Abstract
Clinical relevance:
Incretin-based therapies are revolutionizing the field of human diabetes mellitus (DM) by replacing insulin therapy with safer and more convenient long-acting drugs.
Mechanism of action:
Incretin hormones (glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic peptide [GIP]) are secreted from the intestinal tract in response to the presence of food in the intestinal lumen. GLP-1 delays gastric emptying and increases satiety. In the pancreas, GLP-1 augments insulin secretion and suppresses glucagon secretion during hyperglycemia in a glucose-dependent manner. It also protects beta cells from oxidative and toxic injury and promotes expansion of beta cell mass.
Advantages:
Clinical data have revealed that GLP-1 analog drugs are as effective as insulin in improving glycemic control while reducing body weight in people suffering from type 2 DM. Furthermore, the incidence of hypoglycemia is low with these drugs because of their glucose-dependent mechanism of action. Another significant advantage of these drugs is their duration of action. While insulin injections are administered at least once daily, long-acting GLP-1 analogs have been developed as once-a-week injections and could potentially be administered even less frequently than that in diabetic cats.
Outline:
This article reviews the physiology of incretin hormones, and the pharmacology and use of GLP-1 analogs, with emphasis on recent research in cats. Further therapies that are based on incretin hormones, such as DPP-4 inhibitors, are also briefly discussed, as are some other treatment modalities that are currently under investigation.
Physiology of incretin hormones
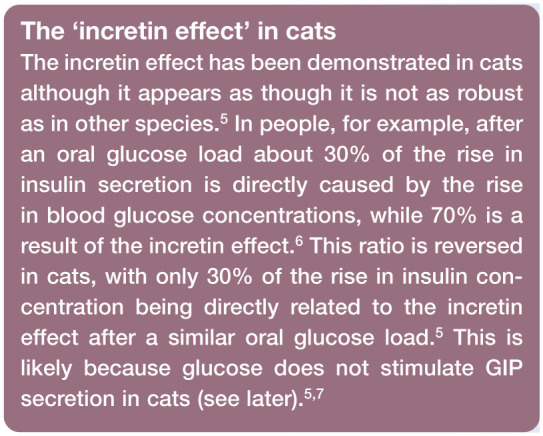
Incretin-secreting cells (K and L cells) are interspersed in the epithelium of the gut at low but varying densities depending on the cell and anatomic location (see later). 1 Their physiological role is to sense the type and quantity of digested nutrients in the gut. 2 They then secrete incretins (and other hormones) as preparatory signals to other remote organs (pancreas, brain, etc). In the pancreas, the main effect of incretin hormones is to increase sensitivity to the stimulatory effect of glucose. This effect is responsible for the observed difference in insulin secretion between oral and intravenous glucose infusions and is defined as the ‘incretin effect’; oral glucose leads to much greater insulin secretion compared with intravenous glucose, even when blood glucose concentrations are equal.3,4
The incretin effect is thought to be exclusively mediated by two peptide hormones: glucagon-like peptide-1 (GLP-1), which is secreted from L cells, and glucose-dependent insulinotropic peptide (GIP), which is secreted from K cells (Figure 1). 2
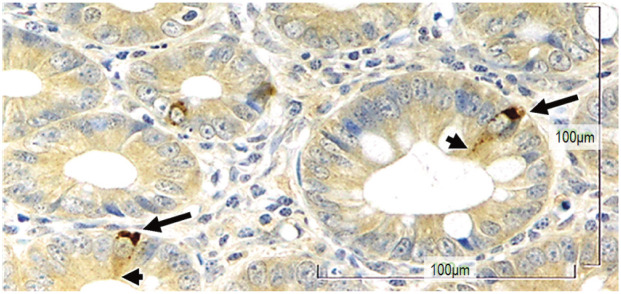
Figure 1.
K cells in the feline ileum. Long arrows indicate the basal side of the cell that is packed with glucose-dependent insulinotropic peptide hormones (brown stain) ready to be secreted to the lamina propria where blood vessels and nerve endings are abundant. Arrowheads indicate the fine extension of the cell on the luminal side, where sensing of nutrients occurs. x 500 magnification
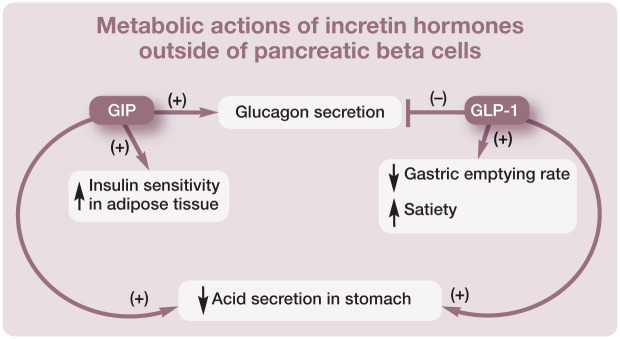
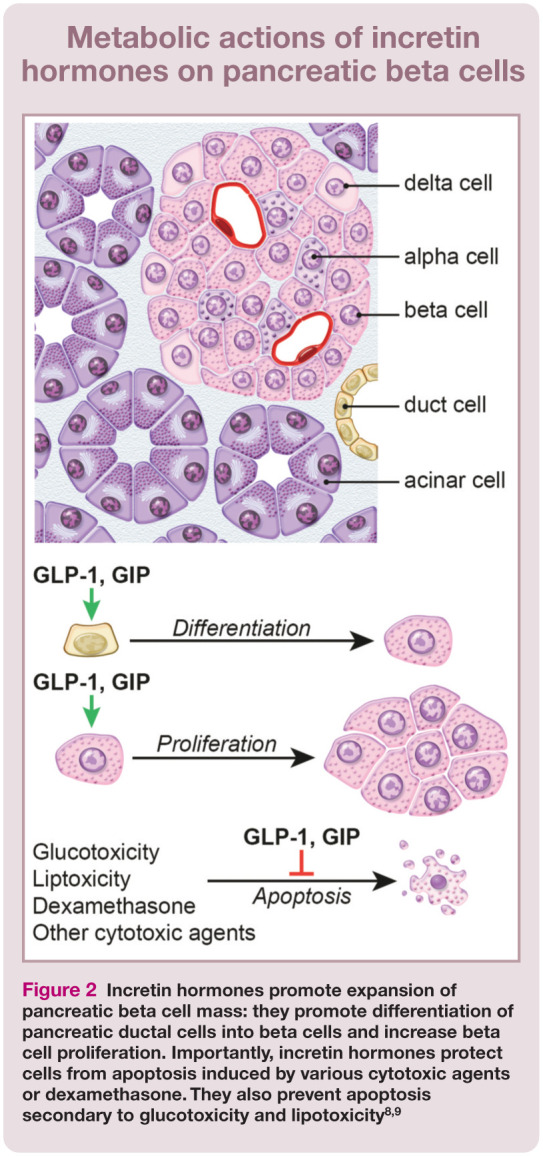
Incretin hormones play a major role in glucose homeostasis via their effect on pancreatic beta cells (Figure 2) and also by affecting a multitude of other tissues.2,8 For example, incretin hormones are important in regulating glucagon secretion (see box below). GLP-1 inhibits glucagon secretion, although it is unclear whether this is a direct effect on alpha cells or whether it is mediated through insulin or other hormones. In contrast, GIP stimulates glucagon secretion and it does so by directly stimulating pancreatic alpha cells. 2 GLP-1 receptors are expressed in several brainstem nuclei involved in appetite regulation. 8 GLP-1 suppresses appetite and energy intake in both normal weight and obese individuals. In animal models of obesity (including minipigs and rodents) GLP-1 analogs reduce feeding frequency and meal size. 10 GLP-1 also promotes satiety and weight loss through decreasing the rate of gastric emptying. In contrast, GIP promotes weight gain and obesity by increasing the sensitivity of adipose tissue to insulin. 2
Figure 2.
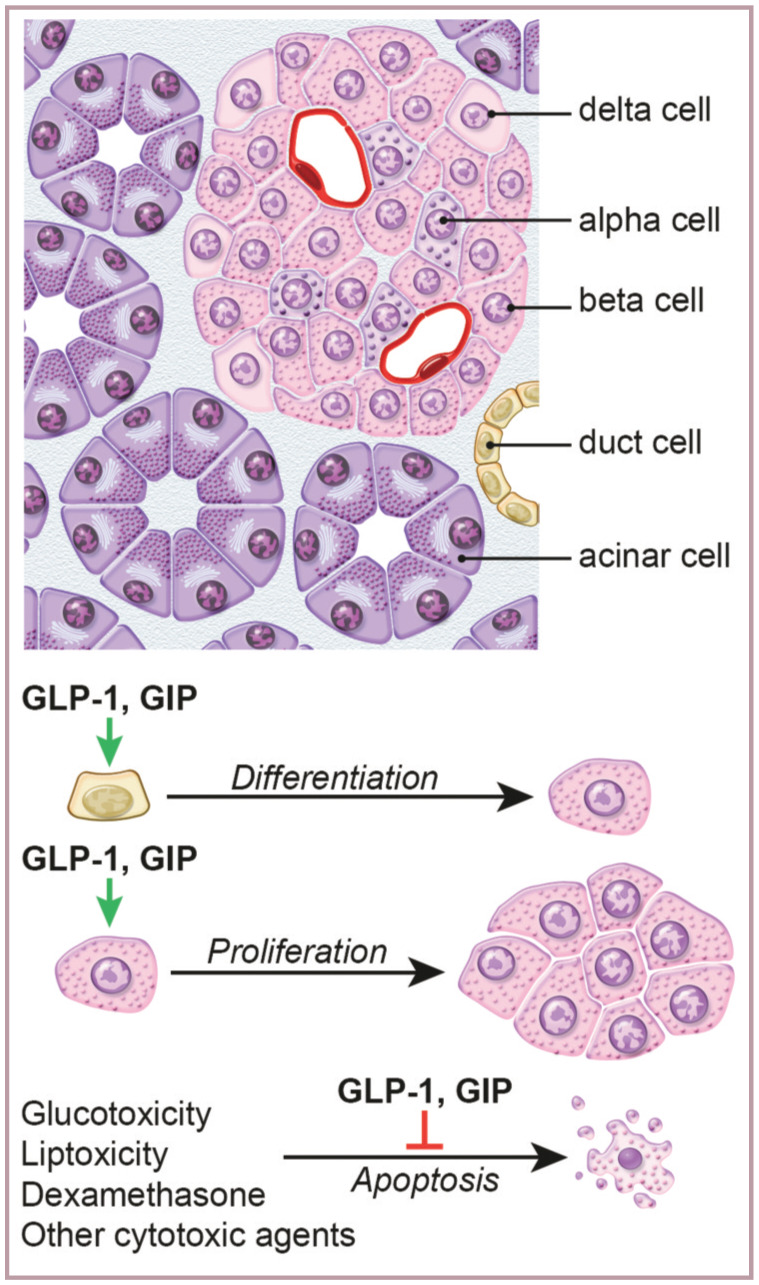
Incretin hormones promote expansion of pancreatic beta cell mass: they promote differentiation of pancreatic ductal cells into beta cells and increase beta cell proliferation. Importantly, incretin hormones protect cells from apoptosis induced by various cytotoxic agents or dexamethasone. They also prevent apoptosis secondary to glucotoxicity and lipotoxicity8,9
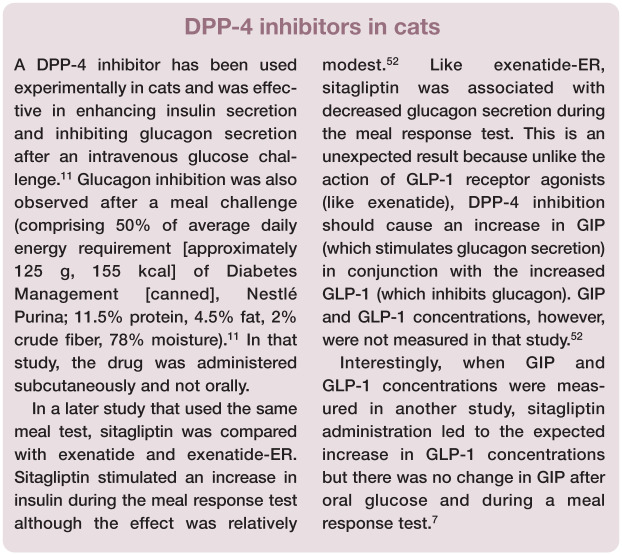
Active GIP and active GLP-1 are degraded by the enzymes dipeptidyl peptidase-4 (DPP-4, also known as CD26) and neutral endopeptidase 24.11 (NEP-24.11) into inactive forms, thereby modifying or inhibiting their activity. 2 DPP-4 and NEP are ubiquitous in tissues, and DPP-4 is also present in soluble form in the blood. Consequently, when injected intravenously, active GLP-1 and active GIP are quickly degraded into inactive forms. This results in a short half-life of both GLP-1 (1–2 mins) and GIP (5 mins) when administered intravenously. The inactive GLP-1 and GIP are quickly cleared by the kidneys.2,8 Although the half-life of GLP-1 and GIP is unknown in cats, the activity of DPP-4 in healthy cats has been indirectly demonstrated: administration of a DPP-4 inhibitor to healthy cats was associated with increased insulin concentrations and decreased glucagon concentrations after an intravenous glucose challenge. 11 In another study, administration of a DPP-4 inhibitor was associated with increased GLP-1 concentrations but, interestingly, GIP concentrations were unaltered whether after oral glucose or after a meal. 7
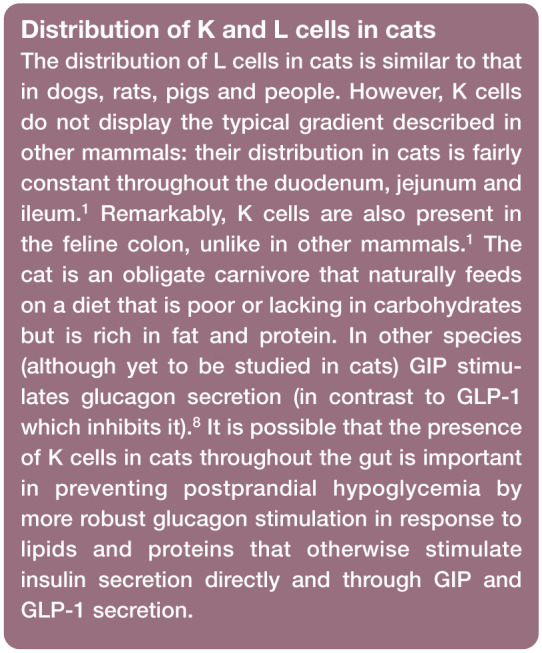
L and K cells are described as open-type cells, reaching the lumen of the gut via a slender apical process that carries their sensing mechanisms (Figure 1). They secrete hormones from their basal aspects which are in close proximity to the vasculature and neurons of the lamina propria. 12 The main stimulus for secretion of hormones from K and L cells is the presence of nutrients in the lumen of the gut (not nutrients in the blood). Lipids and carbohydrates are sensed by specific G protein-coupled receptors (GPRs), although other sensing mechanisms have been described as well: GPR40, GPR120 and GPR119, for example, sense lipids and T1R2/T1R3 (the ‘sweet-taste receptor’) senses simple sugars.13–16 Cats have a deletion mutation in their sweet-taste receptor (T1R2/T1R3) gene and cannot express this receptor. 17 This renders their tongues insensitive to glucose but also affects their ability to sense glucose via their intestinal cells. 18 Whether or not cats have a different G protein- coupled taste receptor or a different mechanism of sensing carbohydrates by intestinal enteroendocrine cells is unknown.
The degree of GIP and GLP-1 stimulation by different nutrients is species-dependent. 19 Fat and carbohydrates are potent stimuli of GIP secretion in humans, dogs, pigs and rodents. Fat is more potent than carbohydrates in stimulating GIP secretion in people and dogs, but the opposite is true in rodents and pigs. 12 In cats, fat is more potent than amino acids in stimulating GIP secretion but oral administration of glucose has no effect on GIP secretion.5,7 Postprandial GLP-1 secretion is typically biphasic and is stimulated by ingested lipid, carbohydrate and protein. 19 In one study in healthy cats, lipids, carbohydrates and amino acids had overall a similar effect on the total amount of GLP-1 secreted, although the temporal pattern differed. 5
Different distributions of L cells and K cells along the intestinal tract in different species determine, in part, the importance of different stimuli of GLP-1 and GIP secretion.1,8,20 Quantitative immunohistochemical studies in dogs, rats, pigs and people have demonstrated that in general there is an opposite gradient to the densities of L and K cells: there are few (if any) L cells in the distal duodenum and their density increases aborally through the jejunum, reaching maximum numbers in the ileum and colon.20–22 In contrast, K cells are at their highest density in the duodenum and their density decreases aborally along the jejunum. K cells are not present in the ileum and colon in dogs, rats, pigs and people.20–22
GLP-1 based treatments in diabetes mellitus
GIP is not considered a beneficial target in the treatment of DM because of the reduced sensitivity of pancreatic beta cells to GIP in the diabetic state and because of the negative effects of GIP in terms of increasing glucagon secretion and promoting obesity by increasing the sensitivity of adipose tissue to insulin. If anything, decreasing GIP secretion or antagonizing its effect outside of beta cells would be beneficial but such therapies are not currently available and will not be discussed further.
In contrast, GLP-1 treatment has many beneficial effects but its very short half-life prevents its widespread use in the clinical setting. There are several treatment modalities that overcome this problem. The first incretin-based drugs to be approved for use in people were orally administered DPP-4 inhibitors that prolong the half-life of GLP-1 and GIP. Later on, the GLP-1 analog exenatide was approved for use, followed by a number of other GLP-1 analogs, with more still being developed. These long-acting, DPP-4-resistant, synthetic GLP-1 receptor agonists are currently the biggest and most widely used group of GLP-1 based drugs. They are all peptides (Figure 3) that are administered as subcutaneous injections, but other delivery systems are being developed (including enteral, pulmonary or sublingual). Also under development are non-peptidic GLP-1 receptor agonists.31,32
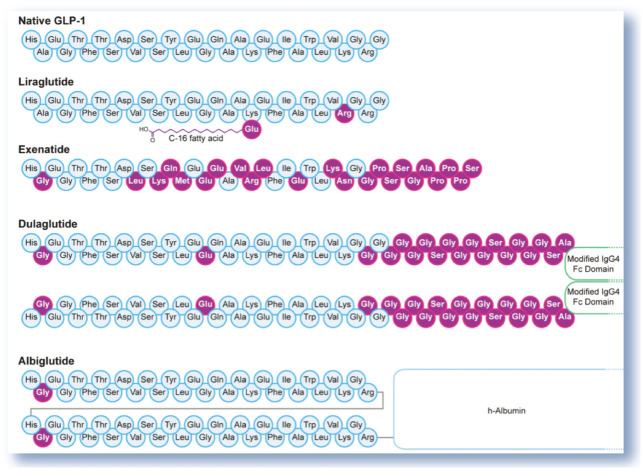
Figure 3.
Amino acid sequence of native GLP-1 and its analogs. The substitution of alanine with glycine at position 2 renders the peptide resistant to DPP-4
Drugs that are commercially available are discussed in the following sections. Other GLP-1 based treatment approaches are described in the box on page 737.
GLP-1 analogs
GLP-1 analogs are peptides derived from mammalian GLP-1 (which is 100% homologous between humans, rodents, cats, dogs, pigs and other mammals) with various modifications that render them resistant to DPP-4 degradation. Some commercially available analogs are discussed below. Non-peptidic GLP-1 receptor agonists are not commercially available and are not discussed.
Exenatide
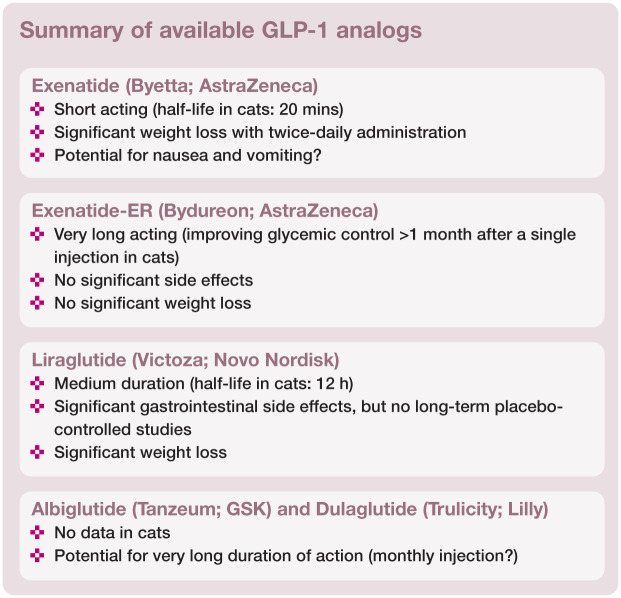
The peptide exendin-4 was first isolated from the poisonous venom of the Gila monster (Heloderma suspectum). Exendin-4 is a 39-amino acid peptide that shares only a 53% sequence homology with GLP-1 but its affinity for the GLP-1 receptor is 1000 times greater than the affinity of GLP-1. Unlike GLP-1, exendin-4 is not a substrate for DPP-4 and NEP. 2 Exenatide is a synthetic exendin-4. Resistant to degradation, exenatide is eliminated by the kidneys and has a half-life of 3–4 h in people. Its biological effect lasts about 8 h after subcutaneous injection and it can be detected in the plasma for up to 15 h. 43
Multiple studies, both in vitro and in vivo, have shown that, in general, exendin-4 has the same physiologic effects as GLP-1 in the pancreas, gastrointestinal tract and brain. 8 Exenatide is associated with improvement in some of the earliest (sometimes years before fasting hyperglycemia is diagnosed) and most fundamental abnormalities of type 2 DM: diminished ‘first-phase insulin response’ and the proinsulin/insulin ratio. Acute administration of exenatide in type 2 diabetic patients corrects the abnormal insulin secretion pattern after an intravenous glucose bolus (first phase and second phase insulin responses) and restores the ability of beta cells to respond to rapid changes in blood glucose concentrations. 8 Exenatide also improves the proinsulin/insulin ratio after 30 weeks of treatment. 44
Exenatide is as effective as insulin glargine in the treatment of DM in humans but with fewer side effects (eg, hypoglycemia and weight gain). 45 In a 2 year follow-up of patients receiving exenatide, sustained and significant reductions in glycosylated hemoglobin were achieved, accompanied by significant weight loss (instead of weight gain commonly seen in diabetics receiving insulin) and improvement in serum liver enzyme activity and blood pressure. Most importantly, treatment with exenatide improved beta cell function as measured by homeostasis model assessment of beta cell function (HOMA-B). 46
Exenatide has minimal side effects in people. It is mostly associated with nausea and less frequently with vomiting. Infrequently, it can cause hypoglycemia. A systematic review and meta-analysis found that severe hypoglycemia (requiring assistance) was reported rarely (only 5/2781 patients) and only in patients who also received sulfonylurea drugs. 47 Antibodies to exenatide developed in 67% of patients but this did not affect outcome and was not associated with side effects. 47 When first discovered, exendin-4 was shown to potentiate amylase release from rat acinar pancreatic cells in response to other hormones such as cholecystokinin. This was shown ex vivo and in high doses. A possible association between GLP-1 analogs and pancreatitis as well as medullary thyroid cancer has been suggested and led to issuing of a warning by the FDA. However, multiple studies have refuted these concerns and it is widely accepted that the proven enormous benefits of GLP-1 analogs outweigh their hypothetical risks.48,49
Exenatide is commercially available in the USA under the trade name Byetta (AstraZeneca) and is used in people as a twice-daily drug. It is marketed as a pre-filled pen for injection, delivering a minimum dose of 5 µg per injection.
Exenatide extended-release (ER)
A long-acting extended-release (ER) formulation of exenatide (Bydureon; AstraZeneca) has recently been approved by the FDA as the first once-weekly subcutaneous injection for treatment of type 2 DM in people. It consists of injectable microspheres of exenatide and poly(D,L-lactic-co-glycolic acid), a common biodegradable medical polymer with established use in absorbable sutures and extended-release pharmaceuticals, which allows gradual drug delivery at controlled rates. In people, exenatide plasma concentrations are sustained at an effective concentration (50 pg/ml) for longer than 60 days after a single injection at doses of 5, 7 or 10 mg. 54
In a clinical trial exenatide-ER was more effective than once-a-day insulin glargine in achieving glycemic control, with decreased risk of hypoglycemia and with reduction (instead of gain) in body weight. 55 It was also more effective than regular (short acting) exenatide (Byetta) in achieving glycemic control, with no increased risk of hypoglycemia. Unlike short acting exenatide, exenatide-ER was associated with decreased fasting blood glucose, an important predictor of long-term complications of DM in people. Compared with short acting exenatide, exenatide-ER also was associated with less frequent side effects like nausea, but with similar reductions in body weight. 56 The similar reduction in body weight is an important finding because, theoretically, weight loss is not expected as much with long acting formulations of GLP-1 analogs.
Continuous exposure to a GLP-1 receptor agonist downregulates the effects of that agonist on gastric emptying, thus decreasing its effect on curbing appetite. 57 This downregulation does not occur with intermittent exposure (as is the case with short acting exenatide) even after many months (and years) of treatment. As a result, the overall effect of GLP-1 agonists on postprandial gastric emptying are greater with intermittent exposure compared with continuous exposure (with long acting formulations such as exenatide-ER). This is seen even when peak drug concentrations are higher during continuous vs intermittent exposure. Conversely, the effect on fasting glucose is decreased with intermittent compared with continuous exposure. 57
Liraglutide
Liraglutide is a synthetic GLP-1 analog with two amino acid substitutions and a fatty acid acyl group (Figure 3) that enables non- covalent binding to albumin, thereby extending the pharmacokinetic profile of the GLP-1 molecule. Liraglutide exhibits a prolonged (relative to unmodified native GLP-1) pharmacokinetic profile after a single injection, as well as all of the actions of native GLP-1. 2
Liraglutide (once-a-day) was compared with exenatide (twice-a-day). Liraglutide provided significantly greater improvements in glycemic control than did exenatide and was generally better tolerated. 62 Liraglutide q24h was also more effective than exenatide-ER once weekly in achieving glycemic control, but nausea, vomiting and diarrhea occurred less frequently with exenatide-ER. 63 Possibly this increased efficacy is secondary to fluctuations in drug levels of liraglutide, preventing downregulation of its direct effects on the gastrointestinal tract (as described above). It is also possible that the hydrophobic properties of liraglutide, conferred by its fatty acid component, allow better penetration through the blood–brain barrier and therefore greater effect on the brain. Liraglutide has been used successfully to treat obesity in non-diabetic patients. 64
Liraglutide is used in people as a once-daily drug marketed under the trade name Victoza (Novo Nordisk). It is provided as a pre-filled pen for injection, delivering a minimum dose of 0.6 µg per injection.
Albiglutide and dulaglutide
Albiglutide (Tanzeum; GSK) and dulaglutide (Trulicity; Lilly) were approved by the FDA in 2014 for treatment of type 2 DM. These are fusion proteins of GLP-1 and large-size molecules that slow absorption from the subcutaneous injection site, protect the active GLP-1 component from degradation by DPP-4 activity, and prolong the half-life considerably by reducing renal clearance. In albiglutide, two GLP-1 molecules are covalently linked to one molecule of human albumin. 66 In dulaglutide, two GLP-1 molecules are covalently linked to a modified human IgG4 Fc fragment (Figure 3). 67
While these fusion molecules exhibit reduced affinity for the GLP-1 receptor, they display a broad spectrum of GLP-1 receptor-dependent actions. Both have been studied extensively and like previously described long-acting GLP-1 receptor agonists they are associated with glucose-dependent insulin stimulation, inhibition of food intake and gastric emptying, and improvement of both fasting and postprandial blood glucose.66,67 They are also associated with a similar profile of transient gastrointestinal side effects. Both molecules have been studied in type 2 diabetic people and are superior to insulin in terms of reducing glycosylated hemoglobin while not promoting weight gain and minimizing the risk of hypoglycemia.66,67 Importantly, they have the potential to be used as a very long-acting drug: albiglutide has a half-life of 6–8 days and dulaglutide has a half-life of 5 days in people.
Albiglutide has been investigated in people for use as a weekly, biweekly and monthly injection (with higher doses given at lower frequency). 66 Monthly injections were as effective in controlling glycemia as weekly and biweekly injections, but the reduced frequency was associated with greater fluctuations in drug levels ad an associated greater frequency of nausea and vomiting. 66 Because cats seem more resistant to the side effects of GLP-1 receptor agonists, it is tempting to speculate that albiglutide and dulaglutide could be used in cats at high doses and low frequency of injection of once a month, with good efficacy and no significant side effects. To date, however, neither drug has been studied in cats.
DPP-4 inhibitors
DPP-4 inhibitors (eg, sitagliptin, vildagliptin) are administered orally. In people, they are well tolerated and not associated with hypoglycemia when used alone. DPP-4 inhibitors in people increase plasma concentrations of GLP-1 and GIP after meal ingestion, enhance glucose-stimulated insulin secretion and reduce ratios of proinsulin:insulin, consistent with an improvement in beta cell function. They are, however, less potent than other oral hypoglycemic drugs. 8
In contrast to GLP-1 analogs, DPP-4 inhibitors are not associated with nausea or vomiting in people, but they are associated with weight gain. DPP-4 inhibitors are also associated with increased risk of nasopharyngitis, urinary tract infections and headaches. 47 Increased risk of infections might be related to the action of DPP-4 in T cells as a co-stimulatory molecule (CD26).
Challenges in managing feline diabetes mellitus and potential uses of GLP-1 based therapies
Insulin-related issues
Treatment of feline DM currently relies upon the administration of exogenous insulin, usually given twice daily, along with dietary modification and treatment of underlying (eg, hypersomatotropism) or concurrent diseases. For many owners, this has a profound impact on their quality of life and the perceived wellbeing of their pet, and it has been estimated that 30% of affected cats are euthanized within a year of diagnosis. 68 Owners often cite insulin-related issues as a major cause of anxiety (including worrying about hypoglycemic events and the inability to have the cat cared for by others). 69 Therefore, eliminating the need for long-term insulin therapy in diabetic cats will impact the survival of countless cats.
Duration of remission
A substantial proportion of diabetic cats (about 30%) enter a state of remission (ie, become insulin independent after being treated with insulin) and no longer require such careful management. 70 It is assumed that exogenous insulin therapy, along with dietary manipulation and mitigation of insulin-resistant disorders, reverses glucotoxicity and enables the pancreatic beta cells to resume function. However, the duration of remission can be short-lived, and exogenous insulin must then be reinstituted. 70
While it is recognized that prompt insulin therapy is needed to achieve remission in cats with overt DM, studies in people and rodent models show that chronic administration of GLP-1 receptor agonists preserves and even increases beta cell mass and the capacity to secrete insulin. 2 Therefore, it seems possible that GLP-1 receptor agonists may prolong remission time in cats and delay, or possibly even prevent, the recurrence of insulin dependence. If that is the case, long acting formulations like exenatide-ER administered once a month would have dramatic effects on survival of cats, and the quality of life of cats and their owners.
Potential for early diagnosis
Traditionally, feline DM is equated to type 2 DM in people and indeed many similarities exist between these diseases. Probably the most striking differences are the criteria for diagnosis and (likely as a result) the requirement for exogenous insulin administration. People are diagnosed with DM and prediabetes when their blood glucose is only mildly elevated and most often years before their hyperglycemia causes any clinical signs. This early diagnosis allows for lifestyle interventions and drug administration that prevent or delay progression from prediabetes to DM and prevent or delay the need for exogenous insulin treatment. During these years of treatment, pancreatic cells are still present in large enough numbers and hyperglycemia can be effectively treated (eg, with GLP-1 based treatments). Control of hyperglycemia is crucial for preventing the complications of DM.
In cats, the diagnosis of DM is made when blood glucose concentrations are much higher, typically higher than the renal threshold for glucose reabsorption (which leads to the typical clinical presentation of polyuria and polydipsia). Presumably, this occurs in much later stages of DM when pancreatic beta cell mass is significantly decreased and the window of opportunity to treat with GLP-1 based treatments has closed or is near closure. The future of research in feline DM may therefore lie with a focus on early diagnosis of DM, and the possibility of preventing clinical DM with pre-emptive treatment with GLP-1 based therapies.
Key points
- The current standard of care in feline diabetes (insulin and diet):
- – Is associated with a relatively low remission rate (about 30%);
- – Has a significant negative impact on the quality of life of cats and their owners.
- Incretin hormones have the following effects on pancreatic beta cells:
- – Increase sensitivity to glucose;
- – Increase glucose-dependent insulin secretion;
- – Increase insulin biosynthesis;
- – Inhibit apoptosis;
- – Stimulate proliferation and differentiation.
GLP-1 is an incretin hormone that retains its positive physiologic effects in diabetics and has an excellent safety profile, even when administered at supraphysiologic concentrations.
- GLP-1 based therapies have the potential to:
- – Improve diabetic remission rates in cats, especially those diagnosed early in the course of their disease;
- – Improve quality of life of cats and their owners by replacing twice-daily injections with once-monthly injections.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Gilor C, Gilor S, Graves TK, et al. Distribution of K and L cells in the feline intestinal tract. Domest Anim Endocrinol 2013; 45: 49–54. [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ, Vilsboll T, deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol 2009; 297: 127–136. [DOI] [PubMed] [Google Scholar]
- 3. McIntyre N, Holdsworth Cd, Turner dS. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab 1965; 25: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498. [DOI] [PubMed] [Google Scholar]
- 5. Gilor C, Graves TK, Gilor S, et al. The incretin effect in cats: comparison between oral glucose, lipids, and amino acids. Domest Anim Endocrinol 2011; 40: 205–212. [DOI] [PubMed] [Google Scholar]
- 6. Holst JJ, Knop FK, Vilsboll T, et al. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011; 34 Suppl 2: S251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishii N, Takashima S, Iguchi A, et al. Effects of sitagliptin on plasma incretin concentrations after glucose administration through an esophagostomy tube or feeding in healthy cats. Domest Anim Endocrinol 2014; 49: 14–19. [DOI] [PubMed] [Google Scholar]
- 8. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008; 60: 470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson RP, Harmon J, Tran Po, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004; 53 Suppl 1: S119–124. [DOI] [PubMed] [Google Scholar]
- 10. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012; 36: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furrer d, Kaufmann K, Tschuor F, et al. The dipeptidyl peptidase IV inhibitor NVP-DPP728 reduces plasma glucagon concentration in cats. Vet J 2010; 183: 355–357. [DOI] [PubMed] [Google Scholar]
- 12. Baggio LL, drucker dJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 13. Parker HE, Habib AM, Rogers GJ, et al. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009; 52: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyauchi S, Hirasawa A, Ichimura A, et al. New frontiers in gut nutrient sensor research: free fatty acid sensing in the gastrointestinal tract. J Pharmacol Sci 2010; 112: 19–24. [DOI] [PubMed] [Google Scholar]
- 15. overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol 2008; 153 Suppl 1: S76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 2007; 104: 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Li W, Wang H, et al. Cats lack a sweet taste receptor. J Nutr 2006; 136 Suppl 7: 1932S–1934S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Batchelor dJ, Al-Rammahi M, Moran AW, et al. Sodium/glucose cotransporter-1, sweet receptor, and disaccharidase expression in the intestine of the domestic dog and cat: two species of different dietary habit. Am J Physiol Regul Integr Comp Physiol 2011; 300: R67–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept 2005; 128: 117–124. [DOI] [PubMed] [Google Scholar]
- 20. damholt AB, Kofod H, Buchan AM. Immunocytochemical evidence for a paracrine interaction between GIP and GLP-1-producing cells in canine small intestine. Cell Tissue Res 1999; 298: 287–293. [DOI] [PubMed] [Google Scholar]
- 21. Kauth T, Metz J. Immunohistochemical localization of glucagon-like peptide 1. Use of poly- and monoclonal antibodies. Histochemistry 1987; 86: 509–515. [DOI] [PubMed] [Google Scholar]
- 22. Eissele R, Goke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 1992; 22: 283–291. [DOI] [PubMed] [Google Scholar]
- 23. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired betacell function? Diabetes 2010; 59: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 25. Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003; 88: 2706–2713. [DOI] [PubMed] [Google Scholar]
- 26. Rask E, olsson T, Soderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001; 24: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 27. Forcada Y, Holder A, Church dB, et al. A polymorphism in the melanocortin 4 receptor gene (MC4R:c.92C>T) is associated with diabetes mellitus in overweight domestic shorthaired cats. J Vet Intern Med 2014; 28: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panaro BL, Tough IR, Engelstoft MS, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab 2014; 20: 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoenig M, Jordan ET, Ferguson dC, et al. Oral glucose leads to a differential response in glucose, insulin, and GLP-1 in lean versus obese cats. Domest Anim Endocrinol 2010; 38: 95–102. [DOI] [PubMed] [Google Scholar]
- 30. Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, et al. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complications 2015; 29: 445–450. [DOI] [PubMed] [Google Scholar]
- 31. Sloop KW, Willard FS, Brenner MB, et al. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes 2010; 59: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eldor R, Kidron M, Greenberg-Shushlav Y, et al. Novel glucagon-like peptide-1 analog delivered orally reduces postprandial glucose excursions in porcine and canine models. J Diabetes Sci Technol 2010; 4: 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gentilcore d, Chaikomin R, Jones KL, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 2006; 91: 2062–2067. [DOI] [PubMed] [Google Scholar]
- 34. Park YM, Heden Td, Liu Y, et al. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J Nutr 2015; 145: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adachi T, Yanaka H, Kanai H, et al. Administration of perilla oil coated with Calshell increases glucagon-like peptide secretion. Biol Pharm Bull 2008; 31: 1021. –1023. [DOI] [PubMed] [Google Scholar]
- 36. Morishita M, Tanaka T, Shida T, et al. Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP-1 secretion. J Control Release 2008; 132: 99–104. [DOI] [PubMed] [Google Scholar]
- 37. Everard A, Lazarevic V, derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011; 60: 2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 2015; 64: 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pories WJ, Swanson MS, Macdonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhee NA, Wahlgren Cd, Pedersen J, et al. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia 2015; 58: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 41. Nergard BJ, Lindqvist A, Gislason HG, et al. Mucosal glucagon-like peptide-1 and gastric inhibitory polypeptide cell numbers in the super-obese human foregut after gastric bypass. Surg Obes Relat Dis 2015; 11: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 42. Baskota A, Li S, dhakal N, et al. Bariatric surgery for type 2 diabetes mellitus in patients with BMI <30 kg/m2: a systematic review and meta-analysis. PLoS One 2015; 10: e0132335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolterman oG, Kim dd, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62: 173–181. [DOI] [PubMed] [Google Scholar]
- 44. deFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 45. Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24: 639–644. [DOI] [PubMed] [Google Scholar]
- 46. Buse JB, Klonoff dC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29: 139–153. [DOI] [PubMed] [Google Scholar]
- 47. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298: 194–206. [DOI] [PubMed] [Google Scholar]
- 48. Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012; 98: 271–284. [DOI] [PubMed] [Google Scholar]
- 49. Chiu WY, Shih SR, Tseng CH. A review on the association between glucagon-like peptide-1 receptor agonists and thyroid cancer. Exp Diabetes Res 2012; 2012: 924168. doI: 10.1155/2012/924168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gilor C, Graves TK, Gilor S, et al. The GLP-1 mimetic exenatide potentiates insulin secretion in healthy cats. Domest Anim Endocrinol 2011; 41: 42–49. [DOI] [PubMed] [Google Scholar]
- 51. Seyfert TM, Brunker Jd, Maxwell LK, et al. Effects of a glucagon-like peptide-1 mimetic (exenatide) in healthy cats. Intern J Appl Res Vet Med 2012; 10: 147–156. [Google Scholar]
- 52. Padrutt I, Lutz TA, Reusch CE, et al. Effects of the glucagonlike peptide-1 (GLP-1) analogues exenatide, exenatide extended-release, and of the dipeptidylpeptidase-4 (DPP-4) inhibitor sitagliptin on glucose metabolism in healthy cats. Res Vet Sci 2015; 99: 23–29. [DOI] [PubMed] [Google Scholar]
- 53. Scuderi M, Snead E, Unniappan S, et al. Safety and efficacy assessment of a GLP-1:glargine combination for feline diabetes [abstract]. J Vet Intern Med 2015; 29: 1172. [DOI] [PubMed] [Google Scholar]
- 54. Fineman M, Flanagan S, Taylor K, et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet 2011; 50: 65–74. [DOI] [PubMed] [Google Scholar]
- 55. diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012; 35: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buse JB, drucker dJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010; 33: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fineman MS, Cirincione BB, Maggs d, et al. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab 2012; 14: 675–688. [DOI] [PubMed] [Google Scholar]
- 58. Rudinsky AJ, Adin CA, Borin-Crivellenti S, et al. Pharmacology of the glucagon-like peptide-1 analog exenatide extended-release in healthy cats. Domest Anim Endocrinol 2015; 51: 78–85. [DOI] [PubMed] [Google Scholar]
- 59. Riederer AR, Fracassi F, Salesov E, et al. Assessment of a glucagon-like peptide-1 analogue (exenatide extended release) in cats with newly diagnosed diabetes mellitus [abstract]. J Vet Intern Med 2015; 29: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niessen SJ, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: beware of the acromegalic imposter. PLoS One 2015; 10: e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Forcada Y, German AJ, Noble PJ, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008; 10: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 63. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013; 381: 117–124. [DOI] [PubMed] [Google Scholar]
- 64. Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009; 374: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 65. Hall MJ, Adin CA, Borin-Crivellenti S, et al. Pharmacokinetics and pharmacodynamics of the glucagon-like peptide-1 analog liraglutide in healthy cats. Domest Anim Endocrinol 2015; 51: 114–121. [DOI] [PubMed] [Google Scholar]
- 66. Rosenstock J, Reusch J, Bush M, et al. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care 2009; 32: 1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giorgino F, Benroubi M, Sun JH, et al. Efficacy and safety of once weekly dulaglutide vs. insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care 2015; 38: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 68. Niessen S, Powney S, Guitian J, et al. Diabetes mellitus and euthanasia: how often and why? [abstract]. J Vet Intern Med 2010; 24: 1568. [DOI] [PubMed] [Google Scholar]
- 69. Niessen SJ, Powney S, Guitian J, et al. Evaluation of a quality-of-life tool for cats with diabetes mellitus. J Vet Intern Med 2010; 24: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 70. Gostelow R, Forcada Y, Graves T, et al. Systematic review of feline diabetic remission: separating fact from opinion. Vet J 2014; 202: 208–221. [DOI] [PubMed] [Google Scholar]