Abstract
Practical relevance Although the surgical management of feline congenital portosystemic shunts (CPSSs) is normally performed at specialist centres, a good knowledge of treatment options and prognosis is important for the general practitioner when advising clients.
Clinical challenges A variety of surgical techniques are described for the correction of CPSSs in cats. Choosing between the different techniques is a challenge, given the limited availability of evidence supporting one technique over another. In addition, postoperative complications, and in particular neurological complications, are seen more frequently in the cat than the dog and thus postoperative monitoring and treatment is critically important in feline patients.
Audience This article summarises current evidence in surgical management and is aimed at practising veterinarians, postgraduate students and specialists alike.
Equipment Surgical management of CPSSs typically requires advanced surgical and critical care facilities. The precise nature will depend to some extent on the technique employed.
Evidence base The evidence base for decision making in the surgical management of CPSSs is relatively sparse. In reviewing the evidence that is available, as well as the areas in which information is still lacking, this article may hopefully serve as a stimulus for further investigation into this condition.
Aims of surgery
Surgery is the recommended treatment for most cats with CPSSs. The purpose of surgery is to identify the shunting vessel and to attenuate it in order to redirect blood flow to the liver and restore normal hepatic function. Thus surgical treatment should theoretically offer the best long-term outcome for the individual. There have not been any studies comparing surgical and medical treatment in the cat. However, a recent study compared surgical and medical treatment of CPSSs in dogs and concluded that, in terms of overall survival time, surgical treatment was preferable. 1 Successful surgery should obviate the need for long-term medical treatment, which is attractive to most owners for reasons of convenience and finance.
Surgical options
Suture attenuation
A number of different procedures have been advocated for the surgical treatment of CPSSs in cats. The originally described technique was attenuation of the shunting vessel using a silk ligature. 2–7 However, in some cats the hepatic vasculature is poorly developed (hypoplastic) and is unable to accommodate the increased hepatic blood flow resulting from acute occlusion of the CPSS. This causes portal hypertension, which is rapidly fatal if the vessel is left occluded. Consequently, complete, acute attenuation of a CPSS is only possible in a proportion of cats (see later). It is thus important to temporarily occlude the shunting vessel to assess for evidence of portal hypertension (see box on page 186) prior to deciding whether complete attenuation will be possible. In cats with either subjective or objective evidence of portal hypertension, a partial attenuation is performed with the aim of producing an acceptable increase in portal pressure that has no, or a minimal, effect on the viscera.
The authors recommend the use of prolene suture material for shunt ligation rather than silk, as it has superior strength and knot security. By contrast, silk loses tensile strength and is slowly absorbed over the course of 2 years. Indeed, there is a report of recurrence of shunting following absorption of a silk ligature in a dog. 19
INVESTIGATION, DIAGNOSIS AND STABILISATION.
A sister article focusing on the pathophysiology of congenital portosystemic shunts, the common historical and clinical examination findings, and the investigation, diagnosis and medical management of affected cats appears on pages 173–184 of this issue of J Feline Med Surg, and at:
doi:10.1016/j.jfms.2011.01.010
Assessment and interpretation of portal hypertension.
Portal hypertension can be assessed using direct measurement of portal pressure using a catheter placed in a mesenteric vessel and connected to a manometer or pressure transducer. Little information on acceptable increases in portal pressures in the cat is published. Normal portal pressures in cats with CPSSs have been reported as 3–13 mmHg or 0–14 cmH2O. 4,6,8–10 The authors would limit any increase in portal pressure following CPSS attenuation in cats to no greater than a 10 cmH2O (8 mmHg) increase or doubling from the pre-occlusion value, and not exceeding 20–23 cmH2O (15–18 mmHg) total; these recommendations are based on values extrapolated from information available in the cat and those reported for clinical use in dogs. 6,10–15
The authors would limit any increase in portal pressure following CPSS attenuation in cats to no greater than a 10 cmH2O (8 mmHg) increase or doubling from the pre-occlusion value, and not exceeding 20–23 cmH2O (15–18 mmHg) total.
Additionally, cats that are coping well with CPSS occlusion should not show any decrease in arterial or central venous pressure or significant increase in heart rate. Portal hypertension can also be assessed subjectively by observing the intestines and pancreas, and this is reported to be as reliable as objective measurements. 10,18
Portal pressure and other objective measurements are influenced by a variety of factors and may not be accurate so they should be interpreted in view of subjective changes and the appearance of the hepatic vasculature on portovenography. 10 Intestinal hypermotility, pulsation in jejunal vessels and cyanosis of the intestines and pancreas are associated with portal hypertension.
Guidelines for intraoperative changes in objective parameters associated with attenuation of CPSSs in cats
Normal portal pressure in cats: 0–14 cmH2O / 3–13 mmHg 4,6,8–10
-
Acceptable increases in portal pressure associated with attenuation:
— Maximum total pressure reading 20–23 cmH2O (15–18 mmHg) 6,10,11,13,15
— Maximum total pressure reading not more than double pre-occlusion level
— Increase in pressure reading not greater than 10 cmH2O (8 mmHg) 11–15
-
Acceptable changes in other cardiovascular parameters associated with attenuation:
— Maximum decrease in systemic blood pressure 5 mmHg 16
— Maximum decrease in central venous pressure 1 cmH2O 17
Full ligation (Fig 1) has been reported to be possible in 29–43% of cats. 6–8,15,20,21 A study of six cats with intrahepatic CPSSs found that only one (17%) of the cats could tolerate a full attenuation. 10 A larger study did not detect a significant difference in the proportion of complete attenuation between intrahepatic and extrahepatic CPSSs. 21
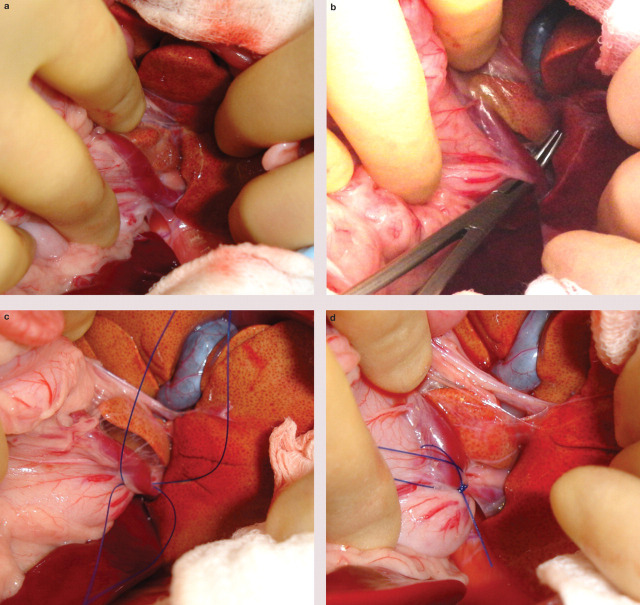
FIG 1.
(a) Exploratory laparotomy in a cat with a left gastric extrahepatic CPSS. The stomach has been retracted caudoventrally to reveal the shunting vessel in the region of the gastric cardia. (b) Mixter forceps are used to carefully dissect around the shunt. (c) Two lengths of polypropylene suture (Prolene; Ethicon) have been passed around the shunting vessel to allow temporary occlusion and subsequent ligation. (d) The cat tolerated complete occlusion of the abnormal vessel and the suture has been tied tightly. The other length of suture will be removed prior to routine abdominal closure
Some reports have described cats with portal atresia, whereby the portal vein was apparently underdeveloped or absent. 5,7,22 Some of these cats were euthanased due to a perceived poor prognosis. Cats with CPSSs are likely to have a degree of underdevelopment (hypoplasia) of the portal vein as a consequence of the shunting vessel. This will vary in severity and in extreme instances the portal vein may appear absent. The authors believe that true portal vein aplasia is extremely uncommon in cats. A very underdeveloped portal vein may not be visible on gross inspection at laparotomy but may become obvious after temporary occlusion of the CPSS. Similarly, studies have shown that although there may be no opacification of the intrahepatic vasculature on portovenography before attenuation, there is a significant improvement following temporary occlusion of the CPSS. 8,20
Surgery is the recommended treatment for most cats with CPSSs.
Several studies have documented that cats with complete CPSS attenuation have a better long-term outcome than those treated with partial attenuation. Cats treated with partial attenuation have shown a high frequency of relapse of clinical signs, which is usually attributable to persistent shunting but may also be due to the development of multiple acquired shunts. 5–7,15,19,22 Nevertheless some cats can have a good outcome following partial attenuation. 5,10,19 It is unclear whether these animals achieve full attenuation with time or whether they cope with a small amount of residual shunting.
A second surgery performed 2–3 months later allows a second attempt at complete attenuation in cats treated initially with partial suture attenuation. 19,23 During this time the hepatic vasculature will have developed, allowing full attenuation in the majority of cats. 6–8,21 This is supported by the findings of a study comparing portovenography at first and second surgeries which showed that the degree of opacification of intrahepatic vessels increased significantly between procedures. 8 The authors routinely perform follow-up surgery in cats that have undergone partial attenuation and leave a length of suture material around the shunt to be tied at the second surgery, thus avoiding further dissection around the shunt. Repeat surgery has been shown to be associated with a negligible rate of postoperative complications. 8
Any cat that can tolerate a complete acute CPSS attenuation (suture or cellophane band) can benefit from this, instead of receiving a gradual, possibly incomplete, CPSS attenuation.
Ameroid constrictors.
Ameroid constrictors consist of a ring of casein with a lumen, surrounded by a stainless steel collar. There is a gap in the ring to allow it to be placed around a vessel and this is closed with a small ‘key’ of casein. In theory, the casein absorbs fluid and expands and thus the lumen is gradually occluded. However, it has been shown experimentally that ameroid constrictors cause vessel occlusion primarily by thrombus formation, that this occlusion may be more rapid than desired and that re-canalisation is possible. 24,25
Ameroid constrictors and cellophane banding
Ameroid constrictors (Fig 2) and cellophane banding (Fig 3) are also used for the surgical treatment of CPSSs in cats with the aim of gradually attenuating the shunt over a period of weeks. This approach is designed to produce complete shunt attenuation gradually without causing portal hypertension, thereby avoiding the need for multiple surgeries in cats that cannot tolerate complete attenuation at the first attempt. However, long-term residual shunting is reported with both techniques and the exact rate of attenuation is unknown.
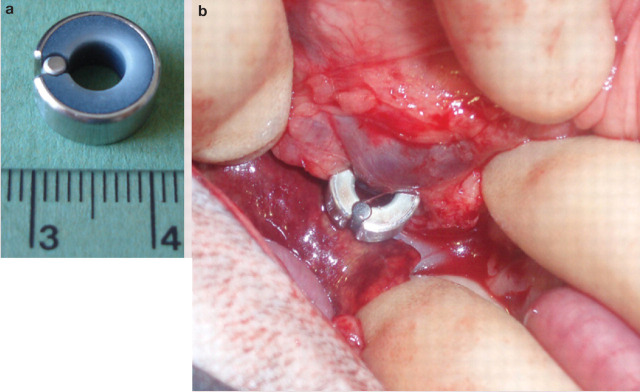
FIG 2.
(a) An ameroid constrictor with the key in place. (b) Intraoperative image of an ameroid constrictor placed on a portocaval shunt in a cat. Image (b) courtesy of Mr Alasdair Hotston Moore
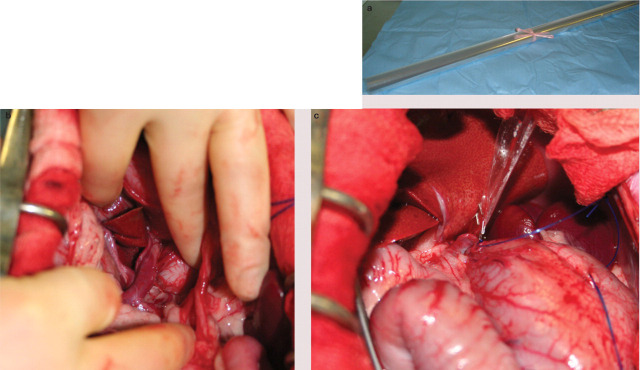
FIG 3.
(a) Roll of (florist's) cellophane for attenuation of a CPSS. The cellophane is cut and folded to form a three layer strip approximately 10 cm long and 4 mm wide. 28 (b) Intraoperative image of a large left gastric shunt in a cat. (c) The shunt has been dissected and a cellophane band has been placed around the vessel. The cellophane has been secured with two haemoclips. The cellophane is cut short prior to abdominal closure. Images (b) and (c) courtesy of Jane Ladlow
Ameroid constrictors were first described for the treatment of extrahepatic CPSSs in two cats. 26 Subsequently, two published studies have reported the outcome for cats with extra-hepatic CPSSs treated with ameroid constrictors, with mixed results (Table 1). 9,27 Information regarding the technique of cellophane banding in cats is limited to two small case series involving five and nine cats, respectively. 28,29
TABLE 1.
Summary of the outcome of surgical management of feline CPSSs, as reported in the literature
| Study | No. of cats | Method of attenuation | Postoperative neurological complication rate | Mortality rate | Short-term outcome | Long-term outcome |
|---|---|---|---|---|---|---|
| White et al (1996) 10 | 6 | Suture (5 partial, 1 full attenuation) | 16.7% 1/6 cats suffered severe neurological complications | 16.7% 1/6 cats died due to neurological complications |
|
|
| Wolschrijn et al (2000) 22 | 15 | Suture | 13.3% 2/15 cats suffered severe neurological complications that resulted in status epilepticus and coma | 20% 3/15 cats died, two due to neurological complications and one euthanased intraoperatively due to portal vein hypoplasia | ||
| Havig and Tobias (2002) 9 | 12 | Ameroid constrictor | 33.3% 4/12 cats suffered neurological complications — however, all had neurological signs before surgery | 0% 0/12 |
|
Excellent in 2/9 (22.2%) cats (10–60 months): Clinically normal |
| ||||||
| Kyles et al (2002) 27 | 23 | Ameroid constrictor | Up to 77% 17/22 cats suffered some form of complication including blindness (10), hyperthermia (6), frantic behaviour (5), encephalopathy (5), seizures (3), focal seizures (1), coagulopathy (1), tremors (1), seroma (1) and transfusion reaction (1). The precise proportion of cats with neurological complications was unclear |
|
|
|
| Hunt et al (2004) 28 | 5 | Cellophane banding |
|
|
|
|
| Lipscomb et al (2007) 21 | 49 | Suture (28 partial, 21 full attenuation) |
|
|
|
|
| Cabassu et al (2011) 29 | 9 | Cellophane banding |
|
|
|
|
Although some surgeons simply place an ameroid constrictor or cellophane band around a CPSS without measuring portal pressure, and without any initial shunt attenuation, others recommend measuring portal pressure and performing temporary complete CPSS occlusion in all cases because of the following advantages:
Cellophane bands.
Cellophane can be placed around the shunt while providing some degree of attenuation depending on evidence of portal hypertension. 28 Alternatively, it can be placed without any shunt attenuation. 29 It is then secured in place with surgical clips. The cellophane causes fibrosis of the vessel, resulting in gradual occlusion over a period of weeks.
Any cat that can tolerate a complete acute CPSS attenuation (using suture or cellophane band) can benefit from this, instead of receiving a gradual, possibly incomplete, CPSS attenuation.
A cat with a very high initial portal pressure (eg, >25 mmHg) following temporary CPSS occlusion may not be a good candidate for an ameroid constrictor; this is because of the risk of development of multiple acquired shunts if the rate of shunt attenuation exceeds the capacity of the intrahepatic portal vasculature to accommodate the increased blood flow. 23
A cellophane band can be placed to provide initial partial attenuation of the CPSS to an acceptable level (similar to partial suture attenuation), with the expectation of further attenuation with time.
Whichever technique is used it is important to perform close postoperative monitoring as it is still possible for acute portal hypertension to develop if an ameroid constrictor suddenly moves and occludes the CPSS, if a cellophane band is applied too tightly or if acute thrombus formation in the CPSS occurs.
Other surgical considerations
Cystic calculi
Consideration should be given to removing cystic calculi from affected cats at the same time as surgical correction of the CPSS. 21 If the surgery is prolonged or complicated then removal of calculi may be postponed until the cat has recovered sufficiently for a second procedure.
Neutering of affected animals
As PSSs are congenital in origin, affected animals should not be used for breeding. The authors recommend neutering of affected cats either during surgical attenuation or at a later date.
Intrahepatic versus extrahepatic shunts
Intrahepatic CPSSs are more challenging to treat surgically than extrahepatic shunts due to their location within the hepatic parenchyma. In one study of six cats with intrahepatic CPSSs it was possible to attenuate the vessel directly either posthepatically in cats with a left divisional CPSS or via intrahepatic dissection in right or central divisional CPSSs, without the need for more complicated intravascular techniques. 10 An anatomical study in cats concluded that patent ductus venosus can be readily ligated in a prehepatic location where the vessel enters an ampulla prior to the caudal vena cava. 30 One report described the successful use of an ameroid constrictor on a Siamese cat with a right divisional intrahepatic CPSS, but it is usually more difficult to apply either an ameroid constrictor or cellophane band to an intrahepatic shunt because there is generally restricted space around the shunt following dissection compared with an extra-hepatic shunt. 31 Intraoperative haemorrhage is much more of a concern with intrahepatic shunts and ideally these cats should be cross-matched to a blood donor cat prior to surgery.
An alternative to surgical management is coil embolisation using interventional radiology, which has been described in one cat with an intrahepatic CPSS. 32
Liver biopsy and histopathology
Liver biopsy is often performed at the same time as surgical correction of CPSSs in cats (Fig 4). This allows assessment of the underlying pathology and identification of any additional pathology. Typical histopathological changes associated with a CPSS include lobular atrophy, hepatocyte degeneration, reduced or absent portal veins, arteriole hyperplasia, biliary hyperplasia, dilated lymphatics and, occasionally, mild portal fibrosis. 4,21,27,33 To date, no studies in cats have investigated the relationship, if any, between the histopathological changes and outcome, although none has been identified in dogs with CPSSs. 34,35
FIG 4.
Photomicrograph of a liver biopsy taken from a cat with a CPSS (x 200 magnification). Note the proliferation of arterioles and biliary tracts and the absence of portal venules
Anaesthesia for cats undergoing surgical CPSS attenuation.
Anaesthesia of cats with CPSSs can be challenging. Agents that are metabolised by the liver should be avoided or used in low doses. Opiates can be used for premedication and analgesia with minimal cardiovascular depression. Anaesthesia can be induced with propofol or alfaxalone, taking extra care to use the minimum amount necessary to achieve intubation. 23 Isoflurane and sevoflurane are minimally metabolised by the liver and are therefore good choices.
As cats are relatively small there is a significant risk of hypothermia. Steps should be taken to minimise heat loss during surgery and an incubator is extremely useful for recovery. With experienced surgical and anaesthetic teams, surgery can be relatively straightforward and surgical times are not particularly long, even with intraoperative portovenography (approximately 60–90 mins). Although hypoglycaemia is not as common in cats as dogs, glucose levels should still be monitored perioperatively and supplemented as necessary.
Surgical complications
Recent data suggest that most cats have a good outcome following surgery, which is comparable to outcomes reported for dogs. 21 However, all long-term outcome data is based on subjective owner assessment. Although objective measures, such as bile acid testing and scintigraphy, have been used in some studies these have assessed short-term outcome (6 months or less). 9,21,27,28 It, therefore, remains unclear whether all cats achieve full shunt attenuation following surgery and what proportion develops multiple acquired shunts. Although it has been shown that cats have a better outcome with full attenuation compared with partial attenuation, in some instances partial attenuation may be associated with a good outcome as assessed by the owner. The authors still recommend complete attenuation in all instances if possible.
Intraoperative complications
Intraoperative complications are uncommon, with iatrogenic pneumothorax described in one cat and haemorrhage from the CPSS in two others. 20,22 Two other studies reported no intraoperative complications. 8,9
Neurological complications
Neurological problems are the most common postoperative complication (Fig 5) and are reported in 13.3–37% of surgically treated cats. 9,21,22,28 An additional study reported a high number of cats with postoperative complications (77%), but the precise proportion of these that were neurological is unclear. 27 There seems to be a greater risk of these complications in cats compared with dogs, in which neurological complications are reported in 0–20.6% of cases. 36–42 It has been shown that these complications do not seem to relate to hepatic encephalopathy (HE) or hypoglycaemia as ammonia has been normal or dramatically reduced postsurgery compared with presurgery levels, and glucose and electrolytes are normal. 8,21,43
FIG 5.
Cat suffering from neurological complications following full suture attenuation of a CPSS. He initially recovered well from surgery but the following day become increasingly uncoordinated and mentally depressed. Neurological signs were a result of the surgery rather than a progression of HE. The cat was treated with phenobarbitone and levetiracetam. His neurological signs gradually improved and he was discharged from the hospital on continued treatment. Courtesy of Miss Sophie Adamantos
Neurological signs can vary in form and severity and include mild tremors or ataxia, central blindness, depression and weakness, hyperaesthesia, seizures and status epilepticus. Seizures are a particularly severe complication and are reported in 6.5–22.4% of cats treated with suture attenuation. 7,10,21,22 Seizures usually occur within 72 h of surgery and it is recommended that cats are monitored closely during this time. 8,21,27,43
Neurological problems are the most common postoperative complication, and are reported in 13.3–37% of surgically treated cats …
In cats treated with ameroid constrictors it might be expected that neurological signs present before surgery would persist for a period of time and that no new neurological signs would occur as attenuation of the CPSS should be gradual. In one study, seizures in one cat and neurological signs in three were still present after surgery. 9 In another study of ameroid constrictors in 23 cats, 45% suffered blindness and 14% seizures postoperatively. 27 Thus neurological complications still occur following treatment with gradual occlusion devices.
Phenobarbitone is the first-line treatment for cats with neurological complications. However, cats with severe or refractory seizures may need boluses of propofol or an infusion to manage their signs initially while the phenobarbitone is loaded (see sister article on investigation, diagnosis and stabilisation, doi:10.1016/j.jfms.2011.01.010). 8,21,43 Levetiracetam could be used in addition, or as an alternative, to phenobarbitone but there is no published information regarding its use in cats with seizures following shunt surgery. Although treatment for HE should be continued in animals that have had a partial or gradual attenuation this is rarely effective in cats with postoperative neurological complications and antiseizure medication should be given as early as possible. Any confounding factors such as hypoglycaemia or gastrointestinal haemorrhage should also be treated (see sister article).
… Despite postoperative neurological problems, cats can still make a full recovery, with 56% having complete resolution of neurological signs and 22% having a good quality of life with medical management of their neurological signs in one study.
Poorly developed intrahepatic vasculature is the only risk factor that has been identified for the occurrence of postoperative neurological signs. There does not seem to be a difference between intrahepatic and extrahepatic CPSSs, full or partial ligation, the age of the cat or the presence of seizures preoperatively.
Unfortunately, a number of cats (4–22.2%) die or are euthanased due to the severity of neurological complications. 21,22,27,29 Other cats never fully recover or require long-term anti-seizure medication. One study identified that cats with poor development of their intrahepatic vasculature (as assessed on portovenography) had an increased risk of developing postoperative neurological complications. 8 However, no other risk factors have been identified for the occurrence of postoperative neurological signs and there does not seem to be a difference between intrahepatic and extrahepatic CPSSs, full or partial ligation, the age of the cat or the presence of seizures pre-operatively. 21 Despite postoperative neurological problems, cats can still make a full recovery, with 56% having complete resolution of neurological signs and 22% having a good quality of life with medical management of their neurological signs in one study. 21
The origin of these postoperative neurological complications is unclear because seizures can occur in cats following surgery despite a postoperative return to normal shunt fractions on scintigraphy and normal bile acid stimulation results. 9 Thus continued neurological signs may be related to primary alterations in the central nervous system. In addition, HE has a profound effect on cerebral metabolism and it is suggested that this chronic abnormal metabolism affects the brain so that it is unable to function normally immediately after the shunt has been corrected. 44 Postsurgical decreases in endogenous inhibitory central nervous system benzodiazepine agonist levels or an imbalance in excitatory and inhibitory neurotransmitters are possible explanations for these neurological signs. 36 It is also possible that in some cats preoperative seizures result in a degree of irreversible damage to the nervous system that, therefore, persists after surgical correction of the CPSS (although this clearly does not account for those cats without seizures prior to surgery).
Portal hypertension
One of the major concerns with surgery is the development of postoperative portal hypertension, which can be fatal. Portal hypertension is characterised by ascites, abdominal pain, hypovolaemic shock and haemorrhagic diarrhoea. Older studies reported a relatively high mortality rate with suture ligation of 10–11%, due to neurological complications and postoperative portal hypertension. 7,14 However, with improvements in anaesthesia and surgery, and more familiarity with the risk of portal hypertension, mortality has decreased. Reported postoperative mortality is 0–4.5% in the more recent studies of treatment with suture ligation or ameroid constrictors. 9,21,27 Another study on partial suture ligation of CPSSs reported a mortality rate of 3/15 cats (20%) although one of these cats was euthanased due to severe portal vein hypoplasia. 22 Mortality in the more recent studies is usually associated with development of neurological complications and no cats died as a result of portal hypertension.
Other, minor postoperative complications
Minor postoperative complications reported include mild to moderate ascites (likely due to subclinical portal hypertension, which is an expected sequela to CPSS attenuation), seroma formation, a clinical coagulopathy, a transfusion reaction and pyrexia. 21,27 One study of the use of ameroid constrictors in cats did not identify any postoperative complications. 9 However, another study found a high rate of complications during the immediate postoperative period (77%). 27
In the more recent studies, mortality has usually been associated with the development of neurological complications — no cats died as a result of portal hypertension.
Prophylactic phenobarbitone — a strategy to consider?
Prophylactic treatment of cats with phenobarbitone has been suggested in order to reduce the likelihood of postoperative seizures. Little information is available to support this concept in the cat. In one study, seizures occurred in one cat despite preoperative phenobarbitone administration and in another study neurological complications occurred despite 83% of cats receiving preoperative antiseizure medication. 9,27 Phenobarbitone is metabolised in the liver and must be used carefully in animals with hepatic insufficiency. For these reasons the authors do not routinely give preoperative pheno-barbitone to cats. They prefer to monitor cats closely for neurological signs in the postoperative period. Anti-seizure (phenobarbitone or propofol) medication is given promptly if neurological signs are detected, even if relatively mild.
Outcome/prognosis following surgery
Follow-up bile acid testing can be used to assess cats following surgery. However, in some cats bile acids may still be mild to moderately increased despite a good clinical outcome. 21 Scintigraphy has also been used to monitor the response to treatment. 27 In instances where cats have had a less than excellent outcome scintigraphy has revealed persistent shunting, presumably due either to failure to achieve complete attenuation or to the formation of multiple acquired shunts. 27 In one study of ameroid constrictors, 57% of cats had persistent shunting 8–10 weeks following surgery. 27 Nevertheless, 75% went on to have an excellent outcome.
Suture attenuation
In the largest case series reporting on cats treated with suture attenuation, 30/36 cats were still alive after a median of 47 months, with only two having been euthanased due to the development of postoperative neurological complications/seizures. 21 Of the 36 surviving cats, 56% were considered to be normal, 19% exhibited very minor clinical signs, 8% had a good quality of life with continued medical management for HE, 11% were treated medically for intermittent seizures and 6% had been euthanased due to persistent seizures. 21 This suggests that surgical treatment is associated with a good long-term outcome (Fig 6).
FIG 6.
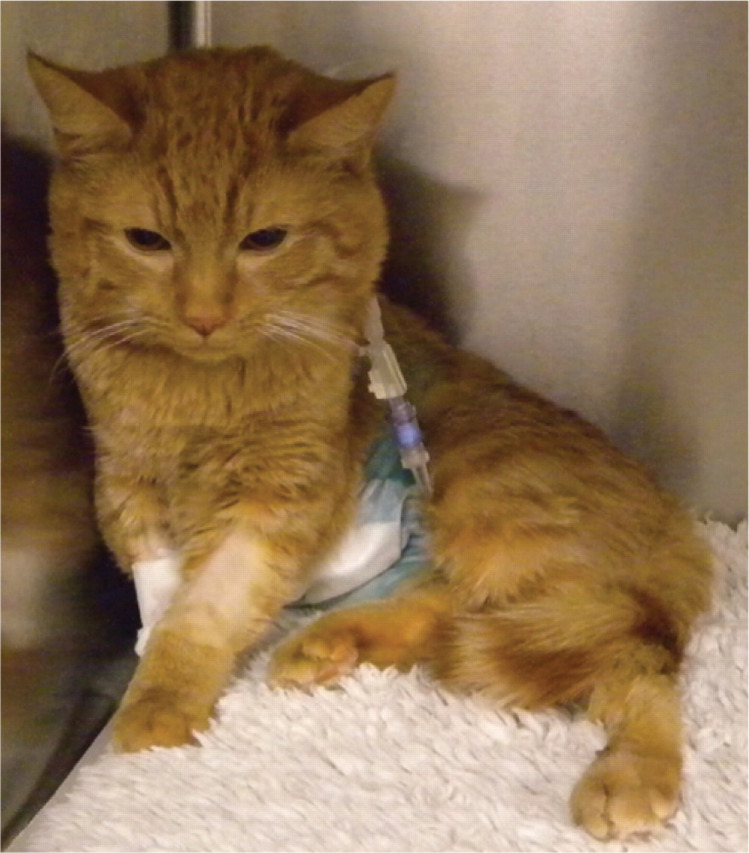
Cat making a normal recovery following full suture attenuation of a CPSS
Ameroid constrictors
Two case series described the outcome of cats treated with ameroid constrictors. 9,27 In one study long-term follow-up was available for 9/12 cats. 9 Four of the cats (45%) were euthanased at a median of 11.5 months after surgery because of progression or persistence of seizures or neurological signs. Three cats (33%) were clinically normal 10 months to 5 years after surgery and the remaining two cats (22%) had progression or recurrence of clinical signs 6 months after surgery. In the other study follow-up was available for 20/21 cats at a mean of 26 months. 27 Three cats (15%) had been euthanased or died due to persistent neurological signs, two cats (10%) had intermittent clinical signs (one was on medical treatment) and 15 cats (75%) were considered to be normal. Studies have also shown persistent portosystemic shunting (as determined by scintigraphy) in a significant proportion of cats treated with ameroid constrictors, suggesting failure of the ameroid to fully occlude the shunt or the development of multiple acquired shunts. 9,26,27
Several studies have shown that cats with complete CPSS attenuation have a better long-term outcome than those treated with partial attenuation.
Cellophane banding
One study described the outcome of cellophane banding in five cats. 28 All of the cats survived the postoperative period although one (20%) developed mild neurological signs which resolved after 7 days. Three cats had normal liver function, as assessed by ammonia or bile acid testing, and two cats had persistent abnormal liver function. Repeat surgery revealed that one cat had developed multiple acquired shunts and the other cat had persistent shunting due to failure of the cellophane band to produce fibrosis. Another study described the use of cellophane banding without intraoperative attenuation in nine cats. 29 Two cats (22.2%) were euthanased post-operatively due to persistent seizures. Postprandial bile acids were normal in all of the five cats tested 3–12 months postsurgery. One cat was euthanased 3.5 months following surgery due to recurrence of clinical signs and another cat required a cystotomy for removal of ammonium biurate stones more than 2 years postoperatively.
KEY POINTS.
Surgical attenuation is the recommended treatment for CPSSs but the method of choice remains controversial. Cats with a simple extrahepatic CPSS that cannot tolerate complete attenuation at surgery have the option of having a staged suture attenuation (two surgeries), placement of an ameroid constrictor or placement of a cellophane band. Substantial long-term data that directly compares these techniques is lacking.
Intrahepatic shunts are more challenging to treat surgically. In some instances, where space around the CPSS is restricted, attenuation may only be achieved by placement of a suture ligature. A cellophane band may be more likely to fit around an intrahepatic shunt than an ameroid constrictor.
Seizures are the most common postoperative complication and the only significant cause of perioperative mortality in recent studies. Prompt and aggressive treatment with phenobarbitone, and a propofol infusion if necessary, is recommended.
References
- 1. Greenhalgh SN, Dunning MD, McKinley TJ, et al. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. J Am Vet Med Assoc 2010; 236: 1215–20. [DOI] [PubMed] [Google Scholar]
- 2. Levesque DC, Oliver JE, Cornelius LM, Mahaffey MB, Rawlings CA, Kolata RJ. Congenital portacaval shunts in 2 cats — diagnosis and surgical correction. J Am Vet Med Assoc 1982; 181: 143–45. [PubMed] [Google Scholar]
- 3. Scavelli TD, Hornbuckle WE, Roth L, et al. Portosystemic shunts in cats — 7 cases (1976–1984). J Am Vet Med Assoc 1986; 189: 317–25. [PubMed] [Google Scholar]
- 4. Berger B, Whiting PG, Breznock EM, Bruhlday R, Moore PF. Congenital feline portosystemic shunts. J Am Vet Med Assoc 1986; 188: 517–21. [PubMed] [Google Scholar]
- 5. Blaxter AC, Holt PE, Pearson GR, Gibbs C, Gruffydd-Jones TJ. Congenital portosystemic shunts in the cat — a report of 9 cases. J Small Anim Pract 1988; 29: 631–45. [Google Scholar]
- 6. VanGundy TE, Boothe HW, Wolf A. Results of surgical management of feline portosystemic shunts. J Am Anim Hosp Assoc 1990; 26: 55–62. [Google Scholar]
- 7. Levy JK, Bunch SE, Komtebedde J. Feline portosystemic vascular shunts. In: Bonagura JD, ed. Kirk's current veterinary therapy XII small animal practice. Philadelphia: WB Saunders, 1995: 743–49. [Google Scholar]
- 8. Lipscomb VJ, Lee KC, Lamb CR, Brockman DJ. Association of mesenteric portovenographic findings with outcome in cats receiving surgical treatment for single congenital portosystemic shunts. J Am Vet Med Assoc 2009; 234: 221–28. [DOI] [PubMed] [Google Scholar]
- 9. Havig M, Tobias KM. Outcome of ameroid constrictor occlusion of single congenital extrahepatic portosystemic shunts in cats: 12 cases (1993–2000). J Am Vet Med Assoc 2002; 220: 337–41. [DOI] [PubMed] [Google Scholar]
- 10. White RN, Forster-van Hijfte MA, Petrie G, Lamb CR, Hammond RA. Surgical treatment of intrahepatic portosystemic shunts in six cats. Vet Rec 1996; 139: 314–17. [DOI] [PubMed] [Google Scholar]
- 11. Martin RA, Freeman LE. Identification and surgical management of porto-systemic shunts in the dog and cat. Semin Vet Med Surg (Small Anim) 1987; 2: 302–6. [PubMed] [Google Scholar]
- 12. Birchard SJ. Surgical management of portosystemic shunts in dogs and cats. Compend Contin Educ Pract Vet 1984; 6: 795–801. [Google Scholar]
- 13. Butler LM, Fossum TW, Boothe HW. Surgical management of extrahepatic portosystemic shunts in the dog and cat. Semin Vet Med Surg (Small Anim) 1990; 5: 127–33. [PubMed] [Google Scholar]
- 14. Schunk CM. Feline portosystemic shunts. Semin Vet Med Surg (Small Anim) 1997; 12: 45–50. [PubMed] [Google Scholar]
- 15. Birchard SJ, Sherding RG. Feline portosystemic shunts. Compend Contin Educ Pract Vet 1992; 14: 1295–301. [Google Scholar]
- 16. Hunt GB, Bellenger CR, Pearson MRB. Transportal approach for attenuating intrahepatic portosystemic shunts in dogs. Vet Surg 1996; 25: 300–8. [DOI] [PubMed] [Google Scholar]
- 17. Swalec KM, Smeak DD. Partial versus complete attenuation of single portosystemic shunts. Vet Surg 1990; 19: 406–11. [DOI] [PubMed] [Google Scholar]
- 18. Mathews K, Gofton N. Congenital extrahepatic portosystemic shunt occlusion in the dog — gross observations during surgical correction. J Am Vet Med Assoc 1988; 24: 387–94. [Google Scholar]
- 19. Burton CA, White RN. Portovenogram findings in cases of elevated bile acid concentrations following correction of portosystemic shunts. J Small Anim Pract 2001; 42: 536–40. [DOI] [PubMed] [Google Scholar]
- 20. White RN, Macdonald NJ, Burton CA. Use of intraoperative mesenteric portovenography in congenital portosystemic shunt surgery. Vet Radiol Ultrasound 2003; 44: 514–21. [DOI] [PubMed] [Google Scholar]
- 21. Lipscomb VJ, Jones HJ, Brockman DJ. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec 2007; 160: 465–70. [DOI] [PubMed] [Google Scholar]
- 22. Wolschrijn CF, Mahapokai W, Rothuizen J, Meyer HP, van Sluijs FJ. Gauged attenuation of congenital portosystemic shunts: results in 160 dogs and 15 cats. Vet Quart 2000; 22: 94–98. [DOI] [PubMed] [Google Scholar]
- 23. Tillson DM, Winkler JT. Diagnosis and treatment of portosystemic shunts in the cat. Vet Clin North Am Small Anim Pract 2002; 32: 881–99. [DOI] [PubMed] [Google Scholar]
- 24. Besancon MF, Kyles AE, Griffey SM, Gregory CR. Evaluation of the characteristics of venous occlusion after placement of an ameroid constrictor in dogs. Vet Surg 2004; 33: 597–605. [DOI] [PubMed] [Google Scholar]
- 25. Adin CA, Gregory CR, Kyles AE, Griffey SM, Kendall L. Effect of petrolatum coating on the rate of occlusion of ameroid constrictors in the peritoneal cavity. Vet Surg 2004; 33: 11–16. [DOI] [PubMed] [Google Scholar]
- 26. Vogt JC, Krahwinkel DJ, Bright RM, Daniel GB, Toal RL, Rohrbach B. Gradual occlusion of extrahepatic portosystemic shunts in dogs and cats using the ameroid constrictor. Vet Surg 1996; 25: 495–502. [DOI] [PubMed] [Google Scholar]
- 27. Kyles AE, Hardie EM, Mehl M, Gregory CR. Evaluation of ameroid ring constrictors for the management of single extrahepatic portosystemic shunts in cats: 23 cases (1996–2001). J Am Vet Med Assoc 2002; 220: 1341–47. [DOI] [PubMed] [Google Scholar]
- 28. Hunt GB, Kummeling A, Tisdall PL, et al. Outcomes of cellophane banding for congenital portosystemic shunts in 106 dogs and 5 cats. Vet Surg 2004; 33: 25–31. [DOI] [PubMed] [Google Scholar]
- 29. Cabassu J, Seim HB, Macphail CM, Monnet E. Outcomes of cats undergoing surgical attenuation of congenital extrahepatic portosystemic shunts through cellophane banding: 9 cases (2000–2007). J Am Vet Med Assoc 2011; 238: 89–93. [DOI] [PubMed] [Google Scholar]
- 30. White RN, Burton CA. Anatomy of the patent ductus venosus in the cat. J Feline Med Surg 2001; 3: 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bright SR, Williams JM, Niles JD. Outcomes of intrahepatic portosystemic shunts occluded with ameroid constrictors in nine dogs and one cat. Vet Surg 2006; 35: 300–9. [DOI] [PubMed] [Google Scholar]
- 32. Weisse C, Schwartz K, Stronger R, Mondschein JI, Solomon JA. Transjugular coil embolization of an intrahepatic portosystemic shunt in a cat. J Am Vet Med Assoc 2002; 221: 1287–91, 66–67. [DOI] [PubMed] [Google Scholar]
- 33. Forster-van Hijfte MA, McEvoy FJ, White RN, Lamb CR, Rutgers HC. Per rectal portal scintigraphy in the diagnosis and management of feline congenital portosystemic shunts. J Small Anim Pract 1996; 37: 7–11. [DOI] [PubMed] [Google Scholar]
- 34. Baade S, Aupperle H, Grevel V, Schoon HA. Histopathological and immunohistochemical investigations of hepatic lesions associated with congenital portosystemic shunt in dogs. J Comp Pathol 2006; 134: 80–90. [DOI] [PubMed] [Google Scholar]
- 35. Parker JS, Monnet E, Powers BE, Twedt DC. Histologic examination of hepatic biopsy samples as a prognostic indicator in dogs undergoing surgical correction of congenital portosystemic shunts: 64 cases (1997–2005). J Am Vet Med Assoc 2008; 232: 1511–14. [DOI] [PubMed] [Google Scholar]
- 36. Hardie EM, Kornegay JN, Cullen JM. Status epilepticus after ligation of portosystemic shunts. Vet Surg 1990; 19: 412–17. [DOI] [PubMed] [Google Scholar]
- 37. Hunt GB, Hughes J. Outcomes after extrahepatic portosystemic shunt ligation in 49 dogs. Aust Vet J 1999; 77: 303–7. [DOI] [PubMed] [Google Scholar]
- 38. Mehl ML, Kyles AE, Hardie EM, et al. Evaluation of ameroid ring constrictors for treatment for single extrahepatic portosystemic shunts in dogs: 168 cases (1995–2001). J Am Vet Med Assoc 2005; 226: 2020–30. [DOI] [PubMed] [Google Scholar]
- 39. Kummeling A, Van Sluijs FJ, Rothuizen J. Prognostic implications of the degree of shunt narrowing and of the portal vein diameter in dogs with congenital portosystemic shunts. Vet Surg 2004; 33: 17–24. [DOI] [PubMed] [Google Scholar]
- 40. Hurn SD, Edwards GA. Perioperative outcomes after three different single extrahepatic portosystemic shunt attenuation techniques in dogs: partial ligation, complete ligation and ameroid constrictor placement. Aust Vet J 2003; 81: 666–70. [DOI] [PubMed] [Google Scholar]
- 41. Tisdall PL, Hunt GB, Youmans KR, Malik R. Neurological dysfunction in dogs following attenuation of congenital extrahepatic portosystemic shunts. J Small Anim Pract 2000; 41: 539–46. [DOI] [PubMed] [Google Scholar]
- 42. Lee KC, Lipscomb VJ, Lamb CR, Gregory SP, Guitian J, Brockman DJ. Association of portovenographic findings with outcome in dogs receiving surgical treatment for single congenital portosystemic shunts: 45 cases (2000–2004). J Am Vet Med Assoc 2006; 229: 1122–29. [DOI] [PubMed] [Google Scholar]
- 43. Heldmann E, Holt DE, Brockman DJ, Brown DC, Perkowski SZ. Use of propofol to manage seizure activity after surgical treatment of portosystemic shunts. J Small Anim Pract 1999; 40: 590–94. [DOI] [PubMed] [Google Scholar]
- 44. Matushek KJ, Bjorling D, Mathews K. Generalized motor seizures after portosystemic shunt ligation in dogs — 5 cases (1981–1988). J Am Vet Med Assoc 1990; 196: 2014–17. [PubMed] [Google Scholar]