Abstract
Practical relevance:
Pyometra is a commonly occurring uterine disease in cats that often leads to loss of breeding potential and, in some cases, can be life threatening. An increased incidence of cystic endometrial hyperplasia (CEH) and pyometra is seen with age. Most queens present with uterine lesions after 5–7 years of age (average 7.6 years, range 1–20 years). Clinical signs most commonly occur within 4 weeks of the onset of oestrus in queens that are either mated, spontaneously ovulate or are induced to ovulate (mechanical stimulation or hormone induction). The disease is most often observed in dioestrus.
Clinical challenges:
Queens with pyometra often go undiagnosed as there may be few or only very mild clinical signs and laboratory changes. For example, the classic sign of mucopurulent bloody vulvar discharge often goes unnoticed. Abdominal ultrasound is the best tool for diagnosis of pyometra and for monitoring response to therapy.
Patient group:
Classically, middle-aged/older nulliparous intact queens present with pyometra. However, so-called ‘stump pyometra’ can occur if ovarian tissue is left behind during ovariectomy or ovariohysterectomy (ovarian remnant syndrome). Queens treated with exogenous steroid hormones such as high doses of megestrol acetate or medroxyprogesterone acetate for oestrus prevention can also develop CEH and pyometra.
Evidence base:
There has been little published to date on CEH, endometritis and pyometra in the queen and most of the currently available information has been extrapolated from studies carried out in the bitch. The queen and the bitch have very different reproductive physiology; thus, further research and investigation into the precise aetiopathogenesis of these disease processes of the uterus in the queen is warranted.
Audience:
This review is aimed at clinicians working in small animal practice, especially those in countries where surgical sterilisation is not practised as commonly as in the United States, Canada or Australasia, and who will therefore see a greater proportion of intact queens.
Introduction
Pyometra is an acute or chronic suppurative inflammation of the uterine wall in intact queens. It is characterised by endometrial hyperplasia with cystic dilation of endometrial glands and accumulation of purulent exudate in the uterine lumen. The disease is most often observed in dioestrus or ‘pseudopregnancy’ in the queen, which is a phase of progesterone dominance that lasts approximately 40 days. The relatively long progesterone-dominated dioestrous phase occurs in queens that undergo ovulation (induced or spontaneous) and predisposes them to the development of cystic endometrial hyperplasia (CEH) and subsequent pyometra caused by infection from bacteria ascending from the vagina. The most common bacterium involved in pyometra is Escherichia coli. Similar to the bitch, regardless of the underlying cause, the presence of progesterone (endogenous or exogenous in source) facilitates the development of pyometra.
The incidence of feline pyometra (see box on page 21) is not well documented.
Pathogenesis and aetiology: what comes first?
Cats are classified as seasonally polyoestrus, coming into oestrus between spring and early autumn with a seasonal anoestrus in winter (long-day breeders). The oestrous period or ‘call’ lasts 6–7 days. If ovulation (either induced or spontaneous) occurs but the queen does not become pregnant, there follows a period of progesterone secretion (from the corpus luteum) for approximately 40 days. This is the dioestrous phase (or so-called pseudopregnancy). Cats that undergo an anovulatory oestrus will have an interoestrus interval of about 8–10 days with baseline progesterone levels.
The pathogenesis of pyometra is incompletely understood – both in the bitch and the queen, but especially the queen being an induced ovulator. In the bitch, pyometra is currently believed to be multifactorial in origin. It is most likely similar in the queen. The aetiology is similar in the two species, with progesterone influence predisposing the uterus to ascending bacterial (most commonly E coli) infection.
Originally CEH and pyometra were defined as one disease entity. It was believed that repeated exposure of the endometrium to high concentrations of oestrogen during proestrus and oestrus, followed by high concentrations of progesterone during the luteal phase (ie, dioestrus), led to the development of CEH (Figure 1). This, in turn, predisposed the uterus to ascending secondary bacterial infection and development of pyometra. More recently, the question has been raised as to whether pyometra and CEH are actually two separate disease entities. Although the conditions have many similarities and can be found as related events, they also have the potential to occur de novo. Any stimulus or irritant in a progesterone-influenced uterus can lead to CEH,7 –9 and thus the presence of CEH in pyometra could merely be the result of a uterine reaction to the bacterial infection. This could explain why we see pyometra in young cats, which are unlikely to have underlying uterine pathology such as CEH. In bitches, it is hypothesised that varying pathogenicity of E coli strains might be responsible for the development of CEH. No studies investigating the effects of bacterial pathogenicity in pyometra have been undertaken in cats.
Figure 1.
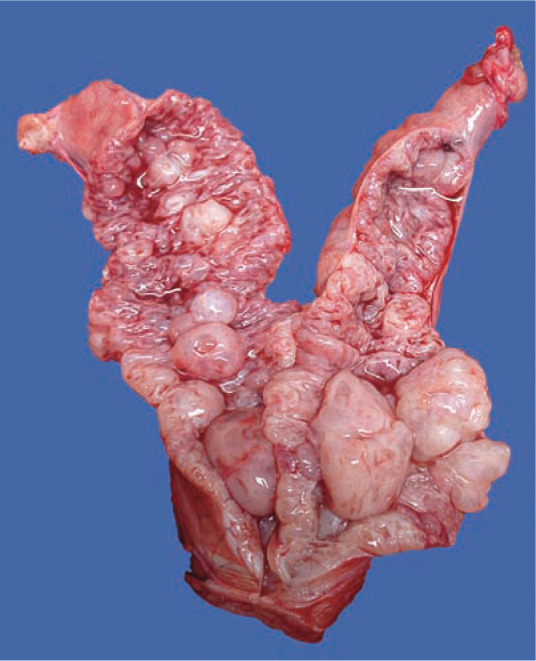
Opened uterine horn from a queen with severe cystic endometrial changes in the uterine wall but no evidence of pyometra. Copyright: Dr Stephanie N Simpson. Source: LORI

What role do reproductive hormones play?
The majority of queens affected by pyometra are presented with clinical signs within 4 weeks of the onset of the latest oestrus. Although there is no evidence that abnormal ovarian hormone concentrations are involved in the pathogenesis of pyometra in queens or bitches, it has been shown that progesterone is necessary to initiate CEH and that oestrogen potentiates the effect by upregulating the expression of progesterone receptors. Therefore, pyometra is believed to be facilitated by an oestrogenic phase that is followed by a relatively long non-pregnant progesterone-dominated phase (dioestrus caused by spontaneous or induced ovulation).
Leukocyte inhibition, decreased myometrial contractions and a closed cervix in the progesterone-influenced uterus facilitate bacterial growth in a non-gravid uterus from ascending infection. Progesterone also stimulates uterine stromal and glandular epithelial proliferation and increases uterine glandular secretions, which are an important source of nutrients for the early developing embryos/fetuses in pregnant queens. These effects are cumulative in spontaneously ovulating cats or cats that experience repeated matings that do not result in pregnancy. Thus, the risk of uterine disease may increase with each non-pregnant oestrous cycle, as the presence of fetuses is effectively protective against the development of pyometra. This finding was first discovered by Dow who reported that nulliparous bitches with pyometra outnumbered multiparous bitches with pyometra by approximately 10-fold. 10 A similar effect is thought to hold true for queens.
However, it has also been shown that progesterone exposure alone, without prior oestrogen priming, can lead to CEH in the queen. 11 The theory that progesterone is critical for the development of pyometra is supported by the fact that the use of exogenous steroid hormones (progestins such as megestrol acetate [MA] or medroxyprogesterone acetate [MPA]) for contraceptive purposes has been shown to induce the disease in both bitches and queens.7,12 Another observation that supports the essential role of progesterone in the disease process, at least in the canine species, is that the incidence of pyometra is similar in ovariectomised and ovariohysterectomised bitches. 13
Which bacteria are commonly involved?
In most cases of pyometra, the bacteria isolated are uropathogenic E coli. Other bacteria, mostly normal vaginal commensals such as Staphylococcus aureus, Klebsiella species, Proteus species and Streptococcus species, have also been reported in cases of pyometra. The uterus is presumed to become infected via ascent of faecal bacteria through the vagina during oestrus when the cervix is relaxed. 14 It has been shown that E coli are capable of establishing an infection in very young healthy dogs, which are unlikely to have underlying CEH changes. 7 This may be another explanation for cases of pyometra in young queens. It is hypothesised that bacteria enter the uterus during proestrus and/or oestrus and act as a mucosal irritant, thus stimulating the development of CEH under the influence of progesterone during dioestrus.
Factors other than bacterial virulence are also likely involved in the pathophysiology of pyometra in the queen, such as deficiencies in the innate immune response and inheritance of susceptibility.
What is the evidence for a genetic predisposition?
Previously, no breed predisposition for pyometra in queens had been reported. However, a retrospective study carried out in Sweden 1 found that Oriental purebred cats have a higher incidence of pyometra than other breeds, with the Sphynx breed having the highest incidence. Other breeds with a predisposition include the Siberian, Ocicat, Korat, Siamese, Ragdoll, Maine Coon and Bengal. 1 Furthermore, in the authors’ experience there are families that have a higher incidence of pyometra. These related queens are often geographically isolated, suggesting a hereditary predisposition to pyometra.
Diagnostic approach
Signalment/history
Risk factors for pyometra in queens include:
Age Typically, middle-aged to older queens (>5–7 years) with a history of oestrus within the previous 4 weeks are affected (although pyometra can be seen in younger queens, Table 1);
Breed Orientals and purebreeds (ie, Siberian, Ocicat, Korat, Siamese, Ragdoll, Maine Coon, Burmese, Birman and Bengal) are predisposed;
Drug therapy A history of treatment with progestins for prevention of oestrus (particularly high-dose regimens of MA [>0.2 mg/kg q24h] or MPA [>0.05 mg/kg q24h] for durations >1 year, 17 especially in older queens), or pharmacological agents to induce ovulation (eg, human chorionic gonadotropin, gonadotropin-releasing hormone [GnRH]), increases the risk.
Table 1.
Summary of clinical, laboratory and diagnostic imaging findings in feline pyometra
| Comments | ||
|---|---|---|
| Clinical presentation | ||
| Signalment | Middle-aged to older queens (>5–7 years of age) | Also young cats, those receiving exogenous hormone treatment and/or with a breed predisposition |
| Clinical signs | Vulvar discharge, depression, lethargy, pyrexia, inappetence, hyporexia/anorexia, vomiting | Often clinical signs are very mild or absent; clinical signs are generally non-specific |
| Laboratory findings | ||
| Complete blood count | White blood cell count >35,000 cells/μl, neutrophilia with left shift ± toxic change | Leukogram may be normal |
| Serum biochemistry | Hyperproteinaemia, hyperglobulinaemia | Often only mild or no changes |
| Progesterone concentration | >2 ng/ml | Can be <2 ng/ml in anoestrus or at end of luteal period (poorer treatment prognosis) |
| Diagnostic imaging | ||
| Ultrasonography | Thick-walled distended tubular uterus filled with hypoechoic/hyperechoic fluid | Often cystic endometrial changes in the uterine wall; amount of intra-luminal fluid depends on patency of cervix and time since ovulation |
| Radiography | Fluid-dense distended tubular uterus in the mid-abdomen | Consider other differentials such as pregnancy, mucometra, hemometra or hydrometra |
Clinical presentation
Presenting complaints include, but are not limited to, haemopurulent vulvar discharge (if the cervix is patent), depression, listlessness, lethargy, hyporexia/anorexia, vomiting and weight loss. Physical examination findings include abdominal distension, dehydration and pyrexia.2,18 Importantly, clinical signs are non-specific, with anorexia and lethargy being the most common presentations. Therefore, pyometra should be ruled out in any ill, intact queen. In contrast to pyometra in bitches, polyuria and polydipsia are not commonly seen in affected queens. Most importantly, clinical signs can be few or mild in queens with pyometra. 2
In many cases the uterus will be palpably enlarged but great care should be taken during abdominal palpation as it can result in uterine rupture if the cervix is closed and the uterus is friable. If the cervix is patent, the uterus may not be as enlarged and only a thickened uterine wall may be appreciated on palpation.
The presence of vulvar discharge is also dependent on the patency of the cervix. In open-cervix pyometra, a haemorrhagic, purulent vulvar discharge may be the only clinical sign. Cats can be fastidious with grooming, which is why a vulval discharge may not be noticed by owners, thereby delaying the diagnosis. Queens with closed-cervix pyometra may not show vulvar discharge and are more commonly systemically ill; absorption of bacterial toxins in these cats can result in endotoxaemia and sometimes bacteraemia.
Laboratory findings
Haematology and biochemistry Remarkably few haematological and biochemical changes are seen in queens with pyometra. 2 The leukogram may show a marked neutrophilia (>35 x 109/l) with a left shift (± toxic changes) but this can be variable and, in some cases, the leukogram may be normal. It is not uncommon to have no other haematological disturbances in queens with pyometra. 2 Hyperproteinaemia, hypokalaemia, azotaemia and an elevation in liver enzymes (alanine aminotransferase and alkaline phosphatase), blood urea nitrogen and creatinine may be noted, especially if sepsis and dehydration are present. However, it is not uncommon to see only mild or no biochemical changes. Queens have significantly less evidence of renal damage associated with pyometra than bitches. In contrast to bitches, biochemical parameters are also not particularly helpful as predictors of disease outcome in queens with pyometra. 2
Serum progesterone Progesterone concentration will commonly be elevated above 2 ng/ml, depending on the length of time since ovulation. If queens are diagnosed with pyometra towards the end of dioestrus, progesterone levels can be relatively low (0.5–2 ng/ml).
Cytology Cytological examination of the vulvar discharge is likely to reveal degenerate polymorphonuclear cells and phagocytosed bacteria (Figure 3).
Culture and sensitivity Bacterial culture of vulvar discharge is not particularly helpful in confirming a diagnosis as normal vaginal flora is most likely to be isolated. However, sensitivity testing is important for making therapeutic decisions as some bacterial strains can be resistant to commonly used antibiotics. Ideally, a sample collected from the uterus would be most diagnostic but obtaining such samples is technically and practically difficult. A sample from the cranial vagina using a guarded swab is the next best option for bacterial culture and sensitivity testing. Samples should be taken before antibiotic therapy is started. Antibiotic treatment should then commence while awaiting results, on the assumption that E coli is the most likely isolate (see later). Once culture and sensitivity results are available, therapy can be modified if needed.
Figure 3.
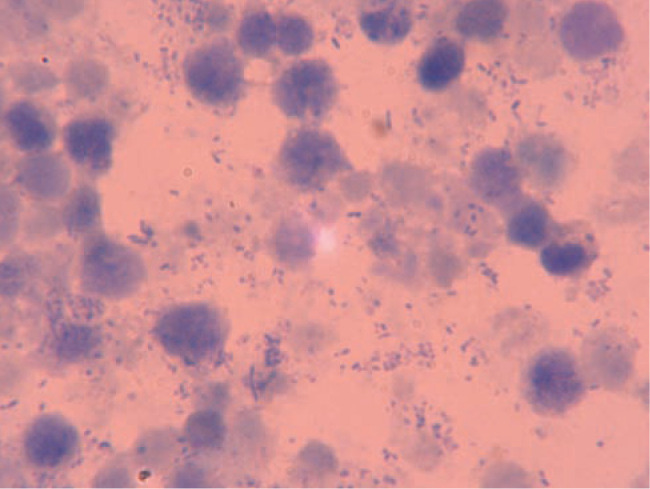
Cytological smear prepared from a queen with pyometra. Note the presence of degenerate neutrophils, epithelial cells and bacteria in the background. Differential interference contrast microscopy, oil immersion, x 1000
Imaging
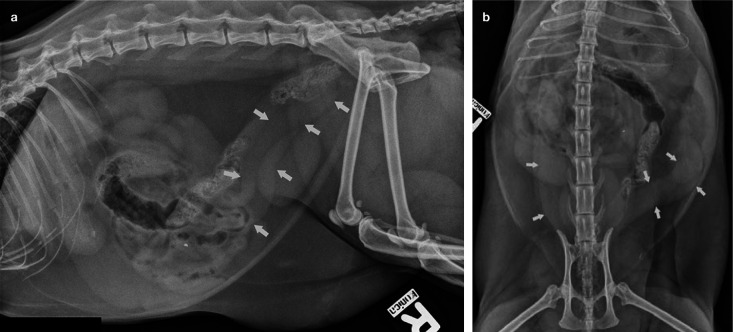
Changes that are observed on abdominal radiography of a queen with pyometra include a distended uterus, which can lead to displacement of the small intestine (Figure 4). These changes are very similar to those seen in early pregnancy prior to fetal skeletal ossification (which starts approximately 40 days after the luteinising hormone [LH] peak). Also, it is often difficult to differentiate pyometra from other causes of uterine enlargement, such as mucometra, hydrometra, hemometra or leiomyoma, which is a further limitation of radiographic examination.
Figure 4.
Lateral (a) and dorsoventral (b) radiographic images of a queen with pyometra. Arrows indicate the enlarged, fluidfilled uterus. Source: LORI
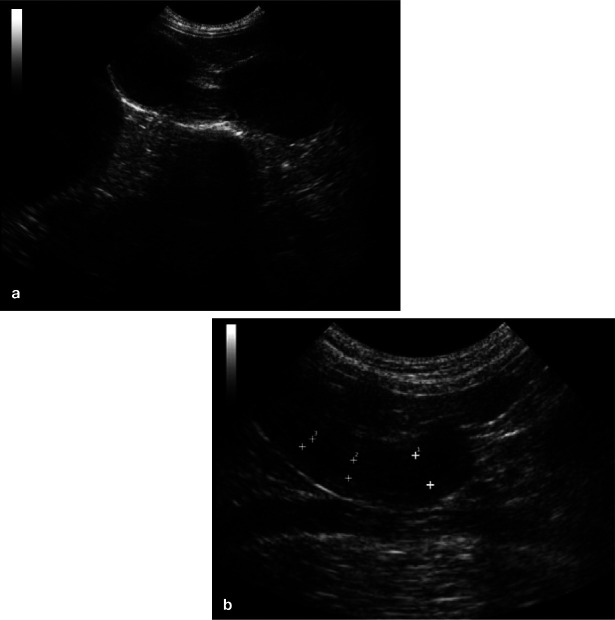
Abdominal ultrasound is the most important diagnostic tool when pyometra is suspected. Early in the disease process, the uterine horns typically appear distended with hypoechoic to hyperechoic fluid, with or without flocculation (Figure 5a). The uterine wall often appears thickened with irregular edges and small hypoechoic areas consistent with cystic changes to the endometrial glands (Figure 5b). However, many queens will present more than 4 weeks after ovulation, and even late in the luteal phase or early anoestrus phase after the cervix has been open for days or weeks. In these cases, there may be no intraluminal fluid detectable and only a thickened uterine wall may be seen (Figure 6).
Figure 5.
Ultrasonographic images of (a) distended fluidfilled loops of uterus of different cross-sectional sizes filling the caudal abdomen in a queen with pyometra diagnosed soon after ovulation; and (b) a queen with an open pyometra diagnosed later in the disease process (approximately 4 weeks postovulation). Note the presence of intraluminal fluid in the uterus and the thickened endometrium with visible cystic lesions. Images courtesy of Dr Cheryl Lopate, Wilsonville Veterinary Clinic, USA
Figure 6.
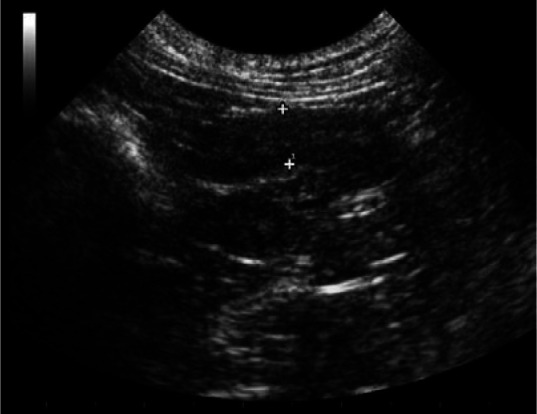
Ultrasonographic image of a cross section of a thickened uterus (with only a trace of intraluminal fluid) in a queen with an open pyometra that presented during the late luteal phase. Note the cysts (cystic endometrial hyperplasia) in the uterine wall. Courtesy of Dr Cheryl Lopate, Wilsonville Veterinary Clinic, USA
Pyometra can cause diffuse or segmental changes and there have even been occasional reports of pyometra in one uterine horn and a pregnancy in the other horn.
Histopathology
Grossly, the uterine horns of a queen with pyometra are usually distended with some degree of annular ring formation on the surface (Figure 7). Protuberant bands are seen on the endometrial surface, which correspond to the annular rings on the serosa. The endometrium of a uterus affected by pyometra is classically described as ‘cobblestone’ in appearance (Figures 1 and 8). The endometrial surface is usually covered by a malodorous, mucopurulent exudate, which can vary in volume (Figure 8).
Figure 7.
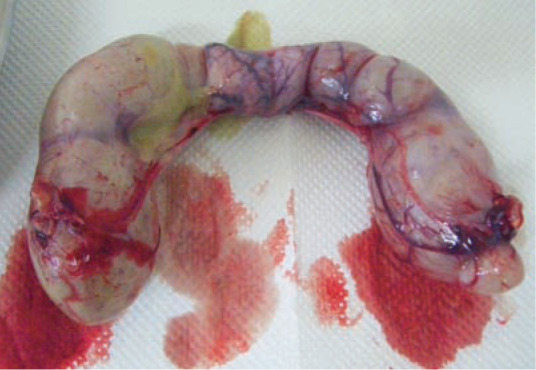
Pus-filled uterus after surgical removal. Note the oozing of purulent material from the friable and stretched uterine wall. Great care is required when handling these pus-filled uteri during ovariohysterectomy
Figure 8.
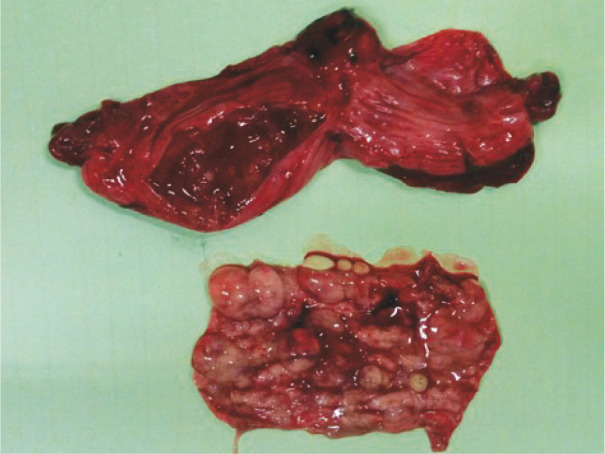
An opened uterine horn from a queen with pyometra. Note the degree of cystic endometrial change in the resected piece of uterine wall. Courtesy of Dr Reto Fritsche, School of Veterinary Medicine, Louisiana State University, USA
Histologically, thickening of the uterine wall is caused by proliferation and dilation of endometrial glands, which occurs throughout the endometrium. These glands contain mucopurulent exudate with large numbers of polymorphonuclear leukocytes (Figure 9). Dense infiltration of neutrophils can also be seen in the superficial stroma under the surface of the endometrium. In some cases, there is evidence of chronic inflammation with infiltrations of predominantly plasma cells and histiocytes in the stroma around the dilated cystic glands.8,9
Figure 9.
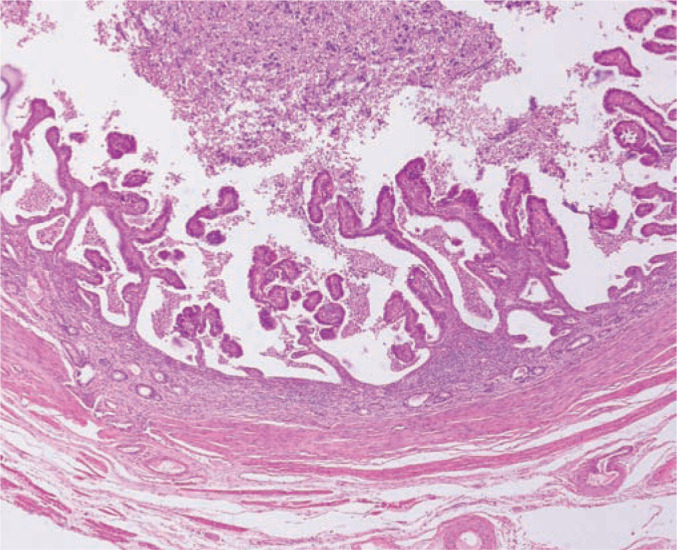
Histopathology of a cross section of the uterine wall in a queen with pyometra. The uterine lumen is dilated and filled with neutrophils and necrotic debris. The myometrium is thin and stretched but the endometrium is approximately trebled in thickness. The luminal surface forms papillary structures and some of the endometrial glands are distended and also contain neutrophils and necrotic debris. Epithelial cells lining the dilated glands and the lumen are hypertrophied and hyperchromatic. The endometrial stroma contains mostly plasma cells, some lymphocytes and scattered neutrophils. Neutrophils are particularly prominent close to the epithelium of dilated glands and the lumen. Courtesy of Dr Rob Foster, Ontario Veterinary College, University of Guelph
Treatment approach
Pyometra can be treated surgically or medically and, in some cases, a combination of the two approaches may be the most effective and safest solution. For example, medical treatment of systemically unwell or older patients to assist with uterine emptying prior to surgery is appropriate to reduce the morbidity and mortality that can be associated with immediate surgical treatment. Medical treatment can allow surgery to be delayed until a time when the queen has been stabilised with intravenous (IV) fluid therapy and IV antibiotics and the anaesthetic risk reduced.
A proportion of pyometra cases in cats spontaneously resolve after the onset of endogenous luteolysis and subsequent cervical opening, which allows drainage before the development of any systemic illness.

Surgical management
Ovariohysterectomy with resection of the entire cervix is the treatment of choice in all queens not intended for breeding. Cats that present in poor condition need to have any acid–base derangements, arrhythmias, hypotension, shock, electrolyte abnormalities and dehydration corrected before undergoing anaesthesia. Fortunately, the majority of cats with pyometra are systemically well at presentation and are good anaesthetic and surgical candidates.
Regardless of presentation, IV fluid therapy and IV antibiotics should be administered. Great care should always be taken in handling the uterus during surgery, as it is often very friable (Figures 7 and 10). Placement of saline-soaked laparotomy sponges in the abdomen is recommended to prevent contamination of the abdominal cavity with purulent material. Removal of the cervix in its entirety is performed in order to avoid leakage of purulent material into the abdomen and prevent the risk of a stump pyometra occurring (Figure 11) if some ovarian tissue is inadvertently left behind (ovarian remnant syndrome).
Figure 10.
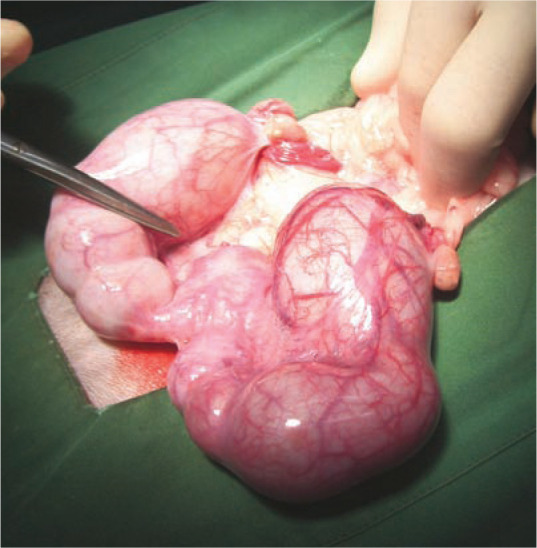
Intraoperative image of an enlarged, fluidfilled and friable uterus in a queen with pyometra
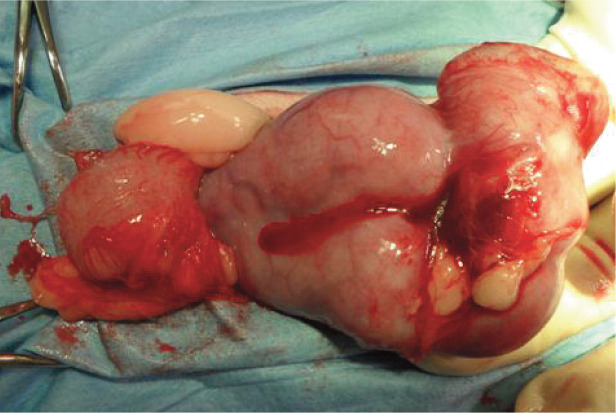
Figure 11.
Intraoperative image of an enlarged, distended, fluid-filled uterus in a queen with stump pyometra secondary to ovarian remnant syndrome
Postoperative monitoring for signs of shock, dehydration, sepsis, electrolyte and acid–base imbalances, hypoproteinaemia, hypoglycaemia and anaemia is required for 24–48 h following surgery.
Medical management
When considering medical treatment of a pyometra, it is important to rule out any concurrent conditions such as peritonitis, kidney disease, reactive hepatitis or disseminated intravascular coagulation (DIC). A full clinical and ultrasound examination, as well as additional haematological and biochemistry assays, should be carried out before commencing treatment. All patients receiving medical treatment for pyometra need to be very carefully monitored and, if systemically well, can be treated as in-house ‘day patients’. However, patients that become unwell or require fluid therapy should be immediately hospitalised. Owners should also be informed of the risk of treatment failure and that, ultimately, surgery may be required.
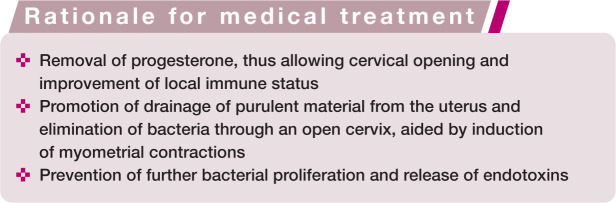
The rationale for medical therapy is three-fold (see box below). Pharmacological options include prostaglandin F2α (PGF), dopamine agonists and progesterone receptor antagonists or antiprogestins (Table 2).
Table 2.
Dosages of commonly used luteolytic, anti-luteotrophic and antiprogestin drugs for treatment of feline pyometra
| Drug name | Dose | Protocol | Actions | |
|---|---|---|---|---|
| Prostaglandin F2α | Dinoprost | 10 µg/kg SC | tid–5x/day x 1day | Luteolysis |
| tromethamine | 25 µg/kg SC | tid–5x/day x 1 day | Myometrial contractions | |
| (Lutalyse; Zoetis) | 50 µg/kg SC | tid–5x/day x 5–7 days* | Cervical opening | |
| If used in combination with aglepristone treatment is given on days 3–7 | ||||
| Cloprostenol | 1 µg/kg SC | sid for 5–7 days or until resolution | ||
| Dopamine agonists | Cabergoline | 5 µg/kg PO | sid | Anti-prolactinic |
| Anti-luteotrophic | ||||
| Bromocryptine | 10–25 µg/kg PO | tid | ||
| Anti-progestins | Aglepristone | 15 mg/kg SC | Days 1, 2, 8 and weekly* | Progesterone receptor antagonist Cervical opening Luteolysis |
Depending on response to treatment
SC = subcutaneous; PO = oral; sid = once a day; tid = three times a day; 5x/day = five times a day
Prostaglandin F2α
Repeated doses of PGF result in luteolysis of the feline corpus luteum. The resultant reduction in progesterone concentrations promotes cervical relaxation and a reduction in uterine secretions. PGF also has ecbolic activity that facilitates drainage of purulent material from the uterus.
There are two forms of PGF: its natural form (dinoprost tromethamine [Lutalyse; Zoetis]) or synthetic derivatives (eg, cloprostenol). Neither form is registered for use in companion animals but both can be used off-label in queens. Cloprostenol has been associated with fewer side effects and requires fewer injections due to its longer half-life. However, natural PGF induces greater myometrial contractions and therefore faster evacuation of purulent material from the uterus compared with synthetic PGF. 19 For this reason, the authors recommend natural PGF for treatment of pyometra in the cat.
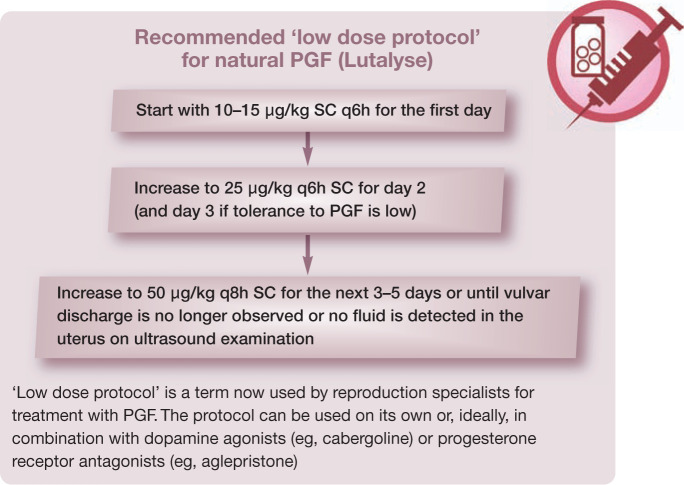
It is paramount, especially in the case of closed-cervix pyometra, that a low starting dose of natural PGF with incremental increases is administered subcutaneously (see ‘low dose protocol’ in the box above). This is in order to minimise the ecbolic effect of the drug and to reduce the risk of uterine rupture, as well as to reduce the side effects associated with higher doses of PGF. Once luteolysis has occurred and the cervix opens, the dose can be increased depending on the individual’s tolerance of the PGF. Doses greater than 50 μg/kg should not be required, which is significantly less than the 200–250 μg/kg reported in the older literature.
It is important to note that the corpus luteum in the queen is more resistant to the luteolytic effects of PGF than that of the bitch. Furthermore, if treatment is started soon after ovulation, the corpus luteum can be refractory to the effects of PGF. Often, higher doses of PGF for longer durations are required to obtain resolution of pyometra, especially if the diagnosis is made early in dioestrus (before day 20 postovulation). During this time, low doses of PGF are poorly effective in inducing complete and definitive luteolysis. 2
Side effects of PGF are dose-dependent and are rarely encountered with the ‘low dose protocol’, mostly limited to a transient hypersalivation. Individual variation in terms of tolerance of PGF is also seen, with some queens tolerating the drug well and requiring a more rapid dose increase. Tolerance of PGF and reduction of side effects is also typically seen after subsequent injections. Reported side effects include tachypnoea, vomiting, diarrhoea, urination and restlessness. Side effects appear about 20 mins after treatment and only last 15–30 mins. Patients should therefore be hospitalised for at least 1 h after treatment to observe for side effects. Systemically well queens can be managed as ‘day patients’ – receiving injections throughout the day while under veterinary supervision but able to go home overnight when no medication is given.
Dopamine agonists
Dopamine agonists can be used for the treatment of pyometra in the queen either alone or in combination with PGF or a progesterone receptor antagonist (see below). Dopamine agonists are ergot-derived alkaloid compounds that act as prolactin antagonists and thus have anti-luteotrophic activity. They are effective from approximately 15–20 days after ovulation when prolactin is present. 2 Therefore, if a queen presents with pyometra soon after oestrus, anti-prolactinic agents are preferred over PGF as they are very effective at inducing luteal arrest and luteolysis in early dioestrus. 2 However, if a queen presents more than 4 weeks after oestrus or mating, use of a dopamine agonist in combination with PGF potentiates the luteolytic effect, causing more rapid luteolysis and leading to cervical opening within 24–48 h.
There are two commonly used dopamine agonists: cabergoline and bromocriptine. Cabergoline is associated with few or no side effects and involves only once daily administration, whereas bromocriptine has a number of side effects including vomiting, anorexia, depression and some behavioural changes, and also requires administration two to three times a day. The recommended dose of cabergoline is 5 µg/kg PO q24h; the dose of bromocriptine is 10–25 µg/kg PO q8h. Both drugs are most commonly used in combination with PGF, with the duration of treatment usually being 7 days.
Progesterone receptor antagonists or antiprogestins
Progesterone receptor antagonists or antiprogestins, such as aglepristone (Alizine; Virbac), are synthetic steroids that competitively bind to progesterone receptors with a greater (9 x in cats) affinity than natural progesterone. This results in a decrease in progesterone activity.20,21 Aglepristone has minimal side effects and is a good choice for the treatment of closed-cervix pyometra as it results in cervical opening with minimal uterine contractions. It also induces luteolysis. However, queens that present with poor liver and/or kidney function should not be treated with aglepristone.
Aglepristone is most effective when used in combination with natural PGF (dinoprost) for 5–10 days to potentiate luteolysis and enhance uterine contractions.22,23 This is particularly important in cats as they are notoriously resistant to luteolysis and removal of the effects of progesterone on the uterus. Furthermore, initiation of aglepristone treatment 48 h before starting PGF treatment can reduce the risk of uterine rupture in a closed-cervix pyometra by slowly opening the cervix without the stimulation of strong uterine contractions. Therefore, when using PGF in combination with aglepristone, treatment with PGF should start on day 3; PGF is then given daily as per the ‘low dose protocol’ described on page 28, except on days when aglepristone is given.
The recommended dosage of aglepristone in the queen is 15 mg/kg SC twice, 24 h apart, and then a single injection on day 8. A higher dose rate is recommended for queens compared with bitches due to reduced bioavailability in the queen. Depending on the patient’s condition, additional injections of aglepristone can be given on days 14 and 28 if resolution of the pyometra has not occurred. In these chronic cases, treatment with aglepristone weekly (mean duration of effect of aglepristone is 6 days) for 2 months has been reported. However, the prognosis with regard to fertility and recurrence rate is significantly poorer in these cases compared with cases that respond after the initial three injections on days 1, 2 and 8. 16
Treatment with aglepristone (15 mg/kg SC q24h) in combination with trimethoprim/sulfadoxine for 7 days resulted in a success rate of 90% (9 out of 10 cats). 24 The authors did not note any recurrences for 2 years after treatment.

Antimicrobial therapy
Antimicrobial therapy should be initiated immediately with a broad spectrum antibiotic. Culture and sensitivity testing should be performed but therapy has to be started at the time of diagnosis on the assumption that E coli is the most likely pathogen. Excellent results have been achieved with amoxicillin/ clavulanic acid (12.5–25 mg/kg PO q12h) or cephalosporins (eg, cefazolin 22 mg/kg IV or IM q8h) and potentiated sulfonamides; care should be taken in using cephalosporins or sulfonamides if renal function is impaired. If oral antibiotics are administered, care must be taken to give the drugs at a different time from the PGF, which might lead to vomiting.
Antimicrobial therapy should be continued for at least 14 days after resolution of vulvar discharge and evacuation of all fluid from the uterine lumen as determined by ultrasound examination.
Assessing the response to therapy and predicting future fertility
There are a number of parameters (see below) that should be assessed throughout the treatment of a queen with pyometra to monitor the response to treatment and determine when luteolytic treatment can cease, as well as to provide an indication of potential future fertility.
A clinical improvement is usually seen within 48 h of initiation of medical therapy. Ideally, resolution of all clinical signs should occur within 7–10 days.
Monitoring tools
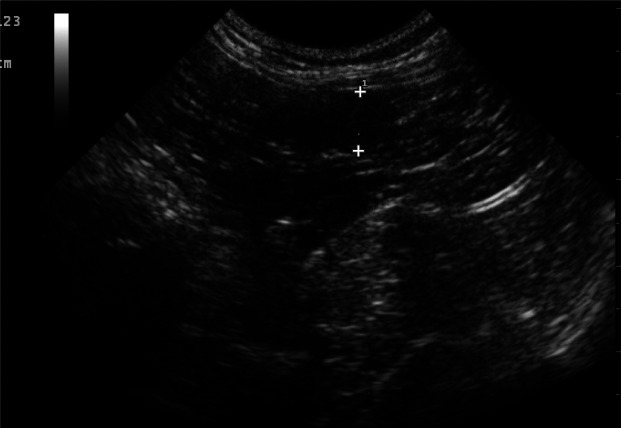
Ultrasound of the uterus Ultrasound examination is the most important monitoring tool. A decrease in uterine size by 50% should be seen 72–96 h after initiation of therapy. If a reduction in uterine size of at least 50% is not observed after 5 days of treatment, the prognosis for future fertility is poor. In cases that respond poorly to luteolytic therapy, surgery is recommended to remove the fluid-filled uterus. 2 In bitches, treatment for longer than 7–10 days can increase the risk of complications such as DIC. This has not been reported in the queen but should be a consideration when undertaking prolonged treatment. Weekly ultrasound examinations are recommended to assess the response to therapy. When the uterine dimensions have returned to normal and there is no fluid present in the uterus, luteolytic treatment can cease. Repeat ultrasound examination 2 weeks after resolution of clinical signs and treatment is advised to assess uterine health (eg, degree of CEH changes) and to confirm the absence of intraluminal fluid (Figure 12). This is especially important when treatment is started in a queen soon after ovulation or in the early luteal phase when the corpus luteum is more refractory to luteolysis and intrauterine fluid accumulation can recur. A reduction in the uterine wall thickness and often also in the degree of CEH changes may be detected on ultrasound after removal of progesterone and resolution of bacterial infection.
Vulvar discharge Vulvar discharge should increase in volume within the first 24 h of treatment and usually ceases about 5–7 days after the onset of treatment. However, in contrast to dogs, pyometra in cats is often slower to resolve. The nature of the vaginal discharge will also gradually change – from purulent (and often blood-tinged) to serosanguineous, before eventually becoming serous.
Vaginal cytology The number of neutrophils seen on vaginal cytology should decrease over the course of treatment.
Leukogram Weekly complete blood cell counts should be performed to evaluate neutrophilia. In most patients, the leukogram will return to normal 2–3 weeks after commencement of medical therapy.
Serum progesterone Measurement of serum progesterone concentration prior to starting medical therapy can be helpful with regard to prognosis. Queens with low progesterone concentrations (<2 ng/ml) or those that are in anoestrus are poor candidates for medical therapy as they usually respond poorly. 16 Measurement of progesterone at weekly intervals can help determine if luteolysis has occurred (indicated by serum progesterone <2 ng/ml). This is particularly valuable if PGF alone is used to treat the pyometra or in refractory cases to help determine whether complete luteolysis has occurred. Progesterone receptor antagonists displace the endogenous progesterone, thus elevating systemic levels initially. Therefore, when using this drug, progesterone concentrations must be interpreted with caution to assess luteolysis. The progesterone concentration 3 weeks after initiation of aglepristone treatment should be <2 ng/ml.
Figure 12.
Ultrasonogram of a uterine horn in a queen treated medically for pyometra several weeks earlier. Note that there is no intraluminal fluid present, but there are marked cystic changes in the thickened uterine wall, indicating a poor prognosis for future fertility. Courtesy of Dr Cheryl Lopate, Wilsonville Veterinary Clinic, USA
Management of breeding queens after medical treatment of pyometra
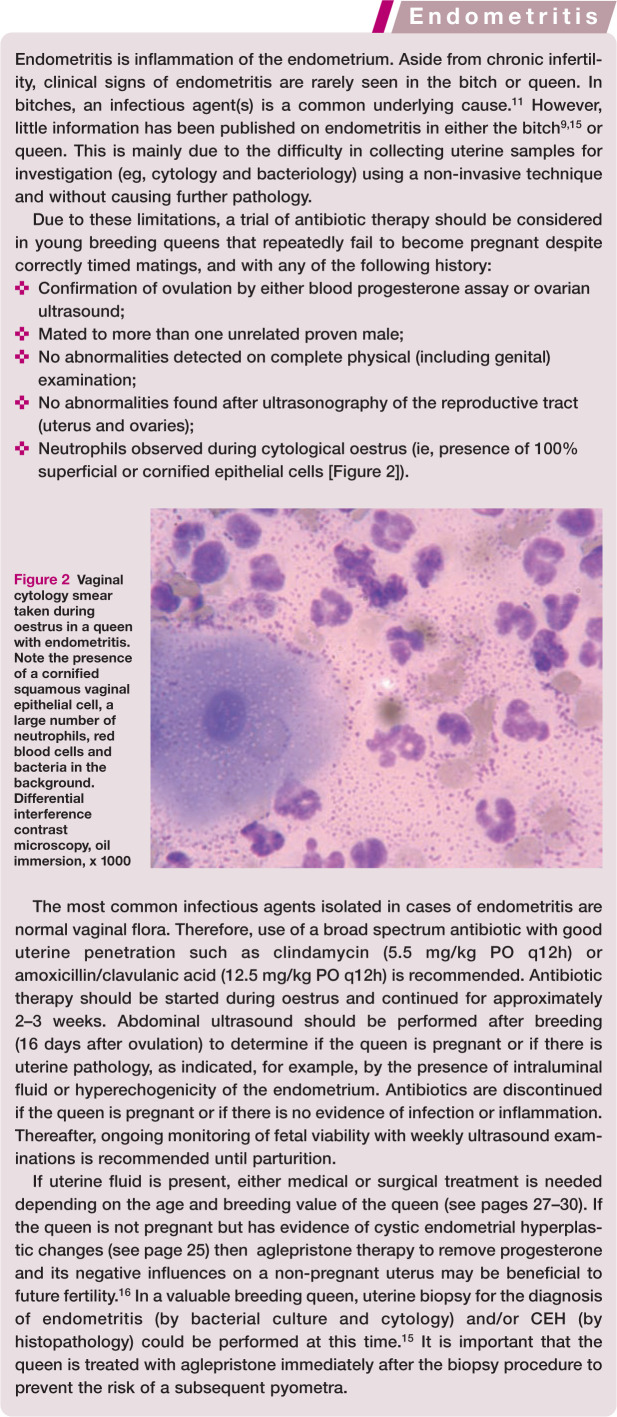
It is optimal that all queens intended for breeding are mated or inseminated on the first oestrus following treatment for pyometra, as a pregnant queen is significantly less likely to develop recurrence of pyometra. Therefore, it is important to manage the oestrus to optimise the likelihood of the queen becoming pregnant. Using a proven, fertile, young tom cat or, if AI is to be carried out, using high-quality fresh semen, is essential, as is optimal timing with the use of ovulation-inducing agents. Observation of multiple matings and confirmation of ovulation by measuring progesterone 48–72 h after calling has ceased or ovulation is induced is recommended. Administration of a broad spectrum antibiotic (amoxicillin and clavulanic acid) during oestrus and the early luteal phase (until pregnancy is confirmed by an early ultrasound examination) is indicated if neutrophils are detected on vaginal cytology during oestrus (Figure 2).
Figure 2.
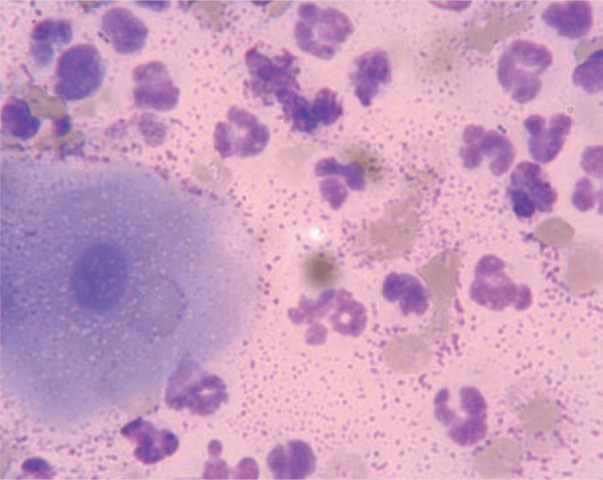
Vaginal cytology smear taken during oestrus in a queen with endometritis. Note the presence of a cornified squamous vaginal epithelial cell, a large number of neutrophils, red blood cells and bacteria in the background. Differential interference contrast microscopy, oil immersion, x 1000
After mating, it is essential to follow queens closely with ultrasound examinations in order to detect any recurrence of pyometra early, before clinical signs associated with systemic illness occur. An early ultrasound examination should be scheduled for 16 days after the LH peak to detect either embryonic vesicles (consistent with pregnancy) or uterine fluid (consistent with recurrence of pyometra).
However, it is often not possible to breed a queen on every oestrus subsequent to treatment for pyometra. Prevention of oestrus in these queens – especially individuals that are known repeatedly to spontaneously ovulate – is something to consider to reduce the risk of pyometra recurrence. Unfortunately, safe and effective methods and pharmaceutical agents for prevention of oestrus in queens are limited. GnRH analogues such as deslorelin implants (Suprelorin; Virbac) are reversible contraceptives that inhibit oestrus by downregulation of the hypothalamic–pituitary–ovarian axis. 28 The effects are not only long term but highly variable. A minimum of 6 months’ suppression of oestrus would be obtained from a 4.7 mg implant and a minimum of 12 months from a 9.4 mg implant. The timing of implantation with regard to season would have an effect on this variability, as well as individual response. In one study, the period of oestrus suppression after implantation with a 4.7 mg Suprelorin implant in 20 female cats ranged from 16–37 months. 29 Suprelorin is not registered for use in queens (or bitches) due to this variability in response. Importantly, in the above-mentioned study, 7/8 queens that were mated after the implant was no longer effective became pregnant and went on to kitten naturally.
Melatonin implants have been reported to safely provide oestrus suppression for up to 4 months in queens.30,31 Synthetic progestins have been widely used for oestrus suppression in the queen, especially in European countries. 17 Great care should be taken when using these agents (eg, MA and MPA) in a queen that already has cystic changes in the uterus as this may predispose to recurrence of pyometra. Use of anabolic steroids such as mibolerone for oestrus prevention is contraindicated in cats.
An alternative management strategy for queens with a history of pyometra is to measure serum progesterone concentration 3–4 weeks after the end of oestrus to evaluate for spontaneous ovulation. If ovulation has occurred (indicated by progesterone concentrations >2–5 ng/ml), treatment with aglepristone in an attempt to prevent pyometra may be considered.
Queens no longer intended for breeding should undergo ovariohysterectomy. Ovariectomy is not recommended in a queen that has had pyometra and previous pregnancies, as the risk of a pyometra recurring in these queens is high if exogenous hormonal therapy (oestrogens and progestins) is administered or if ovarian remnants are inadvertently left behind after ovariectomy.
Key Points
There are many published studies on the prevalence, pathophysiology, treatment and prognosis of pyometra in the bitch. Unfortunately this work has not yet been documented in queens and there has been much extrapolation from the bitch as a model for pyometra in the queen.
A recent large retrospective study indicates that the incidence of pyometra in the queen is potentially much higher than initially assumed. This finding opens up many questions as to the underlying pathogenesis of pyometra in the queen.
Queens are unique in that the corpus luteum is much more resistant to the currently available drugs and protocols available for the medical treatment and management of pyometra. More refractory cases are seen than in bitches and often a more aggressive approach is required to induce luteolysis.
Successful medical treatment and, more importantly, successful breeding of a queen after treatment of a pyometra is ultimately influenced by the selection of suitable candidates for medical therapy.
With the availability of new drugs and protocols for the treatment of pyometra in queens, as well as a greater understanding of appropriate selection of candidates for medical therapy, clinicians are now much more able to facilitate a successful decision by owners of queens that develop a pyometra in regard to ‘spay or not to spay?’.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Contributor Information
Fiona Hollinshead, GlenBred, Matamata Veterinary Services, 26 Tainui Street, Matamata 3400, New Zealand.
Natali Krekeler, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Werribee, VIC 3030, Australia.
References
- 1. Hagman R, Ström Holst B, Möller L, et al. Incidence of pyometra in Swedish insured cats. Theriogenology 2014; 82: 114–120. [DOI] [PubMed] [Google Scholar]
- 2. Verstegen J, Onclin K. The mucometra–pyometra complex in the queen. Proceedings of the North American Veterinary Conference; January 7–11, Orlando, FL, USA, 2006. [Google Scholar]
- 3. Lawler DF, Johnston SD, Hegstad RL, et al. Ovulation without cervical stimulation in domestic cats. J Reprod Fertil Suppl 1993; 47: 57–61. [PubMed] [Google Scholar]
- 4. Pelican KM, Brown JL, Wildt dE, et al. Short term suppres- sion of follicular recruitment and spontaneous ovulation in the cat using levonorgestrel versus a GnRH antagonist. Gen Comp Endocrinol 2005; 144: 110–121. [DOI] [PubMed] [Google Scholar]
- 5. Gudermuth dF, Newton L, Daels P, et al. Incidence of spon-taneous ovulation in group-housed cats based on serum and faecal concentrations of progesterone. J Reprod Fertil Suppl 1997; 51: 177–184. [PubMed] [Google Scholar]
- 6. Verstegen J. Estrous cycle regulation, estrous induction and pregnancy termination in the queen. Proceedings of the Society for Theriogenology; September 16–20, Columbus, OH, USA, 2003, pp 334–339. [Google Scholar]
- 7. Arora N, Sandford J, Browning GF, et al. A model for cystic endometrial hyperplasia/pyometra complex in the bitch. Theriogenology 2006; 66: 1530–1536. [DOI] [PubMed] [Google Scholar]
- 8. Nomura K, Funahashi H. Histological characteristics of canine deciduoma induced by intrauterine inoculation of E coli suspension. J Vet Med Sci 1999; 61: 433–438. [DOI] [PubMed] [Google Scholar]
- 9. Schlafer DH, Gifford AT. Cystic endometrial hyperplasia, pseudo-placentational endometrial hyperplasia, and other cystic conditions of the canine and feline uterus. Theriogenology 2008; 70: 349–358. [DOI] [PubMed] [Google Scholar]
- 10. C Dow. The cystic hyperplasia–pyometra complex in the bitch. Vet Rec 1958; 70: 1102–1110. [Google Scholar]
- 11. Chatdarong K, Rungsipipat A, Axnér E, et al. Hysterographic appearance and uterine histology at different stages of the reproductive cycle and after progestagen treatment in the domestic cat. Theriogenology 2005; 64: 12–29. [DOI] [PubMed] [Google Scholar]
- 12. Hollinshead FK, Krekeler N. Pyometra in the bitch. In: Monnet E. (ed). Small animal soft tissue surgery. Blackwell, 2013, pp 625–635. [Google Scholar]
- 13. van Goethem B, Schaefers-Okkens A, Kirpensteijn J. Making a rational choice between ovariectomy and ovariohysterectomy in the dog: a discussion of the benefits of either technique. Vet Surg 2006; 35: 136–143. [DOI] [PubMed] [Google Scholar]
- 14. Wadås B, Kühn I, Lagerstedt AS, et al. Biochemical phenotypes of Escherichia coli in dogs: comparison of isolates isolated from bitches suffering from pyometra and urinary tract infection with isolates from faeces of healthy dogs. Vet Microbiol 1996; 52: 293–300. [DOI] [PubMed] [Google Scholar]
- 15. Fontaine E, Levy X, Grellet A, et al. Diagnosis of endometritis in the infertile bitch: a new approach. Reprod Domest Anim 2009; 44 Suppl 2: 196–199. [DOI] [PubMed] [Google Scholar]
- 16. Romagnoli S. Practical use of aglepristone. Proceedings of the Southern European Veterinary Conference; October 1–4, Barcelona, Spain, 2009. [Google Scholar]
- 17. Romagnoli S. Progestins to control feline reproduction. Historical abuse of high doses and potentially safe use of low doses. J Feline Med Surg 2015; 17: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenney KJ, Matthiesen DT, Brown NO, et al. Pyometra in cats: 183 cases (1979–1984). J Am Vet Med Assoc 1987; 191: 1130–1132. [PubMed] [Google Scholar]
- 19. Verstegen J, Dhaliwal G, Verstegen-Onclin K. Mucometra, cystic endometrial hyperplasia, and pyometra in the bitch: advances in treatment and assessment of future reproductive success. Theriogenology 2008; 70: 364–374. [DOI] [PubMed] [Google Scholar]
- 20. Arnold S, Hubler M, Reichler I. Canine pyometra: new approaches to an old disease. Proceedings of the World Small Animal Veterinary Association Conference; October 11–14, Prague, Czech Republic, 2006. [Google Scholar]
- 21. Galac S, Kooistra HS, Butinar J, et al. Termination of mid-gestation pregnancy in bitches with aglepristone, a progesterone receptor antagonist. Theriogenology 2000; 53: 941–950. [DOI] [PubMed] [Google Scholar]
- 22. Gobello C, Castex G, Klima L, et al. A study of two protocols combining aglepristone and cloprostenol to treat open cervix pyometra in the bitch. Theriogenology 2003; 60: 901–908. [DOI] [PubMed] [Google Scholar]
- 23. Fieni F. Clinical evaluation of the use of aglepristone, with or without cloprostenol, to treat cystic endometrial hyperplasia–pyometra complex in bitches. Theriogenology 2006; 66: 1550–1556. [DOI] [PubMed] [Google Scholar]
- 24. Nak D, Nak Y, Tuna B. Follow-up examinations after medical treatment of pyometra in cats with the progesterone-antagonist aglepristone. J Feline Med Surg 2009; 11: 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zambelli D, Bini C, Cunto M. Endoscopic transcervical catheterization in the domestic cat. Reprod Domest Anim 2015; 50: 13–16. [DOI] [PubMed] [Google Scholar]
- 26. Declue AE, Delgado C, Chang CH, et al. Clinical and immunologic assessment of sepsis and the systemic inflammatory response syndrome in cats. J Am Vet Med Assoc 2011; 238: 890–897. [DOI] [PubMed] [Google Scholar]
- 27. Ruthrauff CM, Smith J, Glerum L. Primary bacterial septic peritonitis in cats: 13 cases. J Am Anim Hosp Assoc 2009; 45; 268–276. [DOI] [PubMed] [Google Scholar]
- 28. Fontaine C. Long-term contraception in a small implant. A review of Suprelorin (deslorelin) studies in cats. J Feline Med Surg 2015; 17: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goericke-Pesch S, Georgiev P, Atanasov A, et al. Treatment of queens in estrus and after estrus with a GnRH-agonist implant containing 4.7 mg deslorelin; hormonal response, duration of efficacy, and reversibility. Theriogenology 2013; 79: 640–646. [DOI] [PubMed] [Google Scholar]
- 30. Gimenez F, Stornelli MC, Tittarelli CM, et al. Suppression of estrus in cats with melatonin implants. Theriogenology 2009; 72: 493–499. [DOI] [PubMed] [Google Scholar]
- 31. Kutzler MA. Alternative methods for feline fertility control. Use of melatonin to suppress reproduction. J Feline Med Surg 2015; 17: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]