Abstract
Prenatal exposure to nonpersistent chemicals such as phthalates, bisphenols, and organophosphate (OP) pesticides is ubiquitous and occurs in mixtures. So far, epidemiological studies investigating neurodevelopmental consequences of these exposures have mainly been restricted to single-pollutant models. Thus, we studied the association between prenatal exposure to nonpersistent chemical mixtures and child IQ and emotional and behavioral problems. Data came from 782 mother–child pairs. Eleven phthalate, one bisphenol, and five OP pesticide urinary exposure biomarkers were measured three times during pregnancy and averaged. Nonverbal IQ, internalizing and attention problems, aggressive behavior, and autistic traits were assessed at child age 6 years. We used quantile g-computation to estimate the change in each outcome per quartile increase in all chemicals within the mixture. Higher exposure to the mixture was associated with lower nonverbal IQ(−4.0 points (95%CI = −7.0, −1.0), −5.5 points (95%CI = −10.2, −0.9), and −4.6 points (95%CI = −10.8, 1.5) for the second, third, and fourth quartile, respectively, compared to the first quartile). These results were mainly driven by the phthalate mixture. No association was observed with emotional and behavioral problems. Prenatal exposure to nonpersistent chemical mixtures was associated with lower nonverbal IQ in children. Exposure to chemical mixtures during gestation is universal and may impact neurodevelopment.
Keywords: prenatal exposures, vulnerable population, nonpersistent chemicals, endocrine disruptor chemicals, chemical mixtures, neurodevelopment
Graphical Abstract
INTRODUCTION
Since the start of the industrial revolution, a plethora of chemicals have been introduced in the global environment. Of those, more than 5000 chemicals are currently being produced on a large scale.1-3 Many chemicals end up directly in the environment.4,5 In addition, numerous chemicals reach the general population via consumer and food products.6,7 This extensive usage of chemicals has led to a widespread exposure of the general population. Results from biomonitoring studies have shown that three main chemical groups to which humans are continuously exposed are phthalates, bisphenols, and organophosphate (OP) pesticides.8 Phthalates and bisphenols are chemicals typically used as solvents and plasticizers to improve plastic product characteristics and therefore are present in products such as food packaging materials, cosmetics, and flooring materials.9-16 OP pesticides are insecticides, in particular used to protect crops in agricultural settings, which are present in, for example, fruit and vegetables.17-19 Although exposure sources and routes vary between these three chemical groups, diet is an important source of exposure to OP pesticides, phthalates, and bisphenols. These compounds are nonpersistent and, consequently after exposure, rapidly metabolized and excreted.20-22
Studies have shown that these chemicals can reach the fetus when pregnant women are exposed since they are able to cross the placental barrier and the fetal blood brain barrier.23-29 During gestation, the brain is particularly susceptible to chemical insults because many vital biological processes take place to ensure normal brain growth.30 Animal studies have provided ample support for a relation between low-dose exposure to phthalates, bisphenols, and OP pesticides and impaired neurodevelopment and behavior in offspring.31-33 However, epidemiological studies investigating associations between prenatal exposure to these nonpersistent chemicals and neurodevelopmental outcomes, including IQ and emotional and behavioral problems have not been consistent.34-37
In real world situations, fetal exposure to these nonpersistent chemicals co-occurs in exposure combinations. Except for a small number of studies,38-42 the majority of studies investigating the association between prenatal exposure to these chemicals and neurodevelopment in children, including our previous work,43-45 have applied single exposure models, which have important limitations.46 For example, co-occurring chemical exposures may join additively to produce larger effects which cannot be investigated.47-49 Further, single exposure analyses may be biased if potential cochemical confounding exists. Finally, the rate of type I errors may inflate when correlated chemical exposures are modeled separately.50 Examining the overall mixture effect by looking at joint exposures may overcome such limitations and provide effect estimates that more closely resemble real-world exposures.50 Further, adverse effects on neurodevelopment during childhood may affect health later in life. For example, higher IQ in children is predictive of healthy behavior, higher educational achievement, and better employment later in life.51,52 Moreover, children with behavior problems are more likely to have a depression in adulthood.53,54 Therefore, the aim of this study is to expand our previous work by investigating whether prenatal exposure to the overall additive mixture effect of phthalates, bisphenols, and OP pesticides is associated with offspring nonverbal IQ and emotional and behavioral problems.
METHODS
Study Population.
The Generation R Study is a prospective population-based birth cohort designed to investigate early environmental and genetic determinants of development. All pregnant women who lived in the study area, Rotterdam, The Netherlands, were eligible. In total 8879 pregnant women were enrolled between 2002 and 2006.55 Between February 2004 and January 2006, women provided three spot urine samples during ultrasound visits at <18 weeks, 18–25 weeks, and >25 weeks of gestation. In total, 2083 women provided a complete set of three urine samples. At child age 6 years, the mother-child pairs were invited to the clinic to engage in a follow-up study and to collect data including neurobehavioral data. Of the 2083 mothers that provided three urine samples prenatally, 1405 mother-child pairs provided data at the follow-up visit. Of those, 782 mothers had complete data on prenatal exposure biomarkers and neurodevelopment at the child age of 6 years. Mothers provided written informed consent for themselves and for their children. The study protocol underwent human subjects review at Erasmus Medical Center, Rotterdam, The Netherlands (MEC 198.782.2001.31, MEC-2007-413).
Exposure Biomarker Measures.
Specific information regarding urine specimen collection and the process for urine analyses of phthalate metabolites, bisphenols, and OP pesticide metabolites can be found elsewhere.56-58 In short, concentrations of 18 phthalate metabolites were determined by utilizing a solid-phase extraction method followed by enzymatic deconjugation of the glucuronidated phthalate monoesters coupled with high performance liquid chromatography electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS).59 Regarding bisphenols, eight biomarkers were quantified using a liquid–liquid extraction method followed by enzymatic deconjugation of the glucuronidated bisphenols coupled with HPLC-ESI-MS/MS.59 The limits of detection (LOD) for phthalate and bisphenol biomarkers ranged between 0.008–1.11 μg/L. Next, six nonspecific dialkylphosphate (DAP) metabolites of OP pesticides were measured using gas chromatography coupled with tandem mass spectrometry (GC–MS/MS). As a result, three dimethyl (DM) metabolites and three diethyl (DE) metabolites were detected with LODs in the range of 0.06–0.50 μg/L. Finally, creatinine concentrations were also measured to account for urine dilution.60
Supporting Information (SI) Table S1 presents the descriptive statistics of biomarker concentrations included in the analyses by weeks of gestation. We included a total of 17 exposure biomarkers with more than 50% detection rate in the analysis (11 phthalate metabolites, one bisphenol, and five OP pesticides metabolites) including phthalic acid (PA, an end metabolite of all phthalates used as a proxy for unmeasured phthalate metabolites). SI Table S2 presents the descriptive statistics of biomarker concentrations excluded in the analyses by weeks of gestation. Among the 17 exposure biomarkers included in the study, the concentrations below the LOD for the 11 phthalate metabolites and bisphenol A were not estimated by the lab and were therefore imputed by LOD divided by the square root of 2.61 For the five DAP metabolites, the concentrations below the LOD were estimated by the lab and were therefore used.62
Nonverbal IQ.
The nonverbal IQ score was measured at the age of 6 years using the reliable and validated Snijders-Oomen Nonverbal Intelligence Test–Revised (SON-R).63-65 The SON-R test correlates well with the Wechsler Preschool and Primary Scale of Intelligence with a reliability score of 0.9.63-65 The SON-R test was chosen because of the multiethnic composition of the Generation R Study. Spoken or written language is not required for this test and instructions can also be given nonverbally. The SON-R contains six language-independent subtests: Patterns, Mosaics, Puzzels, Situations, Categories, and Analogies. These subtests are classified into two empirical groups: Performance tests (Patterns, Mosaics, Puzzles) and Reasoning tests (Situations, Categories, Analogies). Because of time constraints, one performance subtest, that is, Mosaics, and one reasoning subtest, that is, Categories, was selected to have at least one subtest of each empirical group. These two subtests cover spatial insight (Mosaics) and abstract reasoning abilities (Categories). Subtest raw scores were transformed into a single age-standardized nonverbal IQ score using age-specific reference scores provided in the manual (mean = 100, SD = 15). The correlation between the IQ score based on these two subtests and the full SON-R IQ battery was high (r = 0.86).66
Child Emotional and Behavioral Problems.
Emotional and behavioral problems in children were measured using the primary care giver (mostly mothers)-reported Child Behavior Checklist (CBCL) 1.5–567 at child age 6 years and Social Responsiveness Scale (SRS)68 at child age 7 years. The CBCL is a globally validated and reliable instrument that quantifies emotional and behavioral problems of the preceding 2 months on a continuous scale using 99 items.67 Each item is scored on a 3-point rating scale 0 = “not true”, 1 = “somewhat or sometimes true”, and 2 = “very true or often true”. Emotional problems were assessed using the internalizing problems syndrome scale, which consisted of the summed raw scores of Emotionally Reactive (nine items), Anxious/Depressed (eight items), Somatic Complaints (11 items), and Withdrawn (eight items) syndrome scales, generating a score ranging from 0 to 72 points. Behavioral problems were assessed using the attention problems and the aggressive behavior syndrome scales. The attention problems syndrome scale (five items) generates a score ranging from 0 to 10 points. The aggressive behavior syndrome scale (19 items) generates a score ranging from 0 to 38 points. Syndrome scales are generalizable across 23 countries, including The Netherlands.69
Autistic traits were assessed using a SRS short form.68 The SRS is a valid quantitative measure of subclinical and clinical autistic traits and determines several dimensions of inter-personal behavior, communication and repetitive/stereotypic behavior characteristic of autism.70 The SRS is reported by the mother based on her observation of the child’s social behavior during the previous 6 months. The SRS has excellent correspondence to autism classification according to the Developmental, Dimensional, and Diagnostic interview (3Di) and the Autism Diagnostic Observation Schedule (ADOS).71 In this study, a modified version of the SRS tool, consisted of 18 items, was used to lower the participant burden.72 Each item is scored on a 4-point rating scale 0 = “never true” to 3 = “almost always true”, generating a score ranging from 0 to 72 points. The SRS and the modified version of the SRS have shown to be highly correlated (r > 0.90) among test scores.73
Potential Confounders.
Information on maternal age (year in continuous), maternal prepregnancy body mass index (BMI) (kg/m2 in continuous), maternal education level (low: < 3 years of high school; intermediate: 3+ years of secondary education; and, high: university degree or higher vocational training), maternal ethnicity (Dutch national origin, other-Western, and non-Western), household income (<1200 euro/month, 1200–2000 euro/month, >2000 euro/month), marital status (married/partner, single), parity (0, 1, or 2+), maternal smoking (no smoking, smoked until pregnancy recognized, and continued smoking during pregnancy), maternal alcohol use (no alcohol consumption, alcohol consumption until pregnancy recognized, continued occasionally (<1 glass/week for at least two trimesters), and continued frequently (≥1 glass/week for at least two trimesters)), and maternal psychological dysfunction (score in continuous), using the Brief Symptom Inventory (BSI),74 was collected during pregnancy. Maternal IQ was determined using the computerized Ravens Advanced Progressive Matrices Test, set I75,76 when mother-child pairs visited the 6-year examination (score in continuous). Data on child sex (female, male) was collected at birth and child age at assessment (years in continuous) was reported during the 6-year examination.
Statistical Analyses.
Prior to the main analyses, we used the Multivariate Imputation by Chained Equations (MICE) method in R77,78 to impute 10 times the few biomarker concentrations that were missing due to insufficient volume or machine errors (<2.6% for DETP, and <1% for other biomarkers) and missing confounder data (<13%). The 10th imputed data set was used for the main analyses.
We expressed the biomarker concentrations on a creatinine basis (ug/g creatinine) to correct for urine dilution. We then log10 transformed and averaged the concentrations across pregnancy. Nonpersistent chemicals such as phthalates, bisphenols, and OP pesticides are rapidly metabolized and excreted in urine.79,80 The individual exposure biomarker concentrations may therefore fluctuate over short periods of time as a consequence of contact with varying exposure sources (e.g., different diet). Therefore, the mean of the three concentrations is a better measure of exposure across pregnancy. Next, internalizing problems, attention problems, aggressive behavior, and autistic traits scores were square root transformed to improve normality of the residuals.
In order to estimate the joint effect of phthalate metabolites, bisphenol, and OP pesticide metabolite concentrations on offspring nonverbal IQ and emotional and behavioral problems, we used the quantile-based g-computation method in R.81 Quantile g-computation estimates the effect estimate of simultaneously increasing all exposures within the mixture by a single quantile.81 This approach also permits one to investigate the joint effect of a specific chemical group within the overall mixture (e.g., OP pesticide metabolites) while adjusting for potential confounding by other chemicals in the mixture (e.g., phthalate metabolites and bisphenols). By investigating the joint effect of the mixture, the quantile g-computation decreases multiple testing (i.e., type one error) and deals with copollutant confounding. Further, quantile g-computation allows for the identification of additive and synergistic effects of the mixture and has the ability to model nonlinearity.81
We first investigated the mixture effect of all chemical biomarkers combined (i.e., overall mixture effect) on offspring nonverbal IQ and emotional and behavioral problems. We estimated the change in the outcome (effect estimate) of simultaneously increasing all exposures within the mixture by a single quartile. We explored whether the associations between each biomarker and each outcome were nonlinear by including the squared exposure biomarker concentrations in the models and testing if the model improved using Akaike information criteria. We found that adding a quadratic term for monomethyl phthalate (MMP) in the nonverbal IQ models, for diethylthiophosphate (DETP) in the internalizing problems models, for mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) in the attention problems models, and for mono(3-carboxypropyl) phthalate (MCPP) in the aggressive behavior models the model fit significantly improved (P < 0.05). When one or more quadratic terms are present in the final model, the joint mixture association with a given outcome calculated by the quantile g-computation is determined by a quadratic term estimate and the estimate for the lower-order joint mixture effect, similar to a linear regression. Further, we predicted differences between each quartile and the first quartile based on the estimated outcome (Y) score for each quartile ,
in which is the integer value assigned to each quartile (, 1, 2, 3), is the intercept, is the mixture estimate, and is the quadratic term estimate (if included). Coefficients for each quartile compared to the lowest (reference) quartile were calculated by the difference between the predicted for each quartile, . Second, we investigated the combined exposure effect of chemical biomarkers within each chemical group on offspring nonverbal IQ and emotional and behavioral problems while adjusting for the other chemical groups. All models were adjusted for fetal sex, maternal age, maternal BMI, maternal education level, maternal ethnicity, household income, marital status, parity, maternal smoking, maternal alcohol use, maternal IQ, and child age at assessment. Models for internalizing problems, attention problems, aggressive behavior, and autistic traits were additionally adjusted for maternal psychopathology.
As a secondary analysis, we investigated which chemical biomarker concentrations within the mixture were contributing the most to the overall mixture effect for the chemical exposure-outcome models where we observed an association. Since this question does not have straightforward answers in nonlinear models, we performed this investigation excluding product terms and quadratic terms, thereby assuming linearity. Further, instead of investigating the mixture association with attention problems and aggression behavior separately, one could also investigate the effect on externalizing problems which combines the two scales. We therefore carried out a sensitivity analyses in which we investigated whether prenatal exposure to the mixture is associated with externalizing problems. Next, birth year might predict exposure concentrations of nonpersistent chemical because of the strong temporal patterns of these exposures and because birth year might be a marker of other direct pathways between other unmeasured exposures and neurodevelopment. We therefore carried out a sensitivity analysis in which we additionally adjusted the main models for birth year. Finally, several studies have suggested that sex may be a potential effect estimate modifier in the association of prenatal exposure to phthalates, bisphenols, and OP pesticides and neurodevelopmental outcomes, including mixture studies.38,41,82-84 We therefore explored potential effect modification by sex via stratification.
RESULTS
The median age of pregnant women at enrollment was 31 years (Table 1). The majority of the women were Dutch (58%), were nulliparous (62%), were nonsmokers (77%), had obtained a high level of education (55%), and had a high household income (71%). SI Table S3 compares the sociodemographic and lifestyle characteristics of the study sample with the characteristics of the excluded participants of the Generation R cohort. Mothers of children included in the analysis were more likely to have a Dutch national origin, to be older, and have a higher socioeconomic status compared to mothers excluded from the analyses.
Table 1.
Characteristics of Study Participants (n = 782)
| distributiona | ||
|---|---|---|
| maternal age (years) | 31 (28, 34) | |
| maternal ethnicity | ||
| Dutch | 57.7 | |
| other western | 12.7 | |
| nonwestern | 29.6 | |
| maternal IQ | 100 (90, 107) | |
| missing, n | 19 | |
| maternal education | ||
| low | 14.8 | |
| intermediate | 30.2 | |
| high | 55.0 | |
| missing, n | 24 | |
| household income | ||
| <1200€/month | 12.6 | |
| 1200–2000€/month | 16.5 | |
| >2000€/month | 70.9 | |
| missing, n | 101 | |
| maternal psychopathology | 0.13 (0.08, 0.33) | |
| missing, n | 94 | |
| maternal body mass index (kg/m2) | 22 (21, 25) | |
| missing, n | 97 | |
| maternal parity | ||
| 0 | 62.3 | |
| 1 | 26.6 | |
| ≥2 | 11.1 | |
| missing, n | 4 | |
| maternal marital status | ||
| married/living with partner | 89.8 | |
| no partner | 10.2 | |
| missing, n | 29 | |
| maternal smoking during pregnancy | ||
| no smoking during pregnancy | 77.2 | |
| until pregnancy recognized | 8.9 | |
| continued during pregnancy | 13.9 | |
| missing, n | 63 | |
| maternal alcohol consumption during pregnancy | ||
| no consumption during pregnancy | 36.7 | |
| until pregnancy recognized | 17.5 | |
| continued occasionally | 39.3 | |
| continued frequently | 6.5 | |
| missing, n | 40 | |
| child age at assessment | 5.9 (5.8, 6.0) | |
| child sex (female) | 49.1 |
Median (25th percentile, 75th percentile) for continuous variables and percentage for categorical variables.
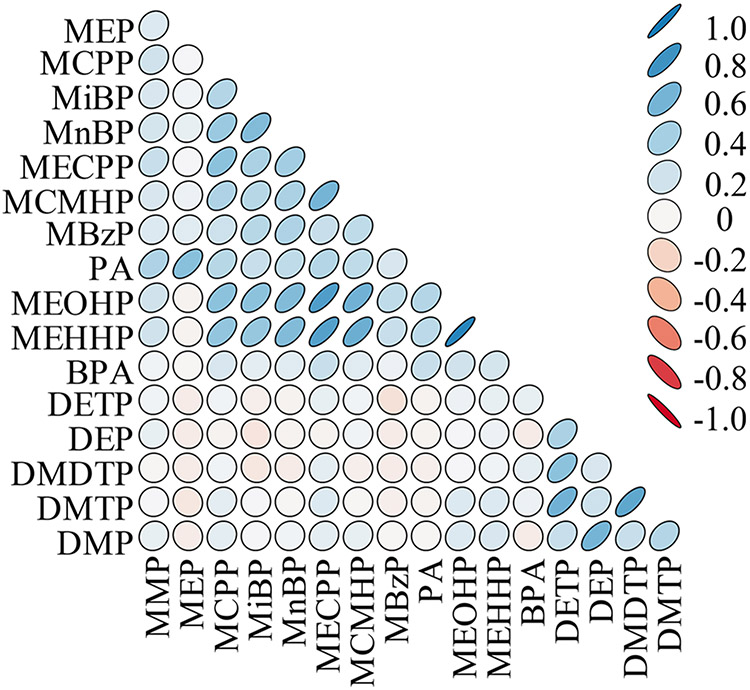
The biomarker concentration distributions are presented in SI Table S4. Pearson and Spearman rank correlations are presented in SI Table S5, and a graphical representation of the correlations can be found in Figure 1. Correlations were generally high within phthalate metabolites and OP pesticide metabolites (e.g., Pearson correlation between (mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) metabolites = 0.76 and between dimethylphosphate (DMP) and diethylphosphate (DEP) metabolites = 0.60) (Figure 1). However, chemical groups had low to moderate correlation between them (e.g., Pearson correlation between bisphenol A and MECPP = 0.25). The distribution of nonverbal IQ and the emotional and behavioral outcomes without square root transformation can be found in SI Table S6.
Figure 1.
Pearson correlation matrix for pregnancy averaged exposure biomarker concentrations. Corresponding data and Spearman rank correlations can be found in SI Table S5. Abbreviations: MMP = monomethyl phthalate, MEP = monoethyl phthalate, MCPP = mono(3-carboxypropyl) phthalate, MiBP = monoisobutyl phthalate, MnBP = mono-n-butyl phthalate, MECPP = mono-(2-ethyl-5-carboxypentyl) phthalate, MCMHP = mono-[(2-carboxymethyl) hexyl] phthalate, MBzP = monobenzyl phthalate, PA = phthalic acid, MEOHP = mono-(2-ethyl-5-oxohexyl) phthalate, MEHHP = mono-(2-ethyl-5-hydroxyhexyl) phthalate, BPA = bisphenol A, DMDTP= dimethyldithiophosphate, DMTP = dimethylthiophosphate, DMP = dimethylphosphate, DETP = diethylthiophosphate, and DEP = diethylphosphate.
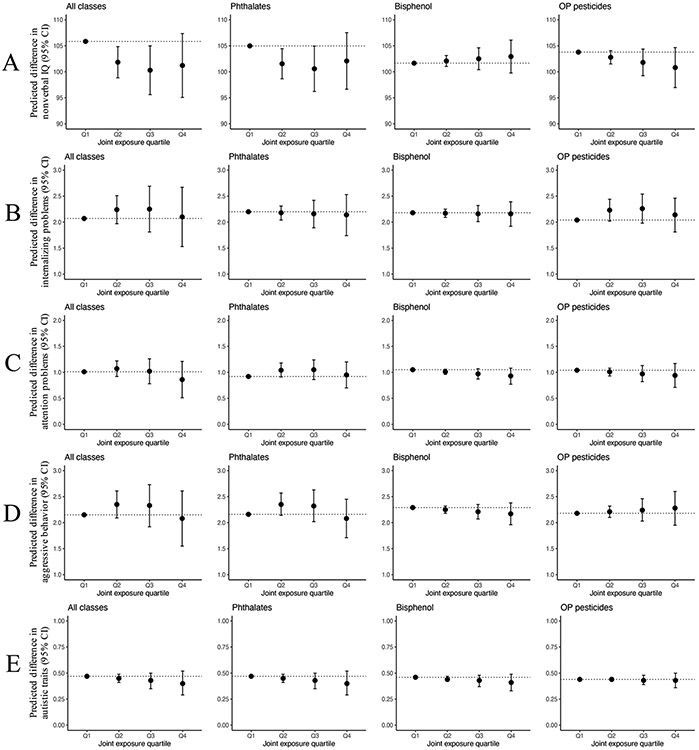
Higher exposure to the overall mixture was associated with lower nonverbal IQ (−4.0 points (95% CI −7.0, −1.0), −5.5 points (95% CI −10.2, −0.9), and −4.6 points (95% CI −10.8, 1.5) for the second, third, and fourth quartile of the overall mixture, respectively, compared to the first quartile) (Figure 2A and SI Table S7). No association was observed between the overall mixture and internalizing problems, attention problems, aggressive behavior, and autistic traits (Figure 2B-E and SI Table S7). Further, when we explored the associations between prenatal exposure to the mixture of each individual chemical group while adjusted for other chemicals, we observed a similar pattern for the association between the phthalate metabolite mixture and nonverbal IQ compared to the association of the overall chemical mixture (Figure 2A and SI Table S7).
Figure 2.
Adjusted difference (95% confidence interval) in estimated outcome for each exposure quartile (Q2–Q4) compared to the lowest quartile (Q1) in all chemicals measured and within each chemical class. Corresponding numeric data are reported in SI Table S7. Each row corresponds to a different mixture model with a different outcome (A: Nonverbal IQ. B: Internalizing problems, C: Attention problems. D: Aggressive behavior. E: Autistic traits). Adjusted for fetal sex, maternal age, maternal prepregnancy bmi, maternal education level, maternal ethnicity, household income, marital status, parity, maternal smoking, maternal alcohol use, maternal IQ, and child age at assessment. Models for internalizing problems, attention problems, aggressive behavior, and autistic traits are additionally adjusted for maternal psychopathology. Models of the phthalate metabolite mixture additionally adjusted for log 10-transformed pregnancy-averaged concentrations of bisphenol A and the OP pesticide metabolites. Models of bisphenol (bisphenol A) additionally adjusted for log 10-transformed pregnancy-averaged concentrations of phthalate and OP pesticide metabolites. Models of the OP pesticide metabolite mixture additionally adjusted for log 10-transformed pregnancy-averaged concentrations of phthalate metabolites and bisphenol A.
Higher exposure to the phthalate metabolite mixture was associated with lower nonverbal IQ (−3.4 points (95% CI −6.3, −0.6), −4.4 points (95% CI −8.8, −0.1), and −2.9 points (95% CI −8.4, 2.6) for the second, third, and fourth quartile of the phthalate mixture, respectively, compared to first quartile). No associations were observed between the bisphenol mixture and the OP pesticide mixture with nonverbal IQ (SI Figure A2 and SI Table S7). No association was observed for each of the individual chemical groups with internalizing problems, attention problems, aggressive behavior, and autistic traits (Figure 2B-E and SI Table S7). Table 2 presents the adjusted effect contribution for each averaged chemical biomarker concentration included in the overall mixture effect on nonverbal IQ. The partial effects provide information on the contributed weight on the overall effect of each chemical biomarker concentration. Overall, the total contribution of chemical exposure biomarkers in the negative direction (−5.4 points of lower nonverbal IQ) was greater than the total contribution of chemical exposure biomarkers in the positive direction (+3.8 points of higher nonverbal IQ). When looking into each chemical group, phthalate metabolites had the highest negative contribution (72.8%) followed by OP pesticide metabolites (27.2%), while bisphenol A did not contribute to the negative association. Among these, MECPP (26.5%) was the metabolite that contributed the most to the negative association.
Table 2.
Adjusted Effect Contribution for Each Averaged Chemical Biomarker Concentration Included in the Overall Mixture Effect on Nonverbal IQ (n = 708)a
| negative effect contribution | weight | positive effect contribution | weight | |||
|---|---|---|---|---|---|---|
| biomarker | B | 95%CI | % | B | 95%CI | % |
| Phthalate Metabolites | ||||||
| MMP | −0.29 | −1.36, 0.78 | 5.3 | |||
| MEP | −0.21 | −1.39, 0.98 | 3.8 | |||
| MCPP | −0.84 | −2.07, 0.38 | 15.5 | |||
| MiBP | −0.77 | −2.08, 0.55 | 14.1 | |||
| MnBP | 0.46 | −0.88, 1.81 | 12.2 | |||
| MECPP | −1.44 | −2.95, 0.07 | 26.5 | |||
| MCMHP | 0.68 | −0.58, 1.93 | 17.9 | |||
| MBzP | 0.41 | −0.68, 1.50 | 10.8 | |||
| PA | −0.41 | −1.70, 0.88 | 7.6 | |||
| MEOHP | 0.94 | −1.31, 3.18 | 24.6 | |||
| MEHHP | 0.38 | −1.70, 2.46 | 10.0 | |||
| total | −3.96 | 72.8 | 2.87 | 75.5 | ||
| Bisphenol | - | - | ||||
| BPA | 0.41 | −0.61, 1.43 | 10.9 | |||
| total | 0.41 | 10.9 | ||||
| OP Pesticide Metabolites | ||||||
| DMDTP | −0.08 | −1.42, 1.27 | 1.4 | |||
| DMTP | −0.79 | −2.35, 0.76 | 14.6 | |||
| DMP | −0.61 | −1.92, 0.70 | 11.2 | |||
| DETP | 0.26 | −1.01, 1.53 | 6.8 | |||
| DEP | 0.26 | −1.05, 1.56 | 6.8 | |||
| total | −1.48 | 27.2 | 0.52 | 13.6 | ||
| Total Mixture | ||||||
| total | −5.44 | 100% | 3.80 | 100% | ||
Models adjusted for fetal sex, maternal age, maternal prepregnancy bmi, maternal education level, maternal ethnicity, household income, marital status, parity, maternal smoking, maternal alcohol use, maternal IQ, and child age at assessment. Abbreviations: MEP = monoethyl phthalate, MMP = monomethyl phthalate, MiBP = monoisobutyl phthalate, MnBP = mono-n-butyl phthalate, MECPP = mono-(2-ethyl-5-carboxypentyl) phthalate, MCMHP = mono-[(2-carboxymethyl) hexyl] phthalate, MEOHP = mono-(2-ethyl-5-oxohexyl) phthalate, MEHHP = mono-(2-ethyl-5-hydroxyhexyl) phthalate, MCPP = mono(3-carboxypropyl) phthalate, mBzP = monobenzyl phthalate, PA = phthalic acid, BPA= bisphenol A, DMDTP = dimethyldithiophosphate, DMTP = dimethylthiophosphate, DMP = dimethylphosphate, DETP = diethylthiophosphate, and DEP = diethylphosphate.
Next, prenatal exposure to the mixture was not associated with externalizing problems (SI Table S8). Further, results additionally adjusting for birth year were similar to the main models (SI Table S9). Finally, sex stratified analyses for the association between the joint mixture effect and nonverbal IQ showed similar effect estimates for boys and girls as compared to the main analyses (SI Table S10). Further, regarding aggressive behavior we observed no association for the overall joint effect among boys. However, girls in the second (B = 0.34, 95%CI = 0.01, 0.67), third (B = 0.48, 95%CI = −0.06, 1.02), and fourth exposure quartile (B = 0.43, 95%CI = −0.31, 1.17) had more aggressive behavior problems as compared to girls in first quartile of the exposure.
DISCUSSION
We observed that exposure to higher concentrations of a mixture of phthalates, bisphenol, and OP pesticides during pregnancy was associated with lower nonverbal IQ in children aged 6 years, and mainly phthalates were driving the association. Also, this association had a nonlinear dose–response relationship with the second, third, and fourth quartiles having very similar differences in nonverbal IQ when compared to the first quartile. No associations were observed between prenatal exposure to the mixture of phthalates, bisphenol, and OP pesticides and emotional and behavioral problems.
The majority of epidemiological studies examining prenatal exposure to nonpersistent chemicals in relation to offspring neurodevelopment have investigated single exposures.85 Although some of these studies have been suggestive of an association, systematic reviews have revealed that the evidence for a relation is inconclusive.34-37 The practice of exploring associations with neurodevelopmental outcomes using separate exposure models has important limitations. First, it increases the chance of false positive associations as an artifact of increasing the number of tests performed and ignores potential cochemical confounding.50,85,86 Moreover, chemicals have the potential to produce additive health effects and this cannot be explored with single exposure models.86,87
To the best of our knowledge, only two previous studies have investigated the effect of chemical mixtures on IQ. All previous studies investigated the overall effect of the mixture using a different method, weighted-quantile sum (WQS) regression, and none have jointly estimated the effect of phthalates, bisphenols, and OP pesticides, but only focused on phthalates or on a different combination of chemicals. Loftus et al. (2021) investigated the overall mixture effect of third trimester phthalate exposure on child IQ. Thirteen phthalate metabolites were measured in pregnant women and cognitive outcomes including full-scale IQ were measured at child age 4–6 years. Contrary to our observation, this study found no evidence for an association. Next, Tanner et al. (2020) explored whether early pregnancy exposure to a mixture of 26 persistent and nonpersistent endocrine-disrupting chemicals (EDCs) was associated with IQ in children aged 7 years. This study found higher exposure to the EDC mixture to be associated with a lower IQ, only in boys. Among a wide variety of EDCs, this study incorporated several phthalate metabolites and bisphenols and found bisphenol F to be the strongest contributor to the mixture effect. Additionally, monoethyl phthalate (MEP) and monobenzyl phthalate (MBzP) metabolites and bisphenol A were also identified as having a substantial contribution to the overall mixture effect. In contrast, we observed that prenatal exposure to a mixture was associated with nonverbal IQ and found no differences in sex specific results when compared to the main result. Further, we found that MEP had only a weak contribution to the negative effect in nonverbal IQ whereas MBzP and bisphenol A had a nonsignificant contribution toward the positive effect. The strongest contributors to the overall negative mixture association on nonverbal IQ were MECPP, MCPP, monoisobutyl phthalate (MiBP), and dimethylthiophosphate (DMTP). In a previous work where we used the same population to investigate phthalate metabolites exposure in association with nonverbal IQ using separate regression analyses, we showed that the averaged concentrations of MCPP and PA across pregnancy had the largest association with nonverbal IQ with larger effect estimates as compared to the estimates observed in this study.45 These different findings indicate that by modeling co-occurring chemical exposures, exposure biomarkers combinations playing a substantial role in the association with a certain outcome might be identified, that are missed in single regression models that do not adjust for or take into account coexposure effects. This observation is consistent with a primary concern of estimating effects of individual exposures, coexposure confounding.
Regarding emotional and behavioral problems, one study investigated the effect of averaged exposure to phthalates across two measurements during pregnancy (16 and 26 weeks of gestation).42 This study assessed children’s emotional and behavioral problems at ages 2, 3, 4, 5, and 8 years using the Behavioral Assessment System for Children-2. Similar to our findings, they did not find an association between prenatal exposure to a mixture of nine phthalate metabolites and emotional and behavioral problems in children.42 Also, Day et al. (2021) investigated whether prenatal exposure to a mixture of nine phthalate metabolites measured in early and late pregnancy was associated with autistic traits and behavior problems in children aged 4–5 years. They found that higher early pregnancy phthalate mixture concentrations were associated with more autistic traits in children and identified MCPP, MBzP, and MEP as the main contributors to the overall effect.41 Although the sample size was relatively small for stratified analyses, this study also observed that higher late pregnancy exposure to a mixture of phthalates was associated with increased externalizing problems only in boys (n = 243), with MCPP and MBzP as the main contributors to the overall effect.41 In contrast, we observed that higher pregnancy exposure to the mixture was associated with more aggressive behavior in girls. However, our sex specific results should be interpreted with caution since the sample sizes were small, not formally tested using an interaction, and not adjusted for multiple testing.
Comparability between studies should be done carefully. Nonpersistent chemicals have short half-lives. Concentrations measured in a single spot urine sample may therefore not be accurate in estimating pregnancy exposure because biomarker concentrations may vary from day to day within each subject resulting in high (within-subject) temporal variability.79,80,88 We averaged the levels measured across the three trimesters of pregnancy to obtain a more precise estimate of exposure across pregnancy. Studies based on a single spot urine measurement might be more prone to measurement error resulting in imprecise effect estimates. Also, the different exposure biomarkers incorporated in the mixture of each study (e.g., only phthalates versus a mixture of different chemical groups), and the potential differences in exposure patterns across different populations could complicate comparisons. Further, different statistical approaches to study exposure mixtures and health outcomes can yield to different results. In our study we used the quantile g-computation method, whereas all other previous studies used the WQS regression to estimate the joint exposure effect. Similar to the quantile g-computation method, the WQS regression estimates the effect by increasing all exposures by a single quantile. Both methods share the simplicity of inference (effect as a whole) and can easily include multiple exposures that co-occur to estimate the additive effects. In regards to the direction and the shape of the dose–response relationship, the WQS regression approach is limited by the assumptions of linearity of the exposure effects and directional homogeneity (i.e., effects of all exposures are zero or in the same direction)81,85 whereas the quantile g-computation is able to incorporate nonlinear effects of individual exposures and the mixture as a whole and does not assume directional homogeneity.81 This allowed us to identify a nonlinear effect in the association between the overall mixture and nonverbal IQ in our study. Several studies exploring chemical exposure effects in both humans and animals indicate that the exposure–disease associations may not be linear.89 For example, Vandenberg et al. (2012) stated in their review that low-dose exposure to EDCs (including phthalates and bisphenols) and nonmonotonic dose–response curves are frequently observed.90,91 Further, in the WQS regression, the direction of the effect estimate associated with the exposures should be specified a priori and should be the same for all, either negative or positive38,40-42 enforced by the assumption of directional homogeneity.81 In contrast, quantile g-computation estimates the health effect in relation to all exposures in the condition that all increase by a single quantile, but without specifying a priori the direction of the associations and allowing them to go into different directions. Therefore, some estimated associations within the overall mixture can be reflected as a weight in the unexpected direction, regardless of statistical significance. For example, in our study we observed that the overall mixture was associated with lower nonverbal IQ, while some exposure biomarkers within the mixture contributed nonsignificantly toward a higher nonverbal IQ (i.e., positive weight). While we would not expect, a priori, for any exposures related to the biomarkers to lead to improved health outcomes, our findings may reflect unknown mechanisms or residual confounding. Therefore, replication studies in populations with different exposure patterns and different confounding structures are warranted to better understand these counterintuitive findings. Next, it is important that the independent effects from these models are interpreted with caution, because the observed association with nonverbal IQ is for the joint exposure of all biomarkers combined. Furthermore, the independent effect estimates come from a model in which we assume linearity whereas the observed association was nonlinear. Finally, none of the independent effects were statistically significant, which shows that the combined effect may be more important. Another innovative method that is able to estimate the additive health effects of exposure to mixtures is the Bayesian kernel machine regression (BKMR). BKMR has many benefits such as the ability to concurrently estimate the effect of chemical classes with high within class correlations next to the calculation of the association of separate chemicals within a chemical class on a health outcome.92 However, the quantile g-computation method and the WQS only provide one or two parameters for a dose response estimation whereas the dose response parameters of the BKMR are not easily interpretable.
The phthalate and bisphenol A concentration levels observed in this study sample were similar as compared to several other studies investigating prenatal exposures. For instance, the median concentrations for MBzP (2–6 ng/mL), MCPP (1–2 ng/mL), MEOHP (6–9 ng/mL), and bisphenol A (2 ng/mL) are somewhat of the same magnitude to concentrations measured in Israel (1, 1, 6, and 2 ng/mL, respectively)93 or Canada (5, 1, 9, and 1 ng/mL, respectively).94 However, in the United States concentrations were slightly higher (e.g., MBzP = 7 ng/mL, MCPP = 2, MEOHP 11 ng/mL.95 DAP metabolite concentrations in this study were higher than most other studies in pregnant women.96-99 Variation in exposure concentration across studies may be due to variation in diet, protocols and timing of urine collection, the use of products, and metabolic rate. Next, correlations were generally high within phthalate metabolites and OP pesticide metabolites. However, chemical groups had low to moderate correlation across groups. Similarly, higher within chemical group correlations as compared to between group correlations have been noted previously in the Human Early-Life Exposome (HELIX) project.100 In this project, correlations for 87 environmental exposures during pregnancy (19 exposure groups) were assessed in six European birth cohorts. The implication of lower correlations between groups as compared to within group may suggest that studies investigating the joint mixture effect of a single chemical group are not greatly affected by copollutant confounding from exposures coming from other chemical groups.100,101 As shown by biomonitoring studies and concentrations observed in this study, exposures to nonpersistent chemicals co-occur. Real life exposure mixtures are complex and may have both similar and mixed modes of action which can result in additive or nonadditive effects, even when correlations between chemical groups are low.
There are some limitations to our study that needs to be considered. First, our study used three spot urine samples during pregnancy to measure the exposure which is most probably a better indicator of exposure across gestation. Nevertheless, measurement error might still have occurred and resulted in imprecise effect estimates.80 Second, the timing of the urine collection varies between 8 am and 8 pm and most likely consist of a combination of first morning and random urine spot samples. This may be of concern given the fact that fluid intake and the time of day may affect concentrations of chemicals, urine volume, and excretion rate.102-104 However, concentrations were adjusted for creatinine and it is unlikely that the timing of urine collection could be a confounder in these associations. Third, mothers of children included in the analysis were more likely to have a Dutch national origin, to be older, and have a higher socioeconomic status as compared to children excluded from the full generation R cohort. Therefore, potential selection bias might have occurred in our study and influenced our results. Unfortunately, at the moment the quantile-g computation method does not allow to include analytical strategies to correct for potential selection bias such as inverse probability censoring weighting. Next, during the data collection wave, the CBCL/1.5–5 was used to measure emotional and behavior problems because it was expected that most children that would participate in the follow up study, would be younger than 6 years. At the end of the assessment 6208 children had sufficient data on CBCL/1.5–5. Of those, 58% were younger than 6 years. As per recommendation of the Achenbach System of Empirically Based Assessment (ASEBA) manual we used the version for younger children if a group comprises children below and above the cutoff. This ensures that the assessment in one examination is uniform. This may have influenced the results since the CBCL 6–18 would be more appropriate to assess emotional and behavioral problems in children of 6 years or older although many items are not appropriate in children at the lower end of the range, for example, “My child smokes, chews, or sniffs tobacco”. However, Cronbach’s alpha values for all scales were the same in children younger and older than 6 years. Therefore, in children older than 6 years, emotional and behavioral problems were also reliably measured.105 Further, by using a single imputed data set we assumed that the imputed value was a true observation in our analyses and did not correct (by pooling) for the uncertainty about the prediction of the missing values. This may have resulted in less precision. However, only few covariates were missing (<13%). Another limitation might be that our study only relied on questionnaires completed by the primary care giver (mostly mothers). This measure can therefore be less accurate as compared to multiple informant measures (both parents, teacher and/or caregivers in preschool or daycare centers report). Next, we used the SRS to measure autistic traits on a continuous spectrum which increases power and reduces the impact of outcome misclassification (e.g., children with symptoms just below the clinical cutoff will be defined as free of having Autism Spectrum Disorder).106 However, the SRS might measure a trait (e.g., affected social cognition) which is shared by another emotional or behavioral problem (e.g., Attention Deficit Hyperactivity Disorder), indicating measurement error or that comorbidity of such behavior problems does exist at these ages.106 Finally, in this study nonadditivity of chemicals in the association was not investigated. Therefore, we may have overlooked potential synergistic effects. However, when exposure biomarkers have a high correlation, the inclusion of a nonlinear term (e.g., a quadratic term) can comprise nonadditive effects in the joint mixture association.107 In this study, in which biomarker correlations vary between low, moderate, and high, it is likely that we (partly) miss nonadditive effects in the joint effect. Nevertheless, the study of Belzak and Bauer (2019) revealsrevealed that it is of importance to bear in mind that nonlinearity in the joint mixture exposure estimates may also comprise nonadditivity, even when interactions between biomarkers are not formally included in the statistical model.
This study has also several strengths, such as the large sample size, three repeated exposure measures of the three chemical groups to asses pregnancy exposure, and in-depth neurodevelopmental data on the children. Further this study is strengthened by the use of a mixture analytical method, the quantile-g computation, which allows for modeling of complex exposure combination corresponding to real world exposure situations, is able to deal with additive and synergistic effects of the exposures, and reduces the number of tests substantially.
In conclusion, higher prenatal exposure to a mixture of phthalates, bisphenol, and OP pesticides was associated with lower nonverbal IQ and phthalates were driving the association. No association was observed between prenatal exposure to the nonpersistent chemical mixture and emotional and behavioral problems in children. Gestational exposure to a combination of chemicals is universal and may be related to neurodevelopment. Future studies are needed to confirm our results, extend these by including more chemicals that share similar exposure sources, and investigating other mixture combinations of chemical groups that may co-occur. The quantile-g computation method might be a valuable tool to investigate these mixture combinations and estimate more closely the real-world neurodevelopmental consequences of co-occurring chemical exposures.
Supplementary Material
ACKNOWLEDGMENTS
The Generation R Study is conducted by the Erasmus Medical Center, Rotterdam in close collaboration with the Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service, Rotterdam Homecare Foundation and Stichting Trombosedienst & Artsenlaboratorium Rijnmond. We thank the contribution of participating parents and their children, general practitioners, hospitals, midwives, and pharmacies.
Funding
This research received financial support from National Institutes of Health (R01ES022972 and R01ES029779), the National Institute of Environmental Health Sciences, National Institutes of Health (HHSN273201500003C and ZIAES101575). The research leading to these results received funding from the European Union Horizon 2020 Research and Innovation Programme under Grant Agreement 733206 (LifeCycle), 874583 (ATHLETE), and 824989 (EUCAN-Connect). Michiel van den Dries was supported by the Ter Meulen grant (KNAWWF/DA/2016/TMB368) and the LifeCycle fellowship (Grant agreement No. 733206). Akhgar Ghassabian is funded by the National Institutes of Health (R01ES032826 and UH3OD023305). Henning Tiemeier was supported by the Netherlands Organization for Scientific Research (NWO) VICI grant (NWO-ZonMW: 016. VICI.170.200). Mònica Guxens is funded by a Miguel Servet fellowship (CPII18/00018) awarded by the Spanish Institute of Health Carlos III. Vincent Jaddoe received grant ERC-2014-CoG-648916 from the European Research Council. Kelly Ferguson was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. We acknowledge support from the Spanish Ministry of Science and Innovation and the State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018–000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c04455.
Ten tables: Table S1 and S2 present the descriptive statistics of biomarker concentrations included and excluded in the analyses by weeks of gestation. Table S3 presents the characteristics of study sample and those excluded from the Generation R cohort. The distributions of the averaged chemical biomarker concentrations (ug/g creatinine) across pregnancy can be found in Table S4 and correlations between averaged exposure biomarkers are presented in Table S5. Table S6 shows the descriptive statistics of nonverbal IQ, internalizing problems, attention problems, aggressive behavior, and autistic traits. Table S7 presents the Adjusted estimates for associations between mixtures of pregnancy averaged biomarker concentrations (ug/g creatinine) and nonverbal IQ, and emotional and behavioral symptoms. Table S8 shows the associations with externalizing problems, Table S9 presents the same models as in Table S7 with additional adjustment for birth year, and Table S10 presents the sex stratified results (PDF)
The authors declare the following competing financial interest(s): The spouse of H.T. is an employee of Eastman Chemical, a company that manufactures substitutes for orthophthalate plasticizers. All other authors declare they have no actual or potential competing financial interests.
Contributor Information
Michiel A. van den Dries, Department of Child and Adolescent Psychiatry, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; The Generation R Study Group, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; ISGlobal, Barcelona 08003, Spain; Pompeu Fabra University, Barcelona 08002, Spain; Spanish Consortium for Research on Epidemiology and Public Health (CIBERESP), Madrid 28029, Spain
Kelly K. Ferguson, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Durham, North Carolina 27709, United States
Alexander P. Keil, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Durham, North Carolina 27709, United States; Department of Epidemiology, University of North Carolina, Chapel Hill, North Carolina 27516, United States
Anjoeka Pronk, Department of Risk Analysis for Products in Development, TNO, Utrecht 3584 CB, The Netherlands Suzanne Spaan.
Suzanne Spaan, Department of Risk Analysis for Products in Development, TNO, Utrecht 3584 CB, The Netherlands.
Akhgar Ghassabian, Department of Pediatrics, New York University School of Medicine, New York City, New York 10016, United States; Department of Environmental Medicine and Department of Population Health, New York University School of Medicine, New York City, New York 10016, United States.
Susana Santos, The Generation R Study Group, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; Department of Pediatrics, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherland.
Vincent W.V. Jaddoe, The Generation R Study Group, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; Department of Pediatrics, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands
Leonardo Trasande, Department of Pediatrics, New York University School of Medicine, New York City, New York 10016, United States; Department of Environmental Medicine and Department of Population Health, New York University School of Medicine, New York City, New York 10016, United States; New York University Wagner School of Public Service, New York City, New York 10012, United States; New York University College of Global Public Health, New York City, New York 10003, United States.
Henning Tiemeier, Department of Child and Adolescent Psychiatry, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, Massachusetts 02115, United States.
Mònica Guxens, Department of Child and Adolescent Psychiatry, Erasmus MC University Medical Center Rotterdam, Rotterdam 3015 CN, The Netherlands; ISGlobal, Barcelona 08003, Spain; Pompeu Fabra University, Barcelona 08002, Spain; Spanish Consortium for Research on Epidemiology and Public Health (CIBERESP), Madrid 28029, Spain.
REFERENCES
- (1).Landrigan PJ; Fuller R; Acosta NJR; Adeyi O; Arnold R; Baldé AB; Bertollini R; Bose-O’Reilly S; Boufford JI; Breysse PN The Lancet Commission on pollution and health. Lancet 2018, 391 (10119), 462–512. [DOI] [PubMed] [Google Scholar]
- (2).Landrigan PJ; Goldman LR Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Affairs 2011, 30 (5), 842–850. [DOI] [PubMed] [Google Scholar]
- (3).Grandjean P; Landrigan PJ Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368 (9553), 2167–2178. [DOI] [PubMed] [Google Scholar]
- (4).Lim X. Tainted water: the scientists tracing thousands of fluorinated chemicals in our environment. Nature 2019, 566 (7742), 26–30. [DOI] [PubMed] [Google Scholar]
- (5).Manisalidis I; Stavropoulou E; Stavropoulos A; Bezirtzoglou E Environmental and health impacts of air pollution: a review. Frontiers in public health 2020, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Li D; Suh S Health risks of chemicals in consumer products: A review. Environ. Int 2019, 123, 580–587. [DOI] [PubMed] [Google Scholar]
- (7).Huang L; Ernstoff A; Fantke P; Csiszar SA; Jolliet O A review of models for near-field exposure pathways of chemicals in consumer products. Sci. Total Environ 2017, 574, 1182–1208. [DOI] [PubMed] [Google Scholar]
- (8).Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019; Dept. of Health and Human Services, Centers for Disease Control and Prevention, 2019. [Google Scholar]
- (9).Lehmler H-J; Liu B; Gadogbe M; Bao W Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3 (6), 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Michałowicz J. Bisphenol A–sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol 2014, 37 (2), 738–758. [DOI] [PubMed] [Google Scholar]
- (11).Vandenberg LN; Maffini MV; Sonnenschein C; Rubin BS; Soto AM Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev 2009, 30 (1), 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sathyanarayana S. Phthalates and Children’s Health. Current Problems in Pediatric and Adolescent Health Care 2008, 38 (2), 34–49. [DOI] [PubMed] [Google Scholar]
- (13).Schierow L-J; Lee MM Phthalates in Plastics and Possible Human Health Effects; Congressional Research Service, Library of Congress, 2008. [Google Scholar]
- (14).Schecter A; Lorber M; Guo Y; Wu Q; Yun SH; Kannan K; Hommel M; Imran N; Hynan LS; Cheng D Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect 2013, 121 (4), 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dodson RE; Nishioka M; Standley LJ; Perovich LJ; Brody JG; Rudel RA Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect 2012, 120 (7), 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Munguia-Lopez EM; Gerardo-Lugo S; Peralta E; Bolumen S; Soto-Valdez H Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit. Contam 2005, 22 (9), 892–898. [DOI] [PubMed] [Google Scholar]
- (17).Mahajan R; Chandel S; Chatterjee S, Environmental Fate of Organophosphate Residues from Agricultural Soils to Fresh Farm Produce: Microbial Interventions for Sustainable Bioremediation Strategies. In Microbes and Enzymes in Soil Health and Bioremediation; Springer, 2019; pp 211–224. [Google Scholar]
- (18).Eaton DL; Daroff RB; Autrup H; Bridges J; Buffler P; Costa LG; Coyle J; McKhann G; Mobley WC; Nadel L; Neubert D; Schulte-Hermann R; Spencer PS Review of the Toxicology of Chlorpyrifos With an Emphasis on Human Exposure and Neurodevelopment. Crit. Rev. Toxicol 2008, 38 (sup2), 1–125. [DOI] [PubMed] [Google Scholar]
- (19).Lu C; Barr DB; Pearson MA; Waller LA Dietary Intake and Its Contribution to Longitudinal Organophosphorus Pesticide Exposure in Urban/Suburban Children. Environ. Health Perspect 2008, 116 (4), 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kishi; Reiko; Grandjean; Philippe Health Impacts of Developmental Exposure to Environmental Chemicals; Springer, 2020; pp 359–373. [Google Scholar]
- (21).Duggan A; Charnley G; Chen W; Chukwudebe A; Hawk R; Krieger RI; Ross J; Yarborough C Di-alkyl phosphate biomonitoring data: assessing cumulative exposure to organophosphate pesticides. Regul. Toxicol. Pharmacol 2003, 37 (3), 382–395. [DOI] [PubMed] [Google Scholar]
- (22).Wang Y; Zhu H; Kannan K A review of biomonitoring of phthalate exposures. Toxics 2019, 7 (2), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bradman A; Barr DB; Claus Henn BG; Drumheller T; Curry C; Eskenazi B Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ. Health Perspect 2003, 111 (14), 1779–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Whyatt RM; Barr DB; Camann DE; Kinney PL; Barr JR; Andrews HF; Hoepner LA; Garfinkel R; Hazi Y; Reyes A; Ramirez J; Cosme Y; Perera FP Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect 2003, 111 (5), 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chou W-C; Chen J-L; Lin C-F; Chen Y-C; Shih F-C; Chuang C-Y Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ. Health 2011, 10, 94–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jensen MS; Nørgaard-Pedersen B; Toft G; Hougaard DM; Bonde JP; Cohen A; Thulstrup AM; Ivell R; Anand-Ivell R; Lindh CH; Jönsson BAG Phthalates and perfluorooctane-sulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ. Health Perspect 2012, 120 (6), 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Philippat C; Wolff MS; Calafat AM; Ye X; Bausell R; Meadows M; Stone J; Slama R; Engel SM Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ. Health Perspect 2013, 121 (10), 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Schönfelder G; Wittfoht W; Hopp H; Talsness CE; Paul M; Chahoud I Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect 2002, 110 (11), A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Silva MJ; Reidy JA; Herbert AR; Preau JL Jr.; Needham LL; Calafat AM Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol 2004, 72 (6), 1226–31. [DOI] [PubMed] [Google Scholar]
- (30).Rice D; Barone S Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect 2000, 108 (Suppl 3), 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Barakat R; Lin P-C; Park CJ; Best-Popescu C; Bakry HH; Abosalem ME; Abdelaleem NM; Flaws JA; Ko C Prenatal exposure to DEHP induces neuronal degeneration and neurobehavioral abnormalities in adult male mice. Toxicol. Sci 2018, 164 (2), 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hoshi H; Ohtsuka T Adult rats exposed to low-doses of di-n-butyl phthalate during gestation exhibit decreased grooming behavior. Bull. Environ. Contam. Toxicol 2009, 83 (1), 62–66. [DOI] [PubMed] [Google Scholar]
- (33).Kougias DG; Cortes LR; Moody L; Rhoads S; Pan Y-X; Juraska JM Effects of perinatal exposure to phthalates and a high-fat diet on maternal behavior and pup development and social play. Endocrinology 2018, 159 (2), 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ejaredar M; Lee Y; Roberts DJ; Sauve R; Dewey D Bisphenol A exposure and children’s behavior: A systematic review. J. Exposure Sci. Environ. Epidemiol 2017, 27 (2), 175–183. [DOI] [PubMed] [Google Scholar]
- (35).Ejaredar M; Nyanza EC; Ten Eycke K; Dewey D Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res 2015, 142, 51–60. [DOI] [PubMed] [Google Scholar]
- (36).Sapbamrer R; Hongsibsong S Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ. Sci. Pollut. Res 2019, 26 (18), 18267–18290. [DOI] [PubMed] [Google Scholar]
- (37).Radke EG; Braun JM; Nachman RM; Cooper GS Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int 2020, 137, 105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Tanner EM; Hallerbäck MU; Wikström S; Lindh C; Kiviranta H; Gennings C; Bornehag C-G Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ. Int 2020, 134, 105185. [DOI] [PubMed] [Google Scholar]
- (39).Daniel S; Balalian AA; Whyatt RM; Liu X; Rauh V; Herbstman J; Factor-Litvak P Perinatal phthalates exposure decreases fine-motor functions in 11-year-old girls: Results from weighted Quantile sum regression. Environ. Int 2020, 136, 105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Loftus CT; Bush NR; Day DB; Ni Y; Tylavsky FA; Karr CJ; Kannan K; Barrett ES; Szpiro AA; Sathyanarayana S Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environ. Int 2021, 150, 106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Day DB; Collett BR; Barrett ES; Bush NR; Swan SH; Nguyen RHN; Szpiro AA; Sathyanarayana S Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ. Int 2021, 147, 106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Li N; Papandonatos GD; Calafat AM; Yolton K; Lanphear BP; Chen A; Braun JM Gestational and childhood exposure to phthalates and child behavior. Environ. Int 2020, 144, 106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Jusko T; van den Dries Michiel A; Pronk A; Shaw P; Guxens M; Spaan S; Jaddoe VW; Tiemeier H; Longnecker MP Organophosphate Pesticide Metabolite Concentrations in Urine during Pregnancy and Offspring Nonverbal IQ at Age 6 Years. Environ. Health Perspect 2019, 127 (1), 017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).van den Dries MA; Guxens M; Pronk A; Spaan S; El Marroun H; Jusko TA; Longnecker MP; Ferguson KK; Tiemeier H Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring attention-deficit hyperactivity disorder and autistic traits. Environ. Int 2019, 131, 105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).van den Dries MA; Guxens M; Spaan S; Ferguson KK; Philips E; Santos S; Jaddoe VWV; Ghassabian A; Trasande L; Tiemeier H Phthalate and Bisphenol Exposure during Pregnancy and Offspring Nonverbal IQ. Environ. Health Perspect 2020, 128 (7), 077009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bopp SK; Barouki R; Brack W; Dalla Costa S; Dorne J-LCM; Drakvik PE; Faust M; Karjalainen TK; Kephalopoulos S; van Klaveren J; Kolossa-Gehring M; Kortenkamp A; Lebret E; Lettieri T; Nørager S; Rüegg J; Tarazona JV; Trier X; van de Water B; van Gils J; Bergman Å Current EU research activities on combined exposure to multiple chemicals. Environ. Int 2018, 120, 544–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Carpenter DO; Arcaro K; Spink DC Understanding the human health effects of chemical mixtures. Environ. Health Perspect 2002, 110 (suppl 1), 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zoeller RT; Brown TR; Doan LL; Gore AC; Skakkebaek NE; Soto AM; Woodruff TJ; Vom Saal FS Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 2012, 153 (9), 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kortenkamp A Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr. Opin. Pharmacol 2014, 19, 105–111. [DOI] [PubMed] [Google Scholar]
- (50).Braun JM; Gennings C; Hauser R; Webster TF What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 2016, 124 (1), A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Batty GD; Deary IJ Early life intelligence and adult health. BMJ. 2004, 329 (7466), 585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wraw C; Der G; Gale CR; Deary IJ Intelligence in youth and health behaviours in middle age. Intelligence 2018, 69, 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wittchen HU; Kessler RC; Pfister H; Höfler M; Lieb R Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatr. Scand 2000, 102, 14–23. [PubMed] [Google Scholar]
- (54).Caspi A; Moffitt TE; Newman DL; Silva PA Behavioral observations at age 3 years predict adult psychiatric disorders: Longitudinal evidence from a birth cohort. Arch. Gen. Psychiatry 1996, 53 (11), 1033–1039. [DOI] [PubMed] [Google Scholar]
- (55).Kooijman MN; Kruithof CJ; van Duijn CM; Duijts L; Franco OH; van Ijzendoorn MH; de Jongste JC; Klaver CCW; van der Lugt A; Mackenbach JP; Moll HA; Peeters RP; Raat H; Rings EHHM; Rivadeneira F; van der Schroeff MP; Steegers EAP; Tiemeier H; Uitterlinden AG; Verhulst FC; Wolvius E; Felix JF; Jaddoe VWV The Generation R Study: design and cohort update 2017. Eur. J. Epidemiol 2016, 31 (12), 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kruithof CJ; Kooijman MN; van Duijn CM; Franco OH; de Jongste JC; Klaver CC; Mackenbach JP; Moll HA; Raat H; Rings EH; Rivadeneira F; Steegers EA; Tiemeier H; Uitterlinden AG; Verhulst FC; Wolvius EB; Hofman A; Jaddoe VW The Generation R Study: Biobank update 2015. Eur. J. Epidemiol 2014, 29 (12), 911–27. [DOI] [PubMed] [Google Scholar]
- (57).Philips EM; Jaddoe VWV; Asimakopoulos AG; Kannan K; Steegers EAP; Santos S; Trasande L Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ. Res 2018, 161, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).van den Dries MA; Pronk A; Guxens M; Spaan S; Voortman T; Jaddoe VW; Jusko TA; Longnecker MP; Tiemeier H Determinants of organophosphate pesticide exposure in pregnant women: A population-based cohort study in the Netherlands. Int. J. Hyg. Environ. Health 2018, 221 (3), 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Asimakopoulos AG; Xue J; De Carvalho BP; Iyer A; Abualnaja KO; Yaghmoor SS; Kumosani TA; Kannan K Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res 2016, 150, 573–581. [DOI] [PubMed] [Google Scholar]
- (60).Butler A. R Jaffe Reaction.2. Kinetic Study of Janovsky Complexes Formed from Creatinine (2-Imino-1-Methylimazolidin-4-One) and Acetone. J. Chem. Soc., Perkin Trans 2 1975, No. 8, 853–857. [Google Scholar]
- (61).Hornung RW; Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 1990, 5 (1), 46–51. [Google Scholar]
- (62).Health Canada. Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 1 (2007–2009); Health, M. o., Ed.; Ottawa, Ontario, 2010. [Google Scholar]
- (63).Jenkinson J; Roberts S; Dennehy S; Tellegen P Validation of the Snijders-Oomen Nonverbal Intelligence Test-Revised 21/2–7 for Australian Children with Disabilities. Journal of Psychoeducational Assessment 1996, 14 (3), 276–286. [Google Scholar]
- (64).Moore C; O’Keefe SL; Lawhon D; Tellegen P Concurrent validity of the Snijders-Oomen Nonverbal Intelligence Test 2 1/2–7–Revised with the Wechsler Preschool and Primary Scale of Intelligence–Revised. Psychological reports 1998, 82 (2), 619–625. [Google Scholar]
- (65).Tellegen PJ, Winkel M, Wijnberg-Williams BJ, Laros JA Snijders-Oomen Nonverbal Intelligence Test. SON-R 21 /2–7 Manual and Research Report. Lisse: Swets & Zeitlinger B.V, 1998; p 267 [Google Scholar]
- (66).Langeslag SJE; Schmidt M; Ghassabian A; Jaddoe VW; Hofman A; van der Lugt A; Verhulst FC; Tiemeier H; White TJH Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Human Brain Mapping 2013, 34 (12), 3299–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Achenbach TM; Rescorla LA Manual for the ASEBA Preschool Forms & Profiles; University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, 2000. [Google Scholar]
- (68).Constantino J; Gruber C The Social Responsiveness Scale; Western Psychological Services: Los Angeles, CA, 2005. [Google Scholar]
- (69).Ivanova MY; Achenbach TM; Rescorla LA; Harder VS; Ang RP; Bilenberg N; Bjarnadottir G; Capron C; De Pauw SSW; Dias P Preschool psychopathology reported by parents in 23 societies: testing the seven-syndrome model of the child behavior checklist for ages 1.5–5. J. Am. Acad. Child Adolescent Psychiatry 2010, 49 (12), 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Constantino JN; Davis SA; Todd RD; Schindler MK; Gross MM; Brophy SL; Metzger LM; Shoushtari CS; Splinter R; Reich W Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev Disord 2003, 33 (4), 427–33. [DOI] [PubMed] [Google Scholar]
- (71).Duvekot J; van der Ende J; Verhulst FC; Greaves-Lord K The Screening Accuracy of the Parent and Teacher-Reported Social Responsiveness Scale (SRS): Comparison with the 3Di and ADOS. Journal of Autism and Developmental Disorders 2015, 45 (6), 1658–1672. [DOI] [PubMed] [Google Scholar]
- (72).Román GC; Ghassabian A; Bongers-Schokking JJ; Jaddoe VWV; Hofman A; Rijke YB; Verhulst FC; Tiemeier H Association of gestational maternal hypothyroxinemia and increased autism risk. Ann. Neurol 2013, 74 (5), 733–742. [DOI] [PubMed] [Google Scholar]
- (73).Constantino JN; Todd RD. Autistic traits in the general population: a twin study. Arch. Gen. Psychiatry 2003, 60 (5), 524–30. [DOI] [PubMed] [Google Scholar]
- (74).De Beurs E. Brief Symptom Inventory Handleiding; Pits Publishers: Leiden, Netherlands, 2003. [Google Scholar]
- (75).McKinzey RK; Prieler J; Raven J Detection of children’s malingering on Raven’s Standard Progressive Matrices. Br J. Clin Psychol 2003, 42 (Pt 1), 95–9. [DOI] [PubMed] [Google Scholar]
- (76).Chiesi F; Ciancaleoni M; Galli S; Primi C Using the Advanced Progressive Matrices (Set I) to assess fluid ability in a short time frame: an item response theory-based analysis. Psychol Assess 2012, 24 (4), 892–900. [DOI] [PubMed] [Google Scholar]
- (77).R core Team A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2015. [Google Scholar]
- (78).van Buuren S; Groothuis-Oudshoorn C MICE: Multivariate Imputation by Chained Equations in R. 2011; Vol. 45. DOI: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- (79).Spaan S; Pronk A; Koch HM; Jusko TA; Jaddoe VWV; Shaw PA; Tiemeier HM; Hofman A; Pierik FH; Longnecker MP Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R study. J. Exposure Sci. Environ. Epidemiol 2015, 25 (3), 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Perrier F; Giorgis-Allemand L; Slama R; Philippat C Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology (Cambridge, Mass) 2016, 27 (3), 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Keil AP; Buckley JP; O’Brien KM; Ferguson KK; Zhao S; White AJ A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect 2020, 128 (4), 047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Casas M; Forns J; Martínez D; Avella-García C; Valvi D; Ballesteros-Gómez A; Luque N; Rubio S; Julvez J; Sunyer J; Vrijheid M Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ. Res 2015, 142, 671–679. [DOI] [PubMed] [Google Scholar]
- (83).Furlong MA; Engel SM; Barr DB; Wolff MS Prenatal Exposure to Organophosphate Pesticides and Reciprocal Social Behavior in Childhood. Environ. Int 2014, 70, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Doherty BT; Engel SM; Buckley JP; Silva MJ; Calafat AM; Wolff MS Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ. Res 2017, 152, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lazarevic N; Barnett AG; Sly PD; Knibbs LD Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ. Health Perspect 2019, 127 (2), 26001–26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect 2007, 115 (Suppl 1), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Gaudriault P; Mazaud-Guittot S; Lavoué V; Coiffec I; Lesné L; Dejucq-Rainsford N; Scholze M; Kortenkamp A; Jégou B Endocrine disruption in human fetal testis explants by individual and combined exposures to selected pharmaceuticals, pesticides, and environmental pollutants. Environ. Health Perspect 2017, 125 (8), 087004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Vernet C; Philippat C; Agier L; Calafat AM; Ye X; Lyon-Caen S; Hainaut P; Siroux V; Schisterman EF; Slama R An Empirical Validation of the Within-subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-based Studies. Epidemiology 2019, 30 (5), 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Calabrese EJ; Baldwin LA U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health 2001, 22 (1), 15–33. [DOI] [PubMed] [Google Scholar]
- (90).Vandenberg LN; Colborn T; Hayes TB; Heindel JJ; Jacobs DR Jr; Lee D-H; Shioda T; Soto AM; vom Saal FS; Welshons WV Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 2012, 33 (3), 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Hill CE; Myers JP; Vandenberg LN Nonmonotonic dose–response curves occur in dose ranges that are relevant to regulatory decision-making. Dose-Response 2018, 16 (3), 1559325818798282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Bobb JF; Valeri L; Claus Henn B; Christiani DC; Wright RO; Mazumdar M; Godleski JJ; Coull BA Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16 (3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Machtinger R; Berman T; Adir M; Mansur A; Baccarelli AA; Racowsky C; Calafat AM; Hauser R; Nahum R Urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in pregnant women in Israel. Environ. Int 2018, 116, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Arbuckle TE; Davis K; Marro L; Fisher M; Legrand M; LeBlanc A; Gaudreau E; Foster WG; Choeurng V; Fraser WD Phthalate and bisphenol A exposure among pregnant women in Canada — Results from the MIREC study. Environ. Int 2014, 68, 55–65. [DOI] [PubMed] [Google Scholar]
- (95).Hyland C; Mora AM; Kogut K; Calafat AM; Harley K; Deardorff J; Holland N; Eskenazi B; Sagiv SK Prenatal Exposure to Phthalates and Neurodevelopment in the CHAMACOS Cohort. Environ. Health Perspect 2019, 127 (10), 107010–107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Eskenazi B; Marks AR; Bradman A; Harley K; Barr DB; Johnson C; Morga N; Jewell NP Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environ. Health Perspect 2007, 115 (5), 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Llop S; Murcia M; Iniguez C; Roca M; Gonzalez L; Yusa V; Rebagliato M; Ballester F Distributions and determinants of urinary biomarkers of organophosphate pesticide exposure in a prospective Spanish birth cohort study. Environ. Health 2017, 16 (1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Cartier C; Warembourg C; Le Maner-Idrissi G; Lacroix A; Rouget F; Monfort C; Limon G; Durand G; Saint-Amour D; Cordier S; Chevrier C Organophosphate Insecticide Metabolites in Prenatal and Childhood Urine Samples and Intelligence Scores at 6 Years of Age: Results from the Mother-Child PELAGIE Cohort (France). Environ. Health Perspect 2016, 124 (5), 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Sokoloff K; Fraser W; Arbuckle TE; Fisher M; Gaudreau E; LeBlanc A; Morisset A-S; Bouchard MF Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada — Results from the MIREC study. Environ. Int 2016, 94, 133–140. [DOI] [PubMed] [Google Scholar]
- (100).Tamayo-Uria I; Maitre L; Thomsen C; Nieuwenhuijsen MJ; Chatzi L; Siroux V; Aasvang GM; Agier L; Andrusaityte S; Casas M The early-life exposome: description and patterns in six European countries. Environ. Health Perspect. Suppl 2018, 123, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Santos S; Maitre L; Warembourg C; Agier L; Richiardi L; Basagana X; Vrijheid M Applying the exposome concept in birth cohort research: a review of statistical approaches. Eur. J. Epidemiol 2020, 35 (3), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Barr DB; Wilder LC; Caudill SP; Gonzalez AJ; Needham LL; Pirkle JL Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect 2005, 113 (2), 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Boeniger MF; Lowry LK; Rosenberg J Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J 1993, 54 (10), 615–27. [DOI] [PubMed] [Google Scholar]
- (104).Cornelis R; Heinzow B; Herber RF; Christensen JM; Poulsen OM; Sabbioni E; Templeton DM; Thomassen Y; Vahter M; Vesterberg O Sample collection guidelines for trace elements in blood and urine. IUPAC Commission of Toxicology. J. Trace Elem. Med. Biol 1996, 10 (2), 103–27. [DOI] [PubMed] [Google Scholar]
- (105).Basten MMGJ; Althoff RR; Tiemeier H; Jaddoe VWV Hofman A; Hudziak JJ; Verhulst FC; van der Ende J The dysregulation profile in young children: empirically defined classes in the Generation R study. Journal of the American Academy of Child & Adolescent Psychiatry 2013, 52 (8), 841–850 e2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Sagiv SK; Kalkbrenner AE; Bellinger DC Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environ. Health 2015, 14, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Belzak WCM; Bauer DJ Interaction effects may actually be nonlinear effects in disguise: A review of the problem and potential solutions. Addictive behaviors 2019, 94, 99–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.