Abstract
Practical relevance:
The reported incidence of hip dysplasia (HD) in cats varies dramatically between studies, but the condition is likely more common than we realise. There is little doubt that cats with HD and associated osteoarthritis (OA) suffer pain, and this warrants appropriate therapy.
Diagnostic challenges:
Clinical signs of HD in cats are often gradual in onset, making them difficult to appreciate, but may include inactivity, pelvic limb lameness, difficulty jumping and climbing stairs, and reluctance to squat to defecate. Often lameness is bilateral, and can be particularly difficult to recognise. The most common radiographic finding is an abnormally shallow acetabulum. Subluxation, however, is not consistently associated with OA in cats and therefore the role that joint laxity plays in disease progression remains uncertain. Degenerative changes of the femoral head and neck seem to develop later than in the dog, and are less marked.
Therapeutic challenges:
The majority of cats respond to non-surgical management with environmental modulation, physical therapy, dietary modulation, weight loss, nutraceuticals and drug therapy. Should non-surgical management not provide sufficient relief, two salvage surgical options are available: femoral head and neck excision (FHNE) and total hip replacement (THR). While there is a risk of complications with micro-THR, the positive outcomes that have been reported indicate that it should be considered in the treatment of coxofemoral pathology in cats in the same way that THR is considered for larger dogs, especially given the inconsistent results associated with FHNE. Monitoring the effect of treatment is challenging as the assessment of pain in cats is complex and there is no validated scoring system or owner-completed questionnaire yet available for cats.
Evidence base:
There is a paucity of clinical reports focusing solely on HD in cats. The author draws on a combination of published studies, in cats, dogs and humans, as well as personal clinical experience.
Introduction
Hip osteoarthritis (OA) is relatively common in the domestic cat, 1 but is not well recognised – either because cat owners do not appreciate the pelvic limb lameness or because cats are better able to compensate for the resulting functional impairment. 2 The reported overall incidence of hip dysplasia (HD) and associated OA in domestic cats varies significantly – ranging from 6.6% in one study of 684 cats 3 to 32% in another study of 78 cats. 4 The incidence is breed dependent, with purebred cats3 –6 having a greater incidence (12.3%) than domestic shorthair (DSH) cats (5.8%); this may partially explain the significant variation in incidence reported, as most of the cats in the aforementioned study of 78 cats were purebred. 4 The different diagnostic criteria used in that study to diagnose HD, including assessment of passive coxofemoral laxity, may also have contributed to the varied incidence. 4
Among purebred cats, the Maine Coon is most likely to be affected, with 18–21% of 284 cats in one study showing radiographic evidence of HD based on standard Orthopedic Foundation for Animals hip radiography. 3 The Persian and Himalayan breeds are also more likely to be affected. 3 These three breeds all have a larger body type, which may be a contributing factor in the development of OA. 3 The high degree of affliction in certain breeds may also be the result of a narrower gene pool. 3
HD is recognised to be an inherited disease in a number of species and the mode of inheritance is generally accepted to be polygenic. 3 With polygenic traits, the environment, while not causing the disease, may exacerbate or modify the phenotypic manifestation. 3 The heritability of feline HD has not been specifically documented, but HD was reported in three DSH cats from the same litter 7 and it is considered likely that genetics plays a role.
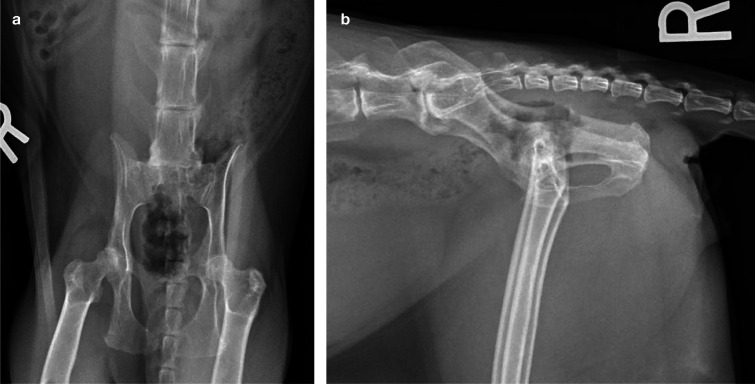
There may be some evidence of a gender predilection in the cat, which has not been noted in the dog, with two studies reporting more cases in female than in male cats.8,9 Concurrent HD and medial patellar luxation has also been noted in cats (Figure 1);8,10,11 a weak association between these conditions exists, 12 with one study showing that cats were three times more likely to have both HD and patellar luxation than they were to have either condition alone. 12 It has long been hypothesized that a luxated patella may produce femoral torsion and alter hip joint forces, and that these altered forces may contribute to the pathophysiology of HD. 13
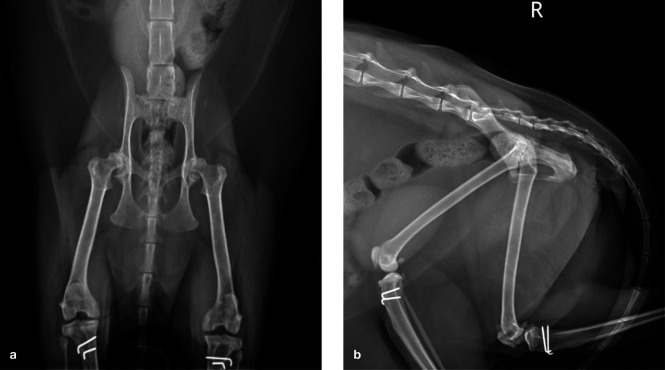
Figure 1.
Ventrodorsal (a) and lateral (b) radiographs of a 7-year-old female domestic shorthair cat that presented for investigation of bilateral pelvic limb lameness. Bilateral medial patellar luxation had been surgically addressed 6 years earlier. A bilateral stiff and stilted gait was noted, with pain on manipulation and reduced range of motion affecting both hips. Both patellae were stable and there was no pain upon stifle manipulation. The radiographs reveal moderate bilateral coxofemoral osteoarthritis (OA) with decreased acetabular coverage of both femoral heads and irregularly marginated shallow acetabulae with osteophytes present along the cranial and caudal margins. A large osteophyte is also present along the caudal margin of the left acetabulum
Clinical presentation and physical examination
Due to the nature of chronic pain associated with OA, which is generally gradual in onset, the accompanying behavioral changes can be subtle and easily missed. 17 Assessment of pain in cats is difficult since they appear less demonstrative than dogs in indicating that they are in pain, with aggression, resentment to handling and lack of responsiveness to human attention being proposed as manifestations of both acute and chronic pain in this species. 18
The challenges of orthopedic examination and gait assessment in cats have been reviewed elsewhere.19,20
Onset and range of signs
Due to the paucity of clinical reports concentrating solely on HD, there is limited evidence regarding the age at which clinical signs become apparent. Based on available data, onset varies between 3 months and 3.5 years of age.11,21
It is generally believed that clinical signs of feline OA include weight loss, anorexia, depression, abnormal elimination habits, poor grooming, coat changes, alterations in claw-sharpening behavior, aggression, increased or decreased interactions with both strangers and owners, changes in posture, vocalization, reluctance to jump and overt lameness.11,22 –25 A strong association has been found between demeanor and the presence or absence of OA in cats, with the suggestion that the presence of radiographic OA is associated with pain and thus an unfriendly temperament. 26 While not specifically associated with hip OA, it has been shown that the presence of generalized OA can impact the inactive behaviors of cats such as sleeping on the bed or lying in the sun. 27 Using accelerometers, cats with hip OA have also been shown to be less active at night when compared with normal cats. 28

Clinical signs reported by owners associated with HD specifically have included inactivity, 29 pelvic limb lameness that is worsened by exercise, 29 and difficulty climbing, 11 reluctance to jump,11,29 inability or reluctance to climb stairs,21,29 walking in a crouched position, 21 howling while resting 21 and reluctance to squat to defecate. 21
Gait abnormalities
Lameness associated with HD in cats can vary from relatively mild to severe, with an inability to walk on the pelvic limbs. 11 In addition, HD is commonly bilateral, 30 and bilateral lameness can be difficult to recognise. 31 With unilateral problems, cats may unload a painful limb, even at rest. 19 Some cats may demonstrate a hip hike, where the hip is elevated when the painful limb strikes the ground. The tail may also be used asymmetrically to shift weight towards the more normal side when the cat is in motion. 19 Where lameness is bilateral, the gait is stiff and stilted with shortened strides bilaterally.
Joint changes and assessment
Negative findings with respect to pain, crepitus, effusion and thickening tend to predict radiographically normal joints. 26 Therefore, screening for the presence of these abnormalities is recommended during physical examination, although appreciation of effusion and thickening around the hip joint can be difficult. The most commonly reported physical examination findings in cats with HD are pain and crepitus upon extension of the hips, and muscle atrophy.11,21,29
An increased range of motion is also associated with decreased odds of radiographic OA being present. 26 Specifically, a reduced range of motion is associated with HD 29 and, therefore, assessment for a restriction in range of motion is recommended when HD and OA are suspected. Goniometry provides a rapid and reliable method of quantifying the range of motion of joints, 32 and results have indicated that goniometric joint measurements in non-sedated and sedated cats are repeatable and valid. 33 Goniometry has been used in human medicine to evaluate the severity of joint injury, and to monitor the progression of disease and the response to treatment,34 –36 and can be performed for similar purposes in cats.
When examining the hip, abduction should be performed in addition to flexion, extension and rotation (Figure 2). In most cats you can easily obtain 90º of pain-free abduction, similar to in dogs. 19 Cats with hip OA generally resent hip abduction, sometimes more so than flexion and extension. 19
Figure 2.
Limits of abduction (a) and extension (b) in a domestic shorthair cat
Testing for the Ortolani sign (an indicator of excessive hip joint laxity) can be attempted, although it is possible for cats with painful hips to overcome this test through muscular forces; therefore, this test should always be repeated under sedation when found to be negative in the conscious cat. To perform the test, the cat can be examined either in lateral or dorsal recumbency. The author’s preference is lateral recumbency. Standing to one side of the cat, one hand is placed over the spine to provide counter-pressure. The other hand supports the limb at the level of the stifle, with the stifle and hip each aligned at approximately 90º of flexion. Pressure is applied up the shaft of the femur, which in cats with coxofemoral laxity will subluxate the hip. The limb is then gently abducted away from midline. In cats with laxity, at a given angle the femoral head will relocate into the correct position as indicated by a palpable, visual or audible click. This constitutes a positive Ortolani sign.

Imaging
Pain on orthopedic examination does not necessarily correlate with radiographic OA. In one study, 67% of apparently painful feline joints had no radiographic signs of OA, 37 and in another only 36% of feline joints with radiographic OA were painful. 38 This disparity between clinical and radiographic signs also extends specifically to HD, with most cats not showing progression of clinical signs despite the progression of radiographic signs. 11 These studies have raised the question of whether there truly is a poor correlation between clinical and radiographic OA or whether OA in cats is associated with less obvious clinical signs that are not being appreciated by owners and veterinarians alike.

Coxofemoral joint assessment
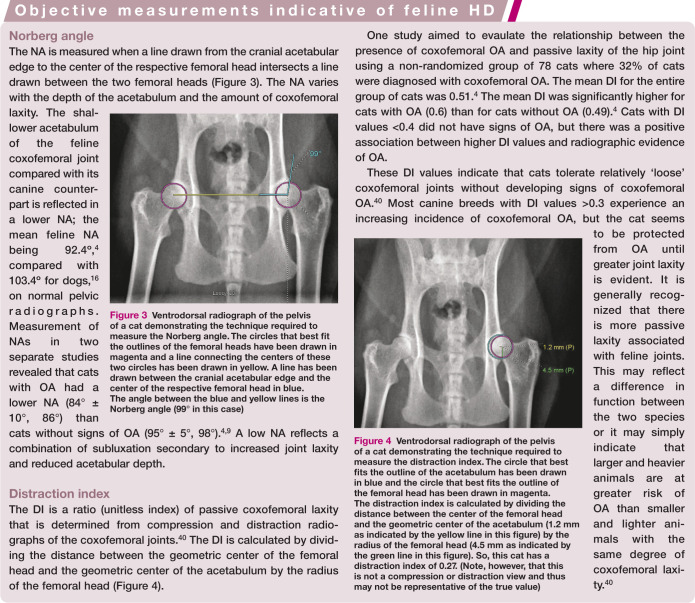
In one study, the normal acetabulum of the cat was generally shallower than that of the dog. 3 In dogs, one of the accepted criteria for normal acetabular depth is coverage of 50% or more of the femoral head. However, even this criterion is not well documented. If it were applied to cats then HD would be overdiagnosed. 3 Numerous cats of advanced age have less than 50% acetabular coverage of the femoral head but no evidence of OA. 3 Objective measurements used in the assessment of coxofemoral joints include the Norberg angle (NA) and the distraction index (DI). 40
Figure 3.
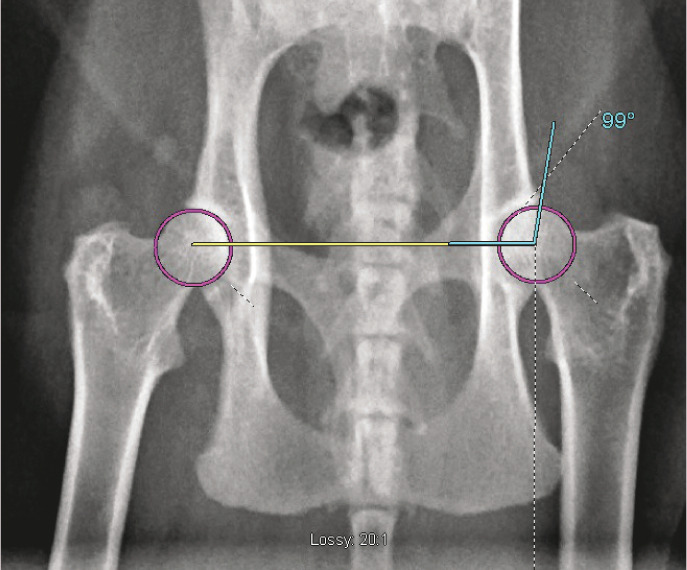
Ventrodorsal radiograph of the pelvis of a cat demonstrating the technique required to measure the Norberg angle. The circles that best fit the outlines of the femoral heads have been drawn in magenta and a line connecting the centers of these two circles has been drawn in yellow. A line has been drawn between the cranial acetabular edge and the center of the respective femoral head in blue. The angle between the blue and yellow lines is the Norberg angle (99° in this case)
Figure 4.
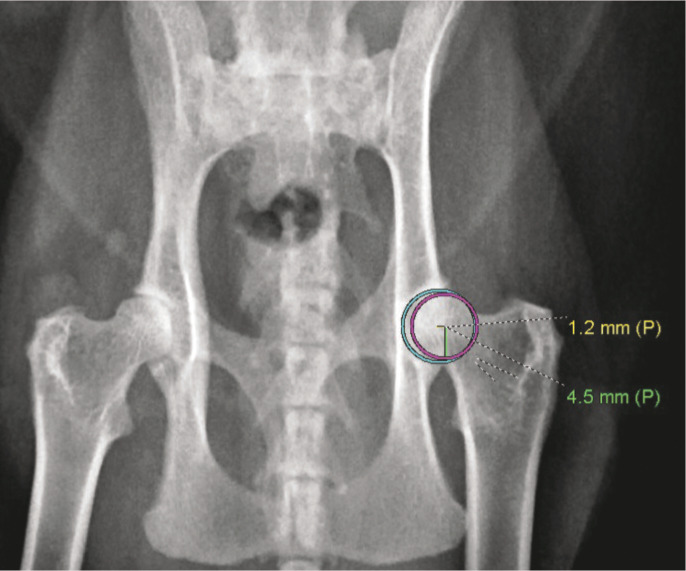
Ventrodorsal radiograph of the pelvis of a cat demonstrating the technique required to measure the distraction index. The circle that best fits the outline of the acetabulum has been drawn in blue and the circle that best fits the outline of the femoral head has been drawn in magenta. The distraction index is calculated by dividing the distance between the center of the femoral head and the geometric center of the acetabulum (1.2 mm as indicated by the yellow line in this figure) by the radius of the femoral head (4.5 mm as indicated by the green line in this figure). So, this cat has a distraction index of 0.27. (Note, however, that this is not a compression or distraction view and thus may not be representative of the true value)
Other radiographic diagnostic criteria
In one study, a shallow acetabulum was the most common (and sometimes only) radiographic abnormality in cats diagnosed as dysplastic. 3 Other radiographic diagnostic criteria for feline HD include signs of coxofemoral subluxation, enthesophyte formation on the acetabular margins, and remodeling and degenerative changes of the femoral head and neck. 40 Subchondral bone sclerosis and joint-associated mineralization have also been associated with coxofemoral OA in cats. 41 Degenerative changes seem to develop later and are less marked than in the dog; also, unlike the condition in dogs, most degenerative changes appear on the craniodorsal acetabular margins (Figure 5), and there is a low incidence of degenerative remodeling reported on the femoral head and neck. 16 In chronic cases the craniodorsal acetabular edge can become markedly deformed, extending caudally and laterally and forming a new dorsal acetabular edge covering the femoral head. 11 These degenerative changes have been interpreted as attempts to buttress or reinforce the acetabular margin, lending support to the hypothesis that a shallow acetabulum is important in development of the disease. 3
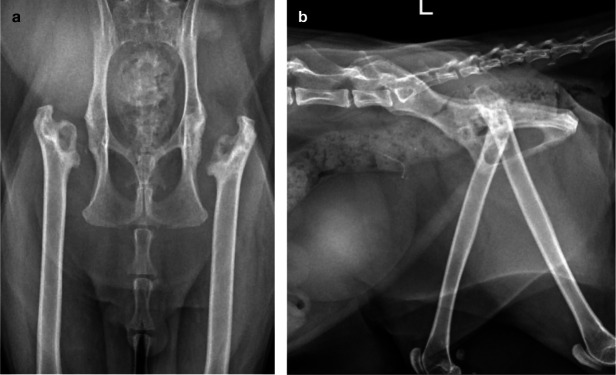
Figure 5.
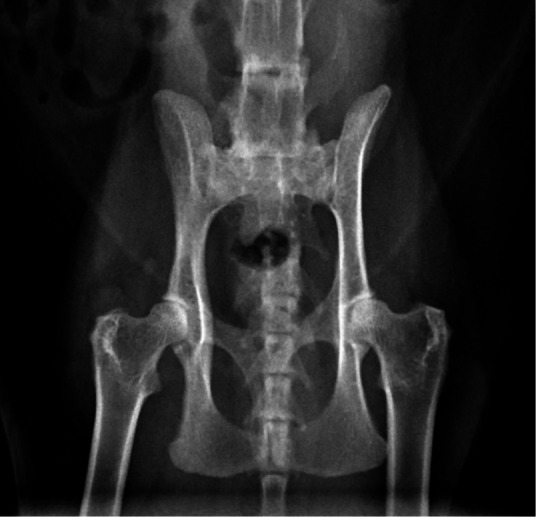
Ventrodorsal radiograph of a 13-year-old domestic shorthair cat that presented for assessment of left pelvic limb lameness. Gait assessment revealed a left hip hike. On orthopedic examination a mild pain response and crepitus were evident upon extension of the coxofemoral joints. The radiograph reveals mild bilateral coxofemoral OA (worse on the left than on the right), with small osteophytes and moderate sclerosis on the left and right acetabular margins
One study reported that a mushroom-shaped, or oval, femoral head was present in cats with radiographic changes associated with HD (Figure 6), but was also visualized in cats (even of advanced age) without remodeling and proliferative changes involving the craniodorsal acetabular margin, shallow acetabulae and/or subluxation. 3 This radiographic change has been reported as a normal variation in cats that do not have radiographic findings associated with OA. 42 When remodeling of the femoral head and neck does occur, this can present as deformation of the femoral head, sometimes with mild flattening, with poor demarcation of the femoral neck. 11 Exostoses can be seen on the craniolateral edge of the femoral head, and fine linear spurs on the lateral aspect of the femoral neck have been noted. 11
Figure 6.
Ventrodorsal (a) and lateral (b) radiographs of a 13-year-old male domestic shorthair cat that presented with an unsteady pelvic limb gait and right pelvic limb lameness. The radiographs demonstrate marked right coxofemoral OA and subluxation. There is moderate rotation on the ventrodorsal view, as evidenced by the asymmetric obturator foramina, and this may affect interpretation. The femoral head is mushroom-shaped with an irregular margin and the femoral neck is shortened as well as markedly thickened. The acetabulum is shallow, irregularly shaped and has marked periarticular new bone formation on the cranial and caudal aspects of the rim. Thickening and subchondral sclerosis are evident on the medial aspect of the right acetabulum. There is an irregularly shaped, smooth margined, crescent-shaped, possibly separate mineral opacity cranial to the greater trochanter on the ventrodorsal view, and multiple rounded, separate mineral opacities caudal to the femur
The degree of osteophytosis, presence of joint-associated mineralizations and joint subluxation have been shown to correlate with the degree of articular cartilage damage in the feline hip. 41 However, macroscopic cartilage damage is commonly found in the hip joints of cats that have no radiographically detectable signs. In one study, 57% of cats with no radiographic signs had macroscopic cartilage damage in the hips. 41 This study showed that radiographic findings did not correlate well with cartilage degeneration in cats and suggested that other imaging modalities should be considered when making a diagnosis of feline OA. 41 While other imaging modalities have not been assessed in great depth, in cases with a clinical presentation suggestive of HD and OA, but where supportive radiographic findings are absent, either further imaging, or potentially a trial treatment of therapy, may be warranted.
In dogs, horses and people, magnetic resonance imaging (MRI) is more sensitive than radiography for assessing OA structural changes such as cartilage lesions, osteophytosis, joint effusion and synovial thickening.43 –45 MRI has also been used to investigate HD and OA in cats. 29 While osteophytes and sclerosis were found using both radiography and MRI, one cat that had no lesions detectable with radiography had several OA lesions identified on magnetic resonance images including bilateral osteophytosis, joint effusion and thinning of the articular cartilage. 29 Use of MRI in two cats with OA revealed bone marrow lesions in the femoral head that were not detected using radiography. 29 Bone marrow lesions are related to involvement of subchondral bone in the etiopathogenesis of OA and are associated with disease progression and pain in people.46 –48
Lumbosacral pathology
When reviewing pelvic radiographs of cats, the lumbosacral region must be evaluated as well as the hips (Figure 7). The lumbosacral area is the second most common region of the axial skeleton to be affected by radiographic OA in cats, 49 and is the area that is most severely affected. 50 While it is unknown how frequently clinical signs are associated with these radiographic changes, clinically relevant lumbosacral intervertebral disc disease has been reported in cats. 51
Figure 7.
Ventrodorsal (a) and lateral (b) radiographs of a 14-year-old male domestic shorthair cat that presented with difficulty ambulating on the left pelvic limb. Gait assessment demonstrated a very stiff and stilted gait bilaterally, and there was mild discomfort upon full hip extension and palpation over the caudal lumbar spine. There were no neurologic deficits. The radiographs demonstrate evidence of L7–S1 chronic intervertebral disc disease with spondylosis deformans at the lumbosacral junction; a significant lateralized component to the left is seen on the ventrodorsal view. The L7–S1 disc space is narrow and the adjacent vertebral end plates are moderately sclerotic. The increased lucency of the left ilial wing relative to the right is likely due to superimposition of gas in the descending colon
The lumbosacral region should be evaluated for osteophytes, spondylosis, disc-associated degeneration (end plate sclerosis, erosion, disc mineralization, disc-space narrowing) and subluxation. 49 Clinical signs associated with lumbosacral disease in cats include reluctance to jump, elimination outside of the litter box, reluctance to ambulate and constipation, 51 as well as, in the author’s experience, pain on hip extension. Thus, careful assessment of radiographs is important as differentiation between lumbosacral disease and HD can be complex based on physical examination findings and history alone.
Non-surgical management
Unalleviated chronic pain is a welfare concern for cats and functional limitations and pain may contribute to behavioral problems (eg, house-soiling, altered social interactions). 52 These, in turn, may cause nuisance, property damage, injury (eg, due to aggression) and loss of the human–animal bond with consequent surrender or even euthanasia. 53 While the clinical signs associated with feline OA are less obvious than those associated with canine OA, primarily because cats are not expected to perform strenuous activities or go on walks with their owners, 54 there is little doubt that cats suffer pain associated with OA 17 and that this warrants appropriate therapy.
Environmental and activity modulation
Arthritic pain causes many significant behavioral changes in cats and modifying the environment in certain ways can help to overcome some of these and thereby improve the physical and psychologic welfare of the animal.17,55 Access to heights is important for cats, 17 and cats with HD and OA have been noted to be less inclined to jump. Moving furniture so as to provide ‘stepped’ access to beds, sofas or window ledges that the cat favors may help. 17 Additionally, access to food, water bowls and litter boxes should be made as easy as possible. 17 Low-edged litter trays may be helpful for some cats, while enclosed litter trays may assist owners in coping with abnormal elimination habits (eg, cats urinating while standing due to a reluctance to posture).
A more complex environment with facilitated access to various levels and areas is also likely to encourage more movement, which is known in other species to be important for maintaining muscle tone and mass, and minimizing pain associated with joint disease. 17 Provision of cat towers, toys and cat-nip, and hiding of food, may also encourage foraging, hunting and play behavior; regular periods of play (with laser pointers, toys, etc) will increase exercise levels, 17 an added bonus of which is that weight gain will be minimized.
Physical therapy
While physical therapy is in the early phases in canine medicine, and has not yet been evaluated for feline patients, it is likely that the same basic principles and benefits will apply to cats.17,56 The specific type of therapy is best designed and supervised by a trained veterinary physiotherapist. Regularly performed low-impact exercise, such as controlled walking, use of a water treadmill or hydrotherapy, helps to maintain supporting muscle strength and joint function, while minimizing joint stresses. Hydrotherapy is used less often in cats than in dogs, but should not be dismissed entirely; following a period of adjustment, many cats will tolerate this modality well. 56 The sit-to-stand exercises, and so on, that are used in dogs to target specific muscle groups are not normally applicable in cats. However, encouraging cats to go up and down stairs or feeding them from a step, such that weightbearing on the pelvic limbs is required, can help to strengthen the pelvic limbs.
Both passive and active range of motion exercises can be used to improve joint range of motion, and promote cartilage metabolism and diffusion of nutrients. 57 These exercises, together with massage techniques, can be taught to owners and can alleviate muscle pain and encourage more interaction between owner and cat, impacting positively on quality of life. 17 Other modalities such as shock wave therapy, laser therapy, and heat and cold therapy may be of benefit in some cases; however, as no controlled studies in cats have been performed it is not possible to state under which circumstances these therapies will be most useful. 17 Further work providing more definitive evidence of beneficial effects is required before firm recommendations can be made.
Dietary modulation
Diets rich in omega-3 fatty acids are recommended for cats with OA. It is not just the level of omega-3 fatty acids that is important but also the ratio of omega-3 to omega-6 fatty acids. In dogs with OA, omega-3 rich diets have been shown to improve weightbearing,58,59 and also allow doses of non-steroidal anti-inflammatory drugs (NSAIDs) to be reduced sooner and to a lower level than when the diets are not employed. 60 The author has had similar experience with cats. One recent study demonstrated an increase in activity levels in cats fed an omega-3 rich diet when compared with cats fed a control diet, based on both subjective owner assessment and objective data from collar-mounted activity monitors. 61 As activity levels have been shown to be reduced in cats with HD and OA, these diets can be recommended in management. Contrary to popular opinion, there is also evidence to suggest that omega-3 rich diets may assist with weight loss.61 –63
Nutraceuticals
Both glucosamine and chondroitin are involved in the metabolism of cartilage matrix proteins and are widely used to treat OA in people, despite conflicting trial results.66 –78 Few studies have shown the use of glucosamine and chondroitin to be useful in cases of canine OA, 79 and a recent small-scale study assessing the use of glucosamine and chondroitin in feline patients with OA did not demonstrate any significant effect. 80 While the patients in this study did improve during the initial 70 days, the improvement was not statistically significant. However, this study only included 30 cats in total, 13 of which received the glucosamine–chondroitin supplement, and so further work is needed in this area. It is possible that a larger study may be more supportive of this nutraceutical, but currently there is no firm data from which to draw conclusions about the use of these supplements in cats with HD or associated OA.
Green lipped mussel extract (GLME) has been shown to have a beneficial effect on clinical signs associated with OA in dogs, 81 but there are no studies to date investigating use of this in feline patients with OA.
There are, nevertheless, anecdotal reports of improvements being seen following nutraceutical use in cats with OA and the author has had positive experience with supplements that contain both GLME and glucosamine and chondroitin. In the absence of evidence to support their use, strong recommendations cannot be made but, in the light of this experience, the author will often trial a joint supplement containing GLME, glucosamine and chondroitin in addition to NSAIDs and an appropriate omega-3 rich diet for treatment of feline HD and OA. One option is Yumove Cat (Lintbells), which contains GLME, glucosamine, chondroitin, manganese, hyaluronic acid and vitamin E, but there are several supplements available from various suppliers. The author’s recommendation is to trial any supplement for a minimum of 8–12 weeks, as results are not anticipated to be immediate. 54 In the absence of any response after this time, continuing with the supplement is not likely to be warranted.
Drug therapy
NSAIDs are the mainstay for managing pain associated with OA in other species and there is a lot of evidence to support their effectiveness in cats.37,38,52,82,83 There are only four NSAIDs licensed for use in cats in the UK: meloxicam, which is licensed for use for an unlimited time; robenacoxib, licensed for use for 6 days; ketoprofen, licensed for 5 days; and tolfenamic acid, licensed for 3 days. More information about these individual drugs, including their formulations and dosing, is available in previous reviews.17,84 The situation becomes more complex in some other countries. For example, in North America no NSAID is licensed for long-term use in cats; meloxicam is only licensed for a single dose and robenacoxib for 3 days.
Given that it is older cats that often suffer from OA, routine blood and urine analyses are advisable to assess liver and kidney status before commencing NSAID therapy. Monitoring of blood pressure is also recommended since inhibition of cyclooxygenase (COX) within the kidneys can exacerbate hypertension.85,86 Detection of abnormalities need not be a contraindication for using the drug but may influence the initial dose that is administered and the frequency of follow-up checks recommended. If there is evidence of renal or hepatic compromise, starting with a lower dose of meloxicam (0.01–0.03 mg/kg) may be appropriate, with gradual increases being permitted if required to control symptoms in the absence of any clinical deterioration.
A common concern in older cats with OA is concomitant chronic kidney disease (CKD). However, recent studies have demonstrated that long-term treatment with meloxicam does not appear to reduce the lifespan of cats with pre-existent stable CKD. 87 Therefore, in cats where OA impacts negatively on quality of life, use of meloxicam should continue to be considered, regardless of the presence of CKD.
There is published and presented evidence supporting the use of NSAIDs – in terms of alleviating pain and enhancing mobility – in painful cases of OA in cats. Meloxicam has proven to be very effective for treating chronic pain in arthritic cats.37,38,52,82,83,87,88 It is palatable and the liquid formulation facilitates accurate dosing, which likely increases owner compliance. In one study, 61% of owners felt that their cat improved following a 4–6 week period of meloxicam treatment. 37 Owners reported changes in their cat’s behavior and lifestyle over time associated with OA and a reversal in the altered behavior patterns was noted when pain relief was given in the form of a 28 day course of meloxicam. 37 The greatest changes noted were in activity levels and ability to jump.37,52 Owners also reported improvements in their cat’s activity levels and quality of life in a study comparing meloxicam with placebo; in this study, actimetry data additionally confirmed an increase in activity levels following administration of meloxicam. 38
Robenacoxib is not licensed for long-term use, in contrast to meloxicam (in the UK), but there is evidence that it specifically targets inflamed tissues. 89 Plasma levels reduce relatively quickly but concentrations in inflamed tissues remain high. The author has had very positive clinical experiences with use of robenacoxib in some cats with OA that had not responded to medication with meloxicam. In cases where longer term therapy is required, pulse therapy with robenacoxib can be considered, with the treatment regimen tailored to the individual patient to maintain comfort.
Multimodal analgesia is now being introduced to feline patients, although experience is relatively lacking at this stage.17,55,90
Stem cell therapy
Adipose-derived mesenchymal stem cell (MSC) therapy is a rapidly growing field of research. It has been shown that stem cells have an affinity for damaged joint tissue, and in vivo studies have confirmed that these cells have the ability to localize and participate in the repair of damaged joint structures including cruciate ligaments, menisci and cartilage lesions. 92 For these reasons, autologous adult stem cells have been used to manage OA. The cells are harvested from adipose tissue, processed and then injected into affected joints several days later. It is known that they can differentiate into several different tissue types and can also supply trophic factors; however, the cellular basis of the improvements seen following injection remains to be elucidated. 17
Two small studies of MSC therapy have been performed in dogs with hip and elbow arthritis, with positive results being shown in some of the outcome measures.93,94 In one of these studies, hip arthritis, lameness, joint range of motion and pain on manipulation all significantly improved over time in treated animals when compared with controls, but statistically significant changes in owner evaluation scores were not noted. 93
This technique has been used in cats, with anecdotal reports of improvement, 17 but no published studies in cats are available at this time.
Surgical management
There are essentially two recommended options for surgical management of feline HD and associated OA: femoral head and neck excision (FHNE) and total hip replacement (THR). Both are salvage options and are only considered appropriate after failure of non-surgical management.
Pectineus myotendonectomy is also mentioned in the more historical literature, 5 but has been reported to result only in temporary improvement in some cases, 21 necessitating FHNE to control ongoing clinical signs. Given the very satisfactory results that can be achieved with the other surgical options available, it is difficult to perceive a convincing role for this procedure in management of feline HD cases today.
Femoral head and neck excision
The salvage procedure of FHNE (Figure 8) is intended to alleviate pain associated with movement of diseased or injured coxofemoral joints.100 –109 Indications include end-stage hip OA secondary to HD. 100 The clinical results reported following this procedure vary significantly depending on the individual study and the outcome measures used. In one study, seven cats underwent FHNE and excellent limb usage was documented in all, based on owner perception assessed by questionnaire postoperatively. 100 No other outcome measures were reported in this study and therefore the clinical examination findings relating to range of motion of the hip joint, and pain associated with this, were not available. Another limitation of the study is that only one of these FHNE procedures was performed for treatment of HD, with the others being performed for femoral fractures (2), acetabular fractures (1) and coxofemoral luxation (3). Therefore, extrapolation of the results to cats with HD may not be appropriate. HD is generally a bilateral condition, while these other conditions are more likely to be unilateral and hence possibly associated with a more favorable prognosis.
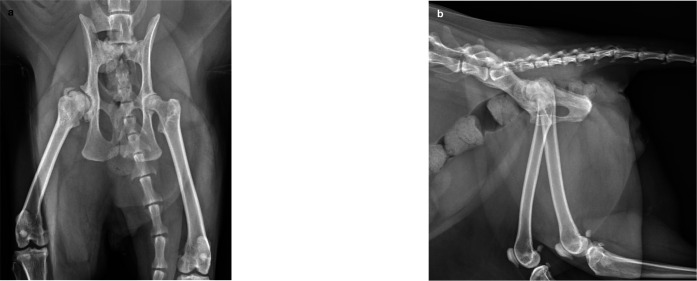
Figure 8.
Ventrodorsal (a) and lateral (b) views of a 4-year-old male domestic shorthair cat that had bilateral FHNE performed 2 years earlier for treatment of HD and associated OA. Pelvic limb lameness and demeanor had improved following this but the cat remained unable to jump, muscle atrophy was evident and a moderate pain response persisted upon full extension or abduction of the hips. The radiographs reveal mild bilateral acetabular and proximal femoral degenerative change. An irregular bony protuberance is evident at the ostectomy edge on the right, but whether this was due to inappropriate ostectomy initially or subsequent remodeling is unknown. Otherwise the ostectomies have been performed appropriately, with the absence of femoral heads and necks and the presence of greater and lesser trochanters bilaterally. The proximal femora are sclerotic and the acetabulae are shallow bilaterally
Following FHNE, high levels of postoperative rehabilitation are often required in order to achieve optimal long-term function.110,111 Successful outcome after FHNE is dependent on sufficient periarticular muscle competency for maintenance of a functional and durable pseudoarthrosis.100 –109
Reported complications after FHNE include ongoing lameness associated with limb shortening, patellar luxation, sciatic neurapraxia, and limitation in range of hip motion accompanied by severe muscle atrophy.108,109,112 –117 The most common reason for poor outcome after FHNE is bone-to-bone contact between the proximal femur and acetabulum, which may or may not be related to inadequate femoral neck resection.101,103,109,112,113 However, several studies have suggested that bone-on-bone contact is common after successful FHNE and is thus not always associated with a suboptimal outcome. 118 The contribution of sciatic nerve impingement to ongoing clinical signs in these cases is unknown. This appears to be a more common complication after use of a biceps femoris or deep gluteal muscle flap in conjunction with FHNE; 119 this is largely of historic interest, however, as the interposition of muscle flaps following FHNE is generally no longer recommended.
There is a common perception that function after FHNE is better in cats and small dogs compared with large dogs. This is based on the presumption that the ability to compensate for the mechanical disadvantages of an absent coxofemoral articulation is dependent on weight, with lighter animals having an advantage.102,105,107,109 Recent work has questioned this presumption, after demonstration of long-term functional disabilities in many small breed dogs and cats after FHNE. 112 For 183 animals (132 dogs and 51 cats), outcome 4 years after surgery following FHNE was good in 38% of cases, satisfactory in 20% and poor in 42%, based on follow-up veterinary examinations supplemented by owner assessment. 112 With functional results only being graded as good in 38% of cases, 112 when recommending FHNE to pet owners it should be emphasized that surgical outcome can be unpredictable, regardless of the weight of the animal. 118
Total hip replacement
Although FHNE has the potential to return some dogs and cats to near-normal function, inconsistent clinical results have fuelled a trend toward choosing THR as the standard-of-care salvage surgery. 103 THR has been available as a salvage procedure for the coxofemoral joint in medium and large breed dogs for over four decades.103,120 Before the introduction of smaller THR prostheses (Micro HIP; Biomedtrix) in 2005, 121 FHNE was the only salvage procedure available for the coxofemoral joint in cats. The rationale for micro-THR use includes the desire to improve quality of life while maintaining normal biomechanical function in cats that are affected by coxofemoral pain and/or dysfunction caused by OA. 122 This is undoubtedly relevant when considering salvage procedures of the coxofemoral joint, as restoration of normal hip joint function is certainly the preferred outcome. 2
THR in dogs is associated with high success rates and relatively low complication rates. Success rates of 92–98% based on both owner assessment and clinical and radiographic evaluation of pain status and functionality have been reported.103,123,124 Ground reaction forces return to normal in large breed dogs after THR.125 –129
Reported complication rates associated with THR range from 7.8–20%.117,123,124,130 –134 Complications include aseptic loosening, septic loosening, improper implant positioning, periprosthetic femoral fracture, luxation, sciatic neurapraxia, pulmonary embolism, femoral medullary infarction, patellar luxation, extraosseous cement granuloma formation and neoplasia.103,114,117,124,131 –135 In dogs, luxation is the most common early complication, with an incidence of 1.1–8.5%,117,123,124,130 –134 and aseptic loosening is the most common late complication, with an incidence of 0.7–2.0%.117,123,124,131,133
Witte et al documented the outcome of four cats (five THRs) 7–27 months postoperatively. 2 All owners reported excellent outcomes, with unlimited exercise and no visible gait abnormalities. Veterinary assessment, performed on three of the four cats, confirmed absence of lameness, no discomfort upon palpation/manipulation of the hip, a normal range of motion and no muscle atrophy. However, it should be noted that these hip replacements were not performed for treatment of HD and OA, but for spontaneous femoral capital physeal fractures. Therefore, it may not be appropriate to extrapolate these results to cats with HD and OA; partly because of the different disease processes and partly because of the high proportion of HD cases that are bilateral, in contrast to the majority of cases in this study where the condition was unilateral.
In a report describing use of the micro-THR system in 49 dogs and eight cats, excellent outcomes were seen in 91% of patients, including all eight cats, with follow-up ranging from 31–223 weeks (mean 96.1 weeks). 122 All animals were partially weightbearing within 24 h of surgery. 122 Marino and others reported the use of cemented THR in two cats, one with dysplasia and one with a femoral neck fracture. 136 Unfortunately the results for these cats cannot be discerned from those of the 35 dogs also included in the study, and follow-up was only available for 3 months. Nevertheless, 92% of animals had excellent limb function 3 months postoperatively and the treatment for both cats was stated to be successful. In a slightly earlier study in cats, outcome (radiographic assessment, passive range of motion, thigh girth, subjective gait and functional assessment) at 11 months (range 9–14 months) after THR was reported to be favorable compared with FHNE. 106
The Biomedtrix modular micro-THR prosthesis includes an acetabular cup, femoral stem and femoral head. The ultra-high molecular weight polyethylene acetabular cup component is available with a 12, 14 or 16 mm outside diameter and an 8 mm inside diameter articular surface. Two femoral stem sizes are available that measure 36 mm (size 2 with a 2.6 mm diameter stem tip) or 46 mm (size 3 with a 3.6 mm diameter stem tip) in length (Figure 9). The stems have a 10 mm long, 4 mm diameter neck with a 2.86° Morse taper. The 8 mm diameter femoral head is available with a +0 or +2 mm neck length. 106
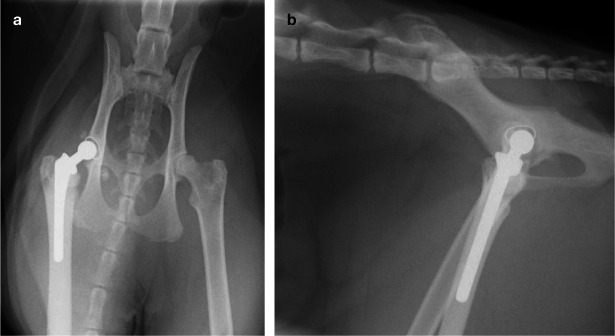
Figure 9.
Ventrodorsal (a) and lateral (b) radiographs of a 2-year-old British Shorthair cat 6 weeks following THR on the right for treatment of hip dysplasia and associated discomfort. Surgery was performed using a 12 mm acetabular prosthesis and a size 3 femoral stem
The lower anatomical limits for implanting Micro-THR components (12 mm cup and size 2 stem – see box) are 11 mm inside dimension from cranial to caudal pole of the acetabulum and 3.5 mm inside diameter of the femoral medullary canal at the isthmus. Note that the normal rule of aiming for a minimum of a 2 mm cement mantle around the entire prosthesis does not apply when using the micro-THR as it is not possible to achieve this when working with these dimensions. 122

Although no objective data comparing functional outcome in cats after FHNE and THR have been reported, it seems likely that cats will benefit from joint replacement in preference to FHNE. 2 Circumstantial evidence to support this statement is provided by Jeffery, 137 who reported that cats with femoral capital physeal fractures functioned better after fracture repair than after FHNE; similarly Liska and others 106 reported better functional outcomes in three cats after THR than in five cats after FHNE. In the report by Witte and others, 2 one cat underwent THR on one side and FHNE on the other; pelvic limb muscle mass disparity was noted between the two sides, with greater muscle mass on the side treated by THR. This would also appear to support the suggestion that cats have a better outcome following THR. There is a risk of complications with micro-THR, as well as cost implications, and surgeons should weigh the risks vs potential benefits. 122
Until further evidence is available, and based on the encouraging initial results of micro-THR in dogs and cats,106,114 THR should be considered as an alternative to FHNE in any cat in which salvage surgery is required.
Prognosis
There is little published information regarding the response rates of cats with HD and associated OA to non-surgical management. In the author’s experience, the majority of these cats do respond to medical management. NSAID therapy does play a key role in this, however, and therefore control of the associated clinical signs in cats where long-term medication with NSAIDs is not applicable, whether due to concomitant health conditions or licensing limitations, may be more difficult. The limited literature available would seem to support a positive prognosis with non-surgical management.11,28,37
In cats that do not respond to non-surgical management, salvage surgeries are available in the form of FHNE or THR. The inconsistent results associated with FHNE, in conjunction with the positive outcomes reported following THR in cats so far, provide evidence that, in most cases, micro-THR should at least be considered in the treatment of coxofemoral pathology in cats – in the same way that THR is considered for larger dogs – as the prognosis following this may be superior. 11
Key points
When examining the hip, abduction should be performed in addition to flexion, extension and rotation. Cats with hip dysplasia (HD) and associated osteoarthritis (OA) generally resent hip abduction, sometimes more so than flexion and extension.
The normal acetabulum of the cat is generally shallower than the acetabulum in the dog.
Degenerative changes develop later and are less marked than in the dog; changes in the cat are often most pronounced on the craniodorsal acetabular margins and sparing to the femoral head and neck.
Although assessment of pain is challenging in cats, there is little doubt that cats suffer pain associated with HD and OA, and that this warrants appropriate therapy.
Non-surgical therapy for HD and associated OA includes environmental modulation, physical therapy, dietary modulation, weight reduction, nutraceuticals and drug therapy.
Femoral head and neck excision (FHNE) and total hip replacement (THR) are salvage surgical options for cats that do not respond satisfactorily to non-surgical management.
Inconsistent results have been reported following FHNE and outcome may be better and more consistent following micro-THR. However, there is a risk of complications and cost implications associated with micro-THR and surgeons must weigh the risks vs the potential benefits carefully.
Footnotes
Funding: The author received no financial support for the research, authorship and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 2. Witte PG, Scott HW, Tonzing MA. Preliminary results of five feline total hip replacements. J Small Anim Pract 2010; 51: 397–402. [DOI] [PubMed] [Google Scholar]
- 3. Keller GG, Reed AL, Lattimer JC, et al. Hip dysplasia: a feline population study. Vet Radiol Ultrasound 1999; 40: 460–464. [DOI] [PubMed] [Google Scholar]
- 4. Langenbach A, Green P, Giger U, et al. Relationship between degenerative joint disease and hip joint laxity by use of distraction index and Norberg angle measurements in a group of cats. J Am Vet Med Assoc 1998; 213: 1439–1443. [PubMed] [Google Scholar]
- 5. Peiffer RL, Jr, Young WO, Jr, Blevins WE. Hip dysplasia and pectineus resection in the cat. Feline Pract 1974; 4: 40–43. [Google Scholar]
- 6. Root CR, Sande RD, Pfleuger S, et al. A disease of Maine Coon cats resembling congenital canine hip dysplasia [abstract]. Proceedings of the 10th Annual Meeting of the American College of Veterinary Radiology; Chicago, USA, 1987. [Google Scholar]
- 7. Rabin KL, De Haan JJ, Ackerman N. Hip dysplasia in a litter of domestic shorthair cats. Feline Pract 1994; 22: 15–18. [Google Scholar]
- 8. Hayes HM, Wilson GP, Burt JK. Feline hip dysplasia. J Am Anim Hosp Assoc 1979; 15: 447–448. [Google Scholar]
- 9. Koeppel E, Ebner J. Hip dysplasia in the cat [article in German]. Kleintierpraxis 1990; 35: 281–298. [Google Scholar]
- 10. Jackson OF. Congenital bone lesions in cats with folded ears. Bull Fel Advis Bur 1974; 14: 2–4. [Google Scholar]
- 11. Patsikas M, Papazoglou L, Komninou A, et al. Hip dysplasia in the cat: a report of three cases. J Small Anim Pract 1998; 39: 290–294. [DOI] [PubMed] [Google Scholar]
- 12. Smith GK, Langenbach A, Green PA, et al. Evaluation of the association between medial patellar luxation and hip dysplasia in cats. J Am Vet Med Assoc 1999; 215: 40–45. [PubMed] [Google Scholar]
- 13. Hauptman J. The hip joint. In: Slatter D. (ed). Textbook of small animal surgery. Philadelphia: WB Saunders, 1985, pp 2153–2179. [Google Scholar]
- 14. Riser WH. Growth and development of the normal canine pelvis. J Am Vet Rad Soc 1973; 14: 24–34. [Google Scholar]
- 15. Lust G. An overview of the pathogenesis of canine hip dysplasia. J Am Vet Med Assoc 1997; 210: 1443–1445. [PubMed] [Google Scholar]
- 16. Smith GK, Gregor TP, Rhodes H, et al. Coxofemoral joint laxity from distraction radiography and its contemporaneous and prospective correlation with laxity, subjective score and evidence of degenerative joint disease from conventional hip-extended radiography in dogs. Am J Vet Res 1993; 54: 1021–1042. [PubMed] [Google Scholar]
- 17. Lascelles BD, Robertson S. DJD-associated pain in cats: what can we do to promote patient comfort? J Feline Med Surg 2010; 12: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lascelles BDX, Waterman AE. Analgesia in cats. In Pract 1997; 19: 203–213. [Google Scholar]
- 19. Kerwin S. Orthopedic examination in the cat: clinical tips for ruling in/out common musculoskeletal disease. J Feline Med Surg 2012; 14: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perry K. The lame cat: optimising orthopaedic examination and investigation. Companion Anim 2014; 19: 518–523. [Google Scholar]
- 21. Holt PE. Hip dysplasia in a cat. J Small Anim Pract 1978; 19: 273–276. [DOI] [PubMed] [Google Scholar]
- 22. Hardie EM. Management of osteoarthritis in cats. Vet Clin North Am Small Anim Pract 1997; 27: 945–953. [DOI] [PubMed] [Google Scholar]
- 23. Godfrey DR. Osteoarthritis in cats: a retrospective series of 31 cases. J Small Anim Pract 2002; 43: 260. [DOI] [PubMed] [Google Scholar]
- 24. Godfrey DR. Osteoarthritis in cats: a prospective series of 40 cases. J Small Anim Pract 2003; 44: 418. [Google Scholar]
- 25. Klinck MP, Frank D, Guillot, et al. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can Vet J 2012; 53: 1181–1186. [PMC free article] [PubMed] [Google Scholar]
- 26. Lascelles BDX, Dong Y-H, Marcellin-Little DJ, et al. Relationship of orthopedic examination, goniometric measurements and radiographic signs of degenerative joint disease in cats. BMC Vet Res 2012; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benito J, Gruen ME, Thomson A, et al. Owner-assessed indices of quality of life in cats and the relationship to the presence of degenerative joint disease. J Feline Med Surg 2012; 14: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grierson J. Hips, elbows and stifles: common joint diseases in the cat. J Feline Med Surg 2012; 14: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillot M, Moreau M, d’Anjou MA, et al. Evaluation of osteoarthritis in cats: novel information from a pilot study. Vet Surg 2012; 41: 328–335. [DOI] [PubMed] [Google Scholar]
- 30. Slingerland LI, Hazewinkel HAW, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 31. Budsberg S. Outcome assessment in clinical trials involving medical management of osteoarthritis in small animals. Vet Clin North Am Small Anim Pract 1997; 27: 815–823. [DOI] [PubMed] [Google Scholar]
- 32. Jaeger G, Marcellin-Little DJ, Levine D. Reliability of goniometry in labrador retrievers. Am J Vet Res 2002; 63: 979–986. [DOI] [PubMed] [Google Scholar]
- 33. Jaeger GH, Marcellin-Little DJ, DePuy V, et al. Validity of goniometric joint measurements in cats. Am J Vet Res 2007; 68: 822–826. [DOI] [PubMed] [Google Scholar]
- 34. Gajdosik RL, Bohannon RW. Clinical measurement of range of motion. Review of goniometry emphasizing reliability and validity. Phys Ther 1987; 67: 1867–1872. [DOI] [PubMed] [Google Scholar]
- 35. Barker KL, Lamb SE, Burns M, et al. Repeatability of goniometer measurements of the knee in patients wearing an Ilizarov external fixator: a clinic-based study. Clin Rehabil 1999; 13: 156–163. [DOI] [PubMed] [Google Scholar]
- 36. Schulte L, Roberts MS, Zimmerman C, et al. A quantitative assessment of limited joint mobility in patients with diabetes. Goniometric analysis of upper extremity passive range of motion. Arthritis Rheum 1993; 36: 1429–1443. [DOI] [PubMed] [Google Scholar]
- 37. Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–445. [DOI] [PubMed] [Google Scholar]
- 38. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain-relief in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 39. Mahoney P. Musculoskeletal imaging in the cat. What’s normal? What’s abnormal? J Feline Med Surg 2012; 14: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allan GS. Radiographic features of feline joint disease. Vet Clin North Am Small Anim Pract 2000; 30: 281–302. [PubMed] [Google Scholar]
- 41. Freire M, Robertson I, Bondell H, et al. Radiographic evaluation of feline appendicular degenerative joint disease vs. macroscopic appearance of articular cartilage. Vet Radiol Ultrasound 2011; 52: 239–247. [DOI] [PubMed] [Google Scholar]
- 42. Morgan JP. Canine hip dysplasia: significance of early bony spurring. Vet Radiol 1987; 28: 2–5. [Google Scholar]
- 43. D’Anjou MA, Moreau M, Troncy E, et al. Osteophytosis, subchondral bone sclerosis, joint effusion and soft tissue thickening in canine experimental stifle osteoarthritis: comparison between 1.5 T magnetic resonance imaging and computed radiography. Vet Surg 2008; 37: 166–177. [DOI] [PubMed] [Google Scholar]
- 44. Peterfy CG, Gold G, Eckstein T, et al. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage 2006; 14 Suppl A: A95–A111. [DOI] [PubMed] [Google Scholar]
- 45. Olive J, D’Anjou MA, Alexander K, et al. Comparison of magnetic resonance imaging, computed tomography, and radiography for assessment of noncartilaginous changes in equine metacarpophalangeal osteoarthritis. Vet Radiol Ultrasound 2010; 51: 267–279. [DOI] [PubMed] [Google Scholar]
- 46. Kwan Tat S, Lajeunesse D, Pelletier JP, et al. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol 2010; 24: 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wenham CY, Conaghan PG. Imaging the painful osteoarthritic knee joint: what have we learned? Nat Clin Pract Rheumatol 2009; 5: 149–158. [DOI] [PubMed] [Google Scholar]
- 48. Harris JE, Dhupa S. Lumbosacral intervertebral disk disease in six cats. J Am Anim Hosp Assoc 2008; 44: 109–115. [DOI] [PubMed] [Google Scholar]
- 49. Taljanovic MS, Graham AR, Benjamin JB, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol 2008; 37: 423–431. [DOI] [PubMed] [Google Scholar]
- 50. Lascelles BDX, Henry JB, 3rd, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 51. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 52. Bennett D, Morton C. A study of owner observed behavioral and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patronek GJ, Glickman LT, Beck AM, et al. Risk factors for relinquishment of cats to an animal shelter. J Am Vet Med Assoc 1996; 209: 582–588. [PubMed] [Google Scholar]
- 54. Bennett D, Zainal Ariffin SM, Johnston P. Osteoarthritis in the cat 2: how should it be managed and treated? J Feline Med Surg 2012; 14: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robertson S, Lascelles BD. Long-term pain in cats: how much do we know about this important welfare issue? J Feline Med Surg 2010; 12: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharp B. Feline physiotherapy and rehabilitation: 1. principles and potential. J Feline Med Surg 2012; 14: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davidson JR, Kerwin S. Common orthopedic conditions and their physical rehabilitation. In: Millis DL, Levine D. (eds). Canine rehabilitation and physical therapy. 2nd ed. Philadelphia, USA: Elsevier Saunders, 2014, pp 543–581. [Google Scholar]
- 58. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc 2010; 236: 67–73. [DOI] [PubMed] [Google Scholar]
- 59. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc 2010; 236: 59–66. [DOI] [PubMed] [Google Scholar]
- 60. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc 2010; 236: 535–539. [DOI] [PubMed] [Google Scholar]
- 61. Lascelles BDX, DePuy V, Thomson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med 2010; 24: 487–495. [DOI] [PubMed] [Google Scholar]
- 62. Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta 2005; 174: 266–286. [DOI] [PubMed] [Google Scholar]
- 63. Brookes PS, Buckingham JA, Tenreiro AM, et al. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlation with standard metabolic rate and phospholipids fatty acid composition. Comp Biochem Physiol B Biochem Mol Biol 1998; 119: 325–334. [DOI] [PubMed] [Google Scholar]
- 64. Bissot T, Servet E, Vidal S, et al. Novel dietary strategies can improve the outcome of weight loss programmes in obese client-owned cats. J Feline Med Surg 2010; 12: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Michel K, Scherk M. From problem to success: feline weight loss programs that work. J Feline Med Surg 2012; 14: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tiraloche G, Girard C, Chouinard L, et al. Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum 2005; 52: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 67. Naito K, Watari T, Furuhata A, et al. Evaluation of the effect of glucosamine on an experimental rat osteoarthritis model. Life Sci 2010; 86: 538–543. [DOI] [PubMed] [Google Scholar]
- 68. Tat SK, Pelletier JP, Vergés J, et al. Chondroitin and glucosamine sulphate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study. Arthritis Res Ther 2007; 9: R1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bruyere O, Reginster J-Y. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging 2007; 24: 573–580. [DOI] [PubMed] [Google Scholar]
- 70. Richy F, Bruyere O, Ethgen O, et al. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med 2003; 163: 1514–1522. [DOI] [PubMed] [Google Scholar]
- 71. Towheed TE, Anastassiades T. Glucosamine therapy for osteoarthritis: an update. J Rheumatol 2007; 34: 1787–1790. [PubMed] [Google Scholar]
- 72. Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of the hip or knee: network meta-analysis. BMJ 2010; 341: c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bruyere O, Pavelka K, Rovati LC, et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage 2008; 16: 254–260. [DOI] [PubMed] [Google Scholar]
- 74. Sawitzke AD, Shi H, Finco MF, et al. The effect of glucosamine and/or chondroitin sulphate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum 2008; 58: 3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hughes R, Carr A. A randomized, double-blind, placebo-controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology (Oxford) 2002; 41: 279–284. [DOI] [PubMed] [Google Scholar]
- 76. Cibere J, Thorne A, Kopec JA, et al. Glucosamine sulfate and cartilage type II collagen degradation in patients with knee osteoarthritis: randomized discontinuation trial results employing biomarkers. J Rheumatol 2005; 32: 896–902. [PubMed] [Google Scholar]
- 77. Vlad SC, LaValley MP, McAlindon TE, et al. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum 2007; 56: 2267–2277. [DOI] [PubMed] [Google Scholar]
- 78. Cibere J, Kopec JA, Thorne A, et al. Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis Rheum 2004; 51: 738–745. [DOI] [PubMed] [Google Scholar]
- 79. McCarthy G, O’Donovan J, Jones B, et al. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet J 2007; 174: 54–61. [DOI] [PubMed] [Google Scholar]
- 80. Sul RM, Chase D, Parkin T, et al. Comparison of meloxicam and a glucosamine-chondroitin supplement in management of feline osteoarthritis. A double-blind randomised, placebo-controlled, prospective trial. Vet Comp Orthop Traumatol 2014; 27: 20–26. [DOI] [PubMed] [Google Scholar]
- 81. Pollard B, Guilford WG, Ankenbauer-Perkins KL, et al. Clinical efficacy and tolerance of an extract of green-lipped mussel (Perna canaliculus) in dogs presumptively diagnosed with degenerative joint disease. N Z Vet J 2006; 54: 114–118. [DOI] [PubMed] [Google Scholar]
- 82. Lascelles BD, Henderson AJ, Hackett IJ. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders. J Small Anim Pract 2001; 42: 587–593. [DOI] [PubMed] [Google Scholar]
- 83. Lascelles BD, Court MH, Hardie EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg 2007; 34: 228–250. [DOI] [PubMed] [Google Scholar]
- 84. Perry KL. The lame cat: the challenge of degenerative joint disease. Companion Anim 2014; 19: 582–590. [Google Scholar]
- 85. Bergh MS, Budsberg SC. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med 2005; 19: 633–643. [DOI] [PubMed] [Google Scholar]
- 86. Khan KN, Venturini CM, Bunch RT, et al. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol Pathol 1998; 26: 612–620. [DOI] [PubMed] [Google Scholar]
- 87. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. King JN, Dawson J, Esser RE, et al. Preclinical pharmacology of robenacoxib: a novel selective inhibitor of cyclooxygenase-2. J Vet Pharmacol Ther 2009; 32: 1–17. [DOI] [PubMed] [Google Scholar]
- 90. Sparkes AH, Heiene R, Lascelles BD, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 519–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scherk M. Experiences in feline practice: incorporating NSAIDs in analgesic therapy. Proceedings of the International Society of Feline Medicine, pre-congress meeting; 2010 June 17; Amsterdam, the Netherlands. Tisbury, Wiltshire: ISFM, 2010, pp 12–19. [Google Scholar]
- 92. Agung M, Ochi M, Yanada S, et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc 2006; 14: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 93. Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 2007; 8: 272–284. [PubMed] [Google Scholar]
- 94. Black LL, Gaynor J, Adams C, et al. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther 2008; 9: 192–200. [PubMed] [Google Scholar]
- 95. Franks JN, Boothe HW, Taylor L, et al. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy. J Am Vet Med Assoc 2000; 217: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 96. Robinson DA, Romans CW, Gordon-Evans WJ, et al. Evaluation of short-term limb function following unilateral carbon dioxide laser or scalpel onychectomy in cats. J Am Vet Med Assoc 2007; 230: 353–358. [DOI] [PubMed] [Google Scholar]
- 97. Romans CW, Conzemius MG, Horstman CL, et al. Use of pressure platform gait analysis in cats with and without bilateral onychectomy. Am J Vet Res 2004; 65: 1276–1278. [DOI] [PubMed] [Google Scholar]
- 98. Lascelles BD, Findley K, Correa M, et al. Kinetic evaluation of normal walking and jumping in cats, using a pressure sensitive walkway. Vet Rec 2007; 160: 512–516. [DOI] [PubMed] [Google Scholar]
- 99. Lascelles BD, Hansen BD, Thomson A, et al. Evaluation of a digitally integrated accelerometer-based activity monitor for the measurement of activity in cats. Vet Anaesth Analg 2008; 35: 173–183. [DOI] [PubMed] [Google Scholar]
- 100. Brezon JL, Howard PE, Covell SJ, et al. A retrospective study of the efficacy of femoral head and neck excisions in 94 dogs and cats. Vet Surg 1980; 9: 88–92. [Google Scholar]
- 101. Montgomery RD, Milton JL, Horne RD, et al. A retrospective comparison of three techniques for femoral head and neck excision in dogs. Vet Surg 1987; 16: 423–426. [DOI] [PubMed] [Google Scholar]
- 102. Gendreau C, Cawley AJ. Excision of the femoral head and neck: the long term results of 35 operations. J Am Anim Hosp Assoc 1977; 13: 605–608. [Google Scholar]
- 103. Olmstead ML, Hohn RB, Turner TM. A five-year study of 221 total hip replacements in the dog. J Am Vet Med Assoc 1983; 183: 191–194. [PubMed] [Google Scholar]
- 104. Gofton N, Sumner-Smith G. Total hip prosthesis for revision of unsuccessful excision arthroplasty. Vet Surg 1982; 11: 134–139. [Google Scholar]
- 105. Lippincott CL. Improvement of excision arthroplasty of the femoral head and neck utilizing a biceps femoris muscle sling. J Am Anim Hosp Assoc 1981; 17: 668–672. [Google Scholar]
- 106. Liska WD, Doyle N, Marcellin-Little DJ, et al. Total hip replacement in three cats: surgical technique, short-term outcome and comparison to femoral head ostectomy. Vet Comp Orthop Traumatol 2009; 22: 505–510. [DOI] [PubMed] [Google Scholar]
- 107. Duff R, Campbell JR. Effects of experimental excision arthroplasty of the hip joint. Res Vet Sci 1978; 24: 174–181. [PubMed] [Google Scholar]
- 108. Mann FA, Tangner CH, Wagner-Mann C, et al. A comparison of standard femoral head and neck excision and femoral head and neck excision using a biceps femoris muscle flap in the dog. Vet Surg 1987; 16: 223–230. [DOI] [PubMed] [Google Scholar]
- 109. Duff R, Campbell JR. Long term results of excision arthroplasty of the canine hip. Vet Rec 1977; 101: 181–184. [DOI] [PubMed] [Google Scholar]
- 110. Davidson JR, Kerwin SC, Millis DL. Rehabilitation for the orthopedic patient. Vet Clin North Am Small Anim Pract 2005; 35: 1357–1388. [DOI] [PubMed] [Google Scholar]
- 111. Piermattei DL, Flo GL, DeCamp CE. The hip joint. In: Piermattei DL, Flo GL, DeCamp CE. (eds). Handbook of small animal orthopaedics and fracture repair. 4th ed. St Louis, MO: Saunders Elsevier, 2006, pp 461–511. [Google Scholar]
- 112. Off W, Matis U. Excision arthroplasty of the hip joint in dogs and cats. Clinical, radiographic, and gait analysis findings from the Department of Surgery, Veterinary Faculty of the Ludwig-Maximilians-University of Munich, Germany. 1997. Vet Comp Orthop Traumatol 2010; 23: 297–305. [PubMed] [Google Scholar]
- 113. Lippincott CL. Excision arthroplasty of the femoral head and neck utilizing a biceps femoris muscle sling: part 2. The caudal pass. J Am Anim Hosp Assoc 1984; 20: 377–384. [Google Scholar]
- 114. Warnock JJ, Dyce J, Pooya H, et al. Retrospective analysis of canine miniature total hip prostheses. Vet Surg 2003; 32: 285–291. [DOI] [PubMed] [Google Scholar]
- 115. Lippincott CL. Femoral head and neck excision in the management of canine hip dysplasia. Vet Clin North Am Small Anim Pract 1992; 22: 721–737. [DOI] [PubMed] [Google Scholar]
- 116. Penwick RC. The variables that influence the success of femoral head and neck excisions in dogs. Vet Med 1992; 87: 325–333. [Google Scholar]
- 117. Massat BJ, Vasseur PB. Clinical and radiographic results of total hip arthroplasty in dogs: 96 cases (1986–1992). J Am Vet Med Assoc 1994; 205: 448–454. [PubMed] [Google Scholar]
- 118. Fitzpatrick N, Pratola L, Yeadon R, et al. Total hip replacement after failed femoral head and neck excision in two dogs and two cats. Vet Surg 2012; 41: 136–142. [DOI] [PubMed] [Google Scholar]
- 119. Jeffery ND. Femoral head and neck excision complicated by ischiatic nerve entrapment in two dogs. Vet Comp Orthop Traumatol 1993; 6: 215–218. [Google Scholar]
- 120. Hoefle WD. A surgical procedure for prosthetic total hip replacement in the dog. J Am Anim Hosp Assoc 1974; 10: 269–276. [Google Scholar]
- 121. Liska WD. Micro total hip replacement for small dogs and cats [abstract]. Proceedings of the 14th ESVOT congress; 2008 Sept 10–14; Munich, Germany, pp 133–134. [Google Scholar]
- 122. Liska WD. Micro total hip replacement for dogs and cats: surgical technique and outcomes. Vet Surg 2010; 39: 797–810. [DOI] [PubMed] [Google Scholar]
- 123. Olmstead ML. Total hip replacement. Vet Clin North Am Small Anim Pract 1987; 17: 943–955. [DOI] [PubMed] [Google Scholar]
- 124. Olmstead ML. The canine cemented modular total hip prosthesis. J Am Anim Hosp Assoc 1995; 31: 109–124. [DOI] [PubMed] [Google Scholar]
- 125. Budsberg S, Chambers J, Van Lue S, et al. Prospective evaluation of ground reaction forces in dogs undergoing unilateral total hip replacement. Am J Vet Res 1996; 57: 1781–1785. [PubMed] [Google Scholar]
- 126. Van Lue SJ, Budsberg SC, Chambers JN. Computer-assisted force plate analysis of the BioMedtrix hip in total hip replacement in the dog; a prospective quantitative analysis of limb function for one year following implantation. Vet Surg 1994; 23: 419. [Google Scholar]
- 127. Dogan S, Manley PA, Vanderby R, Jr, et al. Canine intersegmental hip joint forces and moments before and after cemented total hip replacement. J Biomech 1991; 24: 397–407. [DOI] [PubMed] [Google Scholar]
- 128. Braden TD, Olivier NB, Blaiset MA, et al. Objective evaluation of total hip replacement in 127 dogs utilizing force plate analysis. Vet Comp Orthop Traumatol 2004; 17: 78–81. [Google Scholar]
- 129. Pozzi A, Kowaleski MP, Dyce J, et al. Treatment of traumatic coxo-femoral luxation by cemented total hip arthroplasty. Vet Comp Orthop Traumatol 2004; 17: 198–203. [Google Scholar]
- 130. Paul HA, Bargar WL. A modified technique for canine total hip replacement. J Am Anim Hosp Assoc 1987; 23: 13–18. [Google Scholar]
- 131. Bergh MS, Gilley RS, Shofer FS, et al. Complications and radiographic findings following cemented total hip replacement: a retrospective evaluation of 97 dogs. Vet Comp Orthop Traumatol 2006; 19: 172–179. [PubMed] [Google Scholar]
- 132. Liska WD, Poteet BA. Pulmonary embolism associated with canine total hip replacement. Vet Surg 2003; 32: 178–186. [DOI] [PubMed] [Google Scholar]
- 133. Dyce J, Wisner ER, Wang Q, et al. Evaluation of risk factors for luxation after total hip replacement in dogs. Vet Surg 2000; 29: 524–532. [DOI] [PubMed] [Google Scholar]
- 134. Liska WD. Femur fractures associated with canine total hip replacement. Vet Surg 2004; 33: 164–172. [DOI] [PubMed] [Google Scholar]
- 135. Liska WD, Doyle ND, Schwartz Z. Successful revision of a femoral head ostectomy (complicated by postoperative sciatic neurapraxia) to a total hip replacement in a cat. Vet Comp Orthop Traumatol 2010; 2: 119–123. [DOI] [PubMed] [Google Scholar]
- 136. Marino DJ, Ireifej SJ, Loughin CA. Micro total hip replacement in dogs and cats. Vet Surg 2012; 41: 121–129. [DOI] [PubMed] [Google Scholar]
- 137. Jeffery ND. Internal fixation of femoral head and neck fractures in the cat. J Small Anim Pract 1989; 30: 674–677. [Google Scholar]