Abstract
Breast cancer survivors with obesity are at a high risk of cancer recurrence, comorbidity, and mortality. This review aims to systematically evaluate the effects of combined aerobic and resistance training (CART) on body composition, lipid homeostasis, inflammation, adipokines, cancer-related fatigue, sleep, and quality of life in breast cancer patients and survivors with overweight/obesity. An electronic search was conducted in PubMed, Web of Science, Scopus, Science Direct, Cochrane, and Google Scholar databases from inception up to January 8, 2024. Randomized controlled trials (RCTs) meeting the inclusion criteria were selected for the analysis. The Cochrane risk of bias tool was used to assess eligible studies, and the GRADE method to evaluate the quality of evidence. A random-effects model was used, and data were analyzed using mean (MD) and standardized mean differences (SMD) for continuous variables with 95% confidence intervals (CI). We assessed the data for risk of bias, heterogeneity, sensitivity, reporting bias, and quality of evidence. A total of 17 randomized controlled trials were included in the systematic review involving 1,148 female patients and survivors (mean age: 54.0 ± 3.4 years). The primary outcomes showed significant improvements in body mass index (SMD -0.57 kg/m2, p = 0.04), body fat (SMD -0.50%, p = 0.02), fat mass (SMD -0.63 kg, p = 0.04), hip circumference (MD -3.14 cm, p = 0.02), and fat-free mass (SMD 1.03 kg, p < 0.001). The secondary outcomes indicated significant increases in high-density lipoprotein cholesterol (MD -0.05 mmol/L, p = 0.008), natural killer cells (SMD 0.42%, p = 0.04), reductions in triglycerides (MD -81.90 mg/dL, p < 0.01), total cholesterol (SMD -0.95 mmol/L, p < 0.01), tumor necrosis factor α (SMD -0.89 pg/mL, p = 0.03), and leptin (SMD -0.63 ng/mL, p = 0.03). Also, beneficial alterations were found in cancer-related fatigue (SMD -0.98, p = 0.03), sleep (SMD -1.17, p < 0.001), and quality of life (SMD 2.94, p = 0.02) scores. There was very low to low confidence in the estimated effect of most of the outcomes. The present findings reveal that CART could be considered an adjunct therapy in supporting the conventional clinical approach observed following exercise. However, further high-quality research is needed to evaluate whether CART would be a valuable intervention to lower aggressive pharmacologic use in breast cancer patients with overweight/obesity.
Key points.
Combined aerobic and resistance training exert beneficial changes in anthropometrics, body composition, and lipid metabolism.
Combined aerobic and resistance training reveals advantageous alterations in various cancer-related indicators, such as fatigue, sleep, and quality of life.
Combined aerobic and resistance training may be considered a valuable piece of a multicomponent therapy puzzle in supporting women with breast cancer and concurrent overweight/obesity.
Further research is needed in this area through large-scale randomized controlled trials and higher methodological quality.
Key words: Oncology, muscle strengthening, cardiorespiratory exercise, cardiovascular disease, inflammation, adipokines, fatigue, sleep, quality of life
Introduction
Cancer is a critical global health concern since it is the world's most common non-communicable disease and the second major cause of death (Afolabi et al., 2022b; Meneses-Echávez et al., 2015). More specifically, breast cancer (BC) is the most frequent type of cancer in women, and 25% of BC cases are due to excessive weight and sedentary lifestyle risk factors (Siegel et al., 2020). Thus, obesity affects one out of every three BC survivors (Greenlee et al., 2016), raising the chance of cancer recurrence, comorbidity, and mortality (Feliciano et al., 2019; Hwangbo et al., 2018; Lohmann et al., 2021; Petrelli et al., 2021). Given that fat tissue is an endocrine organ that secretes various inflammatory factors and adipokines (Afolabi et al., 2022a), a significant association between BC and numerous impaired cardiometabolic health-related indices has been documented in women (Chowdhury et al., 2021; Guo et al., 2013; Wu et al., 2009). BC survivors are at a high risk of developing comorbidities, such as hyperlipidemia, sarcopenia, and osteoporosis (Ording et al., 2013), which affect individuals' quality of life (QoL), cardiorespiratory fitness, physical function, and bone health while increasing cancer-related pain and fatigue (Al-Mhanna et al., 2022; Ganz, 2006; Stanton, 2006). Also, excess adiposity is linked to early recurrence on breast cancer survivors after treatment, showing that the management of obesity may be a critical health factor for this cohort (Acevedo et al., 2022; Elreda et al., 2022).
Regular exercise has been reported as a vital tool for reducing the most common and impairing symptoms in BC patients before, during, and after treatment (Mortimer et al., 2010; Mutalub et al., 2022). In BC patients and survivors, exercise is considered an effective non-pharmacologic method for lowering pro-inflammatory biomarkers and cardiovascular disease (CVD) risk factors as well as mental health, and physical function (Campbell et al., 2019; Speck et al., 2010; Van Alsten et al., 2020). However, exercise oncology has not been yet established as an emerging trend among practitioners in the health and fitness community (Newsome et al., 2024). In terms of the mechanism behind the positive role of exercise in BC, exercise-induced reductions in hyperlipidemia and chronic inflammation are believed to inhibit tumor growth by lowering serum cholesterol levels and reducing the exposure of tumor cells to cholesterol, increasing anti-tumor immunity, and regulating tumor vasculature (Betof et al., 2013). In addition, exercise can reduce blood lipid levels by improving skeletal muscle's ability to use lipids instead of glycogen or increasing lecithin-cholesterol acyltransferase and lipoprotein lipase activity (Al-Mhanna et al., 2024a; Al-Mhanna et al., 2024b; Mann et al., 2014). Also, exercise may counteract the pro-tumor impact of hyperlipidemia by reducing chronic inflammation, lowering serum lipids, improving the immune system's ability to identify and destroy tumor cells, and stabilizing the tumor's vascular network (Gonzalo-Encabo et al., 2022).
Although many trials show that exercise may improve body composition in BC patients (Almstedt et al., 2016; Dieli-Conwright and Orozco 2015), there is a lack of evidence synthesis and pooled outcomes via meta-analysis that evaluated the effectiveness of combined aerobic and resistance training (CART) among BC patients with overweight/obesity (Dieli-Conwright et al., 2022; Jones and Courneya, 2002; Milne et al., 2008a). Furthermore, the long-term implications of CART in women with BC remain unclear (de Paulo et al., 2018), indicating that more research is required, aiming to investigate the effects of CART on body composition and several cardiometabolic health aspects as previously reported (de Paulo et al., 2018).. Thus, the present review aimed to assess the effects of CART on anthropometrics, body composition, lipid metabolism, inflammation, adipokines, QoL, sleep quality, and fatigue among women with BC and overweight/obesity.
Methods
Registration
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines (Page et al., 2021). The study protocol was registered in the International Prospective Register of Systematic Reviews (ID: CRD42022308214).
Literature search strategy
Articles were retrieved from PubMed, Web of Science, Scopus, Science Direct, Cochrane Library, and Google Scholar after a systematic electronic search. Four authors (S.A., B.K., A.B., and H.A.) employed a combination of keywords and Boolean operators, specifically "OR" and "AND" in conducting an electronic search of literature up to January 8, 2024. The keywords utilized were ("Breast Cancer" OR "Breast Neoplasm") AND "(Overweight" OR "Obese") AND "(Exercise" OR "Training") to retrieve pertinent material (Table S1). The PICOS was used to formulate the research questions for systematic reviews and meta-analyses as follows: (P) Population: BC patients with overweight or obesity; (I) Intervention: CART; (C) Comparator: standard treatment (ST) (patients who did not perform any exercise); (O) Outcomes: [primary: body weight (BW), body mass index (BMI), waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), body fat (BF), fat mass (FM), fat-free mass (FFM); secondary: total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), C–reactive protein (CRP), Tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-6 (IL-8), natural killer (NK) cells, adiponectin (ADPN), leptin (LEP), cancer-related fatigue (FA), sleep quality (SQ), and QoL]; and (S) Study type: randomized controlled trials (RCTs). The reference lists of included articles were searched for articles that met the inclusion criteria as well as the reference lists of all relevant systematic reviews.
Table S1.
Search strategy.
| # | Database | Algorithm |
|---|---|---|
| 1 | PubMed | (((“Breast cancer” [Title/Abstract]) OR (“Breast Neoplasm” [Title/Abstract])) AND ((“Overweight” [Title/Abstract]) OR (“Obese” [Title/Abstract]))) AND ((“Exercise” [Title/Abstract]) OR (“Training” [Title/Abstract])) |
| 2 | Scopus | TITLE- ABS(“Breast cancer” OR “Breast Neoplasm”) AND TITLE- ABS(“Overweight” OR “Obese”) AND TITLE- ABS(“Exercise” OR “Training”) |
| 3 | Google Scholar | allintitle(“Breast cancer” OR “Breast Neoplasm”) (“Overweight” OR “Obese”) (“Exercise” OR “Training”) |
| 4 | Cochrane Library | (“Breast cancer” OR “Breast Neoplasm”) (“Overweight” OR “Obese”) (“Exercise” OR “Training”) |
| 5 | Web of Science |
|
| 6 | Science Direct | (“Breast cancer” OR “Breast Neoplasm”) (“Overweight” OR “Obese”) (“Exercise” OR “Training”) |
Eligibility criteria
Studies were considered eligible for inclusion if the following criteria were met: (i) participants were BC patients with overweight (BMI 25-29.9 kg/m2) or obesity (BMI ≥30 kg/m2); (ii) no specified age limit for participants; (iii) the intervention used in the studies was CART; (iv) articles provided full-text accessibility and were published in a refereed journal from inception up to 8 January 2024; (v) no language restrictions; and (vi) studies were RCTs. The following were excluded: (i) studies involving a mixed sample of individuals (e.g., underweight or normoweight BC patients or overweight/obese people without BC); (ii) articles where the effects of CART cannot be isolated because exercise training was involved as part of a multicomponent intervention (e.g., diet and exercise intervention); (iii) studies where the control group performed exercise; (iv) articles that did not assess the outcome measures of interest; (v) studies that used an acute exercise intervention (e.g., single bout or duration ≤2 weeks); and (vi) review articles, case reports, studies lacking a control group, and ambiguous or unclear data.
Study selection
The screening process was conducted collaboratively among four authors (S.B.A.L., B.K., A.B., and H.A.), who independently evaluated publications based on titles, abstracts, and full texts (in instances of uncertainty). In cases of conflicts or uncertainties, a fifth author (A.A.I.) was involved in the resolution. While the screening was not conducted in duplicate, it involved thorough evaluation by Multiple authors to ensure robust inclusion and exclusion criteria were applied consistently. Additionally, literature management software (EndNote X9, Clarivate Analytics, Philadelphia, PA, USA) was utilized to manage the records of the literature search.
Data Extraction
Two authors (S.B.A.L. and A.A.I.) independently sampled and extracted data from the relevant studies after reading the full text. The included investigations generated a significant amount of data that was retrieved and published, consisting of the first author, population, publication year, gender, sample size, and exercise intervention details (frequency, intensity, time, and type), study duration, and outcome measures.
Risk of bias assessment
Two authors (S.B.A.L. and A.B.) independently assessed the risk of bias from individual studies according to the Cochrane Handbook for Systematic Reviews of Interventions [35]. The overall risk of bias assessment for each eligible study was judged considering the following factors: (i) random sequence generation; (ii) allocation concealment; (iii) blinding of participants and personnel; (iv) blinding of outcome assessors; (v) completeness of outcome data; (vi) selectivity of outcome reporting; and (vii) other biases as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Table S2). Eligible studies were classified into three levels of risk of bias (e.g., high, some concerns, and low) by the number of factors for which high, unclear, or low risk of bias potentially existed.
Tabel S2.
Risk of bias assessment.
| Bias | Random sequence generation (selection bias) |
Allocation concealment (selection bias) |
Blinding of participants and personnel (performance bias) All outcomes |
Blinding of outcome assessment (detection bias) All outcomes |
Incomplete outcome data (attrition bias) All outcomes |
Selective reporting (reporting bias) | Other bias |
Study |
|---|---|---|---|---|---|---|---|---|
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2018a |
| Support for judgment | Two-arm randomized controlled trial compared a progressive combined-aerobic and resistance-exercise intervention with usual care | Participants were randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists. | Information concerning the blinding of participants was not provided | Information concerning the blinding of the assessor was not provided | Four patients in the intervention group did not complete the study post- intervention: two lost to follow-up and two patients were unreachable. Five patients in the control group did not complete the study post-intervention: Three lost to follow up and two patients were unable to post-test as a result of a work conflict. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2018a |
| Support for judgment | Two-arm randomized controlled trial compared a progressive combined-aerobic and resistance-exercise intervention with usual care | Participants were randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists | Information concerning the blinding of participants was not provided | Information concerning the blinding of the assessor was not provided | Four patients in the intervention group did not complete the study post- intervention: two lost to follow-up and two patients were unreachable. Five patients in the control group did not complete the study post-intervention: Three lost to follow up and two patients were unable to post-test as a result of work conflict. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Dieli- Conwright 2018a |
| Support for judgment | The participants were randomly assigned to either the exercise or control group, and scheduled for the baseline visit. Randomization was performed by the Clinical Investigation Support Office (CISO) at the USC NCCC | To prevent possible bias, study personnel involved in the recruitment did not have access to the randomization lists; the biostatistician who developed the randomization list did not have any patient contacts to influence the recruitment and allocation procedure | The participants were blinding | The assessor was blinding | One patient in the intervention dropped out due to the group lost to follow-up. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2018a |
| Support for judgment | This two-arm randomized controlled trial compared a progressive combined—aerobic and resistance—exercise intervention with usual care | Participants were randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists | Information concerning the blinding of participants were not provided | Information concerning the blinding of the assessor was not provided | four patients of the intervention group did not complete the study post-intervention: two lost to follow-up and two patients were unreachable. Five patients in the control group did not complete the study post- intervention: Three lost to follow up and two patients were unable to post-test as a result of work conflict. Intention-to- treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2019 |
| Support for judgment | This two-arm randomized controlled trial compared a progressive combined—aerobic and resistance—exercise intervention with usual care. | Participants were randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists | Information concerning the blinding of participants were not provided | Information concerning blinding of participants were not provided | All the participants completed the study | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2019 |
| Support for judgment | Randomized control trial comparing a weight loss intervention to usual care. | Participants were randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists | Information concerning the blinding of participants were not provided | Information concerning the blinding of the assessor was not provided | All the participants completed the study | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | High risk | Unclear risk | Low risk | Low risk | Low risk | Dieli- Conwright 2022 |
| Support for judgment | A randomized trial comparing a weight loss intervention to usual care. | Twenty-five participants were randomly assigned to intervention and control groups after baseline testing using concealed randomization lists | Participants were not blinded to intervention assignment | Information concerning the blinding of the assessor was not provided | All the participants completed the study | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Brown 2021 |
| Support for judgment | Participants were randomly assigned in an equal ratio to intervention and control groups | After baseline testing, participants were allocated into two groups and were randomized using a computerized covariate adaptive procedure | Participants were blinding | The assessor was blinding | All the participants completed the study | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Ergun 2013 |
| Support for judgment | Participants were randomly assigned in an equal ratio to intervention and control groups. | After baseline testing, participants were allocated into two groups and were randomized using a computerized covariate adaptive procedure | Participants were not blinded to intervention assignment | The assessor was blinding | All the participants completed the study | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Rogers 2013 |
| Support for judgment | A randomized controlled trial compared an intervention with a control group. Randomization was based on computer-generated numbers, performed in blocks of 4, and revealed in the order in which participants completed baseline testing | Allocation was concealed by central randomization and only revealed after baseline assessment | Participants were blinded | The assessor was blinding | One patient in the intervention group lost to follow-up due to lack of time. One patient in the control group dropped out when asked to repeat a blood draw. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Rogers 2014 |
| Support for judgment | This two-arm randomized controlled trial compared an intervention with a control group using, randomization was done in blocks of four based on computer generated numbers. | Randomization numbers were kept in sealed, opaque envelopes so that study staff and participants were unaware of group allocation until all baseline testing was complete. | Participants were blinded | ALL measures were obtained by individuals who were blinded to the participant's study group allocation | Two participants developed cancer recurrence during the trial (both in the intervention group). These two participants were dropped from the analysis for scientific reasons. Two patients in the control group dropped out due to the lack of time. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Rogers 2014 |
| Support for judgment | This two-arm randomized controlled trial compared an intervention with a control group, using randomization that was conducted in blocks of four based on computer generated numbers | Randomization numbers were kept in sealed, opaque envelopes so that study staff and participants were unaware of group allocation until all baseline testing was complete. | Participants were blinded | ALL measures were obtained by individuals who were blinded to the participant's study group allocation | Two participants developed cancer recurrence during the trial (both in the intervention group). These two participants were dropped from the analysis for scientific reasons. Two patients in the control group dropped out due to the lack of time. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Unclear risk | High risk | Unclear risk | Unclear risk | Low risk | Low risk | Hutnick 2005 |
| Support for judgment | patients with breast cancer were assigned to intervention and control groups. | 36 patients with breast cancer were enrolled in a 6-month moderate exercise program very soon after completing chemotherapy or radiation treatment to the intervention and control group. | Participants were not blinding | Information concerning the blinding of the assessor was not provided. | Seven patients in the intervention group dropped out. Six patients in the control group dropped out after the midpoint of the study. However, the reasons for this were not explained. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Battaglini 2007 |
| Support for judgment | The participants were randomly assigned to exercise or control group | The patients had to choose a number between 1 and 20 for the allocation process. Even-numbered participants were assigned to the experimental group, whereas odd-numbered participants were assigned to the control group | Participants were blinding | The assessor was not blinding. | All the participants completed the study. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Mutrie 2007 |
| Support for judgment | The study was a two-group (intervention and control) by three time points (baseline, 12 weeks, and six-month follow-up) randomized controlled trial. The randomization was stratified by hospital and treatment at baseline and used randomized permuted blocks of length four and six (that is, for sequences of four or six women in each hospital-treatment combination, exactly half were allocated to each group). | After written informed consent and baseline measures, participants were randomly allocated women into one of two groups using randomization list. | Blinding of the participants was not possible | The assessors were blinding | 14 patients in the intervention group did not complete the study: Seven patients were lost to follow-up, four patients did not return the questionnaire, one patient was too ill, and two patients were not contactable. Eight patients in the control group did not complete the study: Four patients were lost to follow-up, two patients did not return the questionnaire, one died, and one withdrew. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Nieman 1995 |
| Support for judgment | Sixteen female breast cancer patients between the ages of 35 and 72 years were recruited for the study | After written informed consent and baseline measures, Subjects were randomly assigned to either an exercise or control group. | The participants were blinding | Information concerning the blinding of the assessor was not provided | Two patients (one from each group did not complete the study, because of the recurrence of the disease. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Ligibel 2008 |
| Support for judgment | 101 sedentary, overweight breast cancer survivors were randomly assigned either to exercise intervention or to a usual care control group | After enrolment, participants were randomly assigned 1:1 to an exercise intervention group or control group | The participants were blinding | Participants underwent a series of anthropometric measurements at the time of study enrolment (baseline), and these were repeated after the 16-week study period by study staff who were not blinded to group assignment | 11 patients in the intervention group did not complete the study for the following reasons: Two lost to follow-up, one had a family emergency, three had too much of a time commitment, one was too ill for final, one had a disease recurrence, one developed unrelated cancer, one withdrew consent, and one need for unrelated surgery. Seven patients in the control group did not complete the study for the following reasons: Three lost to follow-up, two had disease recurrence, one withdrew upon assignment to the control group, and one for family problems. However, intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Ligibel 2008 |
| Support for judgment | 101 sedentary, overweight breast cancer survivors were randomly assigned either to exercise intervention or to a usual care control group | After enrolment, participants were randomly assigned 1:1 to an exercise intervention group or control group | The participants were blinding | Participants underwent a series of anthropometric measurements at the time of study enrolment (baseline), and these were repeated after the 16-week study period by study staff who were not blinded to group assignment | 11 patients in the intervention group did not complete the study for the following reasons: Two lost to follow-up, one had a family emergency, three had too much of a time commitment, one was too ill for final, one disease recurrence, one developed unrelated cancer, one withdrew consent, and one need for unrelated surgery. Seven patients in the control group did not complete the study for the following reasons: Three lost to follow-up, two disease recurrence, one withdrew upon assignment to the control group, and one for family problems. However, intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Ligibel 2019 |
| Support for judgment | Participants were randomized 1:1 to an exercise and control group. All participants were randomized through the Quality Assurance for Clinical Trials (QACT) Core at Dana-Farber, which served as the coordinating center of the study. | After baseline measures 48 participants were randomly assigned to an exercise intervention group or control group | The participants were blinding | The assessor was blinding | One patient in the intervention group was found to be ineligible after randomization and two patients in the control group withdrew consent after randomization. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Jones 2020 |
| Support for judgment | A quasi-randomized design where each group comprised exercising or non-exercising breast cancer survivors of similar age and treatment characteristics | A simple randomization procedure using the number on entry to allocate women as they entered the trial, on a 1:1 basis, was undertaken. Breast cancer survivors with odd numbers began the 12-week exercise treatment protocol immediately, while even-numbered women acted as wait-listed, non- exercising controls. The control women could access the intervention protocol after their testing in week 13. None of the authors were involved in the recruitment of the participants. | The participants were blinding | The assessor was blinding | Three control participants did not complete data collection, one incurred an ankle injury (not intervention-related), one moved to another town, and one chose to withdraw before any baseline measures. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Lee 2019 |
| Support for judgment | This randomized clinical trial compared an aerobic and resistance exercise intervention with usual care. | For each patient recruited into the study, written informed consent is obtained before performing randomization or outcome measure testing. Upon written informed consent, the patient is randomly allocated to either the Exercise or Control groups. To prevent possible bias, study personnel involved in the recruitment and allocation did not have access to the randomization lists | Information concerning the blinding of the participants was not provided. | Information concerning the blinding of the assessor was not provided | Three patients in the intervention group did not complete the study for the following reasons: Two had Work conflicts, and one had disease progression. Three participants in the control group lost to follow-up post-intervention for the reasons: one family emergency, and two unreachable. Intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Saxton 2014 |
| Support for judgment | A total of 85 women treated for breast cancer 3 to 18 months were randomly allocated to a 6-month to exercise and control group. Randomization was performed by an independent researcher at the Clinical Trials Research Unit, University of Leeds | A total of 85 women treated for breast cancer 3 to 18 months were randomly allocated to a 6-month to exercise and control group. Randomization was performed by an independent researcher at the Clinical Trials Research Unit, University of Leeds. The randomization sequence and allocation were not disclosed until patients had completed their baseline assessments | The participants were blinding | All the outcomes’ measurers were assessed by a trained technician who was blinded to the group allocation | Three women were dropped out due to the lost to follow up in each group. Intention-to-treat analysis was used. However, intention-to-treat analysis was applied | Expected outcomes were reported | Other biases have not been identified | |
| Authors’ judgment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Scott 2013 | |
| Support for judgment | Following the assessment of outcome variables at baseline, patients were randomly allocated (1:1 ratio) to one of two groups: (1) intervention or (2) control group. Randomization was performed by an independent researcher at the Clinical Trials Research Unit, University of Leeds. The randomization sequence was not disclosed until patients had completed their baseline assessments. | A total of 90 women were randomly allocated to intervention and control group. The randomization sequence was not disclosed until patients had completed their baseline assessments. | The participants were blinding. | The assessor was blinding | Six patients in the intervention group did not participate in the follow-up for the following reasons: three patients changed their minds at the beginning, one patient due to a change in work conditions, one had a family situation, and one because of loss of contact. Five patients in the control group lost to follow-up for the following reasons: one withdrew at the beginning, two were excluded on medical grounds, and two could not contact with. Intention-to-treat analysis was applied. | Expected outcomes were reported | Other biases have not been identified |
Data analysis
We performed the meta-analyses using Review Manager 5.4 software (Cochrane Collaboration, https://revman.cochrane.org/info) and reported the results of the random-effects model. Thresholds for the interpretation of the I2 statistic can be misleading since the importance of inconsistency depends on several factors. We used the guide to the interpretation of heterogeneity as outlined: 0–40% might not be important; 30-60% may represent moderate heterogeneity; 50-90% may represent substantial heterogeneity; and 75-100% would be considerable heterogeneity (Higgins and Green, 2008). Mean differences (MD) or standardized mean differences (SMD) and 95% confidence intervals (CI) were applied to calculate the effect size. MD was used when the outcome measures were comparable across studies and were measured on the same scale, while SMD was used when the outcome measures were measured on different scales or were using different units across studies. The preference between MD and SMD was dependent on the nature of the outcome measure and the heterogeneity of the included studies.
The choice between fixed effects and random effects models depends on the assumption about the homogeneity of the study populations and protocols. The fixed effects model assumes one study population and protocol, generalizable to them; while the random effects model assumes various populations and protocols, generalizable to the entire universe of studies. In this study, we did not use the fixed effects model because it includes studies from various populations, making the results generalizable to the entire universe of studies. A two-sided p < 0.05 was considered to indicate statistical significance.
Grading quality of evidence
Two authors (S.B.A.L. and A.B.) independently assessed the quality of evidence for primary and secondary outcomes according to GRADE methodology (GRADEpro, 2014) for risk of bias, inconsistency, indirectness, imprecision, and publication bias; classified as very low, low, moderate, or high. In cases of conflicts or uncertainties, a third author (N.H.A.) was involved in the resolution. Evidence may be upgraded based on factors such as large effect size, dose-response gradient, or when all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect. Downgrading factors may include risk of bias, inconsistency of results, indirectness of evidence, imprecision, and publication bias (Table 2).
Table 2.
Summary of quality assessment findings (GRADE).
| Outcome | Certainty assessment | № of patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CART | ST | p-value | Absolute (95% CI) | ||
| BW (kg) | 5 | RCT | not serious | not serious | not serious | seriousb | none | 231 | 224 | 0.95 | SMD 0.04 lower (1.23 lower to 1.15 higher) |
⊕⊕⊕◯ Moderate |
| BMI (kg/m2) | 6 | RCT | seriouse | seriousc | not serious | seriousb | none | 213 | 216 | 0.04 | SMD 0.57 lower (1.12 lower to 0.02 lower) |
⊕◯◯◯ Very low |
| BMI (≤16 wks) | 4 | RCT | seriouse | not serious | not serious | seriousb | none | 179 | 189 | 0.04 | SMD 0.21 lower (0.42 lower to 0) |
⊕⊕◯◯ Low |
| BMI (>16 wks) | 2 | RCT | seriouse | very seriousa | not serious | seriousb | none | 34 | 27 | 0.27 | SMD 2.49 lower (6.91 lower to 1.93 higher) |
⊕◯◯◯ Very low |
| HC (cm) | 2 | RCT | seriousd | not serious | not serious | seriousb | none | 97 | 94 | 0.02 | MD 3.14 lower (4.77 lower to 1.52 lower) |
⊕⊕◯◯ Low |
| WC (cm) | 2 | RCT | seriousd | not serious | not serious | seriousb | none | 97 | 94 | 0.19 | MD 3.63 lower (9.02 lower to 1.77 higher) |
⊕⊕◯◯ Low |
| WHR | 2 | RCT | seriousd | seriousc | not serious | seriousb | none | 60 | 62 | 0.34 | MD 0.05 lower (0.15 lower to 0.05 higher) |
⊕◯◯◯ Very low |
| BF (%) | 6 | RCT | seriousd | seriousc | not serious | seriousb | none | 157 | 148 | 0.02 | SMD 0.5 lower (0.93 lower to 0.07 lower) |
⊕◯◯◯ Very low |
| FM (kg) | 4 | RCT | not serious | seriousc | not serious | seriousb | none | 174 | 173 | 0.04 | SMD 0.76 lower (1.44 lower to 0.08 lower) |
⊕⊕◯◯ Low |
| FFM (kg) | 2 | RCT | seriousd | not serious | not serious | seriousb | none | 56 | 55 | <0.01 | SMD 1.03 higher (0.63 higher to 1.43 higher) |
⊕⊕◯◯ Low |
| TC (mmol/L) | 2 | RCT | not serious | not serious | not serious | seriousb | none | 87 | 83 | <0.01 | SMD 0.95 lower (1.37 lower to 0.52 lower) |
⊕⊕⊕◯ Moderate |
| HDL-C (mmol/L) | 3 | RCT | not serious | very seriousa | not serious | seriousb | none | 133 | 128 | 0.008 | MD 8 higher (2.05 higher to 13.94 higher) |
⊕◯◯◯ Very low |
| LDL-C (mmol/L) | 2 | RCT | not serious | very seriousa | not serious | seriousb | none | 56 | 55 | <0.01 | SMD 1.4 lower (5.22 lower to 2.41 higher) |
⊕◯◯◯ Very low |
| TG (mg/Dl) | 1 | RCT | not seriousa | very seriousa | not serious | seriousb | none | 46 | 45 | <0.01 | MD 81.9 lower (91.21 lower to 72.59 lower) |
⊕◯◯◯ Very low |
| IL-6 (pg/mL) | 6 | RCT | not serious | not serious | not serious | seriousb | none | 177 | 163 | 0.33 | SMD 0.11 lower (0.32 lower to 0.11 higher) |
⊕⊕⊕◯ Moderate |
| IL-8 (pg/mL) | 3 | RCT | not serious | very seriousa | not serious | seriousb | none | 86 | 85 | 0.20 | SMD 1.32 lower (3.33 lower to 0.7 higher) |
⊕◯◯◯ Very low |
| TNF-α (pg/mL) | 4 | RCT | not serious | seriousc | not serious | seriousb | none | 130 | 126 | 0.03 | SMD 0.89 lower (1.7 lower to 0.08 lower) |
⊕⊕◯◯ Low |
| CRP (mg/dL) | 3 | RCT | not serious | very seriousa | not serious | seriousb | none | 113 | 105 | 0.23 | SMD 1.69 lower (4.42 lower to 1.05 higher) |
⊕◯◯◯ Very low |
| ADPN (ug/mL) | 2 | RCT | seriousd | very seriousa | not serious | seriousb | none | 61 | 59 | 0.36 | MD 5.06 higher (5.82 lower to 15.93 higher) |
⊕◯◯◯ Very low |
| LEP (ng/mL) | 4 | RCT | not serious | seriousc | not serious | seriousb | none | 122 | 114 | 0.03 | SMD 0.63 lower (1.2 lower to 0.06 lower) |
⊕⊕◯◯ Low |
| NK cells (%) | 2 | RCT | not serious | not serious | not serious | seriousb | none | 50 | 47 | 0.04 | SMD 0.42 higher (0.01 higher to 0.82 higher) |
⊕⊕⊕◯ Moderate |
| FA (score) | 4 | RCT | seriouse | very seriousa | not serious | seriousb | none | 168 | 180 | 0.03 | SMD 0.98 lower (1.85 lower to 0.11 lower) |
⊕◯◯◯ Very low |
| SQ (score) | 2 | RCT | not serious | seriousf | not serious | seriousb | none | 70 | 72 | 0.01 | SMD 1.17 lower (1.76 lower to 0.57 lower) |
⊕⊕◯◯ Low |
| QoL (score) | 4 | RCT | seriouse | very seriousa | not serious | seriousb | none | 174 | 182 | 0.02 | SMD 2.94 higher (0.46 higher to 5.41 higher) |
⊕◯◯◯ Very low |
ADPN: Adiponectin, AE: Aerobic Exercise, BC: Breast Cancer, BF: Body Fat, BM: Body Weight, BMI: Body Mass Index, BMD: Bone Mineral Density, C: Control Group, CART: Combined Aerobic and Resistance Training, CI: confidence intervals, CRF: Cardiorespiratory Fitness, CRP: C-Reactive Protein, DBP: Diastolic Blood Pressure, DEP: Depression, EX: Exercise Group, FA: Cancer-related Fatigue, FFM: Fat-Free Mass, FG: Fasting Glucose, FI: Fasting Insulin, FM: Fat Mass, HC: hip circumference, HDL-C: High-Density Lipoprotein Cholesterol, HRmax: Maximum Heart Rate, HRR: Heart Rate Reserve, IL: Interleukin, LEP: Leptin, LDL: Low-Density Lipoprotein Cholesterol, MD: mean difference, NK: Natural Killer, RCT: randomized control trial, RT: Resistance Training, SBP: Systolic Blood Pressure, SMD: standardized mean difference, ST: standard treatment, SQ: Sleep Quality, TC: Total Cholesterol, TG: Triglycerides, TNF-α: Tumor Necrosis Factor α, QoL: Quality of Life, WC: Waist circumference, WHR: Waist-to-Hip Ratio, 1-RM: Repetition Maximum, a: there is considerable heterogeneity in the study's outcome, b: the included studies recorded a small sample size for both the control and intervention group, c: there is substantial heterogeneity in the studies, d: The assessor was not blinding, e: participants were aware of all exercise procedures, f: there is moderate heterogeneity in the involved studies.
Subgroup analysis
We conducted a subgroup analysis when the I2 statistic was more than 50%. Subgroup analysis was done on the duration of the intervention on the studies investigated the effect of CART on BMI, whether the studies were conducted for ≤16 weeks or >16 weeks.
Sensitivity analysis
We performed a sensitivity analysis to investigate the impact of the risk of bias for performance bias and detection bias.
Results
Literature search and selection
From the specified databases, PubMed, Web of Science, Scopus, Science Direct, Cochrane Library, and Google Scholar (Figure 1), a total of 21,751 studies were obtained. After removing duplicate articles, the number of studies eligible for further evaluation was reduced to 20,684. Through a review of the titles and abstracts based on predetermined inclusion and exclusion criteria, 20,643 studies were excluded. Subsequently, the full text of the remaining 41 articles was carefully examined, excluding 18 articles with reasons (Table S3). Therefore, 23 records were included in this study. However, six records were subsequent studies of eligible trials included in this review (Table S4). Hence, 17 studies (RCTs) were finally included in this review, and data were extracted from 1,148 patients who met the eligibility criteria.
Figure 1.
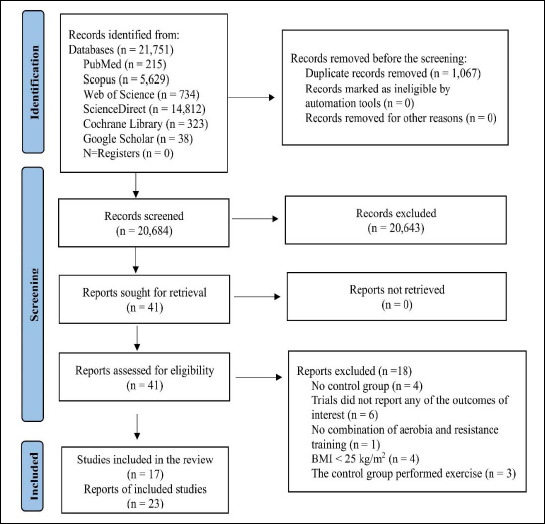
PRISMA flow diagram for the recruiting studies.
Table S3.
Excluded full-text articles with reasons (n=18).
| # | Study | Reason |
|---|---|---|
| 1 2 3 4 5 6 |
Milne et al., 2008a Fernandez-Lao et al., 2012 Sweeney et al., 2012 Bloomquist et al., 2019 Schmitz et al., 2019 May et al., 2008 |
No related outcome measures |
| 7 | Schwartz et al., 2007 | No CART (AT or RT alone) |
| 8 9 10 11 |
Bloomquist et al., 2019 Travier et al., 2014 de Jesus Leite et al., 2021 Courneya et al., 2014a |
No control group |
| 12 13 14 15 |
Milne et al., 2008b Harvie Kim Gómez |
BMI < 25 kg/m2 |
| 16 17 18 |
De Paulo et al., 2018 Paulo et al., 2019 Courneya et al., 2014b |
The control group performed structured exercise |
BMI: body mass index, CART: combined aerobic and resistance training, AT: aerobic training, RT: resistance training.
Table S4.
The subsequent reports of original studies (n =6).
| # | Original Studies | Reports of original studies | Reason for inclusion (reported parameters) |
|---|---|---|---|
| 1 2 3 |
Dieli-Conwright et al., 2018c | Dieli-Conwright et al., 2018a Dieli-Conwright et al., 2018b Dieli-Conwright et al., 2021 |
Cancer-related fatigue Sleep quality Body fat, ADPN, IL-8, and LDL-C |
| 4 | Dieli-Conwright et al., 2019 | Dieli-Conwright et al., 2021 | Quality of life |
| 5 | Ligibel et al., 2008 | Ligibel et al., 2009 | Body fat, hip circumference, LEP, ADPN. |
| 6 | Rogers et al., 2014 | Rogers et al., 2015 | Sleep quality |
ADPN: adiponectin, IL: interleukin, LEP, leptin, LDL: low-density lipoprotein cholesterol
Literature characteristics
Sixteen out of the 17 RCTs were from high-income countries (Battaglini et al., 2007; Brown et al., 2021; Dieli-Conwright et al., 2018; Dieli-Conwright et al., 2022; Dieli-Conwright et al., 2019; Hutnick et al., 2005; Jones et al., 2020; Lee et al., 2019; Ligibel et al., 2008; Ligibel et al., 2019; Mutrie et al., 2007; Nieman et al., 1995; Rogers et al., 2013; Rogers et al., 2014; Saxton et al., 2014; Scott et al., 2013), and one trial from upper-middle income country (Ergun et al., 2013). Thirteen out of the 17 trials recruited their respondents from hospital settings (Battaglini et al., 2007; Dieli-Conwright et al., 2018; Dieli-Conwright et al., 2022; Dieli-Conwright et al., 2019; Ergun et al., 2013; Lee et al., 2019; Ligibel et al., 2008; Ligibel et al., 2019; Mutrie et al., 2007; Rogers et al., 2013; Rogers et al., 2014; Saxton et al., 2014; Scott et al., 2013). Participants in one trial were recruited using a variety of active and passive outreach methods (Brown et al., 2021). In two trials, participants were recruited through advertisements placed in local newspapers (Hutnick et al., 2005; Jones et al., 2020). Meanwhile, in one trial, information regarding the recruitment of participants was not provided (Nieman et al., 1995). Eight out of the 17 trials performed the exercise intervention at health care sites (Battaglini et al., 2007; Dieli-Conwright et al., 2018; Dieli-Conwright et al., 2019; Ergun et al., 2013; Jones et al., 2020; Lee et al., 2019; Saxton et al., 2014; Scott et al., 2013), while nine trials conducted the exercise intervention at both a healthcare site and participants' home (Brown et al., 2021; Dieli-Conwright et al., 2022; Hutnick et al., 2005; Ligibel et al., 2008; Ligibel et al., 2019; Mutrie et al., 2007; Nieman et al., 1995; Rogers et al., 2013; Rogers et al., 2014). The length of the exercise intervention ranged from 8 to 52 weeks (8–12 weeks: 35%; 16–52 weeks: 65%). Table 1 shows the characteristics of the included trials.
Table 1.
Characteristics of included studies.
| References | Patients’ Status/Treatment Stage | Sample Size | Age (yrs)/Country | CART Intervention (training parameters) | Length (wks) | Setting/Cancer Stage | BMI ± SD (kg/m2) | Outcome Measures |
|---|---|---|---|---|---|---|---|---|
| (Dieli-Conwright et al., 2018) | BC survivors After treatment |
N: 91 EX: 46 C: 45 |
EX: 52.8 ± 10.6 CO: 53.6 ± 10.1 United States |
AE: 2–3 d/wk, 150 min, 40%–50% HRR; RT: 3 d/wk, 40%–50% 1-RM |
16 | Supervised/Stage: I, II, III | EX: 33.1 ± 5.7 C: 33.4 ± 5.2 |
HDL-C, TC, TG, CRP, TNF-α, IL-6, LEP, BM, BF, FM, FFM, WC, HC, QoL, FA |
| (Dieli-Conwright et al., 2018) Cont. | BC survivors After treatment |
N: 20 EX: 10 C: 10 |
EX: 53.0 ± 10.0 CO: 55.0 ± 4.5 United States |
Supervised/Stage: I, III | EX: 33.5 ± 5.7 C: 33.3 ± 8.7 |
BF, ADPN, IL-8, LDL | ||
| (Dieli-Conwright et al., 2018) Cont. | BC survivors After treatment |
N: 100 EX: 50 C: 50 |
Total: 52 ± 10.4 United States |
AE: 2 d/wk, 80 min, 65%–80% HRmax; RT: 2–3 d/wk, 60% 1-RM |
16 | Supervised/Stage: 0, III | Total BMI: 33.5 ± 5.5 |
SQ |
| (Dieli-Conwright et al., 2019) | BC survivors After treatment |
N: 56 EX: 29 C: 27 |
EX: 46.9 ± 10.2 C: 46.7 ± 10.0 United States |
AE: 2–3 d/wk, 150 min, 40% –50% HRR; RT: 2–3 d/wk, 80% 1-RM | 16 | Supervised/Stage: I, II, III | EX: 35.1 ± 6.1 C: 34.7 ± 6.4 |
BM, WC, HC, BF, FM, FFM, SBP, DBP, FG, FI, TC, HDL-C, LDL-C, TG, CRP, QoL |
| (Dieli-Conwright et al., 2022) | BC survivors After treatment |
N: 25 EX: 13 C: 12 |
EX: 59.5 ± 6.5 C: 54.1 ± 10.6 United States |
AE+RT, 150 m/wk plus home-based AE 970 min/wk) |
24 | Supervised & home-based/Stage: I, III | EX: 29.5 ± 3.6 C: 36.4 ± 6.1 |
BMI, LEP |
| (Brown et al., 2021) | BC survivors After treatment |
N: 177 EX: 87 C: 90 |
EX: 59.1 ± 8.1 C: 59.0 ± 8.5 United States |
AE: 3–6 d/wk, 180 min/wk, 50%–70% HRmax; RT: 2 d/wk, 2–3 sets x 10 reps. |
52 | Supervised & home-based/Stage: I, III | EX: 34.0 ± 6.2 C: 34.0 ± 5.7 |
BM, FM, BMD |
| (Ergun et al., 2013) | BC survivors After treatment |
N: 40 EX: 20 C: 20 |
EX: 49.7 ± 8.35 C: 50.3 ± 10.4 Turkey |
AE + RT: for 45 min/D for 3d/wk and brisk walking for 30 min/D for 3 d/wk |
12 | Supervised/Stage: N/A | EX: 26.6 ± 4.4 C: 28.6 ± 5.2 |
FA, IL-6, IL-8, TNF-α, QoL, DEP |
| (Rogers et al., 2013) | BC survivors After treatment |
N: 28 EX: 15 C: 13 |
EX: 58.0 ± 6.1 C: 53.7 ± 13.9 United States |
AE: 150 min/wk; RT: 2 d/wk, 60%–70% 1-RM |
12 | Supervised & home-based/Stage: I, II, III | EX: 33.9 ± 7.4 C: 30.3 ±7.11 |
BMI, BF, FM, WHR, FA, IL-1β, IL-6, IL-8, IL-10, TNF-α, LEP |
| (Rogers et al., 2014) | BC survivors After treatment |
N: 42 EX: 20 C: 22 |
EX: 57.2 ± 5.5 C: 55.2 ± 9.1 United States |
AE: 160 min/wk; RT: 2 d/wk, 60%–70% 1-RM |
12 | Supervised & home-based/Stage: I, III | EX: 29.8 ± 4.8 C: 32.6 ± 6.6 |
FA, BMI, BF, IL-6, IL-8, IL-10, TNF-α, SQ |
| (Hutnick et al., 2005) | BC survivors After treatment |
N: 36 EX: 21 C: 15 |
EX: 48.5 ± 10.6 C: 52.3 ± 9.2 United States |
AE+RT (40–90 min), 3 d/wk, 60%–75% HRmax/1-RM |
24 | Supervised & home-based/Stage: I, II, III | EX: 26.7 ± 5.45 C: 26.7 ± 4.5 |
BM, BMI, BF, CRF, IL-6 |
| (Battaglini et al., 2007) | BC patients During treatment |
N: 20 EX:10 C: 10 |
EX 57.5 ± 23.0 C: 56.6 ± 16.0 United States |
AE+RT (60 min), 2 d/wk, 40%–60% HRR/1-RM |
21 | Supervised/Stage: N/A | EX: 77.5 ± 27.3 C: 82.2 ± 25.0 |
FFM, Muscular Strength |
| (Mutrie et al., 2007) | BC patients. During treatment |
N: 181 EX: 82 C: 95 |
EX: 51.3 ± 10.3 C: 51.8 ± 8.7 United Kingdom |
AE+RT (45 min), 2 d/wk, 50%–75% HRR/1-RM |
12 | Supervised & home-based/Stage: 0, III | EX: 27.3 ± 5.2 C: 27.5 ± 6.0 |
BMI, QoL, FA, DEP, Physical Function |
| (Nieman et al., 1995) | BC survivors After treatment |
N: 12 EX: 6 C: 6 |
EX: 60.8 ± 4.0 C: 51.2 ± 4.7 United States |
AE+RT, (60 min), 3 d/wk, 75% HRR/1-RM |
8 | Supervised / Stage: N/A | EX: 67.6 ± 3.7 C: 75.5 ± 9.8 |
CRF |
| (Ligibel et al., 2008) | BC survivors After treatment |
N: 82 EX: 40 C: 42 |
EX: 52.0 ± 9.0 C: 53.0 ± 9.0 United States |
AE: 90 min/wk, 2 d/wk; RT: 50 min/wk, 2 d/wk |
16 | Supervised & home-based/Stage: I, II, III | EX: 30.3 ± 5.9 C: 31.4 ± 6.8 |
BM, BMI, WC, HC, WHR, BF, LEP, ADPN |
| (Ligibel et al., 2019) | BC patients Before treatment |
N: 48 EX: 26 C: 22 |
EX: 52.3 ± 9.6 C: 53.1 ± 7.9 United States |
AE: 180 min/wk; 60%–70% HRmax; RT: 40 min/wk, 60%–70% 1-RM |
16 | Supervised & home-based/Stage: I, II, III | EX: 30.7 ± 6.1 C: 29.1 ± 7.4 |
LEP, CRP, IL-6 |
| (Jones et al., 2020) | BC survivors After treatment |
N: 51 EX: 26 C: 25 |
EX: 55.8 ± 7.2 C: 55.9 ± 7.1 New Zealand |
AE+RT (60 min), 2 d/wk | 12 | Supervised/Stage: I, II, III | EX: 27.8 ± 5.5 C: 27.5 ± 4.8 |
BM, BMI, FM, BF, SBP, DBP, CRF |
| (Lee et al., 2019) | BC survivors After treatment |
N: 91 EX: 46 C: 45 |
Total: 53.5 ±10.4 United States |
AE: ≥150 min, 2–3 d/wk, 65%–80% HRmax; RT: 2–3 d/wk |
16 | Supervised/Stage: I, III | BM1 ≥25.0 or BF ≥30% |
TC, LDL-C, HDL-C, TG |
| (Saxton et al., 2014) | BC survivors After treatment |
N:85 EX: 44 C: 41 |
EX: 55.8 ± 10.0 C: 55.3 ± 8.8 United Kingdom |
AE: 30 min, 65%–85% HRmax, 3 d/wk; RT: 10–15 min, 2–3 d/wk |
24 | Supervised/Stage I, III | EX: 29.7±3.5 C: 31.1 ± 5.7 |
IL-6, TNF-α, NK cell |
| (Scott et al., 2013) | BC survivors After treatment |
N: 83 EX: 43 C: 40 |
EX: 55.6 ± 10.2 C: 55.9 ± 8.9 United Kingdom |
AE: 30 min, 65%–85% HRmax, 3 d/wk; RT: 10–15 min, 2–3 d/wk |
24 | Supervised/Stage: I, III | EX: 29.6 ± 3.5 C: 31.1 ± 5.6 |
CRF, SBP, DBP, FG, CRP, TC, HDL-C, LDL-C, QoL, LEP |
ADPN: Adiponectin, AE: Aerobic Exercise, BC: Breast Cancer, BF: Body Fat, BM: Body Mass, BMI: Body Mass Index, BMD: Bone Mineral Density, C: Control Group, CART: Combined Aerobic and Resistance Training, CRF: Cardiorespiratory Fitness, CRP: C-Reactive Protein, DBP: Diastolic Blood Pressure, DEP: Depression, EX: Exercise Group, FA: Cancer-related Fatigue, FFM: Fat-Free Mass, FG: Fasting Glucose, FI: Fasting Insulin, FM: Fat Mass, HC: hip circumference, HDL-C: High-Density Lipoprotein Cholesterol, HRmax: Maximum Heart Rate, HRR: Heart Rate Reserve, IL: Interleukin, LEP: Leptin, LDL: Low-Density Lipoprotein Cholesterol, NK: Natural Killer, RT: Resistance Training, SBP: Systolic Blood Pressure, SQ: Sleep Quality, TC: Total Cholesterol, TG: Triglycerides, TNF-α: Tumor Necrosis Factor α, QoL: Quality of Life, WC: Waist circumference, WHR: Waist-to-Hip Ratio, 1-RM: Repetition Maximum.
Risk of bias assessments results
The summary of the risk of bias assessment is shown in Figure 2. Details of the risk of bias judgment per domain for each study are provided in Figure S1. In the majority of the trials across most domains, the risk of bias was low or unclear. There was no indication of selective reporting bias. The lack of sufficient random sequence generation in the original study might cause treatment effect bias in both the original studies and the following review findings. The risk of performance bias was presented in seven trials, which was unclear in three trials owing to a lack of details concerning the blinding of the participants, while in four trials the participants were not blinded and hence considered as a high-performance bias. Table S2 details the risk of bias assessment.
Figure 2.
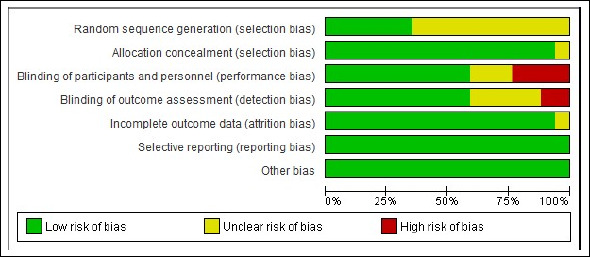
Summary of the risk of bias assessment.
Figure S1.
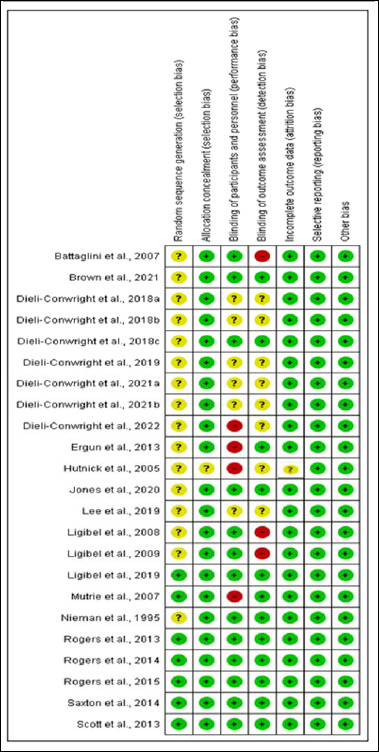
Risk of bias assessment results.
The certainty of findings for the outcomes ranged from very low to low, with the downgrading of evidence primarily due to several factors. These included small sample sizes in both control and intervention groups across the included studies, a high risk of bias, as well as moderate, considerable, and substantial heterogeneities. Table 2 details the summary of quality assessment findings.
Primary outcomes: Anthropometrics
BW and BMI were reported in five and six trials involving 455 and 429 participants, respectively. No difference was found in BW between CART and ST (Figure S2 and Table 2), while CART reduced BMI (SMD -0.57 kg/m2, 95% CI -1.12 to -0.02; I2 = 84%; p = 0.04) compared to ST, showing very low certainty. A subgroup analysis showed that short- [≤16 weeks; (four studies, n = 368); SMD -0.21 kg/m2, 95% CI -0.42 to -0.00; I2 = 0%; p = 0.04], but not long-term [>16 weeks; (two studies, n = 61)] interventions exhibited more favorable effects on BMI than ST (Figure S3 and Table 2). Subgroup analysis is done for BMI. The substantial heterogeneity could not be explained by subgroup analysis according to the duration of the intervention. WC and HC were reported in two trials involving 191 patients. CART induced a significant improvement in HC (Figure S4 and Table 2), demonstrating low certainty (MD -3.14 cm, 95% CI -4.77 to -1.52; I2 = 0%; p = 0.02), but not in WC (Figure S5). WHR was reported in (two studies, n = 122) with no meaningful changes between CART and ST (Figure S6). There were no significant changes in the effect estimate after the sensitivity analysis for Mutrie et al. (2007) was done on the BW, BMI, FA, and QoL parameters. There were no significant changes in the effect estimate after the sensitivity analysis for Hutnick et al. (2005) was done on BW, BMI, BF, and IL-6. However, Sensitivity analysis for WHR outcome in Ligibel et al. (2008) shows changes in the effect estimate (MD - 1.10, 95% CI -0.16 to -0.04, I2 statistics = 0%, p = 0.02).
Figure S2.
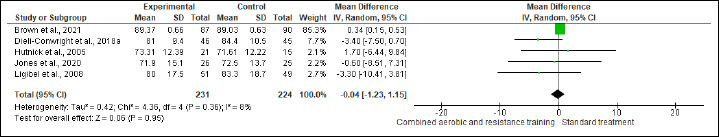
Effects of CART on BM.
Figure S3.
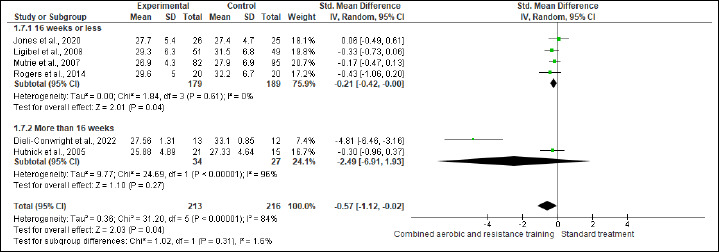
Effects of CART on BMI.
Figure S4.
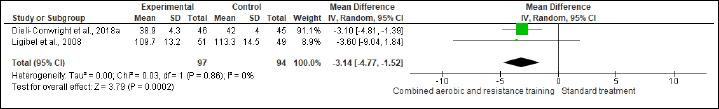
Effects of CART on HC.
Figure S5.
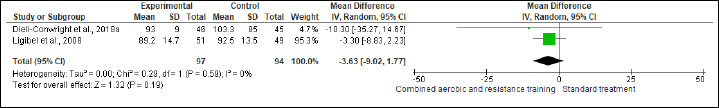
Effects of CART on WC.
Figure S6.

Effects of CART on WHR.
Body composition
BF, FM, and FFM were assessed in (six studies, n=305), (four studies, n = 347), and (two studies, n = 111) trials, respectively. CART induced favorable reductions in BF (SMD -0.50%, 95% CI -0.93 to -0.07; I2 = 68%; p = 0.02; very low certainty) (Figure S7 and Table 2) and FM (SMD -0.63 kg, 95% CI -1.23 to -0.04; I2=83%; p = 0.04; low certainty) compared to ST (Figure S8 and Table 2). In FFM, ST showed a greater increase (SMD 1.03 kg, 95% CI 0.63 to 1.43; I2 = 0%; p < 0.001; low certainty) than CART (Figure S9 and Table 2). There were no significant changes in the effect estimate after the sensitivity analysis for Battaglini et al. (2007) was done on FFM.
Figure S7.
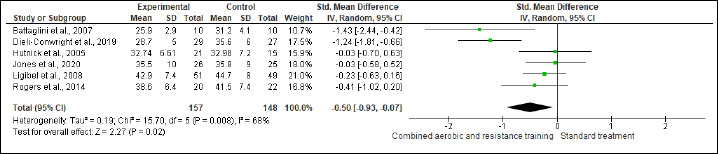
Effects of CART on BF.
Figure S8.
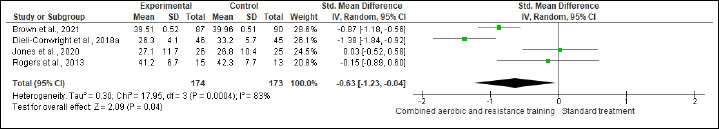
Effects of CART on FM.
Figure S9.

Effects of CART on FFM.
Secondary outcomes: Lipid metabolism
TC, HDL-C, LDL-C, and TG were evaluated in (two studies, n = 170), (three studies, n = 261), (two studies, n = 111), and (one study, n = 91) trials, respectively. CART exhibited significant improvements in TC (SMD -0.95 mmol/L, 95% CI -1.37 to -0.52; I2 = 43%; P < 0.01; moderate certainty), HDL-C (MD -0.05 mmol/L, 95% CI -0.15 to 0.04; I2 = 99%; p = 0.008; very low certainty) (Figure S10 and Table 2) and TG (MD -81.90 mg/dL, 95% CI -91.21 to -72.59; p < 0.01; very low certainty ) (Figure S11 and Table 2), but not in LDL-C (Figure S12 and Table 2).
Figure S10.
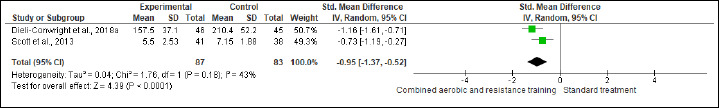
Effects of CART on TC.
Figure S11.
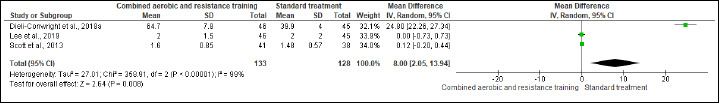
Effects of CART on HDL-C.
Figure S12.
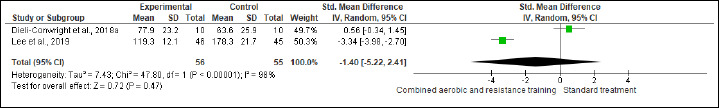
Effects of CART on LDL-C.
Inflammation
IL-6, IL-8, TNF-α, and CRP were investigated in (six studies, n = 340), (three studies, n = 171), (four, n = 256), and (three, n = 218) trials, respectively. No significant changes were found in IL-6, IL-8, and CRP between CART and ST, but CART showed a greater reduction in TNF-α (SMD -0.89 pg/mL, 95% CI -1.70 to -0.08; I2 = 89%; p = 0.03; low certainty) than ST (Figures S13-S16 and Table 2). There were no significant changes in the effect estimate after the sensitivity analysis for Ergun et al. (2013) was done on IL-6, IL-8, and TNF-α. There were no significant changes in the effect estimate after the sensitivity analysis for Ligibel et al. (2008) was done on IL-6, IL-8, TNF-α, CRP, WC, BF,HC, BW and LEP.
Figure S13.
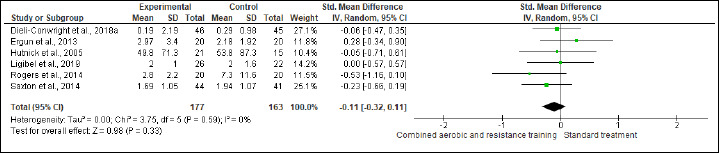
Effects of CART on IL-6.
Figure S14.
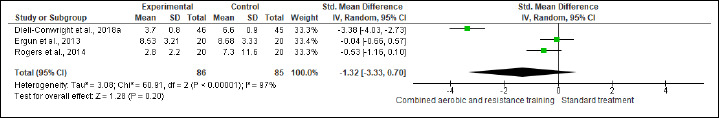
Effect of CART on IL-8.
Figure S15.
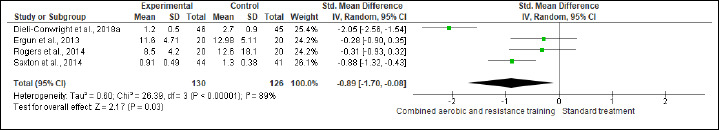
Effects of CART on TNF-α.
Figure S16.
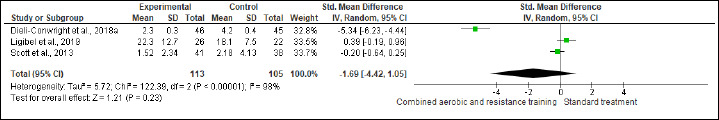
Effects of CART on CRP.
Adipokines
ADPN and LEP were reported in (two studies, n = 120) and (four studies, n=236) trials, respectively. CART induced beneficial alterations in LEP (SMD -0.63 ng/mL, 95% CI -1.20 to -0.06; I2 = 74%; p = 0.03; low certainty), but not in ADPN compared to ST (Figures S17-S18 and Table 2). There were no significant changes in the effect estimate after the sensitivity analysis for Dieli-Conwright et al. (2022) was done on LEP.
Figure S17.

Effect of CART on ADPN.
Figure S18.

Effect of CART on LEP.
Cancer-related indicators
NK cells, FA, SQ, and QoL were assessed in (two studies, n = 97), (four studies, n = 348), (four studies, n = 348) and (four studies, n = 356), respectively. CART more favorable changes in NK cells (SMD 0.42%, 95% CI 0.01 to 0.82; I2 = 0%; p = 0.04; moderate certainty). Also, beneficial alterations were found in FA (SMD -0.98, 95% CI -1.85 to -0.11; I2 = 92%; p = 0.03; very low certainty), SQ (SMD -1.40, 95% CI -2.50 to -0.30; I2 = 87%; p = 0.01; low certainty), and QoL (SMD 2.94, 95% CI 0.46 to 5.41; I2 = 98%; p = 0.02; very low certainty) scores compared to ST (Figure S19-22 and Table 2).
Figure S19.

Effect of CART on NK cells.
Figure S20.
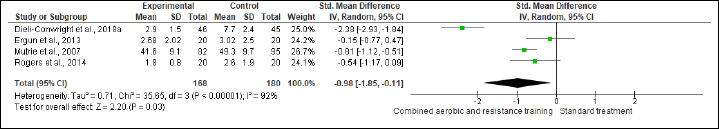
Effect of CART on cancer-related FA.
Figure S21.
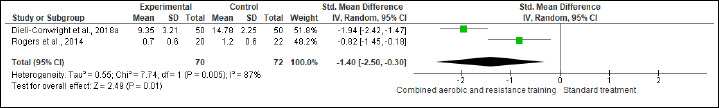
Effects of CART on SQ.
Figure S22.
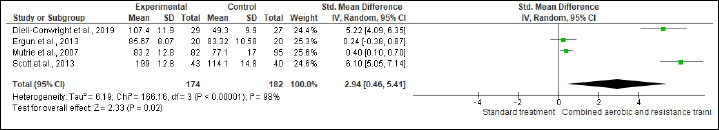
Effect of CART on QoL.
Discussion
In the present review, for the first time to the best of our knowledge, evidence about the efficacy of CART on cardiometabolic risk factors and cancer-related indicators is provided. The main findings reveal that CART induces beneficial alterations in anthropometric characteristics, body composition, lipid metabolism, inflammation, adipokines, and cancer-related outcomes, such as fatigue, sleep, and quality of life in BC patients with overweight/obesity. Given that aerobic and resistance training alone have been documented as effective exercise modalities for provoking positive results in cardiometabolic health-related outcomes among people with excessive weight (Batrakoulis et al., 2022) and/or cancer (Kudiarasu et al., 2023; Yang et al., 2023), our findings suggest that CART may be considered as an effective exercise solution for women with BC and concurrent overweight/obesity commonly present with impaired cardiometabolic health (Simon et al., 2021). However, the present results should be taken into account with caution due to the small number of studies with small participant numbers and low-GRADE ratings included in the current meta-analysis.
Anthropometrics and body composition
The present meta-analysis provides insights into the positive role of CART on specific anthropometric characteristics, such as BMI and HC, but not BM, WC, and WHR in women with BC and concurrent overweight/obesity. However, visceral fat was not assessed and this also is an important category of variables that should be examined further in the future, given that this type of fat adversely affects metabolic health and promotes comorbidity among BC patients and survivors with excessive weight (Anwar et al., 2021). Interestingly, CART as a component of a multimodal lifestyle intervention promotes weight loss in BC survivors (Lake et al., 2022), which is not aligned with our results, given that no meaningful change was reported in BM. However, since women with BC and concurrent excess weight prone to have abdominal obesity linked to glucose metabolism dysregulation (Moore and Shah, 2020), further investigation is needed in this area to determine whether CART alone can induce a significant reduction in BM.
Our results also show considerable improvements in critical body composition markers, such as BF, FM, and FFM, which are in line with CART-induced adaptations reported in people with obesity, but without cancer (Batrakoulis et al., 2022). This is a vital outcome, considering the key role of the obesity epidemic in BC recurrence among those who successfully completed treatment (Acevedo et al., 2022). Likewise, previous research showed similar effects of CART on various anthropometric and body composition indices among BC patients and survivors (Joaquim et al., 2022). Ultimately, exercise training is more beneficial than usual care for increasing FFM in women with BC, both during and after treatment (Fraser et al., 2022). Taking this into account, the present findings corroborate the current evidence underlining the vital role of regular exercise training, with particular emphasis on muscle strengthening activities, regarding the physiological and functional importance of FFM in this cohort (Fraser et al., 2022). However, further high-quality trials are warranted to identify whether CART can elicit favorable changes in visceral adiposity that is associated with lower morbidity and mortality risks (Mulligan et al., 2019).
Lipid metabolism
BC patients and survivors with concurrent overweight/obesity tend to demonstrate lipid metabolism impairments, enhancing the risk of developing CVD (Raychaudhuri et al., 2022). Thus, the relationship between lipid profile and obesity among BC survivors has been widely investigated (de Jesus et al., 2022; Okekunle et al., 2022; Vasseur and Guillaumond, 2022). According to the American Cancer Society guidelines, it is vital for cancer prevention and treatment to maintain a normal lipid profile, aiming to lower the likelihood of comorbidities (Rock et al., 2020). Importantly, the key role of lipid metabolism in promoting BC growth and progression has been documented, showing the strong relationship between obesity and BC (Blucher and Stadler, 2017). In this meta-analysis, CART elicited significant improvements in TC, HDL-C, and TG, but not in LDL-C. The present results support the current findings regarding the beneficial role of CART in improving blood lipids in BC patients (Kong and Gao, 2022). Such a remark cannot be explained here; however, the occurrence of obesity in conjunction with cancer may play some role in the simultaneous management of glucose and lipid homeostasis due to the presence of systemic inflammation (Roxburgh and McMillan, 2014). Noticeably, CART seems to be the optimal exercise solution for inducing favorable effects in lipid metabolism among people with BC and obesity (Kong and Gao, 2022), or obesity alone (Batrakoulis et al., 2022). Also, various traditional and alternative exercise modes appear effective for improving blood lipids in populations with impaired metabolic health and concurrent overweight/obesity (Al-Mhanna et al., 2023; Batrakoulis, 2022a; Batrakoulis, 2022b; Batrakoulis et al., 2021); however, additional studies examining these outcome measures among BC patients and survivors with overweight/obesity are necessary.
Inflammation
Obesity is associated with BC due to chronic adipose tissue inflammation, and thus BC patients with excess weight are likely to demonstrate high inflammatory markers (Kolb and Zhang, 2020). Interestingly, CART has been reported as an effective exercise training modality for improving the inflammatory profile in BC survivors (de Jesus Leite et al., 2018). In the present study, we detected significant CART-induced improvements in TNF-α, but not in IL-6, IL-8, and CRP. Such a substantial reduction in TNF-α is important, given that BC patients and survivors with overweight/obesity commonly present with several cardiometabolic health impairments linked to raised oxidative stress, diminished antioxidant capacity, and insulin dysregulation due to inflamed adipose tissue, altering the immune system (Roxburgh and McMillan, 2014; Simon et al., 2021). However, other inflammatory markers included in this meta-analysis did not exhibit significant changes following CART among BC patients and survivors with overweight/obesity. Hence, our results cannot provide clear evidence regarding the impact of CART on chronic inflammation in this cohort, and therefore further research in this area would be beneficial. Collectively, exercise training appears as a powerful non-pharmacological intervention for lowering circulating cytokines and inhibiting cancer recurrence in BC survivors (Zhou et al., 2022).
Adipokines
Considering that high circulating LEP levels increase the risk of tumor progression in cancer patients, while LEP resistance is common among individuals with obesity, exercise-induced reductions in LEP may be beneficial for BC patients and survivors with obesity (Perego et al., 2021). According to our results, CART showed a substantial reduction in LEP compared to ST. In general, data regarding the effects of CART on LEP among people with cancer and obesity are currently limited. However, a 6-month CART intervention improved various cardiometabolic health-related markers, including adipokines such as LEP and ADPN in middle-aged men with obesity (Brunelli et al., 2015). As for other exercise modes and their impact on LEP, conflicting results are present in the current literature regarding the effectiveness of long-term aerobic exercise. More specifically, aerobic exercise suggests dose-response favorable effects on LEP in premenopausal women at risk for BC (Sturgeon et al., 2016), but a 6-month aerobic-based training protocol did not exhibit a meaningful reduction in LEP among postmenopausal women at risk for BC (Khosravi et al., 2018). In summary, LEP has been documented as a vital mediator for the linkage between obesity and BC, promoting tumor initiation, development growth, and metastasis. That being said, future research on the potential role of CART interventions in circulating LEP levels may be necessary, since LEP is considered a key player in the novel therapeutic strategies for BC treatment (Atoum et al., 2020).
Given that ADPN is involved in glucose and energy homeostasis while being inversely associated with FM, individuals with obesity are commonly presented with low ADPN levels (Kadowaki and Yamauchi, 2005). Taking this into consideration, significant exercise-induced increases in ADPN levels may be critical for BC patients and survivors with a high BMI, since such favorable changes may improve body composition, affecting the risk of developing metastases (Perego et al., 2021). In this meta-analysis, CART did not suggest advantageous changes in ADPN relative to usual care. To date, relevant RCTs examining the role of ADPN in women with BC and obesity are scarce. However, a 4-week CART-like protocol showed positive alterations in ADPN among women with obesity, but without BC (Rejeki et al., 2023). Also, long-term aerobic exercise alone evokes a favorable elevation in ADPN levels while reducing BF in premenopausal women at risk for BC. Noticeably, these beneficial aerobic exercise-induced increases in ADPN were dependent on BF changes in this cohort (Sturgeon et al., 2016). Ultimately, ADPN is vital in both obesity and cancer, and thus exercise interventions suggesting significant increases in ADPN may considered as an important component of a multimodal treatment approach for BC patients and survivors with obesity (Perego et al., 2021).
Cancer-related indicators
Cancer-related FA has been reported as a very common side effect of BC caused by treatment affecting hormones related to fatigue and pain. Therefore, BC patients and survivors demonstrate a likelihood of developing various major mental health complications, such as anxiety, depression, and distress, resulting in reduced sleep and QoL (Cho and Hwang, 2021). Generally, there is a relationship among cancer-related FA, SQ, and QoL among BC patients and survivors. Interestingly, six in 10 of BC patients demonstrate major sleep problems linked to significant reductions in QoL and mood (Fortner et al., 2002). On the other hand, regular involvement in physical activity and exercise may help individuals with obesity improve all these health-related indicators (Bardwell and Ancoli-Israel, 2008; Mendelson et al., 2016). In the present review, CART exhibited significant improvements in cancer-related FA, SQ, and QoL relative to usual care, indicating that such a training modality may be a valuable adjunct therapy option in women with BC and excessive weight. Such beneficial alterations in critical cancer-related indices may also play an important role in adherence and behavioral regulation to exercise during and after treatment, since supervised exercise delivered in a real-world clinical setting should be a priority for patients, clinicians, and practitioners (Kirkham et al., 2018). However, it has been well documented that people with obesity are very likely to show mental health impairments due to body dissatisfaction (Gilyana et al., 2023) linked to sedentarism (Chekroud et al., 2018), resulting in high dropout rates when participating in regular structured exercise (Burgess et al., 2017). Collectively, exercise training seems to be a powerful weapon against poor QoL among BC patients and survivors, and therefore exercise prescription to this particular cohort may be effective for lowering FA, improving SQ, and increasing QoL (Chen et al., 2023). Given that both cancer and obesity adversely affect SQ (Chang and Chang, 2020) and QoL (Heidary et al., 2023; Taylor et al., 2013), the effectiveness of various exercise modes, including CART, is vital for women with BC and excess weight, since CART-induced adaptations and responses may mitigate potential depressive and anxiety symptoms, as well as sleep disturbances frequently present in this cohort before, during, and after treatment without reducing compliance rates compared to usual care. (Wang et al., 2023).
Implications for future research
Considering that the combination of aerobic-based and muscle-strengthening activities is highly recommended for cancer patients and survivors (Campbell et al., 2019) as well as individuals with overweight/obesity (American College of Sports Medicine et al., 2021), further studies are urgently needed to investigate the optimal amount and intensity of CART for this cohort. Future trials should focus on the mechanisms behind the effectiveness of CART on cardiometabolic health and cancer-related indices as well as the association between CART and tumor growth. Also, studies examining the effects of CART on additional cardiovascular disease risk factors (e.g., glucose metabolism, blood pressure, and functional aerobic capacity) and mental health indicators (e.g., depression, anxiety, psychological distress, and mood) would support the current evidence. Furthermore, CART-like protocols should be investigated not only through supervised interventions conducted in a lab-based environment but also under real-world conditions, aiming to evaluate the practicability of CART among BC patients and survivors with overweight/obesity. Lastly, the potential role of CART in immunity among BC cancer patients and survivors also needs to be studied. Such a future approach may comprehend the relationship between CART and immune function as well as the connection between these immunological indicators and clinical benefits in this cohort.
Limitations
The present review has several limitations and thus the findings should be taken into consideration with caution. Eligible studies exhibited inconsistency with regard to the training parameters implemented during the CART interventions, resulting in significant heterogeneity among the included trials. Our study demonstrates that beneficial CART-induced adaptations are existent principally among middle-aged (mean age: 54.0 ± 3.4 years) women. Hence, current outcomes cannot be generalized to men, other age and BMI groups among BC patients and survivors. Considering the outcome measures included in the present study, the role of CART in a wide spectrum of cardiometabolic health still remains unclear due to the lack of data in terms of glucose homeostasis, resting cardiovascular function, oxidative stress, and physical function. Moreover, we discovered substantial heterogeneity in some outcomes in our analysis, but we were unable to explain this due to limited trials. Despite conducting a sensitivity analysis to explore the influence of performance bias and detection bias on the outcomes, in the majority of cases, there were no changes in the estimated effects. This may be attributed to inadequate sample size and biases associated with performance and detection.
Conclusion
The present systematic review and meta-analysis delivers critical outcomes regarding the implementation of CART for BC patients and survivors with concurrent overweight/obesity as an adjunct component of a comprehensive therapy plan. The findings reveal clear evidence that CART has a favorable effect on cardiometabolic health and cancer-related indicators, such as anthropometric characteristics, body composition, lipid homeostasis, inflammation, adipokines, fatigue, sleep, and QoL in BC patients and survivors with concurrent overweight/obesity during and after treatment. More trials with robust methodological design are needed to investigate the dose-response relationship, training parameters configuration, and mechanisms behind these beneficial alterations. This meta-analysis also highlights the rationale for further high-quality RCTs to examine additional outcome measures related to cardiometabolic and mental health, aiming to support the present CART-induced effects for BC patients and survivors with concurrent overweight/obesity.
SUPPLEMANTARY MATERIALS
Acknowledgements
The authors declare that there are no conflicts of interest. The experiments comply with the current laws of the country where they were performed. The data that support the findings of this study are available on request from the corresponding author.
Biographies

Sameer Badri AL-MHANNA
Employment
Department of Exercise Physiology, School of Medical Sciences, Universiti Sains Malaysia, Malaysia
Degree
PhD
Research interests
Clinical Exercise Physiology, Cancer, Obesity, Diabetes Mellitus
E-mail: sameerbadri9@gmail.com

Alexios BATRAKOULIS
Employment
Department of Physical Education and Sport Science, School of Physical Education and Sport Science, Democritus University of Thrace, Greece
Degree
PhD
Research interests
Clinical Exercise Physiology, Obesity, High-Intensity Interval Training, Strength and Conditioning, Health and Fitness Trends
E-mail: abatrako@phyed.duth.gr

Mohd Noor NORHAYATI
Employment
Department of Family Medicine, School of Medical Sciences, Universiti Sains Malaysia, Malaysia
Degree
PhD
Research interests
Maternal Health, Complementary
Medicine
E-mail: hayatikk@usm.my

Mahaneem MOHAMED
Employment
Department of Physiology, School of Medical Sciences, Universiti Sains Malaysia, Malaysia
Degree
PhD
Research interests
Obesity, Diabetes Mellitus
E-mail: mahaneem@usm.my

Clemens DRENOWATZ
Employment
Division of Sport, Physical Activity and Health, University of Education Upper Austria, Austria
Degree
PhD
Research interests
Motor Competence, Exercise, Weight Management
E-mail: Clemens.drenowatz@ph-ooe.at

Ahmad Adebayo IREKEOLA
Employment
Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia, Malaysia
Degree
PhD
Research interests
Viral Infectious Diseases
E-mail: profahmad007@yahoo.com

Hafeez Abiola AFOLABI
Employment
Department of General Surgery, School of Medical Sciences, Hospital University Sains Malaysia, Malaysia
Degree
PhD
Research interests
General Surgery, Cancer, Obesity, Diabetes Mellitus
E-mail: afolabihafeez68@gmail.com

Mehmet GÜLÜ
Employment
Department of Sports Management, Faculty of Sport Sciences, Kirikkale University, Kirikkale, Turkey
Degree
PhD
Research interests
Sports Medicine, Obesity, Physical
Activity
E-mail: mehmetgulu@kku.edu.tr
Nouf H. ALKHAMEES
Employment
Department of Rehabilitation, College of Health and Rehabilitation Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
Degree
PhD
Research interests
Orthopedic Rehabilitation, Holistic Care
E-mail: nhalkhamees@pnu.edu.sa

Wan Syaheedah WAN GHAZALI
Employment
Department of Physiology, School of Medical Sciences, Universiti Sains Malaysia, Malaysia
Degree
PhD
Research interests
Obesity, Diabetes Mellitus
E-mail: syaheeda@usm.my
References
- Acevedo F., Walbaum B., Muniz S., Petric M., Martinez R., Guerra C., Navarro M., Cordova-Delgado M., Pinto M.P., Sanchez C. (2022) Obesity is associated with early recurrence on breast cancer patients that achieved pathological complete response to neoadjuvant chemotherapy. Scientific Reports 12, 21145. https://doi.org/10.1038/s41598-022-25043-2 10.1038/s41598-022-25043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolabi H., Salleh S.M., Zakaria Z., Ch’ng E.S., Mohd Nafi S.N., Abdul Aziz A.A.B., Al-Mhanna S.B., Wada Y., Abdulrahman A.S. (2022a) The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study. Healthcare 10, 1693. https://doi.org/10.3390/healthcare10091693 10.3390/healthcare10091693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolabi H.A., Salleh S.M., Zakaria Z., Ch’ng E.S., Mohd Nafi S.N., Abdul Aziz A.A.B., Irekeola A.A., Wada Y., Al-Mhanna S.B. (2022b) A GNAS gene mutation’s independent expression in the growth of colorectal cancer: a systematic review and meta-analysis. Cancers 14, 5480. https://doi.org/10.3390/cancers14225480 10.3390/cancers14225480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mhanna S.B., Ghazali W.S.W., Mohamed M., Rabaan A.A., Santali E.Y., Alestad J.H., Santali E.Y., Arshad S., Ahmed N., Afolabi H.A. (2022) Effectiveness of physical activity on immunity markers and quality of life in cancer patients: a systematic review. PeerJ 10, e13664. https://doi.org/10.7717/peerj.13664 10.7717/peerj.13664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mhanna S.B., Leão C., Wan Ghazali W.S., Mohamed M., Batrakoulis A., Afolabi H.A., Abubakar B.D., Aldhahi M.I., Gülü M., Yagin F.H., Nikolaidis P.T. (2024a) Impact of Exercise on High-Density Lipoprotein Cholesterol in Adults with Overweight and Obesity: A Narrative Review. Annals of Applied Sport Science 12. [Google Scholar]
- Al-Mhanna S.B., Rocha-Rodriguesc S., Mohamed M., Batrakoulis A., Aldhahi M.I., Afolabi H.A., Yagin F.H., Alhussain M.H., Gulu M., Abubakar B.D., Ghazali W.S.W., Alghannam A.F., Badicu G. (2023) Effects of combined aerobic exercise and diet on cardiometabolic health in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. BMC Sports Science Medicine and Rehabilitation 15, 165. https://doi.org/10.1186/s13102-023-00766-5 10.1186/s13102-023-00766-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mhanna S.B., Wan Ghazali W.S., Batrakoulis A., Alkhamees N.H., Drenowatz C., Mohamed M., Gülü M., Afolabi H.A., Badicu G. (2024b) Impact of Various Types of Exercise on Lipid Metabolism in Patients with Type 2 Diabetes and Concurrent Overweight/Obesity: A Narrative Review. Annals of Applied Sport Science 12. [Google Scholar]
- Almstedt H.C., Grote S., Korte J.R., Beaudion S.P., Shoepe T.C., Strand S., Tarleton H.P. (2016) Combined aerobic and resistance training improves bone health of female cancer survivors. Breast Cancer 5, 274-279. https://doi.org/10.1016/j.bonr.2016.09.003 10.1016/j.bonr.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. Liguori G., Feito Y., Fountaine C., Roy B.A. (2021) ACSM's guidelines for exercise testing and prescription. 11th ed. Philadelphia, PA: Wolters Kluwer Health. [Google Scholar]
- Anwar S.L., Cahyono R., Prabowo D., Avanti W.S., Choridah L., Dwianingsih E.K., Harahap W.A., Aryandono T. (2021) Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer 21, 590. https://doi.org/10.1186/s12885-021-08343-0 10.1186/s12885-021-08343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoum M.F., Alzoughool F., Al-Hourani H. (2020) Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer (Auckl) 14, 1178223419898458. https://doi.org/10.1177/1178223419898458 10.1177/1178223419898458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell W.A., Ancoli-Israel S. (2008) Breast Cancer and Fatigue. Sleep Medicine Clinics 3, 61-71. https://doi.org/10.1016/j.jsmc.2007.10.011 10.1016/j.jsmc.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakoulis A. (2022a) Psychophysiological Adaptations to Pilates Training in Overweight and Obese Individuals: A Topical Review. Diseases 10. https://doi.org/10.3390/diseases10040071 10.3390/diseases10040071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakoulis A. (2022b) Psychophysiological Adaptations to Yoga Practice in Overweight and Obese Individuals: A Topical Review. Diseases 10. https://doi.org/10.3390/diseases10040107 10.3390/diseases10040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakoulis A., Jamurtas A.Z., Fatouros I.G. (2021) High-Intensity Interval Training in Metabolic Diseases: Physiological Adaptations. ACSM's Health & Fitness Journal 25, 54-59. https://doi.org/10.1249/FIT.0000000000000703 10.1249/FIT.0000000000000703 [DOI] [Google Scholar]
- Batrakoulis A., Jamurtas A.Z., Metsios G.S., Perivoliotis K., Liguori G., Feito Y., Riebe D., Thompson W.R., Angelopoulos T.J., Krustrup P., Mohr M., Draganidis D., Poulios A., Fatouros I.G. (2022) Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circulation: Cardiovascular Quality and Outcomes 15, e008243. https://doi.org/10.1161/CIRCOUTCOMES.121.008243 10.1161/CIRCOUTCOMES.121.008243 [DOI] [PubMed] [Google Scholar]
- Battaglini C., Bottaro M., Dennehy C., Rae L., Shields E., Kirk D., Hackney A. (2007) The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Medical Journal 125, 22-28. https://doi.org/10.1590/S1516-31802007000100005 10.1590/S1516-31802007000100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betof A.S., Dewhirst M.W., Jones L.W. (2013) Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, Behavior, and Immunity 30, 75-87. https://doi.org/10.1016/j.bbi.2012.05.001 10.1016/j.bbi.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist K., Adamsen L., Hayes S.C., Lillelund C., Andersen C., Christensen K.B., Oturai P., Ejlertsen B., Tuxen M.K., Møller T. (2019) Heavy-load resistance exercise during chemotherapy in physically inactive breast cancer survivors at risk for lymphedema: a randomized trial. Acta Oncologica 58(12), 1667-1675. https://doi.org/10.1080/0284186X.2019.1643916 10.1080/0284186X.2019.1643916 [DOI] [PubMed] [Google Scholar]
- Blucher C., Stadler S.C. (2017) Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. Frontiers in Endocrinology 8, 293. https://doi.org/10.3389/fendo.2017.00293 10.3389/fendo.2017.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.C., Sarwer D.B., Troxel A.B., Sturgeon K., DeMichele A.M., Denlinger C.S., Schmitz K.H. (2021) A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Research and Treatment 189, 145-154. https://doi.org/10.1007/s10549-021-06284-7 10.1007/s10549-021-06284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli D.T., Chacon-Mikahil M.P.T., Gaspari A.F., Lopes W.A., Bonganha V., Bonfante I.L., Bellotto M.L., Libardi C.A., Cavaglieri C.R. (2015) Combined Training Reduces Subclinical Inflammation in Obese Middle-Age Men. Medicine & Science in Sports & Exercise 47, 2207-2215. https://doi.org/10.1249/MSS.0000000000000658 10.1249/MSS.0000000000000658 [DOI] [PubMed] [Google Scholar]
- Burgess E., Hassmen P., Welvaert M., Pumpa K.L. (2017) Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: a systematic review and meta-analysis. Clinical Obesity 7, 105-114. https://doi.org/10.1111/cob.12180 10.1111/cob.12180 [DOI] [PubMed] [Google Scholar]
- Campbell K.L., Winters-Stone K.M., Wiskemann J., May A.M., Schwartz A.L., Courneya K.S., Zucker D.S., Matthews C.E., Ligibel J.A., Gerber L.H., Morris G.S., Patel A.V., Hue T.F., Perna F.M., Schmitz K.H. (2019) Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Medicine & Science in Sports & Exercise 51, 2375-2390. https://doi.org/10.1249/MSS.0000000000002116 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.P., Chang Y.P. (2020) Meta-Analysis of Changes in Sleep Quality of Women with Breast Cancer before and after Therapy. Breast Care 15, 227-235. https://doi.org/10.1159/000502943 10.1159/000502943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud S.R., Gueorguieva R., Zheutlin A.B., Paulus M., Krumholz H.M., Krystal J.H., Chekroud A.M. (2018) Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry 5, 739-746. https://doi.org/10.1016/S2215-0366(18)30227-X 10.1016/S2215-0366(18)30227-X [DOI] [PubMed] [Google Scholar]
- Chen L., Peng P., Xu Z., Ding X. (2023) The effects of exercise on the quality of life of patients with breast cancer: a systematic review and meta-analysis based on the QLQ-C30 quality of life scale. Gland Surgery 12, 633-650. https://doi.org/10.21037/gs-23-126 10.21037/gs-23-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho O.H., Hwang K.H. (2021) Association between sleep quality, anxiety and depression among Korean breast cancer survivors. Nursing Open 8, 1030-1037. https://doi.org/10.1002/nop2.710 10.1002/nop2.710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F.A., Islam M.F., Prova M.T., Khatun M., Sharmin I., Islam K.M., Hassan M.K., Khan M.A.S., Rahman M.M. (2021) Association of hyperlipidemia with breast cancer in Bangladeshi women. Lipids in Health and Disease 20, 1-9. https://doi.org/10.1186/s12944-021-01480-2 10.1186/s12944-021-01480-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya K.S., McKenzie D.C., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y., Reid R.D., Vallerand J.R., Adams S.C., Proulx C., Dolan L.B., Wooding E., Segal R.J. (2014) Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. British Journal of Cancer 111(9), 1718-1725. https://doi.org/10.1038/bjc.2014.466 10.1038/bjc.2014.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya K.S., Segal R.J., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y., Reid R.D., Jespersen D., Cook D., Proulx C., Trinh L., Dolan L.B., Wooding E., Forbes C.C., McKenzie D.C. (2014) Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Research and Treatment 144(2), 361-369. https://doi.org/10.1007/s10549-014-2883-0 10.1007/s10549-014-2883-0 [DOI] [PubMed] [Google Scholar]
- de Jesus Leite M.A.F., Puga G.M., Arantes F.J., Oliveira C.J.F., Cunha L.M., Bortolini M.J.S., Penha-Silva N. (2018) Effects of combined and resistance training on the inflammatory profile in breast cancer survivors: A systematic review. Complementary Therapies in Medicine 36, 73-81. https://doi.org/10.1016/j.ctim.2017.11.023 10.1016/j.ctim.2017.11.023 [DOI] [PubMed] [Google Scholar]
- de Jesus M., Mohammed T., Singh M., Tiu J.G., Kim A.S. (2022) Etiology and Management of Dyslipidemia in Patients With Cancer. Frontiers in Cardiovascular Medicine 9, 892335. https://doi.org/10.3389/fcvm.2022.892335 10.3389/fcvm.2022.892335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus Leite M.A.F., Mariano I.M., Dechichi J.G.C., Giolo J.S., Gonçalves Á.C., Puga G.M. (2021) Exercise training and detraining effects on body composition, muscle strength and lipid, inflammatory and oxidative markers in breast cancer survivors under tamoxifen treatment. Life Sciences 284, 119924. https://doi.org/10.1016/j.lfs.2021.119924 10.1016/j.lfs.2021.119924 [DOI] [PubMed] [Google Scholar]
- de Paulo T.R., Winters-Stone K.M., Viezel J., Rossi F.E., Simões R.R., Tosello G., Junior I.F.F. (2018) Effects of resistance plus aerobic training on body composition and metabolic markers in older breast cancer survivors undergoing aromatase inhibitor therapy. Experimental Gerontology 111, 210-217. https://doi.org/10.1016/j.exger.2018.07.022 10.1016/j.exger.2018.07.022 [DOI] [PubMed] [Google Scholar]
- Dieli-Conwright C.M., Courneya K.S., Demark-Wahnefried W., Sami N., Lee K., Buchanan T.A., Spicer D.V., Tripathy D., Bernstein L., Mortimer J.E. (2018) Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. Journal of Clinical Oncology 36, 875. https://doi.org/10.1200/JCO.2017.75.7526 10.1200/JCO.2017.75.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli-Conwright C.M., Harrigan M., Cartmel B., Chagpar A., Bai Y., Li F.-y., Rimm D.L., Pusztai L., Lu L., Sanft T. (2022) Impact of a randomized weight loss trial on breast tissue markers in breast cancer survivors. NPJ Breast Cancer 8, 1-7. https://doi.org/10.1038/s41523-022-00396-z 10.1038/s41523-022-00396-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli-Conwright C.M., Orozco B.Z. (2015) Exercise after breast cancer treatment: current perspectives. Breast Cancer: Targets and Therapy 7, 353. https://doi.org/10.2147/BCTT.S82039 10.2147/BCTT.S82039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli-Conwright C.M., Sweeney F.C., Courneya K.S., Tripathy D., Sami N., Lee K., Buchanan T.A., Spicer D., Bernstein L., Mortimer J.E. (2019) Hispanic ethnicity as a moderator of the effects of aerobic and resistance exercise in survivors of breast cancer. Cancer 125, 910-920. https://doi.org/10.1002/cncr.31879 10.1002/cncr.31879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elreda L., Kim A., Malik M. (2022) Mitigating Breast Cancer Disparities by Addressing the Obesity Epidemic. Current Breast Cancer Reports 14, 168-173. https://doi.org/10.1007/s12609-022-00460-4 10.1007/s12609-022-00460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergun M., Eyigor S., Karaca B., Kisim A., Uslu R. (2013) Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. European Journal of Cancer Care 22, 626-637. https://doi.org/10.1111/ecc.12068 10.1111/ecc.12068 [DOI] [PubMed] [Google Scholar]
- Feliciano E.M.C., Chen W.Y., Bradshaw P.T., Prado C.M., Alexeeff S., Albers K.B., Castillo A.L., Caan B.J. (2019) Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. Journal of Clinical Oncology 37, 2528. https://doi.org/10.1200/JCO.19.00286 10.1200/JCO.19.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Lao C., Cantarero-Villanueva I., Fernández-de-Las-Peñas C., del Moral-Ávila R., Castro-Sánchez A.M., Arroyo-Morales M. (2012) Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. The Clinical Journal of Pain 28(2), 113-121. https://doi.org/10.1097/AJP.0b013e318225dc02 10.1097/AJP.0b013e318225dc02 [DOI] [PubMed] [Google Scholar]
- Fortner B.V., Stepanski E.J., Wang S.C., Kasprowicz S., Durrence H.H. (2002) Sleep and quality of life in breast cancer patients. Journal of Pain and Symptom Management 24, 471-480. https://doi.org/10.1016/S0885-3924(02)00500-6 10.1016/S0885-3924(02)00500-6 [DOI] [PubMed] [Google Scholar]
- Fraser S.F., Gardner J.R., Dalla Via J., Daly R.M. (2022) The Effect of Exercise Training on Lean Body Mass in Breast Cancer Patients: A Systematic Review and Meta-analysis. Medicine & Science in Sports & Exercise 54, 211-219. https://doi.org/10.1249/MSS.0000000000002792 10.1249/MSS.0000000000002792 [DOI] [PubMed] [Google Scholar]
- Ganz P.A. (2006) Monitoring the physical health of cancer survivors: a survivorship-focused medical history. Journal of Clinical Oncology 24, 5105-5111. https://doi.org/10.1200/JCO.2006.06.0541 10.1200/JCO.2006.06.0541 [DOI] [PubMed] [Google Scholar]
- Gilyana M., Batrakoulis A., Zisi V. (2023) Physical Activity, Body Image, and Emotional Intelligence Differences in Adults with Overweight and Obesity. Diseases 11. https://doi.org/10.3390/diseases11020071 10.3390/diseases11020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A.M., Martínez C., Fiuza-Luces C., Herrero F., Pérez M., Madero L., Ruiz J.R., Lucia A., Ramírez M. (2011) Exercise training and cytokines in breast cancer survivors. International Journal of Sports Medicine 32(6), 461-467. https://doi.org/10.1055/s-0031-1271697 10.1055/s-0031-1271697 [DOI] [PubMed] [Google Scholar]
- Gonzalo-Encabo P., Christopher C.N., Lee K., Normann A.J., Yunker A.G., Norris M.K., Wang E., Dieli-Conwright C.M. (2022) High Intensity Interval Training Improves Metabolic Syndrome in Women with Breast Cancer Receiving Anthracyclines. Scandinavian Journal of Medicine & Science in Sports 33(4), 475-484. https://doi.org/10.1111/sms.14280 10.1111/sms.14280 [DOI] [PubMed] [Google Scholar]
- GRADEpro G. (2014) Computer program on www.gradepro.org. Version [July, 2016]. McMaster University. [Google Scholar]
- Greenlee H., Shi Z., Molmenti C.L.S., Rundle A., Tsai W.Y. (2016) Trends in obesity prevalence in adults with a history of cancer: results from the US National Health Interview Survey, 1997 to 2014. Journal of Clinical Oncology 34, 3133. https://doi.org/10.1200/JCO.2016.66.4391 10.1200/JCO.2016.66.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-Z., Pan L., Du C.-J., Ren D.-Q., Xie X.-M. (2013) Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asia Pacific Journal of Clinical Oncology 14, 243-248. https://doi.org/10.7314/APJCP.2013.14.1.243 10.7314/APJCP.2013.14.1.243 [DOI] [PubMed] [Google Scholar]
- Harvie M., Pegington M., McMullan D., Bundred N., Livingstone K., Campbell A., Wolstenholme J., Lovato E., Campbell H., Adams J., Speed S., Morris J., Howell S., Howell A. (2019) The effectiveness of home versus community-based weight control programmes initiated soon after breast cancer diagnosis: a randomised controlled trial. British Journal of Cancer 121(6), 443-454. https://doi.org/10.1038/s41416-019-0522-6 10.1038/s41416-019-0522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary Z., Ghaemi M., Hossein Rashidi B., Kohandel Gargari O., Montazeri A. (2023) Quality of Life in Breast Cancer Patients: A Systematic Review of the Qualitative Studies. Cancer Control 30, 10732748231168318. https://doi.org/10.1177/10732748231168318 10.1177/10732748231168318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Green S. (2008) Cochrane handbook for systematic reviews of interventions. https://doi.org/10.1002/9780470712184 10.1002/9780470712184 [DOI] [Google Scholar]
- Hutnick N.A., Williams N.I., Kraemer W.J., Orsega-Smith E., Dixon R.H., Bleznak A.D., Mastro A.M. (2005) Exercise and lymphocyte activation following chemotherapy for breast cancer. Medicine and Science in Sports and Exercise 37, 1827. https://doi.org/10.1249/01.mss.0000175857.84936.1a 10.1249/01.mss.0000175857.84936.1a [DOI] [PubMed] [Google Scholar]
- Hwangbo Y., Kang D., Kang M., Kim S., Lee E.K., Kim Y.A., Chang Y.J., Choi K.S., Jung S.-Y., Woo S.M. (2018) Incidence of diabetes after cancer development: a Korean national cohort study. Journal of Diabetes 4, 1099-1105. https://doi.org/10.1001/jamaoncol.2018.1684 10.1001/jamaoncol.2018.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joaquim A., Leao I., Antunes P., Capela A., Viamonte S., Alves A.J., Helguero L.A., Macedo A. (2022) Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: Evidence from systematic reviews and meta-analyses. Frontiers in Oncology 12, 955505. https://doi.org/10.3389/fonc.2022.955505 10.3389/fonc.2022.955505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.M., Stoner L., Baldi J.C., McLaren B. (2020) Circuit resistance training and cardiovascular health in breast cancer survivors. European Journal of Cancer Care 29, e13231. https://doi.org/10.1111/ecc.13231 10.1111/ecc.13231 [DOI] [PubMed] [Google Scholar]
- Jones L.W., Courneya K.S. (2002) Exercise counseling and programming preferences of cancer survivors. Cancer Practice 10, 208-215. https://doi.org/10.1046/j.1523-5394.2002.104003.x 10.1046/j.1523-5394.2002.104003.x [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Yamauchi T. (2005) Adiponectin and adiponectin receptors. Endocrine Reviews 26, 439-451. https://doi.org/10.1210/er.2005-0005 10.1210/er.2005-0005 [DOI] [PubMed] [Google Scholar]
- Khosravi N., Eskandari Z., Farajivafa V., Hanson E.D., Agha-Alinejad H., Abdollah-Pour A., Haghighat S. (2018) Effect of 6 months of aerobic training on adipokines as breast cancer risk factors in postmenopausal women: A randomized controlled trial. Journal of Cancer Research and Therapeutics 14, 1336-1340. https://doi.org/10.4103/jcrt.JCRT_684_16 10.4103/jcrt.JCRT_684_16 [DOI] [PubMed] [Google Scholar]
- Kim T.H., Chang J.S., Park K.S., Park J., Kim N., Lee J.I., Kong I.D. (2017) Effects of exercise training on circulating levels of Dickkpof-1 and secreted frizzled-related protein-1 in breast cancer survivors: A pilot single-blind randomized controlled trial. Plos One 12(2), e0171771. https://doi.org/10.1371/journal.pone.0171771 10.1371/journal.pone.0171771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham A.A., Bonsignore A., Bland K.A., McKenzie D.C., Gelmon K.A., Van Patten C.L., Campbell K.L. (2018) Exercise Prescription and Adherence for Breast Cancer: One Size Does Not FITT All. Medicine & Science in Sports & Exercise 50, 177-186. https://doi.org/10.1249/MSS.0000000000001446 10.1249/MSS.0000000000001446 [DOI] [PubMed] [Google Scholar]
- Kolb R., Zhang W. (2020) Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue. Cancers 12. https://doi.org/10.3390/cancers12061686 10.3390/cancers12061686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Gao R. (2022) Aerobic exercise combined with resistance exercise training improves cardiopulmonary function and blood lipid of patients with breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 101, e32391. https://doi.org/10.1097/MD.0000000000032391 10.1097/MD.0000000000032391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudiarasu C., Lopez P., Galvao D.A., Newton R.U., Taaffe D.R., Mansell L., Fleay B., Saunders C., Fox-Harding C., Singh F. (2023) What are the most effective exercise, physical activity, and dietary interventions to improve body composition in women diagnosed with or at high-risk of breast cancer? A systematic review and network meta-analysis. Cancer 129, 3697-3712. https://doi.org/10.1002/cncr.35043 10.1002/cncr.35043 [DOI] [PubMed] [Google Scholar]
- Lake B., Damery S., Jolly K. (2022) Effectiveness of weight loss interventions in breast cancer survivors: a systematic review of reviews. British Journal of Sports Medicine Open 12, e062288. https://doi.org/10.1136/bmjopen-2022-062288 10.1136/bmjopen-2022-062288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Tripathy D., Demark-Wahnefried W., Courneya K.S., Sami N., Bernstein L., Spicer D., Buchanan T.A., Mortimer J.E., Dieli-Conwright C.M. (2019) Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncology 5, 710-714. https://doi.org/10.1001/jamaoncol.2019.0038 10.1001/jamaoncol.2019.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel J.A., Campbell N., Partridge A., Chen W.Y., Salinardi T., Chen H., Adloff K., Keshaviah A., Winer E.P. (2008) Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology 26, 907-912. https://doi.org/10.1200/JCO.2007.12.7357. 10.1200/JCO.2007.12.7357 [DOI] [PubMed] [Google Scholar]
- Ligibel J.A., Dillon D., Giobbie-Hurder A., McTiernan A., Frank E., Cornwell M., Pun M., Campbell N., Dowling R.J., Chang M.C. (2019) Impact of a Pre-Operative Exercise Intervention on Breast Cancer Proliferation and Gene Expression: Results from the Pre-Operative Health and Body (PreHAB) Study Exercise Window Trial in Newly Diagnosed Breast Cancer. Clinical Cancer Research 25, 5398-5406. https://doi.org/10.1158/1078-0432.CCR-18-3143 10.1158/1078-0432.CCR-18-3143 [DOI] [PubMed] [Google Scholar]
- Lohmann A.E., Soldera S.V., Pimentel I., Ribnikar D., Ennis M., Amir E., Goodwin P.J. (2021) Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. Journal of the National Cancer Institute 113(11), 1465-1475. https://doi.org/10.1093/jnci/djab023 10.1093/jnci/djab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Beedie C., Jimenez A. (2014) Differential effects of aerobic exercise, resistance training, and combined exercise modalities on cholesterol and the lipid profile: review, synthesis, and recommendations. Sports Medicine 44, 211-221. https://doi.org/10.1007/s40279-013-0110-5 10.1007/s40279-013-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A.M., Van Weert E., Korstjens I., Hoekstra-Weebers J.E., Van Der Schans C.P., Zonderland M.L., Mesters I., Van Den Borne B., Ros W.D. (2008) Improved physical fitness of cancer survivors: a randomised controlled trial comparing physical training with physical and cognitive-behavioural training. Acta Oncologica 47(5), 825-834. https://doi.org/10.1080/02841860701666063 10.1080/02841860701666063 [DOI] [PubMed] [Google Scholar]
- Mendelson M., Borowik A., Michallet A.S., Perrin C., Monneret D., Faure P., Levy P., Pepin J.L., Wuyam B., Flore P. (2016) Sleep quality, sleep duration, and physical activity in obese adolescents: effects of exercise training. Pediatric Obesity 11, 26-32. https://doi.org/10.1111/ijpo.12015 10.1111/ijpo.12015 [DOI] [PubMed] [Google Scholar]
- Meneses-Echávez J.F., González-Jiménez E., Ramírez-Vélez R. (2015) Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer 15, 1-13. https://doi.org/10.1186/s12885-015-1069-4 10.1186/s12885-015-1069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne H.M., Wallman K.E., Gordon S., Courneya K.S. (2008a) Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Research and Treatment 108, 279-288. https://doi.org/10.1007/s10549-007-9602-z 10.1007/s10549-007-9602-z [DOI] [PubMed] [Google Scholar]
- Milne H.M., Wallman K.E., Gordon S., Courneya K.S. (2008b) Impact of a combined resistance and aerobic exercise program on motivational variables in breast cancer survivors: a randomized controlled trial. Annals of Behavioral Medicine 36(2), 158-166. https://doi.org/10.1007/s12160-008-9059-2 10.1007/s12160-008-9059-2 [DOI] [PubMed] [Google Scholar]
- Moore K.J., Shah R. (2020) Introduction to the obesity, metabolic syndrome, and CVD compendium. Circulation Research, 126, 1475-1476. https://doi.org/10.1161/CIRCRESAHA.120.317240 10.1161/CIRCRESAHA.120.317240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J.E., Barsevick A.M., Bennett C.L., Berger A.M., Cleeland C., DeVader S.R., Escalante C., Gilreath J., Hurria A., Mendoza T.R. (2010) Studying cancer-related fatigue: report of the NCCN scientific research committee. Journal of the National Comprehensive Cancer Network 8, 1331-1339. https://doi.org/10.6004/jnccn.2010.0101 10.6004/jnccn.2010.0101 [DOI] [PubMed] [Google Scholar]
- Mulligan A.A., Lentjes M.A.H., Luben R.N., Wareham N.J., Khaw K.T. (2019) Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovascular Disorders 19, 238. https://doi.org/10.1186/s12872-019-1223-z 10.1186/s12872-019-1223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutalub Y.B., Abdulwahab M., Mohammed A., Yahkub A.M., Al-Mhanna S.B., Yusof W., Tang S.P., Rasool A.H.G., Mokhtar S.S. (2022) Gut microbiota modulation as a novel therapeutic strategy in cardiometabolic diseases. Foods 11, 2575. https://doi.org/10.3390/foods11172575 10.3390/foods11172575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutrie N., Campbell A.M., Whyte F., McConnachie A., Emslie C., Lee L., Kearney N., Walker A., Ritchie D. (2007) Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. British Journal of Sports Medicine 334, 517. https://doi.org/10.1136/bmj.39094.648553.AE 10.1136/bmj.39094.648553.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome A.M., Reed R., Sansone J., Batrakoulis A., McAvoy C., Parrott M.W. (2024) 2024 ACSM Worldwide Fitness Trends: Future Directions of the Health and Fitness Industry. ACSM's Health & Fitness Journal 28, 14-26. https://doi.org/10.1249/FIT.0000000000000933 10.1249/FIT.0000000000000933 [DOI] [Google Scholar]
- Nieman D., Cook V., Henson D., Suttles J., Rejeski W., Ribisl P., Fagoaga O., Nehlsen-Cannarella S. (1995) Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. International Journal of Sports Medicine 16, 334-337. https://doi.org/10.1055/s-2007-973015 10.1055/s-2007-973015 [DOI] [PubMed] [Google Scholar]
- Okekunle A.P., Yie G.E., Song S., Kim Z., Youn H.J., Cho J., Min J.W., Kim Y.S., Lee J.E. (2022) Association of lipid profile with obesity among breast cancer survivors: a cross-sectional study. Lipids in Health and Disease 21, 66. https://doi.org/10.1186/s12944-022-01674-2 10.1186/s12944-022-01674-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ording A.G., Garne J.P., Nyström P.M.W., Frøslev T., Sørensen H.T., Lash T.L. (2013) Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis—a Danish nationwide matched cohort study. Plos One 8, e76013. https://doi.org/10.1371/journal.pone.0076013 10.1371/journal.pone.0076013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hrobjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. British Journal of Sports Medicine 372, 71. https://doi.org/10.1136/bmj.n71 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo T.R.S., Rossi F.E., Viezel J., Tosello G.T., Seidinger S.C., Simões R.R., de Freitas R., Jr., Freitas I.F., Jr (2019) The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health and Quality of Life Outcomes 17(1), 17. https://doi.org/10.1186/s12955-019-1090-4 10.1186/s12955-019-1090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego S., Sansoni V., Ziemann E., Lombardi G. (2021) Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines. International Journal of Molecular Sciences 22. https://doi.org/10.3390/ijms22042005 10.3390/ijms22042005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F., Cortellini A., Indini A., Tomasello G., Ghidini M., Nigro O., Salati M., Dottorini L., Iaculli A., Varricchio A. (2021) Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. Journal of Nutrition 4, e213520. https://doi.org/10.1001/jamanetworkopen.2021.3520 10.1001/jamanetworkopen.2021.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S., Dieli-Conwright C.M., Cheng R.K., Barac A., Reding K.W., Vasbinder A., Cook K.L., Nair V., Desai P., Simon M.S. (2022) A review of research on the intersection between breast cancer and cardiovascular research in the Women's Health Initiative (WHI). Frontiers in Oncology 12, 1039246. https://doi.org/10.3389/fonc.2022.1039246 10.3389/fonc.2022.1039246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeki P.S., Pranoto A., Rahmanto I., Izzatunnisa N., Yosika G.F., Hernaningsih Y., Wungu C.D.K., Halim S. (2023) The Positive Effect of Four-Week Combined Aerobic-Resistance Training on Body Composition and Adipokine Levels in Obese Females. Sports (Basel) 11. https://doi.org/10.3390/sports11040090 10.3390/sports11040090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C.L., Thomson C., Gansler T., Gapstur S.M., McCullough M.L., Patel A.V., Andrews K.S., Bandera E.V., Spees C.K., Robien K., Hartman S., Sullivan K., Grant B.L., Hamilton K.K., Kushi L.H., Caan B.J., Kibbe D., Black J.D., Wiedt T.L., McMahon C., Sloan K., Doyle C. (2020) American Cancer Society guideline for diet and physical activity for cancer prevention. Cancer Journal for Clinicians 70, 245-271. https://doi.org/10.3322/caac.21591 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- Rogers L.Q., Fogleman A., Trammell R., Hopkins-Price P., Vicari S., Rao K., Edson B., Verhulst S., Courneya K.S., Hoelzer K. (2013) Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integrative Cancer Therapies 12, 323-335. https://doi.org/10.1177/1534735412449687 10.1177/1534735412449687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.Q., Fogleman A., Trammell R., Hopkins-Price P., Spenner A., Vicari S., Rao K., Courneya K.S., Hoelzer K., Robbs R., Verhulst S. (2015) Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial. Psychooncology 24(3), 302-310. https://doi.org/10.1002/pon.3594 10.1002/pon.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.Q., Vicari S., Trammell R., Hopkins-Price P., Fogleman A., Spenner A., Rao K., Courneya K.S., Hoelzer K.S., Robbs R. (2014) Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Medicine and Science in Sports and Exercise 46, 1077. https://doi.org/10.1249/MSS.0000000000000210 10.1249/MSS.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh C.S., McMillan D.C. (2014) Cancer and systemic inflammation: treat the tumour and treat the host. British Journal of Cancer 110, 1409-1412. https://doi.org/10.1038/bjc.2014.90 10.1038/bjc.2014.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J.M., Scott E.J., Daley A.J., Woodroofe M.N., Mutrie N., Crank H., Powers H.J., Coleman R.E. (2014) Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation, and immune function after early-stage breast cancer: a randomised controlled trial. Breast Cancer Research 16, 1-10. https://doi.org/10.1186/bcr3643 10.1186/bcr3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K.H., Troxel A.B., Dean L.T., DeMichele A., Brown J.C., Sturgeon K., Zhang Z., Evangelisti M., Spinelli B., Kallan M.J., Denlinger C., Cheville A., Winkels R.M., Chodosh L., Sarwer D.B. (2019) Effect of home-based exercise and weight loss programs on breast cancer-related lymphedema outcomes among overweight breast cancer survivors: The WISER Survivor randomized clinical trial. JAMA Oncology 5(11), 1605-1613. https://doi.org/10.1001/jamaoncol.2019.2109 10.1001/jamaoncol.2019.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A.L., Winters-Stone K., Gallucci B. (2007) Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 34(3), 627-633. https://doi.org/10.1188/07.ONF.627-633 10.1188/07.ONF.627-633 [DOI] [PubMed] [Google Scholar]
- Scott E., Daley A., Doll H., Woodroofe N., Coleman R., Mutrie N., Crank H., Powers H., Saxton J. (2013) Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with longterm prognosis after early-stage breast cancer: a randomized controlled trial. Cancer Causes & Control 24, 181-191. https://doi.org/10.1007/s10552-012-0104-x 10.1007/s10552-012-0104-x [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. (2020) Cancer statistics, 2020. Cancer Journal for Clinicians 70, 7-30. https://doi.org/10.3322/caac.21590 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Simon M.S., Hastert T.A., Barac A., Banack H.R., Caan B.J., Chlebowski R.T., Foraker R., Hovsepyan G., Liu S., Luo J., Manson J.E., Neuhouser M.L., Okwuosa T.M., Pan K., Qi L., Ruterbusch J.J., Shadyab A.H., Thomson C.A., Wactawski-Wende J., Waheed N., Beebe-Dimmer J.L. (2021) Cardiometabolic risk factors and survival after cancer in the Women's Health Initiative. Cancer 127, 598-608. https://doi.org/10.1002/cncr.33295 10.1002/cncr.33295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck R.M., Courneya K.S., Mâsse L.C., Duval S., Schmitz K.H. (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship 4, 87-100. https://doi.org/10.1007/s11764-009-0110-5 10.1007/s11764-009-0110-5 [DOI] [PubMed] [Google Scholar]
- Stanton A.L. (2006) Psychosocial concerns and interventions for cancer survivors. Journal of Clinical Oncology 24, 5132-5137. https://doi.org/10.1200/JCO.2006.06.8775 10.1200/JCO.2006.06.8775 [DOI] [PubMed] [Google Scholar]
- Sturgeon K., Digiovanni L., Good J., Salvatore D., Fenderson D., Domchek S., Stopfer J., Galantino M.L., Bryan C., Hwang W.T., Schmitz K. (2016) Exercise-Induced Dose-Response Alterations in Adiponectin and Leptin Levels Are Dependent on Body Fat Changes in Women at Risk for Breast Cancer. Cancer Epidemiology Biomarkers & Prevention 25, 1195-2000. https://doi.org/10.1158/1055-9965.EPI-15-1087 10.1158/1055-9965.EPI-15-1087 [DOI] [PubMed] [Google Scholar]
- Sweeney F.C., Demark-Wahnefried W., Courneya K.S., Sami N., Lee K., Tripathy D., Yamada K., Buchanan T.A., Spicer D.V., Bernstein L., Mortimer J.E., Dieli-Conwright C.M. (2019) Aerobic and resistance exercise improves shoulder function in women who are overweight or obese and have breast cancer: A randomized controlled trial. Physical Therapy 99(10), 1334-1345. https://doi.org/10.1093/ptj/pzz096 10.1093/ptj/pzz096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V.H., Forhan M., Vigod S.N., McIntyre R.S., Morrison K.M. (2013) The impact of obesity on quality of life. Best Practice & Research Clinical Endocrinology & Metabolism 27, 139-146. https://doi.org/10.1016/j.beem.2013.04.004 10.1016/j.beem.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Travier N., Fonseca-Nunes A., Javierre C., Guillamo E., Arribas L., Peiró I., Buckland G., Moreno F., Urruticoechea A., Oviedo G.R., Roca A., Hurtós L., Ortega V., Muñoz M., Garrigós L., Cirauqui B., Del Barco S., Arcusa A., Seguí M.A., Borràs J.M., Gonzalez C.A., Agudo A. (2014) Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Medical Oncology 31(1), 783. https://doi.org/10.1007/s12032-013-0783-5 10.1007/s12032-013-0783-5 [DOI] [PubMed] [Google Scholar]
- Van Alsten S.C., Rabkin C.S., Sawada N., Shimazu T., Charvat H., Yamaji T., Inoue M., Kemp T.J., Pinto L.A., Camargo M.C. (2020) Metabolic syndrome, physical activity, and inflammation: a cross-sectional analysis of 110 circulating biomarkers in Japanese adults. Cancer Epidemiology, Biomarkers & Prevention 29, 1639-1646. https://doi.org/10.1158/1055-9965.EPI-19-1513 10.1158/1055-9965.EPI-19-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur S., Guillaumond F. (2022) Lipids in cancer: a global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis 11, 46. https://doi.org/10.1038/s41389-022-00420-8 10.1038/s41389-022-00420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.C., Chen P.L., Liao W.C., Tsai I.C. (2023) Differential Impact of Exercises on Quality-of-Life Improvement in Breast Cancer Survivors: A Network Meta-Analysis of Randomized Controlled Trials. Cancers 15. https://doi.org/10.3390/cancers15133380 10.3390/cancers15133380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Chou Y., Chou W., Hsu G., Chu C., Yu C., Yu J., Sun C. (2009) Circulating levels of leptin, adiposity, and breast cancer risk. British Journal of Cancer 100, 578-582. https://doi.org/10.1038/sj.bjc.6604913 10.1038/sj.bjc.6604913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Liu L., Zhang X. (2023) Exercise interventions on body composition and quality of life of overweight/obese breast cancer survivors: a meta-analysis. BMC Women's Health 23, 484. https://doi.org/10.1186/s12905-023-02627-2 10.1186/s12905-023-02627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Jia N., Ding M., Yuan K. (2022) Effects of exercise on inflammatory factors and IGF system in breast cancer survivors: a meta-analysis. BMC Women's Health 22, 507. https://doi.org/10.1186/s12905-022-02058-5 10.1186/s12905-022-02058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


