Abstract
Practical relevance While the most common cause of chronic upper respiratory disease signs in cats is viral disease, with subsequent, self-perpetuating inflammation, other, more discrete causes need to be ruled out. These include foreign bodies, bacterial or fungal infections, oral-dental diseases and neoplasia. Any factors contributing to alterations in the structure or function of the upper airways, including inflammation of any cause, will compromise normal function and predispose to chronic damage if the cat is unable to resolve the underlying factors.
Clinical challenges The chronic feline snuffler is a frustrating patient to treat. The longer the course of disease, the more severe the consequences to affected tissues, and the more debilitated the patient becomes. A logical diagnostic plan to differentiate probable etiologies and to rule out non-viral causes results in appropriate therapeutic choices. Even with a viral etiology, therapies to reduce the pathological consequences of infection may modulate and help control the clinical signs. Some novel choices and drug combinations are discussed in this review.
Patient group Cats of all ages may be affected. Cats with conformational (breed or malformation) characteristics, such as short or convoluted nasal passages or very small nostrils, are predisposed to unresolving inflammation. Fungal disease is more relevant in specific geographic regions, making inclusion of a travel history important in history collection. Older cats are more likely to have neoplasia-induced signs.
Audience This review is directed at all veterinarians who see cats with chronic, recurrent upper respiratory disease.

Presentation — what can be deduced?
Chronic, recurrent rhinosinusitis can occur in cats of any age. Cats are presented because of sneezing, nasal discharge and noisy breathing, with or without inappetence. Sneezing occurs because of stimulation of irritant receptors in the nasal and sinus subepithelium. Knowing the timing, onset, duration and frequency of sneezing can be helpful. With chronicity and inflammatory changes this response may be abolished, resulting in accumulation of discharge. Nasal discharge may be serous, mucoid, purulent or sanguinous. It is helpful to know whether the discharge has changed, whether it changes throughout the day or season, and especially whether it is unilateral or bilateral.
Respiratory patterns and sounds may be abnormal. Clients may comment on their cat sounding hoarse or even silent when meowing or that his/her purr is different. In general, sounds heard on inspiration are associated with larger, upper airways whereas expiratory sounds are associated with smaller, lower airways. Snorting occurs with accumulation of discharges in the nasal passages or with secretions coughed into the oropharynx (eg, from pneumonia). A snoring, stertorous sound is associated with proximal upper respiratory occlusion, such as with a polyp or foreign body obstruction or functional inflammatory obstruction. Stridor is an inspiratory wheeze that reflects changes in the larynx. An expiratory wheeze, crackles or rales reflect small airway involvement. A complete lack of bronchovesicular sounds occurs when there is pulmonary consolidation or inflammation.
LOCALIZE THAT LESION!
Inspiratory sounds ⇒ upper/larger airways
Expiratory sounds ⇒ lower/smaller airways
Multimedia
An audio file and a video clip of snuffling cats are included in the online version of this article at doi:10.1016/j.jfms.2010.05.006 Can you identify and distinguish the inspiratory and expiratory sounds?
If the breathing is ‘worse at night’ this could reflect bronchitis or merely the time that the client is at home to observe the cat. Sounds that are worse after exercise or at rest may reflect the severity of the respiratory interference or the movement of secretions. Some cats have seasonal flare-ups, suggesting an allergic or contact irritant component.
Etiologies and pathogenesis
Viruses
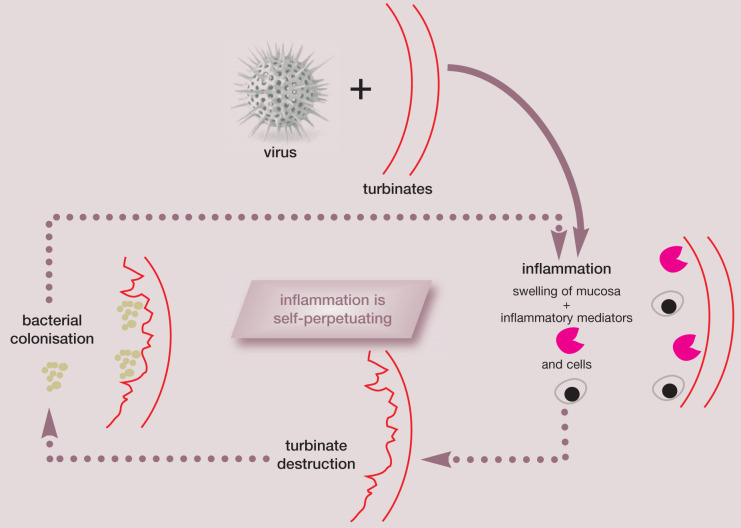
Chronic rhinitis may be a sequela to acute rhinitis but it may be a separate condition altogether. It may represent an ineffective immune response to persistent viral infection. 1 Feline herpesvirus 1 (FHV-1) may be the common denominator, initiating turbinate resorption, with subsequent secondary bacterial infections and unchecked inflammation exacerbating the problem. 2,3 This is especially severe in anatomically predisposed individuals (conformation anomalies, such as the tiny nostrils of the Persian, Fig 1). Irreversible destruction of the turbinates may result in viral or inflammatory mediator-induced cytolysis. Re activation of herpesvirus from infected trigeminal ganglion may result in recurrent destruction. All of these are potential pathogenic strategies, but it is not possible to determine the course/cause in a given individual.
FIG 1.

The Persian, with its compressed nasal passages and tiny nostrils, is anatomically predisposed to unresolving upper airway inflammation. Image, Wikimedia Commons
Calicivirus infection results in a carrier state with continuous shedding for variable periods of time. FHV-1, like other herpesviruses, results in a state of latency and approximately 80% of infected cats are permanent carriers. Latency accounts for recurrence of clinical signs during periods of physiological or psychological stress.
Bacteria
Primary bacterial agents include Bordetella bronchiseptica, which is commonly found as a commensal without causing morbidity. Mycoplasma species may be cultured or amplified by polymerase chain reaction (PCR) from some individuals. They are very common in acutely affected cats and, thus, likely play a role in the chronic syndrome. It is a challenge to identify them in primary care practice due to the difficulty of isolating these fastidious organisms. 4,5
Chlamydophilosis can be a common cause of conjunctivitis in some catteries but is uncommon in client-owned cats with chronic rhinitis. L-forms may also be involved but require specific targeted culture techniques for verification.
In one study of cats with and without chronic rhinosinusitis, aerobic bacteria were cultured from biopsy samples from proportionately more clinically affected cats (4/10, 40%) than controls (2/7, 29%), while anaerobic bacteria were detected only in the affected cats (2/10). 6 Flush samples were collected from the same cats, with aerobes identified in 5/7 controls and 9/10 affected cats; and anaerobes in 3/10 and Mycoplasma species in 2/10 affected cats. Interestingly, FHV-1 was not cultured from any of the cats, but viral DNA was detected in 4/7 control and 3/10 affected cats by PCR, implying that the virus was not viable.
Self-perpetuating cycle of viral-induced inflammation.
The fact that cats on antibiotics often improve clinically, would support the role of bacteria in chronic rhinosinusitis; the fact that signs recur, despite therapy, implies that bacteria are only part of the cause.
Less frequently isolated bacteria worthy of journal publications have included Actinomyces species, Haemophilus species 7 and Capnocytophaga species. 8 Bartonella henselae is commonly detected by serology (antibody titers), yet its true role as an etiologic agent in the chronically snotty cat is not as relevant as its serological exposure implies. 9 One study showed that ‘serological screening for Bartonella antibodies may not be useful for the identification of bacteremic cats (positive predictive value = 46.4%), but the lack of antibodies to B henselae was highly predictive of the absence of bacteremia (negative predictive value = 89.7%).’ 10
The fact that cats on antibiotics often improve clinically would support the role of bacteria; the fact that signs recur, despite therapy, implies that bacteria are only part of the cause of the illness. When antimicrobial therapy of 7–10 days' duration fails to result in resolution of disease, then a thorough diagnostic work-up should be recommended.
Fungi
The main fungal organisms causing chronic upper respiratory disease are Cryptococcus neoformans var neoformans and gattii. These classically cause severe inflammation resulting in facial deformity and skin ulceration, along with nasal discharge (unilateral > bilateral). Aspergillus species 11 and Penicillium species have also been isolated. 12
Neoplasia and other etiologic factors
Any factors contributing to alterations in the structure or function of the upper airways, including inflammation of any cause, will compromise normal function and predispose to chronic damage if the cat is unable to resolve the underlying factors. Trauma, congenital and conformational anomalies, inflammatory polyps, periodontal disease and foreign bodies all predispose to chronic infection. 13 Chondritis and osteomyelitis are often sequelae to infection/inflammation.
Neoplasia further alters function and form, leading to secondary changes, which may be more worrisome to the client than the underlying cancer. If there are concurrent stressors (suboptimal nutrition, social distress, environmental factors) or outright immuno-compromise/suppression (eg, retroviruses), the likelihood of infectious agent involvement and the inability to clear the same is increased.
Most feline nasal tumours are malignant. The most common type is lymphoma, with adenocarcinoma and squamous cell carcinoma representing epithelial origin neoplasms that occur less frequently. 14–17 Tumours tend to be locally invasive (frontal and paranasal sinuses) without metastasizing distantly. Similar to other types of cancer in cats, the older cat is over-represented. 14–17 Clinical signs will vary depending on the location of the tumour. Nasal tumours result in sneezing and unilateral nasal discharge; nasopharyngeal masses are associated with stertorous respiration. Further signs include variable facial deformity, epistaxis and epiphora.
Initial clinical assessment
Assess facial symmetry both face on and from above the head. Palpate the face to look for swelling, invagination or discomfort. Thoroughly evaluate the teeth and alveolar bone for evidence of periodontal disease, abscessation or inflammation. Look at and palpate the hard and soft palate, where feasible, looking for oronasal fistula or mass lesions or ulceration. If a cat retches or yawns, the tonsils may be visualized. Opening the mouth allows neurologic competency to be evaluated — ie, jaw tone (cranial nerve [CN] V), position, movements and symmetry of the tongue (CN XII), and gag reflex (CNs IX and X).
Evaluate nasal passage patency using a small mirror (compact or dental) or a glass slide that has been chilled in the freezer (Fig 2). Wisps of cotton held in front of the nostrils are also helpful. Palpate the trachea to see if this elicits a cough. It is helpful to auscultate the trachea, as well as the lungfields bilaterally at three locations (dorsal cranial, caudal and cranioventral) to define the primary location of the lesion. Occasionally, auscultation of the frontal sinuses may be revealing. For this, a small pediatric bell is used. For pulmonary auscultation, use two heads, a standard bell and a plexiglass scope (eg, Ultrascope), as they provide different sensitivities and frequencies (Fig 3).
FIG 2.

Evaluating patency of the nasal passages with a chilled slide held up to the cat's nose
FIG 3.
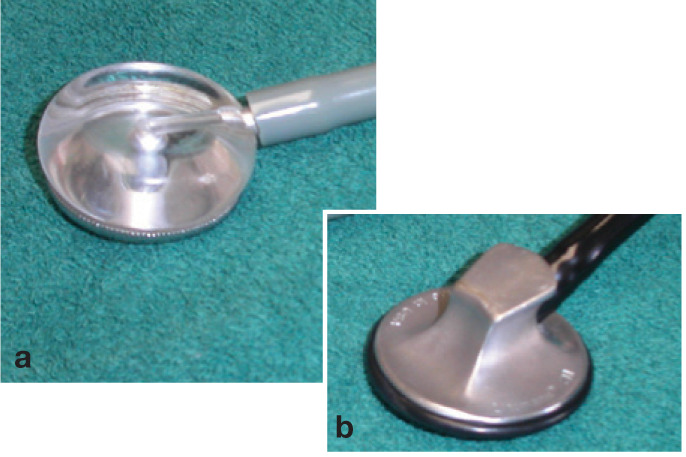
Use of both a plexiglass scope (a) and a standard bell head (b) may be helpful for pulmonary auscultation
A thorough physical examination should be conducted. Fundic examination should be performed to look for Cryptococcus infection, lymphoma and other signs of systemic disease. Retropulsion of the globes is an easy test to assess for retrobulbar abnormalities. Enlargement of regional lymph nodes or generalized node enlargement should be assessed.
Diagnostics
When rhinitis or rhinosinusitis is a recurrent or chronic problem, a logical and thorough diagnostic plan should be followed.
Collection of a minimum database
Start with a minimum database of a complete blood count (CBC), serum biochemistry, retro-viral serology, urinalysis and blood pressure determination, if not already undertaken earlier during the examination. If rhinoscopy is being considered or if epistaxis has been part of the process, a coagulation panel should be performed. Any medications affecting hemostasis (eg, aspirin, α-antagonists) should be temporarily discontinued. Perform Aspergillus and Cryptococcus species serologic antigen titers. While both fungi have a worldwide distribution, Cryptococcus is more prevalent in certain geographic regions (Australia, Pacific Northwest of the United States and Canada), thus should certainly be considered if regionally appropriate. If lymph nodes are enlarged, cytologic specimens should be collected to use for staging in case neoplasia is diagnosed by histopathology.
Imaging
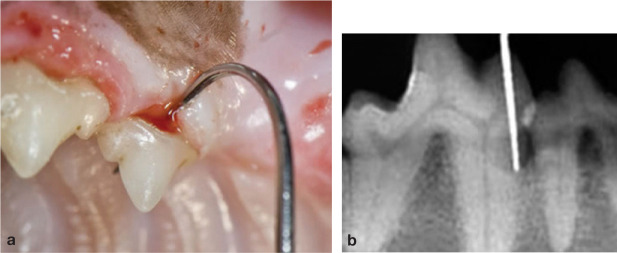
Skull radiography, computed tomography and magnetic resonance imaging for imaging dentition, nasal passages (Fig 4) and sinuses, as well as bone health, will require general anaesthesia. Conventional radiography under-estimates the extent of disease. Probe all periodontal pockets (Fig 5), retract the soft palate to look for polyps (Fig 6) and palpate the soft palate.
FIG 4.
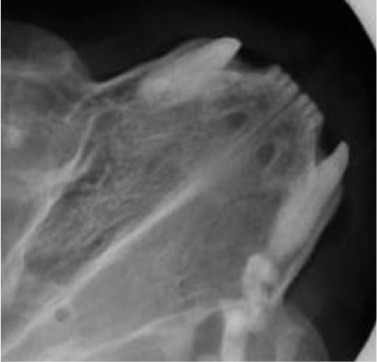
Increased density, with deviation of the nasal septum, indicative of disease infiltrating the nasal passage on the right-hand side of the image
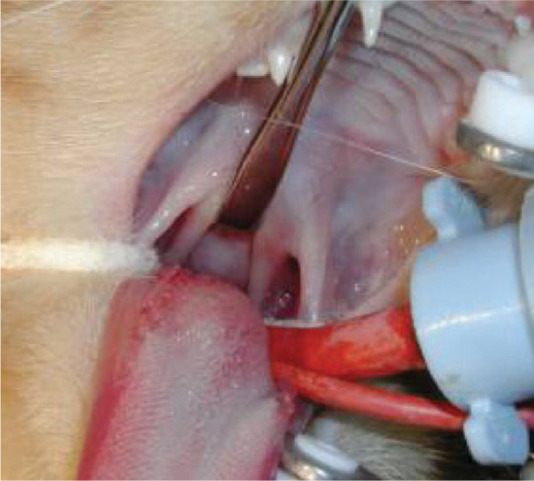
FIG 5.
(a,b) Probing of periodontal pockets
FIG 6.
Retracting the soft palate to look for nasal polyps. Courtesy of Greg Leck
Three standard radiographic views should be exposed using high detail films and screens:
Dorsoventral view — to assess the nasal cavity and bullae (Fig 7). Symmetry is essential for evaluation of changes.
Lateral view — to allow evaluation of the frontal sinuses; if a change is suspected, it may be followed by an oblique lateral view to focus on the sinus in question.
Skyline view — also valuable for assessing the frontal sinuses; this is performed with the cat in dorsal recumbency, pulling the mandible out of the way.
FIG 7.
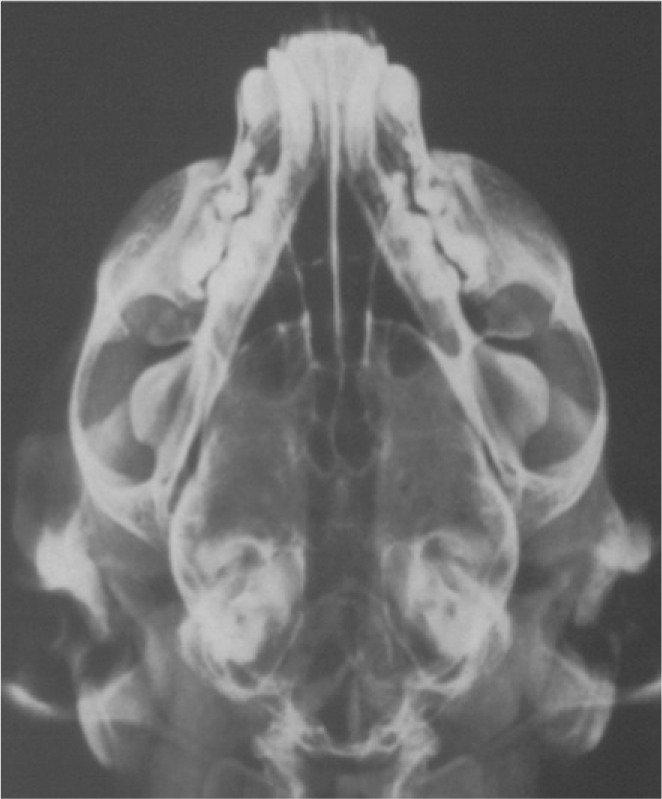
Well positioned, symmetrical dorsoventral view of a skull, showing no radiographically apparent lesions
Cytology (even brush cytology) does not appear to be reliable for the detection of chronic inflammation and evaluation of chronic rhinitis in cats.
Harvesting and analysis of samples
Following imaging, samples should be harvested. Michiels et al evaluated the records of 40 cats that had undergone rhinoscopy for chronic nasal disease to compare relative diagnostic yield. 18 Specimens in 17 cases were collected by brush cytology (higher yield than flush cytology). Concurrent biopsies were collected for histopathologic evaluation. Only 25% of the cases showed agreement. The conclusion was that cytology (even brush cytology) does not appear to be a reliable method for the detection of chronic inflammation and evaluation of chronic rhinitis in cats.
Endoscopic evaluation and biopsy
If unilateral disease is present, endoscopic evaluation of the unaffected side first is recommended. Normal turbinate mucosa should be pale pink and smooth (image a, page 553). Hyperemia, irregular turbinate surfaces and moderate amounts of discharge suggest pathology. Mucus exudation (image b), a polyp (image c) or mass, foreign body (image d) or ‘webbing’/nasopharyngeal stenosis (image e) may be apparent. Fungal plaques may be seen and biopsied. While an adenocarcinoma or sarcoma appears as a discrete mass, lymphoma may present as a mass or as a diffuse infiltrate.
Even if the mucosa looks normal, biopsies should be taken in a cat with chronic disease, as the gross appearance may be misleading. The entire cavity (rostral and caudal) should be examined before biopsying to avoid bleeding, which interferes with visualization. A novel manner of biopsying neoplasia, involving nasal hydropulsion, has been reported. 19 This involved vigorous flushing of the affected side with 20–60 ml of saline using a large (20–60 ml) syringe, repeating the process on the contralateral side. Tissue from the table or from the swabs placed in the oropharynx was submitted for histopathology Diagnostic samples were successfully harvested in all seven cats in this study using this minimally invasive debulking technique. Additional sedation may be desirable on recovery and overnight hospitalization prevents excessive movement, allowing hemostasis to occur.
Sampling for culture.
With a cuffed endotracheal tube in place, pack the oropharynx with a known number of swabs. Take a sterile tom cat catheter and, while still in its packaging, hold it alongside the nose from the nares to the eye. Place the tip of the catheter at the medial canthus and mark the catheter at this point. It is important never to insert the catheter beyond this mark to avoid perforation and penetration of the cribriform plate. Instill a 2 ml aliquot of sterile water as deeply as possible into the unaffected nasal passage first, and collect washings into anaerobic and aerobic culture media. Perform cytology on the flush as well. Repeat on the affected side.
Bacterial culture
Concurrently, samples should be collected for culture (see box above). Aerobic and anaerobic cultures may be set up but results must be interpreted with caution because there are large numbers of normal flora in the nasal cavity. The diagnostic yield can be improved by obtaining cultures from deep within the nasal cavity, avoiding superficial contamination.
Virus identification
FHV-1 or calicivirus can be identified after virus isolation (the laboratory to which the sample is being submitted will advise you regarding handling and submission). However, nucleic acid amplification techniques are often used to prove infection by these organisms. FHV-1 PCR assays are widely available and feline corona virus reverse transcriptase PCR assays are now available as well. PCR assays are also offered for amplification of B bronchiseptica, Chlamydophila felis and Mycoplasma species DNA. A study assessing the relative sensitivity of PCR assays for the detection of FHV-1 DNA in clinical samples and commercial vaccines concluded that none of the assays was able to distinguish between wild-type virus and vaccine virus; 20 this is likely to be a problem with B bronchiseptica and C felis vaccines as well. Additionally, test sensitivity (detection limits and rates) varies greatly between the tests used.
Patient care while under anaesthesia.
Before completion of anaesthesia, flush gently and thoroughly to remove and aspirate the discharge to help the patient during and after recovery. The endotracheal tube must be well cuffed and the oropharynx should be packed with (a known number of) swabs to prevent fluid aspiration.
Each of these five infectious agents can be detected in healthy cats as well as in clinically ill cats. Thus, the positive predictive value for these assays is low (ie, a positive test result does not prove the disease was caused by the agent).
Even if the turbinate mucosa looks normal, biopsies should be taken in a cat with chronic disease, as the gross appearance may be misleading.
Rhinoscopic appearance of nasal passages.
The small size of cats makes scoping challenging. A flexible endoscope may be retroflexed around the soft palate if retraction of the soft palate using a dental mirror was unrevealing. To evaluate the more rostral portions of the nasal passage, a rigid 1.9 mm arthroscope with a 30° viewing angle may be used if a small flexible scope is unavailable. Irrigation with sterile saline is essential for optimal visualization.
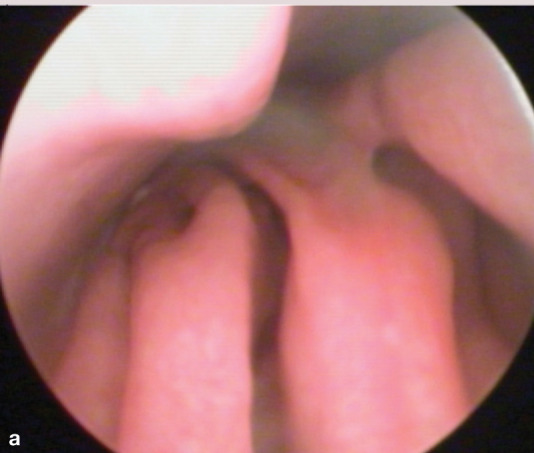
(a) Normal turbinates. Courtesy of Jeff Mayo
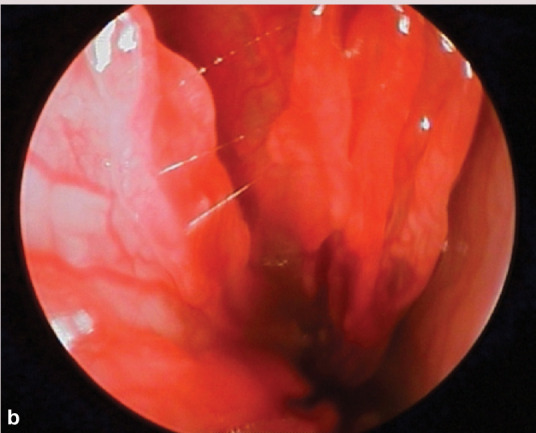
(b) Hyperemia and irregular turbinate surfaces, with mucus. Courtesy of Jeff Mayo
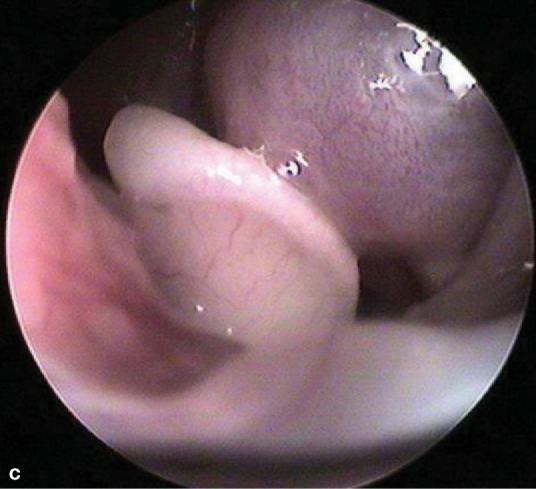
(c) Nasal pharyngeal polyp. Courtesy of Jeff Mayo
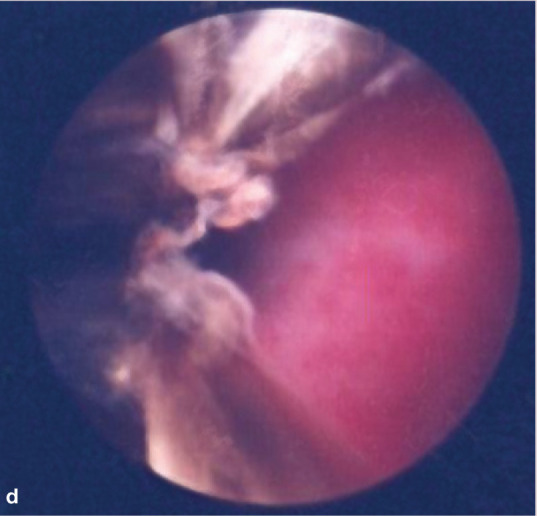
(d) Foreign body (carpet fiber). Courtesy of Jeff Mayo
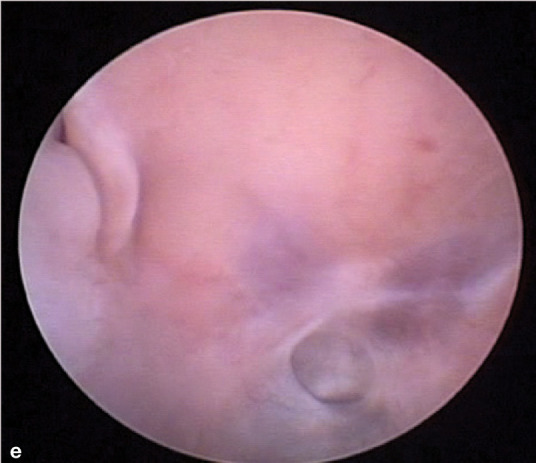
(e) ‘Webbing’/nasopharyngeal stenosis. Courtesy of Todd Morgan
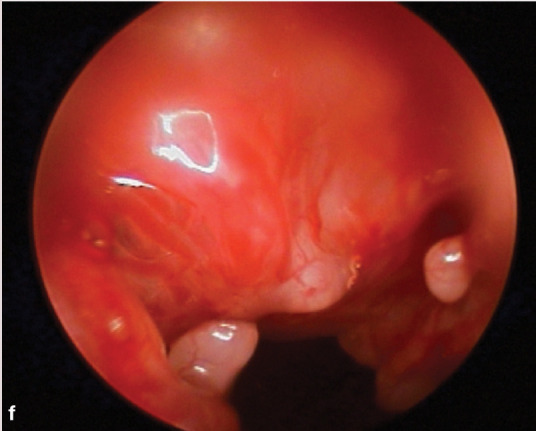
(f) Lymphocytic rhinitis. Courtesy of Jeff Mayo
Therapeutics
Specific therapies
Antibiotics
As practitioners, we frequently choose antibiotics to treat cats with upper respiratory disease — unfortunately without necessarily knowing what organism is involved. If multiple organisms are grown on culture, the significance of the growth is questionable. Should a single bacterial species grow on culture that is not a normal commensal, sensitivity results may be used. Therapy should be continued for 6–8 weeks without changing the antibiotic if there is an initial positive response; hence the antibiotic selected should be safe for long-term use. Also antibiotics should be chosen that reach the site of infection at effective therapeutic concentrations.
Antifungals
Should Cryptococcus or Aspergillus species be cultured, specific antifungal protocols should be followed (discussed elsewhere). 23
Antihistamines
If an allergic component is suspected because of seasonal recurrence, antihistamines (eg, chlorpheniramine maleate) may be considered. Less sedative antihistamines (eg, fexofenadine, loratidine) selectively inhibit peripheral H1 receptors (Table 1).
TABLE 1.
Drugs and recommended dosages for the treatment of the chronic snuffler (see text for further discussion)
Antivirals
For FHV-1 infection, administration of an intranasal herpesvirus and calicivirus vaccine two or three times a year may be beneficial in stimulating local immunity. L-lysine has been used to reduce the frequency of herpesviral recrudescence by competing with arginine, which is needed for viral replication. Recent controlled studies do not, however, support the use of this agent as it has been shown not to prevent or reduce the recurrence of upper respiratory tract infections in shelter cats. 24–26 Oral interferon-α may also help to modulate FHV-1 infection, but controlled studies are lacking. Similarly, ophthalmic administration of interferon-α in saline has been recommended for cats with herpesvirus keratitis or conjunctivitis. Famciclovir is an antiherpetic drug used in humans. It can be used in cats with confirmed herpesvirus infection and started at a low dose (Table 1), with monitoring of the CBC every 2–3 weeks. 27
Immunotherapy
A novel immunotherapeutic protocol was evaluated for the treatment of chronic rhinitis. 1 In a blinded crossover study, cats given intraperitoneal injections of lipid-DNA complexes encoding the feline interleukin-2 gene, administered weekly for 4 weeks, showed an improvement in sneezing and nasal discharge when compared with cats that had received placebo liposome injections. It is hypothesised that this improvement was due to the induction of a Th1-type immune response.
A rational choice of antibiotic?
Antibiotics that penetrate cartilage and bone are of value, making amoxicillin-clavulanic acid, clindamycin and chloramphenicol reasonable choices. Clindamycin, doxycycline and chloramphenicol are effective against Mycoplasma species; metronidazole and doxy cycline modulate the immune response, thereby reducing inflammation somewhat. Doxycycline is effective against Chlamydophila and L-forms. Azithromycin is often chosen because of its long duration of action. However, despite its popularity, it has not been shown to be effective against Chlamydophila even when administered daily; 21 nor was it more effective than amoxicillin in the treatment of suspected bacterial upper respiratory tract infections in a shelter setting. 4 Fluoroquinolones may be considered as rescue therapy for recurrent or stubborn Gram-negative organisms and mycoplasmas. 22 Pulse or intermittent therapy (eg, 1 week per month) predisposes to the development of antibiotic resistance and cannot be recommended. Antibiotic ophthalmic drops may be included in the treatment protocol as direct topical therapy to the nasal passage.
Surgery
Polyps and foreign bodies should be removed. A novel approach to the removal of a polyp originating in a frontal sinus was reported in this journal. 28 Because of the small size of the patient, an endoscope was passed orad through the cardia of the stomach, into the esophagus and oropharynx, allowing retrieval of several polypoid masses. Nasopharyngeal stenosis/‘webbing’ requires surgical resection via a transpalatine approach. Like polyps, webs may recur. Dental disease should be treated, repairing fistulae if present.
Surgical drainage and flushing may be warranted for some patients with chronic sinusitis. After openings are drilled into the frontal sinus, histopathologic samples and bacterial samples may be collected. Trypsin-containing solutions may help to break up heavy mucus. Sinus ablation has also been described, in which the frontal sinus is opened via bone flap, the mucoperiosteal lining and necrotic nasal turbinates are removed, the opening between the sinus and nasal passages is obliterated with a piece of temporal muscle fascia and the frontal sinus is packed with a piece of ventral abdominal fat. 29 This technique has shown success and fat is preferable to the use of polymethylmethacrylate.
Antineoplastic therapy
Feline nasal adenocarcinoma or undifferentiated carcinoma responds well to radiation therapy with or without preceding cyto-reductive surgery. 30–33 Generous excision of squamous cell carcinoma may be curative. Nasal lymphoma responds to chemotherapy (University of Wisconsin 25 week CHOP protocol), but these cats may also achieve long-term control with local irradiation (total radiation dose >32 Gy). There is no reported difference in survival times of cats with nasal lymphoma treated with single versus multi-modality therapy. 17,34–36
Non-specific therapies
Hydration
Maintaining hydration is essential for tissue perfusion, and also to make secretions less viscus and to improve cell function (eg, ability to clear mucus via the cells' mucociliary apparatus). Thus, humidifying the air around patients with chronic airway narrowing is beneficial, be it by steaming the bathroom or instilling saline into the nostrils to stimulate sneezing and clearance of the nasal passages. Therapeutic lavage can be extremely helpful.
Therapeutic lavage.
With the anaesthetized patient in sternal recumbency, a known number of swabs in the oropharynx and a cuffed endotracheal tube in place, gently flush 40–60 ml of saline through each nostril in 3–6 ml aliquots using 3–6 ml syringes. This dislodges inspissated mucus as well as loosely lodged foreign bodies.
Decongestants
Oral decongestants include diphenhydramine hydrochloride, dimenhydrinate and pseudo-ephedrine (Table 1). Nasal decongestant drops (eg, Otrivin Pediatric) are challenging to administer but can be very helpful. As always, handle the cat from behind; tip the head gently upwards and, positioning the dropper bottle or tip of the syringe directly at the nostril opening, drop/squeeze a drop in quickly on one side followed by the other.
Anti-inflammatories
Anti-inflammatories play a role in the treatment of the chronically rhinitic/rhinosinusitic cat. By reducing airway swelling, breathing improves and less secretion is produced, making the patient more comfortable. Glucocorticoids may help by retarding leukocyte function and migration, blocking phospholipase A, decreasing the release of lytic enzymes, and suppressing delayed hypersen-sitivity reactions. This makes them candidates for use in lymphoplasmacytic rhinitis, the most common form of chronic rhinitis. Because the condition itself is not life-threatening, glucocorticoids should be used intermittently rather than continuously long term The author uses prednisolone daily for a week, and reduces to q48h over the following week. The concern with the use of glucocorticoids is the possibility that they might result in recrudescence of viral disease or virus shedding.
Non-steroidal anti-inflammatory drugs (NSAIDs) are an alternative option. Piroxicam or meloxicam may help. Used orally, these NSAIDs should be given with food. Hydration should be optimized to ensure renal and other tissue perfusion and clients counseled to monitor for anorexia, nausea, vomiting or polyuria/polydipsia.
ISFM and AAFP consensus guidelines on long-term use of NSAIDs in cats.
Guidelines to encourage more widespread and appropriate use of NSAIDs in cats that would benefit from treatment are published on pages of 521–538 of this issue of J Feline Med Surg, and at: doi:10.1016/j.jfms.2010.05.004
Leukotriene blockers
Montelukast (Singulair; Merck Sharp & Dohme) and zafirlukast (Accolate; AstraZeneca) may also be considered to reduce inflammatory cell infiltration.
Nutrition
It is critical to pay attention to nutrition, in terms of quality, balance and quantity. In addition to the frequently used anti-histamine/antiserotonin drug cyprohepta-dine, mirtazapine is a newly recognized appetite stimulant for cats (Table 1).
Acupuncture
Acupuncture may be a useful adjunctive therapy. While the author is not educated in this therapeutic modality, it has been shown to be helpful in the management of many chronic problems. There is one citation in the mainstream literature that discusses its use in the treatment of chronic lower/small airway disease. 37 The theory behind the efficacy of acupuncture includes stimulation of the sympathetic nervous system as well as modulation of the inflammatory response.
KEY POINTS.
While FHV-1 is often the initiating cause of chronic upper respiratory disease, any factors contributing to alterations in the structure or function of the upper airways, including inflammation of any cause, will compromise normal function and predispose to chronic damage if the cat is unable to resolve the underlying factors.
The fact that cats on antibiotics often improve clinically supports the role of bacteria; the fact that signs recur, despite therapy, implies that bacteria are only part of the cause of the illness. When antimicrobial therapy of 7–10 days' duration fails to result in resolution of disease, then a thorough diagnostic work-up should be recommended.
The diagnostic work-up should include testing for retroviruses, fungal serology when warranted by geographic region or travel history, imaging, evaluation of the mouth, teeth and periodontal health, and collection of samples. It may not be possible to definitively diagnose the initiating viral cause.
Anesthesia is required to thoroughly evaluate the oropharyngeal cavity both visually and by palpation. Skull radiographs or computed tomography/magnetic resonance imaging are required to image dentition, nasal passages and sinuses, and assess bone health.
Samples should be collected for cytology or, preferably, histopathology. Cytology and cultures must be interpreted with caution. A positive PCR result indicates exposure to an organism (or vaccine antigen), which may or may not be the cause of current disease.
When a specific etiology is determined, treatment should be continued for the full course. Non-specific therapies are directed at alleviation of signs associated with inflammation or its consequences.
It is important that clients understand that a cat with chronic rhinitis/rhinosinusitis will never be cured. With ongoing management, the patient's quality of life can be improved through a reduction in sneezing and nasal discharge.
Prognosis
It is important that clients understand that a cat with chronic rhinitis/rhinosinusitis will never be cured. With ongoing management, the patient's quality of life can be improved through a reduction in sneezing and nasal discharge.
References
- 1. Veir JK, Lappin MR, Dow SW. Evaluation of a novel immunotherapy for treatment of chronic rhinitis in cats. J Feline Med Surg 2006; 8: 400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaskell R, Dawson S, Radford A, Thiry E. Feline herpesvirus. Vet Res 2007; 38: 337–54. [DOI] [PubMed] [Google Scholar]
- 3. Johnson LR, Maggs DJ. Feline herpesvirus type-1 transcription is associated with increased nasal cytokine gene transcription in cats. Vet Microbiol 2005; 108: 225–33. [DOI] [PubMed] [Google Scholar]
- 4. Ruch-Gallie R, Veir JK, Spindel ME, Lappin MR. Efficacy of amoxycillin and azithromycin for the empirical treatment of shelter cats with suspected bacterial upper respiratory infections. J Feline Med Surg 2008; 10: 542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veir JK, Ruch-Gallie R, Spindel ME, Lappin MR. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J Feline Med Surg 2008; 10: 551–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson LR, Foley JE, De Cock HE, Clarke HE, Maggs DJ. Assessment of infectious organisms associated with chronic rhino sinusitis in cats. J Am Vet Med Assoc 2005; 227: 579–85. [DOI] [PubMed] [Google Scholar]
- 7. Milner RJ, Horton JH, Crawford PC, O'Kelley J, Nguyen A. Suppurative rhinitis associated with Haemophilus species infection in a cat. J S Afr Vet Assoc 2004; 75: 103–7. [DOI] [PubMed] [Google Scholar]
- 8. Frey E, Pressler B, Guy J, Pitulle C, Breitschwerdt E. Capnocytophaga sp. isolated from a cat with chronic sinusitis and rhinitis. J Clin Microbiol 2003; 41: 5321–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berryessa NA, Johnson LR, Kasten RW, Chomel BB. Microbial culture of blood samples and serologic testing for bartonellosis in cats with chronic rhinosinusitis. J Am Vet Med Assoc 2008; 233: 1084–89. [DOI] [PubMed] [Google Scholar]
- 10. Chomel BB, Abbott RC, Kasten RW, et al. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titres. J Clin Microbiol 1995; 33: 2445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barrs VR, Beatty JA, Lingard AE, et al. Feline sino-orbital aspergillosis: an emerging clinical syndrome. Aust Vet J 2007; 85: N23. [PubMed] [Google Scholar]
- 12. Tomsa K, Glaus TM, Zimmer C, Green CE.Fungal rhinitis and sinusitis in three cats. J Am Vet Med Assoc 2003; 222: 1380–84, 1365. [DOI] [PubMed] [Google Scholar]
- 13. Henderson SM, Bradley K, Day MJ, et al. Investigation of nasal disease in the cat — a retrospective study of 77 cases. J Feline Med Surg 2004; 6: 245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day MJ, Henderson SM, Belshaw Z, Bacon NJ. An immunohistochemical investigation of 18 cases of feline nasal lymphoma. J Comp Pathol 2004; 130: 152–61. [DOI] [PubMed] [Google Scholar]
- 15. Mukaratirwa S, van der Linde-Sipman JS, Gruys E. Feline nasal and paranasal sinus tumours: clinicopathological study, histo-morphological description and diagnostic immunohistochemistry of 123 cases. J Feline Med Surg 2001; 3: 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox NR, Brawner WR, Jr, Powers RD, Wright JC. Tumors of the nose and paranasal sinuses in cats: 32 cases with comparison to a national database (1977 through 1987). J Am Anim Hosp Assoc 1991; 27: 339–47. [Google Scholar]
- 17. Little L, Patel R, Goldschmidt M. Nasal and nasopharyngeal lymphoma in cats: 50 cases (1989–2005). Vet Pathol 2007; 44: 885–92. [DOI] [PubMed] [Google Scholar]
- 18. Michiels L, Day MJ, Snaps F, Hansen P, Clercx C. A retrospective study of non-specific rhinitis in 22 cats and the value of nasal cytology and histopathology. J Feline Med Surg 2003; 5: 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashbaugh EA, McKiernan BC, Miller CJ, et al. Hydropulsion to biopsy and debulk nasal tumors [abstract]. Proceedings of ACVIM Forum 2008.
- 20. Maggs DJ, Clarke HE. Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. Am J Vet Res 2005; 66: 1550–55. [DOI] [PubMed] [Google Scholar]
- 21. Owen WMA, Sturgess CP, Harbour DA, Egan K, Gruffydd-Jones TJ. Efficacy of azithromycin for the treatment of feline chlamydophilosis. J Feline Med Surg 2003; 5: 305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spindel ME, Veir JK, Radecki SV, Lappin MR. Evaluation of prad-ofloxacin for the treatment of feline rhinitis. J Feline Med Surg 2008; 10: 472–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract 2000; 30: 1029–50. [DOI] [PubMed] [Google Scholar]
- 24. Drazenovich TL, Fascetti AJ, Westermeyer HD, et al. Effects of dietary lysine supplementation on upper respiratory and ocular disease and detection of infectious organisms in cats within an animal shelter. Am J Vet Res 2009; 70: 1391–400. [DOI] [PubMed] [Google Scholar]
- 25. Rees TM, Lubinski JL. Oral supplementation with L-lysine did not prevent upper respiratory infection in a shelter population of cats. J Feline Med Surg 2008; 10: 510–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maggs DJ, Sykes JE, Clarke HE, et al. Effects of dietary lysine supplementation in cats with enzootic upper respiratory disease. J Feline Med Surg 2007; 9: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malik R, Lessels NS, Webb S, et al. Treatment of feline herpesvirus-1 associated disease in cats with famciclovir and related drugs. J Feline Med Surg 2009; 11: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esterline ML, Radlinsky MG, Schermerhorn T. Endoscopic removal of nasal polyps in a cat using a novel surgical approach. J Feline Med Surg 2005; 7: 121–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson GI. The treatment of chronic sinusitis in six cats by ethmoid conchal curettage and autogenous fat graft sinus ablation. Vet Surg 1987; 16: 131–34. [DOI] [PubMed] [Google Scholar]
- 30. Marioni-Henry K, Schwarz T, Weisse C. Cystic nasal adenocarcinoma in a cat treated with piroxicam and chemoembolization. J Am Anim Hosp Assoc 2007; 43: 347–51. [DOI] [PubMed] [Google Scholar]
- 31. Evans SM, Hendrick M. Radiotherapy of feline nasal tumors. A retrospective study of nine cases. Vet Radiol Ultrasound 1989; 30: 128–32. [Google Scholar]
- 32. Mellanby RJ, Herrtage ME, Dobson JM. Long-term outcome of eight cats with non-lymphoproliferative nasal tumours treated by megavoltage radiotherapy. J Feline Med Surg 2002; 4: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Théon AP, Peaston AE, Madewell BR, Dungworth DL. Irradiation of non-lymphoproliferative neoplasms of the nasal cavity and paranasal sinuses in 16 cats. J Am Vet Med Assoc 1994; 204: 78–83. [PubMed] [Google Scholar]
- 34. Haney SM, Beaver L, Turrel J, et al. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986–2006). J Vet Intern Med 2009; 23: 287–94. [DOI] [PubMed] [Google Scholar]
- 35. Milner RJ, Peyton J, Cooke K, et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996–2003). J Am Vet Med Assoc 2005; 227: 1118–22. [DOI] [PubMed] [Google Scholar]
- 36. Sfiligoi1 G, Théon AP, Kent MS. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound 2007; 48: 388–93. [DOI] [PubMed] [Google Scholar]
- 37. Schwartz C. Chronic respiratory conditions and acupuncture therapy. Probl Vet Med 1992; 4: 136–43. [PubMed] [Google Scholar]