Abstract
Newly discovered TT virus (TTV) is widely distributed in human populations. To understand more about the relationship between TTV and its hosts, we tested 400 sera from various nonhuman primates for the presence of TTV DNA by PCR assay. We collected serum samples from 24 different species of nonhuman primates. TTV DNA was determined by PCR with primers designed from the 5′-end region of the TTV genome. Nucleotide sequencing and phylogenetic analysis of viral genomes were also performed. TTV DNA was detected in 87 of 98 (89%) chimpanzees and 3 of 21 (14%) crab-eating macaques. Nucleotide sequences of the PCR products obtained from both animals were 80 to 100% identical between two species. In contrast, the sequences differed from TTV isolates in humans by 24 to 33% at the nucleotide level and 36 to 50% at the amino acid level. Phylogenetic analysis demonstrated that all TTV isolates obtained from simians were distinct from the human TTV isolates. Furthermore, TTV in simians, but not in humans, was classified into three different genotypes. Our results indicate that TTV in simians represents a group different from, but closely related to, TTV in humans. From these results, we tentatively named this TTV simian TTV (s-TTV). The existence of the s-TTV will be important in determining the origin, nature, and transmission of human TTV and may provide useful animal models for studies of the infection and pathogenesis of this new DNA virus.
The known viral agents of hepatitis do not account for all of the cases of hepatitis of purported viral etiology. Specifically, the screening of donated blood for serologic markers of hepatitis C virus has not prevented all cases of non-A, non-B posttransfusion hepatitis, suggesting the existence of a “non-A, non-B, non-C” agent. Recently, the genome of a novel DNA virus, named TT virus (TTV), was isolated from a patient with acute posttransfusion hepatitis by representational difference analysis (8, 9). TTV is an unenveloped, circular, single-stranded DNA virus (having 3,852 nucleotides in the full-length sequence), with an isopycnic density of 1.31 to 1.34 g/ml in CsCl (5, 6). The TTV genome has three possible open reading frames, capable of encoding 770, 202, and 105 amino acids, respectively (5). The genome structure and its banding in buoyant density gradient centrifugation suggest that TTV might be most closely related to Circoviridae viruses among the known animal virus families (5, 14). Despite TTV being a DNA virus, the TTV sequence has a wide range of sequence divergence, allowing classification into several genotypes (1, 6, 14, 15). TTV sequences can be detected in sera and liver tissues from liver disease patients, suggesting that TTV may be responsible for some acute and chronic liver disease of unknown etiology (3, 9). On the other hand, it has been reported previously that TTV infection does not induce significant liver damage (7). We recently reported a very high prevalence of TTV in general populations worldwide, suggesting that this virus may be a common DNA virus with no clear disease association in humans (1). However, the epidemiology, clinical significance, and transmission patterns of TTV remain unclear. In order to establish the nature of TTV and investigate a new host for TTV other than humans, we carried out PCR screening for TTV in various nonhuman primates.
We collected serum samples from various nonhuman primates, including 98 chimpanzees, 1 orangutan, 45 vervet monkeys, 6 de Brazzas' monkeys, 22 blue monkeys, 86 Sykes' monkeys, 21 crab-eating monkeys, 4 stump-tailed monkeys, 3 Assamese monkeys, 2 bonnet monkeys, 4 pigtailed monkeys, 1 Taiwan monkey, 17 Japanese monkeys, 6 hamadryas baboons, 43 anubis baboons, 5 yellow baboons, 13 gray-cheeked mangabeys, 2 patas monkeys, 6 night monkeys, 9 brown capuchins, 1 squirrel monkey, 1 black-handed spider monkey, 2 ring-tailed lemurs, and 2 thick-tailed bush babies. The country of origin is shown in Table 1, and most animals were maintained and housed in an indoor-outdoor facility. All chimpanzees used for this study were born in West Africa and imported into Japan in 1979. We used serum samples that were obtained from the animals immediately after their arrival in Japan at quarantine. The age of most animals was unknown. None had been previously inoculated with human serum, any hepatitis viruses, other serum, or blood products. In addition, to compare the sequence of TTV isolates between animals and humans, we analyzed the TTV DNA in the 5′-end region obtained from 7 Ghanaians, 2 Egyptians, 2 Americans, 2 Vietnamese, 2 Myanmarese, and 2 Bolivians. The serum samples were kept at −40°C or below until tested.
TABLE 1.
Prevalence of TTV DNA in various nonhuman primates
| Common name | Sp. name | Place of capture | No. positive/no. tested (%) |
|---|---|---|---|
| Apes | |||
| Chimpanzee | Pan troglodytes | West Africa | 87/98 (89) |
| Orangutan | Pongo pygmaeus | Southeast Asia | 0/1 |
| Old World monkeys | |||
| Vervet monkey | Cercopithecus aethiops | Africa | 0/45 |
| de Brazzas' monkey | Cercopithecus neglectus | Africa | 0/6 |
| Blue monkey | Cercopithecus mitis stuhlmanni | Africa | 0/22 |
| Sykes' monkey | Cercopithecus mitis kolbi | Africa | 0/86 |
| Crab-eating monkey | Macaca fascicularis | Southeast Asia | 3/21 (14) |
| Stump-tailed monkey | Macaca arctoides | Southeast Asia | 0/4 |
| Assamese monkey | Macaca assamensis | India | 0/3 |
| Bonnet monkey | Macaca radiata | India | 0/2 |
| Pigtailed monkey | Macaca nemestrina | Indonesia | 0/4 |
| Taiwan monkey | Macaca cyclopis | Taiwan | 0/1 |
| Japanese monkey | Macaca fuscata | Japan | 0/17 |
| Hamadryas baboon | Papio hamadryas | Africa | 0/6 |
| Anubis baboon | Papio anubis | Africa | 0/43 |
| Yellow baboon | Papio cynocephalus | Africa | 0/5 |
| Gray-cheeked mangabey | Cercocebus albigena | Africa | 0/13 |
| Patas monkey | Erythrocebus patas | Africa | 0/2 |
| New World monkeys | |||
| Night monkey | Aotus trivirgatus | South America | 0/6 |
| Brown capuchin | Cebus apella | South America | 0/9 |
| Squirrel monkey | Saimiri sciureus | South America | 0/1 |
| Black-handed spider monkey | Ateles geoffroyi | South America | 0/1 |
| Prosimii | |||
| Ring-tailed lemur | Lemur catta | South America | 0/2 |
| Thick-tailed bush baby | Galago crassicaudatus | Southeast Asia | 0/2 |
DNA was extracted from 100 μl of serum samples with a nucleic acid extraction kit (SepaGene RV-R; Sanko Junyaku Co., Ltd., Tokyo, Japan) as directed by the manufacturer. The resulting pellet was resuspended in RNase- and DNase-free water and then subjected to PCR as described previously (1). In brief, the thermocycler was programmed first to preheat at 95°C for 10 min to activate AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.); then followed 55 cycles consisting of 94°C for 20 s, 60°C for 20 s, and 72°C for 30 s with a Perkin-Elmer 9600 or 9700 Thermal Cycler. The sequences of the TTV-specific primers were 5′-GCTACGTCACTAACCACGTG-3′ (T801, sense primer, nucleotides 6 to 25) and 5′-CTBCGGTGTGTAAACTCACC-3′ (T935, antisense primer, nucleotides 185 to 204; B = G, C, or T) as designed by Takahashi et al. (13) in the 5′-end region of the TA278 isolate. The PCR products were detected by electrophoresis on 2% agarose gels, stained with ethidium bromide, and photographed under UV light. Purified PCR products from gels were subjected to direct sequencing from both directions by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer). Sequences of amplified cDNA were determined by using a sequencer (ABI model 373A; Applied Biosystems, Foster City, Calif.).
Nucleotide sequences, not including PCR primer sequence, were multiple aligned with CLUSTAL W, version 1.4. The distance matrix of the nucleotide substitutions among each isolate was estimated by the eight-parameter method (11), and phylogenetic trees were constructed by the neighbor-joining method (12) from the matrix. These procedures were computed with Phylo_win, version 1.2 (4), on a DEC Alpha 2000 server, and the trees were drawn with TreeView, version 1.5 (10). The reliability and topology of each tree branch were tested by bootstrap analysis (2) of the data of 1,000 bootstrap resamplings of the columns in the 5′-end region sequence alignment of TTV. Bootstrap values greater than 60% were considered supportive of the observed groupings. In addition to our isolates in this study, six previously reported TTV sequences isolated from humans were obtained from the GenBank database and used for comparison with the sequence of the isolates in this study.
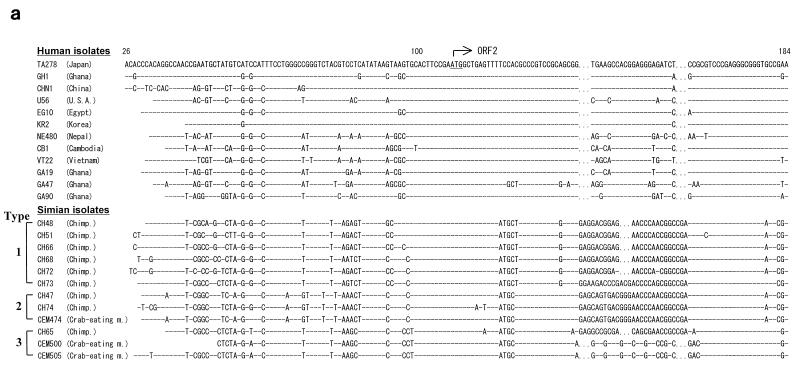
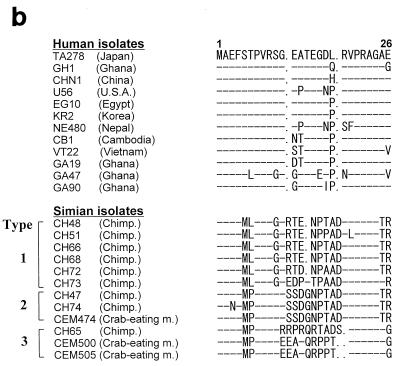
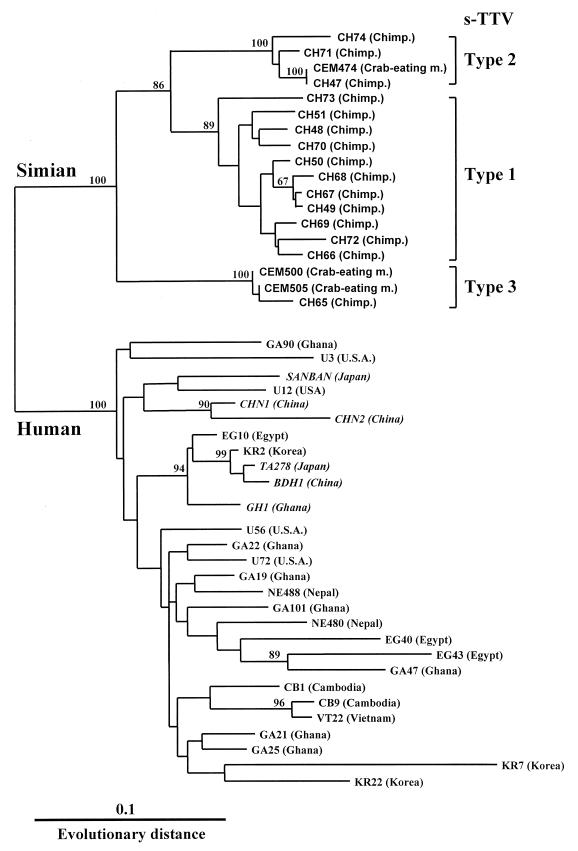
As a result, among 400 serum specimens obtained from nonhuman primates consisting of 24 different species, 87 serum samples from 98 (89%) chimpanzees and 3 from 21 (14%) crab-eating monkeys were TTV DNA positive by PCR assay (Table 1). All infected animals were apparently healthy without evidence of liver diseases. No positive reaction for TTV DNA in the remaining animals was found. To compare the sequence of TTV isolates between simians and humans, we sequenced the amplified PCR products recovered from 15 chimpanzees and 3 crab-eating monkeys. As shown in Fig. 1, the alignment of the nucleotide and amino acid sequence of the representative TTV isolates showed that TTV isolates in simians had a unique arrangement of sequences in several portions compared with those of human isolates. These isolates differed from the TTV genome in humans by 24 to 33% of the nucleotide sequence and 36 to 50% of the amino acid sequence. On the other hand, they had an identity of 80 to 100% at the nucleotide level and 50 to 100% at the amino acid level within TTV isolates from simians. BLAST searches of the DDBJ database with this sequence did not detect significant similarity to any other published sequence, except for the sequence related to the TTV genome. Phylogenetic analysis demonstrated that all TTV isolates obtained from simians were clearly distinct from TTV found in humans, and this difference was strongly supported by bootstrap analysis (Fig. 2). From these results, we have tentatively named this putative virus simian TTV (s-TTV). Furthermore, s-TTV was further divided into three genotypes and showed type 1 as the major genotype. Interestingly, isolates recovered from chimpanzees and crab-eating monkeys belonged to the same genotype. In contrast, there was no significant bootstrap support for genotype classification in the same region of TTV isolates from humans.
FIG. 1.
Alignment of nucleotide (a) and amino acid (b) sequences of TTV and s-TTV isolates. Hyphens indicate identity to the prototype TA278, and dots indicate deletions. U.S.A., United States of America; Chimp., chimpanzee; m., monkey.
FIG. 2.
Phylogram generated by neighbor-joining analysis of genetic distances in the 5′-end region of TTV. The percentages of bootstrap replicates supporting these branches are shown. The database-derived isolates are presented in italics, and their accession numbers are as follows: TA278 (AB008394), GH1 (AF122913), CHN1 (AF079173), CHN2 (AF129887), BDH1 (AF116842), and SANBAN (AB025946). U.S.A., United States of America; Chimp., chimpanzee; m., monkey.
In the present study, we found a high prevalence of s-TTV infection in captured chimpanzees and crab-eating macaques. This finding suggests that s-TTV is widespread among wild chimpanzees living in West Africa. All the animals infected with s-TTV in this study were clinically healthy. Thus s-TTV may be nonpathogenic in chimpanzees and crab-eating monkeys as well as in TTV-infected human individuals, although the pathogenic role of this virus still remains to be investigated. We also sequenced the TTV genomes obtained from human populations in different geographic regions, including Ghana and Southeast Asia. However, our results revealed that there was no sequence similarity of TTV between chimpanzee (born in West Africa) and Ghanaian isolates or crab-eating monkey (born in Southeast Asia) and Southeast Asian isolates. It was noteworthy that s-TTV was classified in a separate group from TTV in humans by phylogenetic analysis. The genetic distance between s-TTV and TTV in humans is too great to allow them to be considered different genotypes. Genomic heterogeneity of s-TTV has been demonstrated, and s-TTV can be divided into genetically distinct genotypes by phylogenetic analysis with bootstrapping. Interestingly, TTVs isolated from chimpanzees and crab-eating monkeys belonged to the same genotypes. This suggests that a single s-TTV strain is prevalent among different species of simians. In addition to the PCR assay with primers in the 5′-end sequence, we have also carried out PCR screening for TTV in simians with primers designed by Okamoto et al. (9) in the ORF1 region of TA278 isolates. However, no positive reaction was found in tested samples (data not shown). This suggests that the s-TTV genome has a different sequence in the ORF1 region from that of TTV in humans. It is also known that the TTV sequence in the ORF1 region has a wide range of sequence divergence despite being a DNA virus (1, 9, 14). To clarify this point, we intend to characterize the extent of the sequence of the s-TTV genome after further study. Studies of the TTV family are important in determining the origin of the virus and may provide useful information and models for studies on the pathogenicity and diversity of this virus.
In conclusion, we identified s-TTV isolates from chimpanzees and crab-eating monkeys that were closely related, but not identical, to the prototype of TTV isolated in human populations.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper have been submitted to the DDBJ, EMBL, and GenBank databases under accession no. AB035154 through AB035171.
Acknowledgments
We thank Takeshi Kurata for continuous encouragement during this study; Ryozaburo Mukai, National Institute of Infectious Diseases, for providing some serum samples; and Mari Yamaguchi and Mitsugu Usui, Sanko Junyaku Co., Ltd., for their collaboration during this study.
REFERENCES
- 1.Abe K, Inami T, Asano K, Miyoshi C, Masaki N, Hayashi S, Ishikawa K, Takebe Y, Win K M, Zayadi A R, Han K-H, Zhang D Y. TT virus infection is widespread in the general populations from different geographic regions. J Clin Microbiol. 1999;37:2703–2705. doi: 10.1128/jcm.37.8.2703-2705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billis D M, Bull J J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol. 1993;42:182–192. [Google Scholar]
- 3.Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998;28:839–842. doi: 10.1002/hep.510280335. [DOI] [PubMed] [Google Scholar]
- 4.Galtier N, Gouy M, Gautier C. SeaView and Phylo_win, two graphic tools for sequence. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 5.Miyata H, Tsunoda H, Kazi A, Yamada A, Khan M A, Murakami J, Kamahora T, Shiraki K, Hino S. Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J Virol. 1999;73:3582–3586. doi: 10.1128/jvi.73.5.3582-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mushahwar I K, Erker J C, Muerhoff A S, Leary T P, Simons J N, Birkenmeyer L G, Chalmers M L, Pilot-Matias T J, Dexai S M. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA. 1999;96:3177–3182. doi: 10.1073/pnas.96.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naoumov N V, Petrova E P, Thomas M G, Williams R. Presence of a new described human DNA virus (TTV) in patients with liver disease. Lancet. 1998;352:195–197. doi: 10.1016/S0140-6736(98)04069-0. [DOI] [PubMed] [Google Scholar]
- 8.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M. Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res. 1998;10:1–16. [Google Scholar]
- 10.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 11.Rzhetsky A N. Tests of applicability of several substitution models for DNA sequence data. Mol Biol Evol. 1995;12:131–151. doi: 10.1093/oxfordjournals.molbev.a040182. [DOI] [PubMed] [Google Scholar]
- 12.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–423. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Hoshino H, Ohta Y, Yoshida N, Mishiro S. Very high prevalence of TT virus (TTV) infection in general population of Japan revealed by a new set of PCR primers. Hepatol Res. 1998;12:233–239. [Google Scholar]
- 14.Takahashi K, Ohta Y, Mishiro S. Partial ∼2.4kb sequence of TT virus genome from eight Japanese isolates: diagnostic and phylogenetic implications. Hepatol Res. 1998;12:111–120. [Google Scholar]
- 15.Tanaka Y, Mizokami M, Orito E, Ohno T, Nakano T, Kato T, Kato H, Mukaide M, Park Y-M, Kim B-S, Ueda R. New genotypes of TT virus (TTV) and a genotyping assay based on restriction fragment length polymorphism. FEBS Lett. 1998;437:201–206. doi: 10.1016/s0014-5793(98)01231-9. [DOI] [PubMed] [Google Scholar]