Abstract
Practical relevance Cats, both young and old, can suffer a variety of weird and wonderful musculoskeletal conditions that are a cause of lameness. These include developmental, metabolic and nutritional bone diseases, ectopic mineralisation disorders, conditions that cause lameness or exercise intolerance and primarily or secondarily affect muscle, and lastly pad conditions.
Clinical challenges These conditions are mostly rare and can be challenging to diagnose. The aim of this review is to bring these conditions to the attention of practitioners so that, if they are encountered, further research around the topic can be undertaken. Radiographic changes and diagnostic tests that can be used to try to confirm diagnoses are described.
Evidence base These unusual causes of lameness are the subject of multiple single case reports or small case series, many of which are relatively old. The evidence presented here is drawn from these articles. However, it is not possible within the scope of this review to discuss all the conditions in as much detail as they may warrant, or to make reference to every article relating to them.
Inherited and developmental disorders
Rickets
Rickets has been recognised in several young kittens presenting with signs related to bone abnormalities or to hypocalcaemia. Rickets is a disorder that occurs due to an absolute deficiency of vitamin D and low or normal calcium levels. Cats are unable to synthesise vitamin D (cholecalciferol) in their skin when exposed to sunlight and so are reliant on an adequate dietary intake. The dietary form is, however, very rare.
Four types of hereditary rickets have been recognised in cats: vitamin D-dependent rickets type 2 (VDDR-2), vitamin D-dependent rickets type 1 (VDDR-1), hypophosphataemic rickets and vitamin D-dependent rickets non-type 1, non-type 2.
In hypophosphataemic rickets the defect is due to loss of phosphate at the level of the renal tubule. 1
VDDR-1 is caused by a defect in the gene encoding the enzyme 25-hydroxyvitamin D-1α-hydroxylase. 2
VDDR-2 is caused by a defect in the vitamin D receptor gene.3–5
VDDR non-type 1, non-type 2 has only recently been reported, and the cause has not been determined. 6
VDDR-1 and VDDR-2 are autosomal recessive conditions. The reported cases are summarised in Table 1.
Table 1.
Summary of reported cases of confirmed rickets in cats
| Rickets type | Age at onset and signalment | History and clinical signs | Physical examination | Haematological abnormalities | Radiographic changes | Reference |
|---|---|---|---|---|---|---|
| Renal hypophosphataemic | 3 months FN DLH 2.27 kg |
Episodes of painful ollapse from 3 months. GI foreign body |
Small stature, abducted elbows, outward rotation of left hind limb, plantigrade stance | Phosphate↓ 25(OH)D3 N Parathyroid hormone↑ |
Diffuse osteopenia, pathological bowing and fractures, lumbar lordosis | 1 |
| VDDR-2 | 4 months M DSH 2.5 kg |
Vomiting, diarrhoea, muscle tremors, mydriasis | Leg tremors when walking | Phosphate↑ Calcium↓ 1,25(OH)2D3↑↑ |
None | 3 |
| VDDR-2 | 4 months F DSH 1.6 kg |
Hunched, reluctance to jump, poor appetite | Stiff, stilted gait, small stature, metaphyseal swelling | Alkaline phosphatase↑ Phosphate↓ 1,25(OH)2D3↑↑↑ Ionised calcium↓ Parathyroid hormone↑ |
Osteopenia, cup-shaped epiphyses (multiple), kyphosis and lordosis | 4 |
| VDDR-2 | 4 months M DSH 2.4 kg |
Inappetence and reduced mobility | Lateral bowing of antebrachium of both thoracic limbs | Calcium ↓/N Alkaline phosphatase↑ 1,25(OH)2D3↑↑↑ |
Cup-shaped epiphyses – distal radius and ulna. Lumbar lordosis | 5 |
| VDDR-1 | 5 months F DSH 1.8 kg |
Generalised pain, reluctance to move, constipation | Small stature, lethargic, pain around spine and tail, reluctant to move, seizures | Calcium↓ Phosphate↑ Alkaline phosphatase↑ Creatine kinase↑ Parathyroid hormone↑↑↑ 1,25(OH)2D3↓ |
Osteopenia, cup-shaped growth plates | 2 |
| Nutritional vitamin D deficiency | (Experimental study) | 1,25(OH)2D3↓ Phosphate↓ Calcium↓ Parathyroid hormone↑ |
Cup-shaped growth plates and irregular mineralisation | 7 | ||
| VDDR non-type 1, non-type 2 | 3 months F Cornish Rex |
Reluctance to move around, bunny hopping, straining during defecation | Swelling of metaphyseal regions, small stature, hunched, stilted gait | Calcium↓ Phosphate↓ 25(OH)D3↓ 1,25(OH)2D3 ↑ Parathyroid hormone↑ |
6 | |
VDDR = vitamin D-dependent rickets, DLH = domestic longhair, DSH = domestic shorthair, GI = gastrointestinal, F = female, FN = female neutered, M = male, MN = male neutered, 1,25(OH)2D3 = calcitriol (1,25-dihydroxycholecalciferol), 25(OH)D3 = calcifediol (25-hydroxycholecalficerol), N = normal
In most of the reported cases of rickets, cats showed signs from 3 months of age, with veterinary attention being sought soon after. The clinical histories vary but often include stiffness, reluctance to move and pain;4–6 or signs that are consistent with hypocalcaemia, such as tremors, seizures or gastrointestinal disturbances. 3 Physical examination findings include stiff joints, swollen epiphyses, small stature and spinal abnormalities such as lordosis or kyphosis.
In cases where rickets is suspected, calcium, phosphate, parathyroid hormone and vitamin D levels should be measured. The urinary fractional clearance of calcium and phosphorus are important when trying to distinguish what type of rickets is present. 1 In classical rickets the radiographic changes are fairly pathognomonic, with widened, cup-shaped growth plates (Figure 1);4–6 but not all cats diagnosed with rickets in the literature have had radiographic changes, 3 so the absence of these changes does not rule out the condition.
Figure 1.

Four-month-old kitten with vitamin D-dependent rickets type 2; note the widened cup-shaped growth plates in the proximal and distal femurs, and proximal tibiae
Treatment usually involves both calcium and vitamin D supplementation. Regular monitoring is advisable by checking blood levels and repeating radiographs to evaluate both the response to treatment and whether therapeutic doses need adjustment. The effective dosage levels for supplements are often considerably higher than those recommended to be given in normal animals. In the published case reports the levels of both supplements were adjusted according to response and a variety of different forms of the drugs were given. It is difficult to give recommendations as to dose rates and precisely which formulation to use as it varies with the specific type of rickets and the severity. The doses for a case of VDDR-2 that was successfully managed are listed in the box below.
The prognosis is variable. It is not possible to make generalisations as only individual case reports are published, and these document varying presentations and clinical signs (Table 1). Some cats seem to make a full recovery, 6 while others do not respond at all to treatment.
Vitamin D and calcium supplementation.
Osteogenesis imperfecta
Osteogenesis imperfecta is an inherited bone disease characterised by poor mineralisation and excessive bone fragility. Several different types are described in humans, each with their own pattern of inheritance, signs and biochemical defect. This heterogeneous syndrome ranges from a mild, subclinical disease to a lethal syndrome leading to stillbirths or neonatal death.
Clinically the disease mimics nutritional secondary hyperparathyroidism. Although osteogenesis imperfecta is rare, it should be considered as a differential diagnosis whenever a kitten is presented for multiple pathological bone fractures or osteopenia, but where a normal diet is being fed.8,9
These animals are very susceptible to pathological fractures 7 and often will have deformed limbs subsequent to malunion or folding fractures. Radiography usually (but not always) reveals poor mineralisation or osteopenia of the skeleton and pathological fractures (Figure 2). Blue sclerae may be seen. The teeth may have a translucent appearance 9 and tooth fractures are also reported; 8 these dental changes are known as dentinogenesis imperfecta. The skin may be thin and hernias are common.
Figure 2.

Eleven-month-old cat with suspected osteogenesis imperfecta. The cat presented after sustaining a second atraumatic tibial fracture. There is diffuse osteopenia and a proximal incomplete diaphyseal tibial fracture and sclerosis of the mid-diaphysis of the same bone, suggestive that a prior fracture had also occurred in this bone. A small bone graft was obtained from the proximal humerus and the cat subsequently developed bilateral humeral fractures while being restricted to a hospital cage
Histologically cortical bone quantity is reduced; the bone is woven and has increased porosity. These diseases probably result from an abnormality of collagen metabolism. Collagen type I constitutes more than 85% of the organic matrix of bone and provides the structural framework for mineralisation. Deficient or abnormal collagen type I results in brittle bones. Definitive diagnosis requires analysis of cultured skin fibroblasts to look for type I collagen abnormalities – a test that is not readily available. 9
Treatment in a kitten was attempted using vitamin C, which is essential in collagen formation and tissue repair; the outcome is unknown as the kitten was euthanased. 9 Bisphosphonates have also been recommended in humans and they have had some efficacy in the treatment of children with osteogenesis imperfecta, but their efficacy has not been proven in cats. 9
Lysosomal storage diseases
Lysosomal storage diseases are rare inherited disorders that occur as a consequence of a lysosomal enzyme deficiency.
Mucopolysaccharidoses
The mucopolysaccharidoses are a group of lysosomal storage diseases that occur as a result of inborn errors of glycosaminoglycan (mucopolysaccharide) metabolism. Numerous different types have been described in man. Types I, VI and VII have been reported in the cat. 10
Classically affected animals usually present at less than a year of age. They have abnormal facial features, an abnormal gait, diffuse neurological disease and dwarfism, and they excrete metabolites in urine. There are characteristic radiographic changes affecting the spine, long bones and joints (Figure 3). The diagnosis is suspected from clinical signs and radiographic changes, and confirmed by urine tests and enzyme assays. 10
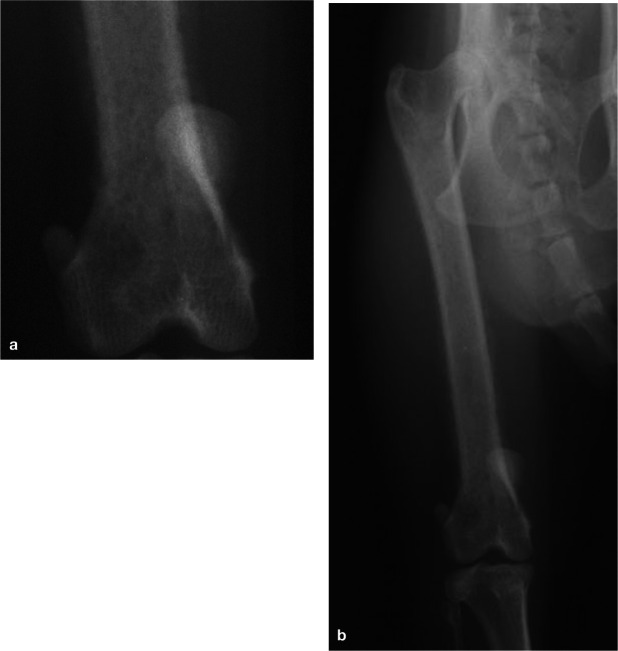
Figure 3.
A cat with mucopolysaccharidosis. Radiographs show (a) osteopenia and hip subluxation, and (b) cervical vertebral fusion with short vertebral bodies and epiphyseal dysplasia
Classically, cats with mucopolysaccharidosis present at less than a year of age with abnormal facial features, an abnormal gait, diffuse neurological disease and dwarfism.
Mucopolysaccharidosis type I
Mucopolysaccharidosis type I (Hurler syndrome) is thought to be an autosomal recessive disease. It has been described in white domestic shorthair cats.11,12 The clinical and radiographic features include facial dysmorphism, corneal clouding and neurological abnormalities. Long bone epiphyseal dysplasia is not a feature of this syndrome and cats are of normal or larger than normal size. Affected cats excrete increased amounts of dermatan and heparin sulphate in the urine associated with a deficiency of alpha-L-iduronidase. Metachromatic granules are not present in the circulating neutrophils. Affected cats can survive comfortably for several years.
Mucopolysaccharidosis type VI
Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome) is an inherited autosomal recessive disease that occurs in Siamese and Siamese-cross cats. 13 Clinical signs develop at an early age, although affected animals may not be presented until they are young adults. Clinical features include broadening of the maxilla, corneal clouding, pectus excavatum and diffuse neurological abnormalities. These animals often have a crouching gait with abduction of the stifles, and pain and crepitus in several joints. The epiphyseal and metaphyseal areas of the long bones are enlarged and irregularly shaped. Neck manipulation is painful, and there is increased muscle tone in the limbs. Most cats have a chronic mucoid ocular discharge and chronic upper respiratory tract infection. Chronic diarrhoea occurs in some cases. Cats may suffer bilateral coxofemoral luxation at an early age.
The radiographic features include bilateral hip luxation/subluxation, coxa valga, fusion of the cervical vertebrae, flaring of the ribs at the costochondral junctions and irregular osseous proliferation of the ends of the long bones.
Diagnosis is confirmed by demonstration of urinary glycosaminoglycans, identification of excessive amounts of dermatan sulphate and confirmation of decreased arylsulphatase B activity. Urinary glycosaminoglycans can be detected with a urine spot test using toluidine blue stain or a commercial reagent. The examination of a blood smear, stained with Wright-Giemsa stain, will demonstrate coarse granular material in over 90% of neutrophils. The granules stain metachromatically blue with toluidine blue.

Mucopolysaccharidosis type VII
Mucopolysaccharidosis type VII (Sly syndrome), associated with a deficiency of β-glucuronidase, has been reported in two cats.14,15 Clinical findings included a small stature, a relatively large head, glossoptosis, peg-shaped and wide-spaced teeth, corneal clouding, hind leg paresis, dorsoventrally compressed rib cage, and limbs that were short and curved. Joints were lax, swollen and crepitant, with hip luxation and patellar luxation. Radiographic changes included platyspondyly (flattened vertebrae), caudal beaking of vertebrae and generalised epiphyseal dysplasia (Figure 3). There were radioucent areas within the femoral heads, irregular acetabula and bilateral coxofemoral luxation. Femoral diaphyses were widened. Peripheral lymphocytes and granulocytes contained cytoplasmic granules that stained metachromatically with toluidine blue. β-glucuronidase was deficient in cultured fibroblasts and chondroitin sulphates were excreted in excess in the urine (positive toluidine blue test).
Mannosidosis
Another form of lysosomal storage disease is typified by intralysosomal accumulation of mannose-rich oligosaccharide due to deficient activity of acidic α-mannosidase. This has been recognised in a litter of Persians 16 and a domestic shorthair cat. 17 Characteristics included dwarfism, limb deformity, ataxia and an intention tremor. Radiographs showed osteolytic lesions in the vertebrae and generalised osteopenia. Corneas were clear but suture line cataracts were present. The α-mannosidase activity of brain tissue of one cat tested was 4.8% of control values and the urine of two cats contained large amounts of mannose-rich oligosaccharides. 16
Mucolipidosis
Accumulation of lipid is the manifestation of mucolipidosis type II, which has been identified in a 7-month-old female domestic shorthair cat. 18 Characteristics included abnormal facial features, retarded growth, progressive hind limb paresis and thickened skin. Radiography revealed a severely deformed spinal column-fused vertebra, bilateral hip luxation and dysplasia, an abnormal shaped skull and decreased bone opacity. Toluidine blue test on urine was negative for glycosaminoglycans. Further biochemical tests revealed deficiency of the enzyme N-acetylglucosamine-1-phosphotransferase in peripheral leukocytes and an elevation of many lysosomal enzymes in the serum, which is diagnostic of mucolipidosis II.
Scottish Fold osteochondrodysplasia.
The Scottish Fold breed (Figure 4) originated in Scotland by crossing naturally occurring spontaneously mutated farm cats with folded ears with British and American Shorthair cats. The breed is not recognised by the Governing Council of the Cat Fancy in the UK and its deliberate breeding is discouraged or banned. There is a strong association with both ear mites and deafness. An autosomal incomplete dominant pattern of inheritance has been demonstrated for Scottish Fold cats. 20
Figure 4.

The characteristic folded ears of the Scottish Fold cat. Courtesy of B Peirone
In addition to the folded ears, autosomal dominant individuals in the breed can suffer from skeletal deformities or osteodystrophy or osteochondrodysplasia. These individuals have a short thick inflexible tail, which usually precedes other problems. Breeders use the presence or absence of the short thickened inflexible tail to determine if the cats are affected with osteodystrophy, but this is not a completely reliable indicator as it is not always present.20,21 Signs of skeletal disease, in addition to the short thick inflexible tail, include lameness, reluctance to jump, a stiff stilted gait, short misshapen distal limbs, and swelling of plantar tarsometatarsal regions.
Radiographic changes include exostosis and secondary arthritis around affected distal joints including the tarsus, metatarsus and carpus, and defective conformation of the phalanges and caudal vertebrae (Figure 5). 22 The pathogenesis is related to disordered endochondral ossification in the epiphysis. Treatment with oral chondroprotective agents such as glucosamine and chondroitin sulphate, 22 pentosan polysulphate, 23 bilateral tarsal arthrodesis 21 and irradiation 24 have all been tried with some success in small numbers of cases.
Figure 5.

Radiograph of a tarsus of a Scottish Fold cat with prominent large exostoses on the plantar aspect. Most of the interphalangeal and metatarsophalangeal joints are deformed, with flattened beaked epiphyses. Courtesy of B Peirone
Appendicular dysostoses
Dysostoses are malformations of individual bones that occur either singularly or in combination. Normal limb development commences with a projection of mesoderm covered by ectoderm that has an inductive ectodermal ridge. Subsequently the individual bones form from a cartilage anlage. Three mesodermal rays contribute to pectoral limb formation, an ulnar, radial and central. Disturbances of one or more rays result in abnormal formation of the corresponding bones and soft tissue. Appendicular dysostoses in cats may be hereditary or occur sporadically due to a spontaneous mutation and they can cause lameness or loss of limb function. The terminology is described in the box on page 36. 10
There are numerous reports usually of single cats or litters affected by a variety of these dysostoses and further information can be found in several textbooks.10,25,26 Figure 6 shows radial hemimelia, which is one of the commonest feline dysostoses. 27 Treatment depends on the precise nature of the dysostosis and the limb function. Arthrodesis or corrective osteotomies have been performed with success in selected cases. The prognosis is good in mild cases, but severe deformities may cause pain or severe disability and amputation may be indicated.
Figure 6.
(a) Radiograph and (b) forelimb bones (after amputation) of a cat with radial hemimelia. The remaining ulna is thickened and bent in the craniocaudal plane. There is also carpal subluxation cranially
Terminology.
Metabolic bone disease
Hypertrophic osteopathy
Hypertrophic osteopathy (also known as pulmonary osteopathy, hypertrophic pulmonary osteoarthropathy and Marie’s disease) usually occurs as a secondary manifestation of space-occupying lesions of the thoracic or abdominal cavity. Primary neoplastic and, less commonly, inflammatory disease has been diagnosed in affected cats. Most affected animals will present with lameness prior to signs of the primary disease such as dyspnoea or coughing.
Hypertrophic osteopathy has been reported in several domestic cats,28–37 most of which have been middle-aged male cats; Persians or Persian crosses are overrepresented. The limbs, especially the metacarpus/metatarsus, were thickened in the majority of cases, and these same animals were lame, with pain reported in just over half of affected animals. The lameness in several cats was attributed to impingement of joints, especially the elbow. Signs were usually bilaterally symmetrical, with firm warm non-oedematous pulsatile swelling of all four limbs and taut skin over the limbs.
Radiographs show marked and profuse irregular lamellated periosteal new bone extending from distal to proximal along the limbs (Figure 7). Phalangeal involvement, which is common in other species, has not often been reported in cats. Laboratory investigations were largely unremarkable or non-specific.
Figure 7.

Radiograph of a cat with hypertrophic osteopathy. There is pallisading profuse periosteal new bone formation on the humerus, antebrachium, carpus and metacarpus in this cat. The primary cause was not identified
A variety of underlying conditions have been reported as the primary cause of hypertrophic osteopathy in cats.28–37 Most cats have had pulmonary neoplastic disease, most often affecting the caudal lung lobe. Benign or non-neoplastic disease has also been reported. Several cases had abdominal neoplasia without metastases. In some cases the underlying cause could not be identified.
Treatment of hypertrophic osteopathy is by resection of the pulmonary or other lesion. Removal of the primary lesion may allow regression of bony lesions (radiographic signs take 3–4 months to resolve). Lameness may resolve in weeks, with pain and swelling decreasing first. If surgery is not a possibility then analgesics can be given in the short term until euthanasia is indicated on humane grounds.
Osteosclerosis
Osteosclerosis (also referred to as osteopetrosis, osteosclerosis fragilis, chalk bones and marble bones) encompasses any abnormal hardening of bones. Sclerosing bone dysplasias, which are characterised by an increase in bone density, are an inherited heterogeneous subset of osteochondrodysplasias. There are several subdivisions of osteosclerosis in humans.
Osteopetrosis is a rare genetic disease identified by abnormally dense bone due to defective osteoclastic resorption of immature bone. The disease is characterised by fragile bones that easily fracture, mental retardation, blood dyscrasia and fetal death. One form of osteopetrosis is less severe and known as delayed, benign or adult-type osteopetrosis.
Most of the reports in cats appear to fit the classification of generalised osteosclerosis or the benign or adult form of osteopetrosis as they have concurrent acquired conditions, the bone is extremely hard and no neurological deficits are present. The osteosclerosis is often an incidental finding 38 in older cats with underlying systemic disease including lymphoblastic anaemia, systemic lupus erythematosus related illness, lymphoma, C-cell tumour, myeloproliferative disorders, chronic renal failure, 39 feline leukaemia virus (FeLV) infection, 40 fibrosarcoma, 41 or anaemia 42 (Figure 8). The bone sclerosis does not appear to cause lameness and, in fact, cats rarely present with signs attributable to the musculoskeletal system. Treatment and prognosis are related to the systemic disease, which is usually the reason the cat was presented to the veterinarian in the first instance.
Figure 8.

Osteosclerosis (increased bone density) of the femora and tibiae of an elderly cat
Ectopic mineralisation disorders
Cats can develop discrete or widespread deposits of bone or mineralisation in their soft tissues and periarticularly. The definitive diagnosis of these lesions is difficult and, hence, the classification is variable. The diseases identified include, or are variably known as, osteochondromas (single or multiple or synovial), myositis ossificans or fibrodysplasia ossificans progressiva.
Osteochondromas
Solitary osteochondromas or osteocartilaginous exostoses occur occasionally in the domestic cat. They are benign slow-growing tumours with a cartilaginous cap that gives rise to cancellous bone by the process of endochondral ossification. In contrast to the condition in dogs, these lesions are more commonly seen in mature cats, often around the elbow (Figure 9) and the presenting complaint is one of lameness. Treatment involves surgical excision but local regrowth and malignant transformation have been observed. 43 Burmese cats seem to be predisposed.44,45
Figure 9.
(a,b) Mineralised periarticular tissue on the caudomedial aspect of the elbow of a middle-aged Burmese cat, which was diagnosed as an osteochondroma
Feline osteochondromatosis
The lesions of multiple osteocartilaginous exostosis or feline osteochondromatosis resemble sarcoma rather than osteochondroma. They occur in young mature cats, and a viral aetiology has been implicated. In decreasing order of involvement, the lesions tend to arise from the rib, scapula (Figure 10), vertebra, skull and pelvis. The prognosis is guarded as the growth of the lesions is continuous. There is no known treatment. Surgery or radiation therapy may be used as a palliative measure in selected cases but this does not seem to alter the outcome. 46 Pool 45 suggests that these lesions are parosteal sarcomas. A test for FeLV is indicated in affected cats. The Siamese breed may be overrepresented.
Figure 10.

Scapular osteochondroma. Reproduced from Montavon PM, Voss K, Langley-Hobbs SJ (eds), Feline Orthopedic Surgery and Musculoskeletal Disease, with permission of Elsevier
Osteochondromas are most commonly seen in mature cats, often around the elbow. The presenting complaint is lameness.
Synovial osteochondromatosis
Ossified nodules located in joints (Figure 11) may represent synovial osteochondromatosis 47 or osteochondrometaplasia. Nodules arise from the articular cartilage and then become mineralised. The lesions may represent end-stage osteoarthritis.
Figure 11.

Multiple joint mice in a cat with degenerative joint disease and synovial osteochondromatosis of the elbow
Fibrodysplasia ossificans progressiva
Generalised
This condition has some features in common with fibrodysplasia ossificans progressiva in man in that it affects skeletal muscle and connective tissue. Typically, weakness, progressive stiffness, decreased limb movement, and muscle pain and enlargement are seen in young to middle-aged cats.48–51 Progression can often be rapid, occurring over weeks to months. 48 This is a disseminated disorder of epimysial connective tissue that is characterised by hyperplasia and ossification causing atrophy and displacement of skeletal muscles, entrapment of vessels and nerves, and progressive immobility. Calcified masses can be palpated in muscles. Radiographs reveal extensive soft tissue mineralisation. There is no effective treatment for the generalised form and the prognosis is poor. 50
Myositis ossificans
The localised form tends to occur in specific muscles such as the biceps femoris or semitendinosus bellies. Myositis ossificans may be related to local trauma or repetitive trauma. This form has a better prognosis than the generalised form in that the lesions stay localised. One cat has been reported as having a fibrotic myopathy of the semitendinosus muscles, 52 and the author has seen three cats with mineralisation affecting primarily the hamstring muscles (Figure 12). Recurrence of the lesion, however, is likely after excision.
Figure 12.
(a,b) Eight-year-old domestic shorthair cat that presented with pelvic limb lameness. There was an area of mineralisation of the gracilis muscle which progressed, as seen on these sequential radiographs taken 8 weeks apart
Nutritional bone disease
Hypervitaminosis A
Hypervitaminosis A is most commonly caused by ingestion of a diet comprised primarily of liver. It is characterised by extensive confluent exostoses of the cervicothoracic vertebrae and appendicular skeleton. 53 The cervical spine is most commonly affected but the forelimbs and, in particular, the elbow joints can also be involved. Vitamin A causes increased sensitivity of the periosteum to trauma. The cervicothoracic region is especially sensitive because of the grooming activity of cats (Figure 13).
Figure 13.

Radiograph (a) and skeleton (b) of a 15-year-old female domestic shorthair cat that presented with urinary incontinence secondary to nerve root compression by the exostoses from hypervitaminosis A. The cervical spine is fused, there is enlargement of the first two ribs and fusion of the sternebrae by confluent exostoses. Reproduced from Montavon PM, Voss K, Langley-Hobbs SJ (eds), Feline Orthopedic Surgery and Musculoskeletal Disease, with permission of Elsevier
Affected animals have ranged in age from 2–9 years and may present with lameness as one of the primary clinical signs. Lameness can be due to bone encroachment on nerve roots or periarticular new bone formation. 54 General signs consist of malaise, anorexia, lethargy, irritability, exophthalmos and a scurfy dull coat. With encroachment of new bone on intervertebral foramina signs can also include those associated with neural compression such as ataxia, paralysis and urinary problems. 54
By removing liver from the diet, progression of the lesions ceases and clinical signs can improve in some cases. 54 However, there is little significant regression of the radiographic signs.
Nutritional secondary hyperparathyroidism
Nutritional secondary hyperparathyroidism is very rarely reported in domestic cats as they are mostly fed balanced commercial diets. However, occasional cases arise due to dietary imbalances in calcium and phosphorus from feeding an all-meat diet or from owners inflicting their dietary preferences on their pet; most reports relate to large cats kept in zoos or safari parks but there are occasional reports of affected domesticated cats.55,56
Affected cats usually present with signs relating to either the hypocalcaemia (seizures and muscle tremors) or the osteopenia (pathological fractures, stiffness and lameness). 55 Radiographs reveal generalised osteopenia, cortical thinning, pathological fractures and lordosis. Pathological fractures most commonly involve cancellous bone where bone turnover is highest, so the spine, pelvis and metaphyseal areas of bone are primarily affected. Biochemical changes are common (Table 2).
Table 2.
| Parameter | Affected cat mean value (range) | Reference interval (4–6-month-old cats) |
|---|---|---|
| Calcium (mmol/l) | 1.97 (1.63–2.44) L | 2.48–2.71 |
| Phosphate (mmol/l) | 2.34 (1.72–3.4) L/N | 2.71–3.21 |
| Alkaline phosphatase (IU/l) | 211 (101–333) H | 92–137 |
| Parathyroid hormone (pg/ml) | 158 (37–271) H | <3–28 |
| 1,25-vitamin D3 (pmol/l) | 445 (263–655) N/H | 192–317 |
| 25-vitamin D3 (nmol/l) | 65 (44–78) L | 126–163 |
L = low, N = normal, H = high
The diets are usually low in calcium and phosphorus, or the ratio between the two minerals is imbalanced. Treatment of the hypocalcaemia and introduction of a normal diet should resolve the condition; success with this treatment regimen provides confirmation of the suspected diagnosis. Cases with neurological signs due to spinal fractures have a guarded prognosis for recovery.55,56
Osteomyelitis
Bone infections can occur secondarily to external trauma (bite wound), after fracture fixation (iatrogenic), as an extension from a local lesion (dental disease) or from systemic spread. 46 Aerobic and anaerobic bacteria, and occasionally fungal organisms, have been isolated. Histopathological evaluation can be useful to differentiate infection from neoplasia. Generally, the author’s impression is that osteomyelitic lesions in cats have less periosteal new bone formation and more pronounced erosive lesions than seen in dogs (Figure 14).
Figure 14.
(a,b) Lytic lesion in the lateral femoral condyle with some adjacent mild periosteal reaction. This was diagnosed as osteomyelitis, and was suspected to be secondary to a cat bite. A good response was seen to antimicrobial therapy
The author’s impression is that osteomyelitic lesions in cats have less periosteal new bone formation and more pronounced erosive lesions than seen in dogs.
Synovial cysts
Elderly cats, with a mean age of 14 years (range 10–16.5 years), will occasionally develop synovial cysts.57–61 Affected cats present with large fluid-filled structures emanating from joints (Figure 15) that show evidence of degenerative joint disease on radiography. The elbow joint is most commonly affected,57,58 but digital cysts have also been reported. 61 The cause of the cysts is unknown; it has been theorised that they are herniations of synovial membrane through the joint capsule related to inflammation and osteoarthritis.
Figure 15.

Large fluid-filled mass diagnosed as an elbow synovial cyst. The cat was elderly and there was concurrent mild elbow degenerative joint disease. The cyst was so large that the skin was ulcerated on the medial aspect. The S-shaped scar is from a previous biopsy
Diagnosis is assisted by radiography, synoviocentesis, ultrasonography and arthrography (see box, right). Computed tomography or magnetic resonance imaging can also be used, although advanced imaging is probably unnecessary.59,60
Temporary alleviation can be achieved by drainage or surgical excision of the cyst, but the condition is likely to recur.57,58 The degenerative joint disease should also be managed. 59
Is this a synovial cyst?
Common findings in cats
Signalment Age >10 years
Physical examination Fluid-filled swelling around a joint (elbow)
Radiography Evidence of degenerative joint disease
Fluid analysis Large volume of viscous clear/yellow/blood-tinged fluid with a low cell count (<3 x 109/l)
Ultrasonography Multiple anechoic spaces
Arthrography Fluid-filled spaces communicating with or adjacent to joint
Histopathology Examination of synovial membrane
Pad problems.
Plasma cell pododermatitis
Plasma cell pododermatitis can cause lameness in cats.62–64 Affected animals have enlarged food pads (most often metacarpal or metatarsal), and the pads have a soft spongy consistency and often a mauve or violet colouration (Figure 16). Ulceration may occur due to the softness of the pad. 62 Haemorrhage from the pad may result in or contribute to anaemia in chronic cases. 63 This is suspected to be an immune-mediated or allergic disease. In some animals there is a seasonal occurrence supporting the allergy supposition. Histologically, the dermis and subcutis are characterised by infiltration of plasma cells. 62 Long-term glucocorticoid therapy is usually effective. 62 Surgical excision may also be effective. 62
Figure 16.

Feet of a Siamese cat that presented with lameness attributable to plasma cell pododermatitis. (a) The right metacarpal pad is unaffected but all the other visible thoracic limb pads are swollen, soft mauve and cracked to a certain degree. (b) The left metatarsal pad is markedly swollen, mauve in colour and soft
Metastatic mineralisation
Mineralisation of pads and the interdigital spaces can be a reason for lameness and veterinary consultation (Figure 17). 65 Most cats have laboratory findings suggestive of renal failure and an imbalance in the calcium/phosphorus ratio. Cytological examination of paw lesions is suggestive of calcinosis. A metastatic pathogenesis is suspected, with a recognised correlation between paw mineralisation and renal failure. Therapy consists of feeding a protein- and phosphorus-restricted diet and prescribing intestinal phosphate-binding agents. Some improvement in paw calcification has been seen in response to such therapy.65,66
Figure 17.

Metastatic calcinosis of the foot in a cat with renal failure. Courtesy of J Declercq
Differential diagnoses for pad problems in cats
Pyogranulomatous lesions (bacterial, fungal, calicivirus, herpesvirus, pox virus)
Metaplastic calcification
Plasma cell pododermatitis
Neoplasia
Foreign body reaction
Key points
Hereditary rickets Consider in young kittens presenting with one or a combination of lameness, stunted growth or trembling or seizures due to hypocalcaemia. Investigations should include radiography and blood work (calcium, phosphate, vitamin D).
Nutritional secondary hyperparathyroidism Seen in kittens presenting with fractures, deformity and lameness. Check diet and measure blood calcium and parathyroid hormone levels.
Hypertrophic osteopathy Massive amounts of symmetrical new bone formation are seen in older cats extending along the limbs from distal to proximal. Search for an underlying thoracic or abdominal cause.
Ectopic mineralisation disorders Cats are prone to various ectopic mineralisation disorders; single lesions tend to have a better prognosis than multiple lesions but biopsies are necessary to rule out neoplasia.
Osteosclerosis Look for an underlying systemic cause.
Hypervitaminosis A Fused cervical vertebrae are seen on radiographs. Check the diet.
Osteogenesis imperfecta Young cats present with multiple fractures and a minimal history of trauma. Bone density may be poor or may appear normal on radiographs.
Lysosomal storage disease Cats have one or a combination of facial changes and neurological disorders, particularly cerebellar signs such as intention tremors and ataxia. Investigations should include radiography, urinalysis, blood work and specialised enzyme assays.
Scottish Fold osteochondrodysplasia Cartilage abnormalities give rise to folded ears and fused deformed joints, particularly the hocks.
Osteomyelitis Often associated with surprisingly little periosteal new bone formation in cats, and mainly lytic.
-
Pad problems causing lameness:
Mineralised pads – check for renal disease or other causes of hypercalcaemia;
Spongy pads – consider plasma cell pododermatitis. Confirm by biopsy.
Funding
The author received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this review article.
Conflict of interest
The author declares that there is no conflict of interest.
References
- 1. Henik RA, Forrest LJ, Friedman AL. Rickets caused by excessive renal phosphate loss and apparent abnormal vitamin D metabolism in a cat. J Am Vet Med Assoc 1999; 215: 1644–49. [PubMed] [Google Scholar]
- 2. Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009; 23: 196–99. [DOI] [PubMed] [Google Scholar]
- 3. Schreiner CA, Nagode LA. Vitamin D-dependent rickets type 2 in a four-month-old cat. J Am Vet Med Assoc 2003; 222: 337–39. [DOI] [PubMed] [Google Scholar]
- 4. Tanner E, Langley-Hobbs SJ. Vitamin-D dependent rickets type 2 with characteristic radiographic changes in a 4-month-old kitten. J Feline Med Surg 2005; 7:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godfrey DR, Anderson RM, Barber PJ, Hewison M. Vitamin D-dependent rickets type II in a cat. J Small Anim Pract 2005; 46:440–44. [DOI] [PubMed] [Google Scholar]
- 6. Phillips AM, Fawcett AC, Allan GS, Wilkinson M, Fraser DR, Malik R. Vitamin D dependent non-type 1, non-type 2 rickets in a 3-month-old Cornish Rex kitten. J Feline Med Surg 2011; 13:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gershoff SN, Legg MA, O’Connor FJ, Hegsted DM. The effect of vitamin D-deficient diets containing various Ca:P ratios on cats. J Nutr 1957; 63:79–93. [DOI] [PubMed] [Google Scholar]
- 8. Cohn LA, Meuten DJ. Bone fragility in a kitten: an osteogenesis imperfecta-like syndrome. J Am Vet Med Assoc 1990: 197:98–100. [PubMed] [Google Scholar]
- 9. Evason MD, Taylor SM, Bebchuk TN. Suspect osteogenesis imperfecta in a male kitten. Can Vet J 2007; 48:296–98. [PMC free article] [PubMed] [Google Scholar]
- 10. Jezyk PF. Constitutional disorders of the skeleton in dogs and cats. In: Newton CD, Nunamaker DM, eds. Textbook of small animal orthopaedics. Philadelphia; JB Lippincott, 1985: 637–54. [Google Scholar]
- 11. Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Alpha-L-iduronidase deficiency in a cat: a model of mucopolysaccharidosis I. Pediatr Res 1979, 13:1294–97. [DOI] [PubMed] [Google Scholar]
- 12. Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis type I. Am J Pathol 1983; 112:27–36. [PMC free article] [PubMed] [Google Scholar]
- 13. Jezyk PF, Haskins ME, Patterson DF, Mellman WJ, Greenstein M. Mucopolysaccharidosis in a cat with arylsulphatase B deficiency: a model of Maroteaux-Lamy syndrome. Science 1977; 198:834–36. [DOI] [PubMed] [Google Scholar]
- 14. Gitzelmann R, Bosshard NU, Superti-Furga A, et al. Feline mucopolysaccharidosis VII due to β-glucuronidase deficiency. Vet Pathol 1994; 31:435–43. [DOI] [PubMed] [Google Scholar]
- 15. Schultheiss PC, Gardner SA, Owens JM, Wenger DA, Thrall MA. Mucopolysaccharidosis VII in a cat. Vet Pathol 2000; 37: 502–5. [DOI] [PubMed] [Google Scholar]
- 16. Maenhout T, Kint JA, Dacremon G, Ducatelle R, Leroy JG, Hoorens JK. Mannosidosis in a litter of Persian cats. Vet Rec 1988; 122:351–54. [DOI] [PubMed] [Google Scholar]
- 17. Blakemore WF. A case of mannosidosis in the cat: clinical and histopathological findings. J Small Anim Pract 1986; 27: 447–55. [Google Scholar]
- 18. Hubler M, Haskins ME, Arnold S, et al. Mucolipidosis type II in a domestic shorthaired cat. J Small Anim Pract 1996; 37: 435–41. [DOI] [PubMed] [Google Scholar]
- 19. Gasper PW, Thrall MA, Wenger DA, et al. Correction of feline arylsulphatase B deficiency (mucopolysaccharidosis VI) by bone marrow transplantation. Nature 1984; 312:467–69. [DOI] [PubMed] [Google Scholar]
- 20. Takanosu M, Takanosu T, Suzuki H, Suzuki K. Incomplete dominant osteochondrodysplasia in heterozygous Scottish Fold cats. J Small Anim Pract 2008; 49:197–99. [DOI] [PubMed] [Google Scholar]
- 21. Mathews KG, Koblik PD, Knoeckel MJ, et al. Resolution of lameness associated with Scottish fold osteodystrophy following bilateral ostectomies and pantarsal arthrodeses: a case report. J Am Anim Hosp Assoc 1995; 31:280–88. [DOI] [PubMed] [Google Scholar]
- 22. Chang J, Jung J, Oh S, et al. Osteochondrodysplasia in three Scottish Fold cats. J Vet Sci 2007; 8:307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malik R, Allan GS, Howlett CR, et al. Osteochondrodysplasia in Scottish Fold cats. Aust Vet J 1999; 77:85–92. [DOI] [PubMed] [Google Scholar]
- 24. Hubler M, Volkert M, Kaser-Hotz B, Arnold S. Palliative irradiation of Scottish Fold osteochondrodysplasia. Vet Radiol Ultrasound 2004; 45:582–85. [DOI] [PubMed] [Google Scholar]
- 25. Langley-Hobbs SJ. Disturbances of growth and bone development. In: Houlton JEF, Cook JL, Innes JF, Langley-Hobbs SJ, eds. BSAVA manual of canine and feline musculoskeletal disorders. Gloucester: BSAVA Publications, 2006: 50–66. [Google Scholar]
- 26. Hubler M, Arnold S, Langley-Hobbs SJ. Hereditary and congenital musculoskeletal diseases. In: Montavon PM, Voss K, Langley-Hobbs SJ, eds. Feline orthopedic surgery and musculoskeletal disease. Edinburgh: Saunders Elsevier, 2009: 41–53. [Google Scholar]
- 27. Winterbotham EJ, Johnson KA, Francis DJ. Radial agenesis in a cat. J Small Anim Pract 1985; 26:393–98. [Google Scholar]
- 28. Carr SH. Secondary hypertrophic pulmonary osteoathropathy in a cat. Feline Pract 1971; 1:25–26. [Google Scholar]
- 29. Richards CD. Hypertrophic osteoarthropathy in a cat. Feline Pract 1977; 7: 41–43. [Google Scholar]
- 30. Roberg J. Hypertrophic pulmonary osteoarthropathy. Feline Pract 1977; 7: 18–22. [Google Scholar]
- 31. Nafe LA, Herron AJ, Burk RL. Hypertrophic osteopathy in a cat associated with renal papillary adenoma. J Am Anim Hosp Assoc 1981; 17:659–62. [Google Scholar]
- 32. Gram WD, Wheaton LG, Snyder PW, Losonsky JM, Whitely HE. Feline hypertrophic osteopathy associated with pulmonary carcinoma. J Am Anim Hosp Assoc 1990; 26:425–28. [Google Scholar]
- 33. Becker TJ, Perry RL, Watson GL. Regression of hypertrophic osteopathy in a cat after surgical excision of an adrenocortical carcinoma. J Am Anim Hosp Assoc 1999; 35:499–505. [DOI] [PubMed] [Google Scholar]
- 34. Grierson JM, Burton CA, Brearley MJ. Hypertrophic osteopathy secondary to pulmonary sarcoma in a cat. Vet Comp Oncol 2003; 1:227–31. [DOI] [PubMed] [Google Scholar]
- 35. Rohr S. Pulmonary origin feline hypertrophic osteopathy. Point Vétérinaire 2003; 35:62–64. [Google Scholar]
- 36. de Melo Ocarino N, Fukushima FB, de Matos Gomes A, Bueno DF, de Oliveira TS, Serakides R. Idiopathic hypertrophic osteopathy in a cat. J Feline Med Surg 2006; 8:345–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mills J. Hypertrophic osteopathy and megaoesophagus in a cat. Vet Comp Orthop Traumatol 2010; 23:218–22. [DOI] [PubMed] [Google Scholar]
- 38. Fujita M, Takaishi Y, Nagae H, et al. Osteopetrosis-like disease in a cat with respiratory distress. J Vet Med Sci 2007; 69: 687–90. [DOI] [PubMed] [Google Scholar]
- 39. Wright MW, Hudson JA, Hathcock JT. Osteopetrosis in cats: clarification of a common misnomer. Abstracts ACVR 2002. Chicago, Illinois. Vet Radiol Ultrasound 2003; 44:106. [Google Scholar]
- 40. Hoover EA, Kociba GJ. Bone lesions in cats with anemia induced by feline leukaemia virus. J Natl Cancer Inst 1974; 53:1277–84. [DOI] [PubMed] [Google Scholar]
- 41. Hanel RM, Graham JP, Levy JK, Buergelt CD, Creamer J. Generalised osteosclerosis in a cat. Vet Radiol Ultrasound 2004; 45:318–24 [DOI] [PubMed] [Google Scholar]
- 42. Kramers P, Flückinger MA, Rahn BA, Cordey J. Osteopetrosis in cats. J Small Animal Pract 1988; 29:153–64. [Google Scholar]
- 43. Langley-Hobbs SJ, Harley R. Humeral osteochondromas in cats. Proceedings of the ECVS Congress. Velbert, Germany, 2001: 307–9. [Google Scholar]
- 44. Hubler M, Johnson KA, Brurling RT, Francis DF, Ratcliffe RC. Lesions resembling osteochondromatosis in two cats. J Small Anim Pract 1986; 27:181–87. [Google Scholar]
- 45. Pool RR. Osteochondromatosis. In: Bojrab MJ, ed. Pathophysiology in small animal surgery. 2nd edn. Philadelphia: Lea & Febiger, 1993: 821–28. [Google Scholar]
- 46. Voss K. Diseases of bone. In: Montavon PM, Voss K, Langley-Hobbs SJ. Feline orthopedic surgery and musculoskeletal disease. Edinburgh: Saunders Elsevier, 2009: 55–62. [Google Scholar]
- 47. Voss K, Langley-Hobbs SJ. Diseases of joints. In: Montavon PM, Voss K, Langley-Hobbs SJ, eds. Feline orthopedic surgery and musculoskeletal disease. Edinburgh: Saunders Elsevier; 2009: 63–74. [Google Scholar]
- 48. Valentine BA, George C, Randolph JF, Center SA, Fuhrer L, Beck KA. Fibrodysplasia ossificans progressiva in the cat. A case report. J Vet Intern Med 1992; 6:335–40. [DOI] [PubMed] [Google Scholar]
- 49. Warren HB, Carpenter JL. Fibrodysplasia ossificans in three cats. Vet Pathol 1982; 21:495–99. [DOI] [PubMed] [Google Scholar]
- 50. Norris AM, Pallett L, Wilcock B. Generalised myositis ossificans in a cat. J Am Anim Hosp Assoc 1980; 16:659–63. [Google Scholar]
- 51. Yabuzoe A, Yokoi S, Sekiguchi M, et al. Fibrodysplasia ossificans progressiva in a Maine Coon cat with prominent ossification in dorsal muscle. J Vet Med Sci 2009; 71:1649–52. [DOI] [PubMed] [Google Scholar]
- 52. Lewis DD. Fibrotic myopathy of the semitendinosus muscle in the cat. J Am Vet Med Assoc 1988; 193:240–41. [PubMed] [Google Scholar]
- 53. Seawright AA, English PB, Gartner RJW. Hypervitaminosis A of the cat. Adv Vet Sci Comp Med 1970; 14:1–27. [PubMed] [Google Scholar]
- 54. Polizopoulou ZS, Kazakos G, Patsikas MN, Roubles N. Hypervitaminosis A in the cat: a case report and review of the literature. J Feline Med Surg 2005; 7:363–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomsa K, Glaus T, Hauser B, et al. Nutritional secondary hyperparathyroidism in six cats. J Small Anim Pract 1999; 40: 533–39. [DOI] [PubMed] [Google Scholar]
- 56. Dimopoulou M, Kipensteijn J, Nielsen DH, Bueland L, Hansen MS. Nutritional secondary hyperparathyroidism in two cats: evaluation of bone mineral density with dual-energy X-ray absorptiometry and computed tomography. Vet Comp Orthop Traumatol 2010; 23:56–61. [DOI] [PubMed] [Google Scholar]
- 57. Stead AC, Else RW, Stead MCP. Synovial cysts in cats. J Small Anim Pract 1995; 36:450–54. [DOI] [PubMed] [Google Scholar]
- 58. Prymak C, Goldschmidt MH. Synovial cysts in five dogs and one cat. J Am Anim Hosp Assoc 1991; 27:151–54. [Google Scholar]
- 59. White JD, Martin P, Hudson D, Clark A, Malik R. What is your diagnosis? J Feline Med Surg 2004; 6:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kligman KC, Kim SE, Winter MD, et al. What is your diagnosis? J Am Vet Med Assoc 2009; 235:9445–46. [DOI] [PubMed] [Google Scholar]
- 61. Hittmair KM, Maedl I, Reifinger M, Mayrhofer E. Synovial cyst of the fifth digit in a cat. J Feline Med Surg 2010; 12: 175–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dias Pereira P, Faustino AM. Feline plasma cell pododermatitis: a study of 8 cases. Vet Dermatol 2003; 14:333–37. [DOI] [PubMed] [Google Scholar]
- 63. Taylor JE, Schmeitzel LP. Plasma cell podermatitis with chronic footpad hemorrhage in two cats. J Am Vet Med Assoc 1990; 197:375–77. [PubMed] [Google Scholar]
- 64. Drolet R, Bernard J. Plasma cell pododermatitis in a cat. Can Vet J 1984; 12:448–49. [PMC free article] [PubMed] [Google Scholar]
- 65. Bertazzolo W, Toscani L, Calcaterra S, Crippa L, Caniatti M, Bonfanti U. Clinicopathological findings in five cats with paw calcification. J Feline Med Surg 2003; 5:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jackson HA, Barber PJ. Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. J Small Anim Pract 1998; 39: 495–97. [DOI] [PubMed] [Google Scholar]