Abstract
Practical relevance:
Diabetes mellitus (DM) is a common endocrinopathy in cats that appears to be increasing in prevalence. The prognosis for affected cats can be good when the disease is well managed, but clinical management presents challenges, both for the veterinary team and for the owner. These ISFM Guidelines have been developed by an independent, international expert panel of clinicians and academics to provide practical advice on the management of routine (uncomplicated) diabetic cats.
Clinical challenges:
Although the diagnosis of diabetes is usually straightforward, optimal management can be challenging. Clinical goals should be to limit or eliminate clinical signs of the disease using a treatment regimen suitable for the owner, and to avoid insulin-induced hypoglycaemia or other complications. Optimising bodyweight, feeding an appropriate diet and using a longer acting insulin preparation (eg, protamine zinc insulin, insulin glargine or insulin detemir) are all factors that are likely to result in improved glycaemic control in the majority of cats. There is also some evidence that improved glycaemic control and reversal of glucose toxicity may promote the chances of diabetic remission. Owner considerations and owner involvement are an important aspect of management. Provided adequate support is given, and owners are able to take an active role in monitoring blood glucose concentrations in the home environment, glycaemic control may be improved. Monitoring of other parameters is also vitally important in assessing the response to insulin. Insulin adjustments should always be made cautiously and not too frequently – unless hypoglycaemia is encountered.
Evidence base:
The Panel has produced these Guidelines after careful review of the existing literature and of the quality of the published studies. They represent a consensus view on practical management of cats with DM based on available clinical data and experience. However, in many areas, substantial data are lacking and there is a need for better studies in the future to help inform and refine recommendations for the clinical management of this common disease.
Introduction
Diabetes mellitus (DM) is a common disease in cats, with some estimates suggesting a prevalence in first opinion practice of around 1:100–1:500.1–3 There is also evidence that the prevalence of feline DM has been increasing, possibly at least partly due to the rise in prevalence of obesity. 4
Managing DM in cats represents a challenge for both owners and the veterinary healthcare team, and considerable support is needed for owners as they continue to care for cats in the home environment. Studies have shown median survival times in cats with DM of between 13 and 29 months, with longer survival times in better stabilised cats, and with many cats dying of diseases other than DM.5–7 The prognosis for cats with DM is thus good when the disease is well managed.
These Guidelines have been developed by an independent, international expert panel of clinicians and academics with the aim of producing practical advice for dealing with the routine diabetic cat, based on currently available best evidence. The Guidelines are not intended to be a comprehensive resource on all aspects of management of DM, and references to other resources are provided where relevant.
Epidemiology, pathogenesis and diagnosis
The majority of cats with DM appear to have a disease bearing similarities to human type 2 DM,8–11 resulting from β-cell dysfunction and insulin resistance; type 1 DM (immune-mediated) is rare in cats. 12 In cats with DM, the β-cell dysfunction usually results in insulin deficiency and is likely to be caused by a number of factors including islet amyloid deposition, glucose toxicity and possibly damage from reactive oxygen species and/or inflammatory cytokines. 13 Many factors may contribute to insulin resistance – obesity is a common cause, but others include concomitant endocrinopathies (eg, acromegaly, hyperadrenocorticism), drug-induced diabetes (eg, corticosteroids, progestagens) and pancreatitis. Importantly, if insulin resistance can be reduced and β-cell function improved, in some cats diabetic remission may be achieved; in other words, exogenous insulin therapy may no longer be needed, although the remission may only be temporary in some cases. 14
Clinical signs and risk factors
Classic clinical signs of DM include polyuria and polydipsia (PU/PD), lethargy, weight loss and polyphagia. Less commonly, weakness, plantigrade stance, depression and anorexia may be seen (the last especially with ketoacidosis).
Numerous studies have identified risk factors for the development of feline DM, although the presence of unidentified concomitant disease (eg, acromegaly) may have influenced the results obtained. Major reported risk factors include:
Obesity This reduces insulin sensitivity and obese cats are up to four times more likely to develop DM than optimal-weight cats.2–4,8,15–19
Increasing age Cats over 7 years old are at greatest risk.2,4,18
Breed Burmese cats have been reported to have a higher risk in studies from Australia, New Zealand and Europe.2,20,21
Physical inactivity Indoor and inactive cats are at increased risk.2,3,20,22
Gender Male cats and neutered cats are at higher risk.2–4,18,20,21,23
Drug treatment Glucocorticoids and progestagens may cause insulin resistance and predispose cats to DM.2,24,25
Diagnosis
Diabetes is usually diagnosed by documenting persistent hyperglycaemia and glucosuria, with consistent clinical signs. Stress hyperglycaemia (and glucosuria) must be excluded prior to initiating therapy – stress uncommonly causes hyperglycaemia >16 mmol/l (288 mg/dl) (although occasionally blood glucose [BG] can be very high) and generally it resolves within a few hours. Repeat blood and urine monitoring will confirm persistent hyperglycaemia with DM, and home monitoring of these parameters may be useful where the diagnosis is in doubt.
Serum fructosamine is indicative of the average BG during approximately the preceding week, 26 and may not be affected by short-term stress hyperglycaemia (depending on its magnitude and duration). Its measurement can be helpful in confirming a diagnosis of DM and in monitoring glycaemic control (see page 245), although it may not be increased in cats with recent-onset and/or mild DM.23,26–28
Evaluation of the diabetic cat
Evaluation of a cat with suspected DM should include:
Thorough history and physical examination.
Routine serum biochemistry.
Complete urinalysis, ideally with culture (especially if there is an active sediment).
Ideally, evaluation would also include:
Complete blood count (CBC).
Serum fructosamine (although not always required for diagnosis and monitoring).
Serum thyroxine in older cats to exclude hyperthyroidism.
Because of the high prevalence of concurrent diseases, including pancreatitis, abdominal ultrasonography and/or determination of serum pancreatic lipase immunoreactivity may be indicated (especially in cats that are depressed or not eating well), although interpretation of these tests may not be straightforward. 29
Diabetic ketoacidosis (DKA) develops in a proportion of untreated diabetic cats, and should be suspected when cats are depressed, anorexic, vomiting, weak, collapsed or moribund. Diagnosis requires these clinical signs plus confirmation of high blood or urine ketone concentrations and metabolic acidosis, in conjunction with persistent hyperglycaemia.
Clinicians should be vigilant and monitor patients for the development of diabetic complications (eg, hypoglycaemia, DKA, diabetic neuropathy) or the presence of concomitant disease, particularly if treatment response is poor or erratic.
Overall goals in managing diabetic cats
Despite the favourable prognosis for well-managed cats, euthanasia is sometimes an outcome due to unmet owner expectations or the impact of disease management on owners’ lives (see box, right). Thus, the main goals of management are twofold:

On occasion, achieving both of these goals can be difficult. Given the negative impact hypoglycaemia can have on cats and the concern this causes owners, 30 it is preferable, in the Panel’s opinion, to prioritise avoiding hypoglycaemia at the expense of allowing periods of hyperglycaemia. That said, in many cats, it may be possible to achieve good glycaemic control safely, particularly with good home monitoring by owners, and this in turn may also improve the prospects of diabetic remission (see below).
Controlling hyperglycaemia
Maintaining blood glucose below the renal threshold (~14 mmol/l [252 mg/dl] in most cats) 31 avoids osmotic diuresis, may reduce the risk of glucose toxicity, 32 and should help minimise metabolic derangements associated with DM, including the risk of DKA. Further, exogenous insulin therapy and good control of glycaemia may result in reduced endogenous insulin requirements and ‘resting’ of the β-cells, which may increase their capacity to regain insulin-secreting ability and ameliorate the effects of glucose toxicity.
Diabetic remission may occur in a proportion of treated cats, and appears more common in cats with better glycaemic control.33–36 Early diagnosis (eg, regular screening of obese and older cats), good management and HMBG (see later) may all potentially help improve glycaemic control and the long-term outcome.33–36
Avoiding hypoglycaemia
Hypoglycaemia is defined as BG <3.0–3.5 mmol/l (54–63 mg/dl). The lower cut-off should be reserved for use when the accuracy and precision of the equipment being used to measure BG is good (eg, a reference laboratory).
Mild hypoglycaemia may be tolerated by the cat and may go unnoticed by the owner, but severe hypoglycaemia can be life-threatening and/or result in reactive hyperglycaemia. 37 Avoiding hypoglycaemia with careful insulin therapy and owner education should, therefore, be a priority.
Role of diet
Practical weight loss in the overweight or obese cat with DM
Arresting DM-associated pathological weight loss is the first goal of nutritional management; thus, initially diabetic cats should be fed ad libitum or multiple meals per day. However, obesity is associated with insulin resistance and, in the face of obesity, managed weight loss is likely to improve glycaemic control and increase the prospects of diabetic remission.15,38–40
Body weight and body condition should be monitored regularly (eg, every 1–2 weeks) in all diabetic cats – by owners at home, or in the clinic if required. Calorie restriction is used to encourage weight loss if the cat is overweight, once moderately good glycaemic control has been achieved. Feeding exclusively wet foods may help with weight loss, as wet food consumption tends to reduce calorie consumption compared with dry foods. 41 Additionally, using wet foods may increase total water intake,42–44 which may be of value in diabetic cats. Close monitoring of BG and insulin requirements is recommended during any period of weight loss.
Choosing an optimum diet for cats with DM
Improved management of cats with DM is likely with restricted dietary carbohydrate (CHO). Two randomised controlled studies suggest a potential benefit of lower CHO diets, with higher diabetic remission rates 45 and improved glycaemic control 46 being reported; note, however, that it is impossible to adjust only one element (the CHO content) of a diet. Although the optimal dietary CHO content has not been determined, diets with restricted CHO content (⩽12% metabolisable energy [ME] or 3 g/100 kcal was suggested by the majority of the Panel) 45 are appropriate pending further studies. Most wet cat foods and therapeutic dry cat foods formulated for management of DM are low in CHO. Other cat foods with a higher CHO concentration (including most dry foods) are not recommended as first-choice diets for diabetic cats.
Studies suggest that both the amount and type of CHO in the diet are important determinants of postprandial insulin and glucose concentrations in cats; and, when it occurs, postprandial hyperglycaemia may also be prolonged.47–54 Although low CHO diets designed to manage DM are therefore the preferred option, good control of DM can still be achieved with insulin therapy and a higher CHO diet, so alternative diets may be used if clinically indicated (eg, due to concomitant disease). 55
Recommended feeding regimen for cats with DM
The optimal feeding regimen for cats with DM has been poorly investigated. 54 However, based mainly on studies in healthy cats, when feeding a low CHO diet (and one with complex CHOs) it appears that the timing of meals does not need to be matched to insulin injections, as clinically relevant postprandial increases in BG are unlikely. In practice, as postprandial changes in glucose may be uncertain, and to reduce possible diet-related increases in glucose, some clinicians prefer to ensure cats are fed at the same time as they receive insulin injections; for some owners, injecting the cat while it is eating may also be easier.
Frequency of feeding is likewise not critical – continuing the normal frequency for the individual cat is generally advised (assuming a minimum of two meals daily), as is maintaining a daily routine. Ad libitum feeding may be acceptable for some cats, but especially with obese cats daily food allowances should be accurately measured.
Where food is withheld (eg, overnight, prior to anaesthesia), and depending on the diet consumed, there may be no significant effect on the morning BG concentration. 54 Nevertheless, it is safer to administer 50% of the normal insulin dose and then monitor BG, supplementing with additional insulin or glucose as required.
Role of oral hypoglycaemic agents
While oral hypoglycaemics are often used in type 2 diabetics in human medicine, there is no good evidence to support their use in preference to insulin therapy in cats. A recent review summarises current knowledge of these agents in cats (Table 1). 56
Table 1.
Use of oral hypoglycaemics in diabetic cats - summary of current knowledge
| Action | Examples | Notes |
|---|---|---|
| Promotion of insulin secretion from the pancreas |
Sulfonylureas • Glipizide • Glyburide • Glimepiride |
Oral glipizide has been used successfully in cats with DM, with benefits being reported in >40% cats,57–59 but transdermal application is unreliable. 60 Adverse effects include cholestasis, hypoglycaemia and vomiting.57–58 Glimepiride stimulates insulin secretion in healthy cats, 61 but has not yet been evaluated in diabetic cats. There is a concern that all these agents may contribute to progression of pancreatic amyloidosis and the underlying disease 62 |
|
Incretins • GLP-1 (glucagon-like peptide 1) • DPP-4 (dipeptidyl peptidase-4) |
GLP-1 and DPP-4 increase insulin secretion in healthy cats,63–65 but have not yet been evaluated in diabetic cats | |
| Inhibition of intestinal glucose absorption |
α-glucosidase inhibitors • Acarbose |
Acarbose inhibits intestinal glucose production and reduces postprandial hyperglycaemia in healthy cats fed a high (but not low) CHO diet. 66 The only published data in cats with DM was an uncontrolled study in cats fed a low CHO diet 39 |
| Inhibition of hepatic glucose production |
Biguanides • Metformin |
Metformin has limited value in diabetic cats. 67 It should be avoided with concomitant kidney disease, and there may be dose-dependent gastrointestinal adverse effects67,68 |
|
Thiazolidinediones • Troglitazone • Darglitazone • Pioglitazone |
Troglitazone has poor bioavailability in cats
69
and is no longer used in humans. Darglitazone and pioglitazone improved insulin sensitivity and lipid metabolism in obese cats,70–71 but have not been evaluated in diabetic cats |
|
| Improvement of peripheral insulin sensitivity | Thiazolidinediones | See above |
|
Transition metals • Chromium • Vanadium |
Chromium supplementation improved glucose tolerance in non-diabetic cats in one study, 72 but not in another. 73 In a small group of diabetic cats, those supplemented with vanadium apparently had lower insulin requirements. 74 However, use of these metals is likely only to significantly benefit cats with chromium or vanadium deficiency |
DM = diabetes mellitus; CHO = carbohydrate
Recommendations on using oral hypoglycaemics
The main indication for using an oral hypoglycaemic is when owners initially refuse insulin treatment. Currently, glipizide is the only agent with sufficient evidence to support its use as sole therapy in cats. It is given at an initial dose of 2.5 mg PO q12h. If there are no adverse effects and glycaemic control has not been achieved after 2 weeks, the dose can be increased to 5 mg q12h. A clinical response, if seen, is usually apparent after 4–6 weeks. (See Table 1 for potential problems with glipizide use.)
Owners frequently change to insulin treatment when glipizide is found to be ineffective. This transition can often be achieved within a few weeks – earlier being valuable to avoid missing the ‘window of opportunity’ for reversal of glucose toxicity and achieving diabetic remission.
Insulin choices
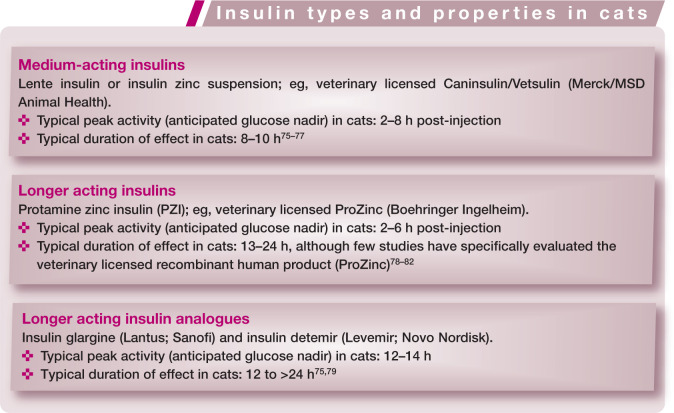
There are many insulin formulations available worldwide, some specifically licensed in cats, which can be used to manage feline DM safely and effectively, especially when combined with an appropriate diet. The choice of insulin used by a clinician will depend on availability, familiarity and the properties of the insulin itself. Additionally, in some countries, regulations may limit the first-line choice to certain veterinary registered products.
Insulin preparations available worldwide and suitable for long-term use in cats with DM fall into three main groups (see box below). The pharmacokinetics of these insulin preparations varies between insulin type, individual cats, and between different formulations of the same type. Additionally, the pharmacokinetics are influenced by the methodology used in different studies. While no available insulin shares the same amino acid sequence as feline insulin, production of anti-insulin antibodies does not appear to be a significant clinical problem in cats. 12
Although in many pharmacodynamic studies of healthy cats, insulin glargine and insulin detemir have been shown to have a duration of activity of over 24 h,75,79 there is evidence from alternative studies that their true clinical duration of activity may be closer to 10–16 h,83,84 while comparative studies for PZI have not been done. Additionally, while a clear BG nadir is seen in some cats with these longer acting insulin analogues, a much smoother curve is seen in others. 84 Insulin glargine, insulin detemir and PZI last longer in cats than lente insulin,79,83 and thus are likely to provide better control of diabetes when used twice daily. Additionally, longer acting insulin preparations may produce a more gradual decline in BG following injection in many cats.
Recommendations for insulin preparation and frequency of dosing
Although good control of DM can be achieved in cats with both intermediate and longer acting insulin preparations, and definitive comparative studies in diabetic cats are lacking, given current knowledge of the pharmacodynamics of insulin preparations in cats, the Panel recommends, whenever possible, the use of longer acting insulin preparations (eg, glargine, detemir or PZI), injected twice daily, for optimal diabetic control.
Rigid adherence to a 12 hourly injection schedule, although ideal, will be unachievable for many owners. Allowing flexibility with dosing (12 h ± 2 h), and/or simply missing an insulin injection when work or social commitments preclude dosing at the correct time, are acceptable compromises.
The primary goal of therapy is to minimise clinical signs associated with DM. The more specific aims of insulin therapy can be defined as:
If control with twice daily administration of insulin is proving difficult, the possibility of more frequent insulin dosing and/or use of a different preparation should be considered.
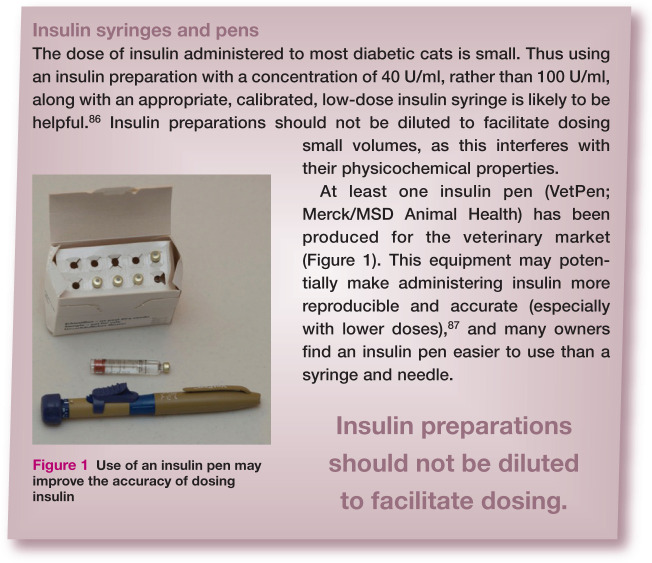
Many insulin preparations contain 100 U/ml, while others (such as Caninsulin/ Vetsulin [Merck/MSD Animal Health] and ProZinc [Boehringer Ingelheim]) are formulated to 40 U/ml, which can be helpful for accuracy of dosing with syringes in cats. It is essential to ensure that insulin syringes or pens are used that are appropriate for the insulin concentration being used.
Manufacturers often state that, once opened, insulin vials should be discarded after 4–6 weeks. With careful handling and refrigerated storage, it has been suggested that at least some insulin preparations can be safely used for between 3 and 6 months. 85 However, any deviation from manufacturers’ recommendations should be undertaken cautiously. If insulin is ever stored and used for longer than recommended, considerable care is needed as repeated needle puncture renders the vials vulnerable to contamination and owners must be advised to discard insulin if it becomes discoloured or more cloudy than usual.
Initial management of the diabetic cat
The starting dose of an intermediate or longer acting insulin preparation in a non-ketotic cat is generally:

Because hyperglycaemia itself causes insulin resistance and β-cell dysfunction, successful treatment may reduce insulin requirements after a variable period of time. Early monitoring of BG is thus aimed mainly at identifying hypoglycaemia, which might require a reduction in insulin dose. Increases in insulin doses (if required) should be made on the basis of persistent clinical signs supported by assessment of glycaemia; for example, serial BG estimates collected either at home or in the clinic, starting approximately 5–7 days after initiating therapy.
Doses should generally not be increased more frequently than every 5–7 days. Rapid escalation in dose is a common cause of hypoglycaemia, rebound hyperglycaemia and poor control.
Figure 1.
Use of an insulin pen may improve the accuracy of dosing insulin
Most cats with uncomplicated DM (ie, clinically well, with no DKA or other major complications) are best initially managed at home with insulin and dietary therapy. Some veterinarians and owners prefer to manage the cat in the clinic for the first few days, to help ensure severe hypoglycaemia does not develop and to assess the initial response to insulin. However, owners should be made aware that stabilisation would not be achieved within these first few days.
In preparation for the cat’s discharge from the clinic, the owner should be educated in the technical aspects of treating a diabetic cat. This should include detailed instructions and demonstrations about:
Transition to optimal diet and feeding.
Using insulin syringes and/or insulin pens.
Correct handling, storage and injection of insulin.
Clinical signs of hypoglycaemia, and how to treat low glucose concentrations.
Written instructions for the owner are invaluable. Additional web-based owner resources are also available (see page 247).
Home (outpatient) stabilisation of the diabetic cat
The following guidelines are offered, but some will vary according to the type of insulin used as indicated.
Five to 10 days post-discharge
The cat should be re-examined in the clinic, either after it has received food and insulin at home, or before the insulin dose is due if the cat eats well when hospitalised.
A thorough physical examination should be performed, the history of clinical response at home, including home records of daily water intake, urine glucose testing, and so on, reviewed, and laboratory parameters re-evaluated as needed.
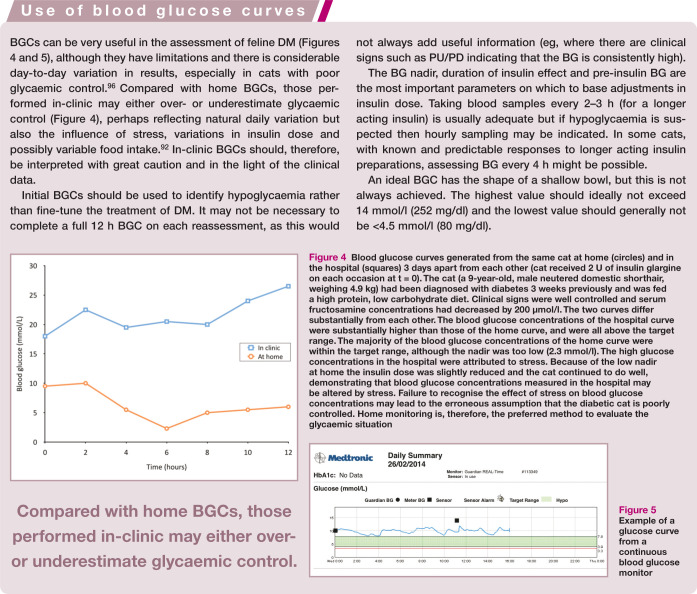
A blood glucose curve (BGC) should be performed, where possible and appropriate (see box on page 242). Ideally, BG is measured every 1–2 h (for lente insulin) or every 2–3 h (for longer acting preparations) for at least 12 h, bearing in mind that BG can vary from day to day within individuals.
As cats are prone to stress hyperglycaemia, the accuracy of data generated from in-hospital curves may be questionable. Serum fructosamine measurement and/or the use of HMBG (see later) can, therefore, be helpful.
Some clinics use a continuous glucose monitoring (CGM) system to evaluate the response to insulin (Figure 2) – this can reduce the risk of stress hyperglycaemia induced by repeat sampling for BG, allow detection of brief periods of hypoglycaemia and facilitate overnight BG monitoring. 88 CGM systems can also be used in the home, but owners must be able to take blood from the cat for calibration of the machine. The dorsal neck may be the most appropriate site for sensor placement. 89
Insulin adjustments are made according to results of clinical monitoring, BG concentrations and clinical signs (Table 2).
Figure 2.
Diabetic cat with a continuous blood glucose monitor in place
Table 2.
Blood glucose (BG) concentrations and possible alterations in insulin after first 5-10 days of therapy
| Blood glucose | Action |
|---|---|
| Nadir BG <4.5 mmol/l (80 mg/dl) | • Reduce insulin dose by 50% • Consider longer acting insulin if peak BG >14 mmol/l (252 mg/dl) |
| BG 4.5-14 mmol/l (80-252 mg/dl) on all samples measured throughout the day | Maintain therapy |
| Peak BG >14 mmol/l (252 mg/dl) and nadir BG 4.5-8.0 mmol/l (80-144 mg/dl) | • Maintain therapy and retest after 1-2 weeks; or • Change insulin (if using lente insulin) to a longer acting insulin; or • Increase dose by 0.5 U/cat q12h (depending on peak and nadir BG) |
| Peak BG >14 mmol/l (252 mg/dl) and nadir BG >8.0 mmol/l (144 mg/dl), with signs of ongoing hyperglycaemia | Increase dose by 0.5-1.0 U/cat q12h |
Three weeks post-discharge (depending on prior response)
The cat is re-evaluated as above, with a BGC performed at home or in the clinic.
HMBG should, where possible and appropriate, be discussed with the owner and support material provided.
Home monitoring of blood glucose
The Panel encourages the use of HMBG, as this helps provide more control over the disease, aids in the identification of hypoglycaemia and may provide better glycaemic control.90,91 Although not all owners are able to perform HMBG, 91 it can be successfully undertaken by most, with sufficient support, and should ideally be introduced early in the management of DM. Assistance (provided by the veterinarian and/ or veterinary nurse/technician) should be readily available for owners.92,93
An experienced technician or veterinarian should teach capillary or marginal ear vein blood sampling using a lancing device and a portable glucose meter validated for use in cats, during an extended consultation (Figure 3). Blood may be obtained interchangeably from the pinnae and metacarpal or metatarsal pads, and the procedure is well tolerated by most cats.94,95 Printed and web-based resources should be made available to owners (see page 247).
Figure 3.
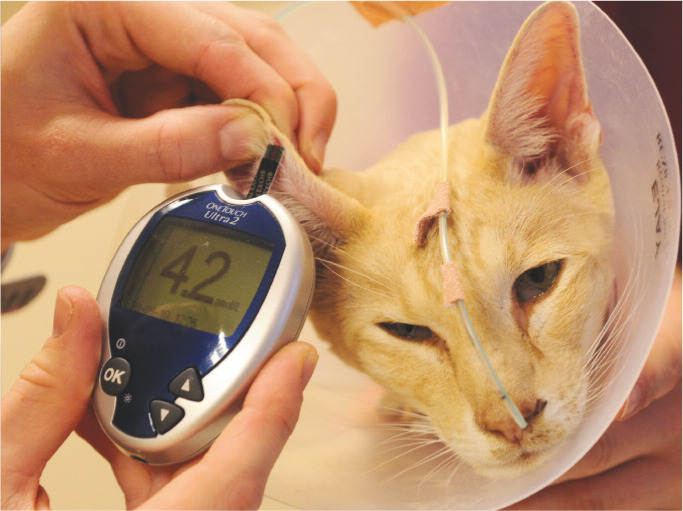
Monitoring blood glucose from the marginal ear vein – a technique that owners can use at home
Figure 4.
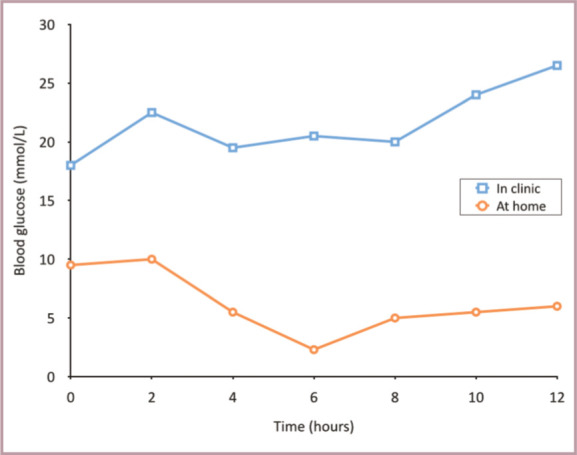
Blood glucose curves generated from the same cat at home (circles) and in the hospital (squares) 3 days apart from each other (cat received 2 U of insulin glargine on each occasion at t = 0).The cat (a 9-year-old, male neutered domestic shorthair, weighing 4.9 kg) had been diagnosed with diabetes 3 weeks previously and was fed a high protein, low carbohydrate diet. Clinical signs were well controlled and serum fructosamine concentrations had decreased by 200 umol/l.The two curves differ substantially from each other. The blood glucose concentrations of the hospital curve were substantially higher than those of the home curve, and were all above the target range. The majority of the blood glucose concentrations of the home curve were within the target range, although the nadir was too low (2.3 mmol/l). The high glucose concentrations in the hospital were attributed to stress. Because of the low nadir at home the insulin dose was slightly reduced and the cat continued to do well, demonstrating that blood glucose concentrations measured in the hospital may be altered by stress. Failure to recognise the effect of stress on blood glucose concentrations may lead to the erroneous assumption that the diabetic cat is poorly controlled. Home monitoring is, therefore, the preferred method to evaluate the glycaemic situation
Figure 5.
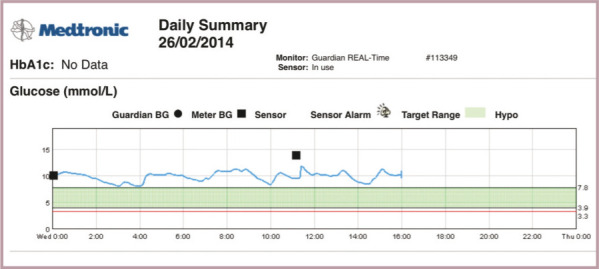
Example of a glucose curve from a continuous blood glucose monitor
A home BGC can be obtained by measuring BG before the morning insulin injection, and every 2–3 h for 12 h (or hourly if hypoglycaemia is suspected). However, owners must be counselled not to make decisions regarding insulin dosage without discussion with the veterinarian.
Intensive management of feline DM
Intensive management of DM has been described using regular HMBG (generally a minimum of three, and on average five, BG samples daily) and appropriate adjustments of insulin,35,36,85 with the aim of maintaining tighter control of BG concentrations and thus keeping BG closer to the physiological range.

There is some evidence from two small studies using twice daily glargine injections in diabetic cats that tighter control of BG may be more likely to result in remission of DM.33,34 There is also some evidence that the longer acting insulin analogues (glargine and detemir) may be less likely to produce clinical hypoglycaemia when aiming for tighter control of BG,33,34,101 although good comparative studies are lacking.
Studies involving intensive management of DM have often recommended aiming for a BG concentration between a low of 2.8–3.0 mmol/l (50–54 mg/dl) and a high of 5.5–11.1 mmol/l (99–200 mg/dl) throughout a 24 h period.34–36,85
However, the Panel believes that:
Undertaking multiple BG measurements every day is not suitable for most owners of diabetic cats.
Such an aggressive approach and tight control of BG is unrealistic for most owners to achieve safely.
While intensive monitoring of diabetes may allow a target of lower peak BG levels (eg, ⩽11 mmol/l [198 mg/dl]), unless and until evidence emerges (through use of randomised controlled clinical studies) of a clear clinical benefit, aiming for a BG nadir <4.5 mmol/l (81 mg/dl) should be avoided.
Long-term management of the diabetic cat
Monitoring at home
Information provided by the cat owner is highly valuable in the assessment of diabetic control, 102 and is especially important if repeated BG monitoring is unavailable (eg, due to financial limitations). Owners should be encouraged to keep a diary (Figure 6) and record the cat’s:
Figure 6.
Example of an owner diary for a cat with DM
Daily overall wellbeing In particular, the cat’s demeanour and activity level.
Daily water intake Average BG corresponds with 24 h water intake 102 and improvements (reductions) in water intake are a useful clinical marker of response to insulin therapy.6,81,102 Daily water intake varies between individual cats, and is also influenced by numerous dietary and environmental factors,43,44, 103–108 in addition to any disease state. In general, cats fed a wet diet will have a higher total water intake than those fed a dry diet, although the volume of water drunk voluntarily (in addition to that consumed in the diet) may be lower.42–44 For these reasons it is impossible to set a target daily voluntary water intake in diabetic cats. Owners can be asked to subjectively assess water intake, but accurate monitoring of daily water consumption is helpful (eg, using a measuring jug with 10 ml increments to record water drunk). If the water bowl is shared between cats, the total volume drunk by all cats can be measured. If water consumption fails to decrease, or increases after being reduced, then re-evaluation of glycaemic control is indicated.
Daily urine production Assessment may be subjective (amount of urine in the litter tray, size/number of urine-soaked clumps of litter) or objective (weight of the litter tray before and after urination).
Daily feeding Amount and type of food offered, and amount of food eaten.
Daily insulin administration Time of administration and dose.
Weekly body weight and body condition score Using accurate scales for weighing (coming into the clinic, if necessary).
Urine glucose Monitoring of glucose in urine samples may be particularly helpful if owners are unable to perform HMBG. Urine can be collected throughout one day per week and owners can use a urine dipstick with pooled urine (collected via non-absorbent litter in the tray or a litter tray with a sieve bottom) or with wet urine-soaked litter. Results should be interpreted cautiously and owners advised not to alter the dose of insulin based on results without prior discussion with their veterinarian. Nevertheless, persistent glucosuria suggests inadequate control, while persistent lack of glucose can reflect variously excellent glycaemic control, diabetic remission or insulin overdose, and further evaluation is required. Urine ketones should also be evaluated (a positive result suggests poor glycaemic control).
- Blood glucose Where good HMBG is possible, ideally the following should be performed and recorded in the diary:
- – BGCs: Weekly until stable and then every 3–4 weeks.
- – Spot BG check: If the owner is concerned about the wellbeing of the cat.
If the owner is attempting intensive management of the DM (see earlier), several daily BG checks may be performed.
Where less frequent HMBG is possible, the following may be performed and recorded in the diary:
– Less frequent BGCs: For example, every 2–6 weeks; and/or
– Spot BG check: If the owner is concerned at any stage; and/or
– BG measurement prior to insulin dosing as frequently as practical.
Together, some or all of these measures may be useful for owners who find performing repeat BGCs difficult or stressful. If BG is measured prior to insulin dosing, insulin can be withheld or a lower dose administered whenever a low reading is obtained, helping to avoid hypoglycaemia. Again, owners must be advised not to adjust insulin dosage without discussion with their veterinarian.
Monitoring in the clinic
Frequent re-evaluations are required initially to slowly titrate the insulin dose, to detect diabetic remission and to identify difficult-to-stabilise cats that require further work-up. The frequency of clinic visits will depend mainly on the response to treatment and the owner’s ability to perform HMBG.
For in-clinic monitoring a guide would be assessment at 1, 2–3, 6–8, 10–12 and 14–16 weeks after initiating treatment. The frequency of clinic re-examinations can then generally be reduced to approximately every 1–4 months depending on how stable the cat is and the conscientiousness/ability of the owner with regard to home monitoring. If diabetic remission seems likely, more frequent checks may be appropriate.
Each re-evaluation will vary according to clinical needs but may include:
Owner diary (see above).
Body weight and body condition.
Physical examination.
In-clinic BGC (eg, after the owner first gives insulin and food at home); this may be desirable if HMBG is not regularly performed.
Measurement of serum fructosamine (see page 245).
Adjusting insulin therapy
Clinical signs and BG measurements (at home or in-clinic, taking into account their limitations) are the most important parameters on which to base adjustments in insulin dose. 102 If clinical signs, such as PU/PD, have resolved and body weight is stable, cats are usually well controlled (although some may also be overdosed). Conversely, persistent clinical signs and weight loss suggest poor glycaemic control and/or concomitant disease.
If there is a discrepancy between clinical signs and results of a BGC, treatment decisions should err on the side of caution – fructosamine concentrations should be re-evaluated, and a BGC may be repeated after a few days before any treatment decision is taken.
During the first 3–4 months of therapy the veterinarian should interpret BGCs, and make decisions on treatment adjustments (Table 3). However, with support from the veterinary healthcare team, during long-term management owners may gain sufficient experience to make (slight) insulin adjustments on their own, according to written guidelines. Dose adjustments should be made no more often than every 5–7 days, except in the case of hypoglycaemia, to allow time for equilibration to a new insulin dose.
Table 3.
Suggested insulin adjustment based on blood glucose (BG) in diabetic cats on long-term management
| Blood glucose | Insulin adjustment |
|---|---|
| Nadir BG <4.5 mmol/l (80 mg/dl) | • Reduce insulin by 0.5-1.0 U/cat q12h if receiving 0.5-3.0 U/cat q12h • Reduce by 25-50% if on a higher insulin dose |
| Nadir BG >8.0 mmol/l (144 mg/dl) | Increase insulin by 0.5-1.0 U/cat q12h |
| Pre-insulin BG 8-10 mmol/l (144-180 mg/dl) | Consider reducing insulin by 0.5 U/cat q12h |
| Pre-insulin BG 4.5-7.9 mmol/l (80-142 mg/dl) | Reduce insulin by at least 0.5 U/cat q12h |
| Pre-insulin BG <4.5 mmol/l (80 mg/dl) | Withhold insulin; if glucose rises appreciably later, give -30-50% of previous dose |
Note: With adjustment of insulin doses it is also valuable to assess the full BGC. The shape of the curve and timing of nadirs may vary between cats and, where available, this information may affect decisions on dose adjustment, the aim being to keep BG between a maximum of 10-14 mmol/l (144-252 mg/dl) and a minimum of 4.5-8 mmol/l (80-144 mg/dl). With glargine and detemir, for example, in some cats the glucose nadir may occur at the time of insulin dosing
Table 4.
Guide to interpretation of fructosamine concentrations in cats with DM
| Fructosamine concentration | General interpretation of glycaemic control |
|---|---|
| <350 μmol/l | Excellent glycaemic control or insulin overdose or diabetic remission |
| 350-450 μmol/l | Good glycaemic control |
| 450-550 μmol/l | Moderate glycaemic control |
| >550 μmol/l | Poor glycaemic control |

The primary goal of management is defined above. Note that:
During initial stabilisation the insulin dose should be increased gradually in steps of 0.5–1.0 U/cat q12h until the glucose nadir is 4.5–8.0 mmol/l (80–144 mg/dl).
If the glucose nadir is in the desired range – but the duration of insulin effect is consistently less than 8–10 h and there are clinical signs of inadequate glycaemic control – the cat should be switched to a longer acting insulin preparation.
Ideally, a BGC should be performed 5–7 days after any adjustment in insulin dose or change in insulin preparation. However, a BGC should be repeated sooner (after 1–3 days) where there is a higher risk of hypoglycaemia (eg, a glucose nadir <4.5 mmol/l [80 mg/dl] or a pre-injection glucose of <8 mmol/l [144 mg/dl]).
Where BGCs are not possible, insulin adjustment should be made with extreme care. The dose should be increased in small steps (0.5 U/cat q12h) no more often than every 7 days until clinical signs resolve and glucosuria is negative or reduced to trace amounts. Serial fructosamine concentrations should also be measured (see box above).
Most cats ultimately require insulin doses between 0.5 and 6.0 U/cat q12h for diabetic control, but if the dose is >1.5 U/kg q12h investigation of causes of insulin resistance should be considered.
Diabetic remission
Detection and management of diabetic remission can be challenging. If urine is persistently negative for glucose, all BG measurements are within the normal range (<~7.5 mmol/l [135 mg/dl]) and/or serum fructosamine is <350 µmol/l, the insulin dose should generally be reduced by 0.25–1.0 U/cat q12h every 1–2 weeks. With low or low-normal BG concentrations, more rapid reduction (or temporary withholding of insulin therapy) may be required.
When a dose of 0.5 U/cat/day (0.5 U/cat q24h or 0.25 U/cat q12h) is reached and BG remains normal, insulin administration should be ceased. If negative glucosuria and/or euglycaemia are maintained for 2–4 weeks without insulin, the cat has likely achieved remission.
If BG measurements cannot be made, and remission is suspected in a well-regulated cat without glucosuria, the insulin dose may be slowly reduced (0.5–1.0 U/cat q12h every 1–2 weeks), either until clinical signs or glucosuria reappear, or until insulin administration can be ceased.
Cats in remission should remain on a low CHO diet and should be monitored closely for recurrence of clinical signs. Regular BG or urine glucose measurements (eg, twice weekly initially) are also recommended and can be performed by the owner at home where possible.
Complications with the management of DM
Diabetic ketoacidosis
Not all cats with ketonaemia and ketonuria will be suffering from DKA, but cats with DKA will have a low blood pH and will be unwell. A ketotic non-acidotic cat can be managed in the same way as a non-ketotic cat, but if DKA is present immediate hospitalisation with intensive treatment and monitoring is required. The main objectives in treatment are to:
Correct dehydration and electrolyte deficits.
Correct acidosis (using bicarbonate only if appropriate fluid therapy is not sufficient).
Provide adequate amounts of insulin to normalise intermediary metabolism (ie, gradually stop ketogenesis and reduce hyperglycaemia).
Provide a parenteral CHO source in vomiting animals and when required during insulin treatment.
Identify precipitating factors (eg, infection) in the disease process.
For these cats, a short-acting insulin preparation (eg, regular/soluble insulin) is generally given either intramuscularly (IM) or by intravenous infusion. 114 If regular/soluble insulin is not available, it has been suggested that glargine may also be given IM or intravenously (IV), 115 although good data on its efficacy by these routes is currently lacking. When the cat is stabilised, insulin treatment is changed to a longer acting insulin via the subcutaneous route and the cat managed as a stable diabetic.
Hypoglycaemia
Hypoglycaemia (BG <3.0 mmol/l [54 mg/dl]) may be more common in diabetic cats than dogs, 116 can be life-threatening and should be treated rapidly. Owners should be advised on signs (eg, seizures, recumbency, anorexia, tremors, vomiting, ataxia, lethargy) and home first aid treatment (including liberal application of honey or glucose to mucous membranes). Ideally, owners should keep dextrose gels (available from human pharmacies) at home in case of hypoglycaemia.
Severe hypoglycaemia appears to be more common in cats receiving doses of insulin >6 U/cat, 116 and requires in-clinic management with parenteral glucose supplementation. A 50% dextrose solution should be diluted 1:2 and an initial dose of 2–4 ml of the 25% solution (ie, 0.5–1.0 g glucose) administered slowly IV over 5–10 mins. Blood glucose should be monitored and further dextrose administered to effect. Euglycaemia should result in rapid clinical improvement, but treatment should still be followed with a 5% dextrose constant rate infusion (CRI) adjusted to maintain euglycaemia with BG being closely monitored. Insulin antagonists, such as corticosteroids, or a glucagon CRI may also be used.
If insulin therapy needs to be re-instituted, this should be done cautiously, with very close monitoring.

The unstable diabetic cat
While detailed discussion of the unstable diabetic cat is outside the scope of these Guidelines, clearly any unstable diabetic should undergo further investigation. The precise nature of this may vary according to the problems encountered, and the presence of other known or previous concurrent diseases.
Investigations to consider include:
Checking the owner’s insulin storage and administration.
- Reviewing the diabetic history to assess:
- – Starting dose of insulin.
- – Type of insulin, duration of effect and BG nadir.
- – Over what time period insulin has been increased and by how much.
- – BGCs (home vs in-clinic curves).
- – Fructosamine results.
- – Monitoring of clinical signs, body weight change, dietary history, and any other medications that the cat is receiving.
- Further assessment for concurrent disease, to include consideration of:
- – Physical examination, including body weight and body condition.
- – Urinalysis and culture.
- – CBC and serum biochemistry.
- – Diagnostic imaging (including evaluation of the pancreas).
- – Evaluation for acromegaly (eg, insulin-like growth factor-1 assay, growth hormone assay).
- – Evaluation of thyroid function (eg, total T4).
- – Evaluation of adrenal function (eg, low dose dexamethasone suppression test).

Supplemental Material
French Version of the guidelines
Acknowledgments
The ISFM would like to thank Boehringer Ingelheim, which helped to support the development of these Guidelines.
Footnotes
Funding: These Guidelines were supported by an educational grant from Boehringer Ingelheim to the ISFM.
The Panel members have no conflicts of interest to declare.
References
- 1. Baral RM, Rand JS, Catt MJ, et al. Prevalence of feline diabetes mellitus in a feline private practice. J Vet Intern Med 2003; 17: 433–434. [Google Scholar]
- 2. McCann TM, Simpson KE, Shaw DJ, et al. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis. J Feline Med Surg 2007; 9: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sallander M, Eliasson J, Hedhammar Å. Prevalence and risk factors for the development of diabetes mellitus in Swedish cats. Acta Vet Scand 2012; 54: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prahl A, Guptill L, Glickman NW, et al. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg 2007; 9: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callegari C, Mercuriali E, Hafner M, et al. Survival time and prognostic factors in cats with newly diagnosed diabetes mellitus: 114 cases (2000–2009). J Am Vet Med Assoc 2013; 243: 91–95. [DOI] [PubMed] [Google Scholar]
- 6. Goossens MM, Nelson RW, Feldman EC, et al. Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985–1995). J Vet Intern Med 1998; 12: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Kraus MS, Calvert CA, Jacobs GJ, et al. Feline diabetes mellitus: a retrospective mortality study of 55 cats (1982–1994). J Am Anim Hosp Assoc 1997; 33: 107–111. [DOI] [PubMed] [Google Scholar]
- 8. Hoenig M. Carbohydrate metabolism and pathogenesis of diabetes mellitus in dogs and cats. Prog Mol Biol Transl Sci 2014; 121: 377–412. [DOI] [PubMed] [Google Scholar]
- 9. Nelson RW, Reusch CE. Animal models of disease: classification and etiology of diabetes in dogs and cats. J Endocrinol 2014; 222: T1–T9. [DOI] [PubMed] [Google Scholar]
- 10. Osto M, Zini E, Reusch CE, et al. Diabetes from humans to cats. Gen Comp Endocrinol 2013; 182: 48–53. [DOI] [PubMed] [Google Scholar]
- 11. Rand JS. Pathogenesis of feline diabetes. Vet Clin North Am Small Anim Pract 2013; 43: 221–231. [DOI] [PubMed] [Google Scholar]
- 12. Hoenig M, Reusch C, Peterson ME. Beta cell and insulin antibodies in treated and untreated diabetic cats. Vet Immunol Immunopathol 2000; 77: 93–102. [DOI] [PubMed] [Google Scholar]
- 13. Webb CB, Falkowski L. Oxidative stress and innate immunity in feline patients with diabetes mellitus: the role of nutrition. J Feline Med Surg 2009; 11: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zini E, Osto M, Moretti S, et al. Hyperglycaemia but not hyperlipidaemia decreases serum amylase and increases neutrophils in the exocrine pancreas of cats. Res Vet Sci 2010; 89: 20–26. [DOI] [PubMed] [Google Scholar]
- 15. Hoenig M, Thomaseth K, Waldron M, et al. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol 2007; 292: R227–R234. [DOI] [PubMed] [Google Scholar]
- 16. Appleton DJ, Rand JS, Sunvold GD. Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain. J Feline Med Surg 2001; 3: 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori A, Sako T, Lee P, et al. Comparison of three commercially available prescription diet regimens on short-term post-prandial serum glucose and insulin concentrations in healthy cats. Vet Res Commun 2009; 33: 669–680. [DOI] [PubMed] [Google Scholar]
- 18. Panciera DL, Thomas CB, Eicker SW, et al. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). J Am Vet Med Assoc 1990; 197: 1504–1508. [PubMed] [Google Scholar]
- 19. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998; 212: 1725–1731. [PubMed] [Google Scholar]
- 20. Lederer R, Rand JS, Jonsson NN, et al. Frequency of feline diabetes mellitus and breed predisposition in domestic cats in Australia. Vet J 2009; 179: 254–258. [DOI] [PubMed] [Google Scholar]
- 21. Rand JS, Bobbermien LM, Hendrikz JK, et al. Over representation of Burmese cats with diabetes mellitus. Aust Vet J 1997; 75: 402–405. [DOI] [PubMed] [Google Scholar]
- 22. Slingerland LI, Fazilova VV, Plantinga EA, et al. Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus. Vet J 2009; 179: 247–253. [DOI] [PubMed] [Google Scholar]
- 23. Crenshaw KL, Peterson ME, Heeb LA, et al. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. J Vet Intern Med 1996; 10: 360–364. [DOI] [PubMed] [Google Scholar]
- 24. Lowe AD, Campbell KL, Graves T. Glucocorticoids in the cat. Vet Dermatol 2008; 19: 340–347. [DOI] [PubMed] [Google Scholar]
- 25. Peterson ME. Effects of megestrol acetate on glucose tolerance and growth hormone secretion in the cat. Res Vet Sci 1987; 42: 354–357. [PubMed] [Google Scholar]
- 26. Link KR, Rand JS. Changes in blood glucose concentration are associated with relatively rapid changes in circulating fructosamine concentrations in cats. J Feline Med Surg 2008; 10: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plier ML, Grindem CB, MacWilliams PS, et al. Serum fructosamine concentration in nondiabetic and diabetic cats. Vet Clin Pathol 1998; 27: 34–39. [DOI] [PubMed] [Google Scholar]
- 28. Reusch CE, Haberer B. Evaluation of fructosamine in dogs and cats with hypo- or hyperproteinaemia, azotaemia, hyperlipidaemia and hyperbilirubinaemia. Vet Rec 2001; 148: 370–376. [DOI] [PubMed] [Google Scholar]
- 29. Forcada Y, German AJ, Noble PJ, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008; 10: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niessen SJ, Powney S, Guitian J, et al. Evaluation of a quality-of-life tool for cats with diabetes mellitus. J Vet Intern Med 2010; 24: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 31. Kruth SA, Cowgill LD. Renal glucose transport in the cat [abstract]. Proceedings of the American College of Veterinary Internal Medicine Forum, 1982, p 78. [Google Scholar]
- 32. Link KR, Allio I, Rand JS, et al. The effect of experimentally induced chronic hyperglycaemia on serum and pancreatic insulin, pancreatic islet IGF-I and plasma and urinary ketones in the domestic cat (Felis felis). Gen Comp Endocrinol 2013; 188: 269–281. [DOI] [PubMed] [Google Scholar]
- 33. Marshall RD, Rand JS, Morton JM. Treatment of newly diagnosed diabetic cats with glargine insulin improves glycaemic control and results in higher probability of remission than protamine zinc and lente insulins. J Feline Med Surg 2009; 11: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nack R, DeClue AE. In cats with newly diagnosed diabetes mellitus, use of a near-euglycemic management paradigm improves remission rate over a traditional paradigm. Vet Q 2014; 25: 1–5. [DOI] [PubMed] [Google Scholar]
- 35. Roomp K, Rand J. Intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine. J Feline Med Surg 2009; 11: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roomp K, Rand J. Evaluation of detemir in diabetic cats managed with a protocol for intensive blood glucose control. J Feline Med Surg 2012; 14: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMillan FD, Feldman EC. Rebound hyperglycemia following overdosing of insulin in cats with diabetes mellitus. J Am Vet Med Assoc 1986; 188: 1426–1431. [PubMed] [Google Scholar]
- 38. Biourge V, Nelson RW, Feldman EC, et al. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med 1997; 11: 86–91. [DOI] [PubMed] [Google Scholar]
- 39. Mazzaferro EM, Greco DS, Turner AS, et al. Treatment of feline diabetes mellitus using an alpha-glucosidase inhibitor and a low-carbohydrate diet. J Feline Med Surg 2003; 5: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effects of weight loss in obese cats on biochemical analytes related to inflammation and glucose homeostasis. Domest Anim Endocrinol 2012; 42: 129–141. [DOI] [PubMed] [Google Scholar]
- 41. Wei A, Fascetti AJ, Villaverde C, et al. Effect of water content in a canned food on voluntary food intake and body weight in cats. Am J Vet Res 2011; 72: 918–923. [DOI] [PubMed] [Google Scholar]
- 42. Buckley CM, Hawthorne A, Colyer A, et al. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br J Nutr 2011; 106 Suppl 1: S128–S130. [DOI] [PubMed] [Google Scholar]
- 43. Carciofi AC, Bazzoli RS, Zanni A. Influence of water content and the digestibility of pet foods on the water balance of cats. Braz J Vet Res Anim Sci 2005; 42: 429–434. [Google Scholar]
- 44. Seefeldt SL, Chapman TE. Body water content and turnover in cats fed dry and canned rations. Am J Vet Res 1979; 40: 183–185. [PubMed] [Google Scholar]
- 45. Bennett N, Greco DS, Peterson ME, et al. Comparison of a low carbohydrate–low fiber diet and a moderate carbohydrate–high fiber diet in the management of feline diabetes mellitus. J Feline Med Surg 2006; 8: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hall TD, Mahony O, Rozanski EA, et al. Effects of diet on glucose control in cats with diabetes mellitus treated with twice daily insulin glargine. J Feline Med Surg 2009; 11: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de-Oliveira LD, Carciofi AC, Oliveira MC, et al. Effects of six carbohydrate sources on diet digestibility and postprandial glucose and insulin responses in cats. J Anim Sci 2008; 86: 2237–2246. [DOI] [PubMed] [Google Scholar]
- 48. Farrow HA, Rand JS, Morton JM, et al. Effect of dietary carbohydrate, fat, and protein on postprandial glycemia and energy intake in cats. J Vet Intern Med 2013; 27: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 49. Hewson-Hughes AK, Gilham MS, Upton S, et al. The effect of dietary starch level on postprandial glucose and insulin concentrations in cats and dogs. Br J Nutr 2011; 106 Suppl 1: S105–S109. [DOI] [PubMed] [Google Scholar]
- 50. Mimura K, Mori A, Lee P, et al. Impact of commercially available diabetic prescription diets on short-term postprandial serum glucose, insulin, triglyceride and free fatty acid concentrations of obese cats. J Vet Med Sci 2013; 75: 929–937. [DOI] [PubMed] [Google Scholar]
- 51. Appleton DJ, Rand JS, Priest J, et al. Dietary carbohydrate source affects glucose concentrations, insulin secretion, and food intake in overweight cats. Nutr Res 2004; 24: 447–467. [Google Scholar]
- 52. Farrow H, Rand JS, Morton JM, et al. Postprandial glycaemia in cats fed a moderate carbohydrate meal persists for a median of 12 hours – female cats have higher peak glucose concentrations. J Feline Med Surg 2012; 14: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kienzle E. Blood sugar levels and renal sugar excretion after the intake of high carbohydrate diets in cats. J Nutr 1994; 124: 2563S–2567S. [DOI] [PubMed] [Google Scholar]
- 54. Martin GJ, Rand JS. Food intake and blood glucose in normal and diabetic cats fed ad libitum. J Feline Med Surg 1999; 1: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zoran DL, Rand JS. The role of diet in the prevention and management of feline diabetes. Vet Clin North Am Small Anim Pract 2013; 43: 233–243. [DOI] [PubMed] [Google Scholar]
- 56. Palm CA, Feldman EC. Oral hypoglycemics in cats with diabetes mellitus. Vet Clin North Am Small Anim Pract 2013; 43: 407–415. [DOI] [PubMed] [Google Scholar]
- 57. Feldman EC, Nelson RW, Feldman MS. Intensive 50-week evaluation of glipizide administration in 50 cats with previously untreated diabetes mellitus. J Am Vet Med Assoc 1997; 210: 772–777. [PubMed] [Google Scholar]
- 58. Ford SL. NIDDM in the cat: treatment with the oral hypoglycemic medication, glipizide. Vet Clin North Am Small Anim Pract 1995; 25: 599–615. [DOI] [PubMed] [Google Scholar]
- 59. Nelson RW, Feldman EC, Ford SL, et al. Effect of an orally administered sulfonylurea, glipizide, for treatment of diabetes mellitus in cats. J Am Vet Med Assoc 1993; 203: 821–827. [PubMed] [Google Scholar]
- 60. Bennett N, Papich MG, Hoenig M, et al. Evaluation of transdermal application of glipizide in a pluronic lecithin gel to healthy cats. Am J Vet Res 2005; 66: 581–588. [DOI] [PubMed] [Google Scholar]
- 61. Mori A, Lee P, Yamashita T, et al. Effect of glimepiride and nateglinide on serum insulin and glucose concentration in healthy cats. Vet Res Commun 2009; 33: 957–970. [DOI] [PubMed] [Google Scholar]
- 62. Hoenig M, Hall G, Ferguson D, et al. A feline model of experimentally induced islet amyloidosis. Am J Pathol 2000; 157: 2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gilor C, Graves TK, Gilor S, et al. The GLP-1 mimetic exenatide potentiates insulin secretion in healthy cats. Domest Anim Endocrinol 2011; 41: 42–49. [DOI] [PubMed] [Google Scholar]
- 64. Padrutt I, Zini E, Kaufmann K, et al. Comparison of the GLP-1 analogues exenatide short-acting, exenatide long-acting and the DPP-4 inhibitor sitagliptin to increase insulin secretion in healthy cats [abstract]. J Vet Intern Med 2012; 26: 1520–1521. [Google Scholar]
- 65. Reusch C, Padrutt I. New incretin hormonal therapies in humans relevant to diabetic cats. Vet Clin North Am Small Anim Pract 2013; 43: 417–433. [DOI] [PubMed] [Google Scholar]
- 66. Singh R, Rand JS, Coradini M, et al. Effect of acarbose on postprandial blood glucose concentrations in healthy cats fed low and high carbohydrate diets. J Feline Med Surg. Epub ahead of print 24 October 2014. DOI: 1098612X14556559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nelson R, Spann D, Elliott D, et al. Evaluation of the oral antihyperglycemic drug metformin in normal and diabetic cats. J Vet Intern Med 2004; 18: 18–24. [DOI] [PubMed] [Google Scholar]
- 68. Michels GM, Boudinot FD, Ferguson DC, et al. Pharmacokinetics of the antihyperglycemic agent metformin in cats. Am J Vet Res 1999; 60: 738–742. [PubMed] [Google Scholar]
- 69. Michels GM, Boudinot FD, Ferguson DC, et al. Pharmacokinetics of the insulin-sensitizing agent troglitazone in cats. Am J Vet Res 2000; 61: 775–778. [DOI] [PubMed] [Google Scholar]
- 70. Clark M, Thomaseth K, Dirikolu L, et al. Effects of pioglitazone on insulin sensitivity and serum lipids in obese cats. J Vet Intern Med 2014; 28: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoenig M, Ferguson DC. Effect of darglitazone on glucose clearance and lipid metabolism in obese cats. Am J Vet Res 2003; 64: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 72. Appleton DJ, Rand JS, Sunvold GD, et al. Dietary chromium tripicolinate supplementation reduces glucose concentrations and improves glucose tolerance in normal-weight cats. J Feline Med Surg 2002; 4: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cohn LA, Dodam JR, McCaw DL, et al. Effects of chromium supplementation on glucose tolerance in obese and nonobese cats. Am J Vet Res 1999; 60: 1360–1363. [PubMed] [Google Scholar]
- 74. Fondacaro JV, Greco DS, Crans DC. Treatment of feline diabetes mellitus with protamine zinc analine insulin (PZI) alone compared with PZI and oral vanadium dipicolinate [abstract]. J Vet Intern Med 1999; 13: 244. [Google Scholar]
- 75. Marshall RD, Rand JS, Morton JM. Insulin glargine has a long duration of effect following administration either once daily or twice daily in divided doses in healthy cats. J Feline Med Surg 2008; 10: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martin GJ, Rand JS. Pharmacology of a 40 IU/ml porcine lente insulin preparation in diabetic cats: findings during the first week and after 5 or 9 weeks of therapy. J Feline Med Surg 2001; 3: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Michiels L, Reusch CE, Boari A, et al. Treatment of 46 cats with porcine lente insulin – a prospective, multicentre study. J Feline Med Surg 2008; 10: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Broussard JD, Peterson ME. Comparison of two ultralente insulin preparations with protamine zinc insulin in clinically normal cats. Am J Vet Res 1994; 55: 127–131. [PubMed] [Google Scholar]
- 79. Marshall RD, Rand JS, Morton JM. Glargine and protamine zinc insulin have a longer duration of action and result in lower mean daily glucose concentrations than lente insulin in healthy cats. J Vet Pharmacol Ther 2008; 31: 205–212. [DOI] [PubMed] [Google Scholar]
- 80. Nelson RW, Lynn RC, Wagner-Mann CC, et al. Efficacy of protamine zinc insulin for treatment of diabetes mellitus in cats. J Am Vet Med Assoc 2001; 218: 38–42. [DOI] [PubMed] [Google Scholar]
- 81. Nelson RW, Henley K, Cole C. Field safety and efficacy of protamine zinc recombinant human insulin for treatment of diabetes mellitus in cats. J Vet Intern Med 2009; 23: 787–793. [DOI] [PubMed] [Google Scholar]
- 82. Wallace MS, Peterson ME, Nichols CE. Absorption kinetics of regular, isophane, and protamine zinc insulin in normal cats. Domest Anim Endocrinol 1990; 7: 509–515. [DOI] [PubMed] [Google Scholar]
- 83. Gilor C, Ridge TK, Attermeier KJ, et al. Pharmacodynamics of insulin detemir and insulin glargine assessed by an isoglycemic clamp method in healthy cats. J Vet Intern Med 2010; 24: 870–874. [DOI] [PubMed] [Google Scholar]
- 84. Gilor C, Graves TK. Synthetic insulin analogs and their use in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 297–307. [DOI] [PubMed] [Google Scholar]
- 85. Roomp K, Rand JS. Management of diabetic cats with long-acting insulin. Vet Clin North Am Small Anim Pract 2013; 43: 251–266. [DOI] [PubMed] [Google Scholar]
- 86. Borin-Crivellenti S, Bonagura JD, Gilor C. Comparison of precision and accuracy of U100 and U40 insulin syringes [abstract]. J Vet Intern Med 2014; 28: 1029. [Google Scholar]
- 87. Burgaud S, Riant S, Piau N. Comparative laboratory evaluation of dose delivery using a veterinary insulin injection pen. Proceedings of the British Small Animal Veterinary Association Congress [poster]; 2012 April 12–15; Birmingham, UK. [Google Scholar]
- 88. Dietiker-Moretti S, Muller C, Sieber-Ruckstuhl N, et al. Comparison of a continuous glucose monitoring system with a portable blood glucose meter to determine insulin dose in cats with diabetes mellitus. J Vet Intern Med 2011; 25: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 89. Hafner M, Lutz TA, Reusch CE, et al. Evaluation of sensor sites for continuous glucose monitoring in cats with diabetes mellitus. J Feline Med Surg 2013; 15: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kley S, Casella M, Reusch CE. Evaluation of long-term home monitoring of blood glucose concentrations in cats with diabetes mellitus: 26 cases (1999–2002). J Am Vet Med Assoc 2004; 225: 261–266. [DOI] [PubMed] [Google Scholar]
- 91. Reusch CE, Kley S, Casella M. Home monitoring of the diabetic cat. J Feline Med Surg 2006; 8: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Casella M, Hassig M, Reusch CE. Home-monitoring of blood glucose in cats with diabetes mellitus: evaluation over a 4-month period. J Feline Med Surg 2005; 7: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Casella M, Wess G, Reusch CE. Measurement of capillary blood glucose concentrations by pet owners: a new tool in the management of diabetes mellitus. J Am Anim Hosp Assoc 2002; 38: 239–245. [DOI] [PubMed] [Google Scholar]
- 94. Wess G, Reusch C. Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration. J Small Anim Pract 2000; 41: 60–66. [DOI] [PubMed] [Google Scholar]
- 95. Zeugswetter FK, Rebuzzi L, Karlovits S. Alternative sampling site for blood glucose testing in cats: giving the ears a rest. J Feline Med Surg 2010; 12: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Alt N, Kley S, Haessig M, et al. Day-to-day variability of blood glucose concentration curves generated at home in cats with diabetes mellitus. J Am Vet Med Assoc 2007; 230: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 97. Zini E, Moretti S, Tschuor F, et al. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilkd 2009; 151: 448–451. [DOI] [PubMed] [Google Scholar]
- 98. Dobromylskyj MJ, Sparkes AH. Assessing portable blood glucose meters for clinical use in cats in the United Kingdom. Vet Rec 2010; 167: 438–442. [DOI] [PubMed] [Google Scholar]
- 99. Domori A, Sunahara A, Tateno M, et al. The clinical utility of two human portable blood glucose meters in canine and feline practice. Vet Clin Pathol 2014; 43: 55–62. [DOI] [PubMed] [Google Scholar]
- 100. Wess G, Reusch C. Assessment of five portable blood glucose meters for use in cats. Am J Vet Res 2000; 61: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 101. Hoelmkjaer KM, Spodsberg EM, Bjornvad CR. Insulin detemir treatment in diabetic cats in a practice setting. J Feline Med Surg 2015; 17: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Martin GJ, Rand JS. Comparisons of different measurements for monitoring diabetic cats treated with porcine insulin zinc suspension. Vet Rec 2007; 161: 52–58. [DOI] [PubMed] [Google Scholar]
- 103. Finco DR, Adams DD, Crowell WA, et al. Food and water intake and urine composition in cats: influence of continuous versus periodic feeding. Am J Vet Res 1986; 47: 1638–1642. [PubMed] [Google Scholar]
- 104. Grant DC. Effect of water source on intake and urine concentration in healthy cats. J Feline Med Surg 2010; 12: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hashimoto M, Funaba M, Abe M, et al. Dietary protein levels affect water intake and urinary excretion of magnesium and phosphorus in laboratory cats. Exp Anim 1995; 44: 29–35. [DOI] [PubMed] [Google Scholar]
- 106. Kane E, Rogers QR, Morris JG. Feeding behavior of the cat fed laboratory and commercial diets. Nutr Res 1981; 1: 499–507. [Google Scholar]
- 107. Kane E, Leung PM, Rogers QR, et al. Diurnal feeding and drinking patterns of adult cats as affected by changes in the level of fat in the diet. Appetite 1987; 9: 89–98. [DOI] [PubMed] [Google Scholar]
- 108. Xu H, Laflamme DP, Long GL. Effects of dietary sodium chloride on health parameters in mature cats. J Feline Med Surg 2009; 11: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Elliott DA, Nelson RW, Reusch CE, et al. Comparison of serum fructosamine and blood glycosylated hemoglobin concentrations for assessment of glycemic control in cats with diabetes mellitus. J Am Vet Med Assoc 1999; 214: 1794–1798. [PubMed] [Google Scholar]
- 110. Norsworthy GD, Lynn R, Cole C. Preliminary study of protamine zinc recombinant insulin for the treatment of diabetes mellitus in cats. Vet Ther 2009; 10: 24–28. [PubMed] [Google Scholar]
- 111. Thoresen SI, Bredal WP. Clinical usefulness of fructosamine measurements in diagnosing and monitoring feline diabetes mellitus. J Small Anim Pract 1996; 37: 64–68. [DOI] [PubMed] [Google Scholar]
- 112. Gilor C, Graves TK, Lascelles BD, et al. The effects of body weight, body condition score, sex, and age on serum fructosamine concentrations in clinically healthy cats. Vet Clin Pathol 2010; 39: 322–328. [DOI] [PubMed] [Google Scholar]
- 113. Reusch CE, Tomsa K. Serum fructosamine concentration in cats with overt hyperthyroidism. J Am Vet Med Assoc 1999; 215: 1297–1300. [PubMed] [Google Scholar]
- 114. Marshall RD, Rand JS, Gunew MN, et al. Intramuscular glargine with or without concurrent subcutaneous administration for treatment of feline diabetic ketoacidosis. J Vet Emerg Crit Care (San Antonio) 2013; 23: 286–290. [DOI] [PubMed] [Google Scholar]
- 115. Rand JS. Diabetic ketoacidosis and hyperosmolar hyperglycemic state in cats. Vet Clin North Am Small Anim Pract 2013; 43: 367–379. [DOI] [PubMed] [Google Scholar]
- 116. Whitley NT, Drobatz KJ, Panciera DL. Insulin overdose in dogs and cats: 28 cases (1986–1993). J Am Vet Med Assoc 1997; 211: 326–330. [PubMed] [Google Scholar]
- 117. Oppliger S, Hartnack S, Riond B, et al. Agreement of the serum Spec fPL and 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6’-methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J Vet Intern Med 2013; 27: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 118. Oppliger S, Hartnack S, Reusch CE, et al. Agreement of serum feline pancreas-specific lipase and colorimetric lipase assays with pancreatic ultrasonographic findings in cats with suspicion of pancreatitis: 161 cases (2008–2012). J Am Vet Med Assoc 2014; 244: 1060–1065. [DOI] [PubMed] [Google Scholar]
- 119. Xenoulis PG, Steiner JM. Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012; 41: 312–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
French Version of the guidelines