Abstract
Overview:
Hepatozoonosis of domestic cats has been reported in several countries, mainly as a subclinical infection.
Disease agent:
Infection has been described mostly in areas where canine infection is present and, in recent years, Hepatozoon felis has been identified as a distinct species by molecular techniques. The vector for feline hepatozoonosis remains unknown and the pathogenesis has not been elucidated.
Infection in cats:
Feline hepatozoonosis is mainly a subclinical infection and few cases have been reported with clinical signs. The diagnosis of hepatozoonosis in cats can be made by observation of parasite gamonts in blood smears, parasite meronts in muscles by histopathology, and detection of parasite DNA in blood and tissue by PCR.
Disease management:
The treatment of choice is not known, but single cases have been treated with doxycycline or oxytetracycline and primaquine. Although the mode of transmission and the type of vector is not known, preventive treatment against blood-sucking vectors (fleas and ticks) is advised.
Agent properties
Hepatozoon species are apicomplexan parasites (family Hepatozoidae) with a blood-sucking arthropod final host and a vertebrate intermediate host. 1 In general, the agent is acquired by ingestion of the infected arthropod (eg, Rhipicephalus sanguineus in H canis and H americanum infection of dogs), but meat eating and hunting are also routes of infection (H americanum), as is transplacental transmission (H canis). 2
More than 340 species of Hepatozoon have been described, not only in mammals but also in amphibians, reptiles, birds and marsupials. The first report in a domestic cat dates from 1908 when the parasite was named Leucocytozoon felis domestici. 3 Later it was reclassified in the genus Hepatozoon species 4 as a result of similarities with the species infecting dogs and wild canids. For some time, reports of the infection in cats referred to Hepatozoon species or Hepatozoon-like species.
More recently, with the use of molecular techniques, H felis was identified as a distinct and predominant species in cat infections;5,6 however, there is also evidence that H canis can infect cats.7 –9
Epidemiology
Feline hepatozoonosis has been reported in several countries worldwide, including India, South Africa, Nigeria, the USA, Brazil, Israel, Spain, France and Portugal.3,6,10 –16 The prevalence of infection varies depending on the geographical area, cat life style and type of samples tested. Two studies showed a high prevalence of infection in Israel. In one study, meronts were found in the myocardium of 36% of cats examined post mortem. 14 In a more recent study, Hepatozoon DNA was found in blood samples of 36% of cats tested. 9 In Spain, in studies using blood PCR, prevalence rates were much lower, but varied depending on the study populations: 0.6% in domestic cats, 16% in a colony of feral cats and 4% in a group of privately owned cats visiting a referral hospital.5,6,17 Two recent studies in Portugal found H felis DNA in blood samples in 15.6% of randomly sampled cats and 8.6% of owned and shelter cats.16,18

A significant association between infection and outdoor access has been reported, but no association with gender or age has been observed. 9 There are conflicting observations as regards retroviral status; one study found no association between feline immunodeficiency virus (FIV) infection and H felis infection, 9 while other studies found a significant association between FIV and feline leukaemia virus (FeLV) infection and hepatozoonosis in cats.15,17,19
The route of transmission has not been fully elucidated yet, but the association with outdoor access suggests transmission by some ubiquitous vectors such as the common flea, mites or ticks, or predation as in other species. The arthropod vectors of H felis remain unknown, but recently H felis DNA was detected in ticks (R sanguineus) in Turkey and Portugal.20,21 Transplacental transmission of H felis has been suggested and could represent an important route of infection. 9
Pathogenesis
There have been no published studies on the pathogenesis of infection in cats. Two forms of the parasite have been found in the cat: intracellular gamonts in neutrophils and monocytes, and meronts in several tissues. H felis usually produces an infection of myocardial and skeletal muscles.14,15 The infection does not lead to significant inflammatory reaction around the parasite meronts, so the cat rarely develops clinical signs.9,14,15 The presence of meronts has been observed in many other tissues as well as skeletal muscle and myocardium; for example, lung, liver, pancreas, bone marrow, lymph node and placenta, as well as in amniotic fluid. 9
The level of parasitaemia is low, with fewer than 1% of neutrophils and monocytes containing H felis gamonts. 19 Some studies have shown no correlation between the presence of gamonts in blood smears and meronts in muscle tissues.7,14,19
Clinical presentation
Feline hepatozoonosis caused by H felis is mostly subclinical; a high proportion of cats appear to be infected with no overt clinical signs. 9
The scant clinical information on the disease that exists is based on three case reports describing systemic disease; liver and/or kidney disease were present and Hepatozoon-like parasites were demonstrated in liver or blood.11,12,22 The remaining reported cases were infected cats with no clinical signs. In a retrospective study of seven cats with Hepatozoon species detected in blood smears, diverse clinical signs (lethargy, fever, weakness, lymphadenopathy) and clinicopathological abnormalities (anaemia and thrombocytopenia) were described. 19 However, all seven cats were suffering from other diseases, which could explain the clinical signs. Four of the cats were co-infected with retrovirus and two with haemotropic mycoplasmas, suggesting that the clinicopathological abnormalities were not associated with Hepatozoon infection. Interestingly, five of the cats had clinicopathological abnormalities suggesting muscular damage (elevated levels of creatine kinase and lactate dehydrogenase). 19
Observation of H felis gamonts in a feline blood smear might be a sign of immunosuppression, which is why retrovirus testing and investigations for other co-infections and diseases is indicated. In an epidemiological study in Barcelona, Spain, four cats that tested positive for H felis were sick (attributed to other diseases) and one had leishmaniosis, 6 suggesting that immunosuppression and/or concurrent disease could be risk factors for Hepatozoon infection.
Diagnosis
In clinical practice, diagnosis is usually based on the observation of Hepatozoon gamonts in the cytoplasm of neutrophils and monocytes in blood smears stained with Diff-Quik or May-Grunwald Giemsa methods. H felis gamonts have an ellipsoidal shape and are 10.5 x 4.7 µm in size (Figure 1). They are less prominent and so are easily missed compared with the larger H canis gamonts in dogs.
Figure 1.

Hepatozoon felis gamont within a neutrophil in a cat blood smear. Courtesy of Professor Gad Baneth, School of Veterinary Medicine, Hebrew University, Jerusalem, Israel
Several studies have shown that blood smears have low sensitivity for diagnosis of Hepatozoon infection compared with PCR detection of DNA. In one study in Thailand, 32% of 300 cats were PCR positive but gamonts were observed in blood smears in only 0.7% of cats. 7 Similarly, in a study in Israel, none of the cats with meronts in the myocardium tested positive when blood smears were examined. 14 Therefore, blood PCR should be considered the diagnostic test of choice for confirming Hepatozoon infection when blood smears do not show parasites, and is the best tool for prevalence and epidemiological studies. However, positive DNA results should be interpreted in the light of the clinical picture, as it is most likely that clinical signs are associated with another infectious agent. A quantitative PCR test has been developed to improve the sensitivity of detection. 8

Meronts (round to oval parasites surrounded by a thick membrane, and measuring 39 x 34.5 µm) in skeletal muscle (Figure 2) might be detected in cats in which muscle biopsies are obtained during investigations of muscle pain or polymyositis, but this scenario has not been reported so far. Meronts in skeletal and myocardial muscle might also be detected as incidental or unexpected findings at necropsy of cats in endemic areas.
Figure 2.
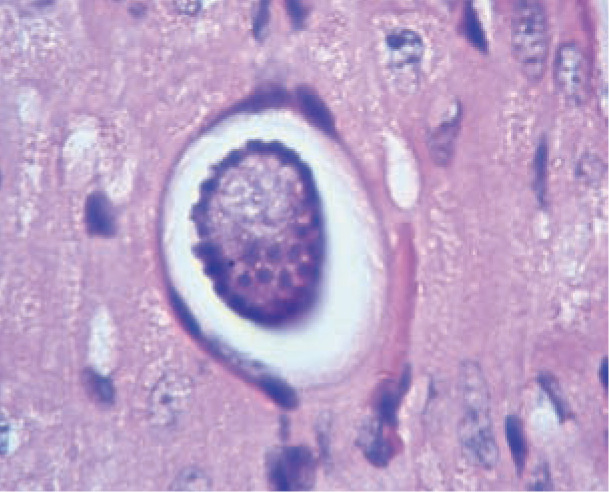
Hepatozoon felis meront within myocardial muscle in a cat. Courtesy of Professor Gad Baneth, School of Veterinary Medicine, Hebrew University, Jerusalem, Israel
Treatment
There have been no prospective controlled studies on the treatment of feline hepatozoonosis and so all information is based on a few historical case reports. Doxycycline was used in one case with no clear results, 12 while a combination of oxytetracycline and primaquine in another case led to a successful outcome [EBM grade IV]. 11 Treatment with drugs that are frequently used in canine hepatozoonosis has not been reported in cats.
Prevention
No clear recommendations on the prevention of infection can be made, as the routes of transmission in cats remain unknown. It is likely that, as in dogs, transmission is related to blood-sucking vectors, as well as the consumption of meat and the transplacental route. Therefore, preventive treatment against external parasites (fleas, ticks, others) is strongly advised in any cat, especially one with outdoor access.
Key Points
Hepatozoonosis in cats has been reported in many countries, mainly as a subclinical infection or an incidental finding.
Ingestion of infected vectors (ticks, fleas) or meat, and transplacental transmission seem to be the most common routes of infection.
H felis is the predominant species in cats, although H canis can also infect cats.
Observation of H felis gamonts in a cat may be a sign of immunosuppression.
Blood smears have low diagnostic sensitivity; blood PCR is the diagnostic method of choice.
No evidence-based treatment protocol exists for cats.
Preventive treatment against ticks and fleas is recommended.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Merial, but is a scientifically independent body and its members receive no stipends from Merial.
The authors do not have any potential conflicts of interest to declare.
References
- 1. Smith TG. The genus Hepatozoon. J Parasitol 1996; 82: 565–585. [PubMed] [Google Scholar]
- 2. Baneth G. Perspectives on canine and feline hepatozoonosis. Vet Parasitol 2011; 181: 3–11. [DOI] [PubMed] [Google Scholar]
- 3. Patton WS. The haemogregarines of mammals and reptiles. Parasitol 1908; 1: 318–321. [Google Scholar]
- 4. Wenyon CM. Protozoology: a manual for medical men, veterinarians and zoologists. William Wood, New York, 1926, pp 1085–1095. [Google Scholar]
- 5. Criado-Fornelio A, Ruas JL, Casado N, et al. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J Parasitol 2006; 92: 93–99. [DOI] [PubMed] [Google Scholar]
- 6. Tabar MD, Altet L, Francino O, et al. Vector-borne infections in cats: molecular study in Barcelona area (Spain). Vet Parasitol 2008; 15: 332–336. [DOI] [PubMed] [Google Scholar]
- 7. Jittapalapong S, Rungphisutthipongse O, Maruyama S, et al. Detection of Hepatozoon canis in stray dogs and cats in Bangkok, Thailand. Ann NY Acad Sci 2006; 1081: 479–488. [DOI] [PubMed] [Google Scholar]
- 8. Criado-Fornelio A, Buling A, Cunha-Filho NA, et al. Development and evaluation of a quantitative PCR assay for detection of Hepatozoon spp. Vet Parasitol 2007; 150: 352–356. [DOI] [PubMed] [Google Scholar]
- 9. Baneth G, Sheiner A, Eyal O, et al. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood from morphology, and possibly transplacental transmission. Parasit Vectors 2013; 6: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leeflang P, Ilemobade AA. Tick-borne disease of domestic animals in northern Nigeria. II. Research summary, 1966 to 1976. Trop Anim Health Prod 1977; 9: 211–218. [DOI] [PubMed] [Google Scholar]
- 11. Van Amstel S. Hepatozoonose in ‘n kat. J S Afr Vet Med Assoc 1979; 50: 215–216. [PubMed] [Google Scholar]
- 12. Ewing GO. Granulomatous cholangiohepatitis in a cat due to a protozoan parasite resembling Hepatozoon canis. Feline Pract 1977; 7: 37–40. [Google Scholar]
- 13. Perez RR, Rubini AS, O’Dwyer LH. The first report of Hepatozoon spp. (Apicomplexa, Hepatozoidae) in domestic cats from Säo Paolo state, Brazil. Parasitol Res 2004; 94: 83–85. [DOI] [PubMed] [Google Scholar]
- 14. Klopfer U, Nobel TA, Neumann F. Hepatozoon-like parasite (schizonts) in the myocardium of the domestic cat. Vet Pathol 1973; 10: 185–190. [DOI] [PubMed] [Google Scholar]
- 15. Beaufils JP, Martin-Granel J, Jumelle P. Hepatozoon spp. parasitemia and feline leukemia virus infection in two cats. Feline Pract 1998; 26: 10–13. [Google Scholar]
- 16. Vilhena H, Martínez-Díaz VL, Cardoso L, et al. Feline vector-borne pathogens in the north and centre of Portugal. Parasit Vectors 2013; 6: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortuño A, Castellà J, Criado-Fornelio A, et al. Molecular detection of a Hepatozoon species in stray cats from a feline colony in North-eastern Spain. Vet J 2008; 177: 134–135. [DOI] [PubMed] [Google Scholar]
- 18. Maia C, Ramos C, Coimbra M, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors 2014; 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baneth G, Aroch I, Tal N, et al. Hepatozoon species infection in domestic cats: a retrospective study. Vet Parasitol 1998; 79: 123–133. [DOI] [PubMed] [Google Scholar]
- 20. Aktas M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet Parasitol 2014; 200: 276–283. [DOI] [PubMed] [Google Scholar]
- 21. Maia C, Ferreira A, Nunes M, et al. Molecular detection of bacterial and parasitic pathogens in hard ticks from Portugal. Ticks Tick Borne Dis 2014; 5: 409–414. [DOI] [PubMed] [Google Scholar]
- 22. Baneth G, Lavy E, Presentey BZ. Hepatozoon spp. parasitemia in a domestic cat. Feline Pract 1995; 23: 1012. [Google Scholar]


