Abstract
In central nervous system (CNS) infectious and inflammatory diseases of known cause, oligoclonal bands represent antibody directed against the causative agent. To determine whether disease-relevant antibodies can be cloned from diseased brain, we prepared an antibody phage display library from the brain of a human with subacute sclerosing panencephalitis (SSPE), a chronic encephalitis caused by measles virus, and selected the library against SSPE brain sections. Antibodies that were retrieved reacted strongly with measles virus cell extracts by enzyme-linked immunosorbent assay and were specific for the measles virus nucleocapsid protein. These antibodies immunostained cells in different SSPE brains but not in control brain. Our data provide the first demonstration that diseased brain can be used to select in situ for antibodies directed against the causative agent of disease and point to the potential usefulness of this approach in identifying relevant antibodies in chronic CNS or systemic inflammatory diseases of unknown cause.
A common feature of chronic infectious and inflammatory diseases of the central nervous system (CNS) is the intrathecal synthesis of oligoclonal immunoglobulin G (IgG). In known CNS infectious diseases, oligoclonal bands (OGBs) are directed against the etiologic agent (reviewed in reference 10). We hypothesize that in CNS inflammatory disease of unknown cause, such as multiple sclerosis (MS), the specificities of OGBs may similarly yield important clues for identifying a putative pathogen and contribute to our understanding of disease pathogenesis.
Standard immunological techniques have not revealed a viral or cellular antigen that consistently reacts with MS OGBs. Recombinant antibody technology combined with phage display offers a novel strategy to identify the disease-relevant IgG in MS cerebrospinal fluid and brain plaques (1). Combinatorial antibody library technology, in which Fab fragments are expressed on the surface of phage, has been used to describe human antibody responses to both infectious agents (2, 5, 8, 9, 15, 19) and autoantigens (4, 11, 12, 16–18). We tested the feasibility of using this technology to clone and characterize intrathecal IgG found in the prototype chronic CNS infectious disease, subacute sclerosing panencephalitis (SSPE), caused by persistent measles virus (MV) infection in brain. Large combinatorial antibody libraries were constructed from heavy and light chain sequences expressed in a single SSPE brain and panned against MV-infected cell extracts. Fabs specific for the MV phosphoprotein and nucleocapsid protein were selected, proving that IgG mRNA expressed in brain during a chronic CNS infection can be used to generate high-affinity antibodies specific for the disease-causing pathogen (6). However, use of this strategy in a CNS inflammatory disease of unknown cause requires that panning be performed in situ with diseased tissue. In this study, we panned for MV-specific Fabs by using SSPE brain tissue sections as a source of antigen.
Construction of the recombinant antibody phage display library used for panning has been described previously (6). This library contains heavy (γ1) and kappa light chain sequences PCR amplified from a cDNA library generated from pathologically verified SSPE brain (SSPE 83) of a 14-year-old boy. IgG extracted from this brain was shown to be oligoclonal by isoelectric focusing and reactive with multiple MV proteins (6, 14). Phage library panning was performed as described previously (8) except for the use of SSPE brain sections (SSPE 81) instead of antigen-coated microtiter wells. Sections of fresh-frozen brain were prepared for panning as follows. Slides were dehydrated under vacuum for 2 h at room temperature (RT), fixed for 10 min in −20°C acetone, hydrated in cold phosphate-buffered saline (PBS) (10 mM sodium phosphate [pH 7.4], 150 mM NaCl), treated with 1% H2O2 in 100% methanol for 10 min at 4°C, washed twice for 1 min at RT in 100% methanol, and then air dried and stored at −20°C. Tissue sections on the slide were shaved to approximate the area of a microtiter well and surrounded by a glue wall to provide a reservoir. Two sets of slides were used: one set was pretreated for 10 min with 0.1 M HCl, pH 2.2 (low-pH elution), and neutralized in PBS, and the other set was untreated (no elution). In a humid chamber, sections were blocked with 75 μl of 3% bovine serum albumin in water for 60 min at 25°C.
After removal of blocking solution, 50 μl of freshly prepared phage suspension was added and sections were incubated for 2 h at RT. Unbound phage were removed by immersing the slides into a petri dish containing PBS–0.5% Tween 20 and agitated for 5 min on a 120-rpm rotary shaker. Washing was repeated four additional times with fresh buffer for each wash. After the final wash, excess buffer was removed, and 50 μl of elution buffer (0.1 M HCl, adjusted to pH 2.2 by the addition of glycine) was added onto the tissue section. After disruption of the tissue with a pipette tip and a 10-min incubation, the eluate was transferred to an Eppendorf tube and adjusted to neutral pH by addition of 6 μl of 1 M Tris base. The tissue was rinsed with 50 μl of PBS, and this material was pooled with the first eluate and reamplified for the next panning round.
After five rounds of panning on SSPE brain sections, phage were converted to a soluble Fab expression system, and 20 individual antibody clones from both panning experiments were examined for MV reactivity in enzyme-linked immunosorbent assay (ELISA) against lysates of either uninfected or MV-infected Vero cells (6, 9). In addition, to further establish that any MV-reactive Fabs were specifically selected during panning, soluble antibody was also generated from 40 clones taken from the unpanned SSPE library. No immunoreactivity was found in any of the unpanned Fabs. However, a single Fab (Fab 1) from the panning against untreated SSPE sections and two Fabs (Fabs 2 and 3) from the panning against acid-treated SSPE sections demonstrated strong immunoreactivity against MV-infected cell extracts, but not uninfected cell extracts (Table 1).
TABLE 1.
Binding of soluble Fabs from panned SSPE library to uninfected and MV-infected cell lysatesa
| Clone | Optical density value for SSPE sections
|
|||||
|---|---|---|---|---|---|---|
| Acid eluted
|
Untreated
|
|||||
| Infected | Uninfected | Background | Infected | Uninfected | Background | |
| 1 | 0.078 | 0.078 | 0.069 | 0.063 | 0.071 | 0.064 |
| 2 | 0.068 | 0.071 | 0.062 | 0.080 | 0.073 | 0.065 |
| 3 | 2.188 | 0.092 | 0.081 | 3.189 | 0.082 | 0.071 |
| 4 | 0.077 | 0.087 | 0.066 | 0.071 | 0.091 | 0.071 |
| 5 | 0.076 | 0.085 | 0.071 | 0.084 | 0.099 | 0.071 |
| 6 | 0.086 | 0.079 | 0.081 | 0.101 | 0.119 | 0.127 |
| 7 | 0.079 | 0.091 | 0.075 | 0.087 | 0.103 | 0.116 |
| 8 | 0.071 | 0.072 | 0.066 | 0.089 | 0.118 | 0.116 |
| 9 | 0.092 | 0.097 | 0.080 | 0.070 | 0.106 | 0.114 |
| 10 | 2.139 | 0.105 | 0.086 | 0.074 | 0.109 | 0.106 |
| 11 | 0.076 | 0.082 | 0.070 | 0.064 | 0.071 | 0.064 |
| 12 | 0.069 | 0.087 | 0.066 | 0.069 | 0.080 | 0.066 |
| 13 | 0.082 | 0.089 | 0.067 | 0.066 | 0.076 | 0.063 |
| 14 | 0.196 | 0.192 | 0.150 | 0.086 | 0.084 | 0.063 |
| 15 | 0.081 | 0.088 | 0.072 | 0.107 | 0.132 | 0.127 |
| 16 | 0.065 | 0.068 | 0.064 | 0.139 | 0.154 | 0.145 |
| 17 | 0.074 | 0.086 | 0.075 | 0.144 | 0.170 | 0.137 |
| 18 | 0.194 | 0.207 | 0.110 | 0.161 | 0.149 | 0.075 |
| 19 | 0.434 | 0.080 | 0.074 | 0.197 | 0.181 | 0.090 |
| 20 | 0.127 | 0.115 | 0.083 | 0.136 | 0.090 | 0.069 |
| IF9b | 1.301 | 0.076 | 0.062 | 1.381 | 0.085 | 0.062 |
ELISA measures binding of soluble Fabs to detergent extracts of MV-infected and uninfected Vero cells. Background binding measures immunoreactivity of secondary antibody to MV-infected cell lysates in the absence of soluble Fab. Optical density was measured at 405 nm after a 60-min incubation with p-nitrophenol phosphate as substrate.
IF9 is an MV phosphoprotein-specific Fab obtained by panning on MV-infected cell lysates (6).
We next determined the heavy chain amino acid sequences of the three MV-specific Fabs. Each contained the same sequence within complementarity-determining region 3 (CDR3) (Table 2). This sequence was also identical to that found in Fab 2B4, a recombinant antibody specific for the MV nucleocapsid protein that was selected by panning against MV-infected cell extracts (6). Importantly, we did not find this heavy chain sequence in the heavy chain sequences of 38 Fab clones randomly selected from the unpanned library (data not shown). A comparison of kappa light chain variable region sequences revealed that Fab 1 had the same light chain as 2B4, whereas Fabs 2 and 3 had a light chain that differed only by a single amino acid substitution in the CDR3. Alignment of these two light chain variable regions showed extensive homology but also revealed several distinct amino acid differences characteristic of clonal variants (data not shown).
TABLE 2.
CDR3 amino acid sequence of heavy chain and associated kappa light sequences of Fabs selected by panning on SSPE brain sections
| Fab | VH
|
Vκ
|
||||
|---|---|---|---|---|---|---|
| FR3 | CDR3 | FR4 | FR3 | CDR3 | FR4 | |
| 1 | …YYCVL | IAGRY | WGQGTL… | …YYC | QQYYSTPLT | FGGGT… |
| 2 | …YYCVL | IAGRY | WGQGTL… | …YYC | QQYFSTPLT | FGGGT… |
| 3 | …YYCVL | IAGRY | WGQGTL… | …YYC | QQYFSTPLT | FGGGT… |
| 2B4a | …YYCVL | IAGRY | WGQGTL… | …YYC | QQYYSTPLT | FGGGT… |
See reference 6.
We next sought to test the reactivity of the selected antibodies against SSPE brain sections compared to normal human brain white matter, acute MS plaques, and a brain infarct. However, the presence of large amounts of endogenous IgG in SSPE and MS brain precluded the use of an antihuman secondary antibody in evaluating recombinant Fab reactivity. To circumvent this problem, we expressed the recombinant antibodies in pFLAG, a modified form of the pComb3H vector (3) in which an 8-amino-acid flag epitope tag (13) is fused to the carboxyl terminus of the heavy chain (Fd region) moiety of the Fab. The flag peptide sequence was introduced into the backbone of the pComb3H phagemid vector between the more downstream of two SfiI sites and the unique NheI site, replacing sequence encoding cpIII with a 38-bp insert created by annealing the oligonucleotide primers HCFLAG(+) (5′-CGG CCC AGA CTA CAA GGA CGA TGA CGA TAA GGG CTA AG-3′) and HCFLAG(−) (5′-CTA GCT TAG CCC TTA TCG TCA TCG TCC TTG TAG TCT GGG CCG GCC-3′). Phagemid DNA recovered following library panning was digested with SfiI, and the excised fragment, containing antibody sequences, was directionally religated into the SfiI sites of pFLAG. Flag-tagged soluble Fab was expressed and purified as described previously (8). The flagged 2B4 antibody (identical in heavy and light chain composition to Fab 1) reacted specifically with MV-infected cells in culture, intensely staining the cytoplasm of multinucleated cells (Fig. 1A). Bound 2B4-flag was detected with a mouse anti-flag monoclonal antibody (Sigma Chemical Co., St. Louis, Mo.) followed by an anti-mouse IgG conjugated to alkaline phosphatase (Vector Labs, Burlingame, Calif.). In the same assay, unflagged 2B4 was negative with MV-infected cells (Fig. 1B).
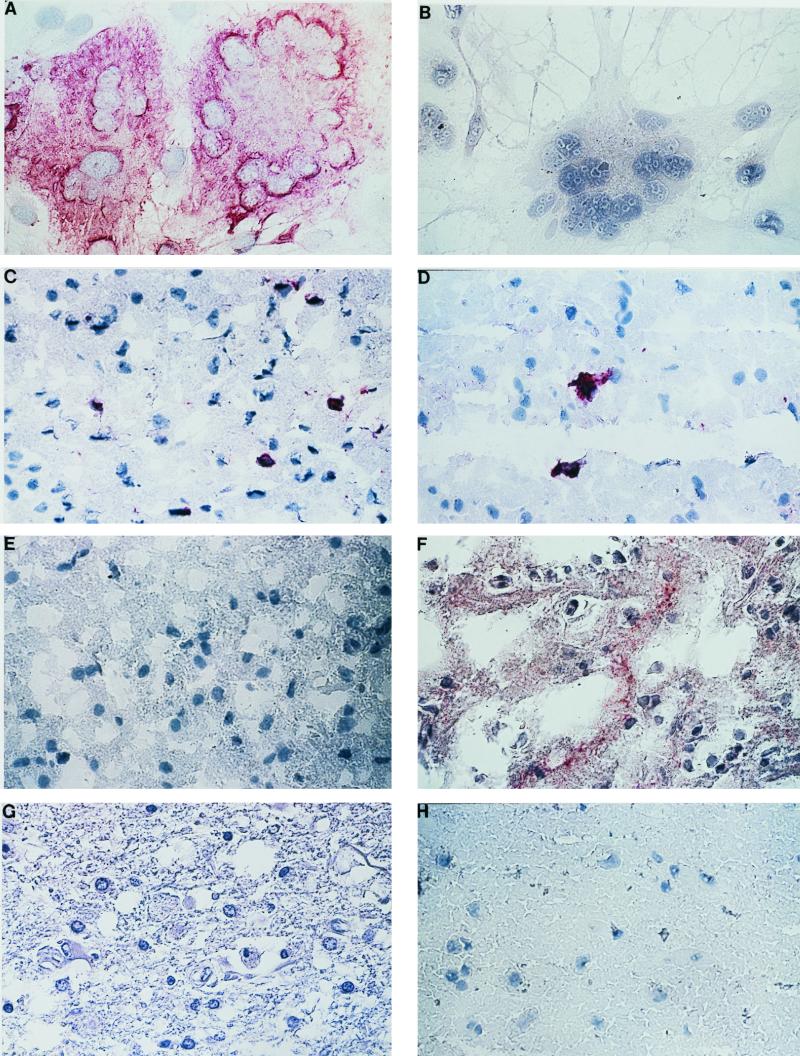
FIG. 1.
Immunostaining of MV-infected cells and SSPE and control brain with Fab 2B4-flag. (A) Staining of MV-infected BSC 1 cells with 2B4-flag. (B) Staining of MV-infected cells is not seen with normal Fab 2B4. (C) Positive staining of scattered cells in SSPE 81 brain with 2B4-flag. (D) Positive staining of individual cells in SSPE brain 81 with rabbit anti-MV polyclonal sera. (E) Staining of cells in SSPE 81 brain is not observed with normal (unflagged) 2B4. (F) Positive staining of SSPE brain (SSPE 2D) with 2B4-flag. (G) Staining of a brain infarct is not observed with Fab 2B4-flag. (H) Staining of normal human white matter is not observed with 2B4-flag. All panels were photographed at a magnification of ×250. Cells were stained with New Fuchsin substrate (red) as described in the text and counterstained with hematoxylin (blue).
To test antibody binding to brain, sections were rinsed in PBS and incubated for 1 h in 10% normal goat serum (NGS) diluted in PBS, before the addition of 5 μg of soluble flagged or unflagged 2B4 Fab or a rabbit anti-MV polyclonal antibody (1:200 dilution in 10% NGS-PBS) per ml. After incubation overnight at 4°C and an additional 2 h at RT, sections were washed five times with PBS. Sections bound with 2B4 antibodies were incubated for 3 h at RT with mouse monoclonal IgG specific for the flag peptide sequence (1:200 dilution in 10% NGS-PBS). After washing in PBS, sections were overlaid with mouse anti-IgG conjugated to alkaline phosphatase (a 1:200 dilution in 2% NGS-PBS) for 1 h at RT and again washed extensively with PBS. Staining was developed with New Fuchsin (DAKO, Carpinteria, Calif.) as substrate, and sections were counterstained with Gill no. 2 hematoxylin (Sigma Chemical Co.) and mounted with aqueous Immunomount (Fisher Scientific, Pittsburgh, Pa.) for light microscopy. Bound rabbit anti-MV IgG was detected with a 1:200 dilution of a goat anti-rabbit IgG conjugated to alkaline phosphatase.
The flagged 2B4 Fab reacted strongly with infected cells in the fresh-frozen acetone-fixed SSPE brain used for panning (Fig. 1C). This staining pattern was also seen when the same SSPE brain was incubated with a polyclonal anti-MV antibody (Fig. 1D). Immunoreactivity was not observed when adjacent sections from the same SSPE brain were stained with unflagged 2B4 (Fig. 1E). A second fresh-frozen paraffin-embedded SSPE brain (SSPE 2D) was also stained by flagged 2B4 (Fig. 1F). In this tissue, cytoplasmic staining of individual cells was accompanied by punctate staining that appeared to be extracellular. No 2B4-flag immunoreactivity was detected in sections from a formalin-fixed paraffin-embedded brain infarct (Fig. 1G) or fresh-frozen acetone-fixed normal human brain white matter (Fig. 1H) or acute MS plaques (data not shown).
Our findings demonstrate that a complex mixture of brain antigens in tissue sections can be used to specifically enrich for immunoreactive Fabs. After five rounds of panning, approximately 10% (3 of 36) of the eluted phage represented a single MV-specific Fab. The heavy chain of this Fab was not detected in 38 random clones sequenced from the unpanned library. Thus, the presence of this Fab after extensive panning does not simply reflect carryover of an abundant heavy chain sequence. Further, the three enriched Fabs all had ELISA values with MV-infected cell lysates 20- to 30-fold above those of uninfected cell lysates, whereas none of the 40 unpanned Fabs assayed displayed any immunoreactivity. The heavy chain of the enriched immunoreactive Fabs was identical to that of 2B4, a nucleocapsid-specific Fab obtained by panning against MV-infected cell extracts (Table 2). One of the three corresponding kappa light chains was also identical to the 2B4 kappa light chain, and the other two differed by a single amino acid in the CDR3 region and 2 amino acids in the framework 3 region. Acid pretreatment of SSPE sections had minimal effect on our panning results. Apparently, sufficient free antigen was available in brain sections to adequately enrich specific Fabs, consistent with our observation that MV antigens in SSPE brain are readily detected with anti-MV antibodies.
The 2B4 Fab not only immunoprecipitates the MV nucleocapsid protein from infected cell extracts and recognizes the denatured form of the protein by immunoblotting (6) but also recognizes the nucleocapsid antigen in different SSPE brains. Staining with both 2B4 and anti-MV IgG revealed that only a small fraction of cells (<1%) in the panned sections harbored MV proteins, and most immunoreactivity was confined to small areas (Fig. 1C). Thus, despite the apparent paucity of MV antigens, modest numbers of MV-specific phage were still recovered.
Phage display of combinatorial antibody libraries is a powerful tool to rapidly generate specific high-affinity antibodies from immune human donors (7). We have extended this technology to infectious inflammatory diseases of the CNS. The selection of specific Fabs with a complex antigen source such as brain sections suggests that this technology may be valuable to demonstrate antibody specificity not only in CNS diseases of unknown cause such as MS but also in other chronic inflammatory systemic disorders of unknown cause such as sarcoidosis, Wegener's granulomatosis, polyarteritis nodosa, and systemic lupus erythematosus.
Acknowledgments
This work was supported in part by Public Health Service grants NS 32623 to D.H.G. and AI 39162 to D.R.B. from the National Institutes of Health.
SSPE brain was kindly provided by the National Neurological Research Specimen Bank, VAMC, Los Angeles, Calif. SSPE-2D and brain infarct were kindly provided by John W. Prineas, Veterans Administration Hospital, East Orange, N.J. We also thank Paul Rota from the Centers for Disease Control and Prevention for giving us the Edmonston strain of MV and Thomas Moench for providing rabbit serum against purified MV. We thank Marina Hoffman for editorial review and Cathy Allen for preparing the manuscript.
REFERENCES
- 1.Barbas C F, III, Kang A S, Lerner R A, Benkovic S J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas C F, III, Collett T A, Amberg W, Roben P, Binley J M, Hoekstra D, Cababa D, Jones T M, Williamson R A, Pilkington G R, Haigwood N L, Cabezas E, Satterthwait A C, Sanz I, Burton D R. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 3.Barbas C F, III, Wagner J. Synthetic human antibodies: selecting and evolving functional proteins. Companion Methods Enzymol. 1995;8:94–103. [Google Scholar]
- 4.Barbas S M, Ditzel H J, Salonen E M, Wang W P, Silverman G J, Burton D R. Human autoantibody recognition of DNA. Proc Natl Acad Sci USA. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binley J M, Ditzel H J, Barbas III C F, Sullivan N, Sodroski J, Parren P W H I, Burton D R. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved motifs in antigen binding. AIDS Res Hum Retrovir. 1996;12:911–924. doi: 10.1089/aid.1996.12.911. [DOI] [PubMed] [Google Scholar]
- 6.Burgoon M P, Williamson R A, Owens G P, Ghausi O, Bastides R B, Burton D R, Gilden D H. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94:204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- 7.Burton D R, Barbas C F., III Human antibodies from combinatorial libraries. Adv Immunol. 1994;57:191–280. doi: 10.1016/s0065-2776(08)60674-4. [DOI] [PubMed] [Google Scholar]
- 8.Burton D R, Barbas III C F, Persson M A A, Koenig S, Chanock R M, Lerner R A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ditzel H J, Barbas S M, Barbas III C F, Burton D R. The nature of the autoimmune antibody repertoire in human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1994;91:3710–3714. doi: 10.1073/pnas.91.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilden D H, Devlin M E, Burgoon M P, Owens G P. The search for virus in multiple sclerosis brain. Mult Scler. 1996;2:179–183. doi: 10.1177/135245859600200403. [DOI] [PubMed] [Google Scholar]
- 11.Graus Y F, de Baets M H, Parren P W H I, Berrih-Aknin S, Wokke J, van Breda Vriesman P J, Burton D R. Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol. 1997;158:1919–1929. [PubMed] [Google Scholar]
- 12.Hexham J M, Furmaniak J, Pegg C, Burton D R, Smith B R. Cloning of a human autoimmune response: preparation and sequencing of a human anti-thyroglobulin autoantibody using a combinatorial approach. Autoimmunity. 1992;12:135–141. doi: 10.3109/08916939209150320. [DOI] [PubMed] [Google Scholar]
- 13.Hopp T P, Prickett K S, Price V L, Libby R T, March C J, Cervetti D P, Urdal D L, Corbon P J. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology. 1988;6:1204–1210. [Google Scholar]
- 14.Owens G P, Burgoon M P, Devlin M E, Gilden D H. Extraction and purification of active IgG from SSPE and MS brain. J Virol Methods. 1997;68:119–125. doi: 10.1016/s0166-0934(97)00118-3. [DOI] [PubMed] [Google Scholar]
- 15.Parren P W H I, Burton D R. Antibodies against HIV-1 from phage display libraries: mapping of an immune response and progress towards antiviral immunotherapy. In: Capra J D, editor. Antibody engineering. S. Basel, Switzerland: Karger; 1997. pp. 18–56. [DOI] [PubMed] [Google Scholar]
- 16.Portolano S, McLachlan S M R B. High affinity, thyroid-specific human autoantibodies displayed on the surface of filamentous phage use V genes similar to other autoantibodies. J Immunol. 1993;151:2839–2851. [PubMed] [Google Scholar]
- 17.Siegel D L. Isolation of human anti-red blood cell antibodies by repertoire cloning. Ann N Y Acad Sci. 1995;764:547–548. doi: 10.1111/j.1749-6632.1995.tb55880.x. [DOI] [PubMed] [Google Scholar]
- 18.Siegel D L. The human immune response to red blood cell antigens as revealed by repertoire cloning. Immunol Res. 1998;17:239–251. doi: 10.1007/BF02786448. [DOI] [PubMed] [Google Scholar]
- 19.Williamson R A, Burioni R, Sanna P P, Partridge L J, Barbas III C F, Burton D R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci USA. 1993;90:4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]