Abstract
Overview:
Leptospirosis is a bacterial disease affecting a variety of domestic and wild animals as well as humans worldwide. Leptospirosis has been reported in over 150 mammalian species. It is considered an emerging infectious disease in humans and in dogs. Subclinically infected wild and domestic animals serve as reservoir hosts and are a potential source of infection for incidental hosts and humans.
Infection:
Reports of leptospirosis in cats are rare, but the importance of cats shedding Leptospira species and serving as a source of infection has recently gained attention. Leptospira species antibodies are commonly present in the feline population, and Leptospira species shedding of cats with outdoor exposure has been demonstrated. Cats mostly become infected through transmission from hunting rodents.
Significance:
The role of healthy carrier cats as a source of contamination, as well as the role of leptospires as a pathogen in cats, are likely underestimated.
Agent properties
Leptospires are mobile, thin, filamentous bacteria belonging to the spirochetes that appear as fine spirals with hook-shaped ends (Figure 1). 1 Leptospires can remain infectious for several months under optimal environmental conditions (temperature around 25°C, moisture, and a neutral soil pH). 2
Figure 1.
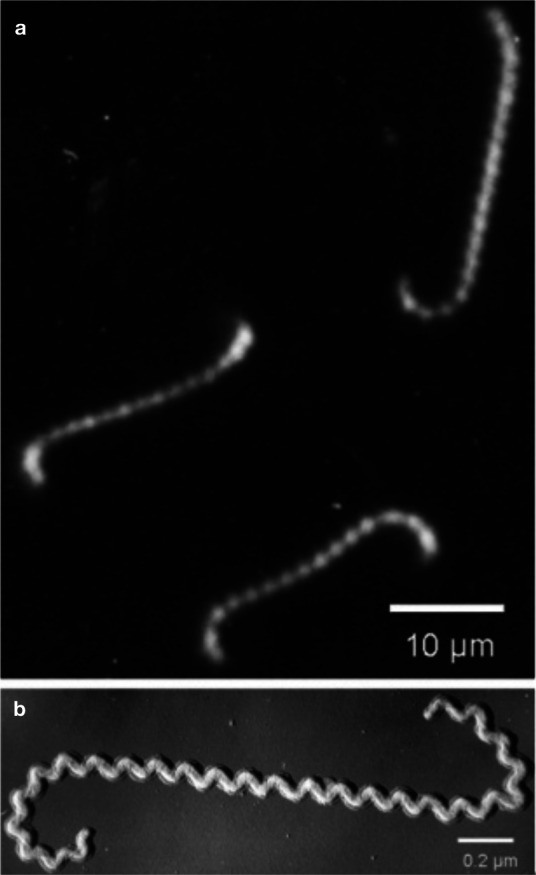
Dark field (a) and shadowed electron (b) photomicrographs of Leptospira species. Courtesy of Ben Adler, Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics, Monash University, Clayton, Australia
There are over 250 pathogenic serovars based on differences in the carbohydrate component of the bacterial lipopolysaccharide. Different serovars are adapted to different wild or domestic animal reservoir hosts. Serovars are further grouped into antigenically related serogroups. Immunity to leptospires is serogroup-specific. Leptospirosis in dogs and humans is caused primarily by serogroup Leptospira interrogans and Leptospira kirschneri. Several serovars of both serogroups also have been reported to cause infections in cats.2,3
In dogs, serovars icterohaemorrhagiae and canicola were responsible for most cases of canine leptospirosis prior to 1960. Since the widespread use of a bivalent serovar-specific vaccine against canicola and icterohaemorrhagiae, there has been an apparent shift to other serovars that are now identified much more commonly in dogs suffering from leptospirosis. 2 Recently, new vaccines have come on to the market for dogs in the USA and several European countries, which contain not only canicola and icterohaemorrhagiae, but also grippotyphosa, and in some vaccines additionally bratislava or pomona.

Epidemiology
Leptospires cause infections in many animal species. Subclinically infected wild and domestic animals serve as reservoir hosts and are a potential source of infection for incidental hosts, including humans. In the incidental hosts, severe clinical signs can develop. Incidental hosts also shed the organism, although generally for a shorter period. 2
Transmission of leptospires occurs by direct or indirect contact. Leptospires can be transmitted directly between hosts in close contact through urine, venereal routes, placental transfer, bites or ingestion of infected tissues as the organism penetrates mucosa or broken skin. Infected animals shed leptospires mainly through urine. Indirect transmission is more frequent than direct transmission in dogs and occurs through exposure of susceptible animals to a contaminated environment (eg, soil, food or bedding). 2 Water contact is the most prevalent means of spread in dogs and humans, and habitats with stagnant or slow-moving warm water favour survival of the organisms. Leptospires in contaminated water invade the host through skin wounds or through intact mucous membranes.
In cats, transmission through water contact is less likely due to the natural aversion of cats to water. However, cats can become infected by feeding on animals harbouring leptospires. Rodents are a natural reservoir for many serovars, and prey–predator transmission between cats and rodents is likely. 4 Cats can also be exposed to urine of cohabiting dogs. Spirochetes have also been shown to survive in insects and other invertebrates but the role of these as potential vectors is still unknown. 2
Antibody prevalence in cats ranges from 0–35%.5 –11 Infections with several serovars have been identified in cats, including icterohaemorrhagiae, canicola, grippotyphosa, pomona, hardjo, autumnalis and ballum.5 –11 Prevalence of different serovars and preference of reservoir hosts differ significantly between geographical regions, and, thus, the most commonly found serovars in cats vary between published studies. Most studies based on antibody detection have used microagglutination tests (MATs). As cross-reactions between serovars can occur in the MATs, results of serovar prevalence studies are sometimes difficult to interpret. No association has been found between the presence of antibodies and sex and breed. However, an association with age has been reported in several studies, with old cats being more likely to have antibodies.5,8,9 Antibodies are more common in outdoor cats, cats living in urban areas, and cats that are known hunters. 5
Several new studies have looked into the shedding of Leptospira species in the urine of cats.5,12 One study detected leptospiruria in shelter cats in Colorado using quantitative real-time polymerase chain reaction (PCR). 12 Ten of 85 stray or feral shelter cats with previous outdoor exposure were shedding pathogenic leptospires in the urine. Thus, prevalence of urinary shedding in cats is comparable to that occurring in healthy dogs, and outdoor cats might potentially be reservoir or incidental hosts in the transmission of leptospires. 12 Urinary shedding and the role of cats as a source of contamination has been underestimated in the past. It has been demonstrated that, with some serovars, animals can even shed the organism in their urine without having detectable antibodies. 4
Wild felids can be infected with Leptospira species as well. A recent study looking at the presence of antibody against Leptospira species in captive felids in Brazil found antibodies in 2/57 healthy felids. 13
Pathogenesis
The pathogenesis of feline leptospirosis is not well understood, but likely is similar to that in dogs and humans. Infection of dogs and humans occurs via direct contact with a host or its urine or indirectly via contaminated soil or water (eg, recreational activity, drinking or bathing in water). After penetration of mucous membranes or abraded and scratched skin, leptospires multiply rapidly upon entering the blood vascular space as early as 1 day after infection. They invade many organs, including the kidneys, liver, spleen, central nervous system, eyes and genital tract, and produce damage to these organs by replicating and causing inflammation. Initial replication mainly causes damage to the kidneys and liver. The extent of damage to internal organs is variable depending on the virulence of the organism and host susceptibility.
The immune response clears the leptospires from most organs except the kidneys, in which leptospires can persist. Shedding can continue for weeks to months in infected dogs.2,3
Clinical signs
In dogs, infection with leptospires results in illness of varying severity, depending on the infecting strain, geographical location and host immune response. Some dogs display mild or no signs of disease, whereas others develop severe illness or even death, often as a result of renal injury. In general, signs of hepatic and renal dysfunction and of coagulation defects are predominant in dogs with leptospirosis. In addition, dogs with leptospirosis can show signs of uveitis, pulmonary haemorrhage, abortion or acute febrile illness.2,3
The presence of antibodies in cats, as well as experimental infections, have demonstrated that cats can be infected with leptospires, but clinical signs seem to be rare and infection is usually clinically inapparent [EBM grade III].6,7 The hypothesis that cats are usually infected without clinical signs is supported by published literature that has shown no significant difference in antibody prevalence between sick and healthy cats, 14 and no evidence of leptospiral infection causing disease.6,15
However, clinical signs can be present in some infected cats. Experimental infection of cats results not only in leptospiraemia and leptospiruria, but also in mild disease with histological evidence of renal and hepatic inflammation. In addition, a few studies have reported an association between Leptospira species infection and clinical signs [EBM grade IV].6,14 –21 In a cat with ascites, enlarged liver and impaired hepatic function, but without icterus, antibodies against serovar hardjo could be detected. 6 In another report, leptospires were isolated from thoracic fluid, aqueous humour and kidneys of a cat, which at necropsy had widespread haemorrhages and straw-coloured fluid in the thoracic and peritoneal cavities. 14 The most common clinical manifestation in cats with leptospirosis seems to be an interstitial nephritis caused directly by the spirochete. In experimental and naturally infected cats, nephritis has been reported.16–18,21 In one study, a relationship was found between polyuria/polydipsia and the presence of antibodies against Leptospira species.15,19 A recently published case series of three cats with leptospirosis reported that all three cats suffered from renal failure, while liver disease was not present. 21
Immunity
In humans and animals immunity to leptospires is serogroup-specific. There is no cross-protection between different serogroups. Recovery from infection depends on production of specific antibodies. As serum antibodies increase, the organism is cleared from most tissues, with the exception of the kidneys. Renal colonisation occurs in most infected animals, and the organism usually persists in renal tubular epithelial cells causing shedding from kidneys for months to years after clinical recovery. The prognosis is highly dependent on conservation of renal function. 2
Diagnosis
Direct detection of the organism is difficult. Thus, usually antibody testing using a MAT is used for diagnosis of infection.
Direct detection of leptospires
Direct identification of the organism can be achieved by several techniques including visualisation in fresh urine by dark-field microscopy or in tissue sections or on air-dried smears by light microscopy, culturing of the organism, or detection of DNA by PCR. All direct methods, however, are only reliable in the case of a positive result, and a negative result never excludes the presence of the infectious agent due to the fact that leptospires are only shed intermittently and sometimes in low numbers. 2

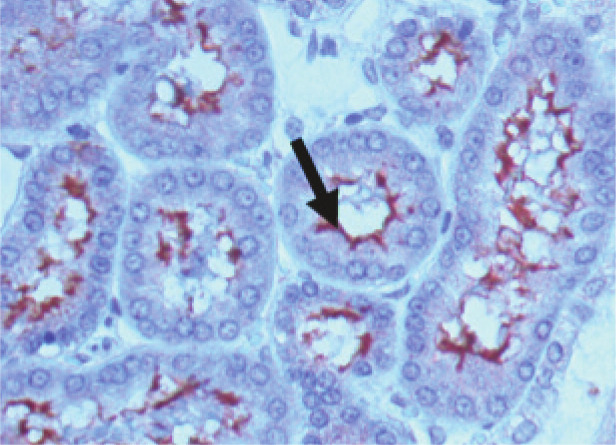
Direct identification of viable leptospires by dark-field microscopy is not reliable and therefore not recommended. A more reliable method is antibody staining (eg, by immunofluorescence), which can be used to identify leptospiral agents in body fluids and imprints of liver or kidneys (Figure 2), if appropriate samples are available. 2
Figure 2.
Immunohistology staining of leptospires in the kidneys of an infected hamster. Leptospires stained with specific antiserum (arrow) are seen lining the proximal renal tubules. Courtesy of Ben Adler, Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics, Monash University, Clayton, Australia
Leptospires can be cultured from blood, urine and cerebrospinal fluid (CSF), but grow very slowly. Culture can take weeks to months before becoming positive. Culturing is difficult and very time consuming 2 and is only reliable if the animal has not been pretreated with antibiotics. Thus, in most cases culturing is not a useful option in practice.
PCR methods have been developed to detect DNA of leptospires in body fluids, including blood, CSF, aqueous humour and urine.22 –25 In humans, PCR was shown to be more reliable than antibody testing or culture in early diagnosis. 26 Due to the higher concentration of leptospires in urine, PCR in urine samples is recommended. Use of PCR to identify the organisms in urine has been experimentally shown to be sensitive and specific, with the ability to establish a diagnosis at a very early stage of infection. PCR, however, is likewise only useful in untreated animals. Using PCR, organisms in subclinical carriers and non-viable Leptospira species will also be detected. Sensitivity and specificity of available PCR tests for the diagnosis of the disease in dogs have not been fully assessed. 2
PCR has been used in a few studies to detect Leptospira species shedding in cats [EBM grade III].5,12
Detection of antibodies
Antibodies can be detected using a MAT or an ELISA, with the MAT being the most common diagnostic method in dogs and humans and also the test that has been used in cats [EBM grade III].5 –11 However, cross-reactive results in MAT can make identification of the infecting serovar difficult.2,3 Laboratory variation and differences in host-specific humoral immune responses sometimes make correct assignment of antibody tests even more difficult. Many different serogroup antigens are tested in the assay but false-negative results occur when the infecting serogroup is not included.
In dogs, antibody titres of 1:800 against non-vaccine serovars or a fourfold increase or decrease of the titre are considered presumptive of leptospirosis when compatible clinical signs are present. 2 Diagnosis of infection by antibody detection in cats might be easier because there is no vaccine in cats [EBM grade IV].
Treatment
In dogs, treatment consists of supportive therapy and antibiotics, and the same therapeutic approach should be followed in cats [EBM grade IV]. Supportive treatment depends on the severity of clinical signs and the presence of renal or hepatic dysfunction and other complicating factors. Treatment of acute renal failure is the most crucial aspect in dogs, and the same is likely true for cats. Acute renal failure with anuria commonly requires renal replacement therapy with haemodialysis that is life saving for many animals with severe anuric lepto-spirosis. Thus, animals should be referred early to facilities with haemodialysis. Haemodialysis is indicated in animals with inadequate urine output that are developing volume overload, hyperkalaemia, or signs of uraemia that are not responsive to medical management. 3 The extent of renal damage after treatment determines the overall prognosis.
Antimicrobial therapy usually consists of two stages of treatment. The first stage aims to immediately inhibit replication of the organism and rapidly reduce fatal complications of infection such as hepatic and renal failure. Penicillin and its derivatives are the anti-biotics of choice for terminating Leptospira species replication [EBM grade IV]. Initially, ampicillin (20 mg/kg q8h IV) or amoxicillin, if available for IV use (20 mg/kg q12h IV), should be given parenterally to the vomiting, uraemic or hepatically compromised animal. These drugs prevent shedding and transmission of the organism within 24 h of initiation of therapy. They are, however, neither able to clear infection from the kidneys nor to eliminate the carrier state and prevent chronic shedding. Thus, other drugs, such as doxycycline, should be administered after use of penicillins to eliminate the carrier state.
Doxycycline (5 mg/kg q12h PO for 3 weeks) is the drug of choice for this second stage of treatment [EBM grade IV]. Doxycycline treatment should start as soon as the clinical condition allows its oral application. Doxycycline is usually given orally as intravenous application can cause shock and vomiting, and subcutaneous injection leads to development of abscesses in cats. Doxycycline can also cause liver toxicity. Thus, it should only be started after the animal stops vomiting and liver enzymes are in the reference range. In cats, use of doxycycline suspension is preferred over tablets or capsules due to the risk of oesophageal strictures.
In animals with no or only mild clinical signs, doxycycline can be used for both initial and elimination therapy. 2 In healthy cats shedding leptospires, treatment with doxycycline should be initiated to eliminate the carrier state.
Prevention
No vaccine is available for cats. The only way to prevent cats from becoming infected would be to avoid them feeding on potentially infected rodents and to avoid contact with stagnant water. Cats that are kept indoors have only a very low risk of becoming infected.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Merial, but is a scientifically independent body.
The authors do not have any potential conflicts of interest to declare.
Key Points
Leptospirosis is a worldwide occurring systemic bacterial disease.
Leptospirosis has been reported in over 150 mammalian species, including humans.
Subclinically infected wild and domestic animals serve as reservoir hosts and are a potential source of infection for other animals and humans.
Leptospirosis is considered an emerging infectious disease in people and dogs, and potentially also in cats.
Clinical leptospirosis is rare in cats, but recently more cases have been reported.
Many cats have antibodies against Leptospira species, indicating that infection is common.
Healthy cats with outdoor exposure can shed Leptospira species in their urine and serve as a source of infection.
Cats mostly become infected through hunting rodents.
An infection can be diagnosed by detecting antibodies using the MAT; shedding of leptospires is detected by urine PCR.
The drug of choice to completely eliminate leptospires is doxycycline.
The role of healthy carrier cats as a source of contamination, as well as the role of leptospires in causing disease in cats, might have been underestimated.
References
- 1. Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol 2010; 140: 287–296. [DOI] [PubMed] [Google Scholar]
- 2. Greene CE, Sykes JE, Brown CA, Hartmann K. Leptospirosis. In: Greene CE. (ed). Infectious diseases of the dog and cat. 3rd ed. Philadelphia: WB Saunders, 2006, pp 402–417. [Google Scholar]
- 3. Sykes JE, Hartmann K, Lunn KF, Moore GE, Stoddard RA, Goldstein RE. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011; 25: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shophet R. A serological survey of leptospirosis in cats. N Z Vet J 1979; 27: 236, 245–246. [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez J, Blais M, Lapointe C, Carioto L, Harel J. Feline leptospirosis: a serologic and urinary PCR survey in healthy cats and in cats with kidney disease. Proceedings of the 30th Annual Congress of the American College of Veterinary Internal Medicine (ACVIM); 30 May to 2 June, 2012; New Orleans, USA, pp 790–791. [Google Scholar]
- 6. Agunloye CA, Nash AS. Investigation of possible leptospiral infection in cats in Scotland. J Small Anim Pract 1996; 37: 126–129. [DOI] [PubMed] [Google Scholar]
- 7. Dickeson D, Love DN. A serological survey of dogs, cats and horses in south-eastern Australia for leptospiral antibodies. Aust Vet J 1993; 70: 389–390. [DOI] [PubMed] [Google Scholar]
- 8. Mylonakis ME, Bourtzi-Hatzopoulou E, Koutinas AF, Petridou E, Saridomichelakis MN, Leontides L, et al. Leptospiral seroepidemiology in a feline hospital population in Greece. Vet Rec 2005; 156: 615–616. [DOI] [PubMed] [Google Scholar]
- 9. Larsson CE, Santa Rosa CA, Hagiwara MK, Paim GV, Guerra JL. Prevalence of feline leptospirosis: serologic survey and attempts of isolation and demonstration of the agent. Int J Zoonoses 1984; 11: 161–169. [PubMed] [Google Scholar]
- 10. Batza HJ, Weiss R. Occurrence of Leptospira antibodies in cat serum samples. Kleintierpraxis 1987; 32: 171–172. [Google Scholar]
- 11. Markovich JE, Ross L, McCobb E. The prevalence of leptospiral antibodies in free roaming cats in Worcester County, Massachusetts. J Vet Intern Med 2012; 26: 688–689. [DOI] [PubMed] [Google Scholar]
- 12. Fenimore A, Carter K, Lunn K. Detection of leptospiruria in shelter cats in Colorado. Proceedings of the 30th Annual Congress of the American College of Veterinary Internal Medicine (ACVIM); 30 May to 2 June, 2012; New Orleans, USA, p 783. [Google Scholar]
- 13. Ullmann LS, Hoffmann JL, de Moraes W, Cubas ZS, dos Santos LC, da Silva RC, et al. Serologic survey for Leptospira spp in captive neotropical felids in Foz do Iguacu, Parana, Brazil. J Zoo Wildl Med 2012; 43: 223–228. [DOI] [PubMed] [Google Scholar]
- 14. Bryson DG, Ellis WA. Leptospirosis in a British domestic cat. J Small Anim Pract 1976; 17: 459–465. [DOI] [PubMed] [Google Scholar]
- 15. Andre-Fontaine G. Canine leptospirosis – do we have a problem? Vet Microbiol 2006; 117: 19–24. [DOI] [PubMed] [Google Scholar]
- 16. Fessler JF, Morter RL. Experimental feline leptospirosis. Cornell Vet 1964; 54: 176–190. [PubMed] [Google Scholar]
- 17. Hemsley LA. Leptospira canicola and chronic nephritis in cats. Vet Rec 1956; 68: 300–301. [Google Scholar]
- 18. Rees HG. Leptospirosis in a cat. N Z Vet J 1964; 12: 64. [Google Scholar]
- 19. Luciani O. Receptivité et sensibilité du chat aux leptospires. Thesis École Nationale Vétérinaire de Nantes, France; 2004. [Google Scholar]
- 20. Mason RW, King SJ, McLachlan NM. Suspected leptospirosis in two cats. Aust Vet J 1972; 48: 622–623. [DOI] [PubMed] [Google Scholar]
- 21. Arbour J, Blais MC, Carioto L, Sylvestre D. Clinical leptospirosis in three cats (2001–2009). J Am Anim Hosp Assoc 2012; 48: 256–260. [DOI] [PubMed] [Google Scholar]
- 22. Merien F, Baranton G, Perolat P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J Infect Dis 1995; 172: 281–285. [DOI] [PubMed] [Google Scholar]
- 23. Bal AE, Gravekamp C, Hartskeerl RA, De Meza-Brewster J, Korver H, Terpstra WJ. Detection of leptospires in urine by PCR for early diagnosis of leptospirosis. J Clin Microbiol 1994; 32: 1894–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harkin KR, Roshto YM, Sullivan JT. Clinical application of a polymerase chain reaction assay for diagnosis of leptospirosis in dogs. J Am Vet Med Assoc 2003; 222: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 25. Harkin KR, Roshto YM, Sullivan JT, Purvis TJ, Chengappa MM. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J Am Vet Med Assoc 2003; 222: 1230–1233. [DOI] [PubMed] [Google Scholar]
- 26. Brown PD, Gravekamp C, Carrington DG, van de Kemp H, Hartskeerl RA, Edwards CN, et al. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. J Med Microbiol 1995; 43: 110–114. [DOI] [PubMed] [Google Scholar]