Abstract
Practical relevance Feline bronchial asthma is one of the most commonly diagnosed respiratory conditions of cats. Clinical signs range from intermittent wheezing and coughing, which can compromise quality of life, to episodes of severe dyspnea that can be life-threatening.
Clinical challenges Feline asthma can be easily disregarded as a simplistic condition. However, much about its pathophysiology remains obscure. There is no gold standard method of diagnosis, and current approaches are associated with various limitations. Also, feline asthma is typically treated with long-term glucocorticoid therapy, which can have significant consequences.
Audience Because of its prevalence, general practitioners encounter asthma regularly. Refractory cases are often managed by veterinary internists and pulmonologists.
Patient group Asthma can be diagnosed in cats of any age but is usually seen in young to middle-aged adults (mean 4 years, range 1–15 years). There is no sex predilection, but the Siamese breed appears to be overrepresented.
Evidence base While the standard clinical approach to feline asthma has changed little in recent years, new research has provided greater insight into many aspects of this complex disease and new strategies are being studied. This article reviews the current literature in order to raise awareness of how advances in the understanding of the pathophysiology, diagnosis and treatment of feline asthma may be determining the future direction of clinical practice.
What do we currently understand by ‘feline asthma’?
Disease classification
The general term of feline bronchial disease is often used to refer to any type of airway pathology distal to the tracheal bifurcation. 1 This term is also frequently applied more specifically to inflammatory conditions of the airways that lack an identifiable etiology. 2,3 A wide variety of clinicopathologic, radiographic and clinical features are associated with this disease category. In particular, several reports have described predominantly neutrophilic bronchoalveolar inflammation in the majority of cases of feline bronchial disease, while eosinophilic or mixed inflammation has also been seen in a significant subset of cats. 1,2,4–6 These findings suggest that feline bronchial disease could be further classified into two phenotypic categories: feline bronchial asthma, characterized primarily by eosinophilic airway inflammation, and chronic bronchitis, identified by neutrophils as being the predominant cell type within the airways.
Is comparison between feline and human asthma appropriate?
Asthma in cats has often been compared with the condition that is well characterized in humans. Although a subset of cats with feline bronchial disease do demonstrate spontaneous bronchoconstriction and airway remodeling, which resemble the changes seen in the human asthma syndrome, the majority of cats with naturally occurring feline bronchial disease described in the veterinary literature display cough as the predominant clinical sign. 1,2,4–6,8 This contrasts with the asthmatic condition in humans, which is characterized by dyspnea as the most common symptom. Furthermore, the inability to perform forced-exhalation spirometry in cats, which is the test of choice for diagnosing asthma in humans, and the deficits in our knowledge about the specific clinical features of feline asthma, impair our ability to make direct comparisons between the feline and human forms of the disease. 8
Clinically, some authors have differentiated these conditions based on the presence of a daily cough in cats with chronic bronchitis and the documentation of reversible airflow limitation in those with bronchial asthma. 7 At present, there are no widely accepted, standardized criteria used to discriminate feline bronchial asthma from chronic bronchitis, and distinguishing these conditions in a clinical setting can be challenging. 3,8
The distinguishing feature of asthma is reversible airflow limitation caused by airway hyperreactivity, increased mucus production and smooth muscle hypertrophy consequent to lower airway inflammation — changes that are postulated to result from a type I hypersensitivity reaction within the airways.
Experimental models — a pivotal advance.
Development of experimental models of feline asthma that exhibit the features of the naturally occurring disease has been a pivotal advance in the study of this condition. In a laboratory setting, cats were artificially sensitized to Bermuda grass allergen, house dust mite allergen or Ascaris suum by parenteral, intranasal and aerosol administration. 9,10 Following sensitization, these cats demonstrated clinical signs histopathological lesions, airway responsiveness, airway cytology changes and radiographic findings consistent with those seen in cats with naturally occurring asthma, providing an accessible model for further study.
Pathophysiology
The distinguishing feature of asthma is reversible airflow limitation caused by airway hyperreactivity, increased mucus production and smooth muscle hypertrophy consequent to lower airway inflammation — changes that are postulated to result from a type I hypersensitivity reaction within the airways. 6,7 This reaction is initiated when antigen becomes bound to the surface of dendritic cells via major histocompatibility complex (MHC) class II molecules (Fig 1). 11 These cells present the antigen to naive CD4+ T cells, which facilitates secretion of interleukins (IL) 4, 5 and 13 from T helper 2 (TH2) cells. IL-4, in particular, induces B lymphocytes to undergo isotype switching and synthesize allergen-specific immunoglobulin E (IgE) molecules that bind to the surface of mast cells. 12
FIG 1.
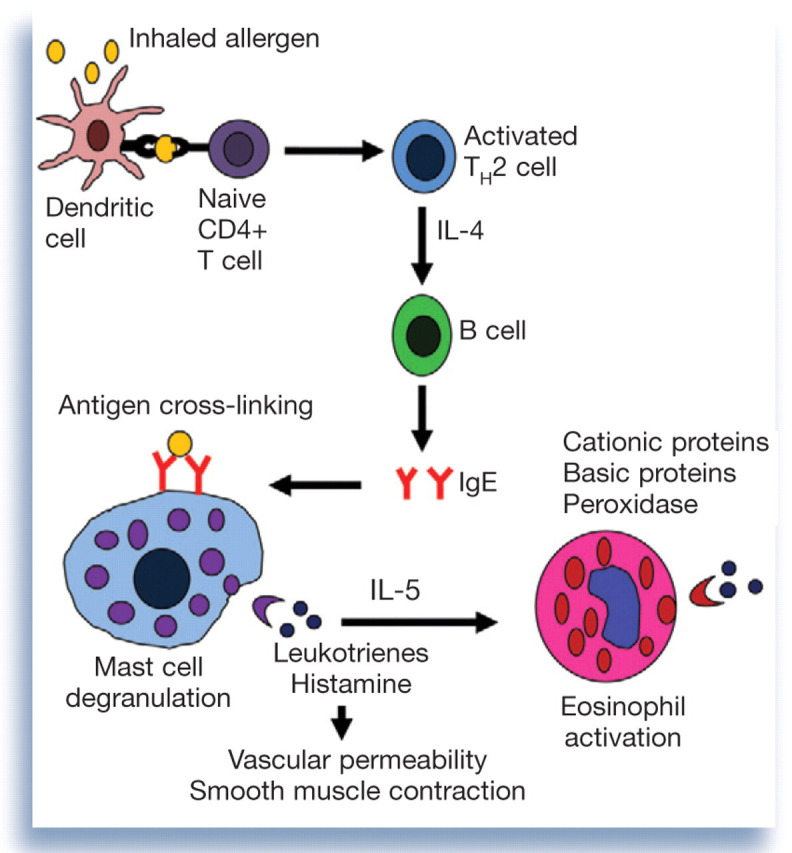
Basic pathophysiology of a type I hypersensitivity reaction within the airways
When allergen is inhaled subsequently, IgE molecules become cross-linked on the surface of mast cells by binding a common antigen particle, and mast cell degranulation occurs. 11 Histamine and leukotrienes released from mast cell granules promote vascular permeability and smooth muscle contraction. Chemokines released from the granules recruit eosinophils to the airways. 12 Cationic protein, peroxidase, major basic protein and eosinophil-derived neurotoxin released from degranulating eosinophils mediate tissue pathology. 13
Novel insights into pathophysiology
Eosinophils: friend or foe?
Eosinophils have been profiled as the primary destructive cells in the asthmatic response, but evidence is surfacing to suggest that eosinophils may in fact modulate the activity of a variety of cell types. 14 For example, human eosinophils secrete IL-4, IL-5 and IL-13, cytokines that are essential for eosinophil recruitment, activation and survival. 11,15,16 These interleukins are also the representative TH2 cytokines, which suggests that eosinophils may participate in reactions mediated by these cells. 11 Likewise, eosinophils may be involved in the responses of T helper 1 (TH1) cells, as human eosinophils express IL-12 and interferon-γ, the representative TH1 cytokines. 17 Eosinophils may even suppress local inflammatory responses by altering the TH1/TH2 balance through the production of cytokines such as IL-10, transforming growth factor and indoleamine. 17,18 Evidence indicates that eosinophils may also function as professional antigen-presenting cells by expressing MHC II on their cell surface. 19 Finally, it is speculated that eosinophils participate in T cell selection in the thymus. 14
Despite the emphasis on the eosinophil as the primary effector cell in the dysregulated immune response that characterizes the human asthmatic condition, much about the inflammatory processes that occur in feline asthma remains to be ascertained. The complexity of the pathophysiology of the feline condition is underscored by the cytological and histopathological findings in experimental models of feline asthma (see above). For example, similar increases in the percentages of both eosinophils and neutrophils in the bronchoalveolar lavage fluid (BALF) of artificially sensitized cats were noted following allergen challenge in one study. 9 Likewise, pulmonary eosinophilic inflammation was not a prominent histopathologic feature in the experimental models described in another study. 10 These results, in conjunction with the findings of primarily neutrophilic airway inflammation in several retrospective studies of cats with naturally occurring bronchial disease, accentuate the need for further investigation into the pathophysiological features that are specific to asthmatic disease in cats.
IgG and IgA
Like eosinophils, IgG and IgA have both beneficial and detrimental effects in the pathophysiology of human asthma. On the one hand, IgG can neutralize antigen by binding it directly before it interacts with IgE, and secretory IgA prevents allergen absorption from mucosal surfaces. 20,21 On the other hand, IgA has been shown to mediate eosinophil degranulation, and some IgG subclasses may incite mast cell degranulation. 22,23 In cats with experimentally induced asthma, Norris et al 24 found a significant increase in the levels of allergen-specific IgG and IgA in the serum and BALF following exposure to aerosolized allergen. These results suggest that IgG and IgA may contribute to the pathophysiology of feline asthma, making the disease process more complex than originally thought.
Advances in diagnosis
Detection of oxidative damage
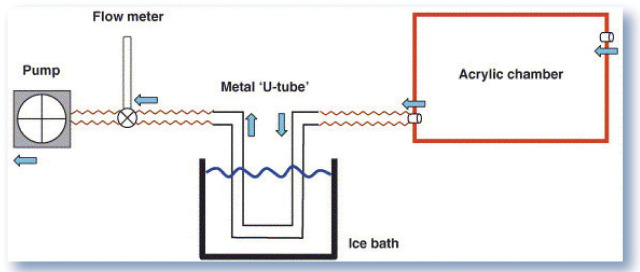
Recognition of an oxidant/antioxidant imbalance in feline asthma has led to the investigation of diagnostic tools such as exhaled breath condensate (EBC) analysis. 25 EBC may be evaluated for markers of oxidative stress including hydrogen peroxide (H2O2). Sparkes et al 26 have described a method of collecting EBC from cats that involves placing a fully awake cat into an acrylic chamber where it is allowed to respire normally. Exhaled air is collected by a tube passing through an ice water bath that condenses the air for analysis (Fig 2). Using this method, Kirschvink et al 27 found a positive correlation between the concentration of H2O2 in the EBC and the percentage of eosinophils in the BALF of cats with experimentally induced asthma. These results indicate that H2O2 in EBC may serve as a biomarker of lower airway inflammation in asthmatic cats and may be measured non-invasively to screen cats for lower airway inflammation.
FIG 2.
Diagrammatic representation of the system used for breath condensate collection (blue arrows indicate flow of air). Reprinted from Sparkes et al, 26 with permission
Identification of allergens
Identifying the specific antigens that incite the asthmatic response is the first step in designing targeted therapeutic strategies. Norris et al 28 have developed a species-specific enzyme-linked immunosorbent assay (ELISA) for measuring allergen-specific IgE in feline serum. With this ELISA, they showed that following artificial sensitization, cats demonstrated increasing levels of serum IgE directed against the allergen used for sensitization. Serum tests for allergen-specific IgE may be a promising means of measuring the degree of IgE response, screening cats for sensitization to particular allergens, and monitoring the response to immunotherapy.
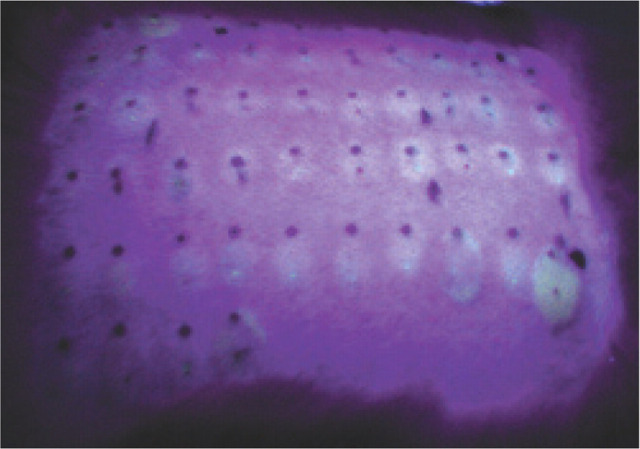
In conjunction with serum IgE measurement, intradermal skin testing (IDST) can be utilized in the diagnosis of atopic dermatitis and may be applied to allergic airway disease (Fig 3). In a pilot study by Moriello et al, 29 IDST and measurement of serum allergen-specific IgE were conducted in cats with idiopathic inflammatory lower airway disease but without evidence of dermatologic disease. Cats with airway disease had significantly more positive serum and skin test reactions than did cats without respiratory or dermatologic disease. However, these positive reactions may have been an indication of allergen exposure only and thus may not have clinical relevance. Also, skin reactivity may not be an accurate reflection of airway reactivity. Another recent study compared IDST with measurement of serum allergen-specific IgE with an ELISA in cats experimentally sensitized to a specific allergen. The results showed IDST to be more sensitive though less specific than serum IgE determination for identifying allergen sensitization. 30
FIG 3.
Wheals resulting from intradermal skin testing visualized under ultraviolet light in a cat. Courtesy of Elaine Striler, Michigan State University
Based on these findings, IDST may have utility as a screening test to detect allergen sensitization, while measurement of serum allergen-specific IgE may be applied as a tool for selecting specific allergens for immunotherapy.
Despite the emphasis on the eosinophil in the dysregulated immune response that characterizes the human asthmatic condition, much about the inflammatory processes that occur in feline asthma remains to be ascertained.
Traditional diagnostic approach.
Currently, no gold standard test exists for the diagnosis of feline asthma. 7,31 Data collected from physical examination, thoracic radiography, bronchoscopy and bronchoalveolar lavage (BAL) analysis have traditionally been used to support a diagnosis in a clinical setting. 31,32 However, these methods are associated with limitations that can obscure the diagnosis and compromise the patient, emphasizing the need for investigation of new techniques.
Physical examination
Abnormalities detected during physical examination of cats with asthma can include dyspnea, wheezing, tachypnea, cyanosis, coughing, sneezing, nasal discharge, expiratory push and palpably decreased compliance of the chest wall. 5,6,32 Thoracic auscultation may reveal expiratory wheezes, crackles and rhonchi. 6,32
Limitations associated with examining a cat suspected to be asthmatic include the risk of exacerbating respiratory distress with handling and restraint. Moreover, the above-mentioned findings can be associated with a variety of disorders such as congestive heart failure, chylothorax, pulmonary parasitism, pneumonia and pulmonary neoplasia. 31,32 Conversely, physical examination and thoracic auscultation may be normal, further confusing the diagnosis. 67
Thoracic radiography
Abnormalities seen on thoracic radiographs of asthmatic cats may include a bronchial, interstitial, alveolar or mixed lung pattern, lung hyperinflation, ill-defined hyperlucencies and atelectasis. 1,6,7,31–33
Limitations of relying on radiography for diagnosis include the lack of specificity of these findings. Also, interobserver variability in interpretation of radiographs of cats with bronchial disease has been documented. 33 Furthermore, radiographs of cats with asthma may reveal no abnormalities in some cases. 6
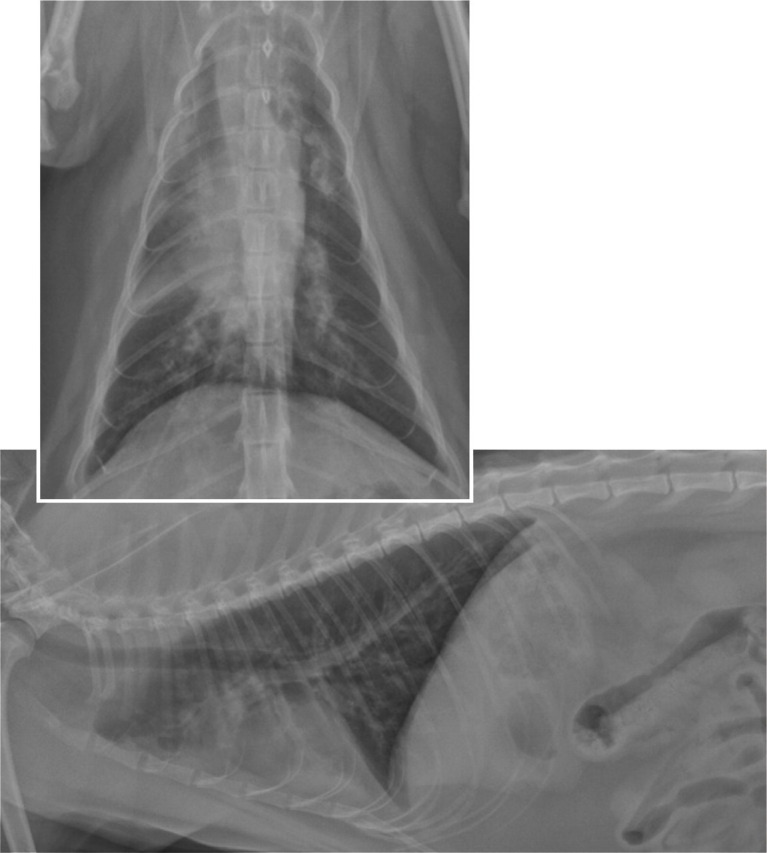
Ventrodorsal and right lateral thoracic radiographs demonstrating a diffuse bronchial pattern with an alveolar pattern in the right cranial and right middle lung lobes in a cat with asthma
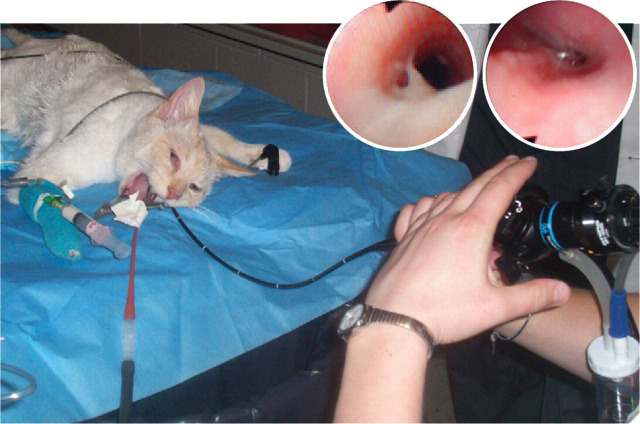
Bronchoscopic examination. (inset images) Mucus accumulation and mucosal hyperemia (left) and airway spasm (right) obtained during bronchoscopy of an asthmatic cat
Bronchoscopy/bronchoalveolar lavage cytology
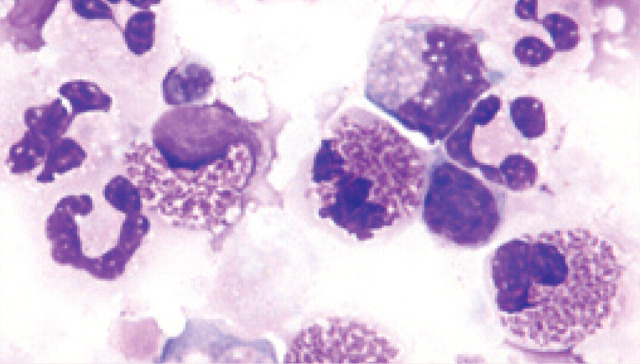
Abnormalities such as hyperemia, edema, mucus accumulation and reduction in airway diameter have been seen during bronchoscopy of feline asthmatics. 32 Bronchoalveolar cytology reveals predominantly eosinophilic inflammation, but increased numbers of neutrophils may also be observed. 1,24–7
Limitations of bronchoscopy that have been documented in asthmatic cats include the risk of inducing severe bronchospasm. 34 This procedure also requires expensive equipment, and expertise. Eosinophils may comprise up to 25% of the cells in BAL samples obtained from healthy cats, making differentiation of asthmatic from healthy cats difficult in some cases. 35 Additionally, eosinophilic airway inflammation may be seen in a variety of conditions including pulmonary parasitism (which has also been documented to elicit predominantly neutrophilic inflammation), fungal or viral pneumonia, hypereosinophilic syndrome and dirofilariasis — and, therefore, is not specific to asthma. 4,36,37
Eosinophilic and neutrophilic inflammation in a bronchoalveolar lavage sample obtained from an asthmatic cat. Courtesy of Michael Scott, Michigan State University, Diagnostic Center for Population and Animal Health
Endotracheal lavage and blind bronchoalveolar lavage.
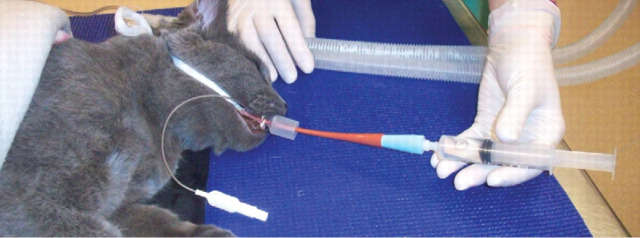
Collection of airway samples for cytological analysis and culture is a key component of the diagnostic evaluation of a cat suspected of having asthma. In general practice, bronchoalveolar or endotracheal lavage can be performed blindly without the use of a bronchoscope to obtain adequate airway samples. The procedures outlined below have been used extensively in both clinical and research settings for the collection of airway samples. 4,38
2–4 h prior to induction of general anesthesia, terbutaline 0.01 mg/kg should be administered subcutaneously to prevent bronchospasm, which may be induced by the procedure. 39
After induction of general anesthesia, the cat is positioned in left lateral recumbency and intubated with a sterile endotracheal tube.
-
Warm sterile 0.9% saline is then infused into the airways by one of the following techniques:
Endotracheal lavage A 5 ml/kg volume of warm sterile saline is infused directly into the lumen of the endotracheal tube using a 35 ml syringe, which is attached to a syringe adapter inserted onto the end of the endotracheal tube. Immediately after infusion of the entire aliquot, suction is applied using the same syringe to retrieve a portion of the fluid. This procedure is repeated twice. 38
Blind bronchoalveolar lavage A 5–10 ml volume of warm sterile saline is infused through a sterile 6 or 8 French gauge red rubber urinary catheter, which has been passed through the endotracheal tube until it lodges within a distal airway. Immediately after infusion of the entire aliquot, suction is applied using the same syringe to retrieve a portion of the fluid. This procedure is repeated once. 4
The caudal half of the cat may be elevated to assist in retrieval of fluid. Retrieval of 40–50% of the infused volume should be expected.
100% oxygen is administered via the endotracheal tube for 5–10 mins following the procedure.
Immediately after collection, the aliquots are pooled, and a small amount is transferred to a sterile collection tube for general aerobic culture and culture for Mycoplasma organisms.
The remainder of the sample should be centrifuged immediately or placed on ice until the samples can be processed.
After centrifugation, a sample of cells from the pellet should be applied to a microscope slide, air-dried, then stained with Wright's stain or Romanowsky-type stains (Diff-Quik).
At least 200 cells per slide should be counted to determine the differential cell count.
Blind BAL being performed
Possibilities for therapy
Allergen-specific immunotherapy
Once the inciting allergens have been identified, allergen-specific immunotherapy (ASIT) can be employed. ASIT induces production of IgE-blocking antibodies, decreases lymphocyte proliferation, and stimulates the T regulatory cells that downregulate TH1 and TH2 responses. 40 Rush immunotherapy (RIT) is a modified form of ASIT that involves administration of increasing doses of allergen over a couple of days, instead of weeks to months (Table 1). 41 Following subcutaneous administration of RIT to cats with experimentally induced asthma, the number of BALF eosinophils was found to be decreased and the concentration of serum allergen-specific IgG increased compared with untreated controls, although structural and functional parameters were not evaluated. 42 Intranasal delivery of RIT has also been evaluated in experimentally sensitized cats, and treatment resulted in complete resolution of clinical signs in all cats in one study. 43
TABLE 1.
Rush immunotherapy schedule in an experimental feline asthma model
| Day | Time | Dose of BGA (μg) | Route |
| 1 | 8 am | 10 | Subcutaneous |
| 1 | 10 am | 20 | Subcutaneous |
| 1 | 12 pm | 40 | Intranodal |
| 1 | 2 pm | 80 | Subcutaneous |
| 1 | 4 pm | 100 | Subcutaneous |
| 1 | 6 pm | 200 | Intranodal∗ |
| 2 | 8 am | 200 | Subcutaneous |
BGA = Bermuda grass allergen
After the second cat underwent RIT and developed anaphylaxis immediately following the second intranodal injection of allergen, this intranodal injection was subsequently changed to a subcutaneous injection
Reproduced from Reinero et al, 42 with permission
In the future, RIT may be an acceptable alternative to chronic glucocorticoid therapy for management of feline asthma.
Traditional therapeutic approach.
A spectrum of clinical signs can be associated with feline asthma — from a chronic, intermittent cough to acute, episodic respiratory distress. 7,31,32 Current therapy is directed towards suppressing the inflammation that leads to these clinical signs and dilating the airways to relieve dyspnea and improve oxygenation. The adverse effects and precautions associated with these therapies underscore the need for alternative therapeutic interventions, which are currently under investigation.
Glucocorticoids
Glucocorticoid therapy is indicated only for cats with clinical signs that occur at least twice weekly.
-
Prednisolone should be administered at an initial dose of 1–2 mg/kg PO q12h to suppress airway inflammation. This dose should be continued for 5–7 days. If clinical improvement is noted, the dose may be gradually tapered over 2–3 months. 7
Adverse effects of systemic glucocorticoid therapy in cats can include polyuria, polydipsia, polyphagia, alopecia, skin atrophy, weight gain, poor wound healing, bruising and increased susceptibility to infection. 44 Conditions such as urolithiasis and obesity may result from chronic systemic steroid use, and pre-existing conditions such as diabetes mellitus and congestive heart failure may be exacerbated. Inhaled glucocortioids represent an appealing alternative to systemic steroids (see page 688); however, the expense associated with these medications may affect clients' willingness to try this form of therapy.
Bronchodilators
-
β2-adrenergic agonists have been used long term in some patients to alleviate the clinical signs associated with bronchospasm. Terbutaline sulfate can be administered orally at a dose of 0.1–0.2 mg/kg PO q8–12 h for chronic management of cats with clinical signs that are difficult to control. Alternatively, inhaled albuterol sulfate (90 μg) may be administered as needed. Albuterol sulfate alone may be sufficient for management of cats with clinical signs that occur less than twice weekly. 7
Adverse effects of β2-adrenergic agonists include tachycardia, central nervous system (CNS) stimulation, tremors and hypokalemia. These medications should be used with caution in patients with pre-existing cardiac disease, diabetes mellitus, hyperthyroidism, hypertension or seizure disorders, or that are being treated with digoxin, sympathomimetic amines, tricyclic antidepressants or monoamine oxidase inhibitors. 7,45 In addition, chronic daily racemic albuterol administration has been associated with increased severity of airway inflammation in experimental models of feline asthma. 46
-
Methylxanthines are phosphodiesterase inhibitors that can be administered chronically to achieve bronchodilation. Theophylline is available in a standard preparation (Theolair; 3M Pharmaceuticals) with a recommended dose of 6–8 mg/kg PO q12h, and an extended release formulation (Slo-bid; Rhône-Poulenc Rorer) with a recommended dose of 25 mg/kg PO q24h given in the evening. 45
Adverse effects associated with methylxanthines include tachyarrhythmias, increased gastric acid secretion and central nervous system stimulation. When administered concurrently with enrofloxacin, toxicity may occur as enrofloxacin inhibits metabolism of theophylline. In addition, theophylline may antagonize the effects of β-adrenergic agonists when administered simultaneously. An increased incidence of seizures may be seen when theophylline is administered with ketamine. 44
Anti-inflammatory therapies
Like ASIT, omega-3 polyunsaturated fatty acids have been used in the management of atopic dermatitis and may be beneficial for the treatment of asthma. The anti-inflammatory effects of these fatty acids are derived from their ability to compete with arachidonic acid for access to lipoxygenase, resulting in decreased production of pro-inflammatory eicosanoids. In addition, they increase production of anti-inflammatory proteins and inhibit expression of pro-inflammatory genes. 47 New Zealand green-lipped mussel lipid extract, a source of these fatty acids, improves clinical, inflammatory and functional parameters in human asthmatics and has been investigated in experimental models of feline asthma. 48 Cats treated with mussel lipid extract did not exhibit a significant reduction in BALF cell counts after therapy, but did demonstrate decreased airway responsiveness and increased BALF concentrations of lipoxin A4, an eicosanoid that mediates resolution of inflammation. Given these results, omega-3 polyunsaturated fatty acids in the form of New Zealand green-lipped mussel lipid extract may be considered as a potential adjunctive therapy in the management of feline asthma.
Antileukotrienes as well as antihistamines and antiserotonergics are unlikely to be the safe and effective alternatives to glucocorticoid therapy that are needed in feline asthma therapy.
Inhibiting eosinophil influx into the airways may be an even more effective means of suppressing the inflammatory response that occurs in asthma. The tripeptide feG is a molecule derived from the submandibular salivary gland that is presumed to downregulate allergen-induced expression of intracellular adhesion molecule-1, a surface receptor necessary for eosinophil diapedesis into the airways. 49 In a study by DeClue et al, 50 artificially sensitized cats were administered feG orally prior to allergen challenge. A significant reduction in the degree of eosinophilic airway inflammation was noted in these cats compared with those administered placebo. However, these results were not noted when cats were treated with feG chronically, suggesting that feG administration may have more utility as a method of preventing acute exacerbations of asthma in situations where allergen exposure is likely. 51 Further studies are necessary to determine whether treatment with feG affects clinical, structural and functional parameters in cats with naturally occurring disease.
Due to their wider availability, pharmaceuticals including antiserotonergics and antihistamines may be a more feasible therapeutic option than feG (Table 2). Serotonin and histamine released during mast cell degranulation contribute to the clinical manifestations of asthma in humans. Initial studies have shown that treatment of experimentally sensitized cats with cyproheptadine, an antihistamine with antiserotonergic properties, and cetirizine, a histamine receptor antagonist, did not significantly reduce the severity of eosinophilic airway inflammation, concentrations of serotonin and histamine in serum or BALF, airway responsiveness, or serum concentration of allergen-specific IgE compared with placebo. 52,53 Further studies may demonstrate that antiserotonergics and antihistamines are effective when used in combination with traditional glucocorticoid therapy. However, at this time, antiserotonergics and antihistamines cannot be advocated as monotherapy for feline asthma.
TABLE 2.
Pharmaceuticals that have been evaluated for management of feline asthma
| Pharmaceutical | Class | Dose | Outcome | References |
| Cyproheptadine | Antiserotonergic | 2–8 mg PO q12h | No difference in functional or inflammatory parameters compared with placebo | Reinero et al 52 Schooley et al 53 |
| Cetirizine | Antihistamine | 5 mg PO q12h | No difference in inflammatory parameters compared with placebo | Schooley et al 53 |
| Zafirlukast | Antileukotriene | 10 mg PO q12h | No difference in inflammatory parameters compared with placebo | Reinero et al 52 |
| Cyclosporine | T cell and mast cell inhibitor | 10 mg/kg PO q12h | Inhibition of airway hyperresponsiveness and cytological and histological alterations but not mast cell degranulation | Padrid et al 54 Mitchell et al 55 |
| Salmeterol/fluticasone | LABA/glucocorticoid | 100/500 μg via inhalation q12h | Significant decrease in functional and inflammatory parameters compared with pretreatment | Leemans et al 56 |
| Racemic albuterol | Short-acting β2-adrenergic agonist | 2 mg/kg via inhalation q12h | Significant increase in inflammatory parameters compared with placebo | Reinero et al 46 |
LABA = long-acting β2-adrenergic agonist
Similarly, antileukotrienes are often used adjunctively in the management of humans with asthma but are unlikely to be valuable in the treatment of the condition in cats when used as the sole therapeutic. In humans, leukotrienes constrict bronchial smooth muscle, impair mucociliary transport, enhance mucus release, potentiate inflammatory cell influx, increase vascular permeability and cause proliferation of smooth muscle. 57 In fact, urinary leukotriene concentrations can be measured as a marker for lower airway inflammation in human patients. Norris et al 58 analyzed the leukotriene:creatinine ratios in the urine and leukotriene:protein ratios in the BALF of cats prior to and following allergen sensitization and found no difference in pre-and post-sensitization values. Moreover, zafirlukast, a leukotriene receptor antagonist, did not significantly alter the BALF eosinophil percentages, airway responsiveness or serum allergen-specific IgE concentrations of artificially sensitized cats. 52 Taken together, these results indicate that antileukotrienes as well as antihistamines and antiserotonergics are unlikely to be the safe and effective alternatives to glucocorticoid therapy that are needed in feline asthma therapy.
Albuterol may be considered for intermittent, short-term asthma intervention, but its long-term use for feline asthma management may be detrimental.
The search for a means of avoiding the adverse effects of systemic glucocorticoid therapy has also led to investigation of the immunosuppressant cyclosporine for treatment of feline asthma. Results of initial studies have been conflicting. Cyclosporine A has been speculated to be effective for asthma therapy due to its ability to inhibit T cell activity and mast cell degranulation in murine models. 59 Padrid et al 54 found that pretreatment of experimentally asthmatic cats with high-dose cyclosporine prevented the development of airway hyperresponsiveness and significant cytological and histological changes within the airways. In contrast, Mitchell et al 55 observed that pretreatment with cyclosporine did not inhibit mast cell degranulation in artificially sensitized cats. Further studies are needed to define the efficacy and safety of cyclosporine for the management of feline asthma in clinical patients.
Another popular strategy that is employed to circumvent the complications inherent in systemic glucocorticoid therapy is the use of inhalant pharmaceuticals. Direct administration of glucocorticoids into the airways (see box) maximizes local therapeutic efficacy while lack of significant systemic absorption minimizes the adverse effects traditionally associated with oral glucocorticoids. Uniform distribution of a radiopharmaceutical in the lungs of cats following nebulization demonstrates that delivery of medications via inhalation is feasible in cats. 60 Experimental cats with mild bronchitis that were treated with inhaled fluticasone exhibited a significant reduction in BALF neutrophil percentage, bronchoscopic and radiographic scores, and bronchial responsiveness compared with pre-treatment values. However, these cats did not demonstrate clinical signs of their disease prior to therapy. 61 Similarly, a significant decrease in BALF eosinophilia has been documented in experimental models of feline asthma following administration of inhaled glucocorticoids such as fluticasone and flunisolide, although improvement in clinical signs was not evaluated. 52,56 Inhaled flunisolide has been shown to cause suppression of the hypothalamic-pituitary-adrenocortical axis but without inducing adverse clinical effects in healthy cats. 62 The use of inhaled glucocorticoids for the management of feline asthma is becoming more widespread and may soon replace systemic steroid therapy as it has in the treatment of asthma in humans.
Inhalant glucocorticoid therapy.
Fluticasone propionate (Flovent; GlaxoSmithKline) is an inhaled glucocorticoid administered by a metered dose inhaler attached to a spacer device and face mask that may be used as an alternative to systemic steroid therapy. Because of its low oral bioavailability, fluticasone is not associated with the adverse effects seen with long-term prednisolone use. 7 Recent work has shown that doses of 44, 110 and 220 μg q12h are equally efficacious in suppressing eosinophilic airway inflammation in experimental models of feline asthma. 63 In moderately affected cats, prednisolone 1–2 mg/kg PO q12h should be administered concurrently for the first 10–14 days of inhalant therapy to allow time for fluticasone to achieve its maximal effect.
Equipment (component parts and assembled) for administration of inhalant pharmaceuticals to cats. When the metered dose inhaler is actuated, the aerosolized medication is dispersed into the spacer device, precluding the need for inhalation by the patient to be coordinated with device actuation
When administering inhalant pharmaceutical to a cat, the face mask should remain tightly on the patient's face for the duration of 7–10 breaths. Photo courtesy of Erin Whalin, Michigan State University
Bronchodilators
In humans with severe asthma, the cornerstone of therapy is inhaled glucocorticoids combined with long-acting bronchodilators, and combination therapy may be on the horizon for feline asthmatics as well. Treatment of experimentally sensitized cats with inhaled salmeterol, a long-acting bronchodilator, combined with fluticasone, an inhaled glucocorticoid, resulted in a significant decrease in airway responsiveness, BALF eosinophil percentage and peripheral eosinophil counts. These results were comparable to those seen with systemic prednisolone therapy. 56
Beta2-adrenergic agonists are also used to treat acute asthmatic crises in cats and humans. Historically, chronic therapy with short-acting β2-adrenergic agonists has been associated with increased mortality in human patients. 64 Racemic albuterol is a commonly prescribed short-acting β2-agonist that consists of a 1:1 mixture of an R-enantiomer and an S-enantiomer. The R-enantiomer has bronchodilatory and anti-inflammatory effects whereas the S-enantiomer promotes airway hyperreactivity and inflammation. Initial results in experimentally sensitized cats treated with S-albuterol and racemic albuterol for 14 days indicate that these formulations exacerbate eosinophilic airway inflammation. 46 As such, albuterol may be considered for intermittent, short-term asthma intervention, but its long-term use for feline asthma management may be detrimental.
Barometric whole-body plethysmography may become the preferred means of monitoring the response to asthma therapy in the future.
Monitoring response to therapy
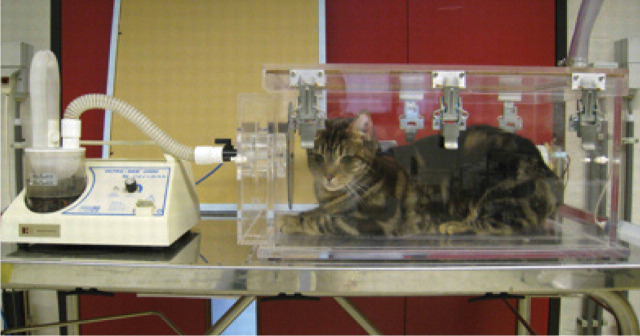
Traditionally, the response to therapy for feline asthma has been judged by monitoring changes in clinical signs. Yet airway inflammation can persist despite clinical improvement. Barometric whole-body plethysmography (BWBP) is a non-invasive procedure that involves placing an awake, spontaneously breathing cat into a transparent, ventilated chamber attached to a pressure transducer (Fig 4). The pressure transducer measures variables generated by the respiratory movements of the cat (Table 3). Gradually increasing concentrations of nebulized carbachol, a parasympathomimetic, are then introduced into the chamber until bronchoconstriction occurs. 65 Using this method to evaluate experimental models of feline asthma, Kirschvink and associates 66 showed that increases in airway responsiveness paralleled increases in the BALF eosinophil count. Airway responsiveness measured by BWBP has also been shown to decrease following treatment with inhaled glucocorticoids. 61
FIG 4.
Cat undergoing barometric whole-body plethysmography. Courtesy of Jerome Leemans, University of Namur
TABLE 3.
Baseline parameters measured by barometric whole-body plethysmography
| Parameter | Normal value ± SEM |
| Respiratory rate | 58 ± 8 breaths/min |
| Peak expiratory flow | 83 ± 12 ml/sec |
| Peak inspiratory flow | 109 ± 8 ml/sec |
| Tidal volume | 58 ± 15 ml |
| Expiratory time | 730 ± 100 msec |
| Inspiratory time | 470 ± 40 msec |
Adapted from Rozanski and Hoffman, 65 with permission
Though experience with this method in cats is limited at this time, BWBP may become the preferred means of monitoring the response to asthma therapy in the future.
What's new in human asthma?
Given various similarities between feline and human asthma, examining some of the current advances in the study of this condition in people is warranted. Recent studies have focused on the increasing incidence of childhood obesity and asthma, and have determined that childhood obesity increases the likelihood of concomitant asthma. 67 Proposed explanations for this association include the presence of common genetic factors giving rise to both conditions, obesity-induced inflammation initiating asthma, and an unhealthy lifestyle promoting the development of these diseases. These findings may be pertinent to veterinary medicine as the incidence of obesity is rising in the feline population.
Dependence on long-term medical therapy is a common feature of the management of refractory disease in both human and feline asthmatics. Bronchial thermoplasty is a therapeutic modality that has been investigated as a means of reducing the contractility of airway smooth muscle. This procedure involves the application of radiofrequency energy to the airway wall using bronchoscopic guidance with the aim of reducing smooth muscle mass and thus the potential for bronchoconstriction. 68 This procedure has been shown to be well tolerated in humans with refractory disease 69 and warrants investigation as a possible intervention in feline asthma to reduce dependence on steroids or to manage severely affected patients.
Interleukin-5 is essential for eosinophil activation, recruitment and survival in the disease mechanisms of both human and feline asthma; mepolizumab is a pharmaceutical consisting of monoclonal antibody directed against this interleukin. In a study by Haldar et al, 70 human patients with refractory asthma treated with mepolizumab once monthly for 1 year experienced significantly fewer exacerbations of their clinical signs and more pronounced reductions in sputum eosinophil counts compared with those treated with placebo. At this time, the use of monoclonal antibodies in veterinary medicine is in the early stages, but it may be an emerging focus in feline asthma research.
Recent studies have have determined that childhood obesity increases the likelihood of concomitant asthma. These findings may be pertinent to veterinary medicine as the incidence of obesity is rising in the feline population.
KEY POINTS.
Recent research using experimental models has expanded our knowledge of feline bronchial asthma.
Eosinophils and immunoglobulins may actually play beneficial roles in mediating the allergic inflammatory response that characterizes feline asthma.
Techniques that detect evidence of oxidative damage and increased serum IgE production may be potential minimally invasive diagnostic tools for detection of feline asthma in a clinical setting.
Avoiding the adverse effects of long-term glucocorticoid therapy is the primary goal of novel therapeutics such as allergen-specific immunotherapy, which is designed to prevent the asthmatic response, and antihistamines, antiserotonergics and antileukotrienes, which are intended to suppress inflammation but have been proven to be ineffective as the sole means of feline asthma management.
Due to proposed similarities between feline and human asthma, investigations into human allergic airway disease will likely dictate the future of feline asthma research.
Ultimately, as more studies are performed, the approach to feline asthma in a clinical setting may be dramatically altered, resulting in improved survival and quality of life for the vast number of cats afflicted with allergic respiratory disease.
Future directions
What is the influence of bronchial infection?
Despite many recent contributions to the understanding of feline and human asthma, much remains to be established. Directions for further research may point towards determining the influence of bronchial infection in the etiopathogenesis of asthma. In human asthmatics, an increased incidence of Mycoplasma and Chlamydophila respiratory infections has been found in patients experiencing acute exacerbations of their disease. 71 Concurrent bacterial infections are documented infrequently in cats with inflammatory bronchopulmonary disease. 2 However, bacterial and viral infections may go undetected as airway samples for culture are not often collected from cats that are treated for asthma empirically. In addition, steroid therapy may predispose cats to secondary infections, which could contribute to the refractory nature of asthma in some cases. Clarifying the role of infection in the pathophysiology of asthma could heighten awareness of impending asthmatic disease in cats with a history of respiratory tract infections and could alter the current approach to therapy.
Is the ‘atopic march’ relevant?
Additional insight into the etiopathogenesis of feline asthma may be gained by investigation of the ‘atopic march’; this is a concept from human medicine that describes the natural progression of the atopic condition, characterized by manifestations such as atopic dermatitis, allergic rhinitis and asthma that peak then subside during different periods of life. 72 Research has shown that coexistent upper and lower airway eosinophilic inflammation is present in human patients with clinical signs indicative of only asthma or allergic rhinitis. 73,74 Studies are underway to determine whether concurrent upper and lower airway inflammation exists in asthmatic cats, and the results may change our current understanding of feline asthma. 75
Acknowledgements
The authors wish to thank Dr Stephan Carey for his assistance in preparing this manuscript.
References
- 1. Moise NS, Wiedenkeller D, Yeager AE, Blue JT, Scarlett J. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986). J Am Vet Med Assoc 1989; 194: 1567–73. [PubMed] [Google Scholar]
- 2. Foster SF, Allan GS, Martin P, Robertson ID, Malik R. Twenty-five cases of feline bronchial disease (1995–2000). J Feline Med Surg 2004; 6: 181–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bay JD, Johnson LR. Feline bronchial disease/asthma. In: King LG, ed. Textbook of respiratory disease in dogs and cats. St Louis: WB Saunders, 2004: 388–95. [Google Scholar]
- 4. Foster SF, Martin P, Braddock JA, Malik R. A retrospective analysis of bronchoalveolar lavage cytology and microbiology (1995–2000). J Feline Med Surg 2004; 6: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dye JA, McKiernan BC, Rozanski EA, et al. Bronchopulmonary disease in the cat: historical, physical, radiographic, clinicopathologic, and pulmonary functional evaluation of 24 affected and 15 healthy cats. J Vet Intern Med 1996; 10: 385–400. [DOI] [PubMed] [Google Scholar]
- 6. Corcoran BM, Foster DJ, Fuentes VL. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats. J Small Anim Pract 1995; 36: 481–88. [DOI] [PubMed] [Google Scholar]
- 7. Padrid P. Chronic bronchitis and asthma in cats. In: Bonagura JD, Twedt DC, eds. Current veterinary therapy XIV. Philadelphia: WB Saunders, 2009: 650–58. [Google Scholar]
- 8. Reinero CR, DeClue AE, Rabinowitz P. Asthma in humans and cats: is there a common sensitivity to aeroallergens in shared environments? Environ Res 2009; 109: 634–40. [DOI] [PubMed] [Google Scholar]
- 9. Kirschvink N, Leemans J, Delvaux F, Snaps F, Clercx C, Gustin P. Functional, inflammatory and morphological characterization of a cat model of allergic airway inflammation. Vet J 2007; 174: 541–53. [DOI] [PubMed] [Google Scholar]
- 10. Norris Reinero CR, Decile KC, Berghaus RD, et al. An experimental model of allergic asthma in cats sensitized to house dust mite or Bermuda grass allergen. Int Arch Allergy Immunol 2004; 135: 117–31. [DOI] [PubMed] [Google Scholar]
- 11. Tizzard IR. Veterinary immunology: an introduction. 6th edn. Philadelphia: WB Saunders, 2000. [Google Scholar]
- 12. Barnes PJ. Pathophysiology of asthma. Eur Respir Mon 2003; 23: 84–113. [Google Scholar]
- 13. Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep 2007; 7: 18–26. [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen EA, Taranova AG, Lee NA, Lee, JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol 2007; 119: 1313–20. [DOI] [PubMed] [Google Scholar]
- 15. Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol 2002; 169: 1021–27. [DOI] [PubMed] [Google Scholar]
- 16. Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol 2001; 166: 4813–17. [DOI] [PubMed] [Google Scholar]
- 17. Lamkhioued B, Gounni AS, Aldebert D, et al. Synthesis of type 1 (IFNγ) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Ann NY Acad Sci 1996; 796: 203–8. [DOI] [PubMed] [Google Scholar]
- 18. Odemuyiwa S, Ghahary A, Li Y, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol 2004; 173: 5909–13. [DOI] [PubMed] [Google Scholar]
- 19. Shi H-Z, Xiao C-Q, Li C-Q, et al. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy 2004; 59: 428–35. [DOI] [PubMed] [Google Scholar]
- 20. Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest 2006; 116: 833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Böttcher MF, Häggström P, Björksten, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy 2002; 32: 1293–98. [DOI] [PubMed] [Google Scholar]
- 22. Peebles RS, Liu MC, Adkinson NF, Lichtenstein L, Hamilton R. Ragweed-specific antibodies in bronchoalveolar lavage fluids and serum before and after segmental lung challenge: IgE and IgA associated with eosinophil degranulation. J Allergy Clin Immunol 1998; 101: 265–73. [DOI] [PubMed] [Google Scholar]
- 23. Van der Zee J, Aalberse R..The role of IgG in immediate-type hypersensitivity. Eur Respir J 1991; 4 (suppl 13): 91s–96s. [PubMed] [Google Scholar]
- 24. Norris CR, Byerly JR, Decile KC, et al. Allergen-specific IgG and IgA in serum and bronchoalveolar lavage fluid in a model of experimental feline asthma. Vet Immunol Immunopathol 2003; 96: 119–27. [DOI] [PubMed] [Google Scholar]
- 25. Hirt RA. Oxidant-antioxidant balance and the role of oxidative stress in feline asthma [abstract]. J Vet Intern Med 2002; 16: 387. [Google Scholar]
- 26. Sparkes AH, Mardell EJ, Deaton C, Kirschvink N, Marlin D. Exhaled breath condensate collection in cats — description of a non-invasive technique to investigate airway disease. J Feline Med Surg 2004; 6: 335–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirschvink N, Marlin D, Delvaux F, et al. Collection of exhaled breath condensate and analysis of hydrogen peroxide as a potential marker of lower airway inflammation in cats. Vet J 2005; 169: 385–96. [DOI] [PubMed] [Google Scholar]
- 28. Norris CR, Decile KC, Byerly JR, et al. Production of polyclonal antisera against feline immunoglobulin E and its use in an ELISA in cats with experimentally induced asthma. Vet Immunol Immunopathol 2003; 96: 149–57. [DOI] [PubMed] [Google Scholar]
- 29. Moriello KA, Stepien RL, Henik RA, Wenholz LJ. Pilot study: prevalence of positive aeroallergen reactions in 10 cats with small-airway disease without concurrent skin disease. Vet Dermatol 2007; 18: 94–100. [DOI] [PubMed] [Google Scholar]
- 30. Lee-Fowler TM, Cohn LA, DeClue AE, Spinka CM, Ellebracht RD, Reinero CR. Comparison of intradermal skin testing (IDST) and serum allergen-specific IgE determination in an experimental model of feline asthma. Vet Immunol Immunopathol 2009; 132: 46–52. [DOI] [PubMed] [Google Scholar]
- 31. Padrid P. Feline asthma: diagnosis and treatment. Vet Clin North Am Small Anim Pract 2000; 30: 1279–93. [DOI] [PubMed] [Google Scholar]
- 32. Byers CG, Dhupa N. Feline bronchial asthma: pathophysiology and diagnosis. Compend Contin Educ Pract Vet 2005; 27: 418–25. [Google Scholar]
- 33. Gadbois J, d'Anjou M, Dunn M, et al. Radiographic abnormalities in cats with feline bronchial disease and intra- and interobserver variability in radiographic interpretation: 40 cases (1999–2006). J Am Vet Med Assoc 2009; 234: 367–75. [DOI] [PubMed] [Google Scholar]
- 34. Kirschvink N, Leemans J, Delvaux F, Snaps F, Clercx C, Gustin P. Bronchodilators in bronchoscopy-induced airflow limitation in allergen-sensitized cats. J Vet Intern Med 2005; 19: 161–67. [DOI] [PubMed] [Google Scholar]
- 35. Padrid PA, Feldman BF, Funk K, Samitz EM, Reil D, Cross CE. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats. Am J Vet Res 1991; 52: 1300–207. [PubMed] [Google Scholar]
- 36. Moon M. Pulmonary infiltrates with eosinophilia. J Small Anim Pract 1992; 33: 19–23. [Google Scholar]
- 37. Bauer T. Pulmonary hypersensitivity disorders. In: Kirk RW, ed. Current veterinary therapy X. Philadelphia: WB Saunders, 1989; 369–77. [Google Scholar]
- 38. Hawkins EC, Kennedy-Stoskopf S, Levy J, Meuten DJ, Cullins L, DeNicola D. Cytologic characterization of bronchoalveolar lavage fluid collected through an endotracheal tube in cats. Am J Vet Res 1994; 55: 795–802. [PubMed] [Google Scholar]
- 39. Johnson LR, Drazenovich TL. Flexible bronchoscopy and bronchoalveolar lavage in 68 cats (2001–2006). J Vet Intern Med 2007; 21: 219–25. [DOI] [PubMed] [Google Scholar]
- 40. Rocklin RE. Immune mechanisms in allergen-specific immunotherapy. Clin Immunol Immunopathol 1989; 53: S119–S131. [DOI] [PubMed] [Google Scholar]
- 41. Mueller RS, Bettenay SV. Evaluation of the safety of an abbreviated course of injections of allergen extracts (rush immunotherapy) for the treatment of dogs with atopic dermatitis. Am J Vet Res 2001; 62: 307–10. [DOI] [PubMed] [Google Scholar]
- 42. Reinero CR, Byerly JR, Berghaus RD, et al. Rush immunotherapy in an experimental model of feline allergic asthma. Vet Immunol Immunopathol 2006; 110: 141–53. [DOI] [PubMed] [Google Scholar]
- 43. Lee-Fowler TM, Cohn LA, DeClue AE, Spinka CM, Reinero CR. Evaluation of subcutaneous versus mucosal (intranasal) allergen-specific rush immunotherapy in experimental feline asthma. Vet Immunol Immunopathol 2009; 129: 49–56. [DOI] [PubMed] [Google Scholar]
- 44. Plumb DC. Plumb's veterinary drug handbook. 5th edn. Ames: Blackwell Publishing Professional, 2005. [Google Scholar]
- 45. Byers CG, Dhupa N. Feline bronchial asthma: treatment. Compend Contin Educ Pract Vet 2005; 27: 426–32. [Google Scholar]
- 46. Reinero CR, Delgado C, Spinka C, DeClue AE, Dhand R. Enantiomer-specific effects of albuterol on airway inflammation in healthy and asthmatic cats. Int Arch Allergy Immunol 2009; 150: 43–50. [DOI] [PubMed] [Google Scholar]
- 47. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leuk Essent Fatty Acids 2006; 75: 197–202. [DOI] [PubMed] [Google Scholar]
- 48. Leemans J, Cambier C, Chandler T, et al. Prophylactic effects of omega-3 polyunsaturated fatty acids and luteolin on airway hyperresponsiveness and inflammation in cats with experimentally-induced asthma. Vet J 2010; 184: 111–14. [DOI] [PubMed] [Google Scholar]
- 49. Dery RE, Ulanova M, Puttagunta L, et al. Frontline: inhibition of allergen-induced pulmonary inflammation by the tripeptide feG: a mimetic of a neuro-endocrine pathyway. Eur J Immunol 2004; 34: 3315–25. [DOI] [PubMed] [Google Scholar]
- 50. DeClue AE, Schooley E, Nafe LA, Reinero CR. feG-COOH blunts eosinophilic airway inflammation in a feline model of allergic asthma. Inflamm Res 2009; 58: 457–62. [DOI] [PubMed] [Google Scholar]
- 51. Eberhardt JM, DeClue AE, Reinero CR. Chronic use of the immunomodulating tripeptide feG-COOH in experimental feline asthma. Vet Immunol Immunopathol 2009; 132: 175–80. [DOI] [PubMed] [Google Scholar]
- 52. Reinero CR, Decile KC, Byerly JR, et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma. Am J Vet Res 2005; 66: 1121–27. [DOI] [PubMed] [Google Scholar]
- 53. Schooley EK, McGee Turner JB, JiJi RD, Spinka CM, Reinero CR. Effects of cyproheptadine and cetirizine on eosinophilic airway inflammation in cats with experimentally induced asthma. Am J Vet Res 2007; 68: 1265–71. [DOI] [PubMed] [Google Scholar]
- 54. Padrid PA, Cozzi P, Leff AR. Cyclosporine A inhibits airway reactivity and remodeling after chronic antigen challenge in cats. Am J Respir Crit Care Med 1996; 154: 1812–18. [DOI] [PubMed] [Google Scholar]
- 55. Mitchell RW, Cozzi P, Ndukwu M, Spaethe S, Leff AR, Padrid PA. Differential effects of cyclosporine A after acute antigen challenge in sensitized cats in vivo and ex vivo. Br J Pharmacol 1998; 123: 1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leemans J, Kirschvink N, Cambier C, Clercx C, Gustin P. Oral and inhaled corticosteroids decrease eosinophilic airway inflammation and bronchial reactivity in Ascaris suum-sensitized and challenged cats. Proceedings of the 26th Annual Veterinary Comparative Respiratory Society Forum; 2008; Oklahoma City, Oklahoma, USA.
- 57. Nicosia S, Capra V, Rovati GE. Leukotrienes as mediators of asthma. Pulm Pharmacol Ther 2001; 14: 3–19. [DOI] [PubMed] [Google Scholar]
- 58. Norris CR, Decile KC, Berghaus LJ, et al. Concentrations of cysteinyl leukotrienes in urine and bronchoalveolar lavage fluid of cats with experimentally induced asthma. Am J Vet Res 2003; 64: 1449–53. [DOI] [PubMed] [Google Scholar]
- 59. Wershil BK, Furuta GT, Lavigne JA, Choudhury AR, Wang ZD, Galli SJ. Dexamethasone or cyclosporine A suppress mast cell-leukocyte cytokine cascades. J Immunol 1995; 154: 1391–98. [PubMed] [Google Scholar]
- 60. Schulman RL, Crochik SS, Kneller SK, McKiernan BC, Schaeffer DJ, Marks SL. Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats. Am J Vet Res 2004; 65: 806–9. [DOI] [PubMed] [Google Scholar]
- 61. Kirschvink N, Leemans J, Delvaux F, et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis. J Feline Med Surg 2006; 8: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reinero CR, Brownlee L, Decile KC, et al. Inhaled flunisolide suppresses the hypothalamic-pituitary-adrenocortical axis, but has minimal systemic immune effects in healthy cats. J Vet Intern Med 2006; 20: 57–64. [DOI] [PubMed] [Google Scholar]
- 63. Cohn LA, DeClue AE, Cohen RL, Reinero CR. Dose effects of fluticasone propionate in an experimental model of feline asthma. Proceedings of the 26th Annual American College of Veterinary Internal Medicine Forum; 2008. June 4–7; San Antonio, Texas, USA.
- 64. Jalba M. Three generations of ongoing controversies concerning the use of short acting beta-agonist therapy in asthma: a review. J Asthma 2008; 45: 9–18. [DOI] [PubMed] [Google Scholar]
- 65. Rozanski EA, Hoffman AM. Lung mechanics using plethysmography and spirometry. In: King LG, ed. Textbook of respiratory disease in dogs and cats. St Louis: Saunders, 2004: 175–81. [Google Scholar]
- 66. Kirschvink N, Leemans J, Delvaux F, Snaps F, Clercx C, Gustin P. Non-invasive assessment of airway responsiveness in healthy and allergen-sensitised cats by use of barometric whole body plethysmography. Vet J 2007; 173: 343–52. [DOI] [PubMed] [Google Scholar]
- 67. Ahmad N, Biswas S, Bae S, Meador KES, Huang R, Singh KP. Association between obesity and asthma in US children and adolescents. J Asthma 2009; 46: 642–46. [DOI] [PubMed] [Google Scholar]
- 68. Cox G, Miller JD, McWilliams A, FitzGerald M, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173: 965–69. [DOI] [PubMed] [Google Scholar]
- 69. Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24: 659–63. [DOI] [PubMed] [Google Scholar]
- 70. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. New Engl J Med 2009; 360: 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest 2007; 132: 1962–66. [DOI] [PubMed] [Google Scholar]
- 72. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003; 112: S118–S127. [DOI] [PubMed] [Google Scholar]
- 73. Gaga M, Lambrou P, Papageorgiou N, et al. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy 2000; 30: 663–69. [DOI] [PubMed] [Google Scholar]
- 74. Braunstahl G-J, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HC, Prins J-B. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy 2003; 33: 579–87. [DOI] [PubMed] [Google Scholar]
- 75. Carey SA, Venema CM, Williams KJ, Gershwin LJ, Reinero CR. Histopathologic and morphometric evaluation of the nasal airways of cats with experimentally induced asthma. Proceedings of the 28th Annual American College of Veterinary Internal Medicine Forum; 2010. June 10–12; Anaheim, California, USA.