Abstract
Objectives
Feline chronic kidney disease (CKD) is characterized by chronic tubulointerstitial nephritis, and inflammation contributes to the progression of renal fibrosis. Mesenchymal stem cells (MSCs) have demonstrated anti-inflammatory and antifibrotic effects in rodent CKD models. However, few randomized trials evaluating the effectiveness of MSC therapy for diseases in companion animals have been reported. The purpose of this study was to evaluate the effectiveness of allogeneic MSCs for the treatment of feline CKD using a randomized, placebo-controlled trial.
Methods
MSCs were isolated from the cryopreserved adipose tissues of specific pathogen-free research cats and culture expanded. CKD cats were enrolled in a randomized, placebo-controlled, blinded one-way crossover clinical study. Four CKD cats were randomized to receive 2 × 106 MSCs/kg intravenously at 2, 4 and 6 weeks. Four CKD cats were randomized to receive placebo, with two cats crossing over to the MSC treatment group and one cat failing to complete the trial. Complete blood counts, chemistry and urinalysis were performed at weeks 0, 2, 4, 6 and 8. Glomerular filtration rate (GFR) via nuclear scintigraphy and urine protein:creatinine ratio (UPC) were determined at weeks 0 and 8.
Results
Six cats received three doses of allogeneic MSC culture expanded from cryopreserved adipose without adverse effects. No significant change in serum creatinine, blood urea nitrogen, potassium, phosphorus, GFR by nuclear scintigraphy, UPC or packed cell volume was seen in cats treated with MSCs. Individual changes in GFR were 12%, 8%, 8%, 2%, –13% and –67% in treated cats compared with 16%, 36% and 0% in placebo-treated cats.
Conclusions and relevance
While administration of MSC culture expanded from cryopreserved adipose was not associated with adverse effects, significant improvement in renal function was not observed immediately after administration. Long-term follow-up is necessary to determine whether MSC administration affects disease progression in cats with CKD.
Introduction
Chronic kidney disease (CKD) is a common medical condition in geriatric cats and is characterized histologically by tubulointerstitial inflammation and fibrosis, with subsequent progressive loss of renal function.1,2 Currently, renal transplant is the only definitive therapy to improve kidney function in cats with CKD. Therefore, novel and effective therapeutic options are highly sought after to provide additional treatment options for cats suffering from this disease.
Mesenchymal stem cells (MSCs) have been proposed as a novel treatment option for the management of CKD. MSCs exert potent anti-inflammatory and antifibrotic effects and may therefore indirectly improve renal function by reducing disease-associated inflammation and fibrosis through paracrine effects.3–7 Demonstrated immunomodulatory effects of MSCs include inhibition of lymphocyte proliferation and cytokine production, suppression of dendritic cell function and suppression of interferon-γ production by natural killer cells. 8 In vitro studies have demonstrated that MSCs can produce growth factors, cytokines and anti-inflammatory mediators, all of which could help maintain or improve renal function and suppress intra-renal inflammation.9–11 The ability of MSCs to suppress inflammation appears to be mediated both by secreted factors and by direct contact with inflammatory cells.10,11 In the majority of studies with experimentally induced CKD in rodents, administration of both adipose-derived and bone marrow-derived MSCs has demonstrated significant renoprotective effects, including reduction of intrarenal inflammatory infiltrate, decreased fibrosis and glomerulosclerosis.3,4,6,7,12 Parameters of renal function and clinical health, including weight, creatinine, blood urea nitrogen (BUN), proteinuria, blood pressure and hematocrit have also been demonstrated to improve as a result of MSC therapy.3,4,6,7,12 Several routes of administration – intraparenchymal, subcapsular, intraperitoneal, intravenous (IV) – have been explored, and all seem to be effective. Efficacy is thought to originate from paracrine effects rather than cell engraftment into the kidney.4,9 Multiple repeated injections of MSCs appear to be even more effective than single injections.3,4 As inflammation appears to be present at all stages of CKD in cats, the immunomodulatory actions of MSC are appealing as a potential means of suppressing intrarenal inflammation and subsequent fibrosis. Thus, MSC therapy may be an effective new approach to slow the progression of CKD and improve renal function.
Previous studies in cats performed by our group have explored the use of MSCs for treatment of feline CKD, but results have been variable.13,14 The purpose of this study was to conduct a placebo-controlled trial to assess the efficacy of adipose-derived allogeneic MSCs to improve renal function in cats with stable CKD. We hypothesized that repeated IV administration of MSCs would be well tolerated and would result in improvement of renal function as measured by serum creatinine and glomerular filtration rate (GFR) by nuclear scintigraphy.
Materials and methods
Study cats
Cats with stable CKD serum creatinine (1.6–5.0 mg/dl) were recruited from the patient population at the Veterinary Teaching Hospital at Colorado State University (CSU). Cats were determined to have stable CKD based on two repeated biochemical evaluations performed at least 2 weeks apart and ultrasonographic evidence of CKD. Stability was defined as no more than a 15% change in serum creatinine, no uncontrolled metabolic disturbances, no change in medication regimen within 2 weeks and no clinical concerns. Pretreatment evaluation included complete blood count (CBC), biochemistry profile, urinalysis, urine culture, blood pressure, total thyroxine, urine protein:creatinine ratio (UPC), feline leukemia/feline immunodeficiency virus serology and a renal ultrasound. Cats were excluded from the study if they had evidence of ureteroliths; pyelonephritis; ureteral obstruction (pelvic dilation >13 mm and/or dilated proximal ureters); anatomic abnormalities such as polycystic kidney disease or masses; uncontrolled hypertension; or concurrent systemic disease. Administration of concurrent supportive therapies was allowed provided there were no changes in therapy 2 weeks prior to or during the study period. The study was approved by the Institutional Animal Care and Use Committee at CSU (#11-2915A), and all owners reviewed and signed consent forms prior to participation in the study.
Predetermined randomization for order of treatment allocation was established using an online random number generator; as cats were enrolled they were assigned consecutively to a treatment group. Treatment group was blinded to the owners during the initial study phase. At the conclusion of the 8 week trial, the owners of cats that were allocated to the placebo group were given the option to crossover to the treatment group.
Adipose-derived MSC preparation and administration
Adipose-derived MSCs were isolated from adipose tissue of specific pathogen-free cats collected from a subcutaneous site on the ventral abdomen just caudal to the umbilicus, as previously described.13,15 For preparation of the adipose tissue for cryopreservation, the tissue was minced and divided into 1 g aliquots in 1 ml freezing medium (11% dimethyl sulfoxide, 14% MSC medium, 75% fetal bovine serum [FBS]), and stored in liquid nitrogen for no longer than 1 year prior to use. Cryopreserved adipose was then later thawed, immediately washed twice with Dulbecco’s Phosphate Buffered Saline (DPBS) and then prepared for culture. For isolation of the stromal vascular fraction, the tissue was minced and digested with 1 mg/ml collagenase (Sigma-Aldrich) for 30 mins at 37°C. The sample was centrifuged, and the stromal vascular fraction was plated in MSC medium (low-glucose Dulbecco’s Modified Eagle Medium [DMEM], 100 U/ml penicillin, 100 µg/ml streptomycin, 2 µM L-glutamine, 1% essential amino acids without L-glutamine, 1% non-essential amino acids, 0.075% sodium bicarbonate [Invitrogen/Gibco] plus 15% FBS [Cell Generation]). The MSCs were incubated until approximately 70% confluent, with media changes every 2–3 days. Cells were harvested by trypsinization at passage 2–3, washed and resuspended in 10 ml DPBS with 200 IU heparin sulfate, and administered as a slow IV push to the cephalic vein over 20 mins. Cats received MSCs at weeks 2, 4 and 6. As previous studies have indicated that some difference in immunomodulatory potential may exist between donors, 16 all three injections received were from different donor cats given in a pre-randomized order. A total of three donor cats, all male, aged 2–7 years, were used to provide MSCs for the study. Placebo-control cats received 10 ml DPBS with 200 IU heparin administered as a slow manual IV push to the cephalic vein over 20 mins at weeks 2, 4 and 6.
Characterization of MSCs
MSCs cultured from cryopreserved adipose tissue were characterized by surface marker expression using flow cytometry and a panel of cross-reactive antibodies specific for surface determinants expressed by MSC from other species. Specifically, feline MSCs were analyzed for surface expression of CD44 (antimouse/human, antibody clone IM7; eBioscience) and CD90 (antihuman, antibody clone eBio5E10; eBioscience). MSCs were also assessed for expression of CD4 (antifeline antibody clone 3-4F4; SouthernBiotech) and major histocompatibility complex (MHC) class II (antihuman antibody clone TU39; BD Biosciences). Samples were analyzed using a CyAn ADP flow cytometer (Beckman Coulter). Approximately 10,000 events were collected for analysis per sample.
In vitro differentiation assays were conducted to confirm the multipotency of feline MSCs, as assessed by their ability to differentiate into three cell lineages (osteoblasts, chondrocytes and adipocytes) that are characteristic of MSCs. For differentiation into adipocytes, MSCs at confluency were incubated with MSC medium supplemented with 0.5 µM dexamethasone, 50 µM indomethacin and 0.5 µM 3-isobutyl-1-methylxanthine (all Sigma- Aldrich) for 3 weeks with media changes every 3–4 days.
Chondrogenic differentiation medium consisted of DMEM 1× (Cellgro) supplemented with 15% FBS (Cell Generation), 10 nM dexamethasone (Sigma-Aldrich), 10 ng/ml transforming growth factor-β (R&D Systems), 50 µg/ml ascorbic acid (Sigma-Aldrich) and 40 µg/ml proline (Sigma-Aldrich).
Osteogenic differentiation medium consisted of MSC medium supplemented with 10 nM dexamethasone, 50 µM ascorbic acid and 20 mM β-glycerophosphate (Sigma-Aldrich). At the end of the differentiation period, cells were fixed with 10% neutral buffered formalin and stained with Oil Red O (Sigma-Aldrich) for the presence of lipid or with Alizarin red (Sigma-Aldrich) for the presence of calcium. Cell pellets from cartilage differentiation were harvested and placed in OCT Compound (Sakura Finetek) and flash frozen prior to staining with toluidine blue (Richard-Allan Scientific) for cartilage matrix. MSCs cultured in MSC medium alone under identical conditions were used as differentiation controls.
Clinical monitoring
Each enrolled cat underwent physical examination, weighing and routine blood work consisting of CBC, serum biochemistry and urinalysis at weeks 0, 2, 4, 6 and 8. Each cat had a UPC performed at weeks 0 and 8. Cats were monitored continuously during MSC injection for any signs of reaction or adverse effects. Additionally, each cat had a GFR by nuclear scintigraphy performed at weeks 0 and 8. For the scintigraphy procedure, all enrolled cats (MSC-treated and placebo-controlled) were sedated with a standard sedation protocol (butorphanol 0.2mg/kg IV once) at a standard time before the procedure. A single nuclear technician performed all of the procedures for each particular cat. For each procedure 1.0 mCi of Tc99m-labeled diethylene triamine penta-acetic acid (Cardinal Health) was injected IV via a catheter placed in a standard location in each cat. Images were obtained using GE Millennium SPECT system applicable for small animal planar, whole body and single-photon emission CT imaging (GE Healthcare). Three blinded board-certified radiologists evaluated the GFR data, and a mean GFR value for each kidney, as well as a global value, was determined using regions of interest drawn on a summed image from the 1–3 mins postinjection time frame. The measurements calculated by the three radiologists were then averaged.
Statistical analysis
Analysis was performed using SAS Proc Mixed (SAS 9.3; SAS Institute). For each variable (GFR total, GFR left, GFR right, creatinine) the change was calculated for each animal taking week 8 – week 0. This change was used as the response variable in further analysis. A model was fit including treatment (placebo or MSC) and a random cat effect was included to account for the fact that two animals received both treatments.
Results
Study cats
Seventy-one cats were screened for entry into the clinical trial. Cats were excluded for the following reasons: concurrent illness (n = 23), end-stage or unstable disease (n = 16), extensive travel required (n = 10), owners thought the study too complex or did not want to participate in a placebo-controlled trial (n = 6), anatomic renal abnormalities (n = 4), and fractious or too stressed by visits (n = 4). Descriptive data for the eight enrolled cats are presented in Table 1. One cat was enrolled and randomized into the study in the placebo group, but between week 0 and 2 the owners elected to discontinue the trial. Data for this cat are not presented.
Table 1.
Summary of demographics of cats enrolled and randomized into the intravenous allogeneic cryopreserved studies
| Cat | Group | Age (years) | Sex | Breed | IRIS CKD stage | Creatinine, mg/dl (mol/l) | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | MSC | 13 | FS | DSH | II | 2.2 (194) | 2 × 106 MSCs/kg IV, three treatments |
| 2 | MSC | 19 | FS | Siamese | III | 3.3 (292) | 2 × 106 MSCs/kg IV, three treatments |
| 3 | MSC | 10 | MC | DLH | III | 3.5 (309) | 2 × 106 MSCs/kg IV, three treatments |
| 4 | MSC | 14 | MC | Siamese | III | 4.5 (398) | 2 × 106 MSCs/kg IV, three treatments |
| 5 | Placebo MSC crossover |
15 | MC | DLH | III | 3.8 (336) | PBS placebo, three treatments; then 2 × 106 MSCs/kg IV, three treatments |
| 6 | Placebo | 11 | FS | Siamese | III | 3.7 (327) | PBS placebo, three treatments |
| 7 | Placebo MSC crossover |
15 | MC | Siamese | II | 2.3 (203) | PBS placebo, three treatments; then 2 × 106 MSCs/kg IV, three treatments |
| 8 | Placebo | 9 | MC | Maine Coon | II | 2.1 (186) | Owners elected not to participate after enrollment and randomization |
IRIS = International Renal Interest Society; CKD = chronic kidney disease; MSC = mesenchymal stem cell; FS = female spayed; MC = male castrated; DSH = domestic shorthair; DLH = domestic longhair; IV = intravenous; PBS = phosphate-buffered saline
Characterization of MSCs
During in vitro culture, feline MSCs were observed to develop into a relatively homogeneous population of plastic-adherent cells with fibroblast-like morphology. Adipose-derived MSC expressed high levels of CD44 and CD90, and were negative for expression of CD4 and MHC class II, as previously reported. 14 MSC cultured from cryopreserved fat were capable of trilineage differentiation (Figure 1).
Figure 1.
Mesenchymal stem cells (MSCs) cultured from cryopreserved adipose were capable of trilineage differentiation. (a) MSCs formed intracellular lipid vacuoles (stained pink with Oil Red O) when incubated in adipocytic differentiation media for 21 days. (b) MSCs stained positive for calcium with alizarin red following differentiation into osteocytic phenotype after 21 days of incubation in differentiation media. (c) Cryosection of pellets of cartilage matrix (stained with toluidine blue) formed by MSCs when exposed to chondrocytic differentiation media for 21 days
Effects of MSC administration on renal function in cats with CKD
Six cats received three infusions of fresh, culture-expanded MSCs at a dose of 2 × 106 MSCs/kg body weight by slow IV infusion without developing clinically apparent adverse effects. No significant change in body weight, serum creatinine (Figure 2), BUN, phosphorus, potassium, packed cell volume, urine specific gravity or UPC was observed in cats treated with MSCs. The UPC in CKD cats treated with MSCs was a median of 0.15 (range 0.1–0.3) prior to administration of MSCs and a median of 0.2 (range 0.1–0.3) at the conclusion of the study period. The UPC in placebo-treated CKD cats was a median of 0.1 (range 0.1–0.4) at the beginning of the study and a median of 0.1 (range 0.1–0.3) at the conclusion of the study period.
Figure 2.
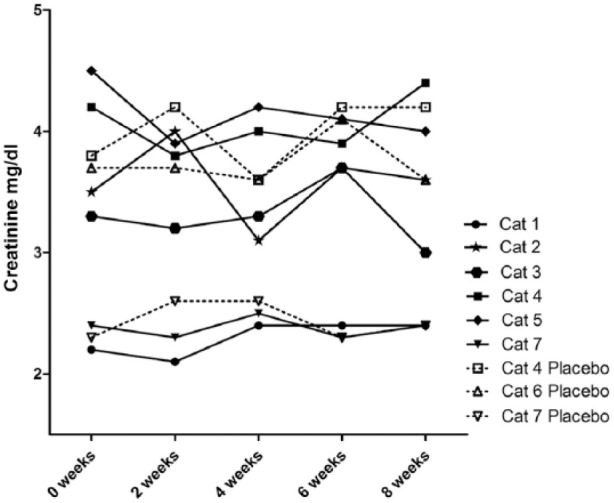
Serum creatinine values for cats that received three doses of 2 × 106 mesenchymal stem cells/kg intravenously 2 weeks apart. No significant difference in creatinine was detected
There was no statistically significant difference in global or individual kidney GFR values as determined by nuclear scintigraphy. Individual changes in global GFR were 12%, 8%, 8% and –67% in MSC-treated cats compared with 16%, 36% and 0% in placebo-treated cats (Figure 3). Cats that were in the placebo group and subsequently crossed over to receive MSC had changes in global GFR of 2% and –13%, respectively, during their MSC treatment period. Discordant creatinine and GFR results were observed in one animal: the MSC-treated cat that experienced the greatest decrease in creatinine also experienced the greatest decline in GFR.
Figure 3.

Results of glomerular filtration rate (GFR) determined by nuclear scintigraphy at 0 and 8 weeks for cats that received 2 × 106 mesenchymal stem cells/kg intravenously at weeks 2, 4 and 6
Discussion
MSCs have demonstrated significant renoprotective effects in rodent models of acute and chronic kidney disease, including reduction of interstitial inflammatory infiltrate, and reduction of fibrosis and glomerulosclerosis. However, few randomized trials evaluating the effectiveness of MSC therapy for diseases in companion animals have been reported. The current study therefore evaluated the safety and efficacy of repeated IV injections of allogeneic adipose-derived MSC for treatment of naturally occurring feline CKD using a randomized placebo-controlled study design. Key findings were that administration of three doses of 2 × 106 MSCs/kg (MSCs expanded from cryopreserved adipose to cats with CKD) was not associated with the development of clinically apparent adverse effects. Also, we did not detect a significant treatment effect (either positive or negative) on renal function in the 6 weeks after MSC treatment.
Previous clinical trials from our research group assessing MSC therapy in feline CKD have had variable results.13,14 For example, we found that intrarenal injections of MSCs appeared safe in CKD cats and resulted in mild improvement in renal parameters. In another study, we determined that repeated IV injections of cryopreserved MSCs in CKD cats resulted in mild improvement in renal parameters in some cats but it was questionable if the degree of improvement was clinically relevant. The clinical trials that have been performed by our research group were designed to emulate the time frame of rodent studies, which is generally short; improvements in clinicopathologic parameters are typically seen in the weeks subsequent to MSC administration, and rodents are sacrificed by 2–5 weeks for histopathologic analysis.3–5,7,12 Notably, none of the studies we have conducted in cats with CKD have replicated the efficacy of MSC treatment reported in rodent models of experimentally induced CKD within this time frame.3–5,7
One explanation for differing results of MSC therapy in cats with CKD is that the chronic nature of feline CKD makes these patients quite different from rodents with experimentally induced disease. The 5/6 nephrectomy model in rats is a commonly used method of experimentally inducing CKD and has been shown to induce histopathologic changes consistent with CKD. 17 One major difference between such models and MSC studies in cats with naturally occurring CKD is that in the majority of rodent studies the MSCs are administered a short period of time after surgical manipulation. For example, time frames for MSC administration range from immediately after nephrectomy (two studies),5,7 to 1 day later (two studies)3,18 and 2 weeks later. 4 Two recent studies evaluated the effect of MSC administered 6 weeks after surgical manipulation; both studies demonstrated improvement in renal functional parameters and amelioration of fibrosis, even when MSCs were given after inflammation and fibrosis was already established.19,20 However, 6 weeks is still a much shorter time frame than is typical in feline CKD patients. In most cases, cats that present to the clinical trials service to participate in MSC studies have a several year history of CKD. Although previous studies in rodent models demonstrate impressive potential, the results of these models should be evaluated with care as administration of MSCs soon after surgical partial nephrectomy is likely not representative of long-standing, naturally occurring disease. Therefore, it needs to be considered that MSCs may not be as effective in an environment of long-standing inflammation and fibrosis, and further studies are needed to explore this question.
There is a growing body of literature supporting the theory that MSCs are adversely affected by uremia.20–24 Recent studies have documented that MSCs obtained from uremic rats have reduced proliferation in culture, loss of regenerative potential, premature senescence, decreased capacity to induce angiogenesis and an altered secretome.20–23 Uremic effects also have been documented in vitro as a reduced capacity of MSCs from uremic individuals to ameliorate renal damage in experimentally induced CKD in comparison with MSCs from healthy rats.20,21 Observations have been mixed as to whether this affects both bone marrow-derived and adipose-derived MSC. However, one study offers evidence that adipose-derived MSCs are not as susceptible to uremic effects as bone marrow-derived MSCs. 25 Although there is a concern regarding the effects of uremia on MSC function, clinical trials performed with allogeneic adipose-derived MSC should circumvent these concerns and give the best opportunity for efficacy. This information does imply, however, that uremic patients are not the best MSC source; a concern for autologous MSC therapy. Little data have been gathered on whether MSCs transplanted into a uremic recipient environment will become compromised. The success of MSC in palliation of acute kidney injury and CKD in rodent models argues against this being an issue. Based on the currently available data, the effect of the uremic environment seems less likely to be the explanation for lack of short-term efficacy seen in the current clinical trial; however, an adverse effect on MSCs when placed in a uremic environment cannot be entirely ruled out.
An unexpected finding in this study was that discordant serum creatinine and GFR results were observed in one cat: the MSC-treated cat that experienced the greatest improvement in creatinine also experienced the greatest decline in GFR. In addition to improved creatinine, this cat gained weight during the clinical trial and was thought by the owner to have done very well clinically, making this result unexpected and difficult to explain. Every effort was made to decrease variability between GFR studies. Studies were performed on the same day of the week, at the same time of day, with the same sedation protocol and technician. However, the potential for inherent variability in the GFR process, the analysis of the images or day-to-day changes in the physiology of the patient still exists. Daily variability in GFR by nuclear scintigraphy has been previously evaluated, but not in cats with CKD. In a previous study in normal young dogs, daily variability was as much as 25%. However, this is probably not applicable to animals with CKD due to impaired autoregulatory mechanisms. 26 Our group previously gained some information on variability in CKD cats by performing GFR studies 1 week apart. In these cats GFR varied by as much as 28% (average 9.6%). 13 Taking these factors into account, GFR likely has to change by >20% to be considered a real change in response to a treatment effect. This inherent variability still does not explain the cat with a –67% change that experienced improvement based on serum creatinine, weight and clinical signs.
Over the course of our 6 year feline stem cell program, approximately 50 GFR studies have been performed, and only three technical failures have occurred in that time. On one occasion the machine broke down and took some time and effort to repair; once the isotope was not labeled by the supplier properly; and once the g camera head orientation was set wrong. In all cases it was immediately obvious that a technical problem had occurred. In the case of the cat in the present study, after careful scrutiny, no plausible explanation for the discordant result could be identified. This brings into question the value of measurement of renal function by this methodology as it requires the patient to be sedated, is expensive and is not a technique routinely used in clinical practice for patient assessment.
Although this was a randomized, blinded, placebo-controlled study, a limitation of this study was the small number of enrolled cats. Enrollment was difficult owing to the strict exclusion criteria and the study design of 50:50 ratio placebo to treatment, with placebo cats receiving the same number of procedures and sedation events, which was unpopular with owners. The latter was ameliorated by allowing placebo cats to cross over to the treatment group. Without definitive evidence of improvement in renal function during the study time frame, and the possibility that the stress of procedures outweighed therapeutic benefit, the decision was made to halt the study to move in a different direction.
Conclusions
Repeated IV injection of allogeneic MSCs expanded from cryopreserved adipose in cats with CKD was not associated with side effects; however, no discernible immediate improvement in renal function was appreciated compared with placebo. Long-term follow-up of patients that have received MSC injections will be necessary to determine if a delayed effect is appreciated. Additional studies are needed to determine if MSCs can be prepared or administered in alternative ways to increase efficacy.
Acknowledgments
We would like to acknowledge Elizabeth Thorne for technical assistance, and Ann Hess for assistance with statistical analysis.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This project was supported by a grant from the Morris Animal Foundation, as well as partially by Frankie’s Fund for Feline Stem Cell Therapy.
Accepted: 17 February 2015
References
- 1. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 2. Chakrabarti S, Syme HM, Brown CA. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 3. Lee SR, Lee SH, Moon JY, et al. Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail 2010; 32: 840–848. [DOI] [PubMed] [Google Scholar]
- 4. Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 2009; 27: 3063–3073. [DOI] [PubMed] [Google Scholar]
- 5. Villanueva S, Ewertz E, Carrion F, et al. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond) 2011; 121: 489–499. [DOI] [PubMed] [Google Scholar]
- 6. Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int 2006; 70: 121–129. [DOI] [PubMed] [Google Scholar]
- 7. Cavaglieri RC, Martini D, Sogayar MC. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc 2009; 41: 947–951. [DOI] [PubMed] [Google Scholar]
- 8. Reinders ME, Fibbe WE, Rabelink TJ. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant 2010; 25: 17–24. [DOI] [PubMed] [Google Scholar]
- 9. Togel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 2007; 292: F1626–F1635. [DOI] [PubMed] [Google Scholar]
- 10. Barry FP, Murphy JM, English K. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev 2005; 14: 252–265. [DOI] [PubMed] [Google Scholar]
- 11. McTaggart SJ, Atkinson K. Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease. Nephrology (Carlton) 2007; 12: 44–52. [DOI] [PubMed] [Google Scholar]
- 12. Villanueva S, Carreno JE, Salazar L, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond) 2013; 125: 199–210. [DOI] [PubMed] [Google Scholar]
- 13. Quimby JM, Webb TL, Gibbons DS. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg 2011; 13: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quimby JM, Webb TL, Habenicht LM. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther 2013; 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg 2012; 14: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel G, Kluba T, Hermanutz-Klein U, et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med 2013; 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleck C, Appenroth D, Jonas P, et al. Suitability of 5/6 nephrectomy (5/6NX) for the induction of interstitial renal fibrosis in rats–influence of sex, strain, and surgical procedure. Exp Toxicol Pathol 2006; 57: 195–205. [DOI] [PubMed] [Google Scholar]
- 18. Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev 2009; 18: 521–529. [DOI] [PubMed] [Google Scholar]
- 19. Donizetti-Oliveira C, Semedo P, Burgos-Silva M, et al. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant 2012; 21: 1727–1741. [DOI] [PubMed] [Google Scholar]
- 20. van Koppen A, Joles JA, Bongartz LG, et al. Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant 2012; 21: 2299–2312. [DOI] [PubMed] [Google Scholar]
- 21. Klinkhammer BM, Kramann R, Mallau M, et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PloS ONE 2014; 9: e92115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noh H, Yu MR, Kim HJ, et al. Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol Dial Transplant 2012; 27: 218–225. [DOI] [PubMed] [Google Scholar]
- 23. Idziak M, Pedzisz P, Burdzinska A, et al. Uremic toxins impair human bone marrow-derived mesenchymal stem cells functionality in vitro. Exp Toxicol Pathol 2014; 66: 187–194. [DOI] [PubMed] [Google Scholar]
- 24. Yamada A, Yokoo T, Yokote S, et al. Comparison of multipotency and molecular profile of MSCs between CKD and healthy rats. Hum Cell 2014; 27: 59–67. [DOI] [PubMed] [Google Scholar]
- 25. Roemeling-van Rhijn M, Reinders ME, de Klein A, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int 2012; 82: 748–758. [DOI] [PubMed] [Google Scholar]
- 26. Kampa N, Bostrom I, Lord P, et al. Day-to-day variability in glomerular filtration rate in normal dogs by scintigraphic technique. J Vet Med A Physiol Pathol Clin Med 2003; 50: 37–41. [DOI] [PubMed] [Google Scholar]



