Abstract
Overview:
In cats, the most serious of adverse effects following vaccination is the occurrence of invasive sarcomas (mostly fibrosarcomas): so-called ‘feline injection-site sarcomas’ (FISSs). These develop at sites of previous vaccination or injection. They have characteristics that are distinct from those of fibrosarcomas in other areas and behave more aggressively. The rate of metastasis ranges from 10–28%.
Pathogenesis:
The pathogenesis of these sarcomas is not yet definitively explained. However, chronic inflammatory reactions are considered the trigger for subsequent malignant transformation. Injections of long-acting drugs (such as glucocorticoids, and others) have been associated with sarcoma formation. Adjuvanted vaccines induce intense local inflammation and seem therefore to be particularly linked to the development of FISS. The risk is lower for modified-live and recombinant vaccines, but no vaccine is risk-free.
Treatment and prevention:
Aggressive, radical excision is required to avoid tumour recurrence. The prognosis improves if additional radiotherapy and/or immunotherapy (such as recombinant feline IL-2) are used. For prevention, administration of any irritating substance should be avoided. Vaccination should be performed as often as necessary, but as infrequently as possible. Non-adjuvanted, modified-live or recombinant vaccines should be selected in preference to adjuvanted vaccines. Injections should be given at sites at which surgery would likely lead to a complete cure; the interscapular region should generally be avoided. Post-vaccination monitoring should be performed.
Introduction
Recently, vaccination of cats has received scientific and public attention linked to the supposition that a range of rare adverse effects can arise following vaccination. In cats, the most serious of these adverse consequences is the occurrence of invasive sarcomas (mostly fibrosarcomas), so-called ‘feline injection-site sarcomas’ (FISSs), that can develop within the skin at sites of previous vaccination. Despite extensive research on the pathogenesis of these sarcomas, there is no definitive causal relationship that explains their occurrence and the direct link to vaccination. The most accepted hypothesis suggests that a chronic inflammatory reaction at the site of injection provides a trigger for subsequent malignant transformation.
Epidemiology and characterisation
In 1991, an increased incidence of tumours in cats that developed at injection sites was first reported in the United States. 1 This observation was connected to an increased use of rabies and feline leukaemia virus (FeLV) vaccinations.2,3 As a consequence, these tumours were first called feline ‘vaccine-associated sarcomas’. However, the subsequent finding that other, non-vaccinal injectables can also cause this type of tumour has led to reclassification of these neoplasms as ‘feline injection-site sarcomas’ (FISSs). These tumours seem to be unique to cats, 4 although comparable tumours have been reported in ferrets 5 and very occasionally in dogs. 6
FISSs occur at sites typically used for vaccination and injections, such as the interscapular region (Figure 1), the lateral thoracic or abdominal wall, the lumbar region, and the area of the semimembranosus and semitendinosus muscles. FISSs are most commonly located in the subcutis, but also can occur intramuscularly.7,8
Figure 1.
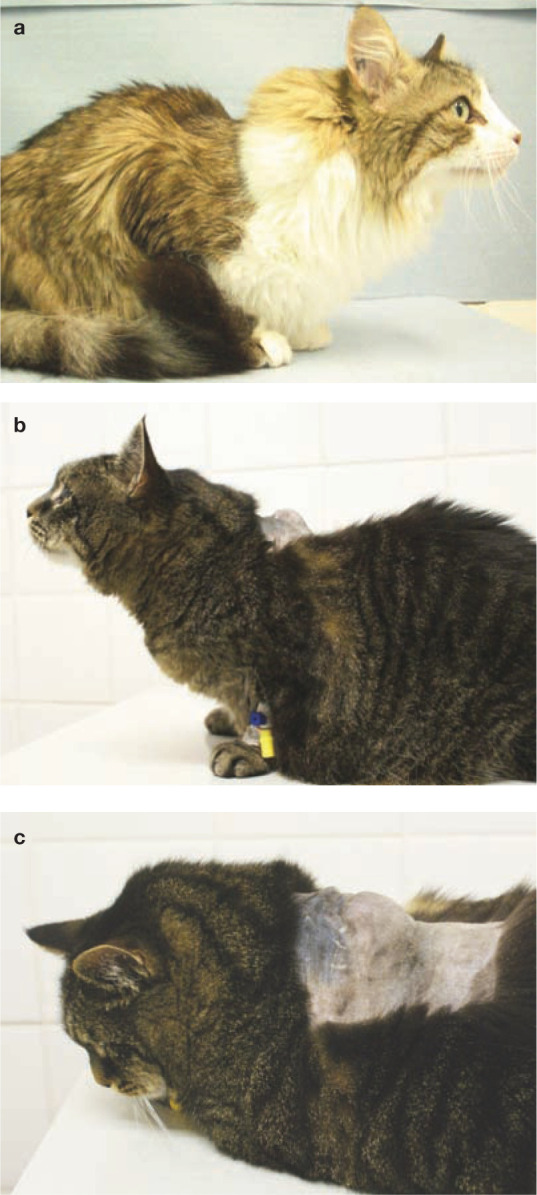
(a–c) Cats with feline injection-site sarcoma. Courtesy of Johannes Hirschberger, Ludwig Maximilians University, Munich, Germany
FISSs can occur as early as 4 months and up to 3 years after an injection. They are characterised by invasive local growth in the subcutis, often with spread along fascial planes. 9 Most FISSs are fibrosarcomas, 10 but other malignancies, such as osteosarcomas, 11 chondrosarcomas, 7 rhabdomyosarcomas, 7 malignant fibrous histiocytomas,7,11 and myofibroblastic sarcomas 8 have also been described.
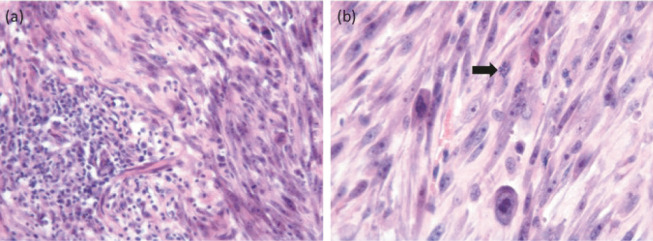
FISSs have histological characteristics that are distinct from those of fibrosarcomas in other areas. Typically there is perivascular infiltration of lymphocytes and macrophages at the tumour periphery, a central area of necrosis, inflammation and local infiltration of tumour cells (Figure 2).10,12 FISSs behave more aggressively than sarcomas at other sites. 13 The rate of metastasis ranges from 10–28%.14,15 The lung is the most common site of metastasis, followed by regional lymph nodes and abdominal organs, such as the kidney, spleen, intestine and liver.16,17
Figure 2.
Histological sections of a 2 cm diameter mass removed from the lateral thorax of a 13-year-old domestic shorthair cat. A similar interscapular mass had been removed from this cat 2 months previously. (a) A focus of lymphoplasmacytic inflammation is contained within the surrounding sarcoma. (b) Higher magnification of the neoplastic tissue reveals a pleomorphic population of neoplastic spindle cells with occasional giant nuclei and irregular mitotic activity (arrow). Haematoxylin and eosin stain. Courtesy of Michael Day, School of Veterinary Sciences, University of Bristol, UK
In the past 20 years, an epidemiological association has been demonstrated between vaccination and the later development of FISS.3,13,18 –21 The incidence of FISS has been estimated at 1–4 in every 10,000 vaccinated cats in the USA,22,23 and the ratio of injection-site to non-injection-site sarcomas increased from 0.5 in 1989 to 4.3 in 1994. 10 In one study in the USA, reported rates of reaction were 0.3 FISSs per 10,000 vaccinations and 11.8 postvaccinal inflammatory reactions per 10,000 vaccinations in cats. 22 If inflammatory reactions are a necessary prelude to FISS, then these rates suggest that 1 in 35–40 inflammatory reactions develop into FISS. In the UK, the incidence of FISSs seems to be relatively low (incidence risk of FISS per year was estimated to be 1 per 16,000–50,000 cats registered by practices, 1 per 10,000–20,000 cat consultations, and 1 per 5000–12,500 vaccination visits). 24 One reason for the low rate might be that rabies vaccination is not a routine procedure for cats in the UK. One study in Canada investigated the annual prevalence of feline postvaccinal sarcomas among 11,609 feline skin mass submissions from 1992 to 2010 and revealed no decrease in disease prevalence or increase in age of affected cats in response to change in vaccination formulation or recommended changes in feline vaccination protocols. 25
Pathogenesis
Despite extensive research, there is no definitive proof of the pathogenesis of FISS. The most widely accepted hypothesis suggests that a chronic inflammatory reaction at the site of an injection acts as a trigger for subsequent malignant transformation. Adjuvanted vaccines seem to be particularly linked to the development of FISS due to the more intense local inflammation associated with such products. This idea is supported by frequent identification of adjuvants in histological or ultrastructural investigations of these sarcomas.12,18
Many data suggest an association between vaccination and FISS in cats. Aluminium, a vaccine adjuvant, has been found in biopsy samples of FISS. 26 In most inactivated vaccines, an adjuvant is added to enhance the inflammation at the site of injection, which is intended and necessary when applying a killed agent in order to trigger the necessary immune response. However, this inflammation might potentially lead to malignant transformation. Traces of adjuvants can be seen in the inflammatory reaction, specifically accumulated within macrophages or multinucleate giant cells, and later in histological sections of FISS in the transformed fibroblast. 18 Intracellular crystalline particulate material was found in an ultrastructural study in 5 of 20 FISSs investigated, and in one of the five cases was identified as aluminium-based. 12 Although no specific vaccine or adjuvant has been incriminated, 27 local irritation from adjuvant is thought to stimulate mainly fibroblasts to the point that malignant transformation occurs.
At first, only rabies and FeLV vaccines were identified as risk factors,3,13,23 but subsequently other vaccines, including vaccines against feline panleukopenia virus (FPV), feline herpesvirus-1 (FHV-1) and feline calicivirus (FCV), were also found to be involved in the development of FISS in some cases.13,23,28 –30 In addition to vaccines, injections such as long-acting glucocorticoids, penicillin, lufenuron,27,31,32 cisplatin 33 and meloxicam 34 have been associated with sarcoma formation. One study found that the frequency of administration of long-acting glucocorticoid injections (dexamethasone, methylprednisolone and triamcinolone) was significantly higher in cats with FISS in the interscapular region than in control cats. 35 Fibrosarcomas were also reported at the site of a deep, non-absorbable suture in one cat; 36 around a surgical swab in the abdomen of one cat; 37 adjacent to the site of microchip implantation in two cats;38,39 and associated with a subcutaneous fluid port device.38,39 This suggests that all inflammatory reactions, theoretically, have the potential to lead to the development of FISS by triggering uncontrolled proliferation of fibroblasts and myofibroblasts, which, in some cases, results in malignant transformation.
Although many causes of inflammation are associated with FISS development, the risk seems to be higher for vaccines compared with other injections; among vaccines, the risk seems to be higher when adjuvanted vaccines are used. Srivastav et al 35 compared associations between vaccine types and other injectable drugs with the development of FISS in a case-control study of 181 cats with soft tissue sarcomas (cases), 96 cats with tumours at non-vaccine regions (control group 1), and 159 cats with basal cell tumours (control group 2). There was a significant association between the administration of various types of vaccines and other injectable products (eg, long-acting corticosteroids) and FISS development. Of 192 cats with sarcoma, 101 had vaccinations at the site of tumour development during the preceding 3 years, and 23 had received other injections. 35 This study also showed that adjuvanted inactivated vaccines were significantly more commonly associated with FISS development than other vaccines; of 35 vaccinated cats with sarcoma on the hindlimb, 25 cats had received adjuvanted vaccines, seven cats had received modified-live virus (MLV) vaccines (FPV, FHV-1 and FCV), and only one cat had received a recombinant vaccine. These findings also indicated that no vaccines are risk-free. 35

The mechanism by which the inflammatory reaction causes tumour formation is not fully understood. Growth factors promote proliferation, can induce malignant transformation, and also can be involved in the regulation of angiogenesis. Overexpression of growth factors and oncogene activation have been demonstrated in cats with FISS and are suspected to play a role in tumour development.40 –42
As vaccination against FeLV is associated with a higher risk of FISS, some studies looked at a possible role of FeLV and its mutant feline sarcoma virus (FeSV) in the development of FISS, but could not detect either FeLV or FeSV in the tumours. 43 Furthermore, no other viruses, including feline immunodeficiency virus, feline foamy virus, polyomaviruses or papillomaviruses were detected in tumour tissues.44 –47 No evidence has been found to implicate replication or expression of endogenous retroviruses in FISS formation.45,46
The observation that not all cats develop FISS after vaccination suggests that there might be a genetic predisposition. It has been suggested that there is a higher incidence of FISS in siblings of affected cats, and that some cats tend to develop more than one FISS. Alterations with unknown relevance such as hyperploidy, 48 translocations 49 and triploidy 50 of oncogene and tumour suppressor loci have been found on extra chromosomes and monosomic chromosomes in affected cats. Mutations have been identified in the tumour suppressor gene p53, which is implicated in cancer initiation and progression in sarcoma tissue of cats with FISS.51 –55 A case-control study (50 domestic shorthair cats with a confirmed diagnosis of FISS and 100 disease-free matched controls) investigating a possible association between polymorphisms in the genomic sequence of the feline p53 gene and a predisposition to FISS, found a strong association between FISS and the presence of specific nucleotides at two of the polymorphic sites. 56 However, another study, conducted in Munich, Germany, could not reproduce these findings and observed no association with the polymorphisms described. 57
Management
Appropriate treatment should first include staging and careful planning of the surgery, because aggressive, radical excision is crucial to avoid tumour recurrence. The prognosis improves if, in addition to radical surgery, adjunctive treatments such as radiotherapy or immunotherapy are used. Preoperatively, (contrast-enhanced) computed tomography (CT) or magnetic resonance imaging (MRI) should be obtained for staging, and to determine the extent of the tumour and the size of the radiation field required to maximise the chance of a successful outcome. 58 It was shown that the actual size of tumours determined by CT could be twice that estimated at physical examination.59,60 Surgeons should attempt to achieve complete, en bloc, surgical tumour resection with at least 3 cm (ideally, 5 cm) margins 61 [EBM grade III] and the removal of one fascial plane underlying the tumour, because incomplete resection can result in recurrence as early as 2 weeks after surgery [EBM grade III].28,62 Treatment using surgical excision alone has a recurrence rate of up to 70%, with tumour regrowth usually occurring in the first 6 months after surgery [EBM grade III]. 13 Tumour-free margins are very important for a longer disease-free interval, which was 700 days when complete tumour excision was accomplished, but only 112 days for incomplete resection [EBM grade III]. 63 However, even with clean surgical margins, the recurrence rate can be as high as 50% [EBM grade III]. 64
Preoperative or postoperative radiation therapy significantly decreases recurrence rates and prolongs remission times,16,63,65 while the benefit of chemotherapy is not proven as large prospective randomised controlled trials are lacking. One non-randomised study found no significant difference between control cats (surgery alone) and cats treated with surgery and doxorubicin [EBM grade III], 66 while a recent study demonstrated chemotherapy benefits compared with historical controls using a combination of neoadjuvant and adjuvant chemotherapy (three epirubicin doses before and after surgery) [EBM grade III]. 67 Chemotherapy mainly remains an option for palliative treatment in cats with non-resectable FISS, when radiation therapy is not available.
Additional immunotherapy appears to be promising.68 –70 Results of prospective randomised controlled studies of cytokine gene transfer techniques for adjuvant-immunological treatment of FISS showed reduced recurrence rates. In cats receiving gene therapy by the peritumoural administration of histo-incompatible Vero cells expressing human interleukin-2 (hIL-2) in addition to surgery and radiation therapy, only 5/16 (31%) had FISS recurrence, while 11/16 control cats (69%) that had surgery and radiation therapy, but no immunotherapy, had FISS recurrence within 16 months [EBM grade I]. 71 Use of neoadjuvant gene therapy using a non-viral vector that expresses feline granulocyte-macrophage colony-stimulating factor (GM-CSF) or a combination of the feline genes GM-CSF, interleukin (IL)-2 and interferon-γ (IFN-γ) was well tolerated by cats [EBM grade I]68,69 and showed promising results. Recombinant feline IL-2 is now commercially available in Europe for the treatment of FISS in combination with surgical excision and radiation therapy. In a randomised controlled clinical trial, administration of a recombinant canarypox virus expressing feline IL-2 was well tolerated and resulted in a significantly longer median time to relapse and a significant reduction in the risk of relapse at 1 year and 2 years [EBM grade I]. 70

Prevention
Prevention consists of three general considerations:
Choice of injection site
In general, injecting distally in a leg aids, where necessary, in the subsequent treatment of sarcoma by amputation of the leg (because these tumours are very difficult to excise completely and often recur after resection). 20 Administration of vaccines (or other injections) between the scapulae is generally contraindicated because tumour resection is almost impossible in this location.
To assess the acceptance of the recommendations of the Vaccine-Associated Feline Sarcoma Task Force (VAFSTF), published in 1996, a study involving 392 cats with FISSs compared the anatomical locations of tumours between cases with FISS diagnosed before and after publication of these recommendations. 72 The proportions of FISS significantly decreased in the interscapular (53% to 40%) and right and left thoracic (10% to 4% and 9% to 1%, respectively) regions, whereas the proportions of FISS significantly increased in the right thoracic limb (1% to 10%) and the combined regions of the right pelvic limb with the right lateral aspect of the abdomen (13% to 25%) and the left pelvic limb with the left lateral aspect of the abdomen (11% to 14%). Thus, while veterinarians are complying with vaccination recommendations to some extent, a high proportion of tumours still developed in the interscapular region. There was also an increase in lateral abdominal FISSs, which could be attributable to aberrant placement of injections intended for the pelvic limbs. It remains the case that only administration of vaccines as distally as possible on a limb allows for complete surgical margins if limb amputation is required [EBM grade III]. 73 Current data in Europe shows a similar situation. In a study examining the location of FISSs in cats presented to the oncology service at the University teaching hospital in Munich, most still occurred between the scapulae (40%), followed by the right (19%) and left thoracic walls (13%). 74
Unfortunately, there is still insufficient clinical information to enable evidence-based vaccine site recommendations. The majority of safety and efficacy data comes from licensing studies in which vaccines are administered subcutaneously in the interscapular region (which should not be used for any injection in the clinical setting). Current research indicates that radical surgical resection of injection-site sarcomas including margins of at least 3 cm, but preferably 5 cm [EBM grade III], 61 is associated with the highest response rate and long-term survival [EBM grade III]. 15 With this in mind, the Feline Vaccination Advisory Panel of the American Association of Feline Practitioners (AAFP) conducted an informal survey of veterinarians whose practices focused on radiation (12), surgical (36), and medical (44) oncology for opinions on what the preferred vaccination sites should be. 62 These experts agreed that distal to the stifle, followed by distal to the elbow, were their preferred sites. Nearly as popular was the tail. Respondents frequently commented that vaccines should be administered as low on the leg as possible. They added that vaccination of cats resting in a crouched position often resulted in inadvertent injection of the skin fold of the flank, leading to tumours that were difficult to resect. 62 This is reflected in a recent paper that found an increase in lateral abdominal injection-site sarcomas since the publication of the VAFSTF’s vaccination recommendations in 1996. 61
Based on these expert opinions, the AAFP now recommends in its new guidelines, 62 consistent with the earlier (2006) guidelines, 75 that vaccines against FPV, FHV-1 and FCV should be administered below the right elbow; FeLV vaccines should be administered below the left stifle; and rabies vaccines should be administered below the right stifle. 62 So far, vaccination in the tail has not been considered a practical option. However, a recent pilot study demonstrated that vaccination in the tail was well tolerated and that tail-vaccinated cats developed an antibody response comparable to that observed following injection of the vaccine distally in the leg [EBM grade II]. 76 Further studies are warranted to confirm whether this would be an alternative option leading to equal protection rates.
Alternative recommendations are made by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association, which recognises the practical difficulties often faced by veterinarians attempting vaccination into limbs or the tail. The advice of the VGG is that a preferred site for vaccine delivery (and surgical resection of a FISS that might arise) is the skin over the lateral abdomen. This is a procedure that appears well tolerated in the majority of cats.
As a general recommendation, recording the sites of injections in the patient’s medical records is important. In addition, post-vaccination monitoring plays a vital role (see box).
Recommendations for reducing inflammatory reactions
In terms of preventing inflammatory reactions at injection sites, there are a few recommendations to follow. Cats should receive as few subcutaneous injections as possible. Intramuscular injections in cats should be avoided because intramuscular tumours develop with a similar frequency, but are more difficult to detect early. Whenever feasible, cats should receive drugs orally or intravenously. The subcutaneous injection of long-acting irritating substances (such as long-acting glucocorticoids) should be avoided.
One study examined potential risk factors when administering vaccines 27 and few factors were associated with the development of FISS. It was observed that the size of the needle and the syringe, the velocity of injection, and whether manual pressure was applied after injection or not, played no role. In contrast, the temperature of the vaccine made a significant difference, with cold vaccines being associated with a higher risk of FISS development than vaccines at room temperature. 27 Thus, vaccines should be taken out of the refrigerator about 15 minutes before injection, but not much longer, to avoid reduction in vaccinal efficacy.
If available, intranasal or oral vaccines would be preferable over injectable vaccines in cats. However, in most countries only injectable vaccines are available. Therefore, vaccines are preferred that cause the least subcutaneous inflammatory reaction. Vaccines without adjuvants should be used rather than adjuvant-containing vaccines, which means that MLV or recombinant vaccines (eg, canarypox-vectored vaccine) without adjuvant are preferred over inactivated vaccines with adjuvants.
It has been shown that recombinant canarypox-vectored vaccines cause less inflammation at the injection site. This was demonstrated in rats, 77 and in a study in cats, in which the typical granulomatous inflammation did not develop at the injection site when using these particular vaccines. 78 An extensive study investigating the subcutaneous tissue response following administration of a single dose of multi-component vaccines confirmed these findings. 79 Three groups of 15 cats were injected with one of three vaccines or saline as a negative control; cats in group A received a non-adjuvanted recombinant canarypox-vectored FeLV vaccine; cats in group B received an FeLV vaccine with a lipid-based adjuvant; and cats in group C were vaccinated with an FeLV vaccine adjuvanted with an alum-Quil A mixture. On days 7, 21 and 62 post-vaccination, significantly less inflammation was associated with administration of the non-adjuvanted recombinant canarypox-vectored vaccine. The inflammation was most severe in the cats receiving the aluminium-based adjuvant. Cats receiving adjuvanted vaccines had evidence of residual adjuvant material accumulated within macrophages even at 62 days post-vaccination. 79 In a case-control study investigating associations between vaccine types and development of FISS, adjuvanted inactivated vaccines were significantly more commonly associated with sarcoma development than other vaccines; of 35 vaccinated cats with sarcoma on the hindlimb, 25 cats had received adjuvanted vaccines, seven cats had received MLV vaccines (FPV, FHV-1 and FCV), while only one cat had received a recombinant canarypox-vectored vaccine [EBM grade III]. 35
Vaccination schedules
Finally, to prevent development of FISS, cats should be vaccinated no more than necessary. Therefore, long vaccination intervals should be applied in adult animals; vaccines (such as rabies vaccines and FPV vaccines) that are licensed for 3 year or even 4 year boosters should be preferred; no FeLV or rabies vaccinations should be administered to indoor-only cats; and immune cats should not be vaccinated (eg, if antibodies are detected). This confirms the necessity of individual vaccination schedules.
Key Points
Vaccination of cats provides essential protection and should not be stopped because of the risk of feline injection-site sarcoma (FISS).
Vaccines are not the only injectable medical products associated with FISS.
An individual vaccination schedule is important. Cats should be vaccinated no more than necessary, in accordance with current guidelines.
Appropriate sites for injection should be selected. The interscapular region should generally be avoided. Vaccines should be injected at a site from which a mass can easily be surgically removed, such as distally on a leg or in the skin of the lateral abdomen.
Vaccines should be brought to room temperature prior to administration, but should not be kept unrefrigerated for hours.
Whenever possible, subcutaneous, rather than intramuscular, injection should be performed.
The preference is for: non-adjuvanted vaccines over those containing adjuvant; modified-live vaccines or recombinant vaccines over inactivated vaccines; and vaccines with a long duration of immunity.
Post-vaccination monitoring should be performed. Any lump at the site of injection that is still present 3 months after vaccination, that is larger than 2 cm in diameter, or that it is increasing in size 1 month after vaccination should be surgically removed.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCd is supported by Merial, but is a scientifically independent body and its members receive no stipends from Merial.
The authors do not have any potential conflicts of interest to declare.
References
- 1. Hendrick MJ, Dunagan CA. Focal necrotizing granulomatous panniculitis associated with subcutaneous injection of rabies vaccine in cats and dogs: 10 cases (1988–1989). J Am Vet Med Assoc 1991; 198: 304–305. [PubMed] [Google Scholar]
- 2. Hendrick MJ, Goldschmidt MH. Do injection site reactions induce fibrosarcomas in cats? J Am Vet Med Assoc 1991; 199: 968. [PubMed] [Google Scholar]
- 3. Kass PH, Barnes WG, Jr, Spangler WL, et al. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc 1993; 203: 396–405. [PubMed] [Google Scholar]
- 4. Carroll EE, Dubielzig RR, Schultz RD. Cats differ from mink and ferrets in their response to commercial vaccines: a histologic comparison of early vaccine reactions. Vet Pathol 2002; 39: 216–227. [DOI] [PubMed] [Google Scholar]
- 5. Munday JS, Stedman NL, Richey LJ. Histology and immunohistochemistry of seven ferret vaccination-site fibrosarcomas. Vet Pathol 2003; 40: 288–293. [DOI] [PubMed] [Google Scholar]
- 6. Vascellari M, Melchiotti E, Bozza MA, et al. Fibrosarcomas at presumed sites of injection in dogs: characteristics and comparison with non-vaccination site fibrosarcomas and feline post-vaccinal fibrosarcomas. J Vet Med A Physiol Pathol Clin Med 2003; 50: 286–291. [DOI] [PubMed] [Google Scholar]
- 7. Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol 1994; 31: 126–129. [DOI] [PubMed] [Google Scholar]
- 8. Dubielzig RR, Hawkins KL, Miller PE. Myofibroblastic sarcoma originating at the site of rabies vaccination in a cat. J Vet Diagn Invest 1993; 5: 637–638. [DOI] [PubMed] [Google Scholar]
- 9. Hirschberger J, Kessler M. Das feline Fibrosarkom. Tierärztliche Praxis 2001; 29: 66–71. [Google Scholar]
- 10. Doddy FD, Glickman LT, Glickman NW, et al. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol 1996; 114: 165–174. [DOI] [PubMed] [Google Scholar]
- 11. Esplin DG, McGill LD, Meininger AC, et al. Postvaccination sarcomas in cats. J Am Vet Med Assoc 1993; 202: 1245–1247. [PubMed] [Google Scholar]
- 12. Madewell BR, Griffey SM, McEntee MC, et al. Feline vaccine-associated fibrosarcoma: an ultrastructural study of 20 tumors (1996–1999). Vet Pathol 2001; 38: 196–202. [DOI] [PubMed] [Google Scholar]
- 13. Hendrick MJ, Shofer FS, Goldschmidt MH, et al. Comparison of fibrosarcomas that developed at vaccination sites and at nonvaccination sites in cats: 239 cases (1991–1992). J Am Vet Med Assoc 1994; 205: 1425–1429. [PubMed] [Google Scholar]
- 14. Couto CG, Macy DW. Review of treatment options for vaccine-associated feline sarcoma. J Am Vet Med Assoc 1998; 213: 1426–1427. [PubMed] [Google Scholar]
- 15. Hershey AE, Sorenmo KU, Hendrick MJ, et al. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J Am Vet Med Assoc 2000; 216: 58–61. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi T, Hauck ML, Dodge R, et al. Preoperative radiotherapy for vaccine associated sarcoma in 92 cats. Vet Radiol Ultrasound 2002; 43: 473–479. [DOI] [PubMed] [Google Scholar]
- 17. Sandler I, Teeger M, Best S. Metastatic vaccine associated fibrosarcoma in a 10-year-old cat. Can Vet J 1997; 38: 374. [PMC free article] [PubMed] [Google Scholar]
- 18. Hendrick MJ, Goldschmidt MH, Shofer FS, et al. Postvaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res 1992; 52: 5391–5394. [PubMed] [Google Scholar]
- 19. Kass PH, Spangler WL, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc 2003; 223: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 20. Macy DW. The potential role and mechanisms of FeLV vaccine-induced neoplasms. Semin Vet Med Surg (Small Anim) 1995; 10: 234–237. [PubMed] [Google Scholar]
- 21. Dean R, Adams V, Whitbread T, et al. Study of feline injection site sarcomas. Vet Rec 2006; 159: 641–642. [DOI] [PubMed] [Google Scholar]
- 22. Gobar GM, Kass PH. World Wide Web-based survey of vaccination practices, postvaccinal reactions, and vaccine site-associated sarcomas in cats. J Am Vet Med Assoc 2002; 220: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 23. Coyne MJ, Reeves NC, Rosen DK. Estimated prevalence of injection-site sarcomas in cats during 1992. J Am Vet Med Assoc 1997; 210: 249–251. [PubMed] [Google Scholar]
- 24. Dean RS, Pfeiffer DU, Adams VJ. The incidence of feline injection site sarcomas in the United Kingdom. BMC Vet Res 2013; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilcock B, Wilcock A, Bottoms K. Feline postvaccinal sarcoma: 20 years later. Can Vet J 2012; 53: 430–434. [PMC free article] [PubMed] [Google Scholar]
- 26. Deim Z, Palmai N, Cserni G. Feline vaccine-associated fibrosarcoma induced by aluminium compound in two cats: short communication. Acta Vet Hung 2008; 56: 111–116. [DOI] [PubMed] [Google Scholar]
- 27. Kass PH, Spangler WL, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc 2003; 223: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 28. Lester S, Clemett T, Burt A. Vaccine site-associated sarcomas in cats: clinical experience and a laboratory review (1982–1993). J Am Anim Hosp Assoc 1996; 32: 91–95. [DOI] [PubMed] [Google Scholar]
- 29. Burton G, Mason KV. Do postvaccinal sarcomas occur in Australian cats? Aust Vet J 1997; 75: 102–106. [DOI] [PubMed] [Google Scholar]
- 30. De Man MM, Ducatelle RV. Bilateral subcutaneous fibrosarcomas in a cat following feline parvo-, herpes- and calicivirus vaccination. J Feline Med Surg 2007; 9: 432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esplin DG, Bigelow M, McGill LD, et al. Fibrosarcoma at the site of a lufenuron injection in a cat. Vet Cancer Soc Newsletter 1999; 23: 8–9. [Google Scholar]
- 32. Gagnon A. Drug injection-associated fibrosarcoma in a cat. Feline Pract 2000; 28: 18–21. [Google Scholar]
- 33. Martano M, Morello E, Iussich S, et al. A case of feline injection-site sarcoma at the site of cisplatin injections. J Feline Med Surg 2012; 14: 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munday JS, Banyay K, Aberdein D, et al. Development of an injection site sarcoma shortly after meloxicam injection in an unvaccinated cat. J Feline Med Surg 2011; 13: 988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Srivastav A, Kass PH, McGill LD, et al. Comparative vaccine-specific and other injectable-specific risks of injection-site sarcomas in cats. J Am Vet Med Assoc 2012; 241: 595–602. [DOI] [PubMed] [Google Scholar]
- 36. Buracco P, Martano M, Morello E, et al. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet J 2002; 163: 105–107. [DOI] [PubMed] [Google Scholar]
- 37. Haddad JL, Goldschmidt MH, Patel RT. Fibrosarcoma arising at the site of a retained surgical sponge in a cat. Vet Clin Pathol 2010; 39: 241–246. [DOI] [PubMed] [Google Scholar]
- 38. Daly MK, Saba CF, Crochik SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg 2008; 10: 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carminato A, Vascellari M, Marchioro W, et al. Microchip-associated fibrosarcoma in a cat. Vet Dermatol 2011; 22: 565–569. [DOI] [PubMed] [Google Scholar]
- 40. Hendrick MJ. Feline vaccine-associated sarcomas: current studies on pathogenesis. J Am Vet Med Assoc 1998; 213: 1425–1426. [PubMed] [Google Scholar]
- 41. Hendrick MJ. Feline vaccine-associated sarcomas. Cancer Invest 1999; 17: 273–277. [DOI] [PubMed] [Google Scholar]
- 42. Nieto A, Sanchez MA, Martinez E, et al. Immunohistochemical expression of p53, fibroblast growth factor-b, and transforming growth factor-alpha in feline vaccine-associated sarcomas. Vet Pathol 2003; 40: 651–658. [DOI] [PubMed] [Google Scholar]
- 43. Ellis JA, Jackson ML, Bartsch RC, et al. Use of immunohistochemistry and polymerase chain reaction for detection of oncornaviruses in formalin-fixed, paraffin-embedded fibrosarcomas from cats. J Am Vet Med Assoc 1996; 209: 767–771. [PubMed] [Google Scholar]
- 44. Kidney BA, Ellis JA, Haines DM, et al. Evaluation of formalin-fixed paraffin-embedded tissues obtained from vaccine site-associated sarcomas of cats for DNA of feline immunodeficiency virus. Am J Vet Res 2000; 61: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 45. Kidney BA, Haines DM, Ellis JA, et al. Evaluation of formalin-fixed paraffin-embedded tissues from vaccine site-associated sarcomas of cats for polyomavirus DNA and antigen. Am J Vet Res 2001; 62: 828–832. [DOI] [PubMed] [Google Scholar]
- 46. Kidney BA, Haines DM, Ellis JA, et al. Evaluation of formalin-fixed paraffin-embedded tissues from vaccine site-associated sarcomas of cats for papillomavirus DNA and antigen. Am J Vet Res 2001; 62: 833–839. [DOI] [PubMed] [Google Scholar]
- 47. Kidney BA, Haines DM, Ellis JA, et al. Evaluation of formalin-fixed paraffin-embedded tissues from feline vaccine site-associated sarcomas for feline foamy virus DNA. Am J Vet Res 2002; 63: 60–63. [DOI] [PubMed] [Google Scholar]
- 48. Kalat M, Mayr B, Schleger W, et al. Chromosomal hyperdiploidy in a feline sarcoma. Res Vet Sci 1991; 51: 227–228. [DOI] [PubMed] [Google Scholar]
- 49. Mayr B, Bockstahler B, Loupal G, et al. Cytogenetic variation between four cases of feline fibrosarcoma. Res Vet Sci 1996; 61: 268–270. [DOI] [PubMed] [Google Scholar]
- 50. Mayr B, Eschborn U, Kalat M. Near triploidy in a feline fibrosarcoma. Zentralbl Veterinarmed A 1991; 38: 617–620. [DOI] [PubMed] [Google Scholar]
- 51. Mayr B, Schaffner G, Kurzbauer R, et al. Mutations in tumour suppressor gene p53 in two feline fibrosarcomas. Br Vet J 1995; 151: 707–713. [DOI] [PubMed] [Google Scholar]
- 52. Banerji N, Kanjilal S. Somatic alterations of the p53 tumor suppressor gene in vaccine-associated feline sarcoma. Am J Vet Res 2006; 67: 1766–1772. [DOI] [PubMed] [Google Scholar]
- 53. Nambiar PR, Jackson ML, Ellis JA, et al. Immunohistochemical detection of tumor suppressor gene p53 protein in feline injection site-associated sarcomas. Vet Pathol 2001; 38: 236–238. [DOI] [PubMed] [Google Scholar]
- 54. Nambiar PR, Haines DM, Ellis JA, et al. Mutational analysis of tumor suppressor gene p53 in feline vaccine site-associated sarcomas. Am J Vet Res 2000; 61: 1277–1281. [DOI] [PubMed] [Google Scholar]
- 55. Mayr B, Reifinger M, Alton K, et al. Novel p53 tumour suppressor mutations in cases of spindle cell sarcoma, pleomorphic sarcoma and fibrosarcoma in cats. Vet Res Commun 1998; 22: 249–255. [DOI] [PubMed] [Google Scholar]
- 56. Banerji N, Kapur V, Kanjilal S. Association of germ-line polymorphisms in the feline p53 gene with genetic predisposition to vaccine-associated feline sarcoma. J Hered 2007; 98: 421–427. [DOI] [PubMed] [Google Scholar]
- 57. Mucha D, Laberke S, Meyer S, et al. Lack of association between p53 SNP and FISS in a cat population from Germany. Vet Comp Oncol 2014; 12: 130–137. [DOI] [PubMed] [Google Scholar]
- 58. Hirschberger J. Principles of treatment for feline lymphoma. In: Ettinger SJ, Feldmann EC. (eds). Textbook of veterinary internal medicine. 2nd ed. Philadelphia: WB Saunders, 2003, pp 98–102. [Google Scholar]
- 59. McEntee MC. The utility of contrast enhanced computed tomography in feline vaccine associated sarcomas: 35 cases. Vet Radiol Ultrasound 2000; 41: 575. [Google Scholar]
- 60. Martano M, Morello E, Buracco P. Feline injection-site sarcoma: past, present and future perspectives. Vet J 2011; 188: 136–141. [DOI] [PubMed] [Google Scholar]
- 61. Phelps HA, Kuntz CA, Milner RJ, et al. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002). J Am Vet Med Assoc 2011; 239: 97–106. [DOI] [PubMed] [Google Scholar]
- 62. Scherk MA, Ford RB, Gaskell RM, et al. 2013 AAFP Feline Vaccination Advisory Panel Report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cronin K, Page RL, Spodnick G, et al. Radiation therapy and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound 1998; 39: 51–56. [DOI] [PubMed] [Google Scholar]
- 64. McEntee MC, Page RL. Feline vaccine-associated sarcomas. J Vet Intern Med 2001; 15: 176–182. [DOI] [PubMed] [Google Scholar]
- 65. Steger-Lieb A, Kostorz A, Hauser B, et al. Einsatz der Strahlentherapie beim vakzineassoziierten Sarkom der Katze, Erfahrungen aus 18 Fällen. Tierärztliche Praxis 2002; 30: 35–40. [Google Scholar]
- 66. Martano M, Morello E, Ughetto M, et al. Surgery alone versus surgery and doxorubicin for the treatment of feline injection-site sarcomas: a report on 69 cases. Vet J 2005; 170: 84–90. [DOI] [PubMed] [Google Scholar]
- 67. Bray J, Polton G. Neoadjuvant and adjuvant chemotherapy combined with anatomical resection of feline injection-site sarcoma: results in 21 cats. Vet Comp Oncol. Epub ahead of print 7 February 2014. DOI 10.1111/vco.12083. [DOI] [PubMed] [Google Scholar]
- 68. Jahnke A, Hirschberger J, Fischer C, et al. Intra-tumoral gene delivery of feIL-2, feIFN-gamma and feGM-CSF using magnetofection as a neoadjuvant treatment option for feline fibrosarcomas: a phase-I study. J Vet Med A Physiol Pathol Clin Med 2007; 54: 599–606. [DOI] [PubMed] [Google Scholar]
- 69. Huttinger C, Hirschberger J, Jahnke A, et al. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: a phase I trial. J Gene Med 2008; 10: 655–667. [DOI] [PubMed] [Google Scholar]
- 70. Jas D, Soyer C, De Fornel-Thibaud P, et al. Adjuvant immunotherapy of feline injection-site sarcomas with the recombinant canarypox virus expressing feline interleukine-2 evaluated in a controlled monocentric clinical trial when used in association with surgery and brachytherapy. Trials Vaccinol 2015; 4: 1–8. [Google Scholar]
- 71. Quintin-Colonna F, Devauchelle P, Fradelizi D, et al. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleukin-2. Gene Ther 1996; 3: 1104–1112. [PubMed] [Google Scholar]
- 72. VAFSTF. Vaccine-Associated Feline Sarcoma Task Force guidelines. Diagnosis and treatment of suspected sarcomas. J Am Vet Med Assoc 1999; 214: 1745. [PubMed] [Google Scholar]
- 73. Shaw SC, Kent MS, Gordon IK, et al. Temporal changes in characteristics of injection-site sarcomas in cats: 392 cases (1990–2006). J Am Vet Med Assoc 2009; 234: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haas J. Klinik, Labordiagnostik und verwendete Impfstoffe bei Katzen mit einem Fibrosarkom: eine Übersicht über die Patienten der Medizinischen Kleintierklinik 1999–2007. Munich: Ludwig Maximilians University; 2009. [Google Scholar]
- 75. Richards JR, Elston TH, Ford RB, et al. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report. J Am Vet Med Assoc 2006; 229: 1405–1441. [DOI] [PubMed] [Google Scholar]
- 76. Hendricks CG, Levy JK, Tucker SJ, et al. Tail vaccination in cats: a pilot study. J Feline Med Surg 2014; 16: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Devauchelle P, Magnol JP. Dynamique de la réaction inflammatoire induite chez le chat par l’administration sous-cutanée d’un vaccin non adjuvé. Proceedings of the CNVSPA Congress; 2001; Lille, France. [Google Scholar]
- 78. Macy DW, Chretin J. Local postvaccinal reactions of a recombinant rabies vaccine. Proceedings of the 1999 Vet Forum, pp 44–49. [Google Scholar]
- 79. Day MJ, Schoon HA, Magnol JP, et al. A kinetic study of histopathological changes in the subcutis of cats injected with non-adjuvanted and adjuvanted multi-component vaccines. Vaccine 2007; 25: 4073–4084. [DOI] [PubMed] [Google Scholar]