Abstract
Practical relevance:
Chronic kidney disease (CKD) is one of the most commonly diagnosed diseases in older cats. In most cats, CKD is also a progressive disease and can be accompanied by a wide range of clinical and clinicopathological changes. These ISFM Consensus Guidelines have been developed by an independent panel of clinicians and academics to provide practical advice on the diagnosis and management of this complex disease.
Clinical challenges:
Although CKD is a common clinical problem in cats, the manifestations of disease vary between individuals. Thus there is a need for careful and repeat evaluation of cats with CKD and adjustment of therapy according to individual needs. In addition to addressing problems arising from CKD and improving quality of life (QoL) for the patient, therapy may also target slowing the underlying progression of disease and hence prolonging life. While maintaining QoL is of paramount importance in our patients, this can be challenging when multiple therapies are indicated. In some cases it is necessary to prioritise therapy, given an understanding of what is likely to most benefit the individual patient.
Evidence base:
In preparing these Guidelines, the Panel has carefully reviewed the existing published literature, and has also graded the quality of evidence for different interventions to help to provide practical recommendations on the therapeutic options for feline CKD. This is a field of veterinary medicine that has benefited from some excellent published clinical research and further research findings will undoubtedly modify the recommendations contained in these Guidelines in the future.
Introduction
Chronic kidney disease (CKD) is a common feline disease. Its prevalence will vary between populations, but a large UK study estimated that the prevalence of feline renal disease in first opinion practices was ~4% (CKD was the seventh most common specific diagnosis made). 1 CKD is more common in older cats,2 –4 and may affect ⩾30–40% of cats over 10 years of age. 4 Renal disease was the most common cause of mortality in cats ⩾5 years of age in a UK study, being the cause of death of >13% of cats at a median age of 15 years. 5
The underlying aetiology of CKD often remains obscure. Most cats investigated have chronic tubulointerstitial nephritis and renal fibrosis on histology (Figure 1)6,7 – lesions thought to be the end phase of a variety of potential underlying aetiologies that may include toxic insults, hypoxia, chronic glomerulonephritis, chronic pyelonephritis, upper urinary tract obstructions, and potentially viral infections involving retroviruses as well as a recently recognised morbillivirus.8 –12 Other specific causes of CKD sometimes recognised include amyloidosis, polycystic kidney disease, renal lymphoma, hypercalcaemic nephropathy and congenital disorders – some of these have breed associations.4,8,13
Figure 1.

Typical histopathology of a kidney from a cat with chronic kidney disease (CKD), characterised by inflammatory infiltrate, tubular loss, increase in extracellular matrix and fibrosis x 20. Courtesy of Shannon McLeland
Other than age, clear risk factors for development of CKD have not been identified in cats,14 –17 but weight loss or poor body condition, polyuria/polydipsia (PU/PD), higher creatinine concentrations, dehydration and potentially lower urine specific gravity (USG) may indicate the presence, or predict development, of CKD.14 –17
The purpose of these Guidelines is to give practitioners an up-to-date, critically assessed overview of the current diagnostic and treatment options to guide in the practical management of CKD.
Diagnosis and assessment of CKD in cats
Routine diagnosis of CKD in cats
CKD in humans is defined as a sustained (⩾3 months) reduction in glomerular filtration rate (GFR, <60 ml/min/1.73 m2) or evidence of sustained (⩾3 months) kidney damage (eg, structural damage, proteinuria). 18 Although CKD has not been clearly defined in cats, similar principles should apply; notably there should be evidence of sustained functional or structural kidney damage (eg, ⩾3 months’ duration).
As CKD is more common in older cats, these patients should be targeted for more detailed and frequent health assessments. Recommendations from International Cat Care/International Society of Feline Medicine, the American Association of Feline Practitioners and the American Animal Hospital Association suggest health checks every 6 months for cats >7 years of age (including evaluation of body weight, body condition score and blood pressure), together with selected diagnostic testing (including haematology, serum biochemistry screening and routine urinalysis) at least annually.19,20
Historical and clinical findings suggestive of CKD, such as weight loss, altered kidney size, unexplained dehydration, PU/PD, systemic hypertension or an unexplained low USG (<1.035–1.040), also justify further investigation.
A simple, accurate biomarker to assess renal function does not currently exist. Thus in clinical practice the combination of azotaemia (increased serum creatinine and/or urea) and an inappropriately low USG are routinely used to diagnose CKD. However, their interpretation is not always straightforward:21,22
Although often measured together, creatinine is preferred over urea as a marker of GFR as its concentration is inversely related to GFR, and is affected by fewer non-renal factors.
Creatinine is an imprecise marker of GFR though; it lacks specificity if reference intervals are set low enough to detect early stage disease, but lacks sensitivity if reference intervals are set higher.
Creatinine concentration is affected by lean tissue mass and hydration.
Creatinine concentrations (and reference intervals) vary between different assays, analysers and laboratories.
The exponential relationship between GFR and creatinine means that substantial early declines in GFR may be accompanied by only small changes in creatinine,while in the latter stages of disease large changes in creatinine may reflect only small changes in GFR.
Bearing in mind these limitations, in clinical practice feline CKD is often diagnosed on the basis of:
An increased serum creatinine concentration >140 µmol/l (>1.6 mg/dl); together with
An inappropriately low USG (<1.035); and
Evidence that these changes are sustained (over several weeks or months) or with a history suggesting sustained clinical signs consistent with CKD.
However, not all cats with CKD will meet these criteria:
Chronic kidney damage evidenced by structural changes to the kidney recognised on diagnostic imaging or persistent renal-origin elevated proteinuria may be present in the absence of azotaemia or an inappropriate USG.
While relatively few healthy cats will produce a USG <1.035, this can be affected by diet, 23 and occasionally some cats with azotaemic CKD will produce a USG ⩾1.035.24,25
Some cats have reduced urine concentrating ability before they develop overt azotaemia.
A persistent and substantial (>15%) increase in serum creatinine from previously determined baseline values in a cat is also likely to indicate reduced renal function.
For these reasons, serial (eg, annual or bi-annual) assessment of serum creatinine or symmetric dimethylarginine (SDMA – see later) and USG may be helpful in older cats (>7 years of age) to determine changes over time, as this may facilitate earlier or more certain diagnosis of CKD.22,26 Additionally, if there is doubt over the diagnosis, additional testing (see page 222) may be desirable.
Routine investigation and staging of CKD in cats
Where CKD is suspected, a minimum routine database should ideally include:
Full history and physical examination;
Routine urinalysis (to include USG, ‘dipstick’ analysis, urine sediment analysis, urine protein:creatinine ratio [UPCR], and culture where indicated);
Routine serum biochemistry, to include a minimum of proteins, urea, creatinine, electrolytes (Na+, K+, Ca2+, Cl_, PO4_), and other analytes (eg, thyroxine in an older cat) as relevant;
Routine haematology;
Systolic blood pressure (SBP);
Diagnostic imaging (renal ultrasonography is generally more valuable than radiography);
In some situations (eg, unexplained renomegaly) a kidney biopsy or fine-needle aspiration may be desirable.
These investigations are aimed at:
Identifying potential underlying aetiologies of the CKD (which may require specific therapy);
Identifying complications that are arising from the CKD;
Identifying concomitant disease that may affect management (eg, hyperthyroidism).
The International Renal Interest Society (IRIS) has established a CKD staging system 27 based on the cat’s fasting creatinine concentration (see box on page 221). This is valuable as the stage (severity) of disease is related to the prognosis for the patient (see later) and can help to focus attention on appropriate treatments. Staging is applicable in cats with confirmed, stable CKD that are well hydrated, and IRIS substaging is based on UPCR and SBP, two important prognostic and therapeutic parameters.
Advanced and emerging tests for feline CKD
Estimation of GFR
The gold standard in renal function testing is direct determination of GFR. Limited- and single-sample plasma clearance methods (eg, using iohexol, inulin, exogenous creatinine or radiolabelled markers) have made GFR assessment easier to undertake in clinical practice, but the reduced numbers of blood samples may yield greater inaccuracy.21,28 Clinical measurement of GFR is mainly used to confirm suspected CKD in non-azotaemic cats.
Symmetric dimethylarginine (SDMA)
SDMA has become available in the veterinary marketplace as a surrogate marker of GFR and, like creatinine, its reciprocal has a linear relationship with GFR. 29 It appears to offer greater sensitivity than creatinine for detection of early CKD 30 and does not appear to be affected by muscle mass. However, further studies are required to fully evaluate its accuracy in clinical patients as SDMA may also be affected by non-renal factors. 31 Although it cannot currently be recommended as a single screening test for CKD, its measurement may be helpful in supporting a diagnosis of CKD or in staging CKD, especially in cats with marked loss of muscle mass. 27
Serum cystatin C
Serum cystatin C is a useful surrogate marker of GFR in human patients. However, in cats its diagnostic value appears compromised by overlap in values between healthy cats and cats with CKD, and the interference of non-renal factors.32,33
Microalbuminuria
Detection of microalbuminuria is important in the diagnosis of CKD in human patients where there is a high prevalence of glomerular disease, but its clinical significance in cats remains unclear. It is measured using a species-specific assay but a benefit of measuring urine albumin:creatinine ratio (UACR) over UPCR in predicting which cats will develop azotaemia has not been demonstrated. 17
Other assays
Studies have demonstrated that development of renal secondary hyperparathyroidism 34 and increased fibroblast growth factor-23 (FGF-23) 35 may precede azotaemia in feline CKD. However, whether there is any diagnostic utility in these assays remains to be determined.
Table 1.
Routine evaluation of feline CKD patients
| Assessment | To include: |
|---|---|
| Full history | Evaluation of progress, complications and owner concerns |
| Evaluation of changes since last assessment | |
| Full clinical examination | Body weight, % change in body weight and body condition score |
| Hydration status | |
| Blood pressure assessment | Systolic blood pressure (Figure 2) and ocular examination (Figure 3) |
| Urinalysis | USG, UPCR, ‘dipstick’ and sediment analysis Bacterial culture if indicated (Figure 4) |
| Routine haematology | Full haematology may not always be required but haematocrit or PCV should be regularly assessed (Figure 5) |
| Serum biochemistry | Proteins, urea, creatinine and electrolytes (Na+, K+, Ca2+, Cl_, PO4_) |
| Other analytes should be measured as necessary, including thyroxine, liver enzymes and acid–base status | |
| Diagnostic imaging | Ultrasonography or radiography (Figures 6 and 7) – to assess for structural changes, obstructions or other lesions – should be part of the initial investigation and may be worth repeating, especially with unexpected deterioration |
USG = urine specific gravity; UPCR = urine protein:creatinine ratio; PCV = packed cell volume
Figure 2.

Blood pressure measurement should be part of the routine evaluation of all cats with proven or suspected CKD. Courtesy of Sarah Caney
Figure 3.

Ocular examination (in this case distant indirect ophthalmoscopy) performed in a dark room is valuable, given the strong association between CKD and hypertension. Courtesy of Sarah Caney
Figure 4.

Collection of a urine sample by lateral cystocentesis for routine monitoring of CKD. Courtesy of Sarah Caney
Figure 5.
Collection of blood for routine monitoring of CKD (note the no restraint technique). Courtesy of Jessica Quimby
Figure 6.
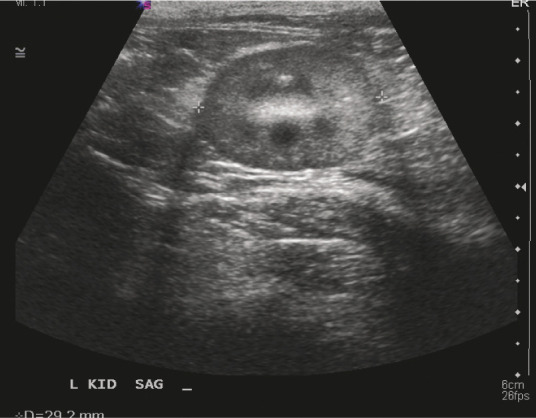
A small kidney and loss of corticomedullary distinction are ultrasound findings consistent with feline CKD. Courtesy of Jessica Quimby
Figure 7.

Radiographs are helpful for identifying abnormalities such as stones within the urinary tract. Courtesy of Jessica Quimby
Table 2.
Studies evaluating IRIS stage and prognosis
Approach to management
Management of CKD is largely focused on supportive and symptomatic therapy with the aim of improving the quality of life (QoL) of affected cats (especially those in CKD stages 3 and 4) and, where possible, slowing the progression of disease (especially in CKD stages 2 and 3). Although beyond the scope of these Guidelines, careful evaluation of cats (as outlined earlier) should also allow identification of certain underlying aetiologies that permit specific intervention such as renal lymphoma, UTIs, nephroliths and ureteroliths.
Because of the chronic nature of the disease, the need for regular monitoring and the potential for various interventions, establishing a good relationship and good communication between the clinic and the cat’s owner is vital. This will facilitate individualised management plans to be created that take into consideration the wishes and ability of the owner, as well as the needs of the cat.
Issues that should be considered are outlined in the box below.
Management of CKD patients
Managing hydration in CKD46,47

CKD is associated with variable obligatory diuresis, and affected cats may be predisposed to dehydration, especially in CKD stages 3 and 4.

Correcting dehydration46,47
Cats with unstable or decompensated CKD may require hospitalisation and intravenous fluid therapy, typically with lactated Ringer’s solution or Hartmann’s. Consideration should also be given to concomitant electrolyte and acid–base disturbances that may need addressing.
The fluid required to correct dehydration (in ml) is calculated from: body weight (kg) × estimated dehydration (%) × 1000, and this (along with maintenance fluids, eg, 50 ml/kg/24 h) is typically provided over 24–48 h, although some cats may tolerate more rapid rehydration.
After rehydration, maintenance fluids can be administered but cats should be monitored carefully to avoid fluid overload. When azotaemia is stable, fluids should be tapered over 2–3 days before the patient is discharged.
Long-term maintenance of hydration
Voluntary water intake Free access to good quality water should be provided at all times (Figure 8), and owners should be advised to offer a variety of water sources (including flavoured waters and running water – eg, a ‘pet fountain’) to encourage drinking. Feeding a wet diet rather than dry diet where possible is important, as it will also increase water intake. 48 Additional water can be added to the food where dehydration is a concern, but it is important to ensure intake of other nutrients is maintained. 49
Use of feeding tubes Water can also be administered via a feeding tube, and this may be preferable to subcutaneous fluids (see below) in many cases. A feeding tube is suitable for long-term maintenance of hydration and is a more physiological approach. It also allows for nutritional support when needed.
Subcutaneous fluid therapy Repeated subcutaneous fluid therapy (75–150 ml every 1–3 days) can be used on an outpatient basis (Figure 9) or by owners at home to maintain hydration. This is most commonly employed in cats with advanced (stages 3 and 4) CKD, but should be considered on a case-by-case basis. Cats should be carefully monitored to ensure there is clinical benefit and to avoid overhydration.
Figure 8.

Multiple sources of fresh water are important for maintaining hydration. Courtesy of Jessica Quimby
Figure 9.
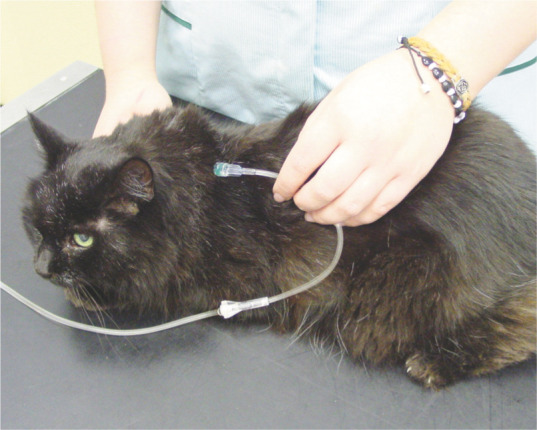
Subcutaneous fluid therapy can be used on an outpatient basis or by owners at home. Courtesy of Sarah Caney
Although a balanced electrolyte solution such as lactated Ringer’s solution is often used, a hypotonic solution (half-strength lactated Ringer’s or 0.45% saline, with added potassium as needed) may be preferable to reduce the sodium load. Fluids can be administered via a needle and giving set, or through an indwelling subcutaneous catheter, although there is the risk of the latter becoming blocked or infected.
Managing diet and mineral/bone disease in CKD
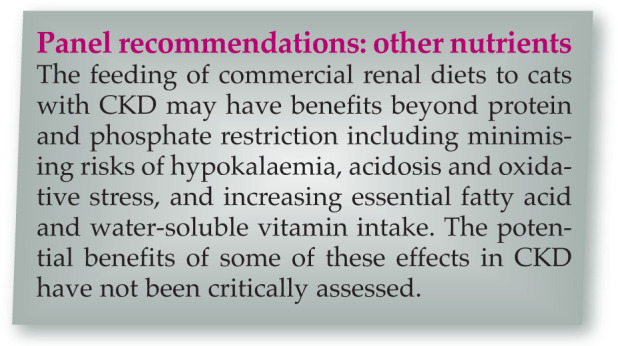
Dietary manipulation is a mainstay of CKD therapy in human 50 and veterinary patients. Renal formulated diets are restricted in both protein and phosphorus, but other features include an increased calorie density, sodium restriction, potassium supplementation, alkalinisation, and supplementation with B vitamins, antioxidants and omega-3 fatty acids.
Protein and phosphate restriction

Protein restriction and phosphate restriction are considered together as they are the main features of commercial renal diets, and are thought to confer the major benefits seen. Feline renal diets typically contain 6–7 g of protein per 100 kcal (above the 5 g/100 kcal recommended allowance for adult cats, 51 but below the 9–10 g/100 kcal commonly seen in maintenance diets). Energy requirements of older (>13 years) cats may increase and severe protein restriction may lead to loss of lean tissue; 52 thus moderate protein restriction is recommended in CKD, together with monitoring of lean body mass, weight and caloric intake (Figure 10). In addition to protein restriction, renal diets contain much less phosphate compared with typical maintenance diets.53,54
Figure 10.

Muscle wasting and dehydration in a cat with CKD. Careful attention to body condition, muscle mass and caloric intake is important. Courtesy of Jessica Quimby
In cats with CKD, renal diets have been shown to reduce clinical signs of uraemia,55 –57 and to significantly prolong longevity (see Table 3), providing a strong rationale for their use.
Table 3.
Studies evaluating effect of renal diets on longevity
Differentiating the effects of protein and phosphate restriction is complex and not always possible, but while moderate protein restriction is thought to help reduce signs of uraemia, there is little evidence that this alone has a major effect on progression of CKD.58 –60 Conversely, hyperphosphataemia is known to be associated with progression of CKD,42,44,45 and phosphate restriction may reduce the severity of renal pathology in CKD;53,61 thus phosphate restriction is thought to be mainly responsible for the improved longevity seen. Furthermore, renal secondary hyperparathyroidism (which may contribute to uraemia and disease progression) can be seen prior to the development of overt hyperphosphataemia or azotaemia, 34 and phosphorus-restricted diets reduce hyperphosphataemia, hyperparathyroidism and FGF-23 (which may indirectly promote hyperparathyroidism).55 –57,62,63

Changing and transitioning diets
Renal diets are generally less palatable than maintenance diets (probably at least partially due to their lower protein content). This can lead to poor acceptance of these diets,24,65 a problem that may be exacerbated by inappetence in cats with more advanced CKD.
Use of phosphate binders

As CKD progresses, serum phosphate tends to increase and may become more refractory to control with dietary phosphate restriction. Where diet alone is insufficient, the use of intestinal phosphate binders is important. Several agents can be used for this purpose (see Table 4).53,67 –69 There are no studies comparing different phosphate binders in cats with CKD, but all are likely to be efficacious. 53 Offering alternative binders when needed may be appropriate, as palatability of the phosphate binders varies.69,70 If calcium-containing phosphate binders are used, monitoring of serum calcium (ideally ionised) is recommended, as hypercalcaemia is occasionally seen as an adverse event. 53
Table 4.
Some common oral phosphate binders used in cats
| Medication | Initial total daily dose* | Possible adverse effects |
|---|---|---|
| Aluminium hydroxide/carbonate | 90 mg/kg | Constipation |
| Calcium carbonate | 90 mg/kg | Hypercalcaemia |
| Calcium acetate | 60–90 mg/kg | Hypercalcaemia |
| Iron, starch, sucrose complex | 0.25–0.5 g/day | Little data available |
| Sevelamer | 90–160 mg/kg | Constipation, impaired vitamin absorption, metabolic acidosis |
| Lanthanum | 30–90 mg/kg | Vomiting |
For all phosphate binders, it is important to split the daily dose and give it mixed with food or at the same time that the cat eats. Doses may have to be increased to achieve the desired effect
Where cats cannot be transitioned to a commercial or home-prepared renal diet with restricted phosphate, phosphate binders can be used with a maintenance diet, but their efficacy is likely to be compromised by the quantity of phosphate in the diet.

Managing serum calcium
Hypercalcaemia is a recognised cause of renal injury, but CKD can also cause changes in serum calcium, although these are generally mild. Ionised hypocalcaemia appears to be most common, and tends to be seen in advanced CKD. 72 An increased calcium–phosphorus product has been linked with disease severity in cats. 73

Calcitriol therapy

Calcitriol (active vitamin D) deficiency may occur with CKD due to various mechanisms including hyperphosphataemia-mediated inhibition of hydroxylation and loss of renal tissue. Calcitriol supplementation can potentially help suppress renal secondary hyperparathyroidism and has been shown to be beneficial in dogs and humans; 74 but despite anecdotal reports of improved QoL, low dose calcitriol has not been shown to have the same benefits in feline CKD. 75 Additionally, formulations of calcitriol can make accurate dosing difficult in cats. Hyperphosphataemia should also be carefully controlled when using this therapy to avoid increasing the serum calcium–phosphate product.

Managing potassium

Feline CKD can lead to excessive kaliuresis, which may be compounded by reduced potassium intake, vomiting and transcellular shifts.55,76 Hypokalaemia may cause or contribute to clinical signs such as lethargy, inappetence, constipation and muscle weakness, and may contribute to development of acidosis, but has not been identified as a risk factor for disease progression or outcome.42,44,45
Although renal diets are typically supplemented with potassium, hypokalaemia may still be seen in some cats. Conversely, hyperkalaemia may occasionally be seen in advanced CKD.

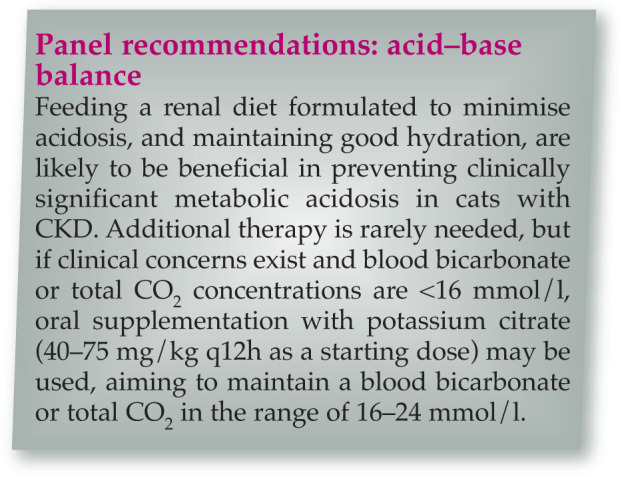
Managing acid–base balance

Metabolic acidosis is multifactorial in CKD 77 and bicarbonate therapy has been shown to improve nutrition (calorie and protein intake, lean body mass) and slow progression in humans with CKD. 78 Metabolic acidosis has been reported to occur in over half of cats with advanced (stage 4) CKD.79,80 However, cats fed commercial renal diets may have higher serum bicarbonate concentrations. 56
Other nutrients

One retrospective study suggested that renal diets with the highest omega-3 fatty acid content were associated with the longest survival times. 54 However, a causal relationship could not be established, and feeding a renal diet may not alter fatty acid profiles. 81
Dietary sodium restriction is recommended for people with CKD to mitigate hypertension and other effects, 82 but evidence for a beneficial effect in older cats, with and without CKD, is generally lacking, and very restricted sodium intake may be deleterious.83 –86
Cats with CKD have evidence of increased oxidative stress compared with healthy cats,87,88 and dietary antioxidant therapy (with vitamins E, C and beta-carotene) may reduce this. 89 Whether this results in any renoprotective effect remains to be determined.
Other dietary supplements, including Chinese rhubarb (Rheum officinale) and a prebiotic/probiotic combination, have not shown any beneficial effects.90,91
Managing hypertension in CKD

Systemic hypertension associated with CKD has a reported prevalence of 19–40% in primary care practices,36,92 and as high as 65% in referral populations. 93 The pathogenesis is not entirely clear, with some cats demonstrating activation of the renin–angiotensin–aldosterone system (RAAS),94 –96 and some having apparent autonomous hyperaldosteronism.96,97
Doppler and high-definition oscillometry are the most reliable non-invasive blood pressure measurement techniques in conscious cats.98 –105 However, even when cats are calm, and a standardised protocol is followed, 105 measurements will vary with the equipment, the operator, the cat and the circumstances.36,106 –111 Current non-invasive techniques are inaccurate for assessing diastolic blood pressure in cats.101,104
Within these limitations, hypertension is usually defined as an SBP persistently >160–180 mmHg,36,112 but has also been defined according to the perceived risk of target organ damage (TOD) (see box on page 221 and Table 5).27,105 Target organs are those particularly susceptible to hypertensive damage – the eyes,93,107,113 heart,114 –116 cerebrovascular tissue113,117 and kidneys. While hypertension is an independent risk factor for progressive CKD in dogs and people,118,119 this has not been proven in cats.37,116,117,120 However, hypertension is associated with the severity of proteinuria (as in people and dogs),37,120 –122 which can be reduced with successful antihypertensive therapy.37,120,123
Table 5.
| SBP (mmHg) | Risk of TOD | Treatment |
|---|---|---|
| <150 | Minimal | No treatment advised |
| 150–159 | Mild | No treatment advised |
| 160–179 | Moderate | Treatment advised if TOD is present Cats with CKD are assumed to have TOD |
| ⩾180 | Severe | Treatment indicated |
SBP = systolic blood pressure
Management of hypertension is aimed at preventing TOD, although the current perceived risk categories (Table 5) are imprecise as data are lacking, risks may vary between individuals and may, for example, depend on how rapidly blood pressure rises. In general, treatment aims to reduce SBP to <150–160 mmHg.27,105
Monotherapy with angiotensin-converting enzyme inhibitors (ACEIs) or atenolol is not effective in most hypertensive cats.95,105,112,124,125 There is some experimental data to suggest the ARB telmisartan at 3 mg/kg q24h may be more effective than benazepril as an anti-hypertensive agent, 126 but further clinical studies are needed. Conversely, the calcium channel blocker amlodipine is an effective monotherapy for most (but not all) cats,97,112,113,115,120,127,128 and may be combined with other drugs if adjuvant therapy is needed (Table 6).
Table 6.
Suggested oral therapy for systemic hypertension
| Drug | Dose | Comments |
|---|---|---|
| Amlodipine (calcium channel blocker) | 0.0625–0.25 mg/kg q24h | Licensed for feline use in some countries. |
| Good efficacy in several studies. Licensed dose in Europe is 0.125–0.25 mg/kg q24h; dose can be doubled (up to 0.5 mg/kg q24h) if needed 127 | ||
| Telmisartan (ARB) | 1–3 mg/kg q24h | Licensed for feline use in some countries as an antiproteinuric agent at 1 mg/kg. Clinical efficacy as antihypertensive uncertain, but higher doses appear to have some efficacy in experimental studies |
| Benazepril (ACEI) | 0.25–0.5 mg/kg q24h | Limited effect on blood pressure used alone; best considered as adjuvant therapy in refractory cases. Dose can be doubled to 0.5–1.0 mg/kg q24h if needed |
| Propranolol Atenolol (beta-blockers) | 2.5–5.0 mg/cat q8h | Limited effect on blood pressure used alone; best considered as adjuvant therapy in refractory cases |
| 6.25–12.5 mg/cat q12h |
ARB = angiotensin receptor blocker; ACEI = angiotensin-converting enzyme inhibitor

Managing anaemia in CKD

Anaemia of varying severity is seen in 30–65% of cats with CKD 38 (Figure 11). A relative lack of erythropoietin (EPO) in CKD produces a non- or poorly-regenerative anaemia, which may be exacerbated by blood loss and/or shortened red blood cell (RBC) survival. 129 Anaemia has been identified as a dependent or independent risk factor for progression of CKD,42 –45 and there is evidence that treatment with erythrocyte-stimulating agents (ESAs) may improve QoL and potentially survival in some cats with CKD. 129
Figure 11.

Anaemia is a recognised risk factor for progression of CKD. Courtesy of Cathy Langston
Figure 12.

Enteral (oesophagostomy) tube in place to support food and fluid intake. Courtesy of Isuru Gajanayake
Blood transfusions and haemoglobin-based oxygen carrying solutions (eg, Oxyglobin; Dechra Veterinary Products) have limited value for the chronic anaemia associated with CKD,130,131 and the use of anabolic steroids is not recommended due to lack of evidence of efficacy and potential adverse events.132,133 In contrast, the use of ESAs (EPO or EPO analogues) has become the standard of care in human medicine.
ESA therapy is designed to elevate the packed cell volume (PCV) to around the lower limit of the reference interval – sufficient to meet tissue oxygen demand. Iron supplementation alone is not effective in managing CKD-associated anaemia, but it enhances the efficacy of ESA therapy in humans, 134 and anecdotal evidence suggests the same is true in cats.
The two ESAs most widely used in cats are recombinant human epoetin alfa (EA, ~80% homology to feline EPO) and darbepoetin alfa (DA, a hyperglycosylated recombinant human EPO analogue). Although often successful, in one study, >40% of cats with CKD-associated anaemia failed to respond or failed to develop a sustained response to ESA therapy. 135 Potential reasons for this include:129,135
Concurrent illnesses (present in most cats that fail to respond to ESA);
Infections or inflammation;
Gastrointestinal bleeding;
Iron deficiency;
Pure red cell aplasia (PRCA) from production of anti-EPO antibodies (Table 7).
Table 7.
Clinical use of human epoetin alfa or darbepoetin alfa in cats with CKD
| Drug | Initial therapy | Maintenance therapy | Efficacy studies in CKD anaemia | Adverse events135,136 |
|---|---|---|---|---|
| Epoetin alfa | 100 U/kg SC 3 x weekly Until PCV ⩾25% |
50–100 U/kg SC 1–2 x weekly Based on PCV |
Effective for cats and dogs 137 | Systemic hypertension (40–50%) Seizures (2–10%) Polycythemia (unlikely) Injection site discomfort Skin reactions (redness) PRCA (25–40%)* |
| Darbepoetin alfa | 1 μg/kg SC 1 x weekly Until PCV ⩾25% |
1 μg/kg SC q2–3 weeks; or Lower dose (eg, 0.5 µg/kg) weekly Based on PCV |
Effective in cats Response tends to occur in 2–3weeks 135 |
Similar adverse event profile to EA but lower incidence of PRCA (<10%)*, so a better choice for therapy in cats |
Anti-erythropoeitin antibodies are produced and PRCA manifests as worsening anaemia, lack of erythrocytosis, and no response to ESA therapy. Diagnosis is supported by a bone marrow aspirate/core and cats become transfusion-dependent for months. 137 SC = subcutaneously; PCV = packed cell volume; PRCA = pure red cell aplasia
Hypertension is also recognised as an adverse effect of ESA therapy, affecting up to 50% of treated cats.129,135
Managing proteinuria in CKD

In human medicine, regardless of the cause of CKD, the severity of proteinuria at the time of diagnosis is an important prognostic indicator, and controlling proteinuria results in slower progression of CKD, largely irrespective of the underlying cause of the CKD. 138 CKD is generally associated with increased intraglomerular capillary pressure and other changes that impair glomerular permselectivity, leading to increased loss of albumin (and other proteins) into tubular fluid; this appears to directly contribute to disease progression by promoting tubular inflammation and fibrosis. 138
Although there may be species differences in pathophysiology, increased proteinuria in cats with CKD (assessed with UPCR and not routine dipsticks, which are inappropriate for assessment of feline proteinuria139,140) is also known to carry a poorer prognosis.37,42,44,120,123 In one study, 37 cats with a UPCR <0.2 were reported to have a median survival time of ~1000 days compared with ~500 days for those with a UPCR of 0.2–0.4, and ~400 days for those with a UPCR >0.4. Very similar findings have also been reported elsewhere. 120 Currently, there is no evidence that measurement of UACR rather than UPCR offers any benefits in cats,37,141 but the urinary proteome in healthy cats and cats with CKD is complex and more studies are needed.142 –146
In humans, treatment with angiotensin receptor blockers (ARBs) or ACEIs is effective in blocking RAAS activation, decreasing glomerular capillary pressure, restoring glomerular permselectivity, reducing proteinuria and slowing the progression of CKD.138,147 –149 These effects are partly as a result of haemodynamic changes and partly through modifying non-haemodynamic remodelling in the kidney. 150
The existing IRIS 27 CKD (see box on page 221) and American College of Veterinary Internal Medicine (ACVIM) proteinuria 151 guidelines suggest cats should be classified as:
Overtly proteinuric: UPCR >0.4
Borderline proteinuric: UPCR 0.2–0.4
Non-proteinuric: UPCR <0.2
Using these criteria, around 50–66% of cats with CKD are likely to be non-proteinuric and around 20% overtly proteinuric.37,42
In cats with CKD, RAAS inhibition with the ACEI benazepril has been shown to significantly reduce the severity of proteinuria.152 –154 More recently, the ARB telmisartan has been licensed in some countries for the management of proteinuric feline CKD. In a large multicentre European study of telmisartan in cats with naturally occurring CKD it was shown to significantly decrease proteinuria at all time points during the 6 months of the study. 155 However, a survival benefit from RAAS blockade in cats has not been demonstrated.152,153 The reasons for the lack of effect on survival are uncertain but may include:
Underpowered clinical trials;
Inadequate duration of clinical trials;
Differences in the pathophysiology between humans and cats with CKD (eg, proteinuria could be a marker of tubular dysfunction in cats rather than a cause of progressive disease);
Differences in the prevalence or severity of proteinuria between humans and cats with CKD;
Inadequate control of proteinuria and/or inappropriate targets for antiproteinuric therapy (while benazepril therapy significantly reduced UPCR in CKD cats compared with placebo in clinical trials, these studies also showed little overall reduction in the UPCR from baseline values within the treatment group152,153).
Further investigations are needed to assess the role of RAAS inhibition in feline CKD and to determine optimal therapy, but currently both ARBs and ACEIs are available and used in cats, and are licensed in some countries (Table 8). There is some (weak) evidence that RAAS blockade may have more beneficial effects (possibly on survival, QoL and appetite) in CKD cats with more severe proteinuria (eg, UPCR ⩾1.0), 152 and currently IRIS and ACVIM guidelines71,151 suggest antiproteinuric therapy should be instituted in CKD cats with a UPCR >0.4.
Table 8.
Suggested oral therapy for managing proteinuria
| Drug | Dose |
|---|---|
| Telmisartan (ARB) | 1 mg/kg q24h |
| Benazepril (ACEI) | 0.25–0.5 mg/kg q12h |
ARB = angiotensin receptor blocker;
ACEI = angiotensin-converting enzyme inhibitor
Managing inappetence, nausea and vomiting in CKD

Cats with CKD can suffer from nausea, vomiting and inappetence as a result of uraemic toxins affecting the central chemoreceptor trigger zone. Inappetence is a significant QoL concern for owners, 156 and in the CKD patient could result in protein and calorie malnutrition with its many adverse consequences. 157 A reduced appetite should therefore be actively managed, along with complications of CKD that can contribute to inappetence, such as dehydration, hypokalaemia, acidosis and anaemia. Centrally acting antiemetics such as maropitant, mirtazapine, ondansetron and dolasetron158 –160 should be considered for management (Table 9). In placebo-controlled trials of cats with stage 2 or 3 CKD, maropitant (given orally for 2 weeks) was shown to reduce vomiting, 160 and mirtazapine (given orally for 3 weeks) reduced vomiting and also increased appetite and weight. 158 Mirtazapine may therefore be a useful adjunct to the nutritional management of cats with CKD.
Table 9.
Suggested therapy for managing inappetence, nausea and vomiting
| Drug | Dose |
|---|---|
| Maropitant (neurokinin-1 receptor antagonist) | 1 mg/kg q24h SC/IV |
| 2 mg/kg q24h PO | |
| Mirtazapine (tetracyclic antidepressant, alpha-2 antagonist) | ~0.5 mg/kg (or 1.88 mg/cat) q48h PO |
| Ondansetron (5-HT3 receptor antagonist) | 0.5–1.0 mg/kg q6–8h SC |
| Dolasetron (5-HT3 receptor antagonist) | 1.0 mg/kg q24h SC |
| Famotidine (H2 blocker) | 0.5–1 mg/kg q12–24h PO |
| Omeprazole (proton pump inhibitor) | 0.5–1 mg/kg q12–24h PO |
SC = subcutaneously; IV = intravenously; PO = orally
There are anecdotal reports of H2 blockers or proton pump inhibitors alleviating inappetence in some feline CKD patients, but the presence and degree of gastric hyperacidity and efficacy of these medications remain unproven. Additionally, although hypergastrinaemia has been reported in feline CKD, 161 gastric ulceration has generally not been observed or reported.73,162 If therapy for hyperacidity in cats is considered, omeprazole appears to be superior to famotidine. 163

Managing UTIs in CKD

Bacterial UTIs in cats with CKD occur at a reported frequency of around 15–30%,39,164 –166 with older female cats having an increased risk. 39
Most (>70%) of these UTIs appear to be subclinical (ie, without lower urinary tract signs [LUTS]), although >85% show changes on urine sediment analysis (>5 white blood cells [WBCs]/hpf, and/or >5 RBCs/hpf, and/or microscopic bacteriuria). 39 Escherichia coli represents 60–75% of isolates, while other organisms include Enterococcus, Streptococcus, Staphylococcus, Enterobacter, Pseudomonas and Klebsiella species.
The presence of LUTS or detection of pyuria (⩾5 WBCs/hpf) in routine urinalysis of CKD patients are indications for bacterial culture of a cystocentesis sample, but whether routine culture of all urine samples should be recommended is controversial, as the significance of subclinical bacteriuria is uncertain. While some clinicians advocate routine treatment of all CKD-associated UTIs (as cats may be at risk for pyelonephritis and deterioration of CKD), recurrent or recrudescent UTIs are common after treatment, 39 the presence of subclinical UTIs has not been associated with disease severity or apparent survival, 39 and unnecessary treatment may risk development of bacterial resistance.
When treated, UTIs should be managed according to international guidelines, 167 selecting antibacterials based on sensitivity testing (note that boric acid tubes should be avoided for urine cultures 168 ) that are excreted unchanged in urine and have a wide therapeutic index (Table 10). If initial empirical therapy is needed, amoxicillin (11–15 mg/kg PO q8h) 167 or potentiated amoxicillin 169 are appropriate choices; 2–4 weeks’ therapy has been recommended, 167 although optimum duration of therapy for CKD-associated UTIs is uncertain. 170 Response to treatment should be monitored with repeat culture 7 days after cessation of treatment.
Table 10.
Considerations when selecting an antibacterial to treat urinary tract infections in cats with CKD
| Consideration | Action | Antibacterials |
|---|---|---|
| Probably safe | No dose adjustment required, due to wide therapeutic index or excretion via extrarenal routes | Chloramphenicol |
| Penicillins (including clavulanate) | ||
| Consider dosage adjustment | Adjust dose in moderate or severe CKD (IRIS stages 3 and 4) | Cephalosporins (most)* |
| Fluoroquinolones† | ||
| Sulphonamides (± trimethoprim) | ||
| Hazardous, avoid if possible | Accumulation of drug or its metabolites in CKD can increase risk of adverse events | Nalidixic acid |
| Nitrofurantoin | ||
| Tetracyclines‡ (except doxycycline) | ||
| Nephrotoxic | Avoid – high-risk drugs will exacerbate CKD | Aminoglycosides |
| Polymyxins |
Some cephalosporins accumulate in renal tubular cells and can cause damage
Avoid enrofloxacin in cats with CKD due to increased risk of retinopathy at standard therapeutic doses
Water soluble tetracyclines (eg, oxytetracycline) depend partly on renal excretion. Tetracyclines also increase protein catabolism, and breakdown products of oxytetracycline have been shown to be nephrotoxic
IRIS = International Renal Interest Society
Other treatments
Anabolic steroids
Information regarding the efficacy of anabolic steroids for cats with CKD is lacking and, as hepatotoxicity has been reported, 133 their use is not currently recommended.
Stem cell therapy
Pilot studies investigating stem cell therapy for feline CKD have not to date demonstrated beneficial effects; and with some techniques adverse effects occur.171,172 Consequently, this treatment is not currently recommended.
Renal transplantation
Kidney transplants from living donors may be available to treat cats with CKD at specialist centres in some regions. This procedure has numerous implications including ethical, financial, welfare and monitoring considerations.173 –176 While it may be viable in some patients, kidney transplantation is beyond the scope of these Guidelines.
Dialysis therapy
Haemodialysis or peritoneal dialysis are techniques that can be successfully applied to cats, although complications may arise. Their main indications are for management of acute kidney injury or acute on chronic kidney disease.177,178

Footnotes
Funding: These Guidelines were supported by an educational grant from Boehringer Ingelheim to the ISFM.
Jonathan Elliott has acted as a paid consultant for CEVA Animal Health, Boehringer Ingelheim, Pfizer (now Zoetis), Bayer, Idexx, Novartis Animal Health, Waltham Centre for Pet Nutrition and Royal Canin; he has research grant funding and contracts to work on kidney disease in cats from CEVA Animal Health, Orion, Zoetis, Royal Canin and Novartis Animal Health (now Elanco Animal Health). Natalie Finch has received research funding from Boehringer Ingelheim. Catherine Langston is a paid consultant for Bayer and for Abaxis. Hervé Lefebvre has received grants/research contracts and/or performs consulting for Royal Canin, Novartis Animal Health, CEVA Animal Health and Bayer.
References
- 1. O’Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary-care veterinary practices in England. Vet J 2014; 202: 286–291. [DOI] [PubMed] [Google Scholar]
- 2. White JD, Norris JM, Baral RM, et al. Naturally-occurring chronic renal disease in Australian cats: a prospective study of 184 cases. Aust Vet J 2006; 84: 188–194. [DOI] [PubMed] [Google Scholar]
- 3. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lulich JP, Osborne CA, O’Brien TD, et al. Feline renal failure: questions, answers, questions. Compen Contin Educ Pract Vet 1992; 14: 127–152. [Google Scholar]
- 5. O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015; 17: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLeland SM, Cianciolo RE, Duncan CG, et al. A comparison of biochemical and histopathologic staging in cats with chronic kidney disease. Vet Pathol 2015; 52: 524–534. [DOI] [PubMed] [Google Scholar]
- 7. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 8. Reynolds BS, Lefebvre HP. Feline CKD: pathophysiology and risk factors – what do we know? J Feline Med Surg 2013; 15 Suppl 1: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White JD, Malik R, Norris JM. Feline chronic kidney disease: can we move from treatment to prevention? Vet J 2011; 190: 317–322. [DOI] [PubMed] [Google Scholar]
- 10. Furuya T, Sassa Y, Omatsu T, et al. Existence of feline morbillivirus infection in Japanese cat populations. Arch Virol 2014; 159: 371–373. [DOI] [PubMed] [Google Scholar]
- 11. Furuya T, Wachi A, Sassa Y, et al. Quantitative PCR detection of feline morbillivirus in cat urine samples. J Vet Med Sci 2016; 77: 1701–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo PC, Lau SK, Wong BH, et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc Natl Acad Sci USA 2012; 109: 5435–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 14. Bartlett PC, Van Buren JW, Bartlett AD, et al. Case-control study of risk factors associated with feline and canine chronic kidney disease. Vet Med Int 2010; 2010: 957570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greene JP, Lefebvre SL, Wang M, et al. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc 2014; 244: 320–327. [DOI] [PubMed] [Google Scholar]
- 16. Hughes KL, Slater MR, Geller S, et al. Diet and lifestyle variables as risk factors for chronic renal failure in pet cats. Prev Vet Med 2002; 55: 1–15. [DOI] [PubMed] [Google Scholar]
- 17. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–813. [DOI] [PubMed] [Google Scholar]
- 18. National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. http://www2.kidney.org/professionals/KdOQI/guidelines_ckd/toc.htm (2002, accessed 4 August 2015).
- 19. AAFP and AFM. American Association of Feline Practitioners/Academy of Feline Medicine Panel Report on Feline Senior Care. J Feline Med Surg 2005; 7: 3–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoyumpa Vogt A, Rodan I, Brown M, et al. AAFP-AAHA: feline life stage guidelines. J Feline Med Surg 2010; 12: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finch N. Measurement of glomerular filtration rate in cats: methods and advantages over routine markers of renal function. J Feline Med Surg 2014; 16: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baral RM, Dhand NK, Morton JM, et al. Bias in feline plasma biochemistry results between three in-house analysers and a commercial laboratory analyser: results should not be directly compared. J Feline Med Surg 2015; 17: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rishniw M, Bicalho R. Factors affecting urine specific gravity in apparently healthy cats presenting to first opinion practice for routine evaluation. J Feline Med Surg 2015; 17: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams LG, Polzin DJ, Osborne CA, et al. Effects of dietary protein and calorie restriction in clinically normal cats and in cats with surgically induced chronic renal failure. Am J Vet Res 1993; 54: 1653–1662. [PubMed] [Google Scholar]
- 25. Minkus G, Reusch C, Hörauf A, et al. Evaluation of renal biopsies in cats and dogs – histopathology in comparison with clinical data. J Small Anim Pract 1994; 35: 465–472. [Google Scholar]
- 26. Baral RM, Dhand NK, Freeman KP, et al. Biological variation and reference change values of feline plasma biochemistry analytes. J Feline Med Surg 2014; 16: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International Renal interest Society. IRIS staging of CKD. http://iris-kidney.com/guidelines/staging.aspx (2013, accessed 4 August 2015).
- 28. Paepe D, Lefebvre HP, Concordet D, et al. Simplified methods for estimating glomerular filtration rate in cats and for detection of cats with low or borderline glomerular filtration rate. J Feline Med Surg 2015; 17: 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braff J, Obare E, Yerramilli M, et al. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med 2014; 28: 1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koch A, Weiskirchen R, Bruensing J, et al. Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm 2013; 2013: 413826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghys LF, Paepe D, Duchateau L, et al. Biological validation of feline serum cystatin C: the effect of breed, age and sex and establishment of a reference interval. Vet J 2015; 204: 168–173. [DOI] [PubMed] [Google Scholar]
- 33. Ghys LF, Paepe D, Lefebvre HP, et al. The effect of feeding, storage and anticoagulant on feline serum cystatin C. Vet J 2015; 206: 91–96. [DOI] [PubMed] [Google Scholar]
- 34. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentration in geriatric cats with various degrees of renal function. J Am Vet Med Assoc 2012; 241: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 35. Finch NC, Geddes RF, Syme HM, et al. Fibroblast growth factor 23 (FGF-23) concentrations in cats with early non zotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013; 27: 227–233. [DOI] [PubMed] [Google Scholar]
- 36. Bijsmans ES, Jepson RE, Chang YM, et al. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med 2015; 29: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 38. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 39. White JD, Stevenson M, Malik R, et al. Urinary tract infections in cats with chronic kidney disease. J Feline Med Surg 2013; 15: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geddes RF, Finch NC, Elliott J, et al. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013; 234–241. [DOI] [PubMed] [Google Scholar]
- 41. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 42. King JN, Tasker S, Gunn-Moore DA, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 906–916. [PubMed] [Google Scholar]
- 43. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor-23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med 2015; 29: 1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 45. Kuwahara Y, Ohba Y, Kitoh K, et al. Association of laboratory data and death within one month in cats with chronic renal failure. J Small Anim Pract 2006; 47: 446–450. [DOI] [PubMed] [Google Scholar]
- 46. Langston C. Managing fluid and electrolyte disorders in renal failure. Vet Clin North Am Small Anim Pract 2008; 38: 677–697. [DOI] [PubMed] [Google Scholar]
- 47. Polzin DJ. Chronic kidney disease. in: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames: Blackwell Publishing, 2011, pp 431–471. [Google Scholar]
- 48. Seefeldt SL, Chapman TE. Body water content and turnover in cats fed dry and canned rations. Am J Vet Res 1979; 40: 183–185. [PubMed] [Google Scholar]
- 49. Wei A, Fascetti AJ, Villaverde C, et al. Effect of water content in a canned food on voluntary food intake and body weight in cats. Am J Vet Res 2011; 72: 918–923. [DOI] [PubMed] [Google Scholar]
- 50. Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev 2009; 2: CD001892. [DOI] [PubMed] [Google Scholar]
- 51. National Research Council. Nutrient requirements of dogs and cats. Washington, DC: National Academies Press, 2006. Doi: 10.17226/10668. [DOI] [Google Scholar]
- 52. Laflamme DP, Hannah SS. Discrepancy between use of lean body mass or nitrogen balance to determine protein requirements for adult cats. J Feline Med Surg 2013; 15: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kidder AC, Chew D. Treatment options for hyperphosphatemia in feline CKD: what’s out there? J Feline Med Surg 2009; 11: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plantinga EA, Everts H, Kastelein AM, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec 2005; 157: 185–187. [DOI] [PubMed] [Google Scholar]
- 55. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 56. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–957. [DOI] [PubMed] [Google Scholar]
- 57. Harte JG, Markwell PJ, Moraillon RM, et al. Dietary management of naturally occurring chronic renal failure in cats. J Nutr 1994; 124: 2660S–2662S. [DOI] [PubMed] [Google Scholar]
- 58. Adams LG, Polzin DJ, Osborne CA, et al. Influence of dietary protein/calorie intake on renal morphology and function in cats with 5/6 nephrectomy. Lab Invest 1994; 70: 347–357. [PubMed] [Google Scholar]
- 59. Finco DR, Brown SA, Brown CA, et al. Protein and calorie effects on progression of induced chronic renal failure in cats. Am J Vet Res 1998; 59: 575–582. [PubMed] [Google Scholar]
- 60. Polzin DJ, Osborne CA, Ross S, et al. Dietary management of feline chronic renal failure: where are we now? In what direction are we headed? J Feline Med Surg 2000; 2: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ross LA, Finco DR, Crowell WA. Effect of dietary phosphorus restriction on the kidneys of cats with reduced renal mass. Am J Vet Res 1982; 43: 1023–1026. [PubMed] [Google Scholar]
- 62. Barber PJ, Rawlings JM, Markwell PJ, et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999; 40: 62–70. [DOI] [PubMed] [Google Scholar]
- 63. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med 2013; 27: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 64. Larsen JA, Parks EM, Heinze CR, et al. Evaluation of recipes for home-prepared diets for dogs and cats with chronic kidney disease. J Am Vet Med Assoc 2012; 240: 532–538. [DOI] [PubMed] [Google Scholar]
- 65. Markovich JE, Freeman LM, Labato MA, et al. Survey of dietary and medication practices of owners of cats with chronic kidney disease. J Feline Med Surg 2015; 17: 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laflamme D, Gunn-Moore D. Nutrition of aging cats. Vet Clin North Am Small Anim Pract 2014; 44: 761–74. [DOI] [PubMed] [Google Scholar]
- 67. Schmidt BH, Dribusch U, Delport PC, et al. Tolerability and efficacy of the intestinal phosphate binder Lantharenol® in cats. BMC Vet Res 2012; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wagner E, Schwendenwein I, Zentek J. Effects of a dietary chitosan and calcium supplement on Ca and P metabolism in cats. Berl Munch Tierarztl Wochenschr 2003; 117: 310–315. [PubMed] [Google Scholar]
- 69. King JN, Delport PC, Luus HG, et al. Efficacy and acceptability of the new oral phosphate binder Lenziaren(®) in healthy cats fed a renal diet. J Vet Pharmacol Ther 2015; 38: 278–289. [DOI] [PubMed] [Google Scholar]
- 70. Bernachon N, Fournel S, Gatto H, et al. Comparative palatability of five supplements designed for cats suffering from chronic renal disease. Ir Vet J 2014; 67: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. International Renal interest Society. IRIS treatment recommendations. http://iris-kidney.com/guidelines/ (2013, accessed 4 August 2015).
- 72. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108–116. [DOI] [PubMed] [Google Scholar]
- 73. McLeland SM, Lunn KF, Duncan CG, et al. Relationship among serum creatinine, serum gastrin, calcium-phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Brito Galvao JF, Nagode LA, Schenck PA, et al. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J Vet Emerg Crit Care (San Antonio) 2013; 23: 134–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hostutler RA, DiBartola SP, Chew DJ, et al. Comparison of the effects of daily and intermittent dose calcitriol on serum parathyroid hormone and ionized calcium concentrations in normal cats and cats with chronic renal failure. J Vet Intern Med 2006; 20: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 76. Deguchi E, Akuzawa M. Renal clearance of endogenous creatinine, urea, sodium, and potassium in normal cats and cats with chronic renal failure. J Vet Med Sci 1997; 59: 509–512. [DOI] [PubMed] [Google Scholar]
- 77. de Morais HA, Bach JF, DiBartola SP. Metabolic acid-base disorders in the critical care unit. Vet Clin North Am Small Anim Pract 2008; 38: 559–574. [DOI] [PubMed] [Google Scholar]
- 78. de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20: 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elliott J, Syme HM, Markwell PJ. Acid-base balance of cats with chronic renal failure: effect of deterioration in renal function. J Small Anim Pract 2003; 44: 261–268. [DOI] [PubMed] [Google Scholar]
- 80. Elliott J, Syme HM, Reubens E, et al. Assessment of acid-base status of cats with naturally occurring chronic renal failure. J Small Anim Pract 2003; 44: 65–70. [DOI] [PubMed] [Google Scholar]
- 81. Tonkin L, Parnell N. Evaluation of serum fatty acids in cats with chronic kidney disease [abstract]. J Vet Intern Med 2015; 29: 1217. [Google Scholar]
- 82. Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 2013; 88: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Buranakarl C, Mathur S, Brown SA. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. Am J Vet Res 2004; 65: 620–627. [DOI] [PubMed] [Google Scholar]
- 84. Xu H, Laflamme DP, Long GL. Effects of dietary sodium chloride on health parameters in mature cats. J Feline Med Surg 2009; 11: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Reynolds BS, Chetboul V, Nguyen P, et al. Effects of dietary salt intake on renal function: a 2-year study in healthy aged cats. J Vet Intern Med 2013; 27: 507–515. [DOI] [PubMed] [Google Scholar]
- 86. Kirk CA, Jewell DE, Lowry SR. Effects of sodium chloride on selected parameters in cats. Vet Ther 2006; 7: 333–346. [PubMed] [Google Scholar]
- 87. Keegan RF, Webb CB. Oxidative stress and neutrophil function in cats with chronic renal failure. J Vet Intern Med 2010; 24: 514–519. [DOI] [PubMed] [Google Scholar]
- 88. Krofič Žel M, Tozon N, Nemec Svete A. Plasma and erythrocyte glutathione peroxidase activity, serum selenium concentration, and plasma total antioxidant capacity in cats with IRIS stages I–IV chronic kidney disease. J Vet Intern Med 2014; 28: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu S, Paetau-Robinson I. Dietary supplements of vitamins E and C and beta-carotene reduce oxidative stress in cats with renal insufficiency. Vet Res Commun 2006; 30: 403–413. [DOI] [PubMed] [Google Scholar]
- 90. Hanzlicek AS, Roof CJ, Sanderson MW, et al. The effect of Chinese rhubarb, Rheum officinale, with and without benazepril on the progression of naturally occurring chronic kidney disease in cats. J Vet Intern Med 2014; 28: 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rishniw M, Wynn SG. Azodyl, a synbiotic, fails to alter azotemia in cats with chronic kidney disease when sprinkled onto food. J Feline Med Surg 2011; 13: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799–1804. [DOI] [PubMed] [Google Scholar]
- 93. Stiles J, Polzin DJ, Bistner SI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994; 30: 564–572. [Google Scholar]
- 94. Mishina M, Watanabe T, Fujii K, et al. Non-invasive blood pressure measurements in cats: clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci 1998; 60: 805–808. [DOI] [PubMed] [Google Scholar]
- 95. Steele JL, Henik RA, Stepien RL. Effects of angiotensin converting enzyme inhibition on plasma aldosterone concentration, plasma renin activity, and blood pressure in spontaneously hypertensive cats with chronic renal disease. Vet Ther 2002; 3: 157–166. [PubMed] [Google Scholar]
- 96. Taugner F, Baatz G, Nobiling R. The renin-angiotensin system in cats with chronic renal failure. J Comp Pathol 1996; 115: 239–252. [DOI] [PubMed] [Google Scholar]
- 97. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med 2014; 28: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT Doppler and oscillometric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005; 7: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Acierno MJ, Seaton D, Mitchell MA, et al. Agreement between directly measured blood pressure and pressures obtained with three veterinary-specific oscillometric units in cats. J Am Vet Med Assoc 2010; 237: 402–406. [DOI] [PubMed] [Google Scholar]
- 100. da Cunha AF, Saile K, Beaufrère H, et al. Measuring level of agreement between values obtained by directly measured blood pressure and ultrasonic Doppler flow detector in cats. J Vet Emerg Crit Care (San Antonio) 2014; 24: 272–278. [DOI] [PubMed] [Google Scholar]
- 101. Martel E, Egner B, Brown SA, et al. Comparison of high-definition oscillometry – a non-invasive technology for arterial blood pressure measurement – with a direct invasive method using radio-telemetry in awake healthy cats. J Feline Med Surg 2013; 15: 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cannon MJ, Brett J. Comparison of how well conscious cats tolerate blood pressure measurement from the radial and coccygeal arteries. J Feline Med Surg 2012; 14: 906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Grandy JL, Dunlop CI, Hodgson DS, et al. Evaluation of the Doppler ultrasonic method of measuring systolic arterial blood pressure in cats. Am J Vet Res 1992; 53: 1166–1169. [PubMed] [Google Scholar]
- 104. Gouni V, Tissier R, Misbach C, et al. Influence of the observer’s level of experience on systolic and diastolic arterial blood pressure measurements using Doppler ultrasonography in healthy conscious cats. J Feline Med Surg 2015; 17: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 106. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998; 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 107. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retino pathy. Am J Vet Res 2004; 65: 245–252. [DOI] [PubMed] [Google Scholar]
- 108. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–62. [DOI] [PubMed] [Google Scholar]
- 109. Lin CH, Yan CJ, Lien YH, et al. Systolic blood pressure of clinically normal and conscious cats determined by an indirect Doppler method in a clinical setting. J Vet Med Sci 2006; 68: 827–832. [DOI] [PubMed] [Google Scholar]
- 110. Sparkes AH, Caney SM, King MC, et al. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–318. [DOI] [PubMed] [Google Scholar]
- 111. Belew AM, Barlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 112. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122–129. [DOI] [PubMed] [Google Scholar]
- 113. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 114. Nelson L, Reidesel E, Ware WA, et al. Echocardiographic and radiographic changes associated with systemic hypertension in cats. J Vet Intern Med 2002; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 115. Snyder PS, Sadek D, Jones GL. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Intern Med 2001; 15: 52–56. [DOI] [PubMed] [Google Scholar]
- 116. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 117. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 118. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003; 222: 322–329. [DOI] [PubMed] [Google Scholar]
- 119. Tozawa M, Iseki K, Iseki C, et al. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41: 1341–1345. [DOI] [PubMed] [Google Scholar]
- 120. Jepson RE, Elliott J, Brodbelt D, et al. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–409. [DOI] [PubMed] [Google Scholar]
- 121. Bacic A, Kogika MM, Barbaro KC, et al. Evaluation of albuminuria and its relationship with blood pressure in dogs with chronic kidney disease. Vet Clin Pathol 2010; 39: 203–209. [DOI] [PubMed] [Google Scholar]
- 122. Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int 1997; 51: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 123. Syme HM. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg 2009; 11: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jensen J, Henik RA, Brownfield M, et al. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997; 58: 535–540. [PubMed] [Google Scholar]
- 125. Lefebvre HP, Toutain PL. Angiotensin-converting enzyme inhibitors in the therapy of renal diseases. J Vet Pharmacol Ther 2004; 27: 265–281. [DOI] [PubMed] [Google Scholar]
- 126. Jenkins TL, Coleman AE, Schmiedt CW, et al. Attenuation of the pressor response to exogenous angiotensin by angiotensin receptor blockers and benazepril hydrochloride in clinically normal cats. Am J Vet Res 2015; 76: 807–813. [DOI] [PubMed] [Google Scholar]
- 127. Huhtinen M, Derré G, Renoldi HJ, et al. Randomized placebo-controlled clinical trial of a chewable formulation of amlodipine for the treatment of hypertension in client-owned cats. J Vet Intern Med 2015; 29: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. O’Neill J, Kent M, Glass EN, et al. Clinicopathologic and MRI characteristics of presumptive hypertensive encephalopathy in two cats and two dogs. J Am Anim Hosp Assoc 2013; 49: 412–420. [DOI] [PubMed] [Google Scholar]
- 129. Chalhoub S, Langston C, Eatroff A. Anemia of renal disease: what it is, what to do and what’s new. J Feline Med Surg 2011; 13: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cowgill Ld. Pathophysiology and management of anemia in chronic progressive renal failure. Semin Vet Med Surg (Small Anim) 1992; 7: 175–182. [PubMed] [Google Scholar]
- 131. Callan MB, Rentko VT. Clinical application of a hemoglobin-based oxygen-carrying solution. Vet Clin North Am Small Anim Pract 2003; 33: 1277–1293. [DOI] [PubMed] [Google Scholar]
- 132. Yang Q, Abudou M, Xie XS, et al. Androgens for the anaemia of chronic kidney disease in adults. Cochrane Database Syst Rev 2014; 10: CD006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Harkin KR, Cowan LA, Andrews GA, et al. Hepatotoxicity of stanozolol in cats. J Am Vet Med Assoc 2000; 217: 681–684. [DOI] [PubMed] [Google Scholar]
- 134. Schiesser D, Binet I, Tsinalis D, et al. Weekly low-dose treatment with intravenous iron sucrose maintains iron status and decreases epoetin requirement in iron-replete haemodialysis patients. Nephrol Dial Transplant 2006; 21: 2841–2845. [DOI] [PubMed] [Google Scholar]
- 135. Chalhoub S, Langston CE, Farrelly J. The use of darbepoetin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Intern Med 2012; 26: 363–369. [DOI] [PubMed] [Google Scholar]
- 136. Langston CE, Reine NJ, Kittrell D. The use of erythropoietin. Vet Clin North Am Small Anim Pract 2003; 33: 1245–1260. [DOI] [PubMed] [Google Scholar]
- 137. Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc 1998; 212: 521–528. [PubMed] [Google Scholar]
- 138. Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol 2012; 23: 1917–1928. [DOI] [PubMed] [Google Scholar]
- 139. Hanzlicek AS, Roof CJ, Sanderson MW, et al. Comparison of urine dipstick, sulfosalicylic acid, urine protein-to-creatinine ratio and a feline-specific immunoassay for detection of albuminuria in cats with chronic kidney disease. J Feline Med Surg 2012; 14: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lyon SD, Sanderson MW, Vaden SL, et al. Comparison of urine dipstick, sulfosalicylic acid, urine protein-to-creatinine ratio, and species-specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc 2010; 236: 874–879. [DOI] [PubMed] [Google Scholar]
- 141. Whittemore JC, Gill VL, Jensen WA, et al. Evaluation of the association between microalbuminuria and the urine albumin-creatinine ratio and systemic disease in dogs. J Am Vet Med Assoc 2006; 229: 958–963. [DOI] [PubMed] [Google Scholar]
- 142. Ferlizza E, Campos A, Neagu A, et al. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet J 2015; 204: 73–81. [DOI] [PubMed] [Google Scholar]
- 143. Miyazaki M, Kamiie K, Soeta S, et al. Molecular cloning and characterization of a novel carboxylesterase-like protein that is physiologically present at high concentrations in the urine of domestic cats (Felis catus). Biochem J 2003; 370: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Miyazaki M, Yamashita T, Hosokawa M, et al. Species-, sex, and age-dependent urinary excretion of cauxin, a mammalian carboxylesterase. Comp Biochem Physiol B Biochem Mol Biol 2006; 145: 270–277. [DOI] [PubMed] [Google Scholar]
- 145. Jepson RE, Syme HM, Markwell P, et al. Measurement of urinary cauxin in geriatric cats with variable plasma creatinine concentrations and proteinuria and evaluation of urine cauxin-to-creatinine concentration ratio as a predictor of developing azotemia. Am J Vet Res 2010; 71: 982–987. [DOI] [PubMed] [Google Scholar]
- 146. Jepson RE, Coulton GR, Cowan ML, et al. Evaluation of mass spectrometry of urinary proteins and peptides as biomarkers for cats at risk of developing azotemia. Am J Vet Res 2013; 74: 333–342. [DOI] [PubMed] [Google Scholar]
- 147. Benigni A, Remuzzi G. Glomerular protein trafficking and progression of renal disease to terminal uremia. Semin Nephrol 1996; 16: 151–159. [PubMed] [Google Scholar]
- 148. Remuzzi A, Remuzzi G. Potential protective effects of telmisartan on renal function deterioration. J Renin Angiotensin Aldosterone Syst 2006; 7: 185–191. [DOI] [PubMed] [Google Scholar]
- 149. Porter AM. Ramipril in non-diabetic renal failure (REIN study) ramipril efficiency in nephropathy study. Lancet 1997; 350: 736; 736–737. [DOI] [PubMed] [Google Scholar]
- 150. Tylicki L, Lizakowski S, Rutkowski B. Renin-angiotensin-aldosterone system blockade for nephroprotection: current evidence and future directions. J Nephrol 2012; 25: 900–910. [DOI] [PubMed] [Google Scholar]
- 151. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 152. King JN, Gunn-Moore DA, Tasker S, et al. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006; 20: 1054–1064. [DOI] [PubMed] [Google Scholar]
- 153. Mizutani H, Koyama H, Watanabe T, et al. Evaluation of the clinical efficacy of benazepril in the treatment of chronic renal insufficiency in cats. J Vet Intern Med 2006; 20: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 154. Watanabe T, Mishina M. Effects of benazepril hydrochloride in cats with experimentally induced or spontaneously occurring chronic renal failure. J Vet Med Sci 2007; 69: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 155. Sent U, Gössl R, Elliott J, et al. Comparison of efficacy of long-term oral treatment with telmisartan and benazepril in cats with chronic kidney disease. J Vet Intern Med 2015; 29: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Reynolds CA, Oyama MA, Rush JE, et al. Perceptions of quality of life and priorities of owners of cats with heart disease. J Vet Intern Med 2010; 24: 1421–1426. [DOI] [PubMed] [Google Scholar]
- 157. Chan DL. The inappetent hospitalised cat: clinical approach to maximising nutritional support. J Feline Med Surg 2009; 11: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Quimby JM, Lunn KF. Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. Vet J 2013; 197: 651–655. [DOI] [PubMed] [Google Scholar]
- 159. Quimby JM, Lake RC, Hansen RJ, et al. Oral, subcutaneous, and intravenous pharmacokinetics of ondansetron in healthy cats. J Vet Pharmacol Ther 2014; 37: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Quimby JM, Brock WT, Moses K, et al. Chronic use of maropitant for the management of vomiting and inappetence in cats with chronic kidney disease: a blinded, placebo-controlled clinical trial. J Feline Med Surg 2015; 17: 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Goldstein RE, Marks SL, Kass PH, et al. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998; 213: 826–828. [PubMed] [Google Scholar]
- 162. Liptak JM, Hunt GB, Barrs VR, et al. Gastroduodenal ulceration in cats: eight cases and a review of the literature. J Feline Med Surg 2002; 4: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med 2015; 29: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Barber PJ, Rawlings JM, Markwell PJ, et al. Incidence and prevalence of bacterial urinary tract infections in cats with chronic renal failure [abstract]. J Vet Intern Med 1999; 13: 251. [Google Scholar]
- 165. Bailiff NL, Westropp JL, Nelson RW, et al. Evaluation of urine specific gravity and urine sediment as risk factors for urinary tract infections in cats. Vet Clin Pathol 2008; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 166. Mayer-Roenne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Feline Med Surg 2007; 9: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int 2011; 2011: 263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Rowlands M, Blackwood L, Mas A, et al. The effect of boric acid on bacterial culture of canine and feline urine. J Small Anim Pract 2011; 52: 510–514. [DOI] [PubMed] [Google Scholar]
- 169. Dorsch R, von Vopelius-Feldt C, Wolf G, et al. Feline urinary tract pathogens: prevalence of bacterial species and antimicrobial resistance over a 10-year period. Vet Rec 2015; 176: 201. [DOI] [PubMed] [Google Scholar]
- 170. Jessen LR, Sørensen TM, Bjornvad CR, et al. Effect of antibiotic treatment in canine and feline urinary tract infections: a systematic review. Vet J 2015; 203: 270–277. [DOI] [PubMed] [Google Scholar]
- 171. Quimby JM, Webb TL, Gibbons DS, et al. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg 2011; 13: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Quimby JM, Webb TL, Habenicht LM, et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther 2013; 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Danielson KC, Hardie RJ, McAnulty JF. Outcome of donor cats after unilateral nephrectomy as part of a clinical kidney transplant program. Vet Surg 2015; 44: 914–919. [DOI] [PubMed] [Google Scholar]
- 174. Roudebush P, Polzin DJ, Ross SJ, et al. Therapies for feline chronic kidney disease. What is the evidence? J Feline Med Surg 2009; 11: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schmiedt CW, Holzman G, Schwarz T, et al. Survival, complications, and analysis of risk factors after renal transplantation in cats. Vet Surg 2008; 37: 683–695. [DOI] [PubMed] [Google Scholar]
- 176. Yeates JW. Ethical considerations in feline renal transplantation. Vet J 2014; 202: 405–407. [DOI] [PubMed] [Google Scholar]
- 177. Langston CE, Cowgill LD, Spano JA. Applications and outcome of hemodialysis in cats: a review of 29 cases. J Vet Intern Med 1997; 11: 348–355. [DOI] [PubMed] [Google Scholar]
- 178. Ross LA, Labato MA. Current techniques in peritoneal dialysis. J Vet Emerg Crit Care (San Antonio) 2013; 23: 230–240. [DOI] [PubMed] [Google Scholar]
- 179. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gowan RA, Baral RM, Lingard AE, et al. A retrospective analysis of the effects of meloxicam on the longevity of aged cats with and without overt chronic kidney disease. J Feline Med Surg 2012; 14: 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. King JN, Strehlau G, Wernsing J, et al. Effect of renal insufficiency on the pharmacokinetics and pharmacodynamics of benazepril in cats. J Vet Pharmacol Ther 2002; 25: 371–378. [DOI] [PubMed] [Google Scholar]
- 183. Ebner T, Schänzle G, Weber W, et al. In vitro glucuronidation of the angiotensin II receptor antagonist telmisartan in the cat: a comparison with other species. J Vet Pharmacol Ther 2013; 36: 154–160. [DOI] [PubMed] [Google Scholar]
- 184. Bartges J, Martin Jimenez T. Drug therapy with renal failure. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames: Wiley-Blackwell, 2011, pp 386–390. [Google Scholar]
- 185. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol 2009; 160: 205–215. [DOI] [PubMed] [Google Scholar]
- 186. Trepanier LA. Pharmacologic management of feline hyperthyroidism. Vet Clin North Am Small Anim Pract 2007; 37: 775–788. [DOI] [PubMed] [Google Scholar]
- 187. Barber PJ, Elliott J. Study of calcium homeostasis in feline hyperthyroidism. J Small Anim Pract 1996; 37: 575–582. [DOI] [PubMed] [Google Scholar]
- 188. Williams TL, Elliott J, Syme HM. Association of iatro-genic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010; 24: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 189. Williams TL, Elliott J, Syme HM. Effect on renal function of restoration of euthyroidism in hyperthyroid cats with iatrogenic hypothyroidism. J Vet Intern Med 2014; 28: 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]