Abstract
Objectives
Anaplasma phagocytophilum is an Ixodes species-transmitted rickettsial organism that is occasionally associated with clinical abnormalities in humans, ruminants, horses, dogs and cats. While serological evidence of A phagocytophilum exposure is common in cats in Ixodes species endemic areas, reports of clinical feline anaplasmosis are few. The objective of this study was to describe the clinical and laboratory abnormalities and treatment responses in 16 cats with A phagocytophilum DNA amplified from blood.
Methods
Commercial laboratory electronic records were searched to find cats that had A phagocytophilum DNA amplified from their blood. Once cases were identified, the primary care veterinarian was interviewed and the medical records were reviewed.
Results
The cats ranged in age from 4 months to 13 years (mean 4.1 years, median 2 years). All cats lived in Ixodes scapularis endemic areas and had potential for exposure. All cats were lethargic, 15 (94%) had elevated body temperature (>39.4°C) and 14 were anorexic on initial physical examination. Other less common clinical findings included hepatosplenomegaly, ataxia, conjunctivitis and elevation of the nictitating membranes. Blood from 11 cats was evaluated by complete blood cell count; abnormalities included lymphopenia in seven (64%) cats, thrombocytopenia in seven (64%), morulae in neutrophils of three (27%), neutropenia in three (27%) and leukopenia in two (18%). Treatment responses were reported for 14 cats, and the clinical abnormalities in these cats resolved when doxycycline was administered.
Conclusions and relevance
This is the first published report describing A phagocytophilum morulae in neutrophils of naturally infected North American cats with infection confirmed by PCR. A phagocytophilum infection should be considered in cats evaluated for lethargy, anorexia and fever living in Ixodes species endemic areas.
Introduction
Anaplasma phagocytophilum is a rickettsial organism that causes granulocytic anaplasmosis in cats, dogs, horses, ruminants and humans. The organism was first described in 1932 in Scottish sheep, 1 and then reported to affect horses, dogs, cats, cattle, camelids and humans. The worldwide distribution of A phagocytophilum follows the geographic distribution of the primary vector, Ixodes species. 2 In North America, the organism is transmitted by Ixodes scapularis in the Northeast and Midwest, and by Ixodes pacificus in the West. 3 Infections are highest in the late spring and autumn when both nymph and adult ticks are most mobile. 4 Transmission to mammals occurs within 24–48 h of tick attachment.5,6
Once transmitted, A phagocytophilum infects circulating neutrophils forming intracellular inclusions (morulae), which can be observed via light microscopy on a Romanowsky or Wright-Giemsa-stained blood smear. In North America, morulae have been identified in the neutrophils of naturally exposed dogs, 4 horses, 7 humans 8 and experimentally infected cats. 9 In Europe, morulae of A phagocytophilum have been described in ruminants, 10 cats, 11 horses 12 and dogs. 13
There are multiple papers describing anaplasmosis in dogs.4,14–16 The first reports of granulocytic anaplasmosis in naturally infected cats primarily reported non-specific clinical signs of lethargy, anorexia, fever and dehydration.11,17 –21 Clinicopathologic abnormalities in infected cats often include neutrophilia, lymphopenia and hyperglycemia with or without thrombocytopenia. Diagnosis of A phagocytophilum can be made based on clinical suspicion, a history of exposure to Ixodes species, identification of morulae within neutrophils, positive serologic results, PCR results and response to treatment. 19 Naturally infected cats produce serologic antibodies to A phagocytophilum and in the acute phase of infection may form morula inclusions in neutrophils identifiable on a blood smear.11,19 There are 20 reported cases identifying morulae in naturally infected cats in Europe: 15 cats in Italy, 19 two cats in Poland 21 and one cat each in Sweden, 11 Switzerland 17 and Finland. 18 To date, only five naturally infected cats with A phagocytophilum have been described in North America. 20
The objectives of this study were to collect retrospectively and describe the clinical and historical findings in cats that were positive by PCR for A phagocytophilum DNA in their blood, and to describe treatment and response. We were also able to characterize intracellular morulae identified in neutrophils on microscopic examination of peripheral blood smears.
Materials and methods
Cats that had A phagocytophilum DNA amplified from a blood sample assayed at a commercial diagnostic laboratory (Antech Diagnostics, Lake Success, NY, USA) between May 2009 and May 2011 were identified by an electronic record search. The laboratory uses EDTA-anticoagulated blood samples to attempt to amplify DNA of A phagocytophilum, Bartonella henselae, Bartonella clarridgeiae, Bartonella quintana, Ehrlichia species, Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’, ‘Candidatus Mycoplasma turicensis’, Rickettsia rickettsii and Rickettsia felis using a proprietary PCR assay (FastPanel) with the standard operating procedures of the laboratory. The main Genbank sequence identification used for the proprietary primer design for A phagocytophilum was gb CP006618.1. The sensitivity of the multiplex primer systems was analyzed using titered, synthesized DNA sequences in bacterial plasmids. These synthetic targets contained exact sequence matches to the primer sequences at the exact sequence distances found in the intended reference target sequences. An internal validation study was performed and assays were determined to be sensitive to 20 copies of their synthetic DNA targets.
The attending veterinarians were then contacted by telephone to obtain clinical information from the case records. Data abstracted from the medical record included history (duration of signs, outdoor activity, tick exposure, use of acaricides and geographic location), physical examination findings, clinicopathologic data, date of the PCR test, diagnostic imaging at the time of diagnosis, treatments administered and response to treatment. Clinicopathologic data came from multiple diagnostic laboratories. Numerical data were evaluated using manually calculated standard descriptive statistics (range, mean, frequency).
Results
Between May 2009 and May 2011, 4334 blood samples from cats were submitted for PCR testing at the commercial laboratory. A phagocytophilium DNA was amplified from the blood of 40 (0.92%) samples. Historical and clinical data were available for 16 cats.
Of the 16 cats, six were spayed females, nine were neutered males and one was an intact male. The median age of the cats was 2 years (mean 4.1 years; range 4 months to 13 years). All cats had access to the outdoors and resided in areas considered endemic for Ixodes species. Of the 16 cats, four had been prescribed fipronil (Frontline and Frontline Plus; Merial) and two had been prescribed selamectin (Revolution; Zoetis).
The cats resided in Connecticut (n = 7), New Jersey (n = 3), New York (n = 3), Massachusetts (n = 2) and Vermont (n = 1). The month of diagnosis was June (five cats), May and October (three cats each) and one cat each in April, July, August, September and November. Duration of clinical signs prior to presentation to a veterinarian was reported for five cats (median 2 days; mean 2.8 days; range 1–7 days). Four cats were presented more than once to a veterinarian prior to diagnosis of A phagocytophilum infection and initiation of specific treatment. Lethargy (16 cats) and elevated body temperature (15 cats) were the most common abnormalities reported the day the blood sample was collected for performance of the PCR assay (Table 1). The body temperatures for the 15 febrile cats ranged from 39.6–41.5ºC, with a mean of 40.3°C.
Table 1.
Clinical abnormalities reported for 16 cats the day blood was submitted and proven to contain Anaplasma phagocytophilum DNA
| Clinical abnormalities | Number of cats (%) |
|---|---|
| Lethargy | 16 (100) |
| Fever | 15 (94) |
| Anorexia | 14 (88) |
| Ocular signs (bilateral ocular discharge, elevated nictitating membranes, mild conjunctivitis) | 7 (44) |
| Abdominal discomfort | 4 (25) |
| Tachycardia | 3 (19) |
| Hepatosplenomegaly | 1 (6) |
| Ataxia | 1(6) |
Of the 16 cats, only one was concurrently positive for DNA of other agents (M haemominutum and B clarridgeiae). Results of a complete blood count and serum biochemical panel were available for 11 cats, performed on or immediately before the day the PCR assay was submitted. Abnormalities are listed in Table 2. These 11 cases had blood smears reviewed by medical technicians and some were additionally reviewed by a board-certified veterinary clinical pathologist (PE). In three cases, cytoplasmic inclusions were identified in 5–20% of neutrophils. The inclusions were basophilic and intracytoplasmic with a coarsely granular texture ranging in size from 1–2 µm (Figure 1), consistent with morulae of Ehrlichia species or Anaplasma species. As the inclusions were not seen in other leukocytes, it was concluded they were most likely those of A phagocytophilum, which was confirmed by PCR assay. The three cats with morula identified were all hyperthermic. One had a body temperature above the mean (41°C), while the other two had lower body temperatures at 39.8°C and 40.2°C at the time of examination. Urinalyses were performed in four cases and proteinuria was identified by dipstick in two cases; quantitative results are not available.
Table 2.
Clinicopathologic abnormalities in cats infected with Anaplasma phagocytophilum
| Parameter | n | Median* | Value or range | Reference interval |
|---|---|---|---|---|
| Total WBC (×109/l) | 1 | NA | 2.3 | 3.5–16.0 |
| RBC (×1012/l) | 2 | NA | 5.88–10.23 | 5.92–9.93 |
| Hematocrit (l/l) | 2 | NA | 0.186–0.245 (18.6–24.5%) | 0.29–0.48 (29–48) |
| Platelets (×109/l) † | 7 | 120 | 68–187 | 200–500 |
| Neutrophils, PMNs (×109/l) | 2 | NA | 1600–2430 | 2500–8500 |
| Lymphocytes (×109/l) | 6 | 498 | 86–936 | 1200–8000 |
| Glucose (mmol/l) | 2 | NA | 10.3–15.6 (186–281 mg/dl) | 3.5–9.4 (64–170) |
| Sodium (mEq/l) | 1 | NA | 144 | 145–158 |
| Cholesterol (mmol/l) | 1 | NA | 10.09 (390 mg/dl) | 1.94–5.69 (75–220) |
| Blood urea nitrogen (mmol/l) | 1 | NA | 9.28 (13 mg/dl) | 10.0–25.7 (14–36) |
| Albumin (g/l) | 1 | NA | 40 (4.0 g/dl) | 25–39 (2.5–3.9) |
A median was not able to be generated when fewer than three animals had the abnormality
Platelet clumping precluded an accurate determination of a platelet count in all seven cats. Five of the seven cats were estimated to have adequate platelets
WBC = white blood cells; RBC = red blood cells; PMN = polymorphonuclear neutrophil; NA = not available
Figure 1.
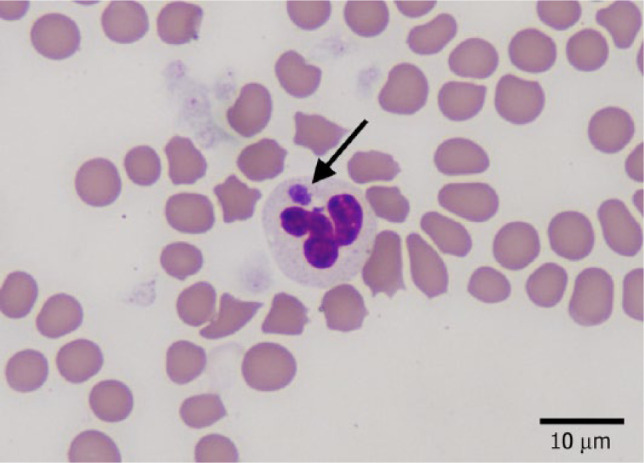
Peripheral blood smear of a cat infected with Anaplasma phagocytophilum, stained with Wright-Giemsa stain. Black arrow shows intracellular morula in the cytoplasm of a neutrophil
Treatment information was available for 15 cats. An antibiotic other than doxycycline (enrofloxacin, clindamycin, amoxicillin with clavulanic acid or ampicillin) was administered to eight cats prior to amplification of A phagocytophilum DNA from blood; for two cats more than one antibiotic was used. Once PCR assay results confirming the presence of A phagocytophilum DNA were available, all 15 cats were administered doxycycline orally. Dosing information was available in four cats (5 mg/kg PO, once or twice daily). Clinical abnormalities resolved after initiating doxycycline therapy in the 14 cats with a known response to treatment. The duration of doxycycline administration varied from 21–45 days, with most cats treated for 21 days. A phagocytophilum serologic test results are unknown and results of repeated laboratory testing and PCR assay are not available. Supportive care was prescribed to seven cats, including subcutaneous or intravenous crystalloid fluids (seven cats), histamine-2 receptor blocker (famotidine; two cats), appetite stimulant (mirtazipine; one cat) and/or analgesics (buprenorphine and oxymorphone; two cats).
Discussion
Cats in endemic areas are commonly exposed to A phagocytophilum based on antibody prevalence rates. 22 For example, in Connecticut, antibodies against A phagocytophilum were detected in 38% of 93 feline sera tested by ELISA. 22 A survey of different areas of the USA showed a prevalence ranging from 0–13.0%, with an overall prevalence of 4.3%. 23 Lastly, antibody prevalence rates in healthy cats and cats with clinical signs (fever, lethargy, inappetence) living in Maine were 3.6% and 15.4%, respectively. 24 The study described here is the largest A phagocytophilum molecular study from feline blood samples reported to date. In contrast to the above-reported prevalence rates,22–24 the prevalence rate of 0.92% in this study (40/4339 cats) is lower. The prevalence reported here likely underestimates the true prevalence rate in specific regions and populations of cats. The samples were submitted for a commercial PCR assay and included samples from all regions of the USA, not exclusively Ixodes species endemic areas. Some veterinarians may have submitted the PCR assay for reasons not specific to A phagocytophilum. The test may have been performed to screen healthy cats as blood donors or to test cats with clinical syndromes not likely to be induced by A phagocytophilum. All 16 of the cats identified in this study were positive for A phagocytophilum DNA in blood, were from an endemic area, had potential exposure to Ixodes species, and the majority had clinical and laboratory evidence of anaplasmosis, as well as apparent responses to doxycycline. Only one cat had molecular evidence of other infectious agents that may have responded to tetracyclines. Thus, we believe that anaplasmosis was the likely diagnosis for these cats. However, the major limitations to the study include the variability in the diagnostic workup, the lack of clinicopathologic data from all cats and differences in clinical management amongst cases. As the study was retrospective, information regarding A phagocytophilum titers or resolution of laboratory abnormalities after treatment could not be determined.
To date, cats with suspected anaplasmosis in the USA have only been described in the Northeast. In contrast, granulocytic anaplasmosis in dogs and humans have been reported in the Midwest, West and Northeast.4,25,26 Causes of this inconsistency in geographic distribution between cats and dogs/humans may include insufficient data, differences in the genetic strains that infect these species and the range of the organism that affects different species. 27 The seasonal distribution of feline infections mimics that seen in other species, with the greatest incidence in the late spring and early autumn. This coincides with the greatest nymphal and adult tick activity.4,28,29
The primary clinical abnormalities of lethargy, elevated body temperature and anorexia are consistent with those found commonly in other infected cats, dogs and humans.8,11,15 Whether the other reported abnormalities presented in Table 1 are related to A phagocytophilum infection is unknown and could not be ascertained in this retrospective study. The ocular changes reported were non-specific and likely the result of systemic inflammation. 30 Abdominal discomfort was reported in 4/16 cats but the cause was not determined. Radiographic hepatosplenomegaly was found in one cat in this study but as hepatic and splenic aspirates were not performed the cause is not known.
With the exception of identifying intracytoplasmic morula within neutrophils, the clinicopathologic abnormalities are non-specific for infection with A phagocytophilum. In this study, anemia was identified in two cats. The cat with the lowest hematocrit (0.186 l/l, 18.6%) was a 4-month-old kitten. As kittens normally have a lower hematocrit than adult cats, the anemia in this case was not as severe as would be expected if present in an adult cat. Anemia has only been described in one other report of cats infected with A phagocytophilum. 21 Lymphopenia has been previously identified,11,18 and in this report was seen in six (55%) cats. Although thrombocytopenia was reported in 7/11 cases, all seven samples had clumped platelets, which falsely decreases the automated platelet counts. 31 The absence of confirmed thrombocytopenia in this study is inconsistent with previous reports where 3/5 cats were reportedly thrombocytopenic; however, one of the three samples had clumped platelets. 20 A more recent report of A phagocytophilum infection in three cats in Poland described thrombocytopenia in all three, though no evaluation of platelet clumping was performed. 21 Though thrombocytopenia is a commonly reported finding of A phagocytophilum infection in dogs,14,16 it may not be as common in cats. Automated platelet counts can falsely lower platelet counts owing to the tendency of feline platelets to aggregate in vitro; 31 therefore, more studies are needed to determine if a true thrombocytopenia exists and how frequently this abnormality occurs. Mild hyperglycemia was identified in two cats and attributed to a normal physiologic stress response. Other mild and non-specific serum biochemical changes were only seen in one cat each, the consequences of which cannot be elucidated based on these few abnormalities.
Morulae were identified in neutrophils in 3/11 (27%) cases in this report, with inclusions in approximately 4–20% of neutrophils. Identification of these inclusions prompted submission of blood for the commercial PCR test, confirming the diagnosis of A phagocytophilum infection. Morulae appear as basophilic intracytoplasmic inclusions that must be carefully differentiated from Döhle bodies. Morulae have been described repeatedly in dogs, 32 horses, 7 sheep 33 and other ruminants, 34 humans, 28 European cats11,17–19 and specific pathogen-free cats infested with wild-caught I scapularis. 9 But, to our knowledge, this is the first report of intracellular morulae being seen in the neutrophils in naturally infected North American cats. The identification of morulae demonstrates the importance of manual examination of a blood smear from any patient where granulocytic anaplasmosis is considered a differential diagnosis.
Co-exposure with Bartonella burgdorferi and A phagocytophilum occurs in dogs, horses, cats, humans and wildlife as both agents have the same vector and can share mammalian hosts. 35 Different prevalences of co-exposure in cats have been reported in different geographical areas. In one study in Connecticut, 16% of cats had evidence of co-exposure to B burgdorferi and A phagocytophilum, 22 whereas in a study in Maine, 5.1% of ill cats and 2.4% of healthy cats had exposure to both infectious agents. 24 The clinical impact of co-exposure or coinfection with B burgdorferi and A phagocytophilum is unknown in cats. In this study, as specific testing was not performed, we could not determine whether B burgdorferi infection contributed to the clinical disease in the cats described.
As previously discussed, only one cat in this study had proven coinfections (B clarridgeiae and ‘Candidatus M haemominutum’). This cat was not anemic, and as hemoplasmas do not always cause clinical illness, 36 the effects of these coinfections is unknown.
As a retrospective study, there was variability in the treatment of each case. Fifteen of the 16 cats were treated with doxycycline once an infection with A phagocytophilum was identified. Where a dose was recorded, cats received 5 mg/kg q12h. The duration of treatment with doxycycline was variable (21–45 days) and clinician dependent. As a retrospective study it is a limitation that an explanation for the duration of treatment is unknown. The majority of cats were treated for 21 days; those that were treated for 45 days were all seen at the same practice. Previous reports in cats have recommended treatment with 5 mg/kg doxycycline PO q12h for 28 days. 20 The ideal duration of treatment with doxycycline in cats is unknown and warrants additional study. One cat received enrofloxacin prior to testing. The cat initially improved with treatment, but had recurrence of clinical signs and tested positive, demonstrating the importance of identification of this infection and appropriate treatment. All other non-tetracycline antibiotics were administered after sample collection while awaiting test results. Owing to the retrospective nature of this study it is unknown if cats responded to non-tetracycline treatment prior to the availability of results. When response to treatment with doxycycline is recorded, all responded without recurrence of clinical signs.
Information regarding persistent or recurrent infections in cats is not available from this study. Persistent infections have been described in dogs, 37 horses 38 and sheep 39 where experimentally induced clinical disease was acute and self-resolving with persistent yet cyclical non-clinical bacteremia. Studies are needed to determine if feline anaplasmosis is a self-resolving disease and to determine if clinical persistent infections occur.
In this report all the cats were in Ixodes species endemic areas with outdoor access. Routine use of tick preventatives in dogs can reduce the incidence of A phagocytophilum transmission. 40 Six cases in this report had a history of parasiticide application; however, the last date of product application is unknown. Based on this information, cats living in an Ixodes species area with exposure to Ixodes species might benefit from monthly treatment with an effective and safe parasiticide.
It is unknown if recurrent infections occur in cats and the most appropriate duration of doxycycline treatment in cats for treating anaplasmosis is still to be elucidated. Other reports have shown feline exposure to A phagocytophilum without known clinical disease;22,24 additional studies are needed to identify how common subclinical infections occur. To date, all cats in the USA with clinical disease have been reported in the Northeast, despite the fact that A phagocytophilum is also present in the Midwest and West. Studies evaluating feline infection of different strains or geographical variants of A phagocytophilum are needed to determine if one strain is more successful at infecting cats than others and which strains cause clinical disease.
Conclusions
A phagocytophilum infection should be included on a differential diagnoses list for any cat that lives in an Ixodes species endemic area with potential tick exposure and presents with acute or intermittent, vague clinical signs of lethargy, fever and anorexia. A phagocytophilum infection can be identified by documenting DNA in peripheral blood by PCR prior to antibiotic administration or by identifying morulae within neutrophils on a direct blood smear examination. Exposure can be documented by demonstrating the presence of antibodies with ELISA and indirect fluorescent antibody serology; a four-fold change in convalescent titers after 14 days indicates an active infection. 8 Treatment without signs of recurrence has been successful with tetracyclines.
Acknowledgments
We would like to thank Sheri Ihle and Pamela Mouser for their editorial support with this paper. All work was performed at Angell Animal Medical Center, Boston MA 02130, USA.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
This research was presented, in part, as an abstract at the Annual Forum of the American College of Veterinary Internal Medicine in Denver, Colorado, June 2011
Accepted: 12 January 2015
References
- 1. Gordon WS, Brownlee A, Wilson DR, et al. Tick-borne fever (a hitherto undescribed disease of sheep). J Comp Pathol Therap 1932; 45: 301–312. [Google Scholar]
- 2. Nicholson WL, Allen KE, McQuiston JH, et al. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010; 26: 205–212. [DOI] [PubMed] [Google Scholar]
- 3. Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol 2010; 167: 108–122. [DOI] [PubMed] [Google Scholar]
- 4. Greig B, Asanovich KM, Armstrong PJ, et al. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol 1996; 34: 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodzic E, Fish D, Maretzki C, et al. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol 1998; 36: 3574–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katavolos P, Armstrong P, Dawson J, et al. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis 1998; 177: 1422–1425. [DOI] [PubMed] [Google Scholar]
- 7. Madigan JE, Gribble D. Equine ehrlichiosis in northern California: 49 cases (1968–1981). J Am Vet Med Assoc 1987; 190: 445–448. [PubMed] [Google Scholar]
- 8. Bakken JS, Dumler S. Human granulocytic ehrlichiosis. Clin Infect Dis 2000; 31: 554–560. [DOI] [PubMed] [Google Scholar]
- 9. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J Vet Diagn Invest 2015; 27: 522–525. [DOI] [PubMed] [Google Scholar]
- 10. Woldehiwet Z. Anaplasma phagocytophilum in ruminants in Europe. Ann NY Acad Sci 2006; 1078: 446–460. [DOI] [PubMed] [Google Scholar]
- 11. Bjöersdorff A, Svendenius L, Owens JH, et al. Feline granulocytic ehrlichiosis – a report of a new clinical entity and characterisation of the infectious agent. J Small Anim Pract 1999; 40: 20–24. [DOI] [PubMed] [Google Scholar]
- 12. Dzięgiel B, Adaszek L, Winiarczyk M, et al. Comparative analysis of 16S RNA nucleotide sequences of Anaplasma phagocytophilum detected in the blood of horses from various parts of Europe. J Med Microbiol 2013; 62: 1891–1896. [DOI] [PubMed] [Google Scholar]
- 13. Domingos MC, Trotta M, Briend-Marchal A, et al. Anaplasmosis in two dogs in France and molecular and phylogenetic characterization of Anaplasma phagocytophilum. Vet Clin Pathol 2011; 40: 215–221. [DOI] [PubMed] [Google Scholar]
- 14. Kohn B, Galke D, Beelitz P, et al. Clinical features of canine anaplasmosis in 18 naturally infected dogs. J Vet Intern Med 2008; 22: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 15. Carrade DD, Foley JE, Borjesson DL, et al. Canine granulocytic anaplasmosis: a review. J Vet Intern Med 2009; 23: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 16. Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007). J Am Vet Med Assoc 2009; 234: 1559–1565. [DOI] [PubMed] [Google Scholar]
- 17. Schaarschmidt-Kiener D, Graf F, von Loewenich FD, et al. Anaplasma phagocytophilum infection in a cat in Switzerland. Schweiz Arch Tierheilkd 2009; 151: 336–341. [DOI] [PubMed] [Google Scholar]
- 18. Heikkilä HM, Bondarenko A, Mihalkov A, et al. Anaplasma phagocytophilum infection in a domestic cat in Finland: case report. Acta Veterinaria Scandinavica 2010; 52: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarello W. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Vet Rec 2005; 156: 772–774. [DOI] [PubMed] [Google Scholar]
- 20. Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc 2004; 225: 893–896. [DOI] [PubMed] [Google Scholar]
- 21. Adaszek L, Górna M, Skrzypczak M, et al. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. J Feline Med Surg 2013; 15: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magnarelli LA, Bushmich SL, IJdo JW, et al. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res 2005; 66: 1895–1899. [DOI] [PubMed] [Google Scholar]
- 23. Billeter SA, Spencer JA, Griffin B, et al. Prevalence of Anaplasma phagocytophilum in domestic felines in the United States. Vet Parasitol 2007; 147: 194–198. [DOI] [PubMed] [Google Scholar]
- 24. Hoyt K, Chandrashekar R, Breitschwerdt E, et al. Anaplasma phagocytophilum and Borrelia burgdorferi antibodies in naturally exposed cats in Maine. Proceedings of the American College of Veterinary Internal Medicine Forum; 2014 Jun 3–6; Nashville, TN, USA. [Google Scholar]
- 25. Foley JE, Foley P, Madigan JE. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am J Vet Res 2001; 62: 1599–1605. [DOI] [PubMed] [Google Scholar]
- 26. Magnarelli LA, Ijdo JW, Anderson JF, et al. Antibodies to Ehrlichia equi in dogs from the northeastern United States. J Am Vet Med Assoc 1997; 211: 1134–1137. [PubMed] [Google Scholar]
- 27. Teglas MB, Foley J. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari:Ixodidae). Exp Appl Acarol 2006; 38: 47–58. [DOI] [PubMed] [Google Scholar]
- 28. Bakken JS, Dumler JS, Chen S, et al. Human granulocytic ehrlichiosis in the upper Midwest United States a new species emerging? J Am Med Assoc 1994; 272: 212–218. [PubMed] [Google Scholar]
- 29. Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg 2011; 85: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiles J, Townsend WM. Feline ophthalmology. In: Gelatt KN. (ed). Veterinary ophthalmology. 4th ed. Ames, IA: Blackwell Publishing, 2007, pp 1101–1102. [Google Scholar]
- 31. Zelmanovic D, Hetherington EJ. Automated analysis of feline platelets in whole blood, including platelet count, mean platelet volume, and activation state. Vet Clin Path 1998; 27: 2–9. [DOI] [PubMed] [Google Scholar]
- 32. Madewell BR, Gribble DH. Infection in two dogs with an agent resembling Ehrlichia equi. J Am Vet Med Assoc 1982; 180: 512–514. [PubMed] [Google Scholar]
- 33. Woldehiwet Z, Scott GR. Stages in the development of Cytoecetes phagocytophilia, the causative agent of tick-borne fever in sheep. J Comp Pathol 1982; 92: 469–474. [DOI] [PubMed] [Google Scholar]
- 34. Nieder M, Silaghi C, Hamel D, et al. Tick-borne fever caused by Anaplasma phagocytophilum in Germany. First laboratory confirmed case in a dairy cattle herd. Tierarztl Prax Ausg G Grosstiere Nutztiere 2012; 40: 101–106. [PubMed] [Google Scholar]
- 35. Nieto NC, Foley JE. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife and Ixodes ricinus-complex ticks. Vector Borne Zoonotic Dis 2009; 9: 93–101. [DOI] [PubMed] [Google Scholar]
- 36. Tasker S, Peters IR, Papasouliotis K. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 2009; 139: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egenvall A, Lilliehöök I, Bjöersdorff A, et al. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet Rec 2000; 146: 186–190. [DOI] [PubMed] [Google Scholar]
- 38. Franzén P, Aspan A, Egenvall A, et al. Molecular evidence for persistence of Anaplasma phagocytophilum in the absence of clinical abnormalities in horses after recovery from acute experimental infection. J Vet Intern Med 2009; 23: 636–642. [DOI] [PubMed] [Google Scholar]
- 39. Thomas RJ, Birtles RJ, Radford AD, et al. Recurrent bacteraemia in sheep infected persistently with Anaplasma phagocytophilum. J Comp Pathol 2012; 147: 360–367. [DOI] [PubMed] [Google Scholar]
- 40. Blagburn B, Spencer J, Billeter S, et al. Use of imidacloprid-permethrin to prevent transmission of Anaplasma phagocytophilum from naturally infected Ixodes scapularis ticks to dogs. Vet Ther 2004; 5: 212–217. [PubMed] [Google Scholar]


