn may be helpful in identifying patients with type 1 MI.
In fact, the baseline hs-cTn level was almost twice as high in patients with type 1 MI compared with those without. This finding was robust in (1) the overall population with multiple hs-cTn assays used, (2) the subgroup testing an hs-cTnT assay (Roche Elecsys), and (3) the subgroup excluding patients with TIA.
In clinical practice, it is important to rapidly identify patients with type 1 MI to allow timely further invasive diagnosis and treatment.4,13 In contrast, current cardiology guidelines from the ESC do not recommend routinely performing immediate angiography in patients with type 2 MI.4,13 Preselection of patients with stroke and a higher likelihood of type 1 MI may therefore help triage patients who should undergo urgent coronary angiography. Of note, hs-cTn elevations beyond the 5-fold ULN also have a high positive predictive value for acute type 1 MI in patients with clinically suspected NSTE-MI.4 In the PRAISE study, the best cutoff values for hs-cTn to diagnose type 1 MI were 5.4 and 10.4 times the ULN. This is considerably higher than the rule-in cutoff values for different assays recommended in the ESC guidelines for suspected NSTE-MI.4 A pragmatic clinical approach might therefore use a higher threshold for hs-cTn values (about 5 times the ULN) to select patients with acute ischemic stroke for urgent coronary angiography. However, based on our results, we cannot yet recommend the use of this cTn threshold to select patients with stroke for coronary angiography in clinical practice. Not only was the discrimination for type 1 MI moderate, but most importantly, it is currently unclear whether patients with acute stroke, elevated hs-cTn, and suspected type 1 MI would benefit from an invasive approach undergoing coronary angiography and potentially percutaneous coronary intervention.
In the PRAISE study, all therapeutic decisions were made at the discretion of the treating physician, and there was no control group of patients who did not receive coronary angiography. Therefore, we do not know whether clinical outcome was modified by the procedure. Future studies evaluating an early invasive vs a conservative approach in preselected patients with stroke, elevated hs-cTn values, and high a priori probability of type 1 MI are warranted to address this question. Moreover, potential risks of coronary angiography need to be taken into account. The most common complication attributable to coronary angiography in our study was major bleeding due to groin hematoma. For this reason, an individual risk-benefit analysis must be carried out for each patient before coronary angiography is performed, taking particular account of the risk of bleeding.
Strengths and Limitations
The PRAISE study has strengths and limitations. The strengths include its prospective multicenter design with prespecified end points, a prespecified statistical analysis plan, blinded central core laboratories, external monitoring, and its independent EAC. The installation of an independent EAC allowed us to determine a diagnosis of MI while blinded for the variable of interest (ie, hs-cTn levels) to avoid circular reasoning. It should be noted that by definition an invasive investigation is not required for the diagnosis of MI. However, the significance and diagnostic specificity of troponin elevation in patients with stroke is less clear than in patients presenting primarily with chest pain. Therefore, in the PRAISE study, we decided to perform invasive coronary angiography in addition to getting laboratory values, ECG, and echocardiography for diagnosis and end point adjudication. This also made it possible to diagnose type 1 MI.
Limitations include the use of different hs-cTn assays at different study sites. Therefore, we standardized hs-cTn values as multiples of the ULN. Another bias may have been caused by the time interval between hospital admission and coronary angiography. An intracoronary thrombus or embolus may have dissolved in the course of the disease and might have been overlooked because of the time delay. We observed that patients eventually diagnosed with MI underwent angiography somewhat earlier than patients without MI. This could indicate that the treating physicians saw higher urgency in these patients. Moreover, treatment with intravenous thrombolysis for ischemic stroke may have altered the clinical course of MI and thereby hs-cTn values. However, we found no differences in the frequency of intravenous thrombolysis treatment between patients with and without MI or between patients with dynamic hs-cTn values and those without, making this hypothesis unlikely. Patients with severe renal insufficiency were not included in PRAISE, so renal insufficiency cannot serve as an alternative explanation for elevated hs-cTn values in our study population.
Inclusion and exclusion criteria limit the generalizability of our study. Because patients had to be able to provide written informed consent, we included patients with mostly mild to moderate strokes. Since we did not measure hs-cTn levels before the stroke occurred, we cannot exclude the possibility that some patients may have developed MI before the stroke. However, we included no patients in PRAISE who had undergone invasive cardiological treatment (including coronary angiography) within 30 days before the stroke.
Conclusions
In the PRAISE study, patients with ischemic stroke and elevated hs-cTn underwent standardized diagnostic procedures, including coronary angiography. Based on a blinded review by an EAC, about half of this study population was diagnosed with MI and 1 in 5 with type 1 MI. Dynamic changes in hs-cTn values were not helpful to diagnose MI. In exploratory analyses, only very high absolute hs-cTn values were associated with a diagnosis of type 1 MI. The results of our study highlight the clinical and pathophysiological relevance of the interplay between heart and brain in acute ischemic stroke. How to select patients with stroke and elevated hs-cTn for coronary angiography requires further exploration.
eMethods. Charters of the endpoint adjudication committee, critical events committee, and data and safety monitoring board
eTable 1. Number of patients with any MI and Type 1 MI
eTable 2. Patient characteristics associated with a diagnosis of myocardial infarction (MI) after exclusion of patients with TIA
eTable 3. Patient characteristics associated with a diagnosis of type 1 myocardial infarction (MI) after exclusion of patients with TIA
eFigure. Diagnostic accuracy of adding hs-cTn changes (0-100%) to predefined regression model for MI after exclusion of patients with TIA
Data sharing statement
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for management of acute ischemic stroke. Stroke. 2018;49(3):e46-e99. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 2018;17(12):1109-1120. doi: 10.1016/S1474-4422(18)30336-3 [DOI] [PubMed] [Google Scholar]
- 3.Scheitz JF, Endres M, Mochmann HC, Audebert HJ, Nolte CH. Frequency, determinants and outcome of elevated troponin in acute ischemic stroke patients. Int J Cardiol. 2012;157(2):239-242. doi: 10.1016/j.ijcard.2012.01.055 [DOI] [PubMed] [Google Scholar]
- 4.Collet JP, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 5.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28(3):220-226. doi: 10.1159/000226773 [DOI] [PubMed] [Google Scholar]
- 6.Scheitz JF, Sposato LA, Schulz-Menger J, Nolte CH, Backs J, Endres M. Stroke-heart syndrome: recent advances and challenges. J Am Heart Assoc. 2022;11(17):e026528. doi: 10.1161/JAHA.122.026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheitz JF, Lim J, Broersen LHA, et al. High-sensitivity cardiac troponin T and recurrent vascular events after first ischemic stroke. J Am Heart Assoc. 2021;10(10):e018326. doi: 10.1161/JAHA.120.018326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellwig S, Ihl T, Ganeshan R, et al. Cardiac troponin and recurrent major vascular events after minor stroke or transient ischemic attack. Ann Neurol. 2021;90(6):901-912. doi: 10.1002/ana.26225 [DOI] [PubMed] [Google Scholar]
- 9.Neumann JT, Weimann J, Sörensen NA, et al. A biomarker model to distinguish types of myocardial infarction and injury. J Am Coll Cardiol. 2021;78(8):781-790. doi: 10.1016/j.jacc.2021.06.027 [DOI] [PubMed] [Google Scholar]
- 10.Scheitz JF, Nolte CH, Laufs U, Endres M. Application and interpretation of high-sensitivity cardiac troponin assays in patients with acute ischemic stroke. Stroke. 2015;46(4):1132-1140. doi: 10.1161/STROKEAHA.114.007858 [DOI] [PubMed] [Google Scholar]
- 11.Nolte CH, von Rennenberg R, Litmeier S, et al. PRediction of acute coronary syndrome in acute ischemic StrokE (PRAISE): protocol of a prospective, multicenter trial with central reading and predefined endpoints. BMC Neurol. 2020;20(1):318. doi: 10.1186/s12883-020-01903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. doi: 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roffi M, Patrono C, Collet JP, et al. ; ESC Scientific Document Group . 2015 ESC guidelines fo the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37(3):267-315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 14.Berger K, Weltermann B, Kolominsky-Rabas P, et al. The reliability of stroke scales: the German version of NIHSS, ESS and Rankin scales [in German]. Fortschr Neurol Psychiatr. 1999;67(2):81-93. doi: 10.1055/s-2007-993985 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann J, Han S, Kraus M, et al. The DZHK research platform: maximisation of scientific value by enabling access to health data and biological samples collected in cardiovascular clinical studies. Clin Res Cardiol. 2023;112(7):923-941. doi: 10.1007/s00392-023-02177-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 17.Mochmann HC, Scheitz JF, Petzold GC, et al. ; TRELAS Study Group . Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the Troponin Elevation in Acute Ischemic Stroke (TRELAS) study. Circulation. 2016;133(13):1264-1271. doi: 10.1161/CIRCULATIONAHA.115.018547 [DOI] [PubMed] [Google Scholar]
- 18.Vranckx P, White HD, Huang Z, et al. Validation of BARC bleeding criteria in patients with acute coronary syndromes: the TRACER trial. J Am Coll Cardiol. 2016;67(18):2135-2144. doi: 10.1016/j.jacc.2016.02.056 [DOI] [PubMed] [Google Scholar]
- 19.Krause T, Werner K, Fiebach JB, et al. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann Neurol. 2017;81(4):502-511. doi: 10.1002/ana.24906 [DOI] [PubMed] [Google Scholar]
- 20.Litmeier S, Meinel TR, von Rennenberg R, et al. Coronary angiography in acute ischemic stroke patients: frequency and determinants of pathological findings in a multicenter cohort study. J Neurol. 2022;269(7):3745-3751. doi: 10.1007/s00415-022-11001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sposato LA, Lam M, Allen B, Shariff SZ, Saposnik G; PARADISE Study Group . First-ever ischemic stroke and incident major adverse cardiovascular events in 93 627 older women and men. Stroke. 2020;51(2):387-394. doi: 10.1161/STROKEAHA.119.028066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Charters of the endpoint adjudication committee, critical events committee, and data and safety monitoring board
eTable 1. Number of patients with any MI and Type 1 MI
eTable 2. Patient characteristics associated with a diagnosis of myocardial infarction (MI) after exclusion of patients with TIA
eTable 3. Patient characteristics associated with a diagnosis of type 1 myocardial infarction (MI) after exclusion of patients with TIA
eFigure. Diagnostic accuracy of adding hs-cTn changes (0-100%) to predefined regression model for MI after exclusion of patients with TIA
Data sharing statement