Abstract
Overview:
Tramadol toxicity has not previously been reported in a cat.
Case summary:
This report describes the clinical signs, diagnosis and treatment of tramadol toxicity, manifesting as serotonin syndrome, in a cat in Australia.
Practical relevance:
For any cat with suspicion of serotonin syndrome, in particular secondary to tramadol overdose, it is recommended that decontamination, monitoring and supportive care are instituted as soon as clinical signs develop. Prolonged hospitalisation may be required in the event of a severe overdose.
Literature review:
The literature relating to the pharmacology of tramadol and tramadol overdose, clinical manifestations of tramadol overdose, and serotonin syndrome in cats, humans and dogs is reviewed. Recommended treatment for tramadol overdose and serotonin syndrome is also discussed.
Introduction
Outpatient pain management is common in cats, with non-steroidal anti-inflammatory drugs (NSAIDs) often being administered in this setting. Due to the potential adverse effects of NSAIDs in cats, alternative therapies may be used, including tramadol.
There is little data on the efficacy and safety of tramadol in cats. 1 The clinical effects of tramadol overdose in people are attributed to serotonin syndrome. 2 Development of serotonin syndrome may be caused by tramadol alone 3 or combined with other medications.2,4 –8 To the authors’ knowledge, clinical tramadol overdose has not been reported previously in a cat.
Clinical report
A 19-year-old, female neutered domestic shorthair cat, weighing 2.5 kg, was administered two doses of 80 mg/kg tramadol PO due to a prescribing error. The intended dose was 4 mg/kg q12h. A few hours after ingestion of the first dose, the owner noted that the cat was agitated, hypersalivating and was displaying a jerky head movement. The referring veterinarian described the cat as agitated and hypertensive. The cat was treated with intravenous fluids of unknown type and volume, which were reported to result in some clinical improvement. A second dose of tramadol was administered later that day as the cat appeared almost normal.
The following day, 36 h after the first tramadol ingestion, the cat was re-presented to the referring veterinarian. It was dehydrated, hypertensive and had altered mentation. Further treatment with intravenous fluids and 0.3 mg/kg acepromazine IV was administered. The cat’s mentation deteriorated throughout the day and it was referred to Animal Accident and Emergency (AAE).
On presentation at AAE, the cat was obtunded, laterally recumbent, tachycardic (200 beats/min), normotensive (mean arterial pressure [MAP] 120 mmHg) and normothermic (rectal temperature 37.9°C). Severe abdominal pain and abdominal distension were noted.
Work-up and presumptive diagnosis
Haematology, biochemistry, serum electro-lytes and blood gas analysis revealed hypochloraemia (115 mmol/l, reference interval [RI] 117–123 mmol/l), hypokalaemia (3.4 mmol/l, RI 4.0–4.5 mmol/l), hypoalbuminaemia (19 g/l, RI 23–39 g/l), decreased urea (5.6 mmol/l, RI 5.7–12.9 mmol/l), hypocholesterolaemia (1.63 mmol/l, RI 1.68–5.81 mmol/l), hypoproteinaemia (51 g/l, RI 57–89 g/l), non-regenerative anaemia (4.36 × 1012 red blood cells [RBC]/l, RI 5–10 × 1012 RBC/l; 0.5% reticulocytes) with a packed cell volume of 22% (RI 24–45%) and total protein of 64 g/l (RI 60–75 g/l). Abdominal radiography revealed faeces and gas in the colon. Rectal examination revealed hard, dry faeces. Overall, blood results were unremarkable for a geriatric cat and did not suggest a definitive cause for the clinical signs.
There was no history of access to anticholinergic toxicant, a sympathomimetic agent or any other medications, so a presumptive diagnosis of serotonin toxicity secondary to ingestion of a large dose of tramadol was made. Meningitis or encephalitis could not be ruled out.
Stabilisation and therapy
The cat was placed on intravenous fluids (Hartmann’s solution) at 10 ml/kg/h with additional potassium chloride (10 mmol/l). It was also treated with cyproheptadine (Periactin; Aspen Pharmacare, Australia, 2 mg PO q24h), buprenorphine (Temgesic; Reckitt Benckiser, Australia, 0.01 mg/kg IV q8h) and a microenema (Microlax; Pharmacia, Australia, 5 ml tube once). The cat defecated after enema administration, which resulted in a reduction in abdominal distension and pain.
Twelve hours after presentation to AAE (60 h post-tramadol ingestion) the cat’s mentation was improving although still altered. The cat was obtunded, but with any stimulus would vocalise and start to paddle uncontrollably. The cat’s blood pressure had increased since admission and was persistently high (MAP 190 mmHg), with tachycardia (220 beats/min) and tachypnoea (60 breaths/min) despite a normal SpO2 on room air (98%). Repeat blood work showed mild hypokalaemia (3.3 mmol/l, RI 4.0–4.5 mmol/l). The potassium supplementation was increased to 20 mmol KCl/l, which normalised the serum potassium level in 24 h. At this time the intravenous fluid rate was reduced to 5 ml/kg/h.
Twenty-four hours after presentation (72 h post-tramadol ingestion) the cat was responsive to voice and would try to right itself to sternal recumbency, but was still unable to maintain this position. Despite its altered mentation, the cat had an intact gag reflex and tolerated syringe feeding using Hill’s a/d. Treatment with maintenance intravenous fluids, buprenorphine and cyproheptadine was continued.
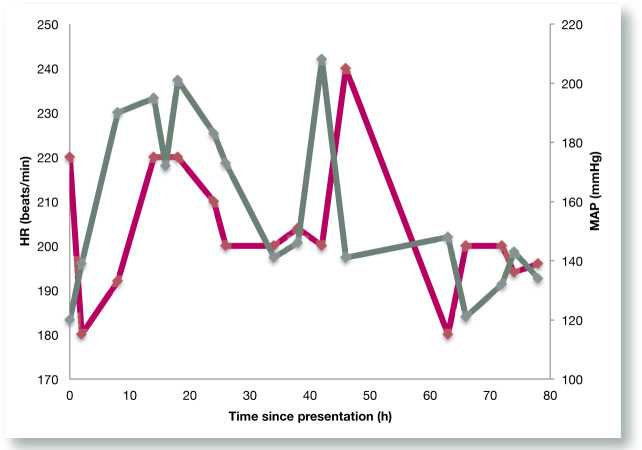
In the first 48 h of hospitalisation, the cat’s blood pressure was erratic (Figure 1), with fluctuations between 120 and 208 mmHg. The cat’s heart rate also varied greatly, with measurements between 180 and 240 beats/min. Episodes of hypertension were generally accompanied by tachycardia. After 48 h, the cat maintained a MAP between 120 and 140 mmHg and a heart rate around 180–200 beats/min for the remainder of its stay in hospital.
Figure 1.
Fluctuations in heart rate and blood pressure for the first 48 h of hospitalisation due to serotonin toxicity causing autonomic hyperactivity. Heart rate and blood pressure stabilised for the remainder of hospitalisation. The dark pink line shows the heart rate (beats/min) and the green line shows the mean arterial pressure (mmHg)
Forty-eight hours after presentation (96 h post-tramadol ingestion) the cat was able to walk with assistance, although was uncoordinated and weak. Abdominal pain was still present and treatment with buprenorphine was continued. Blood pressure had normalised but the cat remained persistently tachycardic. Treatment with intravenous crystalloid fluids and cyproheptadine was continued. The cat was fed two-thirds of resting energy requirements using Hill’s a/d.
By day 4 of hospitalisation, the cat’s tachycardia and hypertension had resolved. Its mentation was almost normal and, although still quiet, it responded normally to stimulation. The cat was able to maintain sternal recumbency for short periods and was eating and drinking without assistance. Intravenous fluid therapy was discontinued. The cat was continued on cyproheptadine.
Discharge and follow-up
Seven days after presentation, the cat had normal mentation and was able to walk a few steps unassisted. All medications were discontinued at this point and the cat was discharged from hospital.
Five days after discharge (14 days post-tramadol ingestion) a follow-up phone call to the owner confirmed that the cat was continuing to improve. Its mentation and appetite were normal, and coordination and strength were improving. The owner felt the cat did not have any permanent changes secondary to tramadol toxicity.
Review of the literature
Mechanism of action of tramadol
Tramadol is supplied as a racemic mixture of two enantiomers: (+)-tramadol and (–)-tramadol. (+)-Tramadol and the metabolite (+)-O-desmethyltramadol (M1) are µ opioid receptor agonists. (+)-Tramadol inhibits neuronal serotonin reuptake and (±)-tramadol inhibits neuronal noradrenaline reuptake, leading to anti-nociceptive effects by enhancing inhibitory effects on pain transmission in the spinal cord. The complementary and synergistic actions of the two enantiomers improve the analgesic efficacy and tolerability profile of the racemate. 9
Tramadol’s affinity for µ opioid receptors is approximately 10-fold less than that of codeine and 6000-fold less than that of morphine, and it has no affinity for δ or κ opioid receptors. 10 (+)-Tramadol has a twofold higher affinity for the µ opioid receptor than (–)-tramadol. 11 Tramadol’s metabolite (+)-M1 binds with about 700-fold higher affinity than tramadol, but still with much lower affinity than morphine. 12 Another metabolite with a higher affinity than (±)-tramadol for the µ opioid receptor is (±)-M5, which due to its polarity does not cross the blood–brain barrier.
(±)-Tramadol inhibits the neuronal reuptake of serotonin; the (+)-enantiomer is about fourfold more potent than the (–)-enantiomer. 13 In addition, (±)-tramadol and its (+)-enantiomer increase serotonin efflux. 14
Tramadol enhances extraneuronal noradrenaline levels by interfering with noradrenaline transporter function. 15 The effect on noradrenaline efflux was smaller than the effect on noradrenaline uptake. 16 (–)-Tramadol is a more potent blocker of noradrenaline reuptake than (+)-tramadol or the M1 metabolite.16,17
Pharmacokinetics of tramadol
Tramadol is metabolised into at least 30 metabolites by O- and N-demethylation and by conjugation reactions forming glucuronides and sulfates. M1 to M5 metabolites are the major metabolites in all species.18 –20 The O-demethylation of tramadol to M1, the main analgesic effective metabolite, is catalysed by cytochrome P450 (CYP) 2D6.21,22 In humans, polymorphism in the CYP 450 enzyme system affects the metabolism and clearance of tramadol. 23 As yet, CYP 450 isoforms have not been well characterised in dogs or cats. 24 In one study, overall CYP activities in cat liver microsomes were lower than in those from dogs or humans, except for CYP2B. 25 Animals with pre-existing renal or hepatic disease may be more susceptible to the effects of medications metabolised via the cytochrome P450 pathways and more likely to develop clinical signs following their ingestion. 26
Eighty-six percent of absorbed tramadol is metabolised in the liver and 90% of tramadol and its metabolites are excreted by the kidneys, 20 with the residual excreted in faeces. Less than 1% of tramadol is eliminated by biliary excretion. 27 The half-life of tramadol is extended in healthy humans over 75 years of age, and in those with impaired hepatic and renal function. 28
Intravenous tramadol has a longer elimination half-life in cats than in dogs or humans, 29 and the half-life is even longer for orally administered tramadol. 30 The difference was proposed to be related to prolonged absorption of tramadol after oral administration and dose dependency.30,31 The lower clearance of tramadol in cats was suspected to indicate a lower capacity of the liver to methylate tramadol. 30
Pharmacokinetic studies in cats show that 2 h after tramadol administration the concentration of M1 is higher than that of the parent compound. 30 M1 levels are also maintained for a longer period in cats than dogs. The persistent M1 metabolite found in cats is likely to be due to slow glucuronidation and consequently slow elimination.
Signs of tramadol overdose in humans and other species
Adverse effects of experimental tramadol overdose in rabbits, dogs, mice and rats include restlessness, hyperactivity, unsteady gait, reduced spontaneous activity, exophthalmos, mydriasis, salivation, vomiting, tremors, convulsions, cyanosis and dyspnoea. 31 Administration of a single oral dose of 450 mg/kg was not fatal in dogs in one experimental study, with dogs recovering completely within an unspecified short time. 31 Dogs produce fewer M1 metabolites and more of the M2 inactive metabolites, which accounts for their shorter recovery, even with much higher tramadol doses.29,32 –34
The high concentrations of M1 in cats administered tramadol can result in opioid-mediated adverse effects in this species. 1 A major adverse effect of opioid analgesics is respiratory depression, mediated by µ opioid receptors. In anaesthetised cats, 1–4 mg/kg tramadol administered intravenously causes ventilatory depression, which is completely reversed with naloxone administration. 35 Other opioid-mediated adverse effects such as sedation, mydriasis, dysphoria or euphoria, constipation and vomiting can occur in cats. 1
The manifestations of tramadol overdose in people are attributed to serotonin syndrome. 2 Development of serotonin syndrome may be caused by tramadol administered alone 3 or in combination with other medications.2,4 –8
Relevant aspects in this case
The cat in this report had constipation and sedation that may be associated with opioid toxicity. Buprenorphine was used instead of naloxone to reverse the potential opioid toxicity but provide some ongoing analgesia. Buprenorphine has high affinity but low intrinsic activity at the µ receptors, displacing other µ opioid agonists from the receptors.55,56
The cat exhibited signs of serotonin syndrome as defined by the Sternbach and Hunter criteria. The autonomic hyperactivity manifested as tachycardia, fluctuating blood pressure and abdominal pain; the neuro-musular signs as hyperreflexia, paresis and inducible clonus. The cat had altered mentation, exhibiting agitation and disorientation. It was treated with intravenous fluids to enhance tramadol excretion, cyproheptadine as a 5-HT2 receptor antagonist, and diazepam to reduce agitation.
The clinical signs associated with serotonin toxicity in this case were prolonged. A number of factors may have contributed to this. There is a suggestion that the pharmacokinetics of tramadol are dose-dependent, with a longer half-life recorded with higher doses. 30 Dehydration was noted on presentation to the referring veterinarian, which may have decreased the rate of drug absorption from the gastrointestinal system and slowed elimination via the kidneys. Polymorphism in the CYP 450 enzyme system affects the metabolism and clearance of tramadol in humans, 23 and may also exist in cats. 24 The half-life of tramadol is extended in healthy humans over 75 years of age 28 and this may have been a factor in this geriatric cat. Although the majority of toxic signs in human tramadol overdose have resolved within 24 h,2,6,57 the average admission period was 2.75 days 6 (range 1–25 days2,6,58). Furthermore, cats have a lower clearance of tramadol, which indicates a lower capacity of the liver to methylate tramadol, 30 likely causing prolonged duration of toxicity in this case.
The cat in this report was administered a 200 mg tablet of tramadol, which may have been a sustained-release tablet that could have caused a protracted duration of serotonin toxicity. 43 Unfortunately we were unable to confirm or rule this out.
Conclusions
This report describes the severe and prolonged clinical signs associated with tramadol overdose in a cat. With supportive care the cat made a full recovery and 2.5 years after discharge the now almost 22-year-old cat is continuing to do well (Figure 2). Tramadol is an increasingly prescribed medication and clinicians must be aware that it has the potential for severe side effects if administered incorrectly or in conjunction with other medications that alter serotonin metabolism.
Figure 2.

The cat pictured close to the time of publication. At almost 22 years of age, she is still going strong, albeit a little arthritic
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Date accepted: 24 April 2014
References
- 1. KuKanich B. Outpatient oral analgesics in dogs and cats beyond nonsteroidal antiinflammatory drugs: an evidence-based approach. Vet Clin North Am Small Anim Pract 2013; 43: 1109–1125. [DOI] [PubMed] [Google Scholar]
- 2. Spiller HA, Gorman SE, Villalobos D, Benson BE, Ruskosky DR, Stancavage MM, et al. Prospective multicenter evaluation of tramadol exposure. Clin Toxicol 1997; 35: 361–364. [DOI] [PubMed] [Google Scholar]
- 3. Takeshita J, Litzinger MH. Serotonin syndrome associated with tramadol. Prim Care Companion J Clin Psychiatry 2009; 11: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goeringer KE, Logan BK, Christian GD. Identification of tramadol and its metabolites in blood from drug-related deaths and drug-impaired drivers. J Anal Toxicol 1997; 21: 529–537. [DOI] [PubMed] [Google Scholar]
- 5. Tjäderborn M, Jönsson AK, Hägg S, Ahlner J. Fatal unintentional intoxications with tramadol during 1995–2005. Forensic Sci Int 2007; 173: 107–111. [DOI] [PubMed] [Google Scholar]
- 6. Shadnia S, Soltaninejad K, Heydari K, Sasanian G, Abdollahi M. Tramadol intoxication: a review of 114 cases. Hum Exp Toxicol 2008; 27: 201–205. [DOI] [PubMed] [Google Scholar]
- 7. Tashakori A, Afshari R. Tramadol overdose as a cause of serotonin syndrome: a case series. Clin Toxicol 2010; 48: 337–341. [DOI] [PubMed] [Google Scholar]
- 8. Jovanović-Cupić V, Martinović Z, Nesić N. Seizures associated with intoxication and abuse of tramadol. Clin Toxicol 2006; 44: 143–146. [DOI] [PubMed] [Google Scholar]
- 9. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43: 879–923. [DOI] [PubMed] [Google Scholar]
- 10. Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 1992; 260: 275–285. [PubMed] [Google Scholar]
- 11. Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, et al. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther 1993; 267: 331–340. [PubMed] [Google Scholar]
- 12. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol 2000; 362: 116–121. [DOI] [PubMed] [Google Scholar]
- 13. Driessen B, Reimann W. Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro. Brit J Pharmacol 1992; 105: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bamigbade TA, Davidson C. Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus. Brit J Anaesth 1997; 79: 352–356. [DOI] [PubMed] [Google Scholar]
- 15. Sagata K, Minami K, Yanagihara N, Shiraishi M, Toyohira Y, Ueno S, et al. Tramadol inhibits norepinephrine transporter function at desipramine-binding sites in cultured bovine adrenal medullary cells. Anesth Analg 2002; 94: 901–906. [DOI] [PubMed] [Google Scholar]
- 16. Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA. Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Brit J Anaesth 1999; 83: 909–915. [DOI] [PubMed] [Google Scholar]
- 17. Driessen B, Reimann W, Giertz H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Brit J Pharmacol 1993; 108: 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu WN, McKown LA, Gauthier AD, Jones WJ, Raffa RB. Metabolism of the analgesic drug, tramadol hydrochloride, in rat and dog. Xenobiotica 2008; 31: 423–441. [DOI] [PubMed] [Google Scholar]
- 19. Wu WN, McKown LA, Liao S. Metabolism of the analgesic drug ULTRAM (tramadol hydrochloride) in humans: API-MS and MS/MS characterization of metabolites. Xenobiotica 2002; 32: 411–425. [DOI] [PubMed] [Google Scholar]
- 20. Lintz W, Erlacin S, Frankus E, Uragg H. Biotransformation of tramadol in man and animal [Article in German]. Arzneimittelforschung 1981; 31: 1932–1943. [PubMed] [Google Scholar]
- 21. Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig 1992; 70: 708–710. [DOI] [PubMed] [Google Scholar]
- 22. Subrahmanyam V, Renwick AB, Walters DG, Young PJ, Price RJ, Tonelli AP, et al. Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos 2001; 29: 1146–1155. [PubMed] [Google Scholar]
- 23. Raffa RB. Tramadol in Japanese population: relative contribution of M1 metabolite as assessed by CYP2D6* 10 genotype. Pharmacol Pharmacy 2012; 3: 337–341. [Google Scholar]
- 24. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Therap 2008; 31: 187–199. [DOI] [PubMed] [Google Scholar]
- 25. van Beusekom CD, Schipper L, Fink-Gremmels J. Cytochrome P450-mediated hepatic metabolism of new fluorescent substrates in cats and dogs. J Vet Pharmacol Therap 2010; 33: 519–527. [DOI] [PubMed] [Google Scholar]
- 26. Thomas DE, Lee JA, Hovda LR. Retrospective evaluation of toxicosis from selective serotonin reuptake inhibitor antidepressants: 313 dogs (2005–2010). J Vet Emerg Crit Care 2012; 22: 674–681. [DOI] [PubMed] [Google Scholar]
- 27. Shipton EA. Tramadol – present and future. Anaesth Intensive Care 2000; 28: 363–374. [DOI] [PubMed] [Google Scholar]
- 28. Scott LJ, Perry CM. Tramadol. Drugs 2000; 60: 139–176. [DOI] [PubMed] [Google Scholar]
- 29. KuKanich B, Papich MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Ther 2004; 27: 239–246. [DOI] [PubMed] [Google Scholar]
- 30. Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther 2008; 31: 52–59. [DOI] [PubMed] [Google Scholar]
- 31. Matthiesen T, Wöhrmann T, Coogan TP, Uragg H. The experimental toxicology of tramadol: an overview. Toxicol Lett 1998; 95: 63–71. [DOI] [PubMed] [Google Scholar]
- 32. Giorgi M, Del Carlo S, Saccomanni G, Łebkowska-Wieruszewska B, Kowalski CJ. Pharmacokinetic and urine profile of tramadol and its major metabolites following oral immediate release capsules administration in dogs. Vet Res Commun 2009; 33: 875–885. [DOI] [PubMed] [Google Scholar]
- 33. Giorgi M, Carlo S, Saccomanni G, Łebkowska-Wieruszewska B, Turini V, Kowalski C. Biopharmaceutical profile of tramadol in the dog. Vet Res Commun 2009; 33: 189–192. [DOI] [PubMed] [Google Scholar]
- 34. McMillan CJ, Livingston A, Clark CR, Dowling PM, Taylor SM, Duke T, et al. Pharmacokinetics of intravenous tramadol in dogs. Can J Vet Res 2008; 72: 325–331. [PMC free article] [PubMed] [Google Scholar]
- 35. Teppema LJ, Nieuwenhuijs D, Olievier CN, Dahan A. Respiratory depression by tramadol in the cat: involvement of opioid receptors. Anesthesiology 2003; 98: 420–427. [DOI] [PubMed] [Google Scholar]
- 36. Tyce GM. Origin and metabolism of serotonin. J Cardiovasc Pharmacol 1990; 16 Suppl 3: S1–7. [PubMed] [Google Scholar]
- 37. Toh CC. Release of 5-hydroxytryptamine (serotonin) from the dog’s gastro-intestinal tract. J Physiol 1954; 126: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerrito F, Raiteri M. Serotonin release is modulated by presynaptic autoreceptors. Eur J Pharmacol 1979; 57: 427–430. [DOI] [PubMed] [Google Scholar]
- 39. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009; 60: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Brit J Anaesth 2005; 95: 434–441. [DOI] [PubMed] [Google Scholar]
- 41. Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28: 205–214. [DOI] [PubMed] [Google Scholar]
- 42. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96: 635–642. [DOI] [PubMed] [Google Scholar]
- 43. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 44. Musshoff F, Madea B. Fatality due to ingestion of tramadol alone. Forensic Sci Int 2001; 116: 197–199. [DOI] [PubMed] [Google Scholar]
- 45. Moore KA, Cina SJ, Jones R, Selby DM, Levine B, Smith ML. Tissue distribution of tramadol and metabolites in an overdose fatality. Am J Forensic Med Pathol 1999; 20: 98–100. [DOI] [PubMed] [Google Scholar]
- 46. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991; 148: 705–713. [DOI] [PubMed] [Google Scholar]
- 47. Pugh CM, Sweeney JT, Bloch CP, Lee JA, Johnson JA, Hovda LR. Selective serotonin reuptake inhibitor (SSRI) toxicosis in cats: 33 cases (2004–2010). J Vet Emerg Crit Care 2013; 23: 565–570. [DOI] [PubMed] [Google Scholar]
- 48. Fitzgerald KT, Bronstein AC. Selective serotonin reuptake inhibitor exposure. Top Companion Anim Med 2013; 28: 13–17. [DOI] [PubMed] [Google Scholar]
- 49. Gwaltney-Brant SM, Albretsen JC. 5-Hydroxy-tryptophan toxicosis in dogs: 21 cases (1989–1999). J Am Vet Med Assoc 2000; 216: 1937–1940. [DOI] [PubMed] [Google Scholar]
- 50. Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Australia 2007; 187: 361–365. [DOI] [PubMed] [Google Scholar]
- 51. Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT) 2A receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890: 23–31. [DOI] [PubMed] [Google Scholar]
- 52. Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13: 100–109. [DOI] [PubMed] [Google Scholar]
- 53. Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43: 155–164. [DOI] [PubMed] [Google Scholar]
- 54. Vahabzadeh M, Moshiri M, Mohammadpour AH, Hosseinzadeh H. Promising effects of intravenous lipid emulsion as an antidote in acute tramadol poisoning. Reg Anesth Pain Med 2013; 38: 425–430. [DOI] [PubMed] [Google Scholar]
- 55. Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther 1988; 247: 47–53. [PubMed] [Google Scholar]
- 56. Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/ naloxone and buprenorphine. Psychopharmacology 2002; 159: 161–166. [DOI] [PubMed] [Google Scholar]
- 57. Marquardt KA. Tramadol exposures reported to statewide poison control system. Ann Pharmacother 2005; 39: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 58. Kitson R, Carr B. Tramadol and severe serotonin syndrome. Anaesthesia 2005; 60: 934–935. [DOI] [PubMed] [Google Scholar]