Abstract
Background
A retrospective cohort study was conducted to collect the data of pregnant women who received hospital delivery in Hangzhou Women's Hospital from January 2018 to December 2020, and who participated in the second trimester (15–20+6 weeks) of free beta human chorionic gonadotropin (free β-hCG). And the study was conducted to explore the relationship between maternal serum free β-hCG and adverse pregnancy outcomes (APO).
Methods
We retrospectively analyzed the clinical data of 1,978 women in the elevated maternal serum free β-hCG group (free β-hCG ≥ 2.50 multiples of the median, MoM) and 20,767 women in the normal group (0.25 MoM ≤ free β-hCG < 2.50 MoM) from a total of 22,745 singleton pregnancies, and modified Poisson regression analysis was used to calculate risk ratios (RRs) and 95% confidence intervals (CI) of the two groups.
Results
The gravidity and parity in the elevated free β-hCG group were lower, and the differences between the groups were statistically significant (all, P < 0.05). The risks of polyhydramnios, preeclampsia, and hyperlipidemia, were increased in women with elevated free β-hCG levels (RRs: 1.996, 95% CI: 1.322–3.014; 1.469, 95% CI: 1.130–1.911 and 1.257, 95% CI: 1.029–1.535, respectively, all P < 0.05), intrauterine growth restriction (IUGR) and female infants were also likely to happen (RRs = 1.641, 95% CI: 1.103–2.443 and 1.101, 95% CI: 1.011–1.198, both P < 0.05). Additionally, there was an association between elevated AFP and free β-hCG levels in second-trimester (RR = 1.211, 95% CI: 1.121–1.307, P < 0.001).
Conclusions
APOs, such as polyhydramnios, preeclampsia, and hyperlipidemia, were increased risks of elevated free β-hCG levels, IUGR and female infants were also likely to happen. Furthermore, there was an association between elevated AFP levels and elevated free β-hCG levels in second-trimester. We recommend prenatal monitoring according to the elevated maternal serum free β-hCG level and the occurrence of APO.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-024-03105-z.
Keywords: Maternal serum, Free beta-subunit human chorionic gonadotropin, Adverse pregnancy outcomes, Risk ratios, Retrospective cohort study
Background
Human chorionic gonadotropin (hCG), a glycoprotein ranging in molecular weight from 36 to 41 kDa of poorly and highly glycosylated forms, is comprised of two non-covalently bound subunits. It has four major isoforms, namely, classical hCG, hyperglycosylated hCG, free β-subunit hCG, and sulfated hCG. This hormone is initially secreted by trophoblasts to promote the secretion of progesterone by the corpus luteum, support the coordinated growth and expansion of the embryo and uterus, mediate signaling in the endometrium, and support the growth and development of the umbilical cord as well as fetal organs [1, 2]. The hCG or free beta-subunit human chorionic gonadotropin (free β-hCG) is synthesized from early pregnancy until 15–20 weeks of pregnancy, after which its synthesis decreases until birth [3, 4].
Maternal serum free β-hCG is a biochemical indicator of aneuploidy, such as trisomy 21, 18 and 13, during second-trimester prenatal screening [5, 6]. There was no agreement on the relationship between serum free β-hCG levels and adverse pregnancy outcomes (APO). Ozdemir et al. [7] reported correlations of the levels of the first- and second-trimester serum markers plasma protein-A (PAPP-A) and alpha-fetoprotein (AFP) with intrauterine growth restriction (IUGR) and macrosomia. In the absence of medically-indicated risk factors for premature delivery, the elevated serum free β-hCG levels of first- and second-trimester pregnant women are associated with a reduced likelihood of spontaneous preterm birth [8]. Likewise, Parry et al. [9] suggested that serum levels, including free β-hCG, in early pregnancy were significantly associated with pregnancy outcomes; however, the assays used to test the analytes did not support their use as clinical biomarkers to predict APO, either alone or in combination with maternal characteristics. Furthermore, Tahe et al. [10] reported that there was a significant difference in β-hCG concentrations between women with preeclampsia and normotensive women, and there was no significant difference in perinatal or maternal outcomes between groups, except in women with β- hCG ≥ 40,000 mIU/mL.
There is an association between abnormal biochemical indicators detected during aneuploidy screening and adverse pregnancy outcomes such as preeclampsia and IUGR. In 2008, the Canadian Association of Obstetricians and Gynecologists made the following recommendations for screening in the second trimester of pregnancy: (i) unexplained maternal serum AFP (> 2.50 MoM), hCG (> 3.00 MoM) and/or inhibin A (≥ 2.00 MoM) levels; and (ii) increased or decreased maternal serum AFP (< 0.25 MoM) and/or unconjugated estriol (< 0.50 MoM) levels, all of which increase the risk of adverse pregnancy outcomes and no specific treatment plan at present [11], long obstetric follow-up is often required.
This retrospective cohort study, which based on the clinical data of 22,745 women in the second trimester of pregnancy (15–20+6 weeks), was conducted to determine the relationship between maternal serum free β-hCG levels and APO.
Materials and methods
Participants
Using a retrospective cohort design, we collected data from 24,001 pregnant women who delivered at the Department of Obstetrics, Hangzhou Women’s Hospital, from January 2018 to December 2020, and participated in maternal serum AFP and free β-hCG screening during the second trimester (15–20+6 weeks). After excluding cases that failed to satisfy the inclusion criteria, a total of 22,608 cases were included, as shown in Fig. 1. Of these, 1,978 cases had elevated maternal serum free β-hCG levels [free β-hCG ≥ 2.50 multiples of the median (MoM)] and 20,767 cases had normal maternal serum free β-hCG levels (0.25 MoM < free β-hCG < 2.50 MoM). This study was approved by the Medical Ethics Committee of Hangzhou Women’s Hospital (2023–002). This research has obtained informed consent from the patients.
Fig. 1.
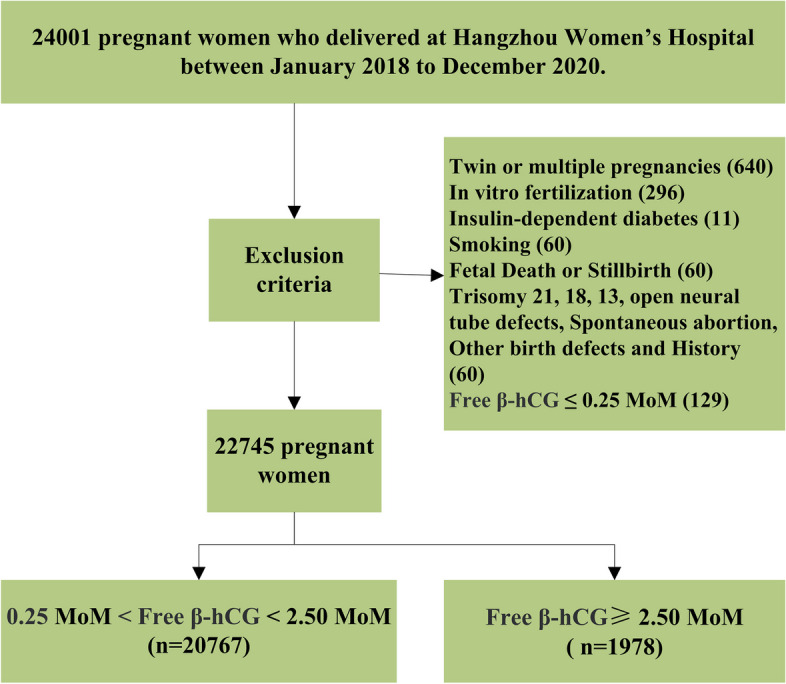
Flow chart of patient selection [12]
Diagnostic and exclusion criteria
APO of complications: gestational hypertension, preeclampsia, gestational diabetes mellitus, intrahepatic cholestasis, thrombocytopenia, hyperlipidemia, arrhythmia, hypothyroidism/hyperthyroidism, premature rupture of membranes, fetal distress, IUGR, premature loss of placenta, oligohydramnios/polyhydramnios, umbilical cord around neck, premature delivery, low birth weight, and macrosomia. All pregnancy complications and pregnancy outcomes were obtained from clinical records diagnosed by obstetricians in hospitals according to the relevant Chinese guidelines [13–16]. Low birth weight referred to a neonatal weight < 2,500 g, whereas macrosomia referred to a newborn weighing > 4,000 g. Premature birth referred to birth days less than 238 days. Oligohydramnios was an amniotic fluid index (AFI) < 5 cm, while the AFI of polyhydramnios was more than 25 cm or a vertical pocket of at least 8 cm. IUGR referred to an estimated weight on ultrasonographic examination below the 10 th percentile adjusted to gestational age [17].
The exclusion criteria were as follows: twin or multiple gestations, in vitro fertilization pregnancies, a history of insulin-dependent diabetes, a history of maternal smoking, fetal death or stillbirth, chromosome abnormalities such as trisomy 18 or 21, fetal open neural tube defects and/or other congenital defects, and free β-hCG ≤ 0.25 MoM in pregnant women with incomplete data [12] (Fig. 1).
Statistical analysis
Statistical analysis was performed with SPSS 21.0 software (IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to determine data normality, and skewed distribution was expressed as median and percentile [M (P2.5—P97.5)]. The Mann–Whitney U test or Chi-Squared test was used for univariate analysis of continuous or categorical data. P < 0.10 was selected as the screening criterion for modified Poisson regression analysis to calculate the risk ratios (RRs) and 95% confidence intervals (CIs) of the influencing factors. The variables were maternal weight, gestational day, AFP MoM, gravidity, parity, hypertension, hyperlipidemia, fetal distress, anemia, postpartum hemorrhage, abnormal amniotic fluid volume, IUGR, and low birth weight and macrosomia, and the main effect method was used to establish the statistical model. APO in pregnant women with elevated free β-hCG levels were assessed by RR. P < 0.05 was considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Basic demographic data
The levels of AFP MoM and free β-hCG MoM in women of the elevated serum free β-hCG group were higher than those in women of the normal serum free β-hCG group (1.13 vs. 1.03, 3.69 vs. 1.08, respectively), whereas gravidity and parity in the elevated serum free β-hCG group were lower than those in the normal serum free β-hCG group (46.30% vs. 49.10%, 27.50% vs. 32.40%, respectively), and the differences were statistically significant (all P < 0.05). No significant difference in maternal age, maternal weight, height, body mass index, gestational age, systolic blood pressure, diastolic blood pressure, mean arterial pressure, mode of delivery, infant weight, infant length, fetal score after birth, and fetal gender was detected between the elevated serum free β-hCG elevated group and the normal serum free β-hCG group (all P > 0.05), as shown in Tables 1 and 2.
Table 1.
Univariate demographic analysis of pregnant in the elevated maternal serum free β-hCG group and normal group n (%)
| Indicators | Groups | Z/x2 | P-values | |
|---|---|---|---|---|
| free β-hCG normal group (n = 20,767) | elevated free β-hCG group (n = 1978) | |||
| AFP MoM | 1.03 (0.55–1.81) | 1.13 (0.58–2.16) | 10.528 | < 0.001* |
| free β-hCG MoM | 1.08 (0.36–2.28) | 3.69 (2.52–7.98) | 73.606 | < 0.001* |
| Maternal age (years old) | 29.00 (23.00–37.00) | 29.00 (23.00–37.00) | 0.235 | 0.814 |
| Categories (Maternal age) (years old) | 0.163 | 0.997 | ||
| 20–24.9 | 10,312 (49.66) | 991 (50.10) | ||
| 25–29.9 | 1460 (7.03) | 138 (6.98) | ||
| 30–34.9 | 7500 (36.11) | 709 (35.84) | ||
| 35–39.9 | 1363 (6.56) | 128 (6.47) | ||
| ≥ 40 | 132 (0.64) | 12 (0.61) | ||
| Maternal weight (kg) | 67.00 (53.00–86.85) | 67.00 (53.00–87.00) | 1.035 | 0.300 |
| Maternal height (cm) | 160.00 (151.00–170.00) | 160.00 (151.00–171.00) | 0.153 | 0.878 |
| BMI (kg/m2) | 25.89 (21.09–32.81) | 25.85 (20.96–33.04) | 1.052 | 0.293 |
| Categories (BMI) | 1.661 | 0.646 | ||
| Thin (< 18.5 kg/m2) | 27 (0.14) | 4 (0.20) | ||
| Obese (25–29.9 kg/m2) | 10,927 (52.62) | 1035 (52.33) | ||
| Obesity (≥ 30 kg/m2) | 2154 (10.37) | 193 (9.76) | ||
| Normal (18.5 kg/m2-25 kg/m2) | 7656 (36.87) | 746 (37.71) | ||
| Gestational age (days) | 273.00 (252.00–287.00) | 273.00 (249.45–287.00) | 1.074 | 0.283 |
| Categories (Gestational days) | 5.027 | 0.081 | ||
| < 259 days | 900 (4.33) | 106 (5.36) | ||
| > 287 days | 240 (1.16) | 19 (0.96) | ||
| Normal (259 days-287 days) | 19,627 (94.51) | 1853 (93.68) | ||
| Systolic blood pressure (SBP) (mmHg) | 118.00 (98.00–138.00) | 118.00 (98.00–138.43) | 0.548 | 0.584 |
| Diastolic blood pressure (mmHg) | 73.00 (60.00–91.00) | 73.00 (60.00–95.00) | 0.964 | 0.335 |
| Arterial pressure difference (MAP) (mmHg) | 87.33 (74.00–105.67) | 87.67 (74.67–108.33) | 0.779 | 0.436 |
| Gravidity | 5.783 | 0.016** | ||
| No(0) | 10,573 (50.90) | 1063 (53.70) | ||
| Yes (≥ 1) | 10,194 (49.10) | 915 (46.30) | ||
| Parity | 20.501 | < 0.001* | ||
| No(0) | 14,034 (67.60) | 1435 (72.50) | ||
| Yes (≥ 1) | 6733 (32.40) | 543 (27.50) | ||
| Mode of delivery | 2.170 | 0.141 | ||
| Vaginal delivery | 14,432 (69.50) | 1343 (67.90) | ||
| Cesarean Section | 6335 (30.50) | 635 (32.10) | ||
Elevated free β-hCG group, 0.25 < free β-hCG MoM < 2.50; free β-hCG normal group, free β-hCG MoM ≥ 2.50
AFP alpha-fetoproteins, free β-hCG free beta-subunit human chorionic gonadotropin, MoM multiples of the median, BMI Body Mass Index, SBP systolic blood pressure, BP Blood Pressure, MAP maternal mean arterial pressure
*P < 0.001
**P < 0.05
Table 2.
Univariate demographic analysis of newborn in the elevated maternal serum free β-hCG and normal groups n (%)
| Indicators | Groups | Z/x2 | P-values | |
|---|---|---|---|---|
| Free β-hCG normal group (n = 20,767) | Elevated free β-hCG group (n = 1978) | |||
| Infant weight | 3300.00 (2450.00–4108.25) | 3300.00 (2194.75–4140.00) | 0.654 | 0.513 |
| Categories (Infant weight) | 18.264 | < 0.001* | ||
| Low birth weight infants (< 2500 g) | 594 (2.86) | 90 (4.55) | ||
| Fetal macrosomia (≥ 4000 g) | 813 (3.91) | 83 (4.20) | ||
| Normal (2500 g-4000 g) | 19,360 (93.22) | 1805 (91.25) | ||
| Infant length | 50.00 (48.00–51.00) | 50.00 (47.00–51.00) | 1.336 | 0.182 |
| Apgar scores | 10.00 (9.00–10.00) | 10.00 (9.00–10.00) | 1.360 | 0.174 |
| Infant gender | 3.803 | 0.149 | ||
| Female | 10,874 (52.36) | 991 (50.10) | ||
| Male | 9893 (47.64) | 987 (49.90) | ||
Elevated free β-hCG group, 0.25 < free β-hCG MoM < 2.50; free β-hCG normal group, free β-hCG MoM ≥ 2.05
Free β-hCG free beta-subunit human chorionic gonadotropin, MoM multiples of the median
*P < 0.001
**P < 0.05
Univariate analysis of related pregnancy complications
Univariate analysis showed that hypertension, hyperlipidemia, anemia, abnormal amniotic fluid volume, fetal distress, postpartum hemorrhage, and IUGR and related categories (infant weight) were correlated with elevated serum free β-hCG levels (all P < 0.10). No significant difference was observed in the risk of influencing factors between the elevated serum free β-hCG MoM group and the normal serum free β-hCG group (all P > 0.10, Tables 3 and 4).
Table 3.
Pregnancy complications of mothers in the elevated maternal serum free β-hCG group and normal group n (%)
| Indicators | Groups | Z/x2 | P-values | |
|---|---|---|---|---|
| Free β-hCG normal group | Elevated free β-hCG group | |||
| (n = 20,767) | (n = 1978) | |||
| Hypertensive disorders of pregnancy (HDP) | 14.316 | < 0.001* | ||
| Preeclampsia | 299 (1.44) | 50 (2.53) | ||
| Gestational hypertension | 717 (3.45) | 71 (3.69) | ||
| Normal blood pressure | 19,751 (95.11) | 1857 (93.88) | ||
| Intrahepatic cholestasis of pregnancy | 0.035 | 0.853 | ||
| No | 20,401 (98.24) | 1942 (98.18) | ||
| Yes | 366 (1.76) | 36 (1.82) | ||
| Hyperlipidaemia | 5.181 | 0.023** | ||
| No | 20,021 (96.41) | 1887 (95.40) | ||
| Yes | 746 (3.59) | 91 (4.60) | ||
| Cesarean scar | 0.508 | 0.476 | ||
| No | 18,260 (87.93) | 1750 (88.47) | ||
| Yes | 2507 (12.07) | 228 (11.53) | ||
| Arrhythmias | 1.217 | 0.270 | ||
| No | 20,703 (99.69) | 1969 (99.54) | ||
| Yes | 64 (0.31) | 9 (0.46) | ||
| Gestational diabetes mellitus | 0.232 | 0.630 | ||
| No | 17,981 (86.58) | 1705 (86.20) | ||
| Yes | 2786 (13.42) | 273 (13.80) | ||
| Thyroid function | 0.063 | 0.969 | ||
| Hyperthyroidism | 45 (0.22) | 4 (0.20) | ||
| Hypothyroidism | 2511 (12.09) | 236 (11.93) | ||
| Normal thyroid function | 18,211 (87.69) | 1738 (87.87) | ||
| Pregnancy with anemia | 3.886 | 0.049** | ||
| No | 15,834 (76.25) | 1469 (74.27) | ||
| Yes | 4933 (23.75) | 509 (25.73) | ||
| Thrombocytopenia | 0.725 | 0.395 | ||
| No | 20,500 (98.71) | 1957 (98.94) | ||
| Yes | 267 (1.29) | 21 (1.06) | ||
| Uterine Inertia | 1.963 | 0.161 | ||
| No | 20,014 (96.37) | 1894 (95.75) | ||
| Yes | 753 (3.63) | 84 (4.25) | ||
| Amniotic fluid volume | 9.981 | 0.007 | ||
| Polyhydramnios | 92 (0.44) | 19 (0.96) | ||
| Oligohydramnios | 1113 (5.36) | 104 (5.26) | ||
| Normal amniotic fluid volume | 19,562 (94.20) | 1855 (93.78) | ||
| Placenta previa | 0.738 | 0.390 | ||
| No | 20,633 (99.35) | 1962 (99.19) | ||
| Yes | 134 (0.65) | 16 (0.81) | ||
| Placental abruption | 0.154 | 0.695 | ||
| No | 20,665 (99.51) | 1967 (99.44) | ||
| Yes | 102 (0.49) | 11 (0.56) | ||
Elevated free β-hCG group, 0.25 < free β-hCG MoM < 2.50; free β-hCG normal group, free β-hCG MoM ≥ 2.50
Free β-hCG free beta-subunit human chorionic gonadotropin, MoM multiples of the median
*P < 0.001
**P < 0.05
Table 4.
Pregnancy outcomes of mothers in the elevated maternal serum AFP group and normal group n (%)
| Indicators | Groups | Z/x2 | P-values | |
|---|---|---|---|---|
| Free β-hCG normal group (n = 20,767) | Elevated free β-hCG group (n = 1978) | |||
| Fetal distress | 2.986 | 0.084 | ||
| No | 18,830 (90.67) | 1770 (89.48) | ||
| Yes | 1937 (9.33) | 208 (10.52) | ||
| Postpartum hemorrhage | 3.836 | 0.050 | ||
| No | 20,707 (99.71) | 1977 (99.95) | ||
| Yes | 60 (0.29) | 1 (0.05) | ||
| Premature rupture of menbranes | 0.949 | 0.330 | ||
| No | 15,960 (76.85) | 1501 (75.88) | ||
| Yes | 4807 (23.15) | 477 (24.12) | ||
| Cord entanglement | 1.844 | 0.174 | ||
| No | 14,329 (69.00) | 1394 (70.48) | ||
| Yes | 6438 (31.00) | 584 (29.52) | ||
| Cesarean delivery | 1.979 | 0.159 | ||
| No | 14,438 (69.52) | 1345 (68.00) | ||
| Yes | 6329 (30.48) | 633 (32.00) | ||
| IUGR | 20.763 | < 0.001* | ||
| No | 20,680 (99.58) | 1955 (98.84) | ||
| Yes | 87 (0.42) | 23 (1.16) | ||
Elevated free β-hCG group, 0.25 < free β-hCG MoM < 2.50; free β-hCG normal group, free β-hCG MoM ≥ 2.50
Free β-hCG free beta-subunit human chorionic gonadotropin, MoM multiples of the median, IUGR intrauterine growth retardation
*P < 0.001
**P < 0.05
Modified Poisson regression analysis
As shown in Table 5, the risk of pregnancy complications, such as polyhydramnios, preeclampsia, and hyperlipidemia, was increased in women with elevated serum free β-hCG levels, with RRs of 1.996, 95% CI: 1.322–3.014; 1.469, 95% CI: 1.130–1.911 and 1.257, 95% CI: 1.029–1.535, respectively (all P < 0.05), and women with elevated serum free β-hCG levels were more likely to have IUGR and female infants, with RRs of 1.641, 95% CI: 1.103–2.443) and 1.101, 95% CI: 1.011–1.198) (both P < 0.05). Additionally, maternal characteristics, such as elevated AFP levels, were correlated with elevated β-hCG levels, with an RR of 1.211, 95% CI: 1.121–1.307, P < 0.001.
Table 5.
Modified Poisson regression analysis of maternal characteristics and pregnancy outcomes
| Indicators | β | Standard error | Wald | df | P-values | RR | 95% CI for RR |
|---|---|---|---|---|---|---|---|
| (intercept) | -1.249 | 0.5161 | 5.858 | 1 | 0.016** | 0.287 | 0.104–0.789 |
| Gravidity | |||||||
| ≥ 1 | 0.048 | 0.0572 | 0.690 | 1 | 0.406 | 1.049 | 0.937–1.173 |
| = 0a | 1 | ||||||
| Parity | |||||||
| ≥ 1 | -0.243 | 0.0646 | 14.189 | 1 | < 0.001* | 0.784 | 0.691–0.890 |
| = 0a | 1 | ||||||
| HDP | |||||||
| Preeclampsia | 0.385 | 0.1341 | 8.226 | 1 | 0.004** | 1.469 | 1.130–1.911 |
| Gestational hypertension | 0.048 | 0.1159 | 0.170 | 1 | 0.680 | 1.049 | 0.836–1.316 |
| Normal blood pressurea | 1 | ||||||
| Hyperlipidaemia | |||||||
| Yes | 0.229 | 0.102 | 5.019 | 1 | 0.025** | 1.257 | 1.029–1.535 |
| Noa | 1 | ||||||
| Fetal distress | |||||||
| Yes | 0.041 | 0.0721 | 0.323 | 1 | 0.570 | 1.042 | 0.905–1.20 |
| Noa | 1 | ||||||
| Pregnancy with anemia | |||||||
| Yes | 0.087 | 0.0491 | 3.117 | 1 | 0.077 | 1.091 | 0.991–1.201 |
| Noa | 1 | ||||||
| Amniotic fluid volume | |||||||
| Polyhydramnios | 0.691 | 0.2103 | 10.800 | 1 | 0.001** | 1.996 | 1.322–3.014 |
| Oligohydramnios | -0.068 | 0.0972 | 0.486 | 1 | 0.486 | 0.934 | 0.772–1.131 |
| Normal amniotic fluid volumea | 1 | ||||||
| IUGR | |||||||
| Yes | 0.496 | 0.2028 | 5.971 | 1 | 0.015** | 1.641 | 1.103–2.443 |
| Noa | 1 | ||||||
| Infant weight (g) | |||||||
| ≥ 4000 g | 0.113 | 0.1098 | 1.056 | 1 | 0.304 | 1.119 | 0.903–1.388 |
| < 2500 g | 0.224 | 0.1325 | 2.872 | 1 | 0.090 | 1.252 | 0.965–1.623 |
| 2500 g-4000 ga | 1 | ||||||
| Gestational days | |||||||
| > 287 days | -0.224 | 0.2229 | 1.009 | 1 | 0.315 | 0.799 | 0.516–1.237 |
| < 259 days | 0.041 | 0.1137 | 0.127 | 1 | 0.721 | 1.041 | 0.833–1.301 |
| 287—259 daysa | 1 | ||||||
| Infant gender | |||||||
| Female | 0.096 | 0.0432 | 4.948 | 1 | 0.026** | 1.101 | 1.011–1.198 |
| Malea | 1 | ||||||
| Maternal weight | -0.005 | 0.0029 | 2.643 | 1 | 0.104 | 0.995 | 0.990–1.001 |
| Gestational days | -0.01 | 0.0041 | 6.015 | 1 | 0.014** | 0.990 | 0.982–0.998 |
| AFP MoM | 0.191 | 0.0392 | 23.798 | 1 | < 0.001* | 1.211 | 1.121–1.307 |
Model: (intercept), gravidity, parity, HDP (gestational hypertension, preeclampsia), Hyperlipidaemia, Fetal distress, Pregnancy with anemia, Amniotic fluid volume, IUGR, Infant gender, Maternal weight, Gestational days, AFP MOM
HDP hypertensive disorders of pregnancy, IUGR intrauterine growth retardation, AFP alpha-fetoproteins, MoM multiples of the median, RR relative risk, CI confidence intervals
*P < 0.001
**P < 0.05
aReference
Discussion
In this study, the pregnancy outcomes of 1978 s-trimester pregnant women with serum free β-hCG ≥ 2.50 MoM and 20,767 s-trimester pregnant women with serum free β-hCG 0.25 MoM < free β-hCG < 2.50 MoM in Hangzhou, China, were analyzed. The results indicated that the risk of pregnancy complications, such as polyhydramnios, preeclampsia, and hyperlipidemia, was increased in women with elevated serum free β-hCG levels, and elevated serum free β-hCG levels were related to APO such as IUGR. Additionally, there was an association between elevated AFP levels and elevated β-hCG levels in the sera of pregnant women.
The results of this study showed that polyhydramnios (RR: 1.996, 95% CI: 1.322–3.014) was higher in pregnant women with high serum free β-hCG levels than that in pregnant women with normal levels of the hormone, consistent with previous studies that reported a correlation of second-trimester non-immune hydrops (including hydramnios) with elevated maternal hCG levels [18] as well as a correlation of placental chorioangioma with early severe polyhydramnios and elevated maternal serum hCG levels [19]. In China, researchers have used proteomic techniques to identify many biomarkers for pregnancy-related pathological conditions, such as β-hCG for Down's syndrome and AFP for trisomy 13,18 syndrome, which were almost derived from or secreted by fetal, maternal circulation, and placental tissues [20]. The cohort study of Tashfeen et al. [21] showed that polyhydramnios could increase the risk of adverse perinatal outcomes, and polyhydramnios was positively correlated with maternal age, diabetes, fetal malformation and macrosomia. Pregnant women with high serum levels of free β-hCG were more likely to give birth to female infants (RR: 1.101, 95% CI: 1.011–1.198), which was similar to the results of a recent review report [22], which pointed out that pregnant women with female fetuses have higher serum β-hCG levels than those with male fetuses.
Free β-hCG is produced by the trophoblast layer outside the villi, and syncytiotrophoblast cells are the main placental source of free β-hCG. The main function of free β-hCG is to promote the synthesis of progesterone in the corpus luteum, maintain the resting state of the uterine muscle layer and pregnancy, until the placenta itself ensures the production of progesterone [23]. The exact time and form of free β-hCG secretion peak in early pregnancy are still not reasonably explained. Placental ischemic diseases such as preeclampsia or IUGR are characterized by abnormal invasion of trophoblasts, which can lead to reduced uterine placental blood flow (detected by Doppler ultrasound) and uterine placental ischemia, resulting in excessive or insufficient production of various pro angiogenic and antiangiogenic factors, which in turn affect the clearance rate or biological activity of free β-hCG, leading to high concentrations of free β-hCG [24, 25]. The results of our study demonstrated that preeclampsia was a risk for APO in pregnant women with elevated serum free β-hCG levels (RR: 1.469, 95% CI: 1.130–1.911), consistent with 2008 SOGC guidelines [11], which stated that hCG > 3.00 MoM was associated with an increased prevalence of APO. However, the guidelines failed to propose a relevant treatment plan. Tancrede et al. [26] confirmed that serum free β-hCG > 2.00 MoM was associated with a risk of delivery before term (RR: 4.60, 95% CI: 2.30–9.10], whereas serum free β- hCG > 2.00 MoM had no effects on some pregnancy outcomes (such as preeclampsia, IUGR, fetal death) after term (RR: 1.10, 95% CI: 0.70–1.70). These findings agree with those of another study in which no differences in pregnancy outcomes between women with serum free β-hCG > 2.00 MoM and those with normal levels of the hormone were reported, although the risks of preeclampsia and low birth weight were higher in the elevated group (cut-off level of incidence, 2.5) than in the control group [27]. This study also showed that IUGR is also a placenta-related factor (RR: 1.641, 95% CI: 1.103–2.443). This was similar to the research findings of Kiyokoba et al. [28], who also pointed out that IUGR and preeclampsia (PE) with IUGR (PE/IUGR) were high-risk perinatal diseases that might involve high levels of hCG and mitochondrial dysfunction, it could also elevated expression of β-hCG and growth differentiation factor mRNA and protein levels in the placenta with both diseases, which played an important role in the pathogenesis of IUGR and PE/IUGR.
The pregnant women were divided into two groups according to second-trimester biomarkers: the high-risk group [patients with AFP and/or hCG above the 75th percentile; odds ratio (OR) of pathological placentation: 2.27] and the low-risk group (patients with AFP and/or hCG below the 25th percentile; OR for pathological placentation: 0.38) [29]. High levels of AFP, free β-hCG, and inhibin A but a low level of unconjugated estriol significantly increased the risk of small for gestational age fetuses [30]. The incidence of APO, such as premature delivery, stillbirth, limb deformities, and chromosomal abnormalities in the maternal serum free β-hCG ≥ 2.00 MoM group, was significantly higher than that in the control group [31, 32], whereas the risk of gestational hypertension (OR: 1.72), premature delivery (OR: 2.50), and stillbirth (OR: 3.93) were increased in the elevated maternal serum β-hCG group [33].
The results of this study revealed an association between hyperlipidemia (RR: 1.257, 95% CI: 1.029–1.535) and elevated maternal serum free β-hCG levels, but few studies support this finding. Only in a previous report mentioned that the concentration of free β-hCG in postmenopausal women was much higher than that in premenopausal women (P < 0.001). Correspondingly, triglyceride (TG) was elevated (P < 0.05) [34]. Therefore, the relationship between hyperlipidemia and maternal serum free β-hCG requires further study.
The results of this study also suggested a correlation between elevated AFP levels (RR: 1.211, 95% CI: 1.121–1.307) and elevated β-hCG levels in second-trimester pregnant women. Chandra et al. [35] reported that, in addition to placental abruption, unexplained elevated maternal serum AFP or hCG levels were independently associated with pregnancy outcomes in both high risk and low risk women, and the RR of fetal death was high (in high risk and low risk women, the RR of AFP or hCG was 4.90, 95%CI: 2.70–8.70), suggesting that antenatal monitoring of patients with elevated AFP and/or hCG levels should be conducted regardless of prenatal risk status.
A previous study has reported that the association between placental-related histopathological changes and pregnancy complications was weak due to unexplained elevated maternal serum AFP and hCG levels [36]. Doppler ultrasound scans of the uterine artery and morphological examinations of the placenta revealed that most biochemical abnormalities in pregnant women resulted in premature delivery and/or perinatal death [37], which led us to recommend placental ultrasound for women with abnormal biochemical indicators when no fetal abnormality is found.A recent report showed that maternal serum free β-hCG concentration was associated with chromosomal structural abnormalities, When the maternal serum free β-hCG ≥ 5.00 MoM, the relative risk of trisomy 21 was higher. When free β-hCG < 0.20 MoM, the probability of trisomy 13, trisomy 18 and Turner syndrome also increased. Therefore, Doppler ultrasonography combined with the concentration of maternal serum free β-hCG can monitor fetal development to some extent [38].
In this study, we retrospectively analyzed APO, such as IUGR and preeclampsia, caused by elevated serum free β-hCG levels in the second trimester. However, there were several limitations. First, this study only included pregnancy outcomes of singleton-pregnant women conceived naturally in Hangzhou, China, from 2018 to 2020. Second, this study explored the relationship between pregnancy outcomes, such as IUGR and preeclampsia with elevated serum free β-hCG levels, and failed to investigate how the physiological mechanisms of IUGR and preeclampsia were affected, such as placental related disease mechanisms and abnormal umbilical artery diastolic blood flow. Lastly, additional studies of longer follow-up periods and larger sample sizes are needed.
Conclusions
In conclusion, this study demonstrated that elevated maternal serum free β-hCG levels were associated with pregnancy complications such as polyhydramnios, preeclampsia, and hyperlipidemia. It is worth noting that the risk of APO could be monitored by the high level of maternal serum free β-hCG during the second trimester.
Supplementary Information
Acknowledgements
The authors are grateful to all of the participants and contributors. We would like to thank Songhe Chen from Hangzhou Women’s Hospital for helping to collect the data. We would also like to thank Yezhen Shi of the Data Analysis Department, Zhejiang Biosan Biochemical Technologies Co., Ltd., for their contribution to data matching. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Authors’ contributions
Y.M. Chen and X.Q. Dai, design and statistical analysis; Bin Wu wrote the first draft of the manuscript. Chen Jiang and Y.X. Yin, provision of study material or patients; X.Q. Dai and Y.X. Yin writing-review & editing. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Authors’ information
Yiming Chen, MBBS, Chief Technician, Master's supervisor in clinical laboratory diagnosis. Published over 102 papers in journals such as Clinical Chimica Acta, including 24 SCI papers. Hosted two projects funded by Zhejiang Natural Science Foundation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Zhejiang Medicine and Health Scientific Research Project [grant number 2021KY258]. The Joint Fund Project of Zhejiang Provincial Natural Science Foundation of China [under Grant No. LBY23H200009].
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
This study has been conducted under the approval of the Human Research Ethics Committee of the Hangzhou Hospital ([2023] Medical Ethics Review A (002)), and the procedures have been performed by the Declaration of Helsinki. This research has obtained informed consent from the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yiming Chen and Xiaoqing Dai are co-first author and contributed equally to this work.
References
- 1.D'Hauterive SP, Close R, Gridelet V, Mawet M, Nisolle M, Geenen V. Human Chorionic gonadotropin and early embryogenesis: Review. Int J Mol Sci. 2022;23(3):1380: 1-16. doi: 10.3390/ijms23031380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogino MH, Tadi P. Physiology, chorionic gonadotropin. 2022. [PubMed] [Google Scholar]
- 3.Friis PJ, Friis-Hansen LJ, Jensen AK, Nyboe AA, Lokkegaard E. Early pregnancy reference intervals; 29 serum analytes from 4 to 12 weeks' gestation in naturally conceived and uncomplicated pregnancies resulting in live births. Clin Chem Lab Med. 2019;57(12):1956–1967. doi: 10.1515/cclm-2019-0495. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Wang X, Chen Y, Ning W, Chen L, Yin Y, et al. Construction and predictive value of risk models of maternal serum alpha-fetoprotein variants and fetal open neural tube defects. Exp Biol Med (Maywood) 2022;247:822–831. doi: 10.1177/15353702221080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Wang X, Li L, Lu S, Zhang Z. New cut-off values for screening of trisomy 21, 18 and open neural tube defects (ONTD) during the second trimester in pregnant women with advanced maternal age. BMC Pregnancy Childbirth. 2020;20(1):776. doi: 10.1186/s12884-020-03464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Chen Y, Shi Y, Ning W, Wang X, Li L, et al. External quality assessment of maternal serum levels of alpha-fetoprotein, free beta-human chorionic gonadotropin, and unconjugated estriol in detecting Down syndrome and neural tube defects in the second trimester of 87 maternal serum samples, based on 105–139 days. Med Sci Monit. 2022;28:e935573. doi: 10.12659/MSM.935573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozdemir S, Sahin O, Acar Z, Demir GZ, Ermin E, Aydin A. Prediction of pregnancy complications with maternal biochemical markers used in Down syndrome screening. Cureus. 2022;14(3):e23115. doi: 10.7759/cureus.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soni S, Krantz DA, Blitz MJ, Vohra N, Rochelson B. Elevated maternal serum-free beta-human chorionic gonadotropin (beta-hCG) and reduced risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med. 2019;32(19):3191–3196. doi: 10.1080/14767058.2018.1459554. [DOI] [PubMed] [Google Scholar]
- 9.Parry S, Carper BA, Grobman WA, Wapner RJ, Chung JH, Haas DM, et al. Placental protein levels in maternal serum are associated with adverse pregnancy outcomes in nulliparous patients. Am J Obstet Gynecol. 2022;227(3):497.e1-497.e13. doi: 10.1016/j.ajog.2022.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taher SI, Alalaf SK. Association between serum beta-human chorionic gonadotropin and preeclampsia and its effects on perinatal and maternal outcomes: a case control study. Arch Gynecol Obstet. 2019;299(3):713–718. doi: 10.1007/s00404-019-05041-y. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon A, Wilson RD. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30(10):918–932. doi: 10.1016/S1701-2163(16)32973-5. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Zhang H, Wu B, Ning W, Chen Y, Chen Y. Correlation between elevated maternal serum alpha-fetoprotein and ischemic placental disease: a retrospective cohort study. Clin Exp Hypertens. 2023;45(1):2175848. [DOI] [PubMed]
- 13.Chinese Medical Association Obstetrics and Gynecology Branch Pregnancy Hypertension Disease Group Guidelines for Diagnosis and Treatment of Hypertension during Pregnancy (2015) Chin J Obstet Gynecol. 2015;50:721–728. [Google Scholar]
- 14.Department of Obstetrics and Gynecology, Chinese Medical Association. Guidelines for diagnosis and treatment of intrahepatic cholestasis of pregnancy. Chinese J Obstet Gynecol. 2015;2015(7):5.
- 15.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehsanipoor R. Premature rupture of membranes: ACOG Practice Bulletin, Number 139. Obstet Gynecol. 2013;122(4):918–930. doi: 10.1097/01.AOG.0000435415.21944.8f. [DOI] [PubMed] [Google Scholar]
- 17.Jiang C, Wen H, Hu T, Liu Y, Dai X, Chen Y. Perinatal characteristics and pregnancy outcomes of advanced maternal age women with gestational diabetes mellitus: a retrospective cohort study. Health Sci Rep. 2024;7(2):e1903. [DOI] [PMC free article] [PubMed]
- 18.Saller DJ, Canick JA, Oyer CE. The detection of non-immune hydrops through second-trimester maternal serum screening. Prenat Diagn. 1996;16(5):431–435. doi: 10.1002/(SICI)1097-0223(199605)16:5<431::AID-PD877>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Bashiri A, Maymon E, Wiznitzer A, Maor E, Mazor M. Chorioangioma of the placenta in association with early severe polyhydramnios and elevated maternal serum HCG: a case report. Eur J Obstet Gynecol Reprod Biol. 1998;79(1):103–105. doi: 10.1016/S0301-2115(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 20.Cen J, Lv L, Wei Y, Deng L, Huang L, Deng X, et al. Comparative proteome analysis of amniotic fluids and placentas from patients with idiopathic polyhydramnios. Placenta. 2020;1(89):67–77. doi: 10.1016/j.placenta.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Tashfeen K, Hamdi IM. Polyhydramnios as a predictor of adverse pregnancy outcomes. Sultan Qaboos Univ Med J. 2013;13(1):57–62. doi: 10.12816/0003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glowska-Ciemny J, Szymanski M, Pankiewicz J, Malewski Z, von Kaisenberg C, Kocylowski R. Influence of selected factors on serum AFP levels in pregnant women in terms of prenatal screening accuracy - literature review. Ginekol Pol. 2023;94(2):158–166. doi: 10.5603/GP.a2022.0148. [DOI] [PubMed] [Google Scholar]
- 23.Guibourdenche J, Leguy MC, Pidoux G, Hebert-Schuster M, Laguillier C, Anselem O, et al. Biochemical screening for fetal trisomy 21: pathophysiology of maternal serum markers and involvement of the placenta. Int J Mol Sci. 2023;24(8):7669. doi: 10.3390/ijms24087669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berndt S, Blacher S, Munaut C, Detilleux J, Perrier d'Hauterive S, Huhtaniemi I, et al. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013;27(4):1309–1321. doi: 10.1096/fj.12-213686. [DOI] [PubMed] [Google Scholar]
- 25.Butler SA, Luttoo J, Freire MO, Abban TK, Borrelli PT, Iles RK. Human chorionic gonadotropin (hCG) in the secretome of cultured embryos: hyperglycosylated hCG and hCG-free beta subunit are potential markers for infertility management and treatment. Reprod Sci. 2013;20(9):1038–1045. doi: 10.1177/1933719112472739. [DOI] [PubMed] [Google Scholar]
- 26.Tancrede S, Bujold E, Giguere Y, Renald MH, Girouard J, Forest JC. Mid-trimester maternal serum AFP and hCG as markers of preterm and term adverse pregnancy outcomes. J Obstet Gynaecol Can. 2015;37(2):111–116. doi: 10.1016/S1701-2163(15)30331-5. [DOI] [PubMed] [Google Scholar]
- 27.Dimitrova V, Chernev T, Vragaleva S, Sluncheva B, Mikhailova E, Kremenski I, Simeonov E. Pregnancy complications with abnormal results of biochemical screening for Down syndrome in second trimester and normal fetal karyotype. Akush Ginekol (Sofiia) 2002;41(6):3–12. [PubMed] [Google Scholar]
- 28.Kiyokoba R, Uchiumi T, Yagi M, Toshima T, Tsukahara S, Fujita Y, et al. Mitochondrial dysfunction-induced high hCG associated with development of fetal growth restriction and pre-eclampsia with fetal growth restriction. Sci Rep. 2022;12(1):4056. doi: 10.1038/s41598-022-07893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berezowsky A, Pardo J, Ben-Zion M, Wiznitzer A, Aviram A. Second trimester biochemical markers as possible predictors of pathological placentation: a retrospective case-control study. Fetal Diagn Ther. 2019;46(3):187–192. doi: 10.1159/000492829. [DOI] [PubMed] [Google Scholar]
- 30.Boonpiam R, Wanapirak C, Sirichotiyakul S, Sekararithi R, Traisrisilp K, Tongsong T. Quad test for fetal aneuploidy screening as a predictor of small-for-gestational age fetuses: a population-based study. BMC Pregnancy Childbirth. 2020;20(1):621. doi: 10.1186/s12884-020-03298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Dongmei. The correlation between maternal serum free β-HCG levels and adverse pregnancy outcomes in mid-pregnancy. Kunming: Master, Kunming Medical College; 2010.
- 32.Li D, Zhao F, Zhang Y, Zhu B, Dong X, Su J, Yin Y. The correlation between the high level of serum free β-HCG in the mother during the middle pregnancy and the adverse pregnancy outcomes was studied. Prog Mod Obstet Gynecol. 2011;20(03):186–189. [Google Scholar]
- 33.Yan S. Predictive value of serum AFP and β-hCG for adverse pregnancy outcomes in pregnant women with midtrimester screening. Master: Suzhou University; 2020. [Google Scholar]
- 34.Liao Y, Chen M, Han Z, Han D, et al. The relationship between human chorionic gonadotrophin and lipoprotein metabolism. Hua Xi Yi Ke Da Xue Xue Bao. 1996;27(2):122–5. [PubMed] [Google Scholar]
- 35.Chandra S, Scott H, Dodds L, Watts C, Blight C, Van Den Hof M. Unexplained elevated maternal serum alpha-fetoprotein and/or human chorionic gonadotropin and the risk of adverse outcomes. Am J Obstet Gynecol. 2003;189(3):775–781. doi: 10.1067/S0002-9378(03)00769-5. [DOI] [PubMed] [Google Scholar]
- 36.Salim R, Okopnik M, Garmi G, Nachum Z, Zafran N, Shalev E. Lack of association between unexplained elevated maternal serum alpha fetoprotein and/or human chorionic gonadotropin and the occurrence of placental thrombotic lesions. Placenta. 2010;31(4):277–281. doi: 10.1016/j.placenta.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Alkazaleh F, Chaddha V, Viero S, Malik A, Anastasiades C, Sroka H, et al. Second-trimester prediction of severe placental complications in women with combined elevations in alpha-fetoprotein and human chorionic gonadotrophin. Am J Obstet Gynecol. 2006;194(3):821–827. doi: 10.1016/j.ajog.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Younesi S, Eslamian L, Khalafi N, Taheri AM, Saadati P, Jamali S, et al. Extreme beta HCG levels in first trimester screening are risk factors for adverse maternal and fetal outcomes. Sci Rep. 2023;13(1):1228. doi: 10.1038/s41598-023-28561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


