Abstract
Disease summary Cryptococcosis, principally caused by Cryptococcus neoformans and Cryptococcus gattii, is the most common systemic mycosis of cats worldwide. Cats may be infected following inhalation of spores from the environment, with the nasal cavity suspected as being the initial site of colonization and subsequent infection. Other sites of infection in cats are the skin, lungs, lymph nodes, central nervous system (CNS), eyes and, occasionally, periarticular connective tissue. Cryptococcosis can be diagnosed using serology (antigen testing), cytologic examination of smears, histopathology or culture. Treatment of localized disease is generally successful using azole antifungal drugs; however, cats with CNS involvement or disseminated disease require additional treatment with amphotericin B, with or without flucytosine. The prognosis is variable, depending on host and pathogen factors. Some cats require long-term (>1 year) treatment or indefinite therapy.
Patient group Cats of any breed, gender and age may be affected. Retroviral status does not appear to be a risk factor for developing cryptococcosis and indoor cats are not protected from disease.
Global importance Feline cryptococcosis occurs worldwide, but is most frequently reported in Australia, western Canada and the western United States. Species and molecular type vary in different geographical regions and may affect clinical presentation and antifungal susceptibility patterns.
Clinical challenges Serologic tests that detect cryptococcal antigen in serum are sensitive and specific, but false negatives can occur in cats with localized disease. Long-term drug therapy can be expensive and has the potential for toxicity. The extent to which the pathogenicity and antifungal susceptibility is affected by molecular type is currently under study.
Evidence base This review draws on recent literature relating to epidemiology, CNS involvement and advanced diagnostic imaging to update clinicians regarding research findings relevant to clinical practice.
Which are the relevant species and molecular types?
Cryptococcosis is the most common systemic mycosis of domestic cats and has been reported in other felids, especially cheetahs. 1–3 The disease is caused by an encapsulated yeast species belonging to the genus Cryptococcus, a dimorphic, basidiomycetous fungus. 4 Infection most commonly results in upper respiratory, cutaneous and central nervous system (CNS) signs. The two most common species infecting cats are Cryptococcus neoformans and Cryptococcus gattii (formerly C. neoformans var. gattii). 4 Other species, including Cryptococcus laurentii and Cryptococcus albidus, can cause disease when associated with immunocompromise. 5 Cryptococcus magnus has been described as the cause of otitis externa in a cat. 6 Because cats are more susceptible than humans to cryptococcosis, they may represent a sentinel for human exposure.
Classically, five serotypes of Cryptococcus (A, B, C, D and AD) have been recognised. C. neoformans var. grubii and C. neoformans var. neoformans comprise of serotypes A and D, respectively, with serotype AD being a hybrid of the two varieties. Serotypes B and C belong to C. gattii. Using molecular typing methods, including polymerase chain reaction (PCR) fingerprinting, amplified fragment length polymorphism (AFLP) analysis and multi-locus sequence typing (MLST), whereby DNA sequence information is obtained from multiple different genes), cryptococcal strains worldwide have now been divided into eight molecular types: VNI and VNII (C. neoformans var. grubii, serotype A); VNIII (hybrid serotype AD); VNIV (C. neoformans var. neoformans, serotype D); and VGI, VGII, VGIII and VGIV (C. gattii, serotypes B and C) (Fig 1). 7
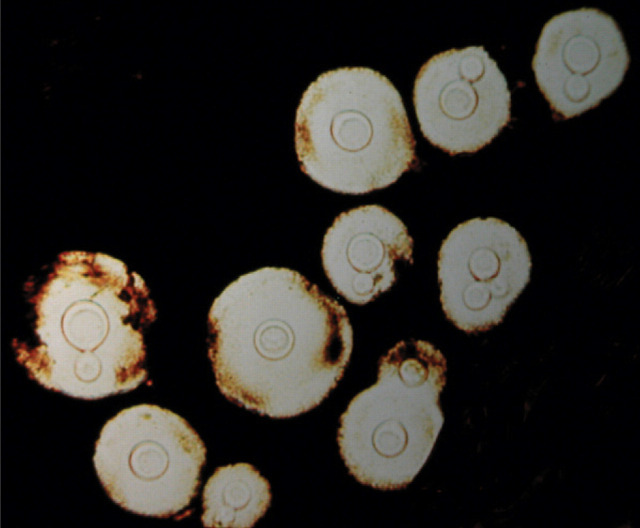
FIG 1.
(a) Molecular typing of cryptococcal veterinary isolates by URA5 RFLP double digestion with the restriction enzymes HhaI and Sau96I. (b,c) Molecular subtyping of the VGII strains by URA5 RFLP triple digestion with the restriction enzymes HhaI, DdeI and BsrGI
Species and molecular type vary in different geographical regions and may affect clinical presentation and antifungal susceptibility patterns. C. neoformans var. grubii is the primary biotype causing disease in humans with acquired immunodeficiency syndrome (AIDS).
Where are Cryptococcus organisms found?
Feline cryptococcosis is most frequently reported in Australia, western Canada and the western United States. 8–10 It is less prevalent, though occurs sporadically, in all other countries worldwide, including the UK.
The primary environmental niche for C. neoformans is thought to be weathered bird (especially pigeon) excreta. 11,12 The organism remains viable for years in environments such as pigeon lofts, where accumulations of guano are protected from drying and sunlight. C. neoformans can also be found in decaying plant matter in the hollows of certain trees. 13
C. gattii was originally associated with tropical and subtropical climates, and with Eucalyptus tree species. However, since 1999, C. gattii infections have been recognized in humans and animals in British Columbia, Canada, and, in the US, in the Pacific Northwest and California. 14–17,18 In these regions, C. gattii can be isolated from the bark of a variety of hardwood tree species, as well as from air, freshwater and seawater. 19 VGII has been subdivided into a large number of subtypes, with VGIIa, VGIIb and VGIIc being the most prevalent ones in the Pacific Northwest. Subtypes VGIIa and VGIIc appear to be especially virulent. Infections of apparently immunocompetent humans and other animal species in British Columbia and the Pacific Northwest have primarily been due to C. gattii VGIIa. To date, VGIIc has only been identified in Oregon. 20
The extent to which the pathogenicity and antifungal susceptibility of different molecular types vary is currently under study.
Geographical variation in species and molecular type.
Eastern Australia 20–30% of affected cats are infected with C. gattii, the remainder being infected with C. neoformans. 8,21 C. gattii isolates from these cats belong mainly to the VGI subtype. 22
Western Australia Approximately equal numbers of cats are infected with C. gattii as are infected with C. neoformans. C. gattii isolates from two cats from western Australia belonged to molecular type VGII. 8
Washington, Oregon and western Canada Cats are generally infected with C. gattii VGIIa and VGIIb.
California In a study, 13/18 cats were infected with C. gattii; 7/8 typed isolates belonged to the major molecular type VGIII, and one belonged to VGIIa. 17,23
Cryptococcal life cycle.
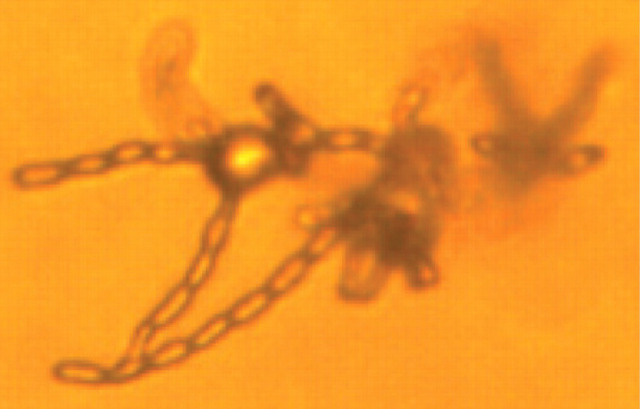
Cryptococcus species undergoes vegetative growth as haploid budding yeasts. This is the way it multiplies in mammalian tissues and on routine synthetic laboratory media. Under stringent laboratory conditions, and using special agar, it can transform to a filamentous form (the corresponding Filobasidiella species), and this is thought to occur in nature in the organism's definitive environmental niche. 4 Two mating types exist, α and a, with the α mating type being much more common in clinical and environmental samples. Under appropriate conditions and in response to mating pheromones, the two mating types can fuse and adopt a dikaryotic filamentous state, also known as the perfect state. This is followed by the production of basidia, which are small, club-shaped structures on which basidiospores form. Asexual reproduction can also occur, a process known as haploid fruiting. 24 Same-sex mating has additionally been described, and is thought to have given rise to cryptococcal strains causing disease in British Columbia. 25
When inhaled, the basidiospores are thought to behave as the infective propagule, first colonizing the mucus of the respiratory tract, and then invading epithelium and subsequently giving rise to disease. 26,27
Basidiospore production by Filobasidiella species
Route of infection
Although still not fully understood, inhalation of air-borne Cryptococcus basidiospores (see life cycle, page 164) is the presumed source of infection. 4 In cats, the nasal cavity is suspected to be the initial site of infection. 28,29 Asymptomatic colonization of the nasal cavity has also been detected. 30,31 Experimentally, intracarotid inoculation of cats with Cryptococcus species has led to distortion and swelling of the nostril, and frontal sinusitis on the inoculated side, suggesting that nasal disease may also occur secondarily to hematogenous spread. 32
Within tissues, the organism converts to a yeast form (blastoconidia). This form is round to oval with a variably sized polysaccharide capsule, which protects it from desiccation and phagocytosis. Reproduction occurs asexually by narrow-based budding (Fig 2). The organism possesses several virulence factors, including its polysaccharide capsule, melanin, mannitol, laccase and other enzymes such as phospholipase and superoxide dismutase, which allow it to survive and multiply in the face of host immune defences. 33
FIG 2.
India ink preparation of an aspirate from a cat's lymph node showing Cryptococcus yeasts. Note the narrow-based budding and the large capsule surrounding the organism. Courtesy of Spencer Jang, University of California, Davis
After infection of the respiratory system, the organism can disseminate hematogenously within macrophages to a variety of tissues. In cats, direct spread of disease within the nasal cavity across the cribriform plate may also lead to meningitis, and infection may track along the optic nerve from the CNS to involve the eye.
The incubation period is variable and can range from months to years. 34 Disease can develop as a result of primary exposure to the pathogen, or develop years later as part of a recrudescent phenomenon (from a constrained focus of infection harboring viable organisms). 34–36 Thus, a thorough travel history should be obtained from any cat suspected of having systemic fungal disease.
Disease can develop as a result of primary exposure to the pathogen, or develop years later as part of a recrudescent phenomenon. Thus, a thorough travel history is important.
What factors predispose cats to cryptococcosis?
Breed, gender, age?
Siamese, Birman and Ragdoll cats were overrepresented breeds in a large study from Australia. 21 A study from California did not find any breed predisposition. 17 No sex predisposition exists. The median age of affected cats is 6 years, 17,29 and young adult cats appear to be at increased risk, although cats of any age may be affected. 17
Retroviral status?
The prevalence of retroviral infections in cats with cryptococcosis is similar to that in the general cat population, suggesting that retrovirus infection is not a risk factor for most cats that develop cryptococcosis. 17,21,29 Cats co-infected with Cryptococcus species and feline leukemia virus (FeLV) may experience more frequent relapses and respond more slowly to treatment. 10 Previous studies have shown that treatment outcome was influenced by feline immunodeficiency virus (FIV) status, with cats seropositive for FIV having a higher likelihood of treatment failure. 10 However, more recent studies have shown that co-infection with FIV may not necessarily be a negative prognostic factor. 37 Additional research is required to understand the clinical implications of co-infection with retroviruses in cats with cryptococcosis.
Concurrent disease?
Underlying neoplasia, such as lymphoma and pulmonary adenocarcinoma, has been identified in a few cats with cryptococcosis, some of which were receiving chemotherapy. 17 Concurrent opportunistic infections such as toxoplasmosis have been reported in other cats. 14 The presence of two opportunistic infections would be unusual in an otherwise immunocompetent individual, and may be suggestive of an unidentified underlying immunodeficiency.
Others?
Cats from British Columbia residing near sites of commercial soil disturbance had a significantly increased risk of developing C. gattii infections. 38 One-quarter of cats with cryptococcosis were housed indoors in one study. 17 Thus, indoor cats are not protected from cryptococcosis. Potential sources of the organism for indoor cats include soil brought into the house on shoes, indoor plants or caged birds. Alternatively, some cats may be infected from outdoor sources before acquisition, and be sub-clinically colonized or infected before developing disease months to years later.
How does cryptococcosis in cats manifest?
The most common sites of cryptococcal infection in cats are the nasal cavity, skin, lymph nodes, brain, meninges and eyes. 17,29,39 CNS involvement in cats results from either cryptococcal meningitis or multifocal granulomatous encephalomyelitis, in which single or (typically) multiple ‘'cryptococcomas’ occur throughout the brain and/or spinal cord. 40 Infection can also involve the lungs and pleura. Less frequently, infection has been identified in the mediastinum, gingiva, spleen, myocardium, liver, thyroid gland, tongue and bone. 17,41 Involvement of the skin (apart from the nasal planum), peri-articular connective tissue, tongue and gingiva tends to indicate widespread hematogenously disseminated disease (Fig 3).
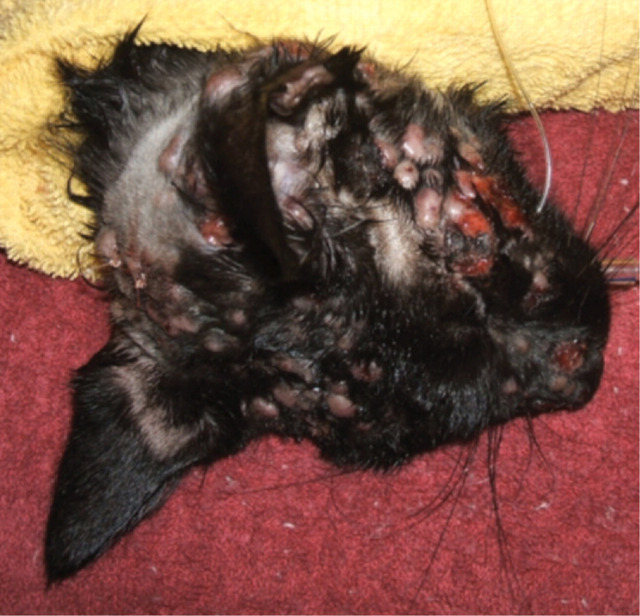
FIG 3.
Multiple cutaneous nodular lesions, some of which are ulcerated, in a cat from southeastern Australia with disseminated cryptococcosis
The majority of cats with cryptococcosis exhibit upper respiratory signs including sneezing, snuffling, stertor, nasal deformities, or mucopurulent, serous or hemorrhagic nasal discharge that is unilateral or bilateral (Fig 4). Signs are usually chronic. A polyp-like mass may be evident in the nostril. Cats with nasopharyngeal cryptococcosis develop stertor, inspiratory dyspnea and, occasionally, otitis media. Lower respiratory signs are less common and include dyspnea or tachypnea. These signs may reflect the presence of cryptococcal pneumonia, pleuritis or a mediastinal mass (Fig 5).
FIG 4.
Four-year-old male neutered domestic longhair cat from California with nasal and cutaneous cryptococcosis associated with nasal deformity, snuffling, stertor, an ulcerated skin lesion above the eye and ocular discharge
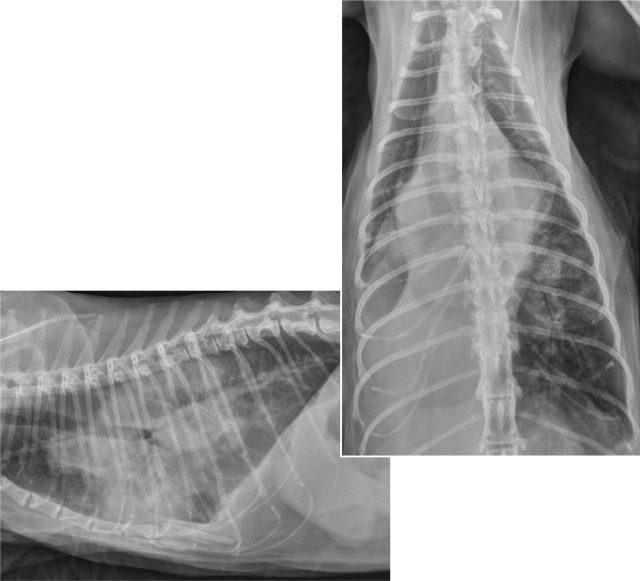
FIG 5.
Lateral and dorsoventral thoracic radiographs showing multiple pulmonary masses, and hilar and mediastinal lymphadenopathy in a 12-year-old female spayed domestic longhair cat from California with disseminated cryptococcosis
Direct spread of disease within the nasal cavity across the cribriform plate may lead to meningitis, and infection may track along the optic nerve from the CNS to involve the eye.
MULTIMEDIA.
A video recording of endoscopic findings in a cat with nasopharyngeal cryptococcosis is included in the online version of this article at doi:10.1016/j.jfms.2011.01.009
Cutaneous lesions often consist of solitary or multiple nodules or ulcerated lesions located on the planum nasale or the bridge of the nose. These ulcerated lesions may be located over, and communicate with, the frontal sinus, and palpation may reveal crepitus due to subcutaneous emphysema. Skin lesions can also occur on the trunk and limbs. Ulcerated or proliferative lesions can develop within the oral cavity. Mild to moderate mandibular lymphade-nomegaly is common.
Occasionally, infection can spread to the retrobulbar tissues, causing exophthalmos and third eyelid prolapse, although this is much more common in invasive feline sino-orbital aspergillosis than cryptococcosis. 42
Geographical variation in clinical presentation.
The clinical presentation of feline cryptococcosis may vary with geographic location, possibly reflecting referral bias or the preponderant cryptococcal molecular types in the region. In a study from California, 10% of cats had localised nasal involvement, 27% had nasal involvement with local extension to adjacent sites (nasal bridge, retrobulbar space, regional lymph nodes, brain) and 16% had nasal and disseminated disease. 17 By contrast, in southeastern Australia, the percentages were 40%, 41%, and 9%, respectively. 21 CNS involvement appears to be more common in cats from California, British Columbia and the Pacific Northwest than in cats from Australia, possibly reflecting the greater virulence of VGII and VGIII isolates. 17,45
Cats with neurological involvement may show one or a combination of the following: obtundation, behavioral changes, hyperesthesia, tremors, seizures, vestibular signs including head tilt, tight circling and nystagmus, head pressing, ataxia, paresis, mydriasis, anisocoria, peripheral or central blindness, or facial twitching. 40 Cranial nerve and proprioceptive deficits may also be identified, and very rarely there are signs of a transverse myelopathy. Despite the presence of meningitis, hyperesthesia and nuchal rigidity are uncommonly detected, but may manifest as pain over the thoracolumbar spine or pelvic limbs. CNS involvement may be present in cats with extraneural cryptococcal disease despite minimal or absent neurologic signs. 40 Signs of upper respiratory tract or cutaneous disease may precede the development of neurologic signs in some cats. Peripheral vestibular signs have been reported in cats from Australia that have cryptococcal otitis media. 43
MULTIMEDIA.
A video recording of a cat with CNS cryptococcosis showing severe mental obtundation is included in the online version of this article at doi:10.1016/j.jfms.2011.01.009
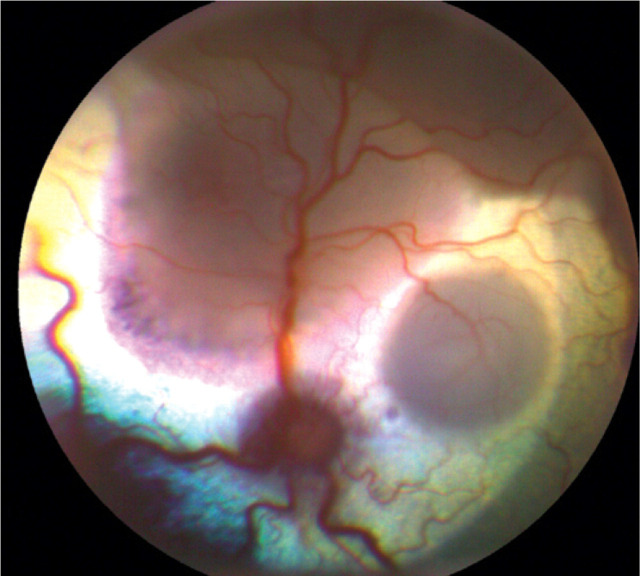
Fundoscopic examination may reveal focal or multifocal chorioretinitis, exudative retinal detachment, signs consistent with optic neuritis, papilloedema and retinal hemorrhage (Fig 6). 17 Severe ocular lesions may result in peripheral blindness with dilated and unresponsive pupils. Ocular involvement is suggestive of concurrent CNS involvement.
FIG 6.
Chorioretinitis with multifocal retinal detachment in a 3-year-old male neutered domestic shorthair cat with disseminated cryptococcosis. Courtesy of J Seth Eaton, University of California, Davis Ophthalmology Service
If infection of the kidneys or bladder is present, cats may exhibit lower urinary tract signs, or polyuria and polydipsia. 17,44 Systemic signs including lethargy, anorexia and weight loss may be present, especially with CNS involvement. Fever is rare and, when it occurs, it tends to be mild (<40°C). Cats with localized upper respiratory involvement often otherwise appear systemically well.
What diagnostic findings might be expected?
Haematology and biochemistry Cats with cryptococcosis show mild and non-specific hematological and serum biochemical abnormalities. Non-regenerative anemia, monocytosis and eosinophilia have been reported in some cats. 29
Radiography Thoracic radiographs are frequently unremarkable. Interstitial to alveolar infiltrates or small nodular lesions may be present in some cats. Infrequently, large pulmonary nodules, hilar lymphadenopathy, a mediastinal mass or pleural effusion are identified (Fig 5). 17
Sonography Abdominal ultrasound findings are normal in over 80% of cats with cryptococcosis. 17 Renal involvement may be accompanied by iso- to hypoechoic mass lesions that sometimes involve the renal pelvis, while other cats with renal involvement have no sonographic changes. 17
Computed tomography Computed tomographic assessment of the sinonasal cavity in cats with nasal cryptococcosis may show soft tissue and fluid opacification of the nasal cavity or frontal sinus, contrast-enhancing mass lesions of the nasal planum, and lysis of the nasal bones or cribriform plate. 17,46
CSF analysis Cerebrospinal fluid analysis in cats with CNS involvement may reveal encapsulated yeasts, mild to moderate hyperproteinemia (usually <1.4 g/l, mean 0.45 g/l) and neutrophilic pleocytosis (mean 200 cells/μl). 40 In one study, yeasts were identified using light microscopy in the CSF of 9/11 cats with cryptococcal meningitis. 40 Occasionally, cryptococcal meningitis can be associated with eosinophilic pleocytosis. 40
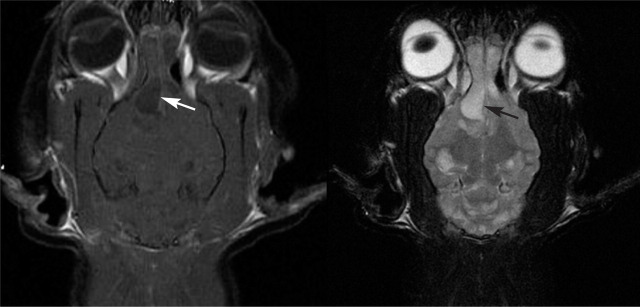
Magnetic resonance imaging Brain MRI findings in cats with CNS involvement include single or multifocal contrast-enhancing mass lesions (cryptococcomas) that tend to be hyperintense on T2W images and hypointense on T1W images (Fig 7). In some cats, lesions appear fluid-filled on T2W images but with more T1W intensity than expected for acellular fluid ('high T2, low T1’ lesions). 40 The lesions may have surrounding T2 hyperintensity, consistent with edema. Lesions have been reported in the cerebrum, cerebellum, thalamus, midbrain, optic chiasm and extradurally in the spinal cord. Mass lesions with peripheral contrast enhancement (ring-enhancing lesions) have also been reported in cats, reflecting the relative lack of inflammation within some feline CNS cryptococcomas. 40 Optic nerve involvement may manifest as optic nerve enlargement, T2 hyperintensity and marked contrast enhancement of the nerve. Mild to moderate meningeal enhancement may also be found, although the relative lack of inflammation present in most cats from California means that MRI is insensitive for detection of meningitis. Some cats with CNS cryptococcosis can have normal MRI scans. 40 Whether the same is true in other geographic locations where different cryptococcal species and molecular types predominate remains to be elucidated. Different cryptococcal strains may have a varying ability to incite an inflammatory response. 47 One report of a cat from Australia with a C. neoformans var. grubii infection revealed a mass lesion with contrast enhancement, suggesting the presence of inflammation. 48
FIG 7.
Post-contrast T1- and T2-weighted MRI scans of the brain of a 3-year-old domestic longhair cat from California with disseminated cryptococcosis. Multiple ‘high T2, low T1’ lesions were identified within the brain, the largest being in the right olfactory bulb (arrows), with smaller foci in the ventral right thalamus and multifocally throughout the cerebellum. The olfactory lesion is causing a moderate leftward falx shift and distorting the shape of the lateral ventricle, and has peripheral contrast enhancement. Right-sided rhinosinusitis is also present
How is the diagnosis confirmed?
Cryptococcosis can be diagnosed by obtaining appropriate samples for cytology or histopathology, with or without fungal culture. Diagnosis can also be made using an immunoassay for detection of the cryptococcal polysaccharide capsular antigen (cryptococcal antigen latex agglutination serology [CALAS]).
Samples for cytology or culture.
Suitable specimens for cytology or culture include nasal swabs or washings, aspirates of mass lesions or enlarged lymph nodes, deep tissue biopsy samples, bronchoalveolar lavage specimens, pleural fluid, CSF or urine.
Serology
Antigen titers are often markedly increased (>1:65,536) in cats, but even a titer of 1:2 is considered significant. 17,49 The test detects all known serotypes and can be applied to serum, plasma, urine or CSF. When performed on serum, the CALAS assay has a sensitivity ranging from 90–100% and a specificity ranging from 97–100%. 17,49,50 False negatives can occur in cats with localized disease, such as those with localized nasal cavity or cutaneous lesions, although false negatives appear to be more common in dogs. 17 When CNS cryptococcosis is suspected but serum titers are negative, determination of CSF antigen titers and fungal culture of the CSF are indicated if cryptococcal organisms are not visible during cytologic examination of the CSF. False positive serum antigen titres are rare in cats, and tend to be ≤1:200. 14 In cats with serum titres ≤1:200, other diagnostic tests (eg, cytology, histopathology, culture) should be performed to confirm a diagnosis of cryptococcosis, because other disease processes mimicking cryptococcosis (such as neoplasia) may be present.
Cytology
On cytological analysis, cryptococcal organisms are seen to have a capsule, which is highly variable in size, and also show narrow-based budding and a thin cell wall (Fig 2). Cytologic examination is sensitive, although negative test results do not eliminate the possibility of cryptococcosis. If no organisms are seen, a portion of the sample can be used for culture, and the remainder processed for histopathology.
Histopathology
On histopathology, lesions may consist almost exclusively of organisms or well-ordered granulomas or pyogranulomas. The primary cellular response includes a mixture of neutrophils, macrophages and giant cells, and fewer plasma cells and lymphocytes. Lesions consisting almost exclusively of organisms may have a gelatinous appearance grossly, because of abundant capsular polysaccharide (Fig 2). Cryptococcal cells can be identified using Mayer's mucicarmine stain, which results in the capsule taking on a rose-red color. Other fungi with similar morphological features (such as Coccidioides, Candida and Histoplasma species) do not stain with this method. 51 Immunohistochemistry has also been used for definitive identification of the organism within tissues. 52
Cats with CNS involvement.
Gross pathologic abnormalities for cats with neurologic involvement have included gray gelatinous mass lesions associated with the brain and spinal cord (cryptococcomas), meningeal edema and congestion, enlargement and softening of the olfactory bulbs, increased meningeal opacity, multifocal parenchymal nodules, cerebral edema and cerebellar herniation through the foramen magnum. 40
On histopathology, the majority of cats with CNS involvement have diffuse meningitis with minimal inflammation, 40 and abundant organisms extending along and forming cystic accumulations within the Virchow-Robin spaces which surround the meningeal arteries perforating the brain parenchyma. These cystic accumulations of cryptococci are called gelatinous pseudocysts. Uncommonly, cats have meningoencephalitis, with inflammatory meningitis and large, multifocal, granulomatous parenchymal lesions with associated necrosis. 40 The degree of inflammation that occurs in response to the organism appears to be less intense in cats than in dogs, which may reflect differences in cryptococcal strains infecting cats versus dogs, or host differences in the inflammatory response to the organism. 33
Gross pathologic and histopathologic abnormalities in cats with neurologic involvement are described in the box on page 168.
Culture
Cryptococcus species can be isolated readily in the laboratory on standard media. The yeast form grows in culture, and is not a hazard to laboratory personnel. Attempts to isolate the organism are recommended prior to treatment, because isolation allows confirmation of the diagnosis, differentiation of C. gattii from C. neoformans, and antifungal susceptibility testing. It also allows subsequent molecular typing to be pursued, which has epidemiologic significance. C. neoformans can be readily differentiated from C gattii using canavanine glycine bromothymol blue agar, 53 a simple method that is being increasingly adopted by veterinary microbiology laboratories. As the nomenclature has changed only recently, clinicians should check with the laboratory to ensure that an isolate reported as being C. neoformans has been differentiated from C. gattii.
What are current treatment recommendations?
Azoles
The major group of antifungal drugs used for the treatment of cryptococcosis is the azoles, including fluconazole, ketoconazole and itraconazole.
Fluconazole
Fluconazole is currently regarded as the initial drug of choice for cats with localized cutaneous or nasal disease. This is because of its relatively low cost compared with itraconazole (now that generic formulations are available), 56 and its good penetration into the brain, eye and urinary tract, with minimal side effects.
As the nomenclature has changed recently, clinicians should check with the laboratory to ensure that an isolate reported as being C. neoformans has been differentiated from C. gattii.
Unfortunately, some cryptococcal isolates may show resistance to fluconazole but susceptibility to other azole antifungal drugs. 57,58
Itraconazole
Although not well distributed into the CNS when compared with fluconazole, itraconazole has been used successfully to treat cats with cryptococcal meningitis. 59,60 Adverse effects of treatment with itraconazole include anorexia, vomiting and hepatocellular damage. These are more likely to occur in cats than dogs, especially cats that are receiving higher doses (>10 mg/kg q24h PO) or cats dosed with ≥5 mg/kg q24h PO of the itraconazole suspension, which has greater oral bioavailability compared with the capsules. 61 A dose reduction to 3 mg/kg q24h PO is recommended for cats receiving the suspension. Liver enzyme activities should be monitored monthly while cats are receiving treatment. The use of some compounded itraconazole formulations may be associated with inadequate blood levels (J Wheat, personal communication).
Ketoconazole
Ketoconazole is inexpensive in North America and may also be an effective treatment in cats with localized disease, although adverse effects are more common than with itraconazole. Nevertheless, it may be a good choice for cats with localized disease associated with fluconazole-resistant isolates, if costs are of concern to the client.
Treatment monitoring and challenges.
Detailed treatment regimens for cats can be found in recent infectious disease texts. 39 Serial antigen titers allow the response to treatment to be monitored, but these must be performed by the same laboratory, as test results differ widely depending on the individual serology kit. 54 Clinical improvement with a decrease in antigen titer suggests adequate treatment. Treatment is continued until the titer reaches zero.
Some cats improve clinically but maintain high antigen titres.
The most common problems relate to the high cost of treatment, and the requirement for multiple hospital visits and prolonged oral azole medication. Some cats improve clinically but maintain high antigen titers, and some cats that become serologically negative subsequently relapse weeks to years later, although the rate at which this occurs is not well understood. Outcomes may vary depending on the host immune response and the cryptococcal strain involved. 55 Reductions in titer typically lag behind clinical improvement and there is no apparent correlation between pretreatment antigen titer and outcome. 49
Other azoles
Other azole drugs that are effective for the treatment of cryptococcosis include voriconazole and posaconazole, although these are currently expensive. These drugs penetrate into the CNS, and thus may be advantageous for the treatment of cryptococcal meningo-encephalitis. 62 Neurologic signs have been described commonly following treatment of cats with voriconazole. 63,64 Posaconazole is well tolerated in humans, and there are reports of it being used without complication in cats. 65–68
Amphotericin B
Cats with CNS involvement or disseminated disease should be treated with amphotericin B, which disrupts fungal cell membranes and is fungicidal rather than fungistatic. Amphotericin B must be given parenterally and can cause nephrotoxicity. Although penetration of the CNS and eye is poor in normal patients, positive treatment responses occur, most likely because of compromise in the blood-brain barrier. Newer forms of amphotericin B such as lipid complex and liposomal preparations are not necessarily more effective but are less nephrotoxic, which may translate into greater efficacy because higher doses can be used, albeit at substantially increased cost.
Protocols have been developed that permit administration of 0.5–0.75 mg/kg amphotericin B subcutaneously in large volumes of 0.45% saline and 2.5% dextrose two or three times each week, with the median cumulative dose of amphotericin B being 16 mg/kg. 69 Such protocols permit outpatient therapy or treatment of the cat at home after initial stabilization in hospital.
Amphotericin B is recommended for treatment of CNS disease in combination with an azole and/or flucytosine. 26
Flucytosine
Flucytosine is a pyrimidine analogue that interferes with fungal nucleic acid synthesis. Monotherapy with flucytosine results in rapid development of resistance, 70 so this drug should never be used alone. Flucytosine can cause bone marrow suppression and gastrointestinal disturbances; furthermore, because it accumulates in patients with renal insufficiency, side effects may be more likely to develop in cats concurrently treated with amphotericin B. Although generally safe and effective in cats, 71 flucytosine is relatively contraindicated in dogs because of a high prevalence of severe cutaneous drug eruptions. 72 Recently, combinations of amphotericin B, flucytosine, and newer azoles (such as posaconazole) have been used to treat refractory cryptococcal meningitis in human patients. 73
Surgery
Surgical intervention may benefit cats with large, surgically accessible cryptococcomas, as penetration of antifungal agents into these masses may be limited. However, in the authors’ experience, some of these cats respond well to antifungal drug therapy without surgery. 4
What prognosis may be offered?
The prognosis for cats with cryptococcosis is variable. In a study from Australia, 60% of cats were cured after an initial course of therapy, but one-third of cats that had a favorable response to treatment subsequently relapsed. 69
CNS involvement adversely affects the prognosis. 9,69 In cats with CNS cryptococcosis from northern California, seizures or high serum CALAS titers were not associated with a poor outcome, but altered mental status was a negative prognostic indicator, 40 as documented in human cryptococcosis. Neurologic signs frequently worsen in cats with CNS involvement within the first 3 days of treatment using amphotericin B, presumably as a result of the inflammatory response to dying yeast organisms.
With supportive care, even severely obtunded or seizuring cats may subsequently recover. Judicious use of anti-inflammatory doses of glucocorticoids in conjunction with ongoing antifungal drug treatment, may improve survival in the first 10 days. 40 If used, the glucocorticoid dose should be tapered over several days and then discontinued once neurologic signs begin to improve, in order to avoid excessive immunosuppression.
KEY POINTS.
Feline cryptococcosis is caused by Cryptococcus neoformans and Cryptococcus gattii. Recent research has demonstrated considerable geographic variation in the prevalence of different cryptococcal molecular types, which may be associated with regional differences in antifungal susceptibility and pathogenicity.
Serological testing can produce false negative results, emphasizing that serology should not be relied on as the sole means of diagnosing cryptococcosis.
Findings on MRI in cats with CNS cryptococcosis include meningitis and pseudocyst lesions that are hypointense on T1-weighted images but hyperintense on T2-weighted images.
CNS involvement adversely affects the prognosis, and cats with CNS involvement generally require treatment with amphotericin B in addition to an azole or flucytosine. Although initial worsening in neurologic signs may occur in the first few days of treatment, cats that survive this phase with supportive care may achieve long-term survival or cure.
Judicious and short-term use of glucocorticoids may improve survival in the first 10 days in cats with CNS involvement.
Cryptococcal typing and susceptibility testing.
Veterinarians interested in typing cryptococcal isolates or performing susceptibility testing should contact Jane Sykes (jesykes@ucdavis.edu) or Wieland Meyer (w.meyer@usyd.edu.au) for information about import permits and other requirements.
References
- 1. Millward IR, Williams MC. Cryptococcus neoformans granuloma in the lung and spinal cord of a free-ranging cheetah (Acinonyx jubatus). A clinical report and literature review. J S Afr Vet Assoc 2005; 76: 228–32. [DOI] [PubMed] [Google Scholar]
- 2. Bolton LA, Lobetti RG, Evezard DN, et al. Cryptococcosis in captive cheetah (Acinonyx jubatus): two cases. J S Afr Vet Assoc 1999; 70: 35–39. [DOI] [PubMed] [Google Scholar]
- 3. Berry WL, Jardine JE, Espie IW. Pulmonary cryptococcoma and cryptococcal meningoencephalomyelitis in a king cheetah (Acinonyx jubatus). J Zoo Wildl Med 1997; 28: 485–90. [PubMed] [Google Scholar]
- 4. Greene CE. Infectious diseases of the dog and cat. Cryptococcosis. 3rd edn. St Louis, Mo: Elsevier Saunders, 2006. [Google Scholar]
- 5. Kano R, Kitagawat M, Oota S, et al. First case of feline systemic Cryptococcus albidus infection. Med Mycol 2008; 46: 75–77. [DOI] [PubMed] [Google Scholar]
- 6. Kano R, Hosaka S, Hasegawa A. First isolation of Cryptococcus magnus from a cat. Mycopathologia 2004; 157: 263–64. [DOI] [PubMed] [Google Scholar]
- 7. Ngamskulrungroj P, Gilgado F, Faganello J, et al. Genetic diversity of the Cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLoS ONE 2009; 4: e5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGill S, Malik R, Saul N, et al. Cryptococcosis in domestic animals in Western Australia: a retrospective study from 1995-2006. Med Mycol 2009; 47: 625–39. [DOI] [PubMed] [Google Scholar]
- 9. Duncan C, Stephen C, Campbell J. Clinical characteristics and predictors of mortality for Cryptococcus gattii infection in dogs and cats of southwestern British Columbia. Can Vet J 2006; 47: 993–98. [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobs GJ, Medleau L, Calvert C, et al. Cryptococcal infection in cats: factors influencing treatment outcome, and results of sequential serum antigen titers in 35 cats. J Vet Intern Med 1997; 11: 1–4. [DOI] [PubMed] [Google Scholar]
- 11. Nielsen K, De Obaldia AL, Heitman J. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell 2007; 6: 949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denton JF, Di Salvo AF. The prevalence of Cryptococcus neoformans in various natural habitats. Sabouraudia 1968; 6: 213–17. [PubMed] [Google Scholar]
- 13. Lazera MS, Pires FD, Camillo-Coura L, et al. Natural habitat of Cryptococcus neoformans var neoformans in decaying wood forming hollows in living trees. J Med Vet Mycol 1996; 34: 127–31. [PubMed] [Google Scholar]
- 14. Bartlett KH, Kidd SE, Kronstad JW. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Curr Infect Dis Rep 2008; 10: 58–65. [DOI] [PubMed] [Google Scholar]
- 15. Fyfe M, MacDougall L, Romney M, et al. Cryptococcus gattii infections on Vancouver Island, British Columbia, Canada: emergence of a tropical fungus in a temperate environment. Can Commun Dis Rep 2008; 34: 1–12. [PubMed] [Google Scholar]
- 16. Stephen C, Lester S, Black W, et al. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J 2002; 43: 792–94. [PMC free article] [PubMed] [Google Scholar]
- 17. Trivedi SR, Sykes JE, Cannon MS, et al. Variation in clinical presentation and epidemiology of cryptococcosis in cats and dogs from California. J Am Vet Med Assoc. In press 2011. [DOI] [PubMed] [Google Scholar]
- 18. Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var gattii. J Clin Microbiol 1990; 28: 1642–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Datta K, Bartlett KH, Baer R, et al. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis 2009; 15: 1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byrnes EJ, 3rd, Li W, Lewit Y, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 2010; 6: e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Brien CR, Krockenberger MB, Wigney DI, et al. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol 2004; 42: 449–60. [DOI] [PubMed] [Google Scholar]
- 22. Campbell LT, Currie BJ, Krockenberger M, et al. Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot Cell 2005; 4: 1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sykes J TS, Samitz E, Meyer W. Epidemiology of cryptococcosis in dogs from California, USA. Proceedings of the European College of Veterinary Internal Medicine — Companion Animals Congress Toulouse, France, 2010: 297.
- 24. Tscharke RL, Lazera M, Chang YC, et al. Haploid fruiting in Cryptococcus neoformans is not mating type alpha-specific. Fungal Genet Biol 2003; 39: 230–37. [DOI] [PubMed] [Google Scholar]
- 25. Fraser JA, Giles SS, Wenink EC, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 2005; 437: 1360–64. [DOI] [PubMed] [Google Scholar]
- 26. Ellis DH, Pfeiffer TJ. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 1990; 336: 923–25. [DOI] [PubMed] [Google Scholar]
- 27. Sukroongreung S, Kitiniyom K, Nilakul C, et al. Pathogenicity of basidiospores of Filobasidiella neoformans var neoformans. Med Mycol 1998; 36: 419–24. [PubMed] [Google Scholar]
- 28. Malik R, Dill-Macky E, Martin P, et al. Cryptococcosis in dogs: a retrospective study of 20 consecutive cases. J Med Vet Mycol 1995; 33: 291–97. [DOI] [PubMed] [Google Scholar]
- 29. Malik R, Wigney DI, Muir DB, et al. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol 1992; 30: 133–44. [DOI] [PubMed] [Google Scholar]
- 30. Duncan C, Stephen C, Lester S, et al. Follow-up study of dogs and cats with asymptomatic Cryptococcus gattii infection or nasal colonization. Med Mycol 2005; 43: 663–66. [DOI] [PubMed] [Google Scholar]
- 31. Malik R, Wigney DI, Muir DB, et al. Asymptomatic carriage of Cryptococcus neoformans in the nasal cavity of dogs and cats. J Med Vet Mycol 1997; 35: 27–31. [PubMed] [Google Scholar]
- 32. Blouin P, Cello RM. Experimental ocular cryptococcosis. Preliminary studies in cats and mice. Invest Ophthalmol Vis Sci 1980; 19: 21–30. [PubMed] [Google Scholar]
- 33. Perfect JR. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol Med Microbiol 2005; 45: 395–404. [DOI] [PubMed] [Google Scholar]
- 34. MacDougall L, Fyfe M. Emergence of Cryptococcus gattii in a novel environment provides clues to its incubation period. J Clin Microbiol 2006; 44: 1851–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Georgi A, Schneemann M, Tintelnot K, et al. Cryptococcus gattii meningoencephalitis in an immunocompetent person 13 months after exposure. Infection 2009; 37: 370–73. [DOI] [PubMed] [Google Scholar]
- 36. Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol 1999; 37: 3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker C, Malik R, Canfield PJ. Analysis of leucocytes and lymphocyte subsets in cats with naturally-occurring cryptococcosis but differing feline immunodeficiency virus status. Aust Vet J 1995; 72: 93–97. [DOI] [PubMed] [Google Scholar]
- 38. Duncan CG, Stephen C, Campbell J. Evaluation of risk factors for Cryptococcus gattii infection in dogs and cats. J Am Vet Med Assoc 2006; 228: 377–82. [DOI] [PubMed] [Google Scholar]
- 39. Sykes J, Malik R. Cryptococcus. In: Greene CE, ed. Infectious disease of the dog and cat. Elsevier Saunders. In press 2011.
- 40. Sykes JE, Sturges BK, Cannon MS, et al. Clinical signs, imaging features, neuropathology and outcome in cats and dogs with CNS cryptococcosis from California. J Vet Intern Med 2010; 6: 1427–38. [DOI] [PubMed] [Google Scholar]
- 41. Lambert RJ, Levin G, Wendelburg K, et al. What is your diagnosis? Cryptococcosis. J Am Vet Med Assoc 2006; 229: 931–32. [DOI] [PubMed] [Google Scholar]
- 42. Malik R, O'Brien C, Whitehead M. The fight against some formidable fungal foes. J Feline Med Surg 2010; 12: 669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beatty JA, Barrs VR, Swinney GR, et al. Peripheral vestibular disease associated with cryptococcosis in three cats. J Feline Med Surg 2000; 2: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chapman TL, Kirk SE. An isolated cryptococcal urinary tract infection in a cat. J Am Anim Hosp Assoc 2008; 44: 262–65. [DOI] [PubMed] [Google Scholar]
- 45. Lester SJ, Kowalewich NJ, Bartlett KH, et al. Clinicopathologic features of an unusual outbreak of cryptococcosis in dogs, cats, ferrets, and a bird: 38 cases (January to July 2003). J Am Vet Med Assoc 2004; 225: 1716–22. [DOI] [PubMed] [Google Scholar]
- 46. Karnik K, Reichle JK, Fischetti AJ, et al. Computed tomographic findings of fungal rhinitis and sinusitis in cats. Vet Radiol Ultrasound 2009; 50: 65–68. [DOI] [PubMed] [Google Scholar]
- 47. Cheng PY, Sham A, Kronstad JW. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect Immun 2009; 77: 4284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Foster SF, Charles JA, Parker G, et al. Cerebral cryptococcal granuloma in a cat. J Feline Med Surg 2001; 3: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malik R, McPetrie R, Wigney DI, et al. A latex cryptococcal antigen agglutination test for diagnosis and monitoring of therapy for cryptococcosis. Aust Vet J 1996; 74: 358–64. [DOI] [PubMed] [Google Scholar]
- 50. Medleau L, Marks MA, Brown J, et al. Clinical evaluation of a cryptococcal antigen latex agglutination test for diagnosis of cryptococcosis in cats. J Am Vet Med Assoc 1990; 196: 1470–73. [PubMed] [Google Scholar]
- 51. Lazcano O, Speights VO, Jr, Strickler JG, et al. Combined histochemical stains in the differential diagnosis of Cryptococcus neoformans. Mod Pathol 1993; 6: 80–84. [PubMed] [Google Scholar]
- 52. Krockenberger MB, Canfield PJ, Kozel TR, et al. An immuno-histochemical method that differentiates Cryptococcus neoformans varieties and serotypes in formalin-fixed paraffin-embedded tissues. Med Mycol 2001; 39: 523–33. [DOI] [PubMed] [Google Scholar]
- 53. Kwon-Chung KJ, Polacheck I, Bennett JE. Improved diagnostic medium for separation of Cryptococcus neoformans var neoformans (serotypes A and D) and Cryptococcus neoformans var gattii (serotypes B and C). J Clin Microbiol 1982; 15: 535–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hamilton JR, Noble A, Denning DW, et al. Performance of cryptococcus antigen latex agglutination kits on serum and cerebrospinal fluid specimens of AIDS patients before and after pronase treatment. J Clin Microbiol 1991; 29: 333–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Flatland B, Greene RT, Lappin MR. Clinical and serologic evaluation of cats with cryptococcosis. J Am Vet Med Assoc 1996; 209: 1110–13. [PubMed] [Google Scholar]
- 56. Wildfeuer A, Laufen H, Schmalreck AF, et al. Fluconazole: comparison of pharmacokinetics, therapy and in vitro susceptibility. Mycoses 1997; 40: 259–65. [DOI] [PubMed] [Google Scholar]
- 57. Varma A, Kwon-Chung KJ. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 2010; 54: 2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chong HS, Dagg R, Malik R, et al. In vitro susceptibility of the yeast pathogen cryptococcus to fluconazole and other azoles varies with molecular genotype. J Clin Microbiol 2010; 48: 4115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Medleau L, Jacobs GJ, Marks MA. Itraconazole for the treatment of cryptococcosis in cats. J Vet Intern Med 1995; 9: 39–42. [DOI] [PubMed] [Google Scholar]
- 60. Medleau L, Greene CE, Rakich PM. Evaluation of ketoconazole and itraconazole for treatment of disseminated cryptococcosis in cats. Am J Vet Res 1990; 51: 1454–58. [PubMed] [Google Scholar]
- 61. Boothe DM, Herring I, Calvin J, et al. Itraconazole disposition after single oral and intravenous and multiple oral dosing in healthy cats. Am J Vet Res 1997; 58: 872–77. [PubMed] [Google Scholar]
- 62. Thompson GR, 3rd, Cadena J, Patterson TF. Overview of anti-fungal agents. Clin Chest Med 2009; 30: 203–15, v. [DOI] [PubMed] [Google Scholar]
- 63. Quimby JM, Hoffman SB, Duke J, et al. Adverse neurologic events associated with voriconazole use in 3 cats. J Vet Intern Med 2010; 24: 647–49. [DOI] [PubMed] [Google Scholar]
- 64. Smith LN, Hoffman SB. A case series of unilateral orbital aspergillosis in three cats and treatment with voriconazole. Vet Ophthalmol 2010; 13: 190–203. [DOI] [PubMed] [Google Scholar]
- 65. Wray JD, Sparkes AH, Johnson EM. Infection of the subcutis of the nose in a cat caused by Mucor species: successful treatment using posaconazole. J Feline Med Surg 2008; 10: 523–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McLellan GJ, Aquino SM, Mason DR, et al. Use of posaconazole in the management of invasive orbital aspergillosis in a cat. J Am Anim Hosp Assoc 2006; 42: 302–7. [DOI] [PubMed] [Google Scholar]
- 67. Barrs V, Gunew M, Beatty JA. Use of posaconazole in multi-modality therapy to treat feline upper respiratory aspergillosis: results of a prospective study. Proceedings of the 1st symposium of the International Society for Companion Animal Infectious Diseases, Toulouse, France, 2010: 20.
- 68. Krockenberger MB, Martin P, Halliday C, et al. Localised Microsphaeropsis arundinis infection of the subcutis of a cat. J Feline Med Surg 2010; 12: 231–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Brien CR, Krockenberger MB, Martin P, et al. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J 2006; 84: 384–92. [DOI] [PubMed] [Google Scholar]
- 70. Block ER, Jennings AE, Bennett JE. 5-fluorocytosine resistance in Cryptococcus neoformans. Antimicrob Agents Chemother 1973; 3: 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moore R. Treatment of feline nasal cryptococcosis with 5-flucytosine. J Am Vet Med Assoc 1982; 181: 816–17. [PubMed] [Google Scholar]
- 72. Malik R, Medeiros C, Wigney DI, et al. Suspected drug eruption in seven dogs during administration of flucytosine. Aust Vet J 1996; 74: 285–88. [DOI] [PubMed] [Google Scholar]
- 73. Esposito V, Viglietti R, Gargiulo M, et al. Successful treatment of cryptococcal meningitis with a combination of liposomal amphotericin B, flucytosine and posaconazole: two case reports. In Vivo 2009; 23: 465–68. [PubMed] [Google Scholar]