Abstract
Overview:
Cytauxzoon species are apicomplexan haemoparasites, which may cause severe disease in domestic cats, as well as lions and tigers. For many years, cytauxzoonosis in domestic cats was only reported in North and South America, but in recent years the infection has also been seen in Europe (Spain, France and Italy).
Infection:
Cytauxzoon felis is the main species; it occurs as numerous different strains or genotypes and is transmitted via ticks. Therefore, the disease shows a seasonal incidence from spring to early autumn and affects primarily cats with outdoor access in areas where tick vectors are prevalent. Domestic cats may experience subclinical infection and may also act as reservoirs.
Clinical signs:
Cytauxzoonosis caused by C felis in the USA is an acute or peracute severe febrile disease with non-specific signs. Haemolytic anaemia occurs frequently; in some cats neurological signs may occur in late stages. The Cytauxzoon species identified in Europe differ from C felis that causes disease in the USA and are probably less virulent. The majority of infected cats have been healthy; in some cases anaemia was found, but disease as it occurs in the USA has not been reported to date.
Diagnosis:
Diagnosis is usually obtained by Cytauxzoon detection in blood smears and/or fine-needle aspirates from the liver, spleen and lymph nodes. PCR assays are able to detect low levels of parasitaemia and may be used for confirmation.
Treatment:
Currently a combination of the antiprotozoal drugs atovaquone and azithromycin is the treatment of choice. Concurrent supportive and critical care treatment is extremely important to improve the prognosis. Cats that survive the infection may become chronic carriers for life.
Prevention:
Cats with outdoor access in endemic areas should receive effective tick treatment.
Introduction
Cytauxzoonosis has been documented in wild felids such as bobcats, Florida panthers and Texas cougars. The first cases in domestic cats were documented in 1976. 1 For many years, cytauxzoonosis in domestic cats was only reported in North America (south eastern and central states and mid-Atlantic regions) and South America, but in recent years the infection has also been documented in Europe.
Agent properties
Cytauxzoon species are apicomplexan haemoparasites (family Theileriidae) of wild and domestic cats, which are transmitted by ticks. Several species have been identified. Cytauxzoon felis is the main species, with numerous different strains or genotypes2,3 producing infection and severe disease in domestic cats, lions and tigers. Wild cats (bobcats, mountain lions, ocelots, spotted cats and jaguars) in North and South America can act as reservoir or incidental hosts. Recent studies have shown that domestic cats can also harbour subclinical infections and may act as reservoirs.4,5 In some endemic areas, the prevalence of subclinical infection in cats may be as high as 30%. 6 Tick vectors for C felis are Amblyomma americanum and Dermacentor variabilis.7 –9
Other species have been identified: Cytauxzoon manul in Pallas cats (Mongolia), Cytauxzoon spp in Iberian lynx and domestic cats in Spain, 10 and C spp in domestic cats in Italy. 11 The tick vectors for the European species are still not known, but most likely are Dermacentor spp or Ixodes ricinus.
Epidemiology
It has been hypothesised that infection in domestic cats involved a species jump from bobcats, in which the prevalence of infection may be high in certain geographic areas. 8 Disease shows a seasonal incidence from spring to early autumn,12,13 associated with peak activity of the tick vectors. There is a significant association between infection and both outdoor access and feral cats in areas where vector ticks are prevalent. 12 No association with gender, breed, age or retroviral status has been found. 11
A hyperendemic focus may be found within endemic areas, but is likely due to tick exposure of cats rather than cat-to-cat transmission, which has never been proven.14,15 In some areas of the USA an increase in cytauxzoonosis diagnoses has been observed in the past decade and it is considered an emerging disease. 13
In recent years, the infection has also been documented in Europe. Cases have been described in the Iberian lynx (Figure 1)10,16,17 and in domestic cats 18 in the south of Spain, and in domestic cats in France. 19 Moreover, a case series was reported in north-eastern Italy (Trieste) and two cases in central Italy.11,20 In the Trieste region, samples from domestic and feral cats showed a 23% prevalence of infection, with a higher prevalence in feral cats (30%). Cytauxzoon species in the European cases is different from C felis, which produces infection and disease in the USA.
Figure 1.
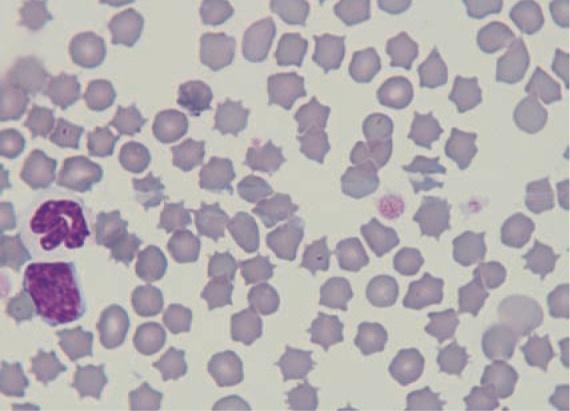
Merozoites within red blood cells in an Iberian lynx from southern Spain. Courtesy of Professor Josep Pastor, Veterinary School of Medicine, Universitat Autònoma de Barcelona, Spain
Pathogenesis
The life cycle and complex pathogenesis has been well described for this infection. 21 Vector ticks ingest merozoite-infected red blood cells from the natural reservoir host (bobcat, lynx or domestic cat). The parasite initiates a process of sexual replication (gametogenesis) in the tick gut and salivary glands. This leads to the formation of sporozoites, which are the infective form and can be transmitted if the tick attaches to a domestic cat. Sporozoites infect endothelial-associated mononuclear cells and undergo asexual replication within the macrophages; these, in turn, develop into large structures known as schizonts – large enough to occlude blood vessels, especially in the liver, spleen and lungs. Widespread dissemination of schizonts results in parasitic thrombosis, circulatory impairment, tissue infection and a severe systemic inflammatory response, which can lead to multi-organ dysfunction and failure and death within 3 weeks of infection. 22 When schizonts rupture in the circulation, large numbers of merozoites are released, infecting red blood cells and additional mononuclear cells. This is late-stage disease, with erythroparasitaemia (piroplasm structures within red blood cells) which can be readily observed in blood smears, and may lead to haemolytic anaemia and erythrophagocytosis.
Recent studies have evaluated systemic and lung immune responses in cats naturally infected with C felis based on serum concentrations of cytokines (TNFα, IL-1β) and serum proteins, immunohistochemical expression of several inflammatory mediators and PCR assay for CD18.23,24 Both studies demonstrated a marked systemic and lung pro-inflammatory response that can contribute to the pathogenesis of the disease; the response was even more pronounced in cats that died compared with survivors.23,24
Clinical presentation
Cytauxzoonosis (C felis) in the USA is typically an acute or peracute severe febrile disease. Clinical signs are non-specific and consist of depression, anorexia, high fever, icterus, dyspnoea, tachycardia, generalised pain and vocalisation. Signs of haemolytic anaemia are frequent (pale mucous membranes, pigmenturia, splenomegaly, hepatomegaly). Some cats may present or evolve to late-stage disease with neurological signs (ataxia, seizures, nystagmus), hypothermia, moribund state and coma. Many cats die within 1 week of the onset of clinical signs.14,25 Veterinarians practising in an endemic area must suspect cytauxzoonosis when faced with any cat with an acute severe disease.
Frequent clinicopathological signs include non-regenerative anaemia, leukopenia with toxic changes, thrombocytopenia, hyperbilirubinaemia, bilirubinuria and an increase in liver enzymes. These changes are associated with erythrophagocytosis and systemic inflammatory response syndrome (SIRS). Coagulation times are usually prolonged due to disseminated intravascular coagulation. Other biochemical abnormalities include hypoalbuminaemia, hyperglycaemia, pre-renal azotaemia, and electrolyte and acid–base disturbances associated with the SIRS state.14,25
Diagnostic imaging reveals non-specific signs consisting of hepatosplenomegaly on abdominal radiography and/or ultrasound, and a pulmonary interstitial–alveolar pattern on thoracic radiography.

Cytauxzoon species infection reported in European cats (Italy, Spain, France) is probably less virulent than C felis infection. The majority of infected cats have been healthy, showing only low-level erythroparasitaemia (merozoites within red blood cells) as an incidental finding. In some cats anaemia was described and one cat died after severe disease of a short duration, but no schizont structures were found in tissues, so cytauxzoonosis was not confirmed.
Diagnosis
In clinical practice, diagnosis is usually obtained by identification of C felis in blood smears and/or fine-needle aspirates from the liver, spleen and lymph nodes using rapid Romanowsky-type stains.
Observation of schizont-infected myeloid cells on blood and/or tissue smears is the diagnostic test of choice because it confirms acute disease. These are seen as very large (50–250 µm diameter) single cells with an eccentric nucleus containing a single prominent nucleolus. The cytoplasm contains variable numbers of basophilic particles (a few to thousands), which are developing merozoites. These cells may be confused with platelet clumps. The sensitivity of blood smears may be low, so fine-needle aspirates and cytology of liver, spleen, lymph nodes and lungs are indicated if blood smears are not diagnostic in a suspected case.
Observation of merozoites (piroplasms) within red blood cells in thin blood smears prepared with Romanowsky-type stains is supportive of a diagnosis of cytauxzoonosis. However, it does not confirm acute disease as merozoites can be an incidental finding in healthy cats, and may also be observed in cats that have survived acute infection or those with clinical signs of another disease. Piroplasms are usually round to oval structures, 1–2 µm in diameter, with a dark purple eccentric nucleus within a pale blue cytoplasm (signet ring shaped), but in some cases may be more elongated with a bipolar nucleus (Figure 2). One to four merozoites may be observed within individual red blood cells. Sensitivity is not very high, as merozoites appear late in the course of the disease; they are either absent or present in very low numbers in probably more than 50% of cats with acute disease. Blood smears should be performed daily because merozoites can appear over the course of the disease. The distal edges of a blood smear are the best place to look for them.
Figure 2.
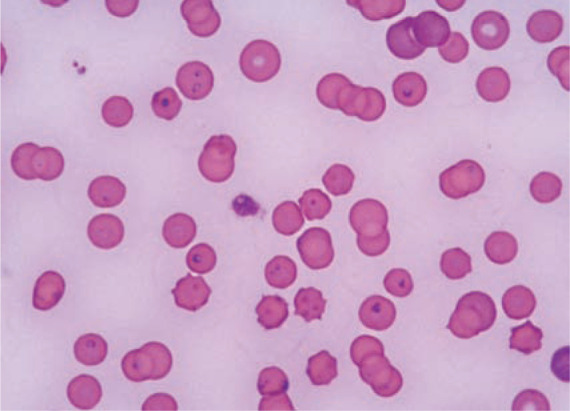
Merozoites within red blood cells in a cat from Trieste (Italy). Courtesy of Dr Erika Carli and Dr Laia Solano-Gallego, Clinica Veterinaria Privata San Marco, Padova, Italy
PCR assays have been developed to confirm the presence of C felis and other Cytauxzoon species,10,11,14 but so far they are not useful as a quick diagnostic tool in practice. It is recommended though that samples from suspected cats are submitted to appropriate laboratories to further confirm the infection. Low levels of parasitaemia can only be detected by PCR assay. 5 In one clinical trial, parasitaemia was determined by qPCR and at significantly lower levels in surviving cats versus non-surviving cats, so qPCR results might be of prognostic value. 26

Treatment
Historically, cytauxzoonosis has been considered a fatal disease, with mortality approaching 100%. With the recent advances in treatment and/or differences in strain pathogenicity, this is no longer true, although the prognosis remains guarded in some cats.27,28
Supportive and critical care treatment (intensive fluid and oxygen therapy, anti-thrombotic therapies such as unfractionated heparin 200 U/kg SC q8h, blood products, antibiotics, analgesics) is extremely important to keep the cat alive while the antiprotozoal drugs and immune system do their work. Many cats deteriorate during the first days and often die; but, if they survive, a gradual improvement is seen over the ensuing days. 26
A variety of antiprotozoal drugs have been used in case reports or experimental studies (diminazene, imidocarb dipropionate, thiacetarsamide sodium, tetracycline, parvaquone, buparvaquone) but efficacy has not been proven [EBM grade IV].27 –29
Imidocarb had been the drug of choice for many years, although it was not known if it provided any advantage over supportive care alone. However, an open-label randomised prospective clinical trial demonstrated better survival rates (60% vs 26%) with the combination of atovaquone (15 mg/kg PO q8h) and azithromycin (10 mg/kg PO q 24h) compared with imidocarb (3.5 mg/kg IM once) in 80 cats with acute disease. 26 Mortality was high (41/80 cats). Most cats died during the first 3 days after presentation, only three cats dying after the third day of treatment. Supportive treatment was the same in all cats, comprising fluid therapy and heparin. This study suggests that this antiprotozoal combination plus supportive treatment is the current approach of choice [EBM grade I]. 26 In some cats, a naso-oesophageal tube may be needed to administer drugs and enteral feeding.
Cats surviving the acute infection may become chronic carriers for life, with piroplasms within the red blood cells. These cats act as reservoirs and may transmit the infection through tick vectors.
A recent study failed to demonstrate efficacy of diminazene at higher doses (4 mg/kg IM) for 5 consecutive days in eliminating or reducing the parasite burden in chronic carrier cats. Moreover, multiple adverse effects appeared, so this treatment is not recommended [EBM grade III]. 30
Prevention
There is currently no vaccine against C felis, although preliminary studies are being conducted. 31
Prevention is based on living indoors or use of effective tick treatment in cats with outdoor access. Efficacy of an acaricide collar (imidacloprid 10% plus flumethrin 4.5%) for prevention of C felis transmission has been proven in a controlled prospective clinical trial. Two groups of cats (with and without a collar) were exposed to ticks (A americanum) infected with C felis. No cats with a collar, vs 90% of the cats with no collar, were infected [EBM grade II]. 32
Testing for the presence of Cytauxzoon species in feline blood donors is advised. Although inoculation of merozoites within red blood cells in a blood transfusion does not lead to the development of schizont structures and disease, cats can become chronic carriers and an infection reservoir.
Prognosis
The prognosis for cats with cytauxzoonosis in the USA should be considered guarded to fair, if proper intensive care is provided and atovaquone is available. It has been suggested that different C felis strains may vary in pathogenicity, as some cats have survived after not receiving antiprotozoal drugs.2,27,33 It is recommended that cats are treated in well-equipped hospitals where the best supportive treatment can be provided.
Cytauxzoon infection in Europe reportedly has a good prognosis: so far, only cats with subclinical infection or signs of mild disease (anaemia, diarrhoea), possibly unrelated to the infection, have been documented.11,20
Key Points
Cytauxzoonosis has been reported worldwide, both in domestic cats and wild cat species.
The parasite is transmitted via ticks, and the prevalence of infection is higher in cats with outdoor access and in feral cats.
In the USA, cytauxzoonosis is typically an acute or peracute, severe febrile disease. Non-regenerative haemolytic anaemia is often present, as are neurological signs, followed by death in nearly 100% of cases.
Cats infected with Cytauxzoon spp have been reported in southern Europe, but clinical signs in those cats were mild and possibly unrelated to the infection.
In practice, diagnosis is often based on blood smears and/or fine-needle aspirates from the liver, spleen and lymph nodes using rapid Romanowsky-type stains.
PCR assays have been developed to confirm the presence of C felis and Cytauxzoon species, but are not useful for a quick diagnosis in practice.
Current treatment of choice is a combination of atovaquone (15 mg/kg PO q8h) and azithromycin (10 mg/kg PO q24h), as well as fluids, heparin and supportive care.
Surviving cats may become chronic carriers.
Prevention is based on living indoors or use of effective tick treatment in cats with outdoor access.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Merial, but is a scientifically independent body and its members receive no stipends from Merial.
The authors do not have any potential conflicts of interest to declare.
References
- 1. Wagner JE. A fatal cytauxzoonosis-like disease in cats. J Am Vet Med Assoc 1976; 168: 585–588. [PubMed] [Google Scholar]
- 2. Brown HM, Berghaus RD, Latimer KS, et al. Genetic variability of Cytauxzoon felis from 88 infected domestic cats in Arkansas and Georgia. J Vet Diagn Invest 2009; 21: 59–63. [DOI] [PubMed] [Google Scholar]
- 3. Shock BC, Birkenheuer AJ, Patton LL, et al. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet Parasitol 2012; 190: 29–35. [DOI] [PubMed] [Google Scholar]
- 4. Haber MD, Tucker MD, Marr HS, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol 2007; 146: 316–320. [DOI] [PubMed] [Google Scholar]
- 5. Brown HM, Latimer KS, Erikson LE, et al. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diagn Invest 2008; 20: 485–488. [DOI] [PubMed] [Google Scholar]
- 6. Brown HM, Lockhart JM, Latimer KS, et al. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol 2010; 172: 311–316. [DOI] [PubMed] [Google Scholar]
- 7. Reichard MV, Edwards AC, Meinkoth JH, et al. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol 2010; 47: 890–896. [DOI] [PubMed] [Google Scholar]
- 8. Shock BC, Murphy SM, Patton LL, et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol 2011; 175: 325–330. [DOI] [PubMed] [Google Scholar]
- 9. Blouin EF, Kocan AA, Glenn BL, et al. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wild Dis 1984; 20: 241–242. [DOI] [PubMed] [Google Scholar]
- 10. Millán J, Naranjo V, Rodríguez A, et al. Prevalence of infection and 18S rRNA gene sequence of Cytauxzoon species in Iberian Lynx (Lynx pardinus) in Spain. Parasitology 2007; 134: 995–1001. [DOI] [PubMed] [Google Scholar]
- 11. Carli E, Trotta M, Chinelli R, et al. Cytauxzoon sp infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol 2012; 183: 343–352. [DOI] [PubMed] [Google Scholar]
- 12. Reichard MV, Baum KA, Cadenhead SC, et al. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet Parasitol 2008; 152: 314–320. [DOI] [PubMed] [Google Scholar]
- 13. Miller J, Davis CD. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet Parasitol 2013; 198: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Birkenheuer AJ, Le JA, Valenzisi AM, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Vet Med Assoc 2006; 228: 568–571. [DOI] [PubMed] [Google Scholar]
- 15. Woods JP. Feline cytauxzoonosis. In: Bonagura and Twedt (eds). Kirk’s current veterinary therapy XV. 15th ed. St Louis, MO: Elsevier Saunders, 2013, pp e405–408. [Google Scholar]
- 16. Luaces I, Aguirre E, García-Montijano M, et al. First report of an intraerythrocytic small piroplasm in wild Iberian lynx (Lynx pardinus). J Wild Dis 2005; 41: 810–815. [DOI] [PubMed] [Google Scholar]
- 17. Millán J, Candela MG, Palomares F, et al. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J 2009; 182: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Criado-Fornelio A, González-del-Rio MA, Buling-Saraña A, et al. The “expanding universe” of piroplasms. Vet Parasitol 2004; 119: 337–345. [DOI] [PubMed] [Google Scholar]
- 19. Criado-Fornelio A, Buling A, Pingret JL, et al. Hemoprotozoa of domestic animals in France: prevalence and molecular characterization. Vet Parasitol 2009; 159: 73–76. [DOI] [PubMed] [Google Scholar]
- 20. Carli E, Trotta M, Bianchi E, et al. Cytauxzoon sp. infection in two free ranging young cats: clinicopathological findings, therapy and follow up. Turkiye Parazitol Derg 2014; 38: 185–189. [DOI] [PubMed] [Google Scholar]
- 21. Kier AB, Wagner JE, Kinden DA. The pathology of experimental cytauxzoonosis. J Comp Pathol 1987; 97: 415–432. [DOI] [PubMed] [Google Scholar]
- 22. Snider TA, Confer AW, Payton ME. Pulmonary histopathology of Cytauxzoon felis infections in the cat. Vet Pathol 2010; 47: 698–702. [DOI] [PubMed] [Google Scholar]
- 23. Frontera-Acevedo K, Balsone NM, Dugan MA, et al. Systemic immune responses in Cytauxzoon felis-infected domestic cats. Am J Vet Res 2013; 74: 901–909. [DOI] [PubMed] [Google Scholar]
- 24. Frontera-Acevedo K, Sakamoto K. Local pulmonary immune responses in domestic cats naturally infected with Cytauxzoon felis. Vet Immunol Immunopathol 2015; 163: 1–7. [DOI] [PubMed] [Google Scholar]
- 25. Hoover JP, Walker DB, Hedges JD. Cytauxzoonosis in cats: eight cases (1985–1992). J Am Vet Med Assoc 1994; 205: 455–460. [PubMed] [Google Scholar]
- 26. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of atavaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med 2011; 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 27. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 2000; 14: 521–525. [DOI] [PubMed] [Google Scholar]
- 28. Greene CE, Latimer K, Hooper E, et al. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc 1999; 215: 497–500. [PubMed] [Google Scholar]
- 29. Motzel SL, Wagner JE. Treatment of experimentally induced cytauxzoonosis in cats with parvaquone and buparvaquone. Vet Parasitol 1990; 35: 131–138. [DOI] [PubMed] [Google Scholar]
- 30. Lewis KM, Cohn LA, Marr HS, et al. Failure of efficacy and adverse effects associated with dose-intense diminazene diaceturate treatment of chronic Cytauxzoon felis infection in five cats. J Feline Med Surg 2014; 16: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tarigo JL, Scholl EH, McK Bird D, et al. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS One 2013; 8: e71233. DOI: 10.1371/journal.pone.0071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reichard MV, Thomas JE, Arther RG, et al. Efficacy of an imidacloprid 10%/flumethrin 4.5% collar (Seresto, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res 2013; 112: 11–20. [DOI] [PubMed] [Google Scholar]
- 33. Walker DB, Cowell RL. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc 1995; 206: 1363–1365. [PubMed] [Google Scholar]


