Abstract
Rationale:
The robust advances in pain management for companion animals underlie the decision of the American Animal Hospital Association (AAHA) and American Association of Feline Practitioners (AAFP) to expand on the information provided in the 2007 AAHA/AAFP Pain Management Guidelines. The 2015 Guidelines summarize and offer a discriminating review of much of this new knowledge.
Relevance:
Pain management is central to veterinary practice, alleviating pain, improving patient outcomes, and enhancing both quality of life and the veterinarian–client–patient relationship. These Guidelines support veterinarians in incorporating pain management into practice, improving patient care.
Approaches:
The management of pain requires a continuum of care that includes anticipation, early intervention, and evaluation of response on an individual patient basis. A team-oriented approach, including the owner, is essential for maximizing the recognition, prevention and treatment of pain in animals.
Evidence base:
The Guidelines include both pharmacologic and non-pharmacologic modalities to manage pain; they are evidence-based insofar as possible and otherwise represent a consensus of expert opinion. Behavioral changes are currently the principal indicator of pain and its course of improvement or progression, and the basis for recently validated pain scores. Post-surgical pain is eminently predictable but a strong body of evidence exists supporting strategies to mitigate adaptive as well as maladaptive forms. Chronic pain is dominated by degenerative joint disease (DJD), which is one of the most significant and under-diagnosed diseases of cats and dogs. DJD is ubiquitous, found in pets of all ages, and inevitably progresses over time; evidence-based strategies for management are established in dogs, and emerging in cats.
Introduction
Pain management is central to veterinary practice, not adjunctive. Alleviating pain is not only a professional obligation (recall the veterinarian’s pledge to ‘the relief of animal pain and suffering’) but also a key contributor to successful case outcomes and enhancement of the veterinarian–client–patient relationship. A commitment to pain management identifies a practice as one that is committed to compassionate care; optimum recovery from illness, injury or surgery; and enhanced quality of life.
These Guidelines continue the trend in all branches of medicine toward evidence-based consensus statements that address key issues in clinical practice. Although not a review article, this compilation is a force multiplier for the busy practitioner, consolidating in a single place current recommendations and insights from experts in pain management. These Guidelines are the product of a collaborative effort by the American Animal Hospital Association (AAHA) and the American Association of Feline Practitioners (AAFP). The recommendations of the Guidelines Task Force are evidence based insofar as possible and otherwise represent a consensus of expert opinion.
These Guidelines are designed to expand on the information contained in the 2007 AAHA/AAFP Pain Management Guidelines for Dogs and Cats.1,2 The 2015 Guidelines differ from the earlier version in several ways. The first sections are general concepts designed to set the stage for the remaining, more specific content. The 2015 Guidelines also discuss the importance of an integrated approach to managing pain that does not rely strictly on analgesic drugs. Because pain assessment in animals has become more scientifically grounded in recent years, various clinically validated instruments for scoring pain in both dogs and cats are described. The extensive list of published references includes numerous studies published within the past 3 years, reflecting the rapid pace of advances in managing pain for companion animals. The 2015 Guidelines summarize and offer a discriminating review of much of this new knowledge.

Types of pain
All types of tissue injury can be generators of pain. Occasionally, pain may occur in the absence of those causative factors. Understanding the mechanisms of pain is the key to its successful prevention and treatment. The pain response is unique to each individual and involves two components:
The sensory component is nociception, which is the neural processing of noxious stimuli;
The affective component is pain perception, which is the unpleasant sensory and emotional experience associated with either actual or potential tissue damage.
Pain is the endpoint of nociceptive input and can only occur in a conscious animal. However, there is also involvement of autonomic pathways and deeper centers of the brain involved with emotion and memory. Hence, pain is a multidimensional experience; it is not just what you feel but also how it makes you feel. 3
Acute pain has been defined as pain that exists during the expected time of inflammation and healing after injury (up to 3 months), and chronic pain is defined as that which exists beyond the expected duration associated with acute pain. Therapy should be focused on the underlying cause of pain (nociceptive, inflammatory or pathological), rather than on arbitrary labels based on duration. 4
Nociceptive pain occurs when peripheral neural receptors are activated by noxious stimuli (eg, surgical incisions, trauma, heat or cold).
Inflammatory pain results gradually from activation of the immune system in response to injury or infection.
Pathological pain, also called maladaptive pain, occurs when pain is amplified and sustained by molecular, cellular and microanatomic changes, collectively termed peripheral and central hypersensitization.
Pathological pain is characterized by hyperalgesia (exaggerated response to noxious stimulus), allodynia (painful response to non-noxious stimuli, such as touch or pressure), expansion of the painful field beyond its original boundaries, and pain protracted beyond the expected time of inflammation and healing. Under some conditions, genomic, phenotypic changes occur that create the condition known as neuropathic pain, whereby pain can be considered a disease of the central nervous system. Those changes are not necessarily chronologic. Maladaptive pain, or the risk for it, can occur within a matter of minutes of certain acute pain conditions (eg, nerve injury, severe tissue trauma, or presence of pre-existing inflammation).
It’s not just about drugs
Classic veterinary medical education places a strong emphasis on treatment of disease through pharmacology and surgery, the esoteric skills that are the domain of the trained clinician. Increasingly, evidence-based data and empirical experience justify a strong role for non-pharmacologic modalities for pain management. A number of those should be considered mainstream options and an integral part of a balanced, individualized treatment plan.
Examples of non-pharmacologic treatments supported by strong evidence include, but are not limited to, cold compression, weight optimization and therapeutic exercise. Treatment options gaining increasing acceptance include acupuncture, physical rehabilitation, myofascial trigger point therapy and therapeutic laser, among other modalities which are discussed later in these Guidelines. In addition, non-pharmacologic adjunctive treatment includes an appreciation of improved nursing care, gentle handling, care-giver involvement, improved home environment, and hospice care. Those methods have the critical advantages of increased care-giver–clinician interaction and a strengthening of the human–pet bond. That shared responsibility promotes a team approach and leads to a more complete and rational basis for pain management decisions. 5

Recognition and assessment of pain
The patient’s behavior is key
Because animals are non-verbal and cannot self-report the presence of pain, the burden of pain assumption, recognition and assessment lies with veterinary professionals. It is now accepted that the most accurate method for evaluating pain in animals is not by physiological parameters but by observations of behavior. Pain assessment should be a routine component of every physical examination, and a pain score is considered the ‘fourth vital sign’, after temperature, pulse and respiration.1,2,6 Obtaining a thorough patient history from the owner can help determine abnormal behavior patterns that may be pain related. Pet owners should be educated in observing any problematic behavioral changes in their pet and to contact their veterinarian in such cases.
Pet owners and practitioners should have an awareness of behavior types that are relevant to pain assessment. Those include the animal’s ability to maintain normal behavior, loss of normal behavior, and development of new behaviors that emerge either as an adaption to pain or a response to pain relief (Figure 2). Because behavioral signs of pain are often overlooked or mistaken for other problems, the healthcare team must be vigilant in recognizing those anomalies in the total patient assessment.
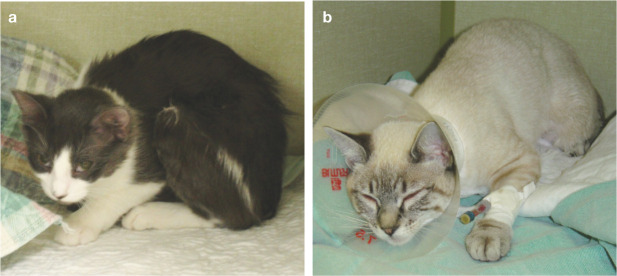
Image 2.
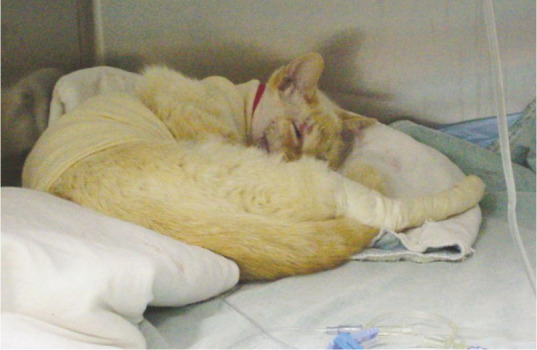
Cats normally sleep in a curled-up position, as seen in this patient, when provided with adequate analgesia. Courtesy of Sheilah Robertson
Pain scoring tools
Although there is currently no gold standard method for assessing pain in dogs and cats, the Guidelines Task Force strongly recommends utilizing pain scoring tools both for acute and chronic pain. It should be noted that those tools have varying degrees of validation, acute and chronic pain scales are not interchangeable, and canine and feline scales are not interchangeable. The use of pain scoring tools can decrease subjectivity and bias by observers, resulting in more effective pain management, which ultimately leads to better patient care.
Acute pain: characteristics and causes
Acute pain involves both nociceptive and inflammatory components and can be caused by trauma, surgery and medical conditions or diseases. These Guidelines will focus on recognition, prevention and treatment of postsurgical pain.
Multifactorial clinical measurement instruments for acute postsurgical pain
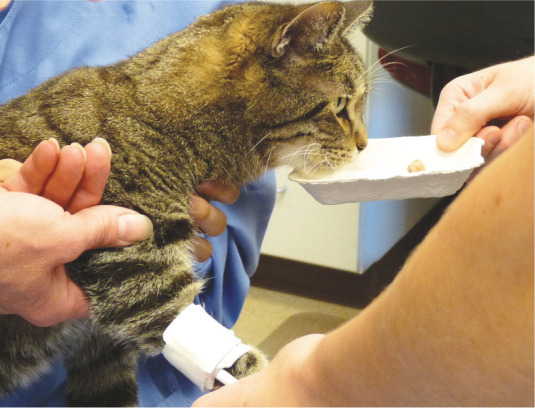
For dogs, a validated, widely used, multifactorial clinical measurement instrument (CMI) for acute pain is the Glasgow Short Form Composite Measure Pain Score. The 4AVet is another composite measure pain score for dogs, reportedly with more interobserver variability than the Glasgow short form, but less biased by sedation.7,8 Simple, online, practice-friendly numerical rating scales (0 to 4) for acute canine and feline pain have been developed (but not yet validated) by Colorado State University. In cats, a currently validated assessment tool is the UNESP-Botucatu Multidimensional Composite Pain Scale.9,10 That scale and video examples of how it is applied in clinical practice can be accessed online, and a description of Colorado State’s acute pain scales are included in Table 1.
Table 1.
Acute postoperative pain scales
| Resource | Internet address | Content |
|---|---|---|
| Colorado State University Canine Acute Pain Scale | www.csuanimalcancercenter.org/assets/files/csu_acute_pain_scale_canine.pdf | • Psychological and behavioral indicators of pain • Response to palpation |
| Colorado State University Feline Acute Pain Scale | csuanimalcancercenter.org/assets/files/csu_acute_pain_scale_feline.pdf | Same as above |
| University of Glasgow Short Form Composite Measure Pain Score | www.newmetrica.com/cmps | • Clinical decision-making tool for dogs in acute pain • Indicator of analgesic requirement • Includes 30 descriptors and six behavioral indicators of pain |
| UNESP-Botucatu Multidimensional Composite Pain Scale | www.animalpain.com.br/en-us/avaliacao-da-dor-em-gatos.php | • Assesses postoperative pain in cats • Includes 10 indicators of pain ranked numerically |
Practical approach to postoperative pain assessment
Validated CMIs are the foundation of rational pain assessment. Those assessment tools provide a simplified approach that encourages regular use by all healthcare members and are based on the following features:
Observing the patient without interaction (ie, the patient’s orientation in the cage, posture, movement, facial expression, activity level and attitude; Images 1 and 2).
Observing the patient while interacting with a care-giver (eg, what occurs when the cage door is opened or an animal is coaxed to move).
Observing the patient’s response to palpation of the surgical site (Image 3).
Assigning a numerical score using a dynamic interactive visual analog scale (eg, from 0 for no pain to 10 for the worst possible pain for that procedure).
Image 1.
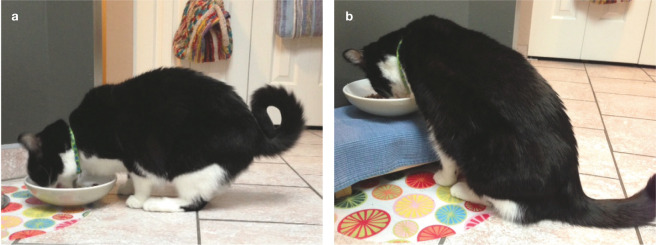
(a,b) Signs of acute pain include squinting, and a hunched or tucked-up position instead of sleeping in a normal curled-up position (see Image 2). Images courtesy of Sheilah Robertson
Image 3.
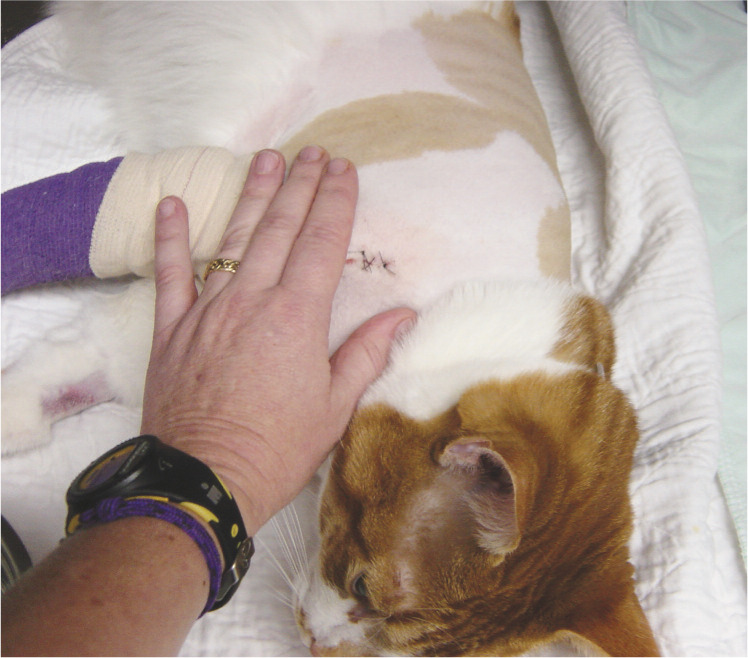
Following assessment from a distance, palpation of the surgical site is performed to further assess acute pain. Courtesy of Sheilah Robertson
The re-evaluation interval will depend on the procedure, expected duration of the chosen intervention, and previous pain score. Variability by different observers can be minimized by having the same team member assess the patient throughout the evaluation period. Ideally, the individual patient’s normal temperament should be known for the purposes of comparison with postsurgical appearance and behavior.
Chronic pain: characteristics and causes
Chronic pain is usually described as either pain that persists beyond the normal healing time or pain that persists in conditions where healing has not or will not occur. In some cases, pain signaling persists in the absence of gross tissue pathology.
The following basic principles are relevant to chronic pain in companion animals:
- Pet owners may not appreciate their pet’s behavior as being an indicator of chronic pain; however, what they might see is increasingly diminished function and mobility that indicate progressive disability. Examples include:
Under-recognized and undermanaged chronic pain can result in premature euthanasia. 11 Conversely, proper recognition and management of chronic pain can be as life preserving as any other medical treatment in veterinary medicine.
Degenerative joint disease (DJD) is the inclusive terminology that includes osteoarthritis (OA). Although DJD and OA are often used interchangeably in the literature and in practice, the broader term, DJD, will be used throughout these Guidelines.
Image 4.
Lack of normal behaviors, such as reduced grooming, is commonly seen in painful cats. Image iStock/jvoisey
Image 5.

Cats may start to perform abnormal behaviors secondary to pain, such as defecating outside of the litter box, either because the cat cannot get downstairs to the box or it cannot jump into the box. Courtesy of Sheilah Robertson
Multifactorial clinical measurement instruments for chronic pain
Observation or reports (eg, in a pre-examination questionnaire) of behavioral changes or abnormalities is the first consideration in recognizing and assessing pain. Thereafter, several standardized, multifactorial CMIs for chronic pain are available to veterinarians, as summarized in Table 2. Such CMIs are chronic pain indices that primarily utilize pet owner observations and input. Ideally, patients with chronic pain should be evaluated with one of the multifactorial CMIs.
Table 2.
Multifactorial CMIs for chronic pain assessment in veterinary medicine
| Helsinki Chronic Pain Index (HCPI) |
| Canine Brief Pain Inventory (CBPI) |
| Cincinnati Orthopedic Disability Index (CODI) |
| Health-Related Quality of Life (HRQL) |
| Liverpool Osteoarthritis in Dogs (LOAD) |
| Feline Musculoskeletal Pain Index (FMPI) |
Pharmacologic intervention of pain
Effective pain management generally involves a balanced or multimodal strategy using several classes of pain-modifying medications. The rationale behind this approach is that it addresses targeting multiple sites in pain pathways, potentially allowing lower doses of each drug and minimizing the potential for side effects associated with any single drug. The choice of medication should be based on anticipated pain levels and individual patient needs. Anticipatory analgesia provided prior to pain onset is more effective than analgesia provided once pain has occurred, contributing to both a dose- and anesthetic-sparing effect.
Opioids
Opioids are the most effective drug class for managing acute pain and can play a role in managing chronic pain. An improved understanding of neuropharmacology and the development of novel formulations of opioids make it incumbent on veterinarians to remain familiar with their modes of action; the various subtypes within this drug class; and the prevention, recognition and treatment of adverse effects.
While a complete discussion of opioids is beyond the scope of these Guidelines, the Task Force makes the following recommendations for using this class of drugs in dogs and cats:
Opioids should be used as a routine preoperative medicant, preferentially in combination with a tranquilizer/sedative (eg, acepromazine, midazolam, diazepam or α-2 adrenergic agonist such as dexmedetomidine), when the patient’s condition warrants their use.
Full µ agonists elicit greater and more predictable analgesia than partial µ agonists or κ agonists. In dogs, the µ antagonist/κ agonist butorphanol, in particular, appears to provide limited somatic analgesia and a very short duration of visceral analgesia.12,13
In a comparison study, buprenorphine administered before surgery and during wound closure provided adequate analgesia for 6 h following ovariohysterectomy in cats, whereas butorphanol did not. 14
In cats, the subcutaneous (SC) route of opioid administration is not recommended. Intramuscular (IM) and intravenous (IV) routes are preferred both pre- and postoperatively. 15 The oral transmucosal or buccal route of administration for buprenorphine may have clinical efficacy as well.16,17
The individual effect of any opioid, including duration, may vary widely from patient to patient. Postoperative re-evaluation should be made frequently to determine ongoing opioid requirements.
- For a patient undergoing major surgery, whereby ongoing opioid administration can be anticipated, the clinician may choose from the following strategies:
- – Periodic readministration of parenteral opioids.
- – Constant or variable rate infusion. Calculators can be found online.
- – Long-acting formulations and technologies. For dogs there is a Food and Drug Administration (FDA) approved transdermal fentanyl product (Recuvyra; Elanco). Given this canine fentanyl product on the market, the Task Force discourages the use of human commercial fentanyl patches in dogs due to highly variable pharmacokinetics, and risk of either accidental or purposeful human exposure, with potential liability for extralabel use. There is not an expert consensus regarding the utility of fentanyl patches in cats. The FDA has more recently approved a concentrated injectable buprenorphine product for cats (Simbadol; Abbott), which has been formulated to provide a 24 h duration of action when administered as directed.
- – Oral opioids. Dogs exhibit a robust first-pass effect of oral opioids. No clinical studies document efficacy, but pharmacokinetics of codeine and hydrocodone suggest possible utility. 18 No comparable studies exist for cats.
Opioids are synergistic with α-2 adrenergic agonists, allowing them to be used in low-dose combinations, either with or without ketamine, to great effect for both sedation and analgesia.
Opioids play a significant role in human medicine for the treatment of chronic pain and may play an underappreciated role in dogs and cats as well, especially for cancer-related pain and in palliative care patients. That said, clinicians must be vigilant with regard to long-term adverse effects such as constipation, drug tolerance and the potential for diversion by clients.
Non-steroidal anti-inflammatory drugs
The majority of conditions that cause pain have an inflammatory component. Non-steroidal anti-inflammatory drugs (NSAIDs) are a mainstay for management of chronic pain as well as for perioperative use. NSAIDs should be used for their central and peripheral effects in both dogs and cats after consideration of risk factors. There is no indication that any one of the veterinary-approved NSAIDs is associated with any greater or lesser incidence or prevalence of adverse events. 19 Canine and feline veterinary-approved NSAIDs have demonstrated acceptable safety profiles, which is in contrast to non-approved NSAIDs such as aspirin, ibuprofen, naproxen and meloxicam for human use.20–22 Long-term use of low-dose meloxicam is approved in cats in many countries other than the USA.
Adverse events related to NSAID use in dogs and cats can be minimized by appropriate use, as outlined in Figure 3. Although the overall incidence and prevalence of NSAID-related toxicity is unknown, it does appear to be very low relative to the number of doses administered. 20
Of the adverse events associated with NSAIDs, gastrointestinal (GI) toxicity is the most common. The GI clinical signs associated with NSAID toxicity in dogs include vomiting, diarrhea and inappetence.20,23–25 In cats, inappetence appears to be the most common adverse event. Although unlikely, it is possible for erosions and ulcers to be silent and occur prior to any clinical signs.23,26 Studies indicate that NSAIDs that spare cyclooxygenase (COX)-1 produce a lower frequency of GI lesions, although the more highly COX-2 selective inhibitors may actually produce more adverse events when underlying gastric damage is already present.19,27
The leading risk factors for NSAID-associated GI perforations are incorrect dosing, concurrent use with other NSAIDs or corticosteroids, and continued use despite GI signs or anorexia.20,24 Signs of GI toxicity usually emerge within 2–4 weeks but can occur at any point during administration.28,29 It is critical that veterinarians communicate NSAID toxicity risk factors to pet owners (eg, providing client information that describes potential side effects, including the commercial circulars provided by drug manufacturers and instruction on when to stop medication and contact a veterinarian).

Another important side effect associated with NSAIDs is nephrotoxicity. When administered before anesthesia in healthy dogs with controlled modest hypotension, no adverse effect on renal function was detected.30,31 However, because some dogs in those studies did develop changes in renal parameters, the importance of maintaining a normotensive state during anesthesia is considered paramount when utilizing preoperative NSAIDs. Preoperative administration in dogs is superior in efficacy to postoperative use, consistent with results of multiple studies performed in humans. 32 Similar studies have not been conducted in cats undergoing anesthesia, but one feline study revealed no alteration in glomerular filtration rate measured by iohexol clearance after 5 days of oral meloxicam. 27 If IV access is not possible and normotension cannot be achieved with certainty, the Task Force recommends limiting the use of NSAIDs to postsurgical administration.
Idiosyncratic hepatocellular necrosis has been reported with various NSAIDs but remains exceedingly rare, at only 1.4 cases/10,000 dogs (0.052%), usually occurring between 2 and 4 weeks after starting treatment. Elevated liver enzymes are not a risk factor. 19 Idiosyncratic hepatocellular necrosis is not a true toxicosis but rather an intrinsic, heritable reaction to the molecule being administered. 20
Highly COX-2 selective NSAIDs have caused delayed bone healing in rabbit and rodent models, and one study in dogs demonstrated delayed healing of experimental tibial osteotomies following long-term NSAID use. 33 That particular study may not be a clinically relevant model, and another study reported that normal tissue healing is rapidly restored once the NSAID is withdrawn. 34 Further, of 299 dogs receiving deracoxib, carprofen and firocoxib in the FDA-approval process, none were reported to have delayed fracture healing or non-union fractures. Finally, no clinically significant bleeding dyscrasias have been reported with the use of veterinary NSAIDs. 20
Local anesthetics
This is the only class of drug that renders complete analgesia. The totality of evidence in human and animal studies reveals the predictable analgesic and anesthetic drug-sparing effects of local anesthetics. In addition, local anesthetics are reported to be antimicrobial and immunomodulating, and can diminish postoperative maladaptive pain states. They do not appear to delay tissue healing. 35 Local anesthetics can be administered either directly at a simple incision site or at a specific nerve to provide analgesia to a large region (or area). A discussion of the many locoregional blocks that can be utilized in dogs and cats is beyond the scope of these Guidelines, but can be found in several readily accessible resources, and most of those blocks can be readily learned by clinicians.
Local anesthetics are considered safe, with adverse events generally limited to very high doses or inadvertent IV administration (bupivacaine especially). The Task Force supports the International Veterinary Academy of Pain Management position that, because of their safety and significant benefit, local anesthetics should be utilized, insofar as possible, with every surgical procedure.
α-2 Adrenergic agonists
α-2 Adrenergic receptors are located with opioid receptors. Thus, used together, opioids and α-2 adrenergic agonists are highly synergistic for sedation and analgesia. α-2 Agonists have a versatile dosing profile. That allows low- and even micro-doses in combination with opioids to be clinically useful and minimizes the cardiovascular effects. Clinicians should be mindful that cardiovascular side effects occur even with very low doses of α-2 adrenergic agonists, that lower doses will have a shorter duration of effect, and that analgesic effects have a shorter duration than the sedative effects.
Ketamine
Ketamine exerts a pain-modifying effect via its N-methyl-D-aspartate receptor antagonist actions. Subanesthetic ketamine constant rate infusion (CRI) in humans prevents pain and has antihyperalgesic and antiallodynic effects.36,37 Studies appear to support a similar clinical effect in dogs,38–40 although the analgesic effect of ketamine has not yet been studied in a feline surgical model. The International Veterinary Academy of Pain Management has adopted a position that the pain-modifying effects and safety profile of subanesthetic doses of ketamine warrant its use as part of a multimodal approach to transoperative pain management, especially in patients with risk factors that may predispose them to either exaggerated or maladaptive pain states.
Systemic lidocaine
There is strong evidence of the safety and beneficial effects of IV lidocaine on pain after abdominal surgery (although not for other surgeries eliciting somatic pain) in humans and possibly in horses, including both analgesia and return of bowel function. 41 IV lidocaine is anesthetic-sparing in dogs and cats, but current evidence for a pain-modifying effect in these species remains inconclusive. 42 Some investigators discourage the use of IV lidocaine in cats due to negative cardiovascular effects, but successful use in clinical practice has been anecdotally reported. 43 Various formulations for a combination of morphine, lidocaine and ketamine CRIs have been described in dogs. 44
Tramadol
In contrast to humans, tramadol in dogs has a very short half-life (1.7 h) and negligible amounts of the opioid M1 metabolite are produced.45–48 Pharmacodynamic studies demonstrate the anesthestic-sparing and pain-modifying effect of parenteral tramadol in dogs.49–53 Convincing evidence for a pain-modifying effect of oral tramadol, however, remains elusive, and already low plasma levels quickly diminish with sequential administration.54–57 One small study of oral tramadol did report a statistically significant increase of mechanical threshold levels in dogs, but only at the 5 and 6 h time points. 48
In contrast to dogs, cats do produce the µ-agonist M1 metabolite. A pain-modifying effect has been demonstrated in both a thermal threshold and clinical surgical model.58,59 There is one case series involving the use of oral tramadol in a flavored compounded form (the drug is otherwise quite bitter). Dose titration, toxicity and safety data are currently lacking in both dogs and cats. 60
Gabapentin
Gabapentin is an anticonvulsant with analgesic properties that may be primarily derived by down-regulating calcium channels. 61 Because of its efficacy and tolerability, gabapentin is widely used in humans with neuropathic and other maladaptive pain conditions. 62 Along with published clinical case reports in animals, the data suggest a strong rationale for using gabapentin in dogs and cats with similar conditions.63,64 One canine study suggested a disease-modifying effect in experimental DJD, but clinical studies are lacking. 65 In cats, one unpublished study demonstrated a benefit of gabapentin in naturally occurring DJD (E Troncy 2013), and one case series of chronic musculoskeletal pain has also been published. 66
There is encouraging evidence to support the use of gabapentin for postsurgical pain in humans,67–72 but not yet in dogs and cats. An 8–12 h dosing interval has been suggested based on one publication. 73 The primary adverse effect in dogs appears to be somnolence (also the case in humans), which usually resolves with patient acclimation over several days, allowing for a tapering-up schedule.
Amantadine
Amantadine exerts a pain-modifying effect as an N-methyl-D-aspartate receptor antagonist and remains a drug of interest for chronic pain (but not specifically for DJD) in humans. 74 One study demonstrated utility as an adjunct to NSAIDs in dogs with refractory DJD, 75 and there is one case report utilizing amantadine to treat neuropathic pain in a dog. 76 Toxicity and pharmacokinetic studies have been performed in humans and cats,77,78 but not in dogs.
Tricyclic antidepressants
As a class, tricyclic antidepressants (TCAs) are the most effective medications for selective neuropathic pain conditions in humans. 79 In dogs, there exists only a single case report where amitriptyline was used for neuropathic musculoskeletal pain. 80
Selective serotonin (norepinephrine) reuptake inhibitors
These compounds exert their effect by increasing serotonin with or without norepinephrine in the synaptic cleft. At least one selective serotonin (norepinephrine) reuptake inhibitor (SS[N]RI), duloxetine, has a chronic pain label indication in humans. In dogs, bioavailability is poor and clinical efficacy is lacking. 81
At this point in the Guidelines, the Task Force wants to emphasize the following:

Acetaminophen
Acetaminophen is contraindicated in cats. In dogs, several early studies revealed a pain-modifying effect in orthopedic surgery, and pharmacokinetic data have been reported.82–84 The literature does not appear to indicate that acetaminophen has a proclivity towards hepatotoxicity in dogs.
Maropitant
Maropitant is a central antiemetic indicated for the treatment of acute canine and feline vomiting, which is often a postsurgical sequela and a contributor to the pain burden. Maropitant works through a blockade of substance-P binding to the neurokinin-1 receptor, which is involved in pain processing. The true pain-modifying effect of maropitant in dogs remains uncertain despite canine studies revealing an anesthetic-sparing effect and a non-inferior effect to morphine in an ovariohysterectomy model.85,86
Bisphosphonates
Administered by IV infusion, this class of drug exerts antiosteoclast activity and can contribute to pain relief in dogs with bone cancer. 87
Corticosteroids
Corticosteroids are not primarily analgesic drugs, but may exert a pain-modifying effect by reducing inflammation. Their utility as an analgesic therapy in dogs and cats has not been reported.88–91
Polysulfated glycosaminoglycans
A parenterally administered polysulfated glycosaminoglycan (PSGAG) product has regulatory approval for the control of signs associated with non-infectious degenerative and/or traumatic arthritis of canine synovial joints. Independent studies support PSGAGs as safe and effective chondroprotectants with possible disease-modifying effects.92–94 The bioavailability and distribution of PSGAGs to inflamed joints in cats has been demonstrated with extra-label SC administration. 95
Nutraceuticals and other oral supplements
Oral nutritional supplements represent a wide spectrum of compounds either as single agents or in combinations. Anecdotal evidence for a pain-modifying effect of those products remains mixed. If nutraceuticals and/or herbal supplements are made part of a treatment plan, the Task Force suggests mindfulness towards product quality control; awareness of the potential for drug interactions with other medications (eg, some over-the-counter joint products and herbal mixtures contain aspirin and some may contain herbs such as St John’s wort that interfere with serotonin release or reuptake); and avoidance of ingredients derived from endangered species.
In the future, evidence for the pain-modifying effect of cannabinoids and/or their commercial drug derivatives may become evident.
Non-pharmacologic modalities for pain management
Weight optimization
Adipose tissue secretes a mixture of cytokines that circulate throughout the body, contributing to the pathology of many diseases, including DJD, and to the hypersensitization process in general. Either maintaining or regaining a lean body condition score is central to the treatment of chronic pain.
Acupuncture
The Guidelines Task Force holds that acupuncture offers a compelling and safe method for pain management in veterinary patients and should be strongly considered as a part of multimodal pain management plans. 96 It is a minimally invasive treatment that, for most animals, is not uncomfortable, often pleasant, and can be used either alone or in addition to other pain treatment modalities (Image 6). Acupuncture has been recognized by the National Institutes of Health since 1998 as having applications in human medicine, especially pain management. There is a solid and still growing body of evidence for the use of acupuncture for the treatment of pain in veterinary medicine to the extent that it is now an accepted treatment modality for painful animals.97–101
Image 6.
Note how comfortable cats usually are for acupuncture therapy. Courtesy of Sheilah Robertson
Physical rehabilitation
Combined modality therapy to decrease pain and restore function is now considered an essential approach for musculoskeletal injury and postsurgical recovery. 102 In the treatment of chronic disease, such as DJD or conformational abnormalities, rehabilitation should be considered an important component of an overall long-term treatment strategy. 103
The foundation of rehabilitation is therapeutic exercise that aims to restore musculoskeletal strength and function, endurance and proprioception, and reduce pain. Most commonly it involves exercise and manual therapy, including joint mobilizations, massage and myofascial release. Energy-based modalities are also often employed, including neuromuscular electrical stimulation, transcutaneous electrical nerve stimulation, cryotherapy with and without compression, therapeutic ultrasound, therapeutic laser and extracorporeal shockwave therapy.104–108
Myofascial pain syndrome (MPS) is increasingly recognized as an important comorbidity in many chronic pain cases in animals. MPS is acknowledged for the important role it plays in the pathology of DJD, repetitive strain injuries in performance dogs, or as a sequela to orthopedic surgery. The pathophysiology of myofascial pain is a complex syndrome involving motor, sensory and autonomic nerve components that is beyond the scope of these Guidelines, but is well described elsewhere. 109 Treatment of MPS is often essential to regain full function of the affected limb, regardless of the underlying cause. 110
Nutrition management
In the overweight patient, the prime nutritional emphasis should be achieving a leaner body condition. Weight control diets fortified with omega-3 fatty acids have been shown to be effective at reducing signs associated with both canine and feline DJD.111–114
Thermal modification
In acute injury, including surgical areas, cold compression has a demonstrable benefit in reducing pain and inflammation, and promoting return to function. 115 In the case of chronic injury, heat can improve comfort and function through a variety of mechanisms. 116
Environmental modifications
There is strong evidence that the stress of hospitalization inhibits normal behaviors in animals, including eating, grooming, sleeping and elimination. 117 Fear, anxiety, stress and distress lead to hyperalgesia in both humans and animals.118–121 Strategies to mitigate hyperalgesia, therefore, include providing bedding, blankets or clothing from home with familiar scents; allowing visitation of hospitalized pets; separating the dogs from the cats; placing cages so that animals do not see each other; using species-specific synthetic pheromones; and proper handling, especially during procedures (see box on page 262).122–126
In patients with DJD, throw rugs and ramps will improve mobility and abilities at home (Image 7).
Image 7.
Environmental management is needed in addition to medical management for cats with DJD. Providing a stool (a,b), ramp or step(s) (c) allows the cat to reach favored areas. Pet steps are commercially available or can be built; those pictured in (c) were built by Dr Robert Wright of The Cat Doctor in Portland ME, USA. Images courtesy of Deb Givin

Chiropractic care
The Guidelines Task Force has not found sufficient, reliable, non-contradictory evidence for the use of chiropractic care for pain management in veterinary medicine at this time. That said, chiropractic care has many well-defined applications in human medicine that have been supported through reliable research.
Homeopathy
Incontrovertible evidence that homeopathy is effective in either human or veterinary medicine for the treatment of pain is lacking. Sole reliance on homeopathy to treat a painful condition is, in essence, withholding pain treatment. Thus, this Task Force discourages the use of homeopathy for the treatment of pain.

Managing surgical pain
The Task Force suggests that pain management for dogs and cats undergoing a surgical procedure includes the following:
A preoperative opioid plus a tranquilizer/sedative (eg, acepromazine and midazolam or diazepam and dexmedetomidine).
Administration of an NSAID either pre- or postoperatively based on patient risk factors and clinician preference.
A local anesthetic.
For patients undergoing procedures with risk factors for more severe, protracted or maladaptive postoperative pain states, the following interventions or drugs should be strongly considered:
Cold compression.
α-2 Adrenergic agonist.
Ketamine CRI.
Lidocaine CRI.
Gabapentin.
Epidural anesthetic(s).
Managing pain associated with DJD
Overview of DJD in companion animals
DJD, including OA, is one of the most significant and underdiagnosed diseases of cats and dogs. DJD is clinically relevant because of its overall prevalence and universal incidence in older animals. Whereas diagnostic approaches for canine DJD are well established, the best tools for diagnosis of feline DJD are still being developed.
The pain treatment continuum for DJD begins with the onset of disease, which usually starts at a very young age in dogs (eg, conformational etiology) and cats (unknown etiology), and persists throughout the animal’s life. Perhaps more so than any other pain condition, the management of DJD benefits from an integration of both pharmacologic and non-pharmacologic treatments. Once a diagnosis is made, treatment goals, expectations and outcome measures should be considered prior to initiating any treatment. The care-giver is an essential part of any treatment program and should be considered a part of the team.
Canine DJD: therapeutic considerations
Because early intervention can delay the onset and severity of DJD, the Task Force emphasizes that chief among all preventive and treatment modalities for canine DJD is weight optimization. Maintaining a lean body condition from an early age demonstrably minimizes DJD development in predisposed breeds.129–131 In overweight patients, weight loss alone, even a modest 6.1–8.85%, improves clinical signs of DJD. 132
There is strong evidence to support the use of NSAIDs for the management of DJD pain in dogs. Data on the safety and efficacy of long-term NSAID administration in dogs appear to suggest an overall benefit from this modality for a sustained period of time at labeled doses and intervals, provided the patient does not have additional risk factors. 22 NSAID therapy should be tailored to suit every individual patient’s needs. Veterinary NSAIDs studied for chronic use (between 28 days and 1 year) demonstrated satisfactory safety profiles in dogs, with 95–97% of dogs able to receive their NSAID at labeled doses and intervals without adverse effects for the duration of the study. 22 There is currently no evidence that a higher risk for NSAID-induced adverse effects exists as the duration of treatment increases. Some dogs may require several weeks of NSAID treatment before clinical improvement is noted. 133
In addition to NSAIDs, there are other options to consider. First, PSGAGs are more likely to have a beneficial effect when given early in the disease process. 92 As mentioned earlier, an FDA-approved product with established efficacy and safety is available. Secondly, data supporting analgesia and functional improvement from therapeutic exercise are well established in humans and are beginning to accrue in dogs. 134 Thirdly, a systematic review analyzing data from several placebo-controlled blinded studies affirmed the utility of diets rich in eicosapentaenoic acid for dogs with DJD. 135 Various other strategies can be (and often are) employed, but their supporting evidence is weak, conflicting or altogether lacking at present.
Feline DJD: therapeutic considerations
Until the early 2000s, little attention was paid to DJD in cats; however, estimates from published studies suggest that 40–92% of all cats may have some clinical signs associated with DJD. 136 Feline DJD is now recognized as a serious welfare problem, particularly in older cats, which is a rapidly growing demographic.136,137 The most frequently affected joints appear to be the hip, stifle, tarsus, elbow, thoracolumbar and lumbosacral area. 137 For each 1 year increase in a cat’s age, the expected total DJD score increases by an estimated 13.6%. Moreover, there is a dramatic increase in the prevalence and burden of DJD at about 10 years of age. A diagnosis of feline DJD is based on a thorough history reflecting changes in behavior and lifestyle, physical exam findings (Image 8), and possible radiographic evidence.
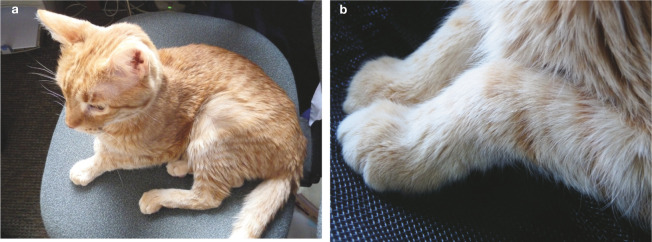
Image 8.
(a) Muscle wasting over affected legs is a common sign of DJD, as seen in this orange tabby, (b) Close-up of the front limbs of the cat, showing deformities secondary to DJD. Images courtesy of Ilona Rodan
Behavioral changes are the most common signs of DJD-associated pain in cats. Feline DJD is usually bilateral – so, although cats rarely limp, they are likely to be stiff, have a less fluid gait, become less active (especially at night) and exhibit decreased jumping frequency or jumping height. 138 Owners often note that their cats are very stiff going up or down stairs. The cat may resist handling, petting, or stroking of the back or limbs. Showing the owner a list of the common pain-related behaviors caused by DJD is helpful in making the diagnosis (Table 3).
Table 3.
Signs of degenerative joint disease (DJD) in cats
| Behavioral component | Indicators of DJD |
|---|---|
| Interaction with others | Withdrawal, hiding, increased ‘clinginess’, irritability when touched, aggression toward other cats or humans |
| Appetite | Declines but cat continues to eat |
| Posture | Hunched, head lowered, sitting or lying abnormally, squinting, facial expression indicating discomfort |
| Grooming | Declines, matting of fur, over-grooming of painful area |
| Litter box use | Decline in bowel movements, house soiling, inability to get into box |
| Play | Reduced overall, reduced jumping |
| Vocalization | Increased but decreased greeting and other pleasant vocalizations, hissing if touched on painful area, squinting if acute pain |
| Mobility | Not jumping as often or as high, hesitant to jump, difficulty going up or down stairs, stiffness, less active, difficulty getting into or out of litter box, sleeping in more easily accessible locations |
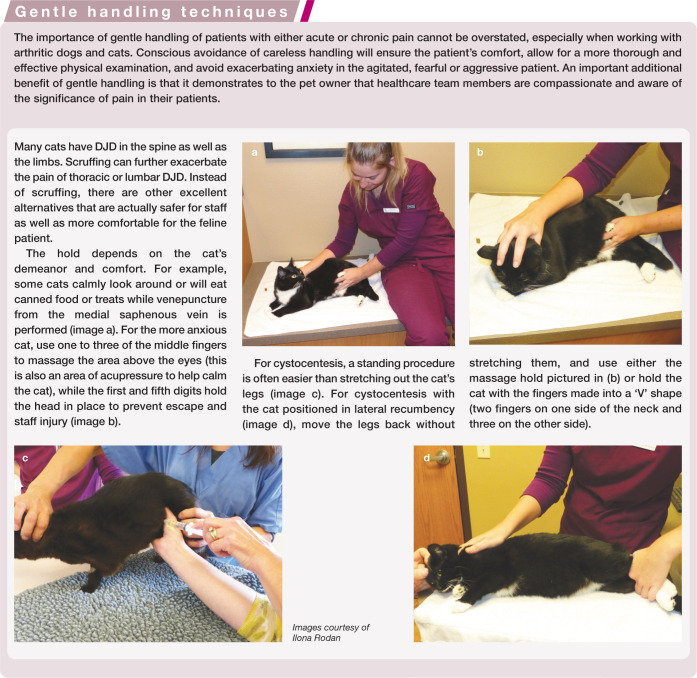
It should be assumed that a senior cat has some DJD, and every effort should be made to incorporate gentle handling techniques (Images 9 and 10) and padded surfaces for the cat to lie on during the exam. A positive clinical response to analgesics will also indicate the presence of DJD. The first validated clinical metrology tool for the evaluation of feline musculoskeletal pain has now been produced and is available for use in practices (see Table 2).139,140
Image 9.
Since DJD is ubiquitous, can occur in cats of all ages, and can be difficult to detect, every effort should be made to incorporate gentle handling techniques for all cats. For example, gently pushing the front leg forward from behind the humerus, instead of pulling on the front foot, will help to prevent elbow pain. Courtesy of Ilona Rodan
Image 10.
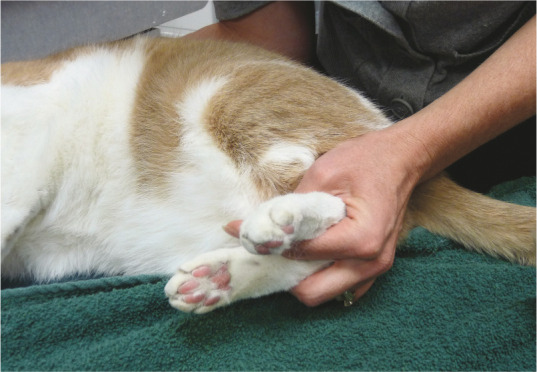
Instead of stretching and pulling hind legs back tightly, gently place one or more fingers between the hind feet and hold without stretching. This prevents exacerbation of lumbar, lumbosacral, knee and hip pain. Courtesy of Ilona Rodan
NSAIDs are the mainstay of pharmacologic treatment for DJD in other species, and there is considerable evidence to support their effectiveness in cats as well.141–145 In the USA, however, NSAIDs are not approved for long-term use in cats, and the potential side effects often deter many clinicians from routinely using them in cats. Renal toxicity is always a consideration with the use of NSAIDs; however, one retrospective study found that long-term use of meloxicam did not reduce the lifespan of cats >7 years of age with pre-existing, stable chronic kidney disease (CKD) compared with cats without CKD. 146
Low-dose meloxicam (ie, 0.01–0.03 mg/kg orally q24h) is effective in treating arthritic cats and is well tolerated, even in cats with CKD provided their clinical status is stable. 145 Meloxicam is effective when administered once q24h and is palatable for most patients, making it easy to administer. In Europe, Australasia and many other countries, meloxicam is approved for long-term use in cats at a dose of 0.05 mg/kg q24h. The oral route of administration and long-term use of meloxicam in cats remain off-label in the USA.
Robenacoxib is a COX-2 selective NSAID approved for surgical pain in cats. It has not been studied for either feline DJD or in older cats but there are long-term safety data in young cats (ie, 5 x the recommended dosage for 6 months and 10 x the recommended dosage for 6 weeks).147,148
Dosing on lean body weight, close monitoring of clinical status (especially appetite), regular laboratory monitoring, and appropriately modifying the treatment plan are recommended for cats receiving NSAIDs. 149 NSAIDs should be used with caution on a case-by-case basis in cats with DJD, and cat owners should be advised that, in the USA, use of NSAIDs for feline DJD is an extra-label treatment.
Treatment of DJD in cats should focus on environmental modification (Images 11 and 12) in addition to pharmacologic therapy. In addition to steps and ramps to facilitate access to favorite elevated areas, additional litter boxes with at least one low side will make access easier. Owners can also provide physical therapy by implementing play times using favorite toys to increase exercise and mobility.
Image 11.
(a) Note this cat’s uncomfortable stance while eating caused by DJD. (b) Raising the food onto a low shelf allows the cat to sit normally and eat comfortably. Images courtesy of Margie Scherk
Image 12.
The gray and white cat in (a) is seen as gregarious and is choosing to climb to higher locations. Image (b) shows the same cat with DJD and the inability to jump. Provision of a step allows the cat in image (c) to perch in a favored place. Images courtesy of Sheilah Robertson
When pain persists: hospice and palliative care
Hospice is designed to provide compassionate comfort and care to patients at the end of their lives and to support their families in the bereavement process. Hospice care for terminally ill patients is recommended when life expectancy is less than 6 months. Palliative care is the active, total care of patients with disease that is not responsive to curative treatment, with pain control being the paramount feature. The goal is achievement of the best quality of life (QoL) for patients and their families. This assumes ongoing assessment of QoL in the terminally ill patient. User-friendly QoL assessment scales are available to help veterinarians, veterinary staff and owners make proper assessments and decisions at the end of a patient’s life. 150 It is generally agreed that the pet’s care-giver is best suited to evaluate QoL, but a team approach (discussed on page 266) emphasizing regular communication is important to provide empathetic support when end-of-life decisions are made.
An integrated approach that includes nonpharmacologic modalities is typically best for palliative care and hospice patients with cancer because their disease is often associated with features of both acute and chronic pain. In cases of palliative radiation, either a smaller number or lower doses of radiation can make treatment protocols more tolerable for the patient and agreeable to the owner. Environmental modification, physical therapy (eg, massage, acupuncture and therapeutic laser) or ultrasound can be useful additions to the pain management plan. Providing nutritional support via feeding tube can be helpful where eating is otherwise difficult or painful.
In cases involving hospice and palliative care, it is important to encourage clients to have realistic expectations of the outcomes involving their pets. As well as explanations of probable outcomes, this involves providing the client with end-of-life choices involving the pet. Euthanasia is an option that relieves pain and suffering and should be discussed as a reasonable and humane alternative at an appropriate point. Euthanasia may be a topic that the veterinary team initiates if the pet owner does not.
A team approach and client education: creating an environment for success
Primary care practices should be committed to educating the healthcare team and its clients about prevention, recognition, assessment and treatment of pain. A team approach and consistent pain management messages directed at clients will help ensure patient comfort at all stages of treatment. The client is often considered the most important member of the healthcare team.
Each healthcare team member should be able to recognize pain-associated behavior in animals, as described earlier in these Guidelines, and know how to respond appropriately. Table 4 provides examples of how healthcare team members should respond to patients experiencing pain.
Table 4.
Healthcare team member responsibilities for pain management
| Assess pain in every patient regardless of appointment type (eg, wellness, sick, follow-up) | |
|---|---|
| Veterinarian | Develop standard operating procedures for the practice to prevent pain, including the following: • Weight optimization and prevention of dental disease • Handling and hospitalization to prevent fear and pain • PLATTER (see Figure 1) to follow up and modify plan |
| Provide staff education on: • Effective client communication and education • Preventive pain strategies • Recognition and assessment of pain • Drug interactions and adverse effects | |
| Technician | Obtain medication history Anticipate painful procedures Recognize signs of pain and alert veterinarian Treat as directed by a veterinarian and update records Assess postoperative patients and record pain score Assess chronic pain patients and record pain score Maintain effective client communication and education |
| Patient-care personnel | Prior to examination: • Note possible causes of pain • Note any patient behavioral changes |
| During the examination: • Proper handling • Other stress/anxiety-relieving techniques | |
| Following the examination: • Monitor patient’s behavior • Contact client about questions or concerns • Set follow-up appointment | |
| Housing should be stress/anxiety-relieving |
Table 5.
Summary of appropriate interventions for pain in dogs and cats
| Approved NSAIDs | Other analgesic drugs | Opioid premed ± tranquilizer/sedative | Local and/or regional anesthetic | Chondroprotectants (GAGs) | Acupuncture | Therapeutic joint diets | Therapeutic exercise | Weight management | Lifestyle/environmental change | Optimal surgical technique | Patient warming perioperative | Other non-pharma interventions | Comments/details | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DJD dog | X | X | X(1) | X | X | X | X | X | X | X | ||||
| DJD cat (with CKD) | X(2) | X | X(1) | X | X | X | X | X | X | X | ||||
| Soft tissue abdominal surgery | X | X(3) | X | X | X | X | X | X | ||||||
| Dental surgery | X | X(3) | X | X | X | X | X | X | ||||||
| Orthopedic procedure | X | X | X | X | X(4) | X | X(4) | X(4) | X(4) | X(4) | X | X | X | |
| Hospital procedures: | ||||||||||||||
| IV catheterization | X(5) | X(8) | X(6) | X | Consider local anesthetic cream | |||||||||
| Urinary catheterization | X | X(9) | X(10) | X(6) | X | X | ||||||||
| Bone marrow aspiration | X | X(9) | X | X(6) | X | X | Consider general anesthesia | |||||||
| Radiography (painful and/or arthritic patient) | X(9) | X | ||||||||||||
| Anal sac expression | X(9) | |||||||||||||
| Ear cleaning | X(7) | X(7) | X(7) | X | Consider general ear cleaning | |||||||||
| Thoracocentesis and/or abdominocentesis | X | X(9) | X(6) | X | ||||||||||
Notes:
Local or regional analgesia may be useful in localization of pain and short term relief of significant DJD pain
See discussion on pages 256-258 concerning the use of non-steroidal anti-inflammatory drugs (NSAIDs) in cats
The addition of other analgesic drugs will depend on patient characteristics and extent of the procedure
These interventions will be helpful pre- and postoperatively for the relief and/or prevention of postoperative and chronic pain
Ideally premedications should precede other preparations for general anesthesia such as placement of an IV catheter
These are invasive procedures and should be treated as such to optimize patient care and minimize trauma/tissue damage and post-procedural pain
The level of intervention will be tailored to the invasiveness of the procedure. Deep ear cleaning will require more significant intervention than superficial cleaning in most cases
In non-emergency settings (eg, routine pre-surgical application)
Chemical restraint in lieu of manual restraint when patient is fractious, distressed or otherwise intolerant of the procedure
Sterile lidocaine lubricant; caution in cases of urethral or bladder mucosal damage GAGs = glycosaminoglycans, CKD = chronic kidney disease, DJD = degenerative joint disease
Staff training and education
Ideally, every healthcare team member should have a defined role in managing animal pain. Staff and client education should address conditions associated with pain; its prevention and treatment; and appropriate interaction, handling and nursing care involving the patient. Medical rounds and staff meetings are effective tools in making sure that all staff members are aware of the individualized pain management needs of every hospitalized patient. Having a patient advocate for each hospitalized animal will enable a highly accurate and individualized evaluation of the patient and ensure successful treatment. Recall that Table 1 lists pain indices relying on observation and input by clinical personnel. Those assessment tools complement the pain-scoring instruments based on owner observation and input, which are listed in Table 2.
Client education and instructions
With each pain management plan, it is important that the client be given specific instructions, both verbally and in writing. Potential adverse drug effects and action to be taken should be emphasized. It is advisable to provide a hands-on demonstration of how to administer medications and handle the pet at home. To reinforce verbal information about pain assessment, provide handouts that discuss general information about animal pain and any side effects of medications. Compliance will improve if the pet owner understands the treatment schedule and a demonstration of how to administer oral medications is given. Clients should be encouraged to address their concerns about the pet’s condition and treatment plan via e-mail, phone or follow-up consultations.
Summary Points
Effective pain management is an essential component of companion animal medicine. It reduces disease morbidity, facilitates recovery, enhances quality of life, and solidifies the relationship between the veterinarian, client and pet.
Behavioral changes are the principal indicator of pain and its resolution, for which there are now several validated clinical scoring instruments.
Pain is not an isolated event but instead exists either as a continuum of causation, progression and resolution or as a chronic condition. Thus, treatment of pain should consist of a continuum of care in the form of anticipatory analgesia through the anticipated pain period followed by longer term or even chronic treatment that relies on periodic reassessment of the patient’s response.
Effective pain management is integrative in two respects. First, it does not rely solely on pharmacologic methods but also uses a variety of non-pharmacologic modalities; not least of those is gentle handling and nursing care of the patient in the context of a stress-free physical environment. When considering either non-pharmacologic methods or hospice care that may be outside the immediate skills or services provided by the primary practice, the veterinarian should have a list of experts for referral in place. A second aspect of integrative pain management is the multimodal use of medications that either block or modify multiple pain pathways. A multimodal approach also reduces reliance on any single agent, minimizing the potential for adverse drug events.
Pain management in clinical practice is a team effort, with the pet owner functioning as an integral part of the team. All healthcare team members should have a defined role in the practice’s approach to providing compassionate care to its patients. That enables the practice to speak with one voice and in a consistent manner in the implementation of pain management protocols.
Client education is a key component that enables the pet owner to manage pain in the home setting. Direct involvement of the client in pain management efforts is consistent with the continuum of care concept and a demonstration of the practice’s commitment to the pet’s quality of life.
A fully integrated approach to pain management, involving recognition and systematic assessment, pharmacologic and non-pharmacologic methods, and including both healthcare team members and the pet owner, ensures that everything possible has been done to relieve a patient’s pain once it enters the practice’s care.
Acknowledgments
These Guidelines were prepared by a Task Force of experts convened by the American Animal Hospital Association and the American Association of Feline Practitioners. The AAHA secured sponsorship of an educational grant in accordance with their policies from Abbott Animal Health, Elanco Companion Animal Health, Merial and Zoetis. This report was subjected to review in accordance with both AAFP and AAHA policies.
Footnotes
Funding: These Guidelines were supported by an educational grant to AAHA from Abbott Animal Health, Elanco Companion Animal Health, Merial and Zoetis.
Mark Epstein has previously consulted for Abbott, Elanco and Merial. Sheilah Robertson is a key opinion leader for Novartis Animal Health.
Contributor Information
Mark E Epstein, TotalBond Veterinary Hospitals PC, 3200 Union Road, Gastonia, NC 28056, USA.
Ilona Rodanm, Cat Care Clinic and Feline-Friendly Consultations, 322 Junction Road, Madison, WI 53717, USA Email: care4cats@gmail.com.
Gregg Griffenhagen, Colorado State University School of Veterinary Medicine, 300 West Drake Road, Fort Collins, CO 80523, USA.
Jamie Kadrlik, Pet Crossing Animal Hospital and Dental Clinic, 10861 Bloomington Ferry Road, Bloomington, MN 55438, USA.
Michael C Petty, Arbor Pointe Veterinary Hospital/Animal Pain Center, 42043 Ford Road, Canton, MI 48187, USA.
Sheilah A Robertson, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48824, USA.
Wendy Simpson, Morrisville Cat Hospital, 100 Keybridge Drive, Suite A, Morrisville, NC 27560, USA.
References
- 1. American Animal Hospital Association; American Association of Feline Practitioners; AAHA/AAFP Pain Management Guidelines Task Force Members, Hellyer P, Rodan I, Brunt J, et al. AAHA/AAFP pain management guidelines for dogs & cats. J Am Anim Hosp Assoc 2007; 43: 235–248. [DOI] [PubMed] [Google Scholar]
- 2. AAHA/AAFP Pain Management Guidelines Task Force Members, Hellyer P, Rodan I, Brunt J, et al. AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2007; 9: 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reid J, Scott M, Nolan A, et al. Pain assessment in animals. In Pract 2013; 35: 51–56. [Google Scholar]
- 4. Woolf CJ. What is this thing called pain? J Clin Invest 2010; 120: 3742–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shanan A. A veterinarian’s role in helping pet owners with decision making. Vet Clin North Am Small Anim Pract 2011; 41: 635–646. [DOI] [PubMed] [Google Scholar]
- 6. American Animal Hospital Association-American Veterinary Medical Association Preventive Healthcare Guidelines Task Force. Development of new canine and feline preventive healthcare guidelines designed to improve pet health. J Am Anim Hosp Assoc 2011; 47: 306–311. [DOI] [PubMed] [Google Scholar]
- 7. Mahler SP, Reece JL. Electrical nerve stimulation to facilitate placement of an indwelling catheter for repeated brachial plexus block in a traumatized dog. Vet Anaesth Analg 2007; 34: 365–370. [DOI] [PubMed] [Google Scholar]
- 8. Guillot M, Rialland P, Nadeau MÈ, et al. Pain induced by a minor medical procedure (bone marrow aspiration) in dogs: comparison of pain scales in a pilot study. J Vet Intern Med 2011; 25: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 9. Brondani JT, Luna SPL, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res 2011; 72: 174–183. [DOI] [PubMed] [Google Scholar]
- 10. Brondani JT, Mama KR, Luna SPL, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawler DF, Evans RH, Larson BT, et al. Influence of lifetime food restriction on causes, time, and predictors of death in dogs. J Am Vet Med Assoc 2005; 226: 225–231. [DOI] [PubMed] [Google Scholar]
- 12. Grimm KA, Tranquilli WJ, Thurmon JC, et al. Duration of nonresponse to noxious stimulation after intramuscular administration of butorphanol, medetomidine, or a butorphanol-medetomidine combination during isoflurane administration in dogs. Am J Vet Res 2000; 61: 42–47. [DOI] [PubMed] [Google Scholar]
- 13. Sawyer DC, Rech RH, Durham RA, et al. Dose response to butorphanol administered subcutaneously to increase visceral nociceptive threshold in dogs. Am J Vet Res 1991; 52: 1826–1830. [PubMed] [Google Scholar]
- 14. Warne LN, Beths T, Holm M, et al. Evaluation of the perioperative analgesic efficacy of buprenorphine, compared with butorphanol, in cats. J Am Vet Med Assoc 2014; 245: 195–202. [DOI] [PubMed] [Google Scholar]
- 15. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 16. Robertson SA, Taylor PM, Sear JW. Systemic uptake of buprenorphine by cats after oral mucosal administration. Vet Rec 2003; 152: 675–678. [DOI] [PubMed] [Google Scholar]
- 17. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 18. KuKanich B. Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine-6-glucuronide in healthy Greyhound dogs. J Vet Pharmacol Ther 2010; 33: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. KuKanich B, Bidgood T, Knesl O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet Anaesth Analg 2012; 39: 69–90. [DOI] [PubMed] [Google Scholar]
- 20. Monteiro-Steagall BP, Steagall PV, Lascelles BD. Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs. J Vet Intern Med 2013; 27: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 21. Khan SA, McLean MK. Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42: 289–306. [DOI] [PubMed] [Google Scholar]
- 22. Innes JF, Clayton J, Lascelles BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec 2010; 166: 226–230. [DOI] [PubMed] [Google Scholar]
- 23. Stanton ME, Bright RM. Gastroduodenal ulceration in dogs. Retrospective study of 43 cases and literature review. J Vet Intern Med 1989; 3: 238–244. [DOI] [PubMed] [Google Scholar]
- 24. Lascelles BD, Blikslager AT, Fox SM, et al. Gastrointestinal tract perforation in dogs treated with a selective cyclooxygenase-2 inhibitor: 29 cases (2002–2003). J Am Vet Med Assoc 2005; 227: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 25. Neiger R. NSAID-induced gastrointestinal adverse effects in dogs – can we avoid them? J Vet Intern Med 2003; 17: 259–261. [PubMed] [Google Scholar]
- 26. Wooten JG, Lascelles BD, Cook VL, et al. Evaluation of the relationship between lesions in the gastroduodenal region and cyclooxygenase expression in clinically normal dogs. Am J Vet Res 2010; 71: 630–635. [DOI] [PubMed] [Google Scholar]
- 27. Goodman LA, Brown SA, Torres BT, et al. Effects of meloxicam on plasma iohexol clearance as a marker of glomerular filtration rate in conscious healthy cats. Am J Vet Res 2009; 70: 826–830. [DOI] [PubMed] [Google Scholar]
- 28. Hampshire VA, Doddy FM, Post LO, et al. Adverse drug event reports at the United States Food And Drug Administration Center for Veterinary Medicine. J Am Vet Med Assoc 2004; 225: 533–536. [DOI] [PubMed] [Google Scholar]
- 29. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 30. Crandell DE, Mathews KA, Dyson DH. Effect of meloxicam and carprofen on renal function when administered to healthy dogs prior to anesthesia and painful stimulation. Am J Vet Res 2004; 65: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 31. Boström IM, Nyman GC, Lord PE, et al. Effects of carprofen on renal function and results of serum biochemical and hematologic analyses in anesthetized dogs that had low blood pressure during anesthesia. Am J Vet Res 2002; 63: 712–721. [DOI] [PubMed] [Google Scholar]
- 32. Lascelles BD, Cripps PJ, Jones A, et al. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg 1998; 27: 568–582. [DOI] [PubMed] [Google Scholar]
- 33. Ochi H, Hara Y, Asou Y, et al. Effects of long-term administration of carprofen on healing of a tibial osteotomy in dogs. Am J Vet Res 2011; 72: 634–641. [DOI] [PubMed] [Google Scholar]
- 34. Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and non-selective COX2 inhibitors and experimental fracture healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am 2007; 89: 114–125. [DOI] [PubMed] [Google Scholar]
- 35. Waite A, Gilliver SC, Masterson GR, et al. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth 2010; 104: 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richebe P, Rivat C, Rivalan B, et al. Low doses ketamine: antihyperalgesic drug, non-analgesic. Ann Fr Anesth Reanim 2005; 24: 1349–1359. [DOI] [PubMed] [Google Scholar]
- 37. Bell RF, Dahl JB, Moore RA, et al. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev 2006; 1: CD004603. [DOI] [PubMed] [Google Scholar]
- 38. Slingsby LS, Waterman-Pearson AE. The postoperative analgesic effects of ketamine after canine ovariohysterectomy – a comparison between pre- and post-operative administration. Res Vet Sci 2000; 69: 147–152. [DOI] [PubMed] [Google Scholar]
- 39. Sarrau S, Jourdan J, Dupuis-Soyris F, et al. Effects of postoperative ketamine infusion on pain control and feeding behaviour in bitches undergoing mastectomy. J Small Anim Pract 2007; 48: 670–676. [DOI] [PubMed] [Google Scholar]
- 40. Wagner AE, Walton JA, Hellyer PW, et al. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. J Am Vet Med Assoc 2002; 221: 72–75. [DOI] [PubMed] [Google Scholar]
- 41. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010; 70: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 42. Smith LJ, Bentley E, Shih A, et al. Systemic lidocaine infusion as an analgesic for intraocular surgery in dogs: a pilot study. Vet Anaesth Analg 2004; 31: 53–63. [DOI] [PubMed] [Google Scholar]
- 43. Pypendop BH, Ilkiw JE. Assessment of the hemodynamic effects of lidocaine administered IV in isoflurane-anesthetized cats. Am J Vet Res 2005; 66: 661–668. [DOI] [PubMed] [Google Scholar]
- 44. Muir WW, 3rd, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res 2003; 64: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 45. KuKanich B, Papich MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Therap 2004; 27: 239–246. [DOI] [PubMed] [Google Scholar]
- 46. McMillan CJ, Livingston A, Clark CR, et al. Pharmacokinetics of intravenous tramadol in dogs. Can J Vet Res 2008; 72: 325–331. [PMC free article] [PubMed] [Google Scholar]
- 47. Giorgi M, Saccomanni G, Lebkowska-Wieruszewska B, et al. Pharmacokinetic evaluation of tramadol and its major metabolites after single oral sustained tablet administration in the dog: a pilot study. Vet J 2009; 180: 253–255. [DOI] [PubMed] [Google Scholar]
- 48. KuKanich B, Papich MG. Pharmacokinetics and antinociceptive effects of oral tramadol hydrochloride administration in Greyhounds. Am J Vet Res 2011; 72: 256–262. [DOI] [PubMed] [Google Scholar]
- 49. Itami T, Tamaru N, Kawase K, et al. Cardiovascular effects of tramadol in dogs anesthetized with sevoflurane. J Vet Med Sci 2011; 73: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 50. Itami T, Kawase K, Tamaru N, et al. Effects of a single bolus intravenous dose of tramadol on minimum alveolar concentration (MAC) of sevoflurane in dogs. J Vet Med Sci 2013; 75: 613–618. [DOI] [PubMed] [Google Scholar]
- 51. Kongara K, Chambers JP, Johnson CB. Effects of tramadol, morphine or their combination in dogs undergoing ovariohysterectomy on peri-operative electroencephalographic responses and post-operative pain. N Z Vet J 2012; 60: 129–135. [DOI] [PubMed] [Google Scholar]
- 52. Neves CS, Balan JA, Pereira DR, et al. A comparison of extradural tramadol and extradural morphine for postoperative analgesia in female dogs undergoing ovariohysterectomy. Acta Cir Bras 2012; 27: 312–317. [DOI] [PubMed] [Google Scholar]
- 53. Teixeira RC, Monteiro ER, Campagnol D, et al. Effects of tramadol alone, in combination with meloxicam or dipyrone, on postoperative pain and the analgesic requirement in dogs undergoing unilateral mastectomy with or without ovariohysterectomy. Vet Anaesth Analg 2013; 40: 641–649. [DOI] [PubMed] [Google Scholar]
- 54. Davila D, Keeshen TP, Evans RB, et al. Comparison of the analgesic efficacy of perioperative firocoxib and tramadol administration in dogs undergoing tibial plateau leveling osteotomy. J Am Vet Med Assoc 2013; 243: 225–231. [DOI] [PubMed] [Google Scholar]
- 55. Rialland P, Authier S, Guillot M, et al. Validation of orthopedic postoperative pain assessment methods for dogs: a prospective, blinded, randomized, placebo-controlled study. PLoS One 2012; 7: e49480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matthiesen T, Wöhrmann T, Coogan TP, et al. The experimental toxicology of tramadol: an overview. Toxicol Lett 1998; 95: 63–71. [DOI] [PubMed] [Google Scholar]
- 57. Malek S, Sample SJ, Schwartz Z, et al. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet Res 2012; 8: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pypendop BH, Siao KT, Ilkiw JE. Effects of tramadol hydrochloride on the thermal threshold in cats. Am J Vet Res 2009; 70: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 59. Brondani JI, Loureiro Luna SP, Beier SL, et al. Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 2009; 11: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ray J, Jordan D, Pinelli C, et al. Case studies of compounded Tramadol use in cats. Int J Pharm Compd 2012; 16: 44–49. [PubMed] [Google Scholar]
- 61. Longmire DR, Jay GW, Boswell MV. Neuropathic pain. In: Boswell MV, Cole BE. (eds). Weiner’s pain management. A practical guide for clinicians. 7th ed. Boca Raton, FL: Taylor & Francis, 2006, p 305. [Google Scholar]
- 62. Solak O, Metin M, Esme H, et al. Effectiveness of gabapentin in the treatment of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2007; 32: 9–12. [DOI] [PubMed] [Google Scholar]
- 63. Plessas IN, Rusbridge C, Driver CJ, et al. Long-term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari-like malformation and syringomyelia. Vet Rec 2012; 171: 501. [DOI] [PubMed] [Google Scholar]
- 64. Rusbridge C, Heath S, Gunn-Moore DA, et al. Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. J Feline Med Surg 2010; 12: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boileau C, Martel-Pelletier J, Brunet J, et al. Oral treatment with PD-0200347, an alpha2delta ligand, reduces the development of experimental osteoarthritis by inhibiting metalloproteinases and inducible nitric oxide synthase gene expression and synthesis in cartilage chondrocytes. Arthritis Rheum 2005; 52: 488–500. [DOI] [PubMed] [Google Scholar]
- 66. Lorenz ND, Comerford EJ, Iff I. Long-term use of gabapentin for musculoskeletal disease and trauma in three cats. J Feline Med Surg 2013; 15: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain – a systematic review of randomized controlled trials. Pain 2006; 126: 91–101. [DOI] [PubMed] [Google Scholar]
- 68. Hurley RW, Cohen SP, Williams KA, et al. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med 2006; 31: 237–247. [DOI] [PubMed] [Google Scholar]
- 69. Reid P, Pypendop BH, Ilkiw JE. The effects of intravenous gabapentin administration on the minimum alveolar concentration of isoflurane in cats. Anesth Analg 2010; 111: 633–637. [DOI] [PubMed] [Google Scholar]
- 70. Wagner AE, Mich PM, Uhrig SR, et al. Clinical evaluation of perioperative administration of gabapentin as an adjunct for postoperative analgesia in dogs undergoing amputation of a forelimb. J Am Vet Med Assoc 2010; 236: 751–756. [DOI] [PubMed] [Google Scholar]
- 71. Aghighi SA, Tipold A, Piechotta M, et al. Assessment of the effects of adjunctive gabapentin on postoperative pain after intervertebral disc surgery in dogs. Vet Anesth Analg 2012; 39: 636–646. [DOI] [PubMed] [Google Scholar]
- 72. Pypendop BH, Siao KT, Ilkiw JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res 2010; 71: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 73. Vollmer KO, von Hodenberg A, Kölle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung 1986; 36: 830–839. [PubMed] [Google Scholar]
- 74. Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage 2000; 20: 358–373. [DOI] [PubMed] [Google Scholar]
- 75. Lascelles BDX, Gaynor J, Smith ES. Evaluation of amantadine as part of a multimodal analgesic regimen for the alleviation of refractory canine osteoarthritis pain. Proceedings of the 32nd World Small Animal Veterinary Association World Congress; 2007 Aug 19–23; Sydney, Australia. p 42. [Google Scholar]
- 76. Madden M, Gurney M, Bright S. Amantadine, an N-Methyl-D-Aspartate antagonist, for treatment of chronic neuropathic pain in a dog. Vet Anaesth Analg 2014; 41: 440–441. [DOI] [PubMed] [Google Scholar]
- 77. Vernier VG, Harmon JB, Stump JM, et al. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol 1969; 15: 642–665. [DOI] [PubMed] [Google Scholar]
- 78. Siao KT, Pypendop BH, Stanley SD, et al. Pharmacokinetics of amantadine in cats. J Vet Pharmacol Ther 2011; 34: 599–604. [DOI] [PubMed] [Google Scholar]
- 79. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005: 118: 289–305. [DOI] [PubMed] [Google Scholar]
- 80. Cashmore RG, Harcourt-Brown TR, Freeman PM, et al. Clinical diagnosis and treatment of suspected neuropathic pain in three dogs. Aust Vet J 2009; 87: 45–50. [DOI] [PubMed] [Google Scholar]
- 81. Patel DS, Deshpande SS, Patel CG, et al. A dual action antidepressant. Indo Global J Pharm Sci 2011; 1: 69. [Google Scholar]
- 82. KuKanich B. Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine-6-glucuronide in healthy Greyhound dogs. J Vet Pharmacol Ther 2010; 33: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mburu DN, Mbugua SW, Skoglund LA, et al. Effects of paracetamol and acetylsalicylic acid on the post-operative course after experimental orthopaedic surgery in dogs. J Vet Pharmacol Ther 1988; 11: 163–170. [DOI] [PubMed] [Google Scholar]
- 84. Mburu DN. Evaluation of the anti-inflammatory effects of a low dose of acetaminophen following surgery in dogs. Vet Pharmacol 1991; 14: 109–111. [DOI] [PubMed] [Google Scholar]
- 85. Alvillar BM, Boscan P, Mama KR, et al. Effect of epidural and intravenous use of the neurokinin-1 (NK-1) receptor antagonist maropitant on the sevoflurane minimum alveolar concentration (MAC) in dogs. Vet Anaesth Analg 2012; 39: 201–205. [DOI] [PubMed] [Google Scholar]
- 86. Boscan P, Monnet E, Mama K, et al. Effect of maropitant, a neurokinin 1 receptor antagonist, on anesthetic requirements during noxious visceral stimulation of the ovary in dogs. Am J Vet Res 2011; 72: 1576–1579. [DOI] [PubMed] [Google Scholar]
- 87. Fan TM, de Lorimier LP, Charney SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med 2005; 19: 74–80. [DOI] [PubMed] [Google Scholar]
- 88. Pelletier JP, DiBattista JA, Raynauld JP, et al. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Invest 1995; 72: 578–586. [PubMed] [Google Scholar]
- 89. Pelletier JP, Martel-Pelletier J. In vivo protective effects of prophylactic treatment with tiaprofenic acid or intraarticular corticosteroids on osteoarthritic lesions in the experimental dog model. J Rheumatol Suppl 1991; 27: 127–130. [PubMed] [Google Scholar]
- 90. Pelletier JP, Martel-Pelletier J. Protective effects of corticosteroids on cartilage lesions and osteophyte formation in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum 1989; 32: 181–193. [DOI] [PubMed] [Google Scholar]
- 91. Pelletier JP, Mineau F, Raynauld JP, et al. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum 1994; 37: 414–423. [DOI] [PubMed] [Google Scholar]
- 92. Fujiki M, Shineha J, Yamanokuchi K, et al. Effects of treatment with polysulfated glycosaminoglycan on serum cartilage oligomeric matrix protein and C-reactive protein concentrations, serum matrix metalloproteinase-2 and -9 activities, and lameness in dogs with osteoarthritis. Am J Vet Res 2007; 68: 827–833. [DOI] [PubMed] [Google Scholar]
- 93. Altman RD, Howell DS, Muniz OE, et al. The effect of glycosaminoglycan polysulfuric acid ester on articular cartilage in experimental arthritis: effects on collagenolytic enzyme activity and cartilage swelling properties. J Rheumatol 1987; 14: 127–129. [PubMed] [Google Scholar]
- 94. Lust G, Williams AJ, Burton-Wurster N, et al. Effects of intramuscular administration of glycosaminoglycan polysulfates on signs of incipient hip dysplasia in growing pups. Am J Vet Res 1992; 53: 1836–1843. [PubMed] [Google Scholar]
- 95. Heidrich JE, Fox SM, Royer R, et al. Fluorescein-labeled polysulfated glycosaminoglycan in a feline acute traumatic knee model. Proceedings of the 35th Annual Conference of the Veterinary Orthopedic Society; 2008 March 8–15; Big Sky, MT. [Google Scholar]
- 96. Fry LM, Neary S, Sharrock J, et al. Acupuncture for analgesia in veterinary medicine. Top Comp Anim Med 2014; 29: 35–42. [DOI] [PubMed] [Google Scholar]
- 97. Groppetti D, Pecile AM, Sacerdote P, et al. Effectiveness of electroacupuncture analgesia compared with opioid administration in a dog model: a pilot study. Br J Anaesth 2011; 107: 612–618. [DOI] [PubMed] [Google Scholar]
- 98. Cassu RN, da Silva DA, Filho TG, et al. Electroanalgesia for the postoperative control of pain in dogs. Acta Cirugica Brasileira 2012; 21: 43–48. [DOI] [PubMed] [Google Scholar]
- 99. Gakiya HH, Silva DA, Gomes J, et al. Electroacupuncture versus morphine for the postoperative control of pain in dogs. Acta Cirurgica Brasiliera 2011; 25: 346–351. [DOI] [PubMed] [Google Scholar]
- 100. Petermann U. Comparison of pre- and post-treatment pain scores of twenty one horses with laminitis treated with acupoint and topical low level impulse laser therapy. AJTCVM 2011; 6: 13–25. [Google Scholar]
- 101. Laim A, Jaggy A, Forterre F, et al. Effects of adjunct electroacupuncture on severity of postoperative pain in dogs undergoing hemilaminectomy because of acute thoracolumbar intervertebral disc disease. J Am Vet Med Assoc 2009; 234: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 102. Au KK, Gordon-Evans WJ, Dunning D, et al. Comparison of short- and long-term function and radiographic osteoarthrosis in dogs after postoperative physical rehabilitation and tibial plateau leveling osteotomy or lateral fabellar suture stabilization. Vet Surg 2010; 39: 173–180. [DOI] [PubMed] [Google Scholar]
- 103. Edge-Hughes L. Hip and sacroiliac disease: selected disorders and their management with physical therapy. Clin Tech Small Anim Pract 2007; 22: 183–194. [DOI] [PubMed] [Google Scholar]
- 104. Johnson JM, Johnson AL, Pijanowski GJ. Rehabilitation of dogs with surgically treated cranial cruciate ligament deficient stifles by use of electrical stimulation of muscles. Am J Vet Res 1997; 58: 1473–1478. [PubMed] [Google Scholar]
- 105. Rexing J, Dunning D, Siegel AM, et al. Effects of cold compression, bandaging and microcurrent electrical therapy after cranial cruciate ligament repair in dogs. Vet Surg 2010; 39: 54–58. [DOI] [PubMed] [Google Scholar]
- 106. Mueller M, Bockstahler B, Skalicky M, et al. Effects of radial shockwave therapy on the limb function of dogs with hip osteoarthritis. Vet Rec 2007; 160: 762–765. [DOI] [PubMed] [Google Scholar]
- 107. Mueller MC, Gradner G, Hittmair KM, et al. Conservative treatment of partial gastrocnemius muscle avulsions in dogs using therapeutic ultrasound. Vet Comp Orthop Traumatol 2009; 22: 243–248. [DOI] [PubMed] [Google Scholar]
- 108. Rochkind S. The role of laser phototherapy in nerve tissue regeneration and repair: research development with perspective for clinical application. Proceedings of the World Association of Laser Therapy; Sao Paulo, Brazil, 2004. [Google Scholar]
- 109. Dommerholt J, Huijbregts P. Myofascial trigger points: pathophysiology and evidence-informed diagnosis and management. Sudberry, MA; Jones and Bartlett, 2011. [Google Scholar]
- 110. Wright B. Management of soft tissue pain. Topical Review 2010; 25: 1: 26–31. [DOI] [PubMed] [Google Scholar]
- 111. Corbee RJ, Barnier MM, van de, Lest CH, et al. The effect of dietary long-chain omega-3 fatty acid supplementation on owner’s perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013; 97: 846–853. [DOI] [PubMed] [Google Scholar]
- 112. Moreau M, Troncy E, Del Castillo JR, et al. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013; 97: 830–837. [DOI] [PubMed] [Google Scholar]
- 113. Rialland P, Bichot S, Lussier B, et al. Effect of a diet enriched with green-lipped mussel on pain behavior and functioning in dogs with clinical osteoarthritis. Can J Vet Res 2013; 77: 66–74. [PMC free article] [PubMed] [Google Scholar]
- 114. Lascelles BD, DePuy V, Thomson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med 2010; 24: 487–495. [DOI] [PubMed] [Google Scholar]
- 115. Drygas KA, McClulre SR, Goring RL, et al. Effect of cold compression therapy on postoperative pain, swelling, range of motion, and lameness after tibial plateau leveling osteotomy in dogs. J Am Vet Med Assoc 2011; 238: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 116. Nadler SF, Weingand K, Kruse RJ. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 2004; 7: 395–399. [PubMed] [Google Scholar]
- 117. Griffin B, Hume KR. Recognition and management of stress in housed cats. In: August J. (ed). Consultations in feline internal medicine. 5th ed. St Louis, MO; Saunders Elsevier, 2006, p 717. [Google Scholar]
- 118. Benedetti F, Amanzio M, Vighetti S, et al. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci 2006; 26: 12014–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain 2009; 142: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Khasar SG, Burkham J, Dina OA, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci 2008; 28: 5721–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stella J, Croney C, Buffington CAT. Effects of stressors on the behavior and physiology of domestic cats. Appl Anim Behav Sci 2013; 143: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim YM, Lee JK, Abd el-aty AM, et al. Efficacy of dog-appeasing pheromone (DAP) for ameliorating separation-related behavioral signs in hospitalized dogs. Can Vet J 2010; 51: 380–385. [PMC free article] [PubMed] [Google Scholar]
- 123. Stella JL, Lord LK, Buffington CAT. Sickness behaviors in response to unusual external events in healthy cats and cats with feline interstitial cystitis. J Am Vet Med Assoc 2011; 238: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kronen PW, Ludders JW, Erb HN, et al. A synthetic fraction of feline facial pheromones calms but does not reduce struggling in cats before venous catheterization. Vet Anaesth Analg 2006; 33: 258–265. [DOI] [PubMed] [Google Scholar]
- 125. Pageat P, Gaultier E. Current research in canine and feline pheromones. Vet Clin North Am Small Anim Pract 2003; 33: 187–211. [DOI] [PubMed] [Google Scholar]
- 126. Ellis S, Rodan I, Carney HC, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg 2013; 15: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Carney HC, Little S, Brownlee-Tomasso D, et al. AAFP and ISFM feline-friendly nursing care guidelines. J Feline Med Surg 2012; 14: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc 2002; 220: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 130. Kealy RD, Lawler DF, Ballam JM, et al. Evaluation of the effect of limited food consumption on radiographic evidence of osteoarthritis in dogs. J Am Vet Med Assoc 2000; 217: 1678–1680. [DOI] [PubMed] [Google Scholar]
- 131. Smith GK, Paster ER, Powers MY, et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J Am Vet Med Assoc 2006; 229: 690–693. [DOI] [PubMed] [Google Scholar]
- 132. Marshall WG, Hazewinkel HA, Mullen D, et al. The effect of weight loss on lameness in obese dogs with osteoarthritis. Vet Res Commun 2010; 34: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Autefage A, Palissier FM, Asimus E, et al. Long-term efficacy and safety of firocoxib in the treatment of dogs with osteoarthritis. Vet Rec 2011; 168: 617. [DOI] [PubMed] [Google Scholar]
- 134. Krontveit RI, Trangerud C, Sævik BK, et al. Risk factors for hip-related clinical signs in a prospective cohort study of four large dog breeds in Norway. Prev Vet Med 2012; 103: 219–227. [DOI] [PubMed] [Google Scholar]
- 135. Vandeweerd JM, Coisnon C, Clegg P, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med 2012; 26: 448–456. [DOI] [PubMed] [Google Scholar]
- 136. Lascelles BDX, Henry JB, 3rd, Brown J, et al. Cross-sectional study evaluating the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 137. Lascelles BDX, Robertson S. DJD associated pain in cats – what can we do to promote patient comfort? J Feline Med Surg 2010; 12: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Guillot M, Moreau M, Heit M, et al. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013; 196: 360–367. [DOI] [PubMed] [Google Scholar]
- 139. Benito J, Depuy V, Hardie E, et al. Reliability and discriminatory testing of a client-based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet J 2013; 196: 368–373. [DOI] [PubMed] [Google Scholar]
- 140. Benito J, Hansen B, Depuy V, et al. Feline musculoskeletal pain index: responsiveness and testing of criterion validity. J Vet Intern Med 2013; 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 141. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 142. Lascelles BDX, Henderson AJ, Hackett IJ, et al. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders. J Small Anim Pract 2001; 42: 587–593. [DOI] [PubMed] [Google Scholar]
- 143. Lascelles BDX, Court MH, Hardie EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg 2007; 34: 228–250. [DOI] [PubMed] [Google Scholar]
- 144. Thoulon F, Narbe R, Johnston L, et al. Metabolism and excretion of oral meloxicam in the cat [abstract]. J Vet Intern Med 2009; 23: 695. [Google Scholar]
- 145. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long term dosing with meloxicam on renal function in aged cats with degenerative joint disease.J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gowan RA, Baral RM, Lingard AE, et al. A retrospective analysis of the effects of meloxicam on the longevity of aged cats with and without overt chronic kidney disease. J Feline Med Surg 2012; 14: 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Robenacoxib Freedom of Information Summary. NADA 141-320. Available at: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-av-gen/documents/document/ucm256758.pdf. (2011, accessed December 4, 2014).
- 148. King JN, Hotz R, Reagan EL, et al. Safety of oral robenacoxib in the cat. J Vet Pharmacol Ther 2012; 35: 290–300. [DOI] [PubMed] [Google Scholar]
- 149. Sparkes AH, Heiene R, Lascelles BD, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Reid J, Wiseman-Orr ML, Scott EM, et al. Development, validation and reliability of a web-based questionnaire to measure health-related quality of life in dogs. J Small Anim Pract 2013; 54: 227–233. [DOI] [PubMed] [Google Scholar]