Abstract
Objectives
Hyperthyroidism is common in cats, but there are no reports that evaluate its severity or underlying thyroid tumor disease based on disease duration (ie, time from original diagnosis). The objective of this study was to compare serum thyroxine (T4) concentrations and thyroid scintigraphic characteristics of cats referred for radioiodine treatment based on disease duration.
Methods
This was a cross-sectional study of 2096 cats with hyperthyroidism. Cats were divided into five groups based on time from diagnosis: ⩽1 year (n = 1773); >1–2 years (n = 169); >2–3 years (n = 88); >3–4 years (n = 35); and >4–6.1 years (n = 31). Methimazole, administered to 996 (47.5%) cats, was stopped at least 1 week prior to examination to allow for serum T4 testing. Each thyroid scintiscan was evaluated for pattern (unilateral, bilateral, multifocal), location (cervical, thoracic inlet, chest) and size (small, medium, large, huge) of the thyroid tumor, as well as features suggesting malignancy.
Results
Median serum T4 concentration increased with increasing disease duration from 100 nmol/l (⩽1 year) to 315 nmol/l (>4–6.1 years) (P <0.001). Prevalence of unilateral thyroid disease decreased, whereas multifocal disease (three or more tumor nodules) increased (P <0.001) with increasing disease duration. Median tumor volume in the five groups increased from 1.6 cm3 (⩽1 year) to 6.4 cm3 (>4–6.1 years). Prevalence of large (4–8 cm3) and huge (>8 cm3) thyroid tumors increased from 5.1% (⩽1 year) to 88.6% (>4–6.1 years), while the prevalence of intrathoracic tumor tissue increased from 3.4% (⩽1 year) to 32.3% (>4–6.1 years). Prevalence of suspected thyroid carcinoma (characterized by severe hyperthyroidism; huge, intrathoracic, multifocal tumors; refractory to methimazole treatment) increased with increasing disease duration from 0.4% (⩽1 year) to 19.3% (>4–6.1 years).
Conclusions and relevance
Our results indicate that the prevalence of severe hyperthyroidism, large thyroid tumors, multifocal disease, intrathoracic thyroid masses and suspected malignant disease all increase with disease duration in cats referred for radioiodine therapy.
Introduction
Hyperthyroidism is the most common endocrine disease in the cat, affecting approximately 10% of senior and geriatric cats.1,2 Most hyperthyroid cats have benign thyroid adenomatous hyperplasia (or adenoma) involving one or both thyroid lobes; thyroid carcinoma occurs in <2% of cases.1–5
Although the exact cause(s) for feline hyperthyroidism remains unclear, major characteristics of the underlying adenomatous thyroid disease are autonomous growth and hyperfunction independent of stimulation by thyroid-stimulating hormone (TSH).4,6,7 Adenomatous thyroid tissue transplanted from hyperthyroid cats into nude mice or placed into culture in a TSH-free media will continue to grow and secrete thyroid hormone outside of the cat.4,8,9 Because such autonomous growth is a primary feature of neoplasia, the thyroid pathology associated with feline hyperthyroidism is most consistent with a true endocrine tumor (ie, thyroid adenoma or carcinoma rather than hyperplasia).10,11
As with any benign or malignant neoplasm, growth of thyroid tumors might be expected over time, but no reports of thyroid pathology or thyroid size in a series of cats with long-term hyperthyroidism have been reported. However, it has been our clinical observation that cats treated with methimazole over the long-term appear to have more severe hyperthyroid disease, larger tumor nodules and a higher prevalence of suspected thyroid carcinoma than do cats with recently diagnosed hyperthyroidism. 12
In a recent study, we characterized the thyroid scintigraphic findings of 2096 consecutive hyperthyroid cats that were referred for radioiodine treatment over a 3.5 year period. 5 In that study, most hyperthyroid cats had bilateral disease (60%) or unilateral (35%) thyroid lobe involvement, but a few (5%) cats did have multifocal disease (defined as three or more distinct thyroid tumor nodules with increased radionuclide uptake). Inasmuch as the thyroid gland is normally composed of only two lobes, multifocal disease by definition implies that these cats have either ectopic thyroid neoplasia or thyroid carcinoma to account for the additional discrete nodular areas of increased radionuclide uptake.5,13,14 However, in that study we did not evaluate whether more severe pathology occurs as a function of disease duration.
Therefore, in this analysis, we sought to characterize the severity and extent of thyroid disease in this same large cohort of hyperthyroid cats, based on the time interval from diagnosis to evaluation for radioiodine treatment. We compared the proportions of cats with unilateral, bilateral and multifocal thyroid disease; the proportions of cats with small, medium, large and huge thyroid tumors; the proportions of cats with intrathoracic disease; and, finally, the proportions of cats with suspected thyroid carcinoma. We hypothesized that cats with long-standing hyperthyroidism will have more advanced thyroid disease, as reflected by higher serum thyroid hormone concentrations, larger thyroid tumors, and a higher prevalence of multifocal disease, intrathoracic disease, and suspected thyroid carcinoma.
Materials and methods
Cats
This prospective, cross-sectional study was conducted from January 2009 to June 2012. All cats referred to our clinics for radioiodine treatment were potentially eligible for the study. Inclusion criteria included cats with a confirmed diagnosis of hyperthyroidism, established on the basis of consistent clinical signs (eg, weight loss despite a good appetite), a palpable thyroid nodule on physical examination, finding of high serum concentrations of thyroxine (T4) and/or free T4, and determination of a high thyroid-to-salivary gland (T/S) ratio calculated from the results of thyroid scintigraphy.5,15,16
Details of the cats’ specific thyroid scintigraphic findings have previously been described. 5 For the purposes of this study, we divided the cats into five groups based on disease duration, defined as the time interval between diagnosis and scintigraphy. Group 1 consisted of cats that been diagnosed within 1 year, whereas groups 2–5 consisted of cats diagnosed >1–2 years, >2–3 years, >3–4 years and >4–6.1 years, respectively. Of these, 673 (38%) cats in group 1 and all cats in groups 2–5 were treated with methimazole prior to referral, but methimazole was discontinued at least 1 week prior to our examination to allow for evaluation of thyroid status with serum thyroid hormone testing and scintigraphy.
Protocol for thyroid scintigraphy
Thyroid scintigraphy was performed in all cats by injecting 111–148 megabecquerel (MBq) (3–4 millicurie) of sodium pertechnetate (99mTcO4−) injected intravenously or subcutaneously, as previously described. 5 Thyroid images were obtained with a large-field-of-view scintillation camera fitted with a low-energy, all-purpose collimator, integrated to a dedicated imaging computer running dedicated nuclear medicine software (NucLear Mac; Scientific Imaging, Inc) and analyzed using DICOM image-processing software (OsiriX Imaging Software; Pixmeo 17 ).
Classification of the scintigraphic patterns of hyperthyroid disease
Abnormal scintigraphic findings indicative of thyroid gland disease were classified as unilateral disease, bilateral disease or multifocal disease (three or more separate tumor nodules or areas of distinct increased radionuclide uptake), as previously described.5,14 When extra-cervical thyroid disease was detected, this classification was extended to include ectopic thyroid tumor or suspected thyroid carcinoma. Cats with multifocal disease had, by definition, either ectopic thyroid tumors or suspected thyroid carcinoma. 5
Location of the hyperfunctional thyroid tumor nodules
In each cat, the number and location of each area of increased 99mTcO4− uptake (ie, hyperfunctional or ‘hot’ thyroid tumor nodules) were recorded. To facilitate defining the location of each area of increased radionuclide uptake, a straight line was drawn between the points of the cats’ shoulders in the region of the thoracic inlet on the ventral thyroid image, as previously described. 18 In most cats, this line was halfway between the middle of the caudal pole of the zygomatic/molar salivary glands and the center of the heart. Areas of uptake above this line were classified as cervical, those touching this line were classified as thoracic inlet and those below this line were classified as intrathoracic (mediastinal); uptake extending from the cervical to intrathoracic location was classified as increased radionuclide uptake in all three locations.
Thyroid tumor size and calculated thyroid tumor volume
After collection of the thyroid scintigraphic image, each cat’s hyperfunctional thyroid tumor nodule(s) was(were) measured using a visually dependent thresholding method. First, the ventral thyroid image was windowed to the ‘full dynamic range’ setting in the DICOM image-processing software, defined as a window width (WW) at its maximum or widest setting (0–100% full range of pixel values), with a window level at 50% of WW.17,19 The full dynamic range setting was used to normalize the image display, compensating for variations in thyroid uptake between cats and reducing the effects of ‘radiation scatter’ (ie, blooming artefact), 20 which may be observed in very intense (hot) thyroid tumor nodules.19,21 Such blooming effects can result in areas of radionuclide uptake that appear much larger than they actually are, resulting in an overestimation of the anatomical volume of thyroid tumor.22–24
Second, to identify and measure the length and width of the thyroid tumor nodule(s) consistently, we used a customized color look-up table, with a cutoff between black and white set at a threshold of 20% of the full dynamic range of the image. 25 Finally, image interpolation during zooming was disabled to prevent blurring of the edges of the tumor. The validity of these measurements was established using 99mTc-labeled phantoms with a known volume and known dimensions (mimicking an enlarged thyroid tumor). All thyroid volume measurements were made by a single observer (MEP) to reduce inter-operator variability and ensure reproducibility across a large number of cases.
Thyroid volume was estimated using the equation for a prolate spheroid, V = 4/3 πa2c. 26 Total thyroid volume was estimated by summing the volume estimations of all identified tumor nodules. Based on the total calculated thyroid tumor volume, tumor size was then classified as small (<2.0 cm3), median (2–4 cm3), large (4.1–8.0 cm3) or huge (>8 cm3).
Statistical analysis
Data were assessed for normality by the D’Agostino-Pearson test and by visual inspection of graphical plots. 27 The assumption of Gaussian distribution was not met and therefore non-parametric testing was used.28,29 All results are reported as the median and interquartile range (IQR), and are represented graphically as box plots or bar graphs. 30
All statistical analyses were performed using proprietary statistical software (GraphPad Prism, version 6.0; GraphPad Software). The binomial and sign test was used to compare the proportion of male to female cats, with an expected equal proportion of 0.5. 31 The Kruskall–Wallis test was used to compare mean ranks of serum T4 concentrations, T/S ratios or thyroid tumor volumes between the five groups of cats. This test was also used to compare mean ranks of serum T4 concentrations or T/S ratios of cats with small, medium and large/huge thyroid tumors. Correlations between disease duration and serum T4 concentrations, T/S ratios or thyroid tumor volume were determined by the Spearman rank correlation coefficient. The χ2 test was used to compare proportions of cats in the five groups with different patterns of thyroid disease (ie, unilateral, bilateral or multifocal), locations of the tumor nodules, thyroid tumor size and prevalence of thyroid carcinoma. For all statistical analyses, values of P <0.05 were considered significant.
Results
Groups of hyperthyroid cats based on time from diagnosis
We identified 2096 client-owned adult cats that met the inclusion criteria. The cats ranged in age from 2–23 years (median 13 years; IQR 11–15 years); 1138 (54%) of the cats were spayed females and 958 (46%) were neutered males (P <0.001). Of the 2096 cats, 1883 (89.8%) were of mixed breeding (domestic shorthair, mediumhair, and longhair); 20 other breeds were represented. 5
Group 1 consisted of 1773 cats, group 2 consisted of 169, group 3 consisted of 88, group 4 consisted of 35 and group 5 consisted of 31 cats. Of these, 673 cats in group 1 and all cats in groups 2–5 had been treated with methimazole (Table 1).
Table 1.
Clinical and scintigraphic characteristics of 2096 hyperthyroid cats, divided into five groups based on disease duration
| Time from diagnosis | All cats | Group 1 (⩽1 year) | Group 2 (>1–2 years) | Group 3 (>2–3 years) | Group 4 (>3–4 years) | Group 5 (>4–6.1 years) |
|---|---|---|---|---|---|---|
| Number of cats (%) | 2096 (100.0) | 1773 (84.5) | 169 (8.1) | 88 (4.2) | 35 (1.7) | 31 (1.5) |
| Methimazole (%) | 996 (47.5) | 673 (38.0) | 169 (100.0) | 88 (100.0) | 35 (100.0) | 31 (100.0) |
| Tumor pattern (%) | ||||||
| Unilateral | 665 (31.7) | 599 (33.8) | 39 (23.1) | 18 (20.5) | 6 (17.1) | 3 (9.7) |
| Bilateral | 1317 (62.8) | 1107 (62.4) | 113 (66.9) | 59 (67.0) | 20 (57.2) | 18 (58.0) |
| Multifocal | 81 (3.9) | 48 (2.7) | 13 (7.7) | 8 (9.1) | 5 (14.3) | 7 (22.6) |
| Unclassified | 33 (1.6) | 19 (1.1) | 4 (2.4) | 3 (3.4) | 4 (11.4) | 3 (9.7) |
| Tumor location (%) | ||||||
| Cervical | 2057 (98.1) | 1744 (98.4) | 166 (98.2) | 85 (96.6) | 33 (94.3) | 29 (93.4) |
| Thoracic inlet | 282 (13.5) | 162 (9.1) | 48 (28.4) | 33 (37.5) | 22 (62.9) | 17 (54.8) |
| Thorax | 115 (5.5) | 63 (3.6) | 17 (10.1) | 15 (17.0) | 10 (28.6) | 10 (32.2) |
| Tumor size (%) | ||||||
| Small | 1135 (54.2) | 1101 (62.1) | 26 (15.4) | 7 (8.0) | 1 (2.9) | 0 (0) |
| Medium | 682 (32.5) | 581 (32.8) | 69 (40.8) | 25 (28.4) | 3 (8.6) | 4 (12.9) |
| Large | 230 (11.0) | 82 (4.6) | 64 (37.9) | 47 (53.4) | 21 (60.0) | 16 (51.6) |
| Huge | 49 (2.3) | 9 (0.5) | 10 (5.9) | 9 (10.2) | 10 (28.6) | 11 (35.5) |
| Ectopic (%) | ||||||
| All ectopic locations | 79 (3.8) | 58 (3.3) | 11 (6.5) | 5 (5.7) | 3 (8.6) | 2 (6.4) |
| Sublingual | 8 (0.4) | 7 (0.4) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Cervical | 6 (0.3) | 5 (0.3) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Mediastinal | 64 (3.1) | 46 (2.6) | 8 (4.7) | 5 (5.7) | 3 (8.6) | 2 (6.4) |
| Both sublingual and mediastinal | 1 (0.05) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Suspected carcinoma (%) | ||||||
| All suspected cases | 35 (1.7) | 9 (0.5) | 6 (3.6) | 6 (6.8) | 6 (17.1) | 8 (25.8) |
| SHIM-RAD subgroup* | 30 (1.4) | 7 (0.4) | 6 (3.6) | 6 (6.8) | 5 (14.3) | 6 (19.3) |
SHIM-RAD = subgroup of cats with thyroid tumors that have the following five features: (1) severe hyperthyroidism (serum thyroxine >300 nmol/l); (2) huge tumors; (3) intrathoracic tumor location; (4) multifocal distribution of radionuclide uptake; and (5) refractory to treatment with antithyroid drugs
Serum T4 concentrations and T/S ratios
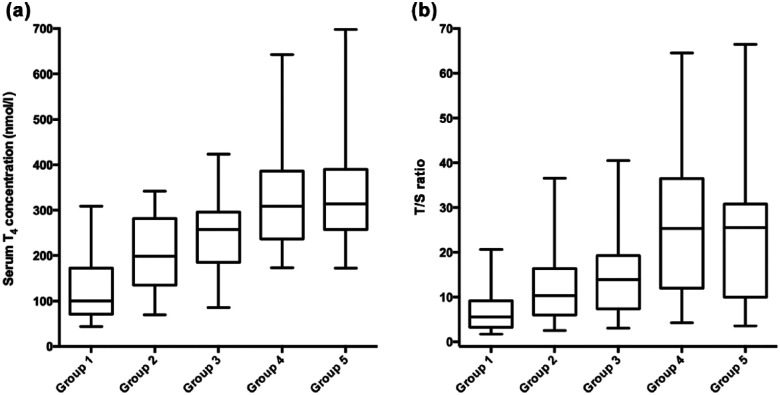
Serum T4 concentration increased with disease duration from 100 nmol/l (IQR 71–172 nmol/l) in group 1 up to 315 nmol/l (IQR 257–390 nmol/l) in group 5 (P <0.001) (Figure 1a). There was a significant positive correlation between increasing disease duration and the serum T4 concentration in these cats (r = 0.47; P <0.0001).
Figure 1.
Box plots of (a) serum thyroxine (T4) concentrations and (b) thyroid to salivary (T/S) ratios in 2096 cats with hyperthyroidism, divided into the following five groups based on the time interval from diagnosis of hyperthyroidism to thyroid scintigraphy: group 1 (n = 1773) ⩽1 year; group 2 (n = 169) >1–2 years; group 3 (n = 88) >2–3 years; group 4 (n = 35) >3–4 years; and group 5 (31 cats) >4–6.1 years. In each graph, the box represents the interquartile range (ie, 25th–75th percentile range or the middle half of the data). The horizontal bar in the box represents the median value. For each box plot, the T-bars represent the 2.5–97.5th percentile range
Similarly, the calculated T/S ratio increased from a median value of 5.5 (IQR 3.3–9.2; reference interval 0.5–1.5) in group 1 to 26 (IQR 10–30) in group 5 (P <0.001) (Figure 1b). 32 There was also a significant positive correlation between increasing disease duration and the magnitude of the T/S ratio in these cats (r = 0.39; P <0.0001).
Patterns of thyroid disease
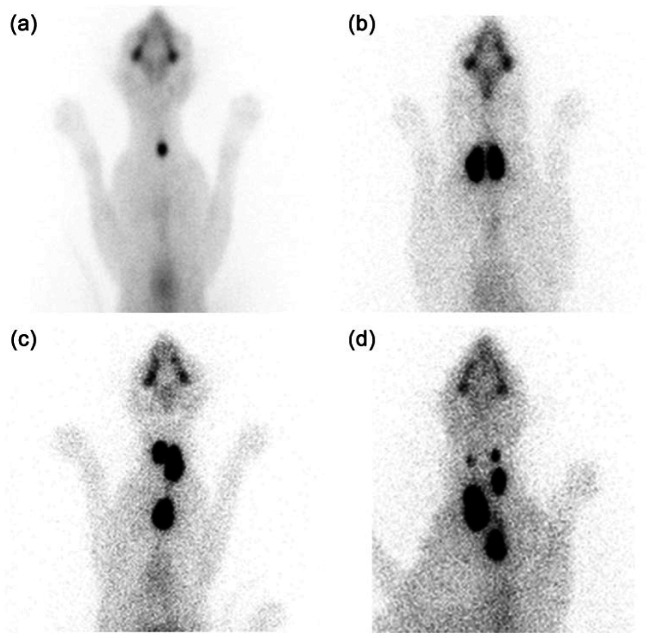
We identified unilateral disease in 665 cats, bilateral disease in 1317 cats and multifocal disease in 81 cats (Table 1; Figure 2a–c). In 33 cats, increased radionuclide uptake patterns could not be classified; of these, 28 had ectopic thyroid tumors and five had suspected thyroid carcinoma. Of the 81 cats with multifocal disease, 51 were considered to have ectopic thyroid tumors and 30 were suspected to have thyroid carcinoma (Table 1; Figure 2c,d).
Figure 2.
Thyroid scintigrams illustrating the major patterns of thyroid disease with one to six areas of sodium pertechnetate uptake in the 2096 cats. (a) One nodule (unilateral disease). (b) Two nodules (bilateral cervical disease). (C) Three nodules (bilateral disease with third ectopic midline nodule in thorax). (d) Six nodules (multifocal disease resulting from suspected thyroid carcinoma)
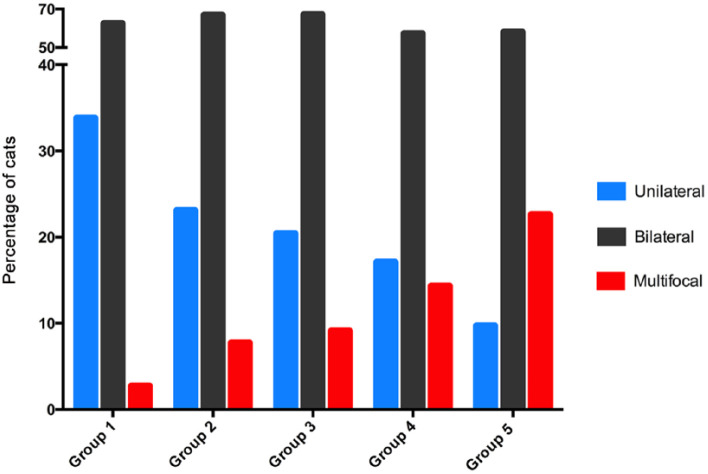
Prevalence of unilateral thyroid disease decreased with increasing disease duration from 33.8% of cats in group 1 to only 9.7% of cats in group 5 (P <0.0001) (Table 1; Figure 3). No significant difference in the prevalence of bilateral disease was detected between the five groups of cats, but the prevalence of multifocal disease increased with increasing disease duration from 2.7% of cats in group 1 up to 22.6% of cats in group 5 (P <0.0001) (Table 1; Figure 3). Similarly, prevalence of cats with unclassified disease increased with increasing disease duration from 1.1% of cats in group 1 to 9.7% of cats in group 5 (Table 1).
Figure 3.
Bar graphs depicting the percentage of hyperthyroid cats with unilateral, bilateral and multifocal (three or more nodules) thyroid disease, in each of five groups, based on the time from diagnosis: group 1, ⩽1 year; group 2, >1–2 years; group 3, >2–3 years; group 4, >3–4 years; and group 5, >4–6.1 years
Location of hyperfunctional thyroid tumor nodules
Cats had a median of two areas of increased radionuclide uptake (hyperfunctional thyroid tumor nodules) (range 1–6), with 81 cats having three or more (multifocal disease) (Table 1). These were located in the neck in 2057, in the thoracic inlet in 282 and in the thoracic cavity in 115 of the cats (Table 1); 59 (2.8%) cats had increased radionuclide uptake in all three locations (neck, thoracic inlet, thorax).
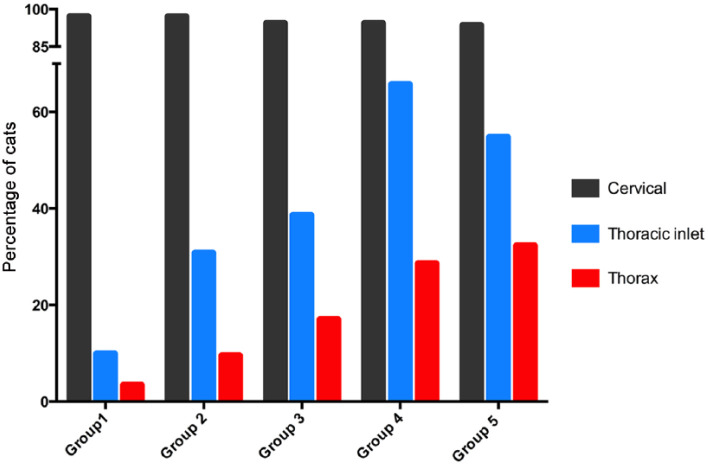
The prevalence of thyroid tumor in the region of the thoracic inlet and thorax increased with increasing disease duration (P <0.0001) (Table 1; Figure 4). Over 60% of the cats in group 4 had tumor nodules within the thoracic inlet, a rate that fell slightly in group 5 cats as the prevalence of intrathoracic tumors continued to increase (Table 1; Figure 4). Only 3.6% of the cats in group 1 had intrathoracic thyroid tumor nodules, a prevalence that increased to 32.2% of cats in group 5 (Table 1; Figure 4).
Figure 4.
Bar graphs depicting the percentage of cats with thyroid tumor nodules in the cervical, thoracic inlet and intrathoracic locations in each of the five groups, based on the time from diagnosis: group 1, ⩽1 year; group 2, >1–2 years; group 3, >2–3 years; group 4, >3–4 years; and group 5, >4–6.1 years
Thyroid tumor size and calculated thyroid tumor volume
Thyroid tumor volume increased with increasing disease duration from 1.6 cm3 (IQR 0.96–2.60 cm3) in group 1 cats up to 6.4 cm3 (IQR 4.5–11.0 cm3) in group 5 cats (P <0.001) (Figure 5). There was a significant correlation between increasing disease duration and the magnitude of the thyroid tumor volume in these cats (r = 0.58; P <0.0001).
Figure 5.
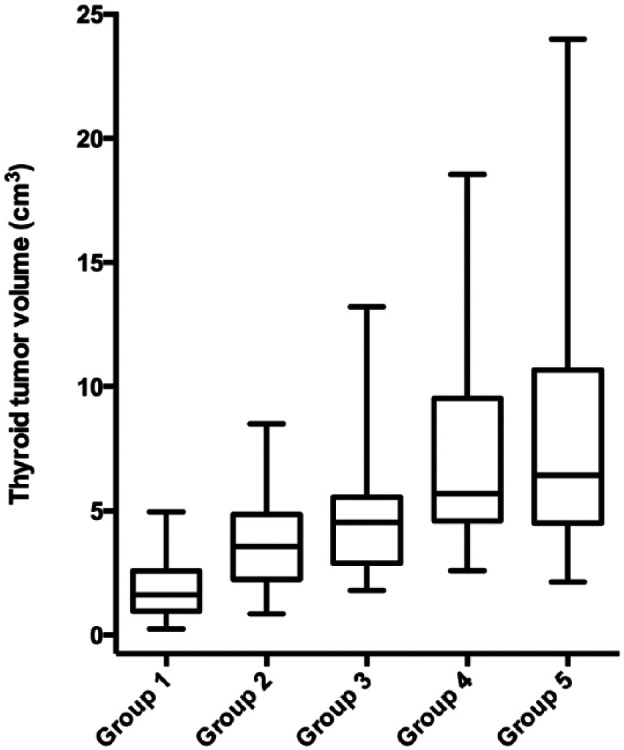
Box plots of the calculated volume of thyroid tumor tissue in 2096 cats with hyperthyroidism, divided into the following five groups based on the time from diagnosis: group 1, ⩽1 year; group 2, >1–2 years; group 3, >2–3 years; group 4, >3–4 years; and group 5, >4–6.1 years
Thyroid tumors were small (<2.0 cm3) in 1135, medium-sized (2–4 cm3) in 682, large (4–8 cm3) in 230 and huge (>8 cm3) in 49 cats (Table 1). Cats with large and huge thyroid tumors had more severe hyperthyroid disease than did the cats with smaller tumors. Cats with large and huge tumors had higher serum T4 concentrations (300 nmol/l) than did cats with medium-sized (165 nmol/l) or small (81 nmol/l) thyroid tumors (P <0.0001). Cats with large and huge thyroid tumors also had higher T/S ratios (16.0) than did cats with medium-sized (9.1) or small (3.9) thyroid tumors (P <0.0001).
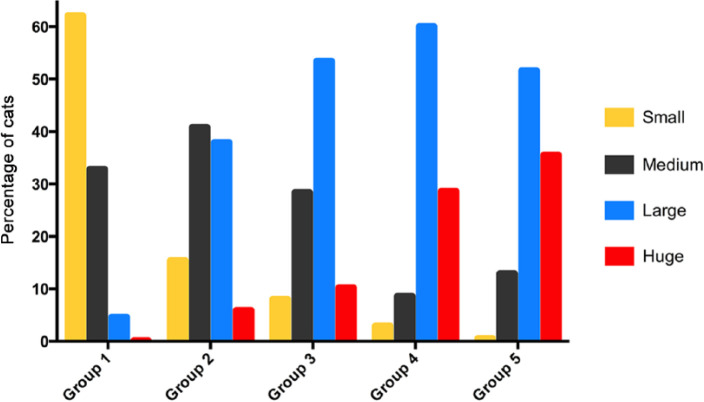
The prevalence of small tumors decreased with increasing disease duration from 62.1% of group 1 cats to only 2.9% of group 4 cats (P <0.0001); none of the 31 cats in group 5 had small thyroid tumors (Table 1; Figure 6). Conversely, the prevalence of large thyroid tumors increased with increasing disease duration from 4.6% of group 1 cats up to 60% of group 4 cats (P <0.0001) (Table 1; Figure 6). Similarly, prevalence of huge thyroid tumors increased with increasing disease duration from 0.5% of group 1 cats up to 35.5% of group 5 cats (P <0.0001) (Table 1; Figure 6).
Figure 6.
Bar graphs depicting the percentage of hyperthyroid cats with small, medium, large and huge thyroid tumors in each of five groups, based on the time from diagnosis: group 1, ⩽1 year; group 2, >1–2 years; group 3, >2–3 years; group 4, >3–4 years; and group 5, >4–6.1 years
Ectopic thyroid disease
We identified ectopic disease in 79 cats; of these, 64 had intrathoracic (mediastinal) ectopic tumors (Table 1; Figure 2c), eight had sublingual tumors, and one cat had ectopic tumor nodules in both intrathoracic and sublingual locations. The remaining six cats had three areas of increased radionuclide uptake (hyperfunctional thyroid tumor nodules) in the cervical region; these cats were considered to have bilateral disease, with an ectopic cervical tumor. 5
In the cats with ectopic disease, the calculated thyroid size was small in 17 (21%), medium-sized in 38 (46.9%), large in 26 (28.4%) and huge in three (3.7%). The median serum T4 concentration in these 81 cats was 195 nmol/l (IQR 142–300 nmol/l).
The prevalence of ectopic disease did not vary significantly across the five groups of cats, ranging from 3.3% in cats of group 1 to 7.1%, 5.7%, 8.6% and 6.5% in groups 2–5, respectively (Table 1; Figure 7).
Figure 7.
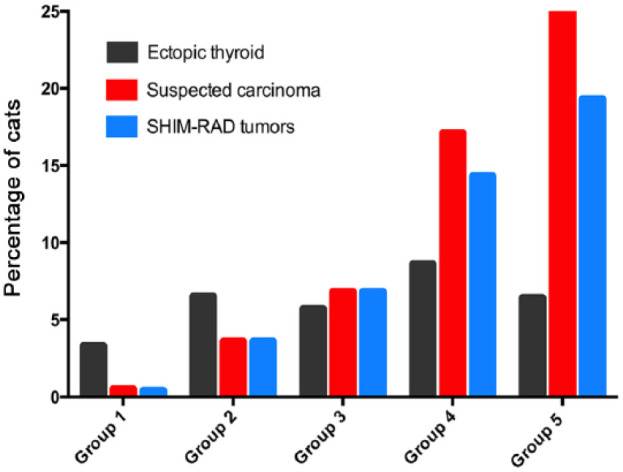
Bar graphs depicting the percentage of hyperthyroid cats with ectopic thyroid tumor tissue, suspected thyroid carcinoma, or SHIM-RAD tumors in each of five groups, based on the time from diagnosis: group 1, ⩽1 year; group 2, >1–2 years; group 3, >2–3 years; group 4 >3–4 years; and group 5, >4–6.1 years. Cats in the SHIM-RAD subgroup had the following five features: (1) severe hyperthyroidism (serum thyroxine >300 nmol/l); (2) huge tumors; (3) intrathoracic tumor location; (4) multifocal distribution of radionuclide uptake; and (5) refractory to treatment with antithyroid drugs
Suspected thyroid carcinoma (SHIM-RAD tumors)
We diagnosed suspected thyroid carcinoma in 35 cats. 5 These cats had 2–6 areas of increased thyroid uptake (median 4 areas) that extended beyond the margins of the thyroid tumor(s) (Figure 2d). All of these tumors were hyperfunctional; non-functional (ie, cold) thyroid tumors were not detected in any of these cats. The calculated thyroid size was very large in four (11.4%) cats and huge in 31 (88.6%) of these cats, with extension of tumor from the cervical region through the thoracic inlet (four cats) or into the thorax (31 cats). Two cats had evidence of metastasis to regional lymph nodes. Of the 35 cats, 32 had been treated with methimazole; all had shown an initial response (serum T4 normalizing), followed weeks-to-months later by a gradual loss of thyroid control despite an increasing daily methimazole dosage.
Of the 35 cats with suspected carcinoma, 30 cats could be characterized as a distinct subgroup (acronym SHIM-RAD) based on having all five of the following clinical and scintigraphic characteristics: (1) severe hyperthyroidism (serum T4 >300 nmol/l); (2) huge tumor size; (3) intrathoracic tumor nodule(s); (4) multifocal disease; and (5) refractory response to treatment with antithyroid drugs. The remaining five cats each fulfilled 4/5 features but lacked one of the SHIM-RAD characteristics: one had tumor masses in both cervical and thoracic inlet locations but did not have intrathoracic involvement; two had bilateral but not multifocal disease; and two had never been treated with methimazole and therefore could not be classified as being refractory to treatment.
The prevalence of suspected thyroid carcinoma increased with increasing disease duration from 0.5% of group 1 cats to 25.8% of group 5 cats (P <0.001) (Table 1; Figure 7). Similarly, prevalence of the subgroup of cats having huge, intrathoracic, multifocal disease refractory to antithyroid drugs (SHIM-RAD group) increased with increasing disease duration from 0.4% of group 1 cats to 19.3% of group 5 cats (P <0.001) (Table 1; Figure 7).
Discussion
Our study, in a large cohort of cats with hyperthyroidism referred for radioiodine therapy, shows that the prevalence of moderate-to-severe hyperthyroid disease increases with disease duration, despite ongoing administration of antithyroid drugs. Serum T4 concentrations (tested after stopping methimazole) and T/S ratios (another predictor of the metabolic status of the thyroid gland 32 ) both increased proportionally with disease duration. Additionally, the size, volume, number of functional thyroid tumor nodules and the prevalence of suspected thyroid carcinoma increased proportionally with disease duration. These findings might be expected, given that antithyroid drugs inhibit thyroid hormone secretion but do not destroy the thyroid tumor pathology,1,2,33 allowing the thyroid adenoma to grow and evolve.
Several estimates of disease severity increased in parallel in our five groups of cats with increased disease duration. As tumor volume and number of thyroid tumor nodules increased, the number of thyroid tumors located within the thoracic inlet and anterior thorax also increased. No cause for this was identified, but we speculate that this might be due to gravitational forces pulling the larger tumor mass ventrally. Additionally, and possibly more importantly, the prevalence of suspected thyroid carcinoma was very low in recently diagnosed cats (0.5%), but accounted for >25% of tumors in group 5 cats. Worse outcomes for cats with suspected thyroid carcinoma highlight the importance of preventing malignant transformation, if possible.3,12,34,35
While we might expect to see a higher thyroid tumor volume in cats with more chronic hyperthyroidism, we did not expect to see an increase in the number of thyroid tumor nodules as disease duration increased. In our cats, the prevalence of bilateral or multifocal disease increased, while prevalence of unilateral thyroid disease progressively decreased as disease duration increased. These findings further support our premise that feline hyperthyroidism is a progressive disease that cannot be arrested by maintaining euthyroidism with antithyroid drugs. Although not addressed in this cross-sectional study, this observation suggests that development of new thyroid tumor nodules over time might explain why a subset of cats treated with unilateral thyroidectomy or radioiodine and initially cured later experience relapse of their hyperthyroid disease.1,2,36–38
One question that comes up as a result of this study is whether the increased disease severity that we observed in cats with longer disease duration was the result of time alone, allowing the thyroid tumor to grow and evolve naturally, or if the chronic use of methimazole enhanced disease progression. In rats, long-term methimazole can induce thyroid nodular hyperplasia and thyroid neoplasia (both follicular cell adenomas and adenocarcinomas).39,40 However, there is marked species variation in the ability of methimazole to induce thyroid carcinogenesis, 41 and the World Health Organization has failed to find sufficient evidence to classify methimazole as a carcinogen in human patients. 42 Obviously, this study was not designed to determine whether or not long-term administration of methimazole directly enhanced thyroid tumor growth or carcinogenesis in cats, and further studies are needed to resolve this question.
In addition to the drug’s possible ability to induce thyroid nodular hyperplasia and neoplasia, methimazole treatment lowers serum T4 and T3 concentrations, thereby increasing circulating concentrations of TSH into or above the reference interval in some cats.43–45 These persistently elevated serum TSH concentrations might also contribute to advancing thyroid tumor growth and disease progression.46,47 It is also possible that high circulating TSH concentrations could induce changes in intrathyroidal methimazole uptake or metabolism, contributing to the antithyroid drug resistance seen in some of our cats. Again, further studies are needed to address these questions.
Historically, investigators have had difficulty in accurately measuring thyroid volume by use of thyroid scintigraphy.22–24,48,49 With scintigraphy, the apparent size of the thyroid gland is related to the degree of 99mTcO4− uptake (ie, the count intensity or T/S ratio), as well as to the actual volume of the gland or tumor.24,50 As a result, thyroid tumor nodules of hyperthyroid cats that show intense 99mTcO4− thyroid uptake can appear much larger than tumor nodules of similar size that have a lower count density. This phenomenon, referred to in digital imaging as the bloom effect, 20 produces fringes of brightness extending from the borders of intense tumor nodules on a scintigraphic image and results in falsely high measured thyroid volumes. 22 In order to correct this overestimation in volume, the use of various filtering and thresholding methods has been suggested.48,51 Most studies in both humans and cats have used a region-of-interest threshold value of 20–30% to improve the accuracy of thyroid volume calculations,24,48,51–53 similar to the cutoff value of 20% used in this study. We believe that our method of thyroid tumor measurement, followed by use of an equation (prolate spheroid) suitable for thyroid tumors that are spherical-to-cylindrical, 26 provided an acceptable estimate of tumor volume; in support of that, the tumor volumes reported in the 2096 cats of this study (median 1.8 cm3; mean 2.4 cm3) compare fairly well with those of another recent study of 32 hyperthyroid cats in which ultrasound was used as the gold standard to determine thyroid volume (mean tumor volume 2.01 cm3). This is in contrast to the study of Forrest et al, 54 in which thyroid volume was calculated in 80 hyperthyroid cats by measuring the thyroid scintigraphic image without first adjusting the image window settings to the full dynamic range or using a threshold cutoff limit; in that study, the mean thyroid tumor volume (10.4 cm3), which we believe to be a falsely high estimate owing, at least in part, to the bloom effect, was more than four-fold greater than the mean volume reported in the present study.
In this and our previous study, 5 we identified 35 cats with suspected thyroid carcinoma based on scintigraphic features suggestive of malignant disease, including massive multifocal disease, heterogeneous areas of 99mTcO4− uptake, and irregular, spiculated tumor margins (suggesting extension beyond the thyroid capsule and/or soft tissue invasion by the tumor tissue).3,5,34,55 However, only two of these cats had evidence of metastatic disease, the most reliable indicator of thyroid malignancy. Unfortunately, histopathology was not used to confirm thyroid carcinoma in any of these 35 cats because of their severe and uncontrolled hyperthyroidism, which put them at high risk for anesthesia and surgical biopsy. Therefore, it is possible that these cats simply had advanced, severe benign thyroid disease, although the presence of multifocal disease is then difficult to explain after exclusion of ectopic thyroid disease. As previously reported, distinguishing ectopic from carcinomatous nodules is relatively straightforward, as ectopic tumor nodules tend to be small-to-moderate in size and are classically located exactly on the ventral midline, along the tract of the descending thyroglossal duct, whereas infiltrative or metastatic masses are typically much larger and located off the ventral midline.3,5,10,13,56
Although we do not have histological proof of malignant disease in our cats with suspected carcinoma, these cats tended to show a more advanced clinical presentation and more severe scintigraphy changes than the other hyperthyroid cats in this study. To that end, we developed a combination of five clinical and scintigraphic features to distinguish these cats with suspected carcinoma from all other hyperthyroid cats (not suspected of having carcinoma) in this study. These included the following criteria: (1) the presence of severe hyperthyroidism (defined as a serum T4 concentration >300 nmol/l); (2) huge tumor size or volume (on both palpation and by scintigraphy); (3) intrathoracic location of thyroid masses; (4) multifocal disease (more than three distinct tumor nodules); and (5) a refractory response to treatment with high doses of antithyroid drugs, despite the fact that these drugs generally had successfully controlled hyperthyroidism earlier in the course of the cats’ disease. Of our 35 cats with suspected carcinoma, 30 cats met all five of these criteria, so we believe this a useful clinical classification scheme to identify these cats. As as alternative to the use of the term ‘suspected carcinoma’, we propose the acronym ‘SHIM-RAD’ for this subgroup of hyperthyroid cats, indicating severe, huge, intrathoracic, multifocal disease, refractory to antithyroid drugs. This allows us to classify these cats to discuss a likely clinical course and best treatment, even when results of thyroid histopathology are not available.
However, whatever we call or label this subset of hyperthyroid cats, the following clinical points are very clear. The cats have severe (often life-threatening) hyperthyroidism that can no longer be managed medically with antithyroid drugs. Surgical thyroidectomy is difficult or impossible owing to the multifocal nature and intrathoracic tumor involvement, but even if excision was possible, these uncontrolled hyperthyroid cats represent a very high anesthetic risk. Radioiodine can be effective treatment in these cats, but the standard doses generally administered (eg, 100–200 mBq) will almost always fail; extremely high doses (5–10-fold higher than the typical 131I doses) are almost always needed.3,34,35 For these reasons, we believe that it is important to categorize these cases separately (and label them either as suspected carcinoma or SHIM-RAD). It is also clinically important to note that the prevalence increases dramatically with longer disease duration, as the thyroid disease is allowed to progress. The pathogenesis of SHIM-RAD thyroid tumors is unknown but likely involves a progression from smaller, benign, unilateral or bilateral adenomatous disease, given the very long disease duration in most of the SHIM-RAD cats.6,7 Again, as noted above, the elevated serum TSH concentrations that can develop with long-term methimazole treatment could contribute to development of these SHIM-RAD tumors.
Overall, we do not believe that classifying a cat as having thyroid adenoma vs carcinoma is as important as staging of disease based on tumor volume, number of tumor nodules, location of disease and local infiltration/invasion as almost all cats with suspected thyroid carcinoma (or SHIM-RAD) have a slow, indolent, relatively benign, biologic course, with metastasis being rare. Again, most of these cats had advanced disease but had been hyperthyroid for many months to many years. In addition, the fact that cats with suspected thyroid carcinomas almost always show a complete clinical response to high-dose radioiodine therapy, with total ablation of all neoplastic thyroid tissue (including the 35 cats of this report), supports this reasoning.3,34,35,57
The results of this cross-sectional study represent the prevalence rates for cats that are referred for radioiodine therapy but may not reflect what happens in all cats treated long-term with antithyroid drugs. Although many of our cats that were diagnosed within a year of our evaluation had not been treated, all of the cats diagnosed more than a year before evaluation had been treated medically with antithyroid drugs. Most cats with chronic hyperthyroid disease were referred because of the need for increasing dosages of methimazole or the failure of medical management to control their hyperthyroid state. 12 The reason for such failure in medical management was likely associated with the thyroid tumor becoming too large for the antithyroid drugs to block thyroid hormone secretion adequately or, in some cats, with the thyroid adenoma undergoing malignant transformation into carcinomas. Therefore, it is possible that some of our hyperthyroid cats represent a subpopulation with larger-than-average thyroid tumors, and that these cats may not represent the ‘average’ cat treated with long-term methimazole. Put another way, most owners who decided to treat their hyperthyroid cat with radioiodine >2 years after initial diagnosis do so mostly because the antithyroid drugs are no longer as effective in maintaining euthyroidism; other cats treated long-term with antithyroid drugs would be much less likely to be referred for radioiodine treatment if the drugs are still effective.
Conclusions
Overall, our study provides evidence that feline hyperthyroidism is a progressive disease, with thyroid pathology that cannot be arrested by management with chronic antithyroid drugs. Over a period of months to years, the thyroid tumors continue to grow and malignant transformation appears possible. What percentage of hyperthyroid cats treated medically show escape from long-term control, necessitating an increase in the daily methimazole dose, is unknown.
Furthermore, progressive dose increments of antithyroid medications warrant re-evaluation of the underlying tumor pathology. Only thyroidectomy or radioiodine can remove or destroy the hyperfunctional thyroid tumor(s) and cure the disease. Neither management with antithyroid drugs nor feeding a low-iodine diet is curative, as both options leave the thyroid adenoma intact to continue to grow and evolve with time. Such sequelae should be considered when discussing treatment options with cat owners, especially in younger cats that will require years of treatment.
Acknowledgments
Work on this study was carried out both at the Animal Endocrine Clinic and Advanced Veterinary Medical Imaging.
Footnotes
This study was presented as a research abstract at the 22nd ECVIM-CA Congress, Maastricht, the Netherlands, September 8, 2012.
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 19 January 2015
References
- 1. Baral R, Peterson ME. Thyroid gland disorders. In: Little SE. (ed). The cat: clinical medicine and management. Philadelphia, PA: Elsevier Saunders, 2012, pp 571–592. [Google Scholar]
- 2. Mooney CT, Peterson ME. Feline hyperthyroidism. In: Mooney CT, Peterson ME. (eds). Manual of canine and feline endocrinology. 4th ed. Quedgeley: British Small Animal Veterinary Association, 2012, pp 199–203. [Google Scholar]
- 3. Turrel JM, Feldman EC, Nelson RW, et al. Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986). J Am Vet Med Assoc 1988; 193: 359–364. [PubMed] [Google Scholar]
- 4. Gerber H, Peter H, Ferguson DC, et al. Etiopathology of feline toxic nodular goiter. Vet Clin North Am Small Anim Pract 1994; 24: 541–565. [DOI] [PubMed] [Google Scholar]
- 5. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2,096 cats with hyperthyroidism. Vet Radiol Ultrasound 2015; 56: 84–95. [DOI] [PubMed] [Google Scholar]
- 6. Peterson M. Hyperthyroidism in cats: what’s causing this epidemic of thyroid disease and can we prevent it? J Feline Med Surg 2012; 14: 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peterson ME, Ward CR. Etiopathologic findings of hyperthyroidism in cats. Vet Clin North Am Small Anim Pract 2007; 37: 633–645. [DOI] [PubMed] [Google Scholar]
- 8. Peter HJ, Gerber H, Studer H, et al. Autonomy of growth and of iodine metabolism in hyperthyroid feline goiters transplanted onto nude mice. J Clin Invest 1987; 80: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peter HJ, Gerber H, Studer H, et al. Autonomous growth and function of cultured thyroid follicles from cats with spontaneous hyperthyroidism. Thyroid 1991; 1: 331–338. [DOI] [PubMed] [Google Scholar]
- 10. Carpenter JL, Andrews LK, Holzworth J. Tumors and tumor-like lesions. In: Holzworth J. (ed). Diseases of the cat: medicine and surgery. Philadelphia, PA: WB Saunders, 1987, pp 406–596. [Google Scholar]
- 11. Derwahl M, Studer H. Hyperplasia versus adenoma in endocrine tissues: are they different? Trends Endocrinol Metab 2002; 13: 23–28. [DOI] [PubMed] [Google Scholar]
- 12. Broome MR, Peterson ME. Treatment of severe, unresponsive, or recurrent hyperthyroidism. In: Little SE. (ed). August’s consultations in feline internal medicine. Philadelphia, PA: Elsevier., 2015, 241–250. [Google Scholar]
- 13. Ibrahim NA, Fadeyibi IO. Ectopic thyroid: etiology, pathology and management. Hormones (Athens) 2011; 10: 261–269. [DOI] [PubMed] [Google Scholar]
- 14. Nykamp SG, Dykes NL, Zarfoss MK, et al. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990–2002). J Am Vet Med Assoc 2005; 226: 1671–1675. [DOI] [PubMed] [Google Scholar]
- 15. Peterson ME, Melian C, Nichols R. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease. J Am Vet Med Assoc 2001; 218: 529–536. [DOI] [PubMed] [Google Scholar]
- 16. Peterson ME. More than just T4: diagnostic testing for hyperthyroidism in cats. J Feline Med Surg 2013; 15: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004; 17: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harvey AM, Hibbert A, Barrett EL, et al. Scintigraphic findings in 120 hyperthyroid cats. J Feline Med Surg 2009; 11: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ott RJ, Flower MA, Marsden PK. The physics of radioisotope imaging. In: Webb S. (ed). The physics of medical imaging. New York: Taylor & Francis Group, 1988, pp 142–318. [Google Scholar]
- 20. Spencer G, Shirley P, Zimmerman K, et al. Physically-based glare effects for digital images. In: Cook R. (ed). Annual Conference on Computer Graphics (SIGGRAPH), 1995, pp 325–334. [Google Scholar]
- 21. Oberholzer M, Ostreicher M, Christen H, et al. Methods in quantitative image analysis. Histochem Cell Biol 1996; 105: 333–355. [DOI] [PubMed] [Google Scholar]
- 22. Tannahill AJ, Hooper MJ, England M, et al. Measurement of thyroid size by ultrasound, palpation and scintiscan. Clin Endocrinol (Oxf) 1978; 8: 483–486. [DOI] [PubMed] [Google Scholar]
- 23. Zaidi H. Comparative methods for quantifying thyroid volume using planar imaging and SPECT. J Nucl Med 1996; 37: 1421–1426. [PubMed] [Google Scholar]
- 24. Wesche MF, Tiel-van Buul MM, Smits NJ. Ultrasonographic versus scintigraphic measurement of thyroid volume in patients referred for 131I therapy. Nucl Med Commun 1998; 19: 341–346. [DOI] [PubMed] [Google Scholar]
- 25. Daniel GB. Digital image processing. In: Daniel GB, Berry CR. (eds). Textbook of veterinary nuclear medicine. 2nd ed. Harrisburg, PA: American College of Veterinary Radiology, 2006, pp 79–120. [Google Scholar]
- 26. Weisstein EW. MathWorld – A Wolfram web resource. http://mathworld.wolfram.com/Volume.html (2014, accessed August 30, 2014).
- 27. D’Agostino RB. Tests for normal distribution. In: D’Agostino RB, Stephens MA. (eds). Goodness-of-fit techniques. New York: Macel Dekker, 1986, pp 367–420. [Google Scholar]
- 28. Conover WJ. Practical nonparametric statistics. 3rd ed. New York: John Wiley & Sons, 1999. [Google Scholar]
- 29. Corder GW, Foreman DI. Nonparametric statistics for non-statisticians: a step-by-step approach. Hoboken, NJ: John Wiley & Sons, 2009. [Google Scholar]
- 30. Simpson RJ, Jr, Johnson TA, Amara IA. The box-plot: an exploratory analysis graph for biomedical publications. Am Heart J 1988; 116: 1663–1665. [DOI] [PubMed] [Google Scholar]
- 31. Abdi H. Binomial distribution: binomial and sign tests. In: Salkind NJ. (ed). Encyclopedia of measurement and statistics. Thousand Oaks, CA: SAGE, 2007, pp 87–89. [Google Scholar]
- 32. Daniel GB, Sharp DS, Nieckarz JA, et al. Quantitative thyroid scintigraphy as a predictor of serum thyroxin concentration in normal and hyperthyroid cats. Vet Radiol Ultrasound 2002; 43: 374–382. [DOI] [PubMed] [Google Scholar]
- 33. Peterson ME, Kintzer PP, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med 1988; 2: 150–157. [DOI] [PubMed] [Google Scholar]
- 34. Guptill L, Scott-Moncrieff CR, Janovitz EB, et al. Response to high-dose radioactive iodine administration in cats with thyroid carcinoma that had previously undergone surgery. J Am Vet Med Assoc 1995; 207: 1055–1058. [PubMed] [Google Scholar]
- 35. Hibbert A, Gruffydd-Jones T, Barrett EL, et al. Feline thyroid carcinoma: diagnosis and response to high-dose radioactive iodine treatment. J Feline Med Surg 2009; 11: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swalec KM, Birchard SJ. Recurrence of hyperthyroidism after thyroidectomy in cats. J Am Anim Hosp Assoc 1990; 26: 433–437. [Google Scholar]
- 37. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995; 207: 1422–1428. [PubMed] [Google Scholar]
- 38. Birchard SJ. Thyroidectomy in the cat. Clin Tech Small Anim Pract 2006; 21: 29–33. [DOI] [PubMed] [Google Scholar]
- 39. Todd GC. Induction and reversibility of thyroid proliferative changes in rats given an antithyroid compound. Vet Pathol 1986; 23: 110–117. [DOI] [PubMed] [Google Scholar]
- 40. Owen NV, Worth HM, Kiplinger GF. The effects of long-term ingestion of methimazole on the thyroids of rats. Food Cosmet Toxicol 1973; 11: 649–653. [DOI] [PubMed] [Google Scholar]
- 41. Dybing E, Sanner T. Species differences in chemical carcinogenesis of the thyroid gland, kidney and urinary bladder. In: Capen CC, Dybing E, Rice JM, et al. (eds). IARC scientific publications No 147. Lyon,: World Health Organization, International Agency for Research on Cancer (IARC) Press, 1999, pp 15–32. [PubMed] [Google Scholar]
- 42. IARC Working Group. Some thyrotropic agents. IARC monogrographs on the evaluation of carcinogenic risks to humans. Lyon: World Health Organization, International Agency for Research on Cancer (IARC) Press, 2001, pp 51–142. [Google Scholar]
- 43. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010; 24: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 44. Fischetti AJ, Drost WT, DiBartola SP, et al. Effects of methimazole on thyroid gland uptake of 99mTC-pertechnetate in 19 hyperthyroid cats. Vet Radiol Ultrasound 2005; 46: 267–272. [DOI] [PubMed] [Google Scholar]
- 45. Chciuk K, Behrend EN, Martin L, et al. Evaluation of thyroid-stimulating hormone, total thyroxine, and free thyroxine concentrations in 65 hyperthyroid cats receiving methimazole therapy. J Vet Intern Med 2013; 27: 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hood A, Liu YP, Gattone VH, 2nd. Sensitivity of thyroid gland growth to thyroid stimulating hormone (TSH) in rats treated with antithyroid drugs. Toxicol Sci 1999; 49: 263–271. [DOI] [PubMed] [Google Scholar]
- 47. Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect 1998; 106: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Isselt JW, de Klerk JM, van Rijk PP, et al. Comparison of methods for thyroid volume estimation in patients with Graves’ disease. Eur J Nucl Med Mol Imaging 2003; 30: 525–531. [DOI] [PubMed] [Google Scholar]
- 49. Vieira LO, Kubo R, Sapienza MT, et al. Correlation between thyroid volume determined by the method of ultrasound versus scintigraphy and its implication in the dosimetric calculations radioiodine therapy in Graves’ disease. Arq Bras Endocrinol Metab 2011; 55: 696–700. [DOI] [PubMed] [Google Scholar]
- 50. Wisner ER, Theon AP, Vet M, et al. Ultrasonographic examination of the thyroid gland of hyperthyroid cats: comparison to 99mTcO−4 scintigraphy. Vet Radiol Ultrasound 1994; 35: 53–58. [Google Scholar]
- 51. Huang JY, Lin KJ, Chen YS. Fully automated computer-aided volume estimation system for thyroid planar scintigraphy. Comput Biol Med 2013; 43: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 52. Pant GS, Kumar R, Gupta AK, et al. Estimation of thyroid mass in Graves’ disease by a scintigraphic method. Nucl Med Commun 2003; 24: 743–748. [DOI] [PubMed] [Google Scholar]
- 53. Volckaert V, Vandermeulen E, Saunders JH, et al. Scintigraphic thyroid volume calculation in hyperthyroid cats. J Feline Med Surg 2012; 14: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forrest LJ, Baty CJ, Metcalf MR, et al. Feline hyperthyroidism: efficacy of treatment using volumetric analysis for radioiodine dose calculation. Vet Radiol Ultrasound 1996; 37: 141–145. [Google Scholar]
- 55. Daniel GB, Brawnier WR. Thyroid scintigraphy. In: Daniel GB, Berry CR. (eds). Textbook of veterinary nuclear medicine. 2nd ed. Harrisburg, PA: American College of Veterinary Radiology, 2006, pp 181–199. [Google Scholar]
- 56. Jones TC, Hunt RD, King NW. Endocrine glands. In: Jones TC, Hunt RD, King NW. (eds). Veterinary pathology. 6th ed. Baltimore, MD: Lippincott, Williams & Wilkins, 1997, pp 1249–1250. [Google Scholar]
- 57. Peterson ME, Broome MR. Radioiodine for feline hyperthyroidism. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy XV. Philadelphia, PA: Saunders Elsevier, 2014, pp e112–e122. [Google Scholar]