Abstract
Practical relevance Although a relatively uncommon condition, the investigation, diagnosis and initial medical management of feline congenital portosystemic shunts is often undertaken within general practice. Early recognition and appropriate treatment are important to ensure a good outcome.
Clinical challenges Clinical signs associated with CPSSs in cats are extremely variable and can be intermittent. Signs can affect a variety of organ systems including the nervous system, and gastrointestinal and urinary tracts. Thus, the differential diagnosis list may be very long and a CPSS may not be suspected initially. More specific diagnostic tests and diagnostic imaging are indicated but may not be 100% accurate and may not be readily available to the general practitioner.
Audience This review highlights challenging aspects of the investigation and management of CPSSs for the practising veterinarian, but is also relevant to postgraduate students and provides a practical summary for specialists.
Patient group In practice, domestic shorthairs make up the majority of cats with CPSSs. However, Siamese, Persian and Himalayan cats may be more commonly affected than other purebreeds. While cats with CPSSs are typically under 6 months old, the condition is seen in mature animals, which may not have exhibited clinical signs for months or years.
Evidence base Despite several retrospective studies of cats with CPSSs, the evidence base for management of the condition remains limited.
Congenital portosystemic shunts (CPSSs) are uncommonly seen in cats, with a reported incidence of 2.5 per 10,000 cats treated in referral practice. 1 Cats present with non-specific or vague clinical signs, making recognition of the problem difficult. There is no overall consensus as to the optimum therapy and successful treatment can be challenging.
The first report of a single extrahepatic CPSS in a cat was published in 1980. 2 This was followed by a number of case reports and small case series, the findings of which were summarised by Birchard and Sherding in 1992. 2–13 Since that time, a number of larger retrospective case series have been published. 1,14–18 However, the fact remains that the evidence base for the management of cats with CPSSs is relatively sparse.
Classification of shunts
Portosystemic shunts (PSSs) can be congenital or acquired in origin. Acquired PSSs develop secondarily to underlying liver disease and are beyond the scope of this review. A congenital PSS is an abnormal communicating vessel between the portal vasculature and the systemic vasculature. The abnormal vessel results in the diversion of blood away from the portal blood system into the systemic system, thus bypassing the liver. A congenital PSS can take the form of an extrahepatic or intrahepatic vessel. Cats with extrahepatic CPSSs have an abnormal vessel branching from the portal vein or one of its tributaries before it enters the liver (ie, the CPSS lies outside the liver parenchyma). By contrast, an intrahepatic CPSS arises from an intrahepatic portal vein branch and thus lies partly or wholly within the hepatic parenchyma.
A congenital portosystemic shunt is an abnormal communicating vessel between the portal vasculature and the systemic vasculature.
SURGICAL MANAGEMENT AND PROGNOSIS.
A sister article exploring the options for surgical management of, and the short- and long-term prognosis for, cats with congenital portosystemic shunts appears on pages 185–194 of this issue of J Feline Med Surg, and at:
doi:10.1016/j.jfms.2011.01.011
Extrahepatic CPSSs are the most common form, accounting for 73–100% of CPSSs in cats in published studies. 1,4,14,16,17,19–22 Extrahepatic CPSSs represent an abnormal functional communication between the vitelline and cardinal venous systems which persists following development. 23 They can take a variety of forms, the most common of which is an abnormal vessel connecting the left gastric vein to the caudal vena cava. 14,15,19,24 Other extrahepatic shunt morphologies are described including portoazygous, portocaval (Fig 1) and shunts connecting the colonic vein or gastrosplenic vein and caudal vena cava. 3,5,15–17,21
FIG 1.
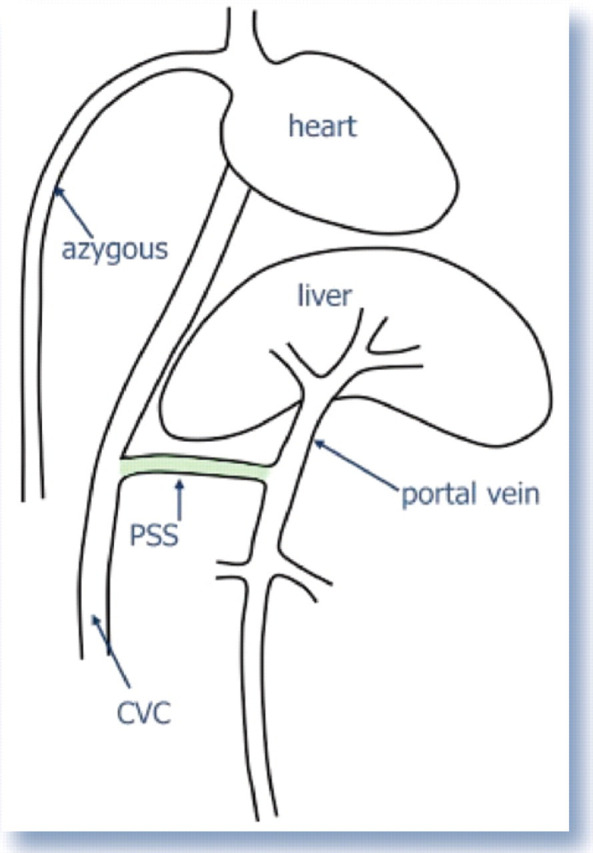
Simple anatomical diagram of a congenital portosystemic shunt (PSS). The shunt joins the portal vein to the caudal vena cava (CVC) — a so-called portocaval shunt. Thus blood flows from the portal vein directly into the CVC (systemic circulation) and bypasses the liver
Intrahepatic CPSSs in cats have been broadly divided into three forms depending on the liver lobe involved: right divisional (right lateral and caudate liver lobes), central divisional (right medial and quadrate liver lobes) and left divisional (left medial and lateral liver lobes). Left divisional intrahepatic shunts in cats are almost exclusively a patent ductus venosus (PDV). 25,26 The ductus venosus is a normal structure which, in the embryo, allows the oxygen-rich umbilical venous blood to bypass the liver and travel straight to the heart. Functional closure of the ductus venosus normally occurs following birth. A PDV results from failure of this normal process. The origin of other forms of intrahepatic CPSSs is unclear. The majority of intrahepatic CPSSs in cats are left divisional. 14,16,19,26
Pathophysiology
The liver performs many important functions, including the metabolism of toxic or harmful substances absorbed from the gastrointestinal tract, which in a normal animal have been transported to the liver via the portal vein. In cats with CPSSs the blood containing these substances is diverted along the shunt vessel, resulting in high levels of waste products (such as ammonia) being present in the systemic circulation. This leads to signs of hepatic encephalopathy (HE, see page 175).
Additionally, the liver relies on blood flow and factors derived from the gut and pancreas for stimulation of normal growth and development. Cats with CPSSs have a reduced blood supply via the portal vein and, therefore, the liver is not stimulated to develop in a normal fashion. This results in a small, underdeveloped liver and resultant functional hepatic insufficiency. Although many reports describe this as ‘liver atrophy’ it may be more correct to consider it as hepatic hypoplasia. Thus, cats with CPSSs may also show signs associated with hepatic insufficiency.
Clinical signs
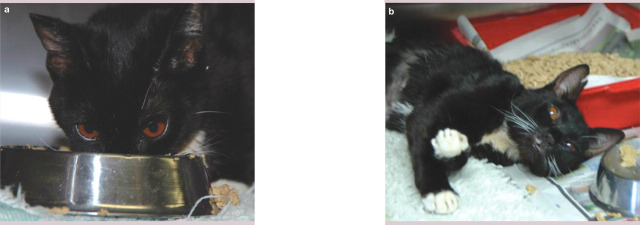
The clinical signs associated with CPSSs can be variable and non-specific. Signs often wax and wane, and can be intermittent. Indeed some cats can essentially appear ‘normal’ and the condition may only be identified as a result of abnormalities seen on routine blood tests in older cats. However, the vast majority of cats (93–100%) are presented with some degree of neurological abnormality including seizures, ataxia, central blindness, behavioural changes, aggression, lethargy, head pressing and tremors. 4,14–16,18 These neurological signs are typically intermittent and can worsen following feeding. 1,4,6 Another hallmark of a CPSS is ptyalism (hypersalivation), which is reported in 67–84% of cats (Fig 2). 4,15,16,21 These neurological signs are caused by HE and reflect a prosencephalic localisation. 28
FIG 2.

Cat with a CPSS showing evidence of ptyalism, a common clinical sign. Courtesy of Miss Zoe Halfacree
Signalment.
Age The majority of cats that exhibit clinical signs are under 6 months of age and may be as young as 6 weeks. 1,3,6 However, some cats seem to cope well with the condition at a young age and may not display any clinical signs until mature. Reports have described the age of cats treated for CPSSs as ranging between 4 months and 10 years. 5,7,14–16,18,21,26 Therefore, it is not possible to rule out a CPSS based on the age of the cat.
Gender Several case series have suggested that male cats are affected more frequently than female cats. 1,4,7,16
Breed Although in most reports the majority of cats are domestic shorthairs, a number of pedigree breeds have been suggested as being predisposed to CPSSs, including the Persian, Himalayan and Siamese. 1,14,15,17,18,21,22,26,27
Hepatic encephalopathy.
Hepatic encephalopathy is a complex syndrome of neurological signs that can develop in cats suffering from hepatic insufficiency (due to either acquired or congenital liver disorders). Common causes are hepatic lipidosis, end-stage liver disease and CPSSs. Clinical signs associated with HE relate to the central nervous system and include behavioural changes, aggression, ataxia, pacing, compulsive circling, head pressing, cortical blindness, tremors, seizures and coma. An episode of HE can be triggered by a high protein meal (Fig 4), gastrointestinal bleeding, azotaemia or the administration of anaesthetic drugs. As mentioned, neurological signs relating to HE are extremely common in cats with CPSSs. Signs may be intermittent, however, and cats with HE secondary to a CPSS may have relatively long periods of normal behaviour.
Cats with HE secondary to a CPSS may have relatively long periods of normal behaviour with intermittent clinical signs.
The pathogenesis of HE is poorly understood and it is likely that multiple factors are involved. The syndrome results from failure of the liver to remove toxic products of gut metabolism from the portal blood and also from failure of the liver to produce factors important for normal brain function. 29 One of the major factors contributing to HE is ammonia, which is often increased in cats with CPSSs. The principal source of ammonia is the gastrointestinal tract, in particular the colon. Anaerobic bacteria and coliforms degrade proteins and produce urease, which converts urea to ammonia. Ammonia is removed from the portal circulation by the liver and converted into urea. In cats with CPSSs, the ammonia is not metabolised by the liver because it enters the systemic circulation directly.
However, ammonia is not the sole cause of HE and ammonia levels can be normal in cats with CPSSs. 6,11,15,30 Various other factors have been implicated in the pathogenesis of HE including increased aromatic amino acids (phenylalanine, tyrosine, tryptophan and methionine), increased central nervous system inhibitors (gamma-aminobutyric acid [GABA] and GABA receptors), increased mercaptans and increased short-chain fatty acids. 28,29 It is believed that several of these factors act synergistically in a given animal with HE and this may partly explain the variation in clinical signs between individuals. 29
For further information, and a detailed discussion of the pathophysiology of HE, readers are referred to a recent article in this journal. 28
FIG 4.
Cat with a CPSS eating (a) and showing signs of HE following the meal (b). Note also the copper-coloured irises. Courtesy of Dr Stephen Baines
A large proportion of cats may be small or stunted with a poor body condition score; however, normal stature does not rule out a CPSS. 1,6,14,16,18,21 In addition, 13–64% of cats with CPSSs are reported to have copper-coloured irises (Fig 3). 15,16,18,21,24 The reason for this is unclear. A high rate of cryptorchidism in male cats with CPSSs has also been reported. 1
FIG 3.

Copper-coloured irises are commonly seen in cats with CPSSs. This cat has an extrahepatic CPSS
Other clinical manifestations may include gastrointestinal signs, urinary tract signs, and polyuria and polydipsia. 1,14–16 However, these signs (polyuria and polydipsia, in particular) are not as commonly seen in cats as in dogs. Gastrointestinal signs, including vomiting, diarrhoea, inappetence and weight loss, are reported in 24–71% of cats. 16,18 Urinary tract signs include dysuria and haematuria and are seen in 8–39% of cats and relate to ammonium urate urolithiasis. 7,14,16,18,21
Intolerance to sedatives or anaesthetics has also been described as a clinical sign. 1,14,15 This can range in severity from a prolonged recovery from anaesthesia to the precipitation of an encephalopathic crisis.
Diagnostic tests
Biochemistry and haematology
Routine haematology and biochemistry are useful in the investigation of cats with a suspected CPSS, particularly to rule out other differential diagnoses. However, biochemical and haematological changes are typically mild and non-specific and may be largely unremarkable. Normal haematology and biochemistry findings do not rule out the presence of a CPSS.
Many biochemical changes are reported in cats with CPSSs and these tend to relate to decreased liver function as a consequence of the shunting of blood. The most common abnormality is low urea, seen in 61–100% of cats with a CPSS. 11,15,21,24 Urea is synthesised in the liver as the waste product of ammonia metabolism (Krebs-Henseleit urea cycle). If more than 70% of liver function is lost then this affects ammonia metabolism and thus urea levels will be reduced. Fluid therapy, polyuria/polydipsia or a restricted protein diet may also contribute to low urea levels. 24 Creatinine may additionally be reduced as a reflection of low muscle mass in small or stunted cats.
Although decreased albumin and total protein are frequently reported in dogs with CPSSs this is less common in cats, with hypoalbuminaemia reported in 0–74% of feline cases. 15,21,24 Albumin and most globulins are synthesised in the liver, and so decreased proteins are likely to be due to hepatic insufficiency. Levels of alkaline phosphatase (ALP) and alanine aminotransferase are normal or may be moderately increased. 21,31 It is suggested that this is due to reduced hepatic perfusion leading to hypoxic cellular damage and enzyme leakage. 24 Increases in ALP may also be due to isoenzyme induction in very young cats. Hypoglycaemia is uncommonly seen in cats with CPSSs. However, it is important to assess glucose levels as severe hypoglycaemia can cause neurological signs which could be confused with HE.
Approximately 27–54% of cats have a microcytosis. 1,15,21,24 Unlike dogs, this is only accompanied by anaemia in a small proportion (approximately 0–15%) of cats. 15,21,24 Lymphopenia and lymphocytosis are also seen but these are very non-specific findings. 1 Reports have described clotting abnormalities in dogs with CPSSs. 32,33 As the liver is responsible for the production of the majority of clotting factors there is a concern regarding coagulopathy in cats with shunts. There have been no studies examining this in the cat but perioperative haemorrhage has not been reported as a common problem.
Serum bile acid testing.
A serum sample is collected and the animal is fed. A postprandial sample is taken after 2 hours. Some studies have shown that cats with CPSSs may have normal preprandial bile acids but increased postprandial bile acids, highlighting the importance of the latter sample. 21
Urinalysis
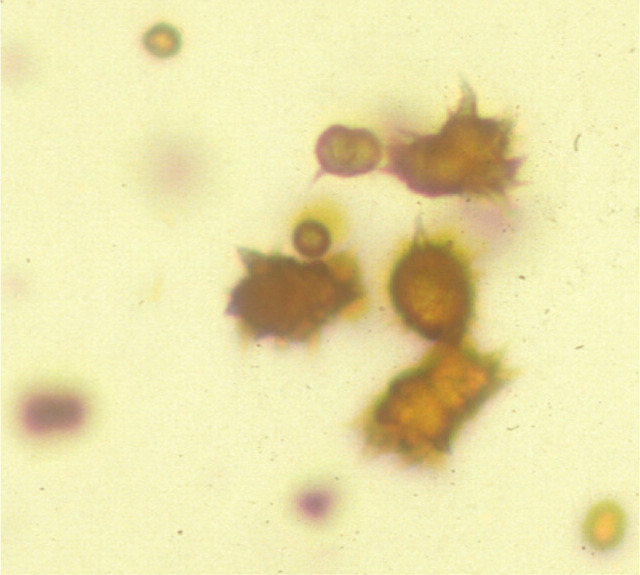
Urinalysis may reveal low urine specific gravity, particularly in cats with polyuria/polydipsia. 1 There may also be evidence of ammonium biurate crystals on sediment examination, although this is uncommon (20–43%). 3,6,11,15,21,24 Ammonium bi-urate crystals are typically golden in colour with a thorn apple shape and can be seen on light microscopy at × 400 magnification (Fig 5). In a normal animal, uric acid, which is a by-product of purine metabolism, is metabolised in the liver. In cats with CPSSs the liver's ability to metabolise uric acid is reduced, leading to an increase in serum ammonia and uric acid, which is excreted in the urine. As uric acid is relatively insoluble ammonium biurate crystals form.
FIG 5.
Examination of urine sediment from a cat with a CPSS. Note the characteristic ammonium biurate crystals. Courtesy of RVC Diagnostic Laboratory
The presence of ammonium biurate crystals increases the suspicion of CPSS. In one study, 12% of cats had ammonium biurate calculi. 1
Hepatic function tests
Assessment of hepatic function is necessary to investigate the suspicion of CPSS further. Two blood tests are commonly used: blood ammonia and the bile acid stimulation test.
As suggested above, fasting ammonia is increased in the majority of cats with CPSSs. 3,11,14,15,21,30 However, ammonia can occasionally be normal and thus a normal result should not rule out a diagnosis of a CPSS. 6,11,15,30 The sensitivity and specificity of ammonia for the diagnosis of a CPSS has been reported as 83% and 86%, respectively. 30 As ammonia is normally metabolised by the liver, resting ammonia levels represent a very crude estimate of liver function. Ammonia is very labile and so samples must be frozen and analysed very quickly. An alternative is to use a ‘point of care’ ammonia analyser, which has been shown to have potential in cats. 34
A bile acid stimulation test is the most accurate test available to measure liver function for the diagnosis of hepatobiliary disease. It is very reliable for the diagnosis of portosystemic shunting, although it is not able to differentiate between a CPSS and other forms of liver disease on its own. It has been shown that bile acid testing alone gives the best sensitivity, specificity and predictive values for the diagnosis of a CPSS in the cat. 35,36 The sensitivity of preprandial and postprandial bile acids has been reported as 58–100% and 100%, respectively, with a specificity of 84% for fasting bile acids. 1,15,21,30,35
Bile acids are synthesised from cholesterol in the liver. Even in liver failure there remains a vast reserve capacity for the production of bile acids and, hence, insufficient production is never a concern. Bile acids are conjugated to an amino acid (taurine or glycine) and stored in the gall bladder. After a period of fasting, bile acids are low in the serum of normal cats. Ingestion of food results in the release of cholecystokinin, which stimulates the gall bladder to contract and release bile acids into the duodenum. Most bile is reabsorbed by active transport in the ileum, although a small amount is passively absorbed throughout the intestine. Bile is extremely well conserved through enterohepatic recycling, with less than 5% lost in the faeces.
A normal liver will take up the bile from the blood very rapidly following absorption from the intestines. Therefore, only minor and transient increases in serum bile acids are seen in a normal animal. However, in an animal with a CPSS or multiple acquired shunts, bile acids that are reabsorbed from the intestine are diverted into the systemic circulation rather than continuing to the liver via the portal vein, thereby producing prolonged and increased serum bile acid concentrations.
Differential diagnosis
Cats with CPSSs may present with an array of non-specific clinical signs, so the initial list of differential diagnoses may be quite extensive. As outlined earlier, the vast majority of cats exhibit neurological signs and, therefore, other neurological diseases may be high on the list of possibilities. Liver function tests (ammonia and dynamic bile acids) are key to ruling in or out a suspicion of liver disease, including a CPSS. Once portosystemic shunting is suspected, further investigations, usually including some form of imaging, are necessary to identify the cause.
In cats, the main differential diagnoses for a CPSS include multiple acquired PSSs (secondary to severe liver disease), arteriovenous (A/V) fistula and primary portal vein hypoplasia (PVH - previously called microvascular dysplasia). In cats the most common diagnosis is a CPSS; A/V fistulae and PVH are extremely rare. 20,37,38 Primary PVH is described as microscopic portosystemic shunting within the liver, and results in similar clinical signs to those exhibited by animals with a gross CPSS. In some animals this is associated with hepatic fibrosis and portal hypertension and can lead to the development of multiple acquired shunts. This is a specific condition and should not be confused with underdevelopment (hypoplasia) of the extra-hepatic portal vein.
Bile acid testing alone gives the best sensitivity, specificity and predictive values for the diagnosis of CPSSs in the cat.
Imaging
Although surgery allows the direct diagnosis of a CPSS, in most instances some form of preoperative imaging is performed in order to clarify the suspicion of a CPSS and to acquire further information regarding the nature of the shunt to help with surgical planning (Table 1).
TABLE 1.
Comparison of different imaging modalities for the diagnosis of CPSSs
| Imaging modality | Advantages | Disadvantages |
| Radiography |
|
|
| Ultrasonography |
|
|
| Scintigraphy |
|
|
| Magnetic resonance imaging/computed tomography |
|
|
| Intraoperative mesenteric portovenography |
|
|
| Preoperative cranial mesenteric angiography/splenoportography |
|
|
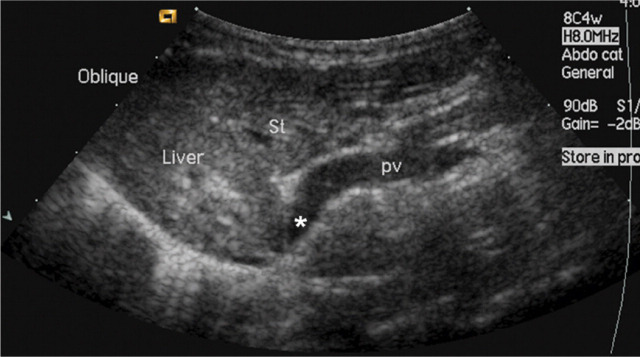
Abdominal ultrasonography is extremely useful, particularly as it is relatively inexpensive and non-invasive. It can be used to directly identify the portosystemic shunt and provide information regarding the type and location of the vessel (Fig 6). 18 Ultrasonography has been reported to have a sensitivity and specificity of 100% in one study, with 13/14 cats correctly identified with the type of CPSS. 18 However, the biggest drawback of ultrasonography for CPSS identification is that it is highly operator dependent. In other studies this imaging modality has only identified approximately 47–50% of CPSSs in cats. 1,21 Although ultrasonography may diagnose a CPSS it may not be able to accurately identify the precise nature of the abnormality (ie, intra-versus extrahepatic). In addition, failure to identify a shunt on ultrasonography does not rule out the possibility that one is present.
FIG 6.
Abdominal ultrasound image from a cat with an extrahepatic CPSS. St = stomach, pv = portal vein, ∗ = shunt branching from portal vein and bypassing the liver. Courtesy of Professor Chris Lamb
Ultrasonography has been reported to have a sensitivity and specificity of 100%, but in other studies it has identified only 47–50% of CPSSs in cats. The biggest drawback is that it is highly operator dependent.
Abdominal radiographs may be performed as part of a diagnostic work-up but add little to the diagnosis of a CPSS. Plain abdominal radiographs may show microhepatica and loss of abdominal detail in underweight cats, but these findings are not consistent. 1,4
Per rectum portal scintigraphy with technetium pertechnetate can be very reliable in the diagnosis of CPSSs in cats, with good sensitivity and specificity. 1,15,39,40 Its use is limited in the UK (and some other countries), however, by radiation safety guidelines and availability. With the advent of ultrasound, scintigraphy has been used less commonly, particularly as it provides limited anatomical information regarding the shunt, which makes it less useful for pre-operative surgical planning. However, it may be of value in the diagnosis of more complicated or occult vascular anomalies. It also has a role in the follow-up of surgically treated cats to assess for continued shunting. 40
Many different techniques involving contrast imaging have been described, including cranial mesenteric angiography, splenoportography and mesenteric portovenography. 27,41,42 Intraoperative mesenteric portovenography using fluoroscopy (see page 179) allows the imaging to be performed at the same time as surgical attenuation of the shunt and avoids the necessity of moving the cat into radiography for the imaging or the requirement for two procedures. 26
One study examined the extent of intrahepatic vasculature development using portovenography. 14 The degree of vascular development at first surgery was significantly associated with age, duration of and response to medical treatment, the probability of neurological complications, the clinical response, the existence of neurological signs at follow-up and decrease in bile acids at follow-up. Thus portovenography can also be used as a prognostic indicator. Logically, cats with well developed intrahepatic vasculature are likely to show a better response to treatment with fewer complications.
There have been reports of the use of computed tomography (CT) angiography and magnetic resonance angiography (MRA) for diagnosing CPSSs in dogs. 43,44 Although only one report of the use of CT angiography in a cat with a CPSS has been published it is likely that both techniques will be used increasingly to provide a definitive diagnosis and detailed information regarding shunt morphology and intrahepatic vasculature development prior to surgery. 45
Intraoperative mesenteric portovenography.
While intraoperative mesenteric portovenography is not essential for every case, the authors perform it in all cats with a suspected CPSS because it provides several advantages:
It allows definitive diagnosis of the CPSS (or, just as importantly, definitive confirmation that a CPSS is not present in animals with other liver conditions in which a CPSS was suspected).
It defines the exact location and morphology of the shunt (particularly useful for intrahepatic shunts or any shunt that is not easily identified on initial abdominal exploration).
It rules out the presence of a second congenital shunt (very rare) or multiple acquired shunts (these may be mistaken for a CPSS if one is much larger than all the others).
It can be used following temporary complete attenuation of the shunting vessel to confirm correct identification of the shunt and all its branches.
It allows an assessment of the degree of intrahepatic portal vascular branching before and after temporary complete attenuation, which may be useful to help decide if a CPSS will tolerate complete permanent attenuation.
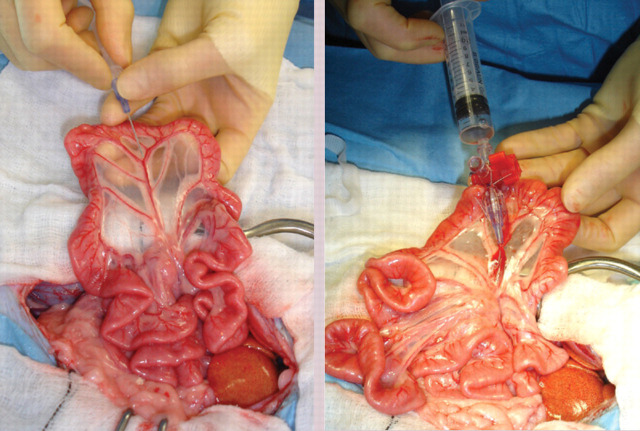
Catheter being placed in a jejunal vein for portal pressure measurement and administration of contrast for portovenography
Intraoperative image showing C-arm positioned for portovenography. Note the sterile cover
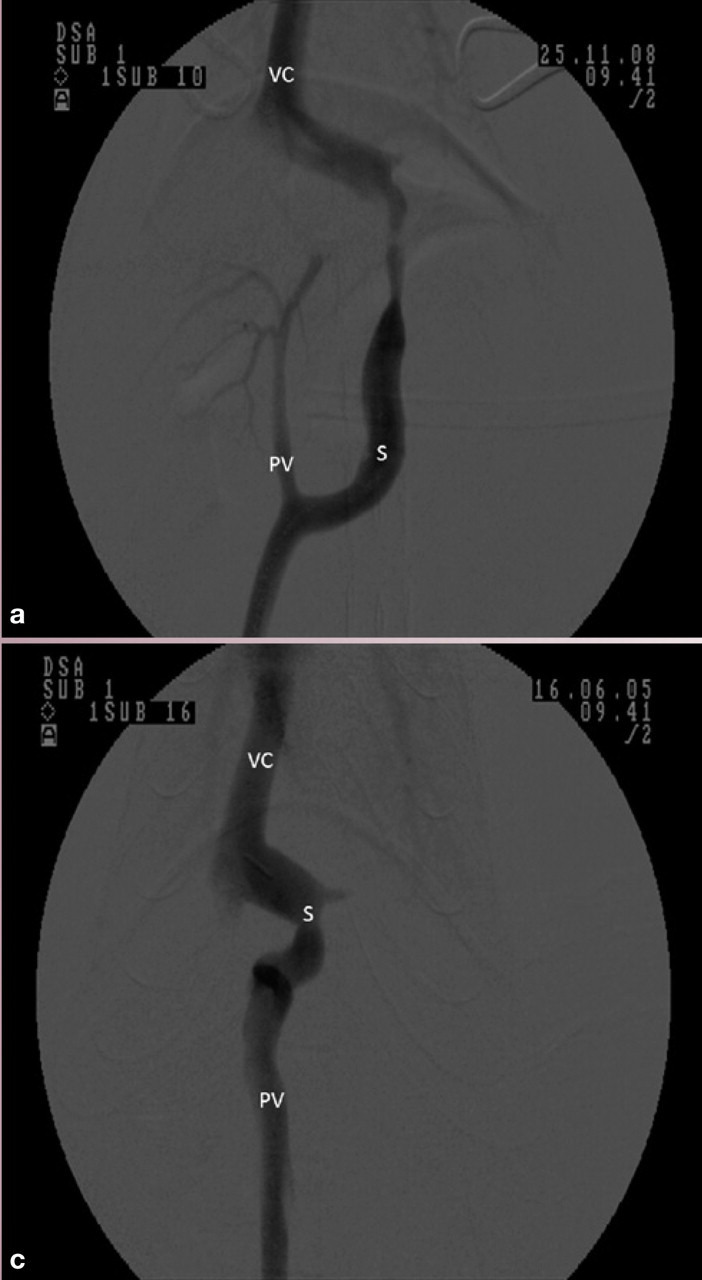
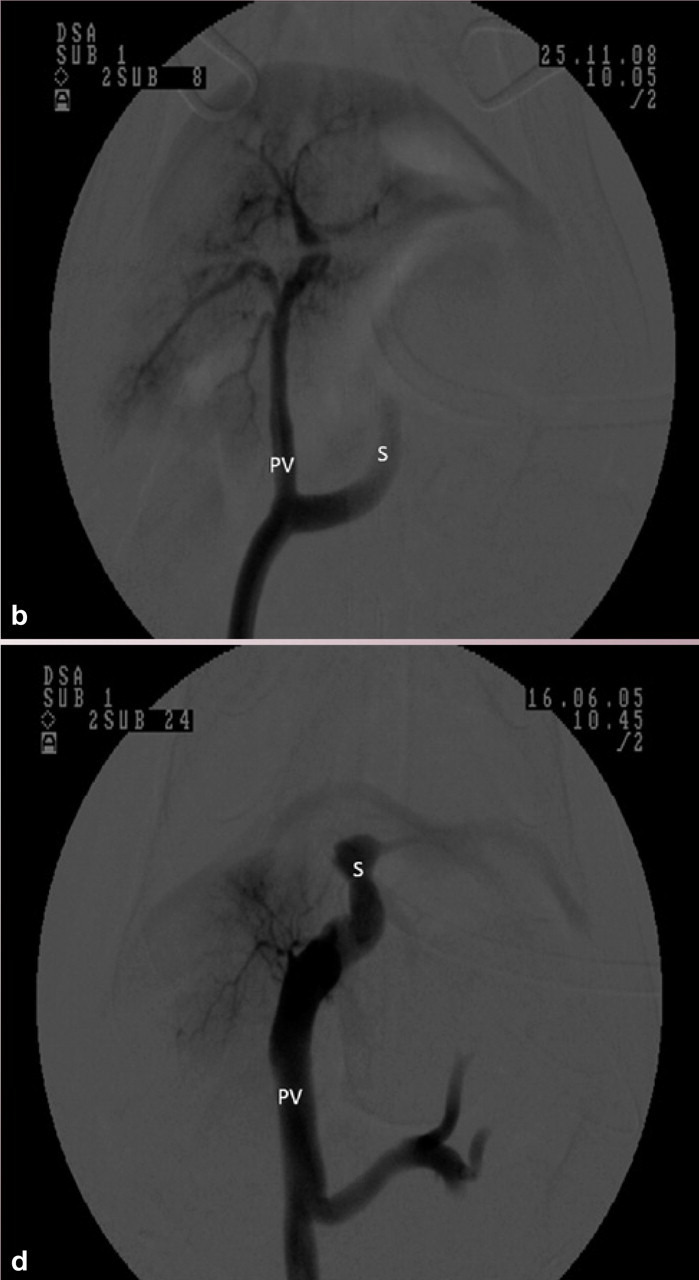
Ventrodorsal portovenograms from two cats with a CPSS (cranial is at the top of each image). VC = vena cava, PV = portal vein, S = shunt. (a) Intraoperative portovenogram in a cat with a left gastric extrahepatic CPSS. Contrast has been injected through a jejunal vein under fluoroscopic guidance with digital subtraction. The majority of the contrast, and hence blood, flows through the large shunt vessel on the right of the image. The portal vein and intrahepatic vasculature appear faintly on the left. (b) Repeat portovenogram following temporary occlusion of the shunting vessel. Note the increased opacification of the liver and lack of contrast flow through the abnormal vessel. (c) Intraoperative portovenogram in a cat with a left divisional intrahepatic CPSS. Note that there is no intrahepatic vasculature visible. (d) Repeat portovenogram following temporary occlusion of the shunting vessel. Note the increased opacification of the liver and lack of contrast flow through the abnormal vessel
Medical management
Many cats with a CPSS show relatively severe clinical signs at the time of diagnosis. Medical therapy is used to stabilise their condition prior to definitive surgical treatment. The authors recommend a minimum period of 2 weeks’ medical management before attempting surgical treatment. Medical management of cats with CPSSs treats the signs of HE but does not correct the underlying portosystemic shunting. Medical therapies (Table 2) aim to reduce ammonia (and other toxin) levels, which usually significantly improves the clinical signs of HE.
TABLE 2.
Recommendations for medical therapy
| Medical treatment | Recommended dose and frequency |
|---|---|
| Lactulose | 0.5–2 ml orally q8-12h (ideally 2–3 soft stools daily) |
| Antibiotics | |
| Ampicillin | 10–20 mg/kg orally q8h |
| Amoxicillin/clavulanate | 12.5 mg/kg orally q12h |
| Metronidazole | 10 mg/kg orally q12h |
| Antiseizure medication (if seizures present) | |
| Phenobarbitone | 1.5–3 mg/kg orally q12h |
| Serum phenobarbitone levels should be measured 2 weeks after starting therapy and the dose adjusted accordingly. Dose may be tapered following a prolonged period (eg, 3–6 months) without seizures | |
| Levetiracetam | 20 mg/kg orally q8h |
Dietary modification
Dietary modification is very important in the management of HE. The ideal diet should:
Be highly digestible to reduce the residue reaching the colonic bacteria;
Contain protein of high biological value with high levels of branched-chain amino acids and arginine, and low levels of aromatic amino acids and methionine;
Have a highly available carbohydrate as the primary source of calories;
Contain physiological levels of vitamins and minerals;
Be highly palatable. 46
A dietary source of arginine is very important in cats as it is used in the metabolism of ammonia to urea. 46 Protein modification is the most important part of dietary management, but also potentially the most challenging aspect. Cats have a high obligatory rate of protein oxidation due to a low capacity to down-regulate protein catabolism in the face of decreased protein intake. 47 As many cats afflicted with HE are small and underweight, excessive protein restriction may exacerbate the signs of HE due to accelerated protein oxidation and ammonia production. Protein levels in the diet must, therefore, meet the animal's needs without being excessively high. This balance is difficult to predict in individual cats and the level of restriction must be titrated to the highest tolerated amount based on clinical signs.
The amount of protein available in the diet is not the sole consideration as growing animals have additional nutrient requirements such as calcium and phosphorus. There are a variety of commercially available hepatic diets which may be suitable for cats with CPSSs; however, these diets would ideally require modification in order to meet calcium and phosphorus requirements. In animals that have already undergone the critical phases of growth, typically after 6 months, the relative importance of meeting calcium requirements is decreased and commercial hepatic diets are acceptable for managing these cases.
In cases where commercial diets cannot meet the nutritional needs of the animal, individualised formulations can be produced by a veterinary nutritionist. In some instances, a mixture of different diets (eg, a hepatic diet supplemented with unsalted cottage cheese) may be sufficient, while in other cases, a complete and balanced home-made diet is necessary, but, again, this requires the expertise of a veterinary nutritionist.
Lactulose
Lactulose is a synthetic disaccharide that is neither hydrolysed nor absorbed in the small intestine. 48 In the colon it is hydrolysed by enteric bacteria (primarily Bacteroides species) to lactic, acetic and formic acids. 46 These organic acids reduce the pH of the colonic contents and osmotically increase faecal water loss. This acidification of colonic contents results in the production of more ammonium (NH4 +) than ammonia (NH3). Ammonia is freely diffusible across the mucosa, whereas ammonium is not. Hence, acidification reduces the amount of ammonia absorbed.
Reported response rates to medical management.
In one study of 25 cats managed medically prior to surgery there was no response to medical treatment in 12% of cats, a partial response in 56% of cats, and 32% showed complete resolution of their clinical signs. 14 While it is clear that medical management may be effective in stabilising the clinical signs associated with HE in the short term, there is very little information regarding its use as the sole treatment long term in the cat. One study reported that two cats managed medically for an intrahepatic CPSS were euthanased at 4 weeks and 30 months due to persistent or recurrent HE. 3 In the same study, two cats that had partial ligation of an extra-hepatic CPSS suffered recurrence of HE at 12 and 15 months postoperatively associated with persistent shunting, and these cats failed to respond to medical management and were euthanased.
The authors recommend a minimum period of 2 weeks’ medical management to stabilise the condition of cats with CPSSs before attempting surgical treatment.
Medical management treats the signs of HE but does not correct the underlying portosystemic shunting. Therapies aim to reduce ammonia (and other toxin) levels.
Lactulose also decreases intestinal transit time and hence reduces the production and absorption of ammonia and other toxins. In addition it increases faecal nitrogen content via the incorporation of ammonia into bacterial proteins. 46 Lactulose is used in combination with antibiotics and can be synergistic.
Antibiotics
Antibiotics are used in the treatment of CPSSs to reduce the enteric flora that produce many of the toxins implicated in HE during digestion of a meal. Ideally, these antibiotics should not be absorbed in the intestine and should be effective against ammonia-producing bacteria. Ampicillin, amoxicillin, amoxicillin/clavulanate, neomycin and metronidazole have all been used to treat HE in cats. 28,46
Antiseizure medication
Cats with neurological signs that fail to respond to medical therapy for HE, or those with uncontrolled seizures or status epilepticus, should be started on antiseizure medication (eg, phenobarbitone). Phenobarbitone is metabolised by the liver so as low a dose as possible should be used and cats on long-term treatment should be carefully monitored. Levetiracetam is an alternative treatment to phenobarbitone and has the advantage of a more rapid onset of action. It is important to note that potassium bromide is not recommended for use in cats due to the high incidence of severe complications, including lower airway disease.
Readers are referred to a recent article in this journal for further information on the management of seizures in cats. 49
Approach to the severely encephalopathic/collapsed cat
In some instances cats can be presented collapsed, comatose or in status epilepticus due to severe HE. On occasion this may have been precipitated by anaesthetic episode or other insult in a cat already being medically managed or with occult disease.
Hepatic encephalopathy can be exacerbated by a number of factors. Increased protein, either in the diet or due to gastrointestinal haemorrhage, can result in increased ammonia production by intestinal bacteria and constipation allows increased absorption of ammonia. Cats with CPSSs often suffer vomiting and this can lead to acid/base abnormalities (particularly metabolic alkalosis), electrolyte abnormalities (hypokalaemia), dehydration and possibly hypovolaemia. Alkalosis results in the formation of ammonia rather than ammonium, which diffuses more readily into the central nervous system. Hypokalaemia results in increased renal ammonia formation and potentiates alkalosis. Hypovolaemia can cause pre-renal azotaemia and hence increased urea, resulting in increased ammonia production.
Cats with severe neurological signs or in status epilepticus should be treated as an emergency.
Minimum database, fluid therapy
A minimum database (including blood glucose and serum electrolytes) should be obtained to rule out other potential causes of seizures or collapse, such as electrolyte abnormalities or hypoglycaemia, and to identify any complicating factors. Cats should be given intravenous fluid therapy with appropriate supplementation to correct any hypo-perfusion, dehydration or metabolic abnormalities that may be potentiating the problem.
Lactulose and antibiotic therapy
Medical therapy should be commenced, as described above, with lactulose and antibiotics. Clearly, in a comatose or seizuring cat it will not be possible to administer drugs orally. Parenteral antibiotics can be given, although they are likely to be less efficacious than oral antibiotics; the authors recommend amoxicillin/clavulanate intravenously. Alternatively, neomycin can be given rectally. Lactulose can be administered as a retention enema. A warm water cleansing enema should be given prior to medicated enemas. A cleansing enema will remove faeces and, hence, reduce ammonia absorption from the colon. If there is evidence of gastrointestinal haemorrhage then omeprazole (a proton pump inhibitor) should be given.
Antiseizure medication
Cats can suffer seizures for a variety of reasons and will often receive diazepam as a first-line treatment. However, there is some controversy over the use of diazepam in cats with CPSSs due to concerns over increased GABA and GABA receptors in cats with HE. Cats that do not respond to diazepam and aggressive treatment of HE should be treated with phenobarbitone intravenously (see box on page 182 for more information on phenobarbitone loading). Once the cat is stabilised, oral phenobarbitone can be started.
However, phenobarbitone takes time to reach therapeutic concentrations and so will not stop seizures immediately. In some instances, therefore, it is necessary to treat the cat with propofol, either by bolus or infusion, to control the seizures initially. Propofol infusions should be used with care in cats as prolonged use is associated with delayed recoveries and may in rare cases cause Heinz body anaemia. Cats treated with phenobarbitone and propofol infusions should be closely monitored with regular measurement of pulse rate, respiratory rate, temperature, blood pressure, and so on. Endotracheal intubation may be necessary in some instances.
Levetiracetam is a relatively new antiseizure medication that has been used in cats, although there are no published reports of its use in cats with HE. 49
In cats with severe HE cerebral oedema can develop and may contribute to neurological signs. In these situations a single bolus of mannitol can be given to see if this brings about an improvement in clinical signs. Mannitol should not be used in severely dehydrated or hypoperfused animals.
Monitoring the progression of severely encephalopathic cats, including response to therapy, may be facilitated by the use of a modified Glasgow Coma Scale, as described in dogs. 50
Treatment protocol for the collapsed/seizuring cat.
Physical examination and minimum database
Check for other causes of neurological signs, not related to a CPSS; in particular, electrolyte abnormalities or hypoglycaemia
Give intravenous fluid therapy to correct acid/base deficits, dehydration and/or hypovolaemia. Supplement with glucose and potassium as necessary
If the cat is conscious give oral medication
Lactulose: 0.5–2 ml q8–12h
Antibiotics: ampicillin (10–20 mg/kg q8h), amoxicillin/clavulanate (12.5 mg/kg q12h) or metronidazole (10 mg/kg q12h)
If the cat is not conscious and able to take oral medication provide parenteral/rectal drugs
Cleansing warm water enema: 10 ml/kg
Lactulose: retention enema — 20 ml/kg of a 3:7 dilution with water for 30 mins q6–8h
Antibiotics: intravenous — amoxicillin/clavulanate 20 mg/kg q8h (recommended) retention enema — neomycin 15 mg/kg diluted with water q6h
Mannitol: 0.25–0.5 g/kg intravenously over 30 mins (indicated in severe cases with cerebral oedema)
If the cat is seizuring
(Diazepam: 0.5–1 mg/kg IV, repeated up to three times)
Phenobarbitone: a loading dose of up to a maximum of 8–12 mg/kg should be given as 2–3 mg/kg boluses IV as necessary to control the seizures (allow at least 20–30 mins between doses and reassess the cat prior to dosing). Once loaded, phenobarbitone should be continued at 1.5–3 mg/kg q12h
Propofol: 0.5–1 mg/kg bolus IV followed by 0.05–0.4 mg/kg/min infusion titrated to effect
Levetiracetam: 20 mg/kg IV or as a retention enema q8h followed by same dose orally once recovered
KEY POINTS.
Cats with a CPSS can present with varying types and severity of clinical signs, which sometimes may be relatively mild or nonspecific. Therefore, recognising that portosystemic shunting is a differential diagnosis in any particular case, and initiating further investigations when appropriate, may be challenging for the veterinarian.
There is not a 100% reliable imaging technique that allows a presurgical diagnosis of the presence and type (intra-versus extrahepatic) of a CPSS without sedation or anaesthesia. Ultrasonography is extremely useful in the hands of experienced operators (and often requires sedation to perform a good study). Advanced imaging (MRI and CT) techniques represent the way forward in terms of definitive diagnosis and providing accurate presurgical CPSS anatomical information, but are more expensive and require general anaesthesia.
It is important to stabilise affected cats with appropriate medical management prior to surgical therapy; particularly cats with severe HE or those suffering seizures. The authors would recommend a minimum period of 2 weeks’ medical management before attempting surgical treatment.
References
- 1. Levy JK, Bunch SE, Komtebedde J. Feline portosystemic vascular shunts. In: Bonagura JD, ed. Kirk's current veterinary therapy XII small animal practice. Philadelphia: WB Saunders, 1995: 743–49. [Google Scholar]
- 2. Vulgamott JC, Turnwald GH, King GK, Herring DS, Hansen JF, Boothe HW. Congenital portocaval anomalies in the cat: two case reports. J Am Anim Hosp Assoc 1980; 16: 915–19. [Google Scholar]
- 3. Blaxter AC, Holt PE, Pearson GR, Gibbs C, Gruffydd-Jones TJ. Congenital portosystemic shunts in the cat — a report of 9 cases. J Small Anim Pract 1988; 29: 631–45. [Google Scholar]
- 4. Birchard SJ, Sherding RG. Feline portosystemic shunts. Compend Contin Educ Pract Vet 1992; 14: 1295–301. [Google Scholar]
- 5. Rothuizen J, Vandeningh TSGAM, Voorhout G, Vanderluer RJT, Wouda W. Congenital porto-systemic shunts in sixteen dogs and three cats. J Small Anim Pract 1982; 23: 67–81. [Google Scholar]
- 6. Scavelli TD, Hornbuckle WE, Roth L, et al. Portosystemic shunts in cats — 7 cases (1976–1984). J Am Vet Med Assoc 1986; 189: 317–25. [PubMed] [Google Scholar]
- 7. VanGundy TE, Boothe HW, Wolf A. Results of surgical management of feline portosystemic shunts. J Am Anim Hosp Assoc 1990; 26: 55–62. [Google Scholar]
- 8. Bighignoli B, Owens SD, Froenicke L, Lyons LA. Blood types of domestic cats. In: August JR, ed. Consultations in feline internal medicine 6. Saunders, 2010: 628–38. [Google Scholar]
- 9. Gandolfi RC. Hepatoencephalopathy associated with patent ductus venosus in a cat. J Am Vet Med Assoc 1984; 185: 301–2. [PubMed] [Google Scholar]
- 10. Carr SH, Thornburg LP. Congenital portacaval shunt in 2 kittens. Feline Pract 1984; 14: 43–45. [Google Scholar]
- 11. Berger B, Whiting PG, Breznock EM, Bruhlday R, Moore PF. Congenital feline portosystemic shunts. J Am Vet Med Assoc 1986; 188: 517–21. [PubMed] [Google Scholar]
- 12. Hawe RS, Mullen HS. An unusual portacaval anomaly as a cause of hepatic encephalopathy in a cat. J Am Anim Hosp Assoc 1984; 20: 987–93. [Google Scholar]
- 13. Levesque DC, Oliver JE, Cornelius LM, Mahaffey MB, Rawlings CA, Kolata RJ. Congenital porta-caval shunts in 2 cats — diagnosis and surgical correction. J Am Vet Med Assoc 1982; 181: 143–45. [PubMed] [Google Scholar]
- 14. Lipscomb VJ, Lee KC, Lamb CR, Brockman DJ. Association of mesenteric portovenographic findings with outcome in cats receiving surgical treatment for single congenital portosystemic shunts. J Am Vet Med Assoc 2009. 15; 234: 221–28. [DOI] [PubMed] [Google Scholar]
- 15. Havig M, Tobias KM. Outcome of ameroid constrictor occlusion of single congenital extrahepatic portosystemic shunts in cats: 12 cases (1993–2000). J Am Vet Med Assoc 2002; 220: 337–41. [DOI] [PubMed] [Google Scholar]
- 16. Lipscomb VJ, Jones HJ, Brockman DJ. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec 2007; 160: 465–70. [DOI] [PubMed] [Google Scholar]
- 17. Wolschrijn CF, Mahapokai W, Rothuizen J, Meyer HP, van Sluijs FJ. Gauged attenuation of congenital portosystemic shunts: results in 160 dogs and 15 cats. Vet Quart 2000; 22: 94–98. [DOI] [PubMed] [Google Scholar]
- 18. Lamb CR, Forster-vanHijfte MA, White RN, McEvoy FJ, Rutgers HC. Ultrasonographic diagnosis of congenital portosystemic shunt in 14 cats. J Small Anim Pract 1996; 37: 205–9. [DOI] [PubMed] [Google Scholar]
- 19. White RN, Macdonald NJ, Burton CA. Use of intraoperative mesenteric portovenography in congenital portosystemic shunt surgery. Vet Radiol Ultrasound 2003; 44: 514–21. [DOI] [PubMed] [Google Scholar]
- 20. Van den Ingh TSGAM, Rothuizen J, Meyer HP. Circulatory disorders of the liver in dogs and cats. Vet Quart 1995; 17: 70–76. [DOI] [PubMed] [Google Scholar]
- 21. Kyles AE, Hardie EM, Mehl M, Gregory CR. Evaluation of ameroid ring constrictors for the management of single extrahepatic portosystemic shunts in cats: 23 cases (1996–2001). J Am Vet Med Assoc 2002; 220: 1341–47. [DOI] [PubMed] [Google Scholar]
- 22. Hunt GB. Effect of breed on anatomy of porto-systemic shunts resulting from congenital diseases in dogs and cats: a review of 242 cases. Aust Vet J 2004; 82: 746–49. [DOI] [PubMed] [Google Scholar]
- 23. Payne JT, Martin RA, Constantinescu GM. The anatomy and embryology of portosystemic shunts in dogs and cats. Semin Vet Med Surg (Small Anim) 1990; 5: 76–82. [PubMed] [Google Scholar]
- 24. Center SA, Magne ML. Historical, physical examination, and clinicopathological features of portosystemic vascular anomalies in the dog and cat. Semin Vet Med Surg (Small Anim) 1990; 5: 83–93. [PubMed] [Google Scholar]
- 25. Lamb CR, White RN. Morphology of congenital intrahepatic portacaval shunts in dogs and cats. Vet Rec 1998; 142: 55–60. [DOI] [PubMed] [Google Scholar]
- 26. White RN, Forster-van Hijfte MA, Petrie G, Lamb CR, Hammond RA. Surgical treatment of intrahepatic portosystemic shunts in six cats. Vet Rec 1996; 139: 314–17. [DOI] [PubMed] [Google Scholar]
- 27. Schunk CM. Feline portosystemic shunts. Semin Vet Med Surg (Small Anim) 1997; 12: 45–50. [PubMed] [Google Scholar]
- 28. Kent M. The cat with neurological manifestations of systemic disease. Key conditions impacting on the CNS. J Feline Med Surg 2009; 11: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hardy RM. Pathophysiology of hepatic encephalopathy. Semin Vet Med Surg (Small Anim) 1990; 5: 100–6. [PubMed] [Google Scholar]
- 30. Ruland K, Fischer A, Hartmann K. Sensitivity and specificity of fasting ammonia and serum bile acids in the diagnosis of portosystemic shunts in dogs and cats. Vet Clin Pathol 2010; 39: 57–64. [DOI] [PubMed] [Google Scholar]
- 31. Maddison JE. Canine congenital portosystemic encephalopathy. Aust Vet J 1988; 65: 245–49. [DOI] [PubMed] [Google Scholar]
- 32. Kummeling A, Teske E, Rothuizen J, Van Sluijs FJ. Coagulation profiles in dogs with congenital portosystemic shunts before and after surgical attenuation. J Vet Intern Med 2006; 20: 1319–26. [DOI] [PubMed] [Google Scholar]
- 33. Niles JD, Williams JM, Cripps PJ. Hemostatic profiles in 39 dogs with congenital portosystemic shunts. Vet Surg 2001; 30: 97–104. [DOI] [PubMed] [Google Scholar]
- 34. BSAVA Manual of haematology and transfusion medicine. Gloucester, British Small Animal Veterinary Association. [Google Scholar]
- 35. Center SA, Baldwin BH, Erb H, Tennant BC. Bile acid concentrations in the diagnosis of hepatobiliary disease in the cat. J Am Vet Med Assoc 1986; 189: 891–96. [PubMed] [Google Scholar]
- 36. Center SA, Baldwin BH, de Lahunta A, Dietze AE, Tennant BC. Evaluation of serum bile acid concentrations for the diagnosis of portosystemic venous anomalies in the dog and cat. J Am Vet Med Assoc 1985; 186: 1090–94. [PubMed] [Google Scholar]
- 37. Legendre AM, Krahwinkel DJ, Carrig CB, Michel RL. Ascites associated with intrahepatic arteriovenous fistula in a cat. J Am Vet Med Assoc 1976; 168: 589–91. [PubMed] [Google Scholar]
- 38. d'Anjou MA, Penninck D, Cornejo L, Pibarot P. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Vet Radiol Ultrasound 2004; 45: 424–37. [DOI] [PubMed] [Google Scholar]
- 39. Koblik PD, Hornof WJ. Transcolonic sodium pertechnetate Tc-99m scintigraphy for diagnosis of macrovascular portosystemic shunts in dogs, cats, and potbellied pigs — 176 cases (1988–1992). J Am Vet Med Assoc 1995; 207: 729–33. [PubMed] [Google Scholar]
- 40. Forster-van Hijfte MA, McEvoy FJ, White RN, Lamb CR, Rutgers HC. Per rectal portal scintigraphy in the diagnosis and management of feline congenital portosystemic shunts. J Small Anim Pract 1996; 37: 7–11. [DOI] [PubMed] [Google Scholar]
- 41. Tillson DM, Winkler JT. Diagnosis and treatment of portosystemic shunts in the cat. Vet Clin North Am Small Anim Pract 2002; 32: 881–99. [DOI] [PubMed] [Google Scholar]
- 42. Moon ML. Diagnostic imaging of portosystemic shunts. Semin Vet Med Surg (Small Anim) 1990; 5: 120–26. [PubMed] [Google Scholar]
- 43. Zwingenberger A. CT diagnosis of portosystemic shunts. Vet Clin North Am Small Anim Pract 2009; 39: 783–92. [DOI] [PubMed] [Google Scholar]
- 44. Seguin B, Tobias KM, Gavin PR, Tucker RL. Use of magnetic resonance angiography for diagnosis of portosystemic shunts in dogs. Vet Radiol Ultrasound 1999; 40: 251–58. [DOI] [PubMed] [Google Scholar]
- 45. Brown JC, Chanoit G, Reeder J. Complex extrahepatic portocaval shunt with unusual caval features in a cat: computed tomographic characterisation. J Small Anim Pract 2010; 51: 227–30. [DOI] [PubMed] [Google Scholar]
- 46. Taboada J. Medical management of animals with portosystemic shunts. Semin Vet Med Surg (Small Anim) 1990; 5: 107–19. [PubMed] [Google Scholar]
- 47. Green AS, Ramsey JJ, Villaverde C, Asami DK, Wei A, Fascetti AJ. Cats are able to adapt protein oxidation to protein intake provided their requirement for dietary protein is met. J Nutr 2008; 138: 1053–60. [DOI] [PubMed] [Google Scholar]
- 48. Bircher J, Muller J, Guggenheim P, Haemmerli UP. Treatment of chronic portal-systemic encephalopathy with lactulose. Lancet 1966; 1: 890–92. [DOI] [PubMed] [Google Scholar]
- 49. Smith Bailey K, Dewey CW. The seizuring cat. Diagnostic work-up and therapy. J Feline Med Surg 2009; 11: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Platt SR, Radaelli ST, McDonnell JJ. The prognostic value of the modified Glasgow coma scale in head trauma in dogs. J Vet Intern Med 2001; 15: 581–84. [DOI] [PubMed] [Google Scholar]