Summary
Ovarian clear cell carcinoma (OCCC) is a gynecological cancer with a dismal prognosis; however, the mechanism underlying OCCC chemoresistance is not well understood. To explore the intracellular networks associated with the chemoresistance, we analyze surgical specimens by performing integrative analyses that combine single-cell analyses and spatial transcriptomics. We find that a chemoresistant OCCC subpopulation with elevated HIF activity localizes mainly in areas populated by cancer-associated fibroblasts (CAFs) with a myofibroblastic phenotype, which is corroborated by quantitative immunostaining. CAF-enhanced chemoresistance and HIF-1α induction are recapitulated in co-culture assays, which show that cancer-derived platelet-derived growth factor (PDGF) contributes to the chemoresistance and HIF-1α induction via PDGF receptor signaling in CAFs. Ripretinib is identified as an effective receptor tyrosine kinase inhibitor against CAF survival. In the co-culture system and xenograft tumors, ripretinib prevents CAF survival and suppresses OCCC proliferation in the presence of carboplatin, indicating that combination of conventional chemotherapy and CAF-targeted agents is effective against OCCC.
Keywords: ovarian clear cell carcinoma, chemoresistant niche, HIF-1α, cancer-associated fibroblasts, PDGF
Graphical abstract
Highlights
-
•
HIF-1α-positive cancer cells and CAFs form chemoresistant niches of OCCC
-
•
PDGF from HIF-1α-positive cancer cells activates CAFs
-
•
Activated CAFs induce HIF-1α expression and chemoresistance in cancer cells
-
•
Inhibition of PDGF signaling in CAFs augments chemosensitivity of cancer cells
Through an integration of spatial analyses and co-culture studies, Mori et al. demonstrate that feedback activation between HIF-1α-positive cancer cells and cancer-associated fibroblasts (CAF) contributes to chemoresistance of ovarian clear cell carcinoma. Targeted inhibition of CAF activation by a receptor tyrosine kinase inhibitor augmented chemosensitivity, suggesting possible application for CAF-targeted therapy.
Introduction
Although molecular-targeted and immune-based therapies have made a substantial impact on refractory cancers, many tumors eventually acquire chemoresistance. Such resistance can be attributed to intratumor cell-cell networks, which enable cancer cells to survive and proliferate after therapy.1 Notably, cancer cells gain chemoresistance by forming cellular networks with non-tumor cells, such as cancer-associated fibroblasts (CAFs).2,3 Therefore, to understand the biological basis of resistance, it is important to gain a global picture of intratumor heterogeneity and the cell-cell networks by which resistant cancer cells survive.
While both genetic and epigenetic alterations contribute to tumor heterogeneity, gene expression analyses in a variety of clinical tumors at the single-cell level have led to marked progress in understanding the epigenetic heterogeneity of clinical tumors.4,5 Transcriptome analyses using single-cell RNA sequencing (scRNA-seq) or single-nucleus RNA-seq (snRNA-seq) have revealed the cellular composition of clinical tumors and the gene expression profiles of each population.6 In addition, recent progress in spatial transcriptomics and other spatial omics approaches has enabled researchers to visualize gene expression in histological specimens and to stratify histological regions based upon these expression profiles.7,8,9 In particular, integrative analyses of spatial transcriptomics using single-cell analysis has allowed the location of identified subpopulations to be visualized within tissue samples.10
Epithelial ovarian cancer (EOC) is a major gynecological malignancy.11 Among the major subtypes of EOC, ovarian clear cell carcinoma (OCCC) at advanced stages is associated with a poor prognosis, which is mainly attributed to chemoresistance to standard regimens that include paclitaxel and carboplatin.12,13 While alternative treatments for OCCC, such as immune checkpoint inhibitors or molecular targeted therapy against receptor tyrosine kinases, are in clinical trials, no effective alternative to standard chemotherapy has been developed.14 Therefore, to develop an effective therapy for advanced OCCC is one of the major unmet medical needs in the field of gynecological oncology.
Previous studies examined the cellular heterogeneity of high-grade serous ovarian cancer (HGSOC), a major subtype of EOC, by performing scRNA-seq of patient ascites and found extensive inter-patient variability both in the tumor and non-tumor populations.15 In another report, clinical specimens of HGSOC were examined by spatial transcriptomics to assess differences in spatial composition between poor responders and excellent responders to adjuvant chemotherapy.16 Despite these pioneering studies, the cellular networks that define chemoresistance of HGSOC remain unclear, and no integrative studies of EOC, including OCCC, have been reported.
In this paper, to elucidate the cell-cell networks responsible for chemoresistance of OCCC, we performed integrative analyses of surgical specimens by combining snRNA-seq and spatial transcriptomics. By comparing results from chemosensitive and chemoresistant cases of OCCC, we identified a chemoresistant subpopulation that was localized in CAF-populated areas and associated with HIF-1α activation. Further investigations combining quantitative analyses of immunostaining, in vitro co-culture studies, and tumor xenograft models suggested that a feedback activation loop between HIF-1α-activated cancer cells and platelet-derived growth factor (PDGF)-activated CAF is the biological basis of OCCC chemoresistance. Furthermore, we demonstrated that inhibition of PDGF-mediated CAF activation, in combination with carboplatin, blocks OCCC proliferation, indicating effectiveness of CAF-targeting therapy for refractory cancers.
Results
Identification of a cancer cell subpopulation associated with chemoresistance of OCCC
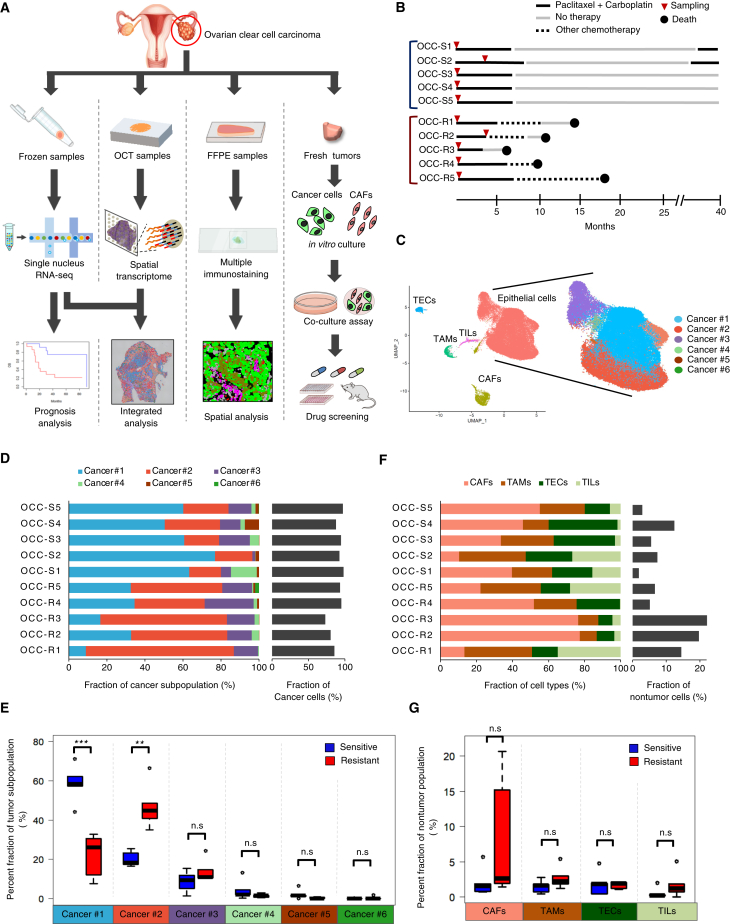
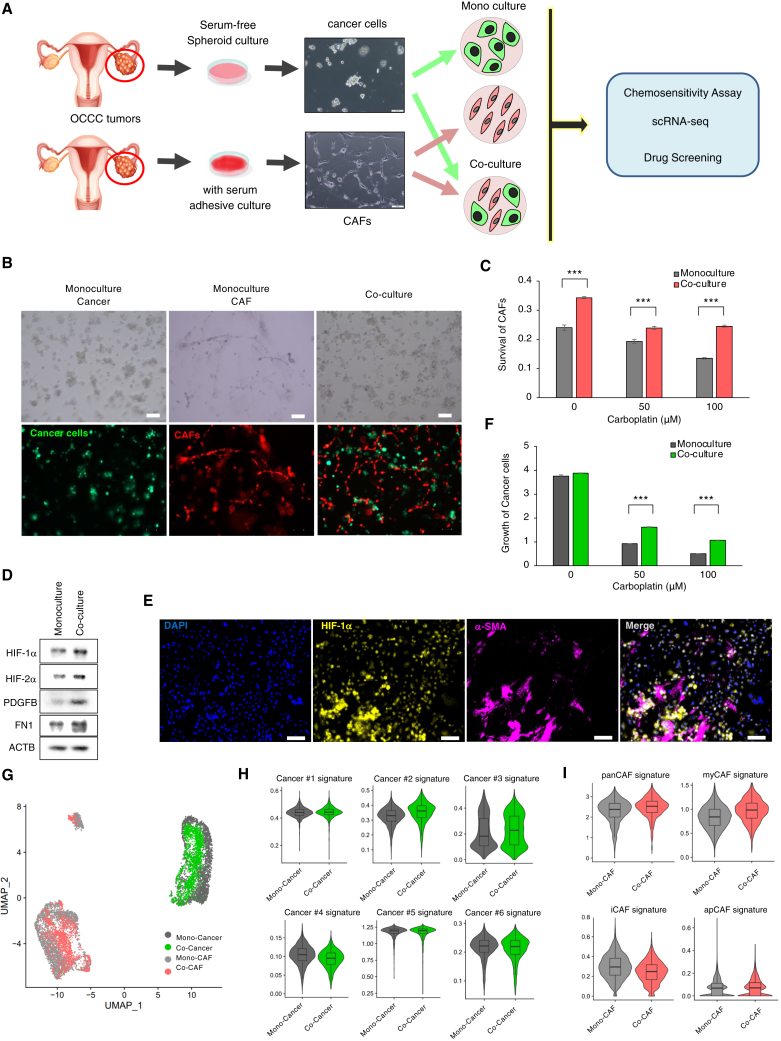
To identify intratumor networks responsible for chemoresistance of OCCC, we obtained frozen samples from surgical specimens and performed integrative analyses that combine single-cell analyses and spatial transcriptomics; subsequently, we extended the analyses by multicolor quantitative immunostaining, in vitro co-cultures, and mouse xenograft experiments (Figure 1A).
Figure 1.
Identification of a cancer cell subpopulation associated with chemoresistance of OCCC
(A) Study design based on surgical specimens of OCCC.
(B) Timeline presentation of 10 patients whose tumors were used for snRNA-seq, spatial transcriptome, and immunostaining analyses.
(C) UMAP presentation of snRNA-seq data obtained from chemoresistant (OCC-R1-5) and chemosensitive (OCC-S1-5) OCCC. Original snRNA-seq data from the 10 tumors (Figure S1A) were subjected to anchoring prior to data integration. Subsequently, the identity of cells in each cluster was determined based upon expression of marker genes,17 as shown in Figure S1C. TEC, tumor endothelial cells; CAF, cancer-associated fibroblast; TAM, tumor-associated macrophage; TIL, tumor-infiltrating lympohcyte. The epithelial tumor population was further classified into six subpopulations (Cancer #1–6), denoted by the indicated colors.
(D) Stacked bar graph displaying the distribution of each cancer cell subpopulation (Cancer #1–6) in each tumor (left) and the percentage of cancer cells (right).
(E) Boxplots showing the fraction of the indicated cancer subpopulations in the chemoresistant and chemosensitive tumors shown in (D). The horizontal bars within the boxes indicate the median value. The top and bottom bars of the box denote the 25th and 75th percentiles, respectively. ∗∗∗p < 0.001; n.s., not significant.
(F) Stacked bar graph displaying the distribution of non-tumor cell populations within each tumor (left) and the percentage of non-tumor cells (right).
(G) Boxplots showing the fractions of the indicated non-tumor populations. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. p values were determined by Student’s t test.
To obtain single-cell transcriptome data from frozen samples, we performed snRNA-seq6 of chemosensitive (n = 5, OCC-S1–5) and chemoresistant (n = 5, OCC-R1–5) cases (Figure 1B; Table S1). The snRNA-seq data were then subjected to dimensionality reduction using uniform manifold approximation and projection (UMAP). Clustering of single-nucleus data presented on UMAP indicated that cells were stratified mainly according to clinical case, which was presumably caused by batch effects (Figure S1A). To eliminate batch effects and integrate individual datasets, we performed an anchoring procedure so that we could compare cell identity across samples18 (Figure S1B).
After anchoring the data, we found that major cell types (epithelial cancer cells, CAFs, endothelial cells, and immune cells) formed distinct clusters upon UMAP presentation (Figures 1C, S1C, and S1D). Cells from both chemoresistant and chemosensitive cases were distributed within each cell type (Figure S1E). In the EpCAM+ epithelial population, non-tumor cells were barely detectable based on an estimation of copy number alterations by InferCNV analyses19 (Figure S1F), which was presumably attributed to careful elimination of non-tumor tissues during sample preparation.
To examine potential links between chemoresistance and oncogenic activations, we evaluated genomic alterations in major oncogenes and tumor suppressor genes using the NCC Oncopanel (Figure S1G). As reported previously, mutation of ARID1A and PIK3CA was identified in a large proportion of the samples (7 of 10 and 4 of 10 cases, respectively). However, there was no clear association with chemoresistance because both of these mutations occurred in both chemosensitive and chemoresistant cases (Figure S1G).
Next, to examine whether a chemoresistant cancer subpopulation exists, we stratified the EpCAM+ tumor population into six subpopulations (Cancer #1–6) (Figures 1C and 1D). Remarkably, we found that a fraction of the Cancer #2 subpopulation was higher in chemoresistant cases than in chemosensitive ones (Figures 1D and 1E). On the other hand, evaluation of non-tumor cell types revealed no significant difference in numbers between chemoresistant and chemosensitive cases (Figures 1F and 1G).
The chemoresistant population of OCCC is associated with HIF activation and a poor prognosis
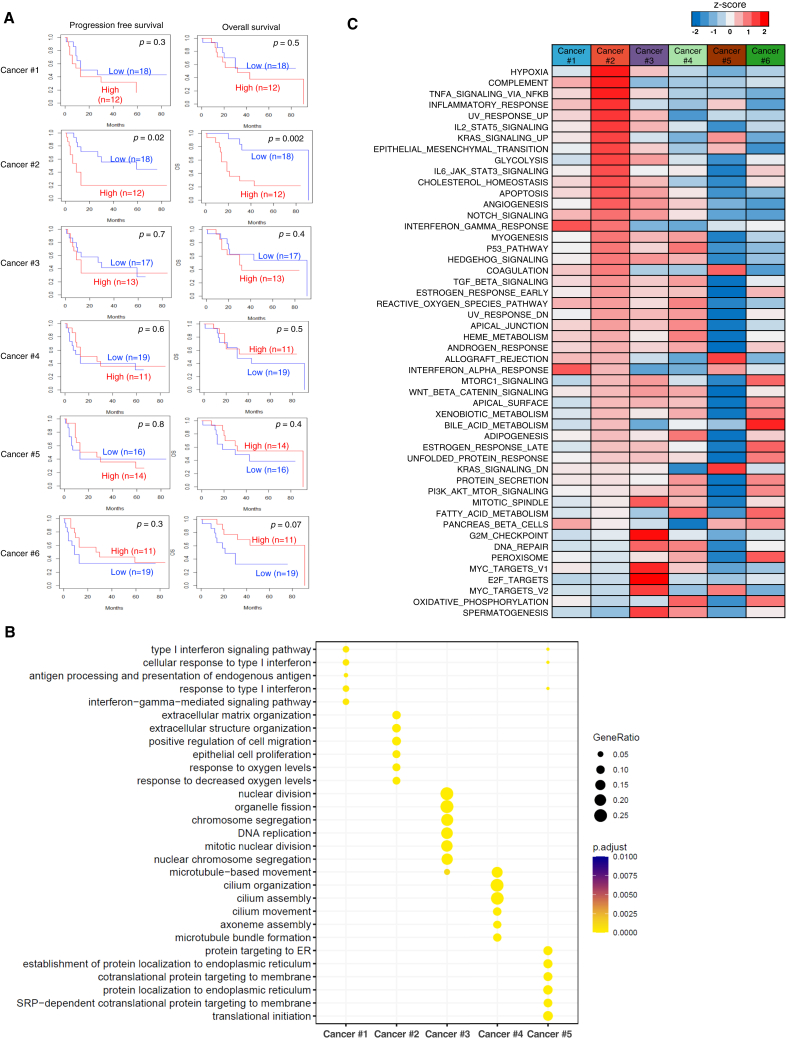
To determine the gene expression profiles of each cancer subpopulation, we isolated preferentially expressed signature genes (Figure S2A; Table S2). Examination of signature gene expression in advanced OCCC cases (n = 30) indicated that the signature of the Cancer #2 subpopulation, but not that of other subpopulations, was associated with shortened progression-free or overall survival (Figure 2A), linking the chemoresistance-associated Cancer #2 subpopulation to a poor prognosis.
Figure 2.
A chemoresistant subpopulation of OCCC is associated with HIF activation and a poor prognosis
(A) Kaplan-Meier plots of advanced-stage OCCC (stages II–IV, n = 30). The top 20 genes highly expressed by each cancer subpopulation (Cancer #1–6) were selected as signature genes and used to classify the 30 cases of advanced OCCC (stages II–IV) into groups with high or low expression of signature genes. Kaplan-Meier curves show progression-free survival and overall survival in each group. p values were calculated using log rank test.
(B) Dot plot presentation of Gene Ontology (GO) terms specifically enriched in cancer subpopulations (Cancer #1–5).
(C) Heatmap of normalized ssGSEA enrichment scores for Hallmark pathways in cancer subpopulations (Cancer #1–6).
Next, we performed Gene Ontology (GO) enrichment analyses of the subpopulations to investigate their biological characteristics. The results showed that the Cancer #2 subpopulation was associated with hypoxic responses and with the extracellular matrix (Figure 2B). In accordance, single-sample gene set enrichment analyses (ssGSEA) of Hallmark signature gene sets revealed that hypoxia pathways were activated specifically in the Cancer #2 subpopulation (Figures 2C and S2B). On the other hand, enrichment analyses of the other major subpopulations indicated that the Cancer #1 and #3 subpopulations were associated with interferon response pathways and cell-cycle-related pathways (Figures 2B and 2C), respectively, suggesting that the Cancer #3 subpopulation is a cycling population.
Next, we performed virtual inference of protein activity by enriched regulon analysis to investigate transcriptional regulators associated with each cluster. In agreement with the findings of an elevated hypoxic response, the Cancer #2 subpopulation showed elevated activity of HIF1A (HIF-1α) and EPAS1 (HIF-2α) (Figures S2C and S2D). Of note, the top five transcription factors activated in the Cancer #2 subpopulation (HIF1A, EGR-1, ATF-2, EPAS1(HIF-2A), and SP-1) mediate hypoxic responses.20 We found extensive overlap of target genes that were upregulated in the Cancer #2 subpopulation (Figure S2E), suggesting that these transcription factors co-operate to induce hypoxic responses. Taken together, these results indicate that the #2 chemoresistant subpopulation is associated with a poor prognosis and HIF-mediated hypoxic responses.
Chemoresistant cells are localized in CAF-populated areas of OCCC
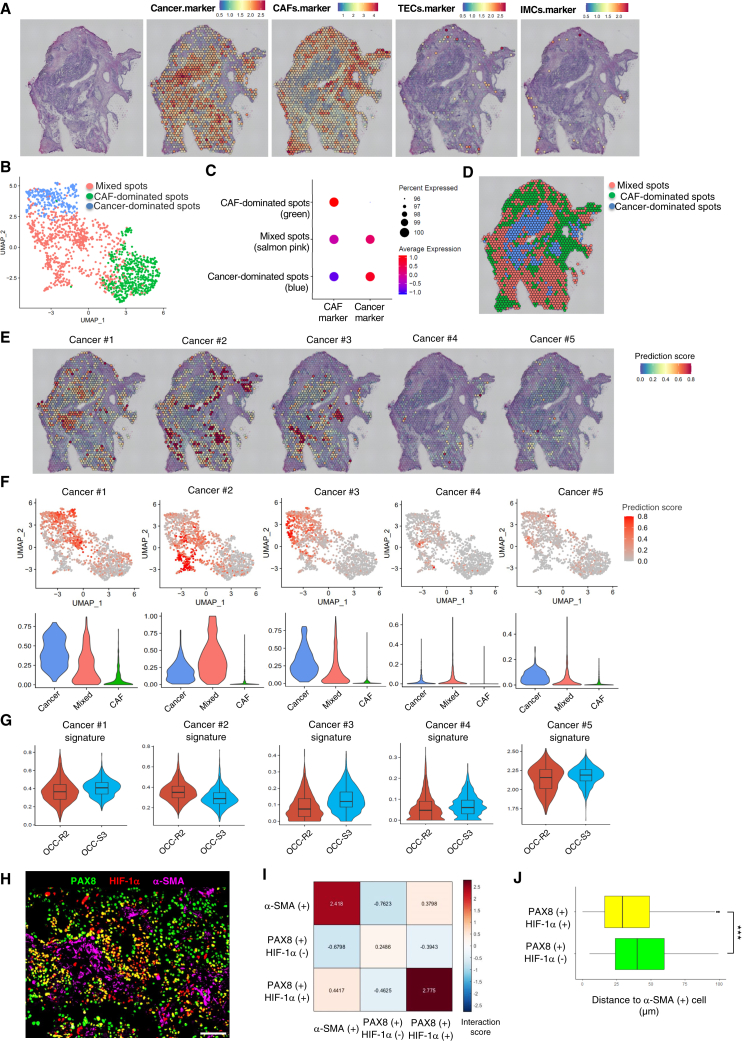
Next, we attempted to determine the histological localization of the Cancer #2 chemoresistant cancer subpopulation by performing spatial transcriptomics analysis. Surgical specimens from chemoresistant (OCC-R2) and chemosensitive (OCC-S3) cases were subjected to Visium spatial gene expression analyses. Specific markers for epithelial cancer cells, CAFs, endothelial cells, and immune cells were used to determine the location of these cells within tumors. Hematoxylin and eosin (H&E) staining of serial sections indicated that the specimens were roughly segregated into cancer- or CAF-dominated regions (Figures 3A and S3A, far left). Consistent with this, we found that the tissue distribution of cancer cells and CAFs depicted by Visium analyses corresponded approximately to the cancer cell- or CAF-dominated regions visualized by H&E staining (Figures 3A and S3A). Indeed, Visium spots could be classified into three groups based upon gene expression profiles (Figures 3B and S3B): cancer-dominated, CAF-dominated, and cancer/CAF-mixed (Figures 3C and S3C). The distribution of the three types of spots corresponded approximately to that of cancer cells and CAFs observed in the H&E images (Figures 3D and S3D).
Figure 3.
HIF-1α-positive chemoresistant cells reside near CAFs in chemoresistant OCCC
(A) Far left: H&E staining of OCC-R2 tissue sections. Right: Visium spatial transcriptomics (ST) spots on H&E-stained sections were overlaid with spatial feature plots of ovarian cancer cell markers (EPCAM, PAX8, and KRT7), CAF markers (COL1A1, COL1A2, DCN, and VIM), endothelial cell (TEC) markers (VWF, CDH5, and PECAM1), or an immune cell marker (PTPRC).
(B) UMAP plots of the Visium ST spots shown in (A). ST spots were classified into three clusters by unsupervised clustering and are denoted by different colors.
(C) Dot plots showing average expression of CAF markers and ovarian cancer cell markers by the indicated clusters. Three clusters shown in (B) were designated as cancer-dominated spots, CAF-dominated spots, and mixed spots, based upon expression of these markers.
(D) Spatial presentation of the three cluster in OCC-R2 tissue sections. Tissue localization of the clustered ST spots was visualized and is denoted by the indicated colors.
(E) Spatial feature plots of the prediction scores for the indicated cancer subpopulations (Cancer #1–5) in OCC-R2 tissue sections.
(F) Top: UMAP feature plots of ST spots of OCC-R2. Prediction scores for the indicated cancer subpopulations are shown in red. Bottom: violin plots of the prediction scores for the cancer subpopulations shown in the top columns. ST spots were classified into three groups as shown in (D), and the prediction scores for each group are presented.
(G) Violin plots showing the average expression values for the signature genes expressed by the indicated cancer subpopulations (Cancer #1–5) in ST spots of OCC-R2 and OCC-S3. In the boxplots inside the violin plots, the top and bottom bars of the boxes represent the 25th and 75th percentile, respectively, and the horizontal bars within the box indicate the median value.
(H) Representative magnified images of HIF-1α(+) cancer cells near α-SMA(+) CAFs in a chemoresistant tumor (OCC-R3). Scale bar, 100 μm.
(I) Nearest-neighbor analysis of the image shown in (H).
(J) Boxplot showing the average distance from PAX8(+)/HIF-1α(+) cells or PAX8(+)/HIF-1α(−) cells to the closest α-SMA(+) cell, calculated using the image shown in (H). ∗∗∗p < 0.001. p values were determined by Student’s t test.
Next, to localize the major cancer subpopulations (#1–5), we performed anchor-based integration of snRNA-seq data and Visium data (STAR Methods) and then calculated a prediction score for each subpopulation in each Visium spot (Figures 3E and S3E). In both chemoresistant and chemosensitive cases, visualization of cancer subpopulations based upon the prediction scores revealed that the Cancer #2 subpopulation was localized mainly in cancer/CAF-mixed spots. By contrast, the Cancer #1 and #3 populations were localized mainly in cancer-dominated spots (Figures 3F and S3F).
In agreement with the snRNA-seq data, the Cancer #2 signature, but not the other signatures, was expressed to a greater extent in OCC-R2 than in OCC-S3 (Figure 3G), thereby supporting an association between cancer cells harboring the Cancer #2 signature and chemoresistance.
HIF-1α-induced cancer cells reside near CAFs in chemoresistant OCCC
Next, we immunostained HIF-1α-positive cancer cells to further investigate the location of the Cancer #2 chemoresistant populations in cancer/CAF-mixed areas. Remarkably, co-immunostaining of chemoresistant tumors (OCC-R1-5) with antibodies specific for HIF-1α, PAX8 (a marker for ovarian cancer cells), and α-SMA (a marker for CAFs) revealed widespread distribution of PAX8-positive cancer cells that co-express HIF-1α (Figure S4A). By contrast, the fraction of cancer cells co-expressing detectable HIF-1α in chemosensitive tumors (OCC-S1-5) was much lower than in chemoresistant tumors (Figure S4A). In fact, quantification of stained cells using QuPath (Figure S4B) indicated that the fraction of the HIF-1α-positive population in chemoresistant tumors was, on average, three times higher than that in the chemosensitive tumors (33.2% vs. 11.0%, respectively) (Figure S4C).
We observed that HIF-1α-positive cancer cells in chemoresistant tumors frequently localized near α-SMA-positive cells (Figures 3H and S4D). Indeed, evaluation of relative distance among HIF-1α-positive cancer cells, HIF-1α-negative cancer cells, and α-SMA-positive cells by nearest-neighbor analyses (CytoMAP) indicated the localization of HIF-1α-positive cancer cells near α-SMA-positive cells (Figures 3I and S4E). Accordingly, calculation of average distance from PAX8(+)/HIF-1α(+) cells or PAX8(+)/HIF-1α(−) cells to the nearest α-SMA(+) cells (QuPath) confirmed the localization of PAX8(+)/HIF-1α (+) cells near α-SMA(+) cells (Figures 3J and S4F). These data suggest that localization of HIF-1α-induced cancer cells near CAF is a hallmark of chemoresistant OCCC.
CAFs in chemoresistant tumors show a myofibroblastic phenotype
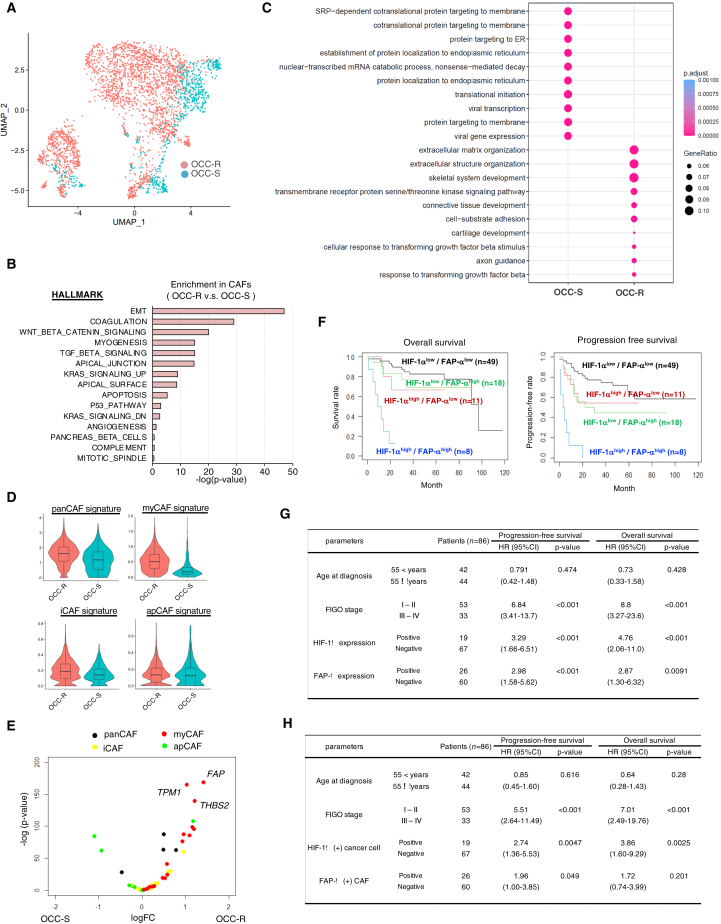
Close localization of CAFs to chemoresistant cells suggests that CAFs may play a functional role in enhancing the chemoresistance of OCCC. To examine a subpopulation of CAFs that may exist in chemoresistant OCCC, we used the snRNA-seq data to stratify the CAF population shown in Figure 1C. Contrary to stratification of the cancer population, however, we did not find a subpopulation that exists preferentially in chemoresistant OCCC (Figures 4A, S5A, and S5B).
Figure 4.
CAFs in chemoresistant tumors are associated with myofibroblastic phenotype
(A) UMAP plot of CAFs, color-coded as chemoresistant (OCC-R1-5) or chemosensitive (OCC-S1-5) cases.
(B) Bar chart showing enrichment of specific biological pathways in CAFs from resistant tumors. Enrichment of a pathway is calculated by comparing the average ssGSEA values of Hallmark gene sets in CAFs from five chemoresistant and five chemosensitive tumors. p values for the top 15 Hallmark terms enriched in CAFs from chemoresistant cases were determined by Student’s t test. ∗∗p < 0.01.
(C) Dot plot of biological GO terms upregulated in CAFs from chemoresistant (OCC-R) and chemosensitive (OCC-S) cases.
(D) Violin plots of the signature scores for the indicated CAFs from chemoresistant and chemosensitive cases.
(E) Volcano plots showing preferential induction of myCAF signature genes in CAFs from chemoresistant cases. Average expression values for the indicated CAF signature genes22 expressed by CAFs from chemoresistant and chemosensitive cases were calculated, and the relative ratios are shown. The horizontal and vertical axes represent the log10 values of fold changes and p values, respectively. Red dots, myCAF signature genes; yellow dots, iCAF signature genes; green dots: apCAF signature genes; black dots, panCAF signature genes. A list of the signature genes is presented in Table S3.
(F) Kaplan-Meier plots of OCCC (stages I–IV, n = 86). The patients were stratified into 4 groups: HIF-1αlow/FAP-αlow (n = 49), HIF-1αhigh/FAP-αlow (n = 11), HIF-1αlow/FAP-αhigh (n = 18), and HIF-1αhigh/FAP-αhigh (n = 8). Kaplan-Meier curves of progression-free survival and overall survival in each group are shown.
(G and H) Association of HIF-1α and FAP-α expression with poor prognosis. Association of the indicated parameters with progression-free survival or overall survival was evaluated by univariate (G) and multivariate (H) analyses (86 cases of OCCC). Hazard ratios (HRs), 95% confidence intervals, and p values were calculated with Cox’s proportional hazards regression model. ∗∗∗p < 0.001.
As an alternative approach, we used the snRNA-seq data to examine whether CAFs from chemosensitive cancers were associated with any particular biological features. It has been reported that CAFs comprise heterogeneous populations, including inflammatory CAFs (iCAFs), antigen-presenting CAFs (apCAFs), and myofibroblastic CAFs (myCAFs), and that myCAFs reside close to cancer cells.2,21 Comparison of CAFs from chemoresistant and chemosensitive tumors by ssGSEA and GO term analyses indicated that CAFs from chemoresistant OCCC were associated with epithelial-mesenchymal transition (EMT) and extracellular matrix organization (Figures 4B and 4C), phenotypes associated with myCAFs. Strikingly, CAFs from chemoresistant OCCC showed elevated expression of the myCAF gene signature (Figure 4D) and the myCAF-related genes FAP, TPM1, and THBS2 (Figure 4E). In accordance, visualization of each CAF signature supported dominant distribution of myCAFs in chemoresistant but not in chemosensitive tumors (Figure S5C). Furthermore, immunostaining studies revealed higher levels of FAP-α (a protein encoded by the FAP gene) in chemoresistant tumors than in chemosensitive ones (Figure S5D). Collectively, these data indicate that the myCAF population is increased in chemoresistant cancer and that HIF-1α-induced OCCC cells and myCAFs constitute a cancer microenvironment associated with chemoresistant OCCC.
To establish the clinical importance of HIF1-α-positive cancer cells or FAP-α-expressing myCAFs in chemoresistant OCCC, we next examined their presence in a large set of formalin-fixed paraffin-embedded specimens of treatment-naive OCCC (86 cases: stage I, 45; stage II, 8; stage III, 24; stage IV, 9) by performing tyramide-based multicolor immunofluorescence for HIF-1α, PAX8, FAP-α, and α-SMA (Table S4; Figure S5E). To examine whether increased fractions of HIF-1α-positive cancer cells or FAP-α-expressing myCAFs affect the clinical course of OCCC, we calculated the fraction of HIF-1α-positive cells among PAX8-positive cancer cells and FAP-α-positive cells among α-SMA-positive CAFs. A fraction of HIF-1α-positive cancer cells were weakly associated with that of FAP-α-positive CAFs (Figure S5F), and we observed modest increases of fractions of these cells in advanced stages (Figure S5G). Of note, calculation of the relative distance from FAP-α-positive cells to the nearest HIF-1α-positive or HIF-1α-negative cancer cells (QuPath) indicated the localization of HIF-1α-positive cancer cells near FAP-α-positive cells (Figure S5H), suggesting localization of HIF-1α-induced cancer cells near active CAFs.
Remarkably, Kaplan-Meier analyses revealed that a higher fraction of these cells in tumors was associated with poor prognosis and that patients with both high fractions (>10%) of HIF-1α-positive cancer cells (HIF-1αhigh) and high fractions (>80%) of FAP-α-positive CAFs (FAP-α high) suffered from significantly worse prognoses than the other groups (Figure 4F). In accordance, univariate analyses showed that fractions of HIF-1α- and FAP-α -positive cells were associated with progression-free and overall survival (Figure 4G). Furthermore, multivariate analyses indicated that both fractions were prognostic factors independent of advanced stages (Figure 4H). These data indicate that expansion of HIF-1α-positive cancer cells and myCAFs negatively affects the clinical courses of OCCC, presumably as a result of aggravated chemoresistance.
In vitro co-cultivation of CAFs with chemoresistant OCCC cells recapitulates the chemoresistant niche
Co-localization of the chemoresistant subpopulation of cancer cells and CAFs implies mutual crosstalk between these cells in chemoresistant niches. To examine potential crosstalk between these cells, we established an in vitro co-culture system. First, we established cancer spheroids and CAFs separately from fresh surgical OCCC specimens. Second, we retrospectively selected chemoresistant cancer-derived spheroids based upon extensive expression of HIF-1α in cancer cells in the original surgical specimens (data not shown). The identities of the selected cancer spheroids and CAFs were confirmed by expression of specific markers: PAX8 and KRT7 for OCCC and α-SMA and collagen I for CAF (Figure S6A). Third, the spheroids and CAFs were labeled with GFP and mCherry, respectively, and then cultivated alone or together (at a 1:1 ratio) to examine cell proliferation and phenotypic alterations induced by co-cultivation (Figures 5A and 5B). Indeed, co-cultivation increased the survival rate of CAFs (Figure 5C) and induced expression of HIF-1α and HIF-2α in cancer spheroid cells (Figures 5D and 5E). Remarkably, co-culture increased chemoresistance to carboplatin (Figure 5F), indicating that the presence of CAFs contributes to chemoresistance of OCCC.
Figure 5.
Co-cultivation of CAFs with chemoresistant OCCC cells recapitulates the chemoresistant niche in vitro
(A) Experimental design of the in vitro co-culture system. Cancer spheroid cells and CAFs were derived from surgical specimens of HIF-1α-positive OCCC. The established cancer cells and CAFs were labeled with GFP/Luc2 and mCherry/hRluc, respectively; cultivated either alone or in combination; and subjected to a chemosensitivity assay, scRNA-seq, or drug screening.
(B) Bright-phase images (top) and fluorescence images (bottom) of the indicated cells cultivated under organoid conditions for 7 days. Scale bars, 100 μm.
(C) Survival of CAFs upon monoculture or co-culture with cancer cells (OVN-48) for 7 days. Cultured cells were grown in the absence or presence of the indicated concentrations of carboplatin, and cell survival was evaluated by measuring hRLuc activity. The data are presented as mean ± SD (n = 3). p values were determined by Student’s t test. ∗∗∗p < 0.001.
(D) Western blot analyses of cancer cells that were sorted by fluorescence-activated cell sorting (FACS) after incubation under monoculture or co-culture conditions for 3 days.
(E) Representative image of immunostaining of HIF-1α and α-SMA in cancer cells and CAFs co-cultured for 3 days. Scale bars, 100 μm.
(F) Cancer cell growth (OVN-48) upon monoculture or co-culture with CAFs for 7 days. Cultured cells were grown in the absence or presence of the indicated concentrations of carboplatin, and cancer cell proliferation was evaluated by measuring Luc2 activity (n = 3). ∗∗∗p < 0.001.
(G) UMAP plot of scRNA-seq data from cancer cells (OVN-48) and CAFs incubated under monoculture and co-culture conditions for 3 days.
(H) Violin plots of the signature scores for the cancer subpopulations (Cancer #1–6) grown under the monoculture and co-culture conditions in (G).
(I) Violin plots of the indicated signature genes in CAFs grown under the monoculture and co-culture conditions shown in (G). Statistically significant differences are indicated: ∗∗∗p < 0.001.
Next, to examine changes in gene expression induced after co-culture, we performed scRNA-seq of cells cultivated under monoculture or co-culture conditions (Figures 5G and S6B). When we compared the gene expression profiles of cancer spheroids under co-culture and monoculture conditions by ssGSEA, we found that four of five top Hallmark signatures (EMT, tumor necrosis factor alpha signaling via nuclear factor κB, inflammatory response, and hypoxia) induced after co-culture were identical to those upregulated in the Cancer #2 subpopulation (Figures 2C and S6C). In addition, co-culture with CAFs caused specific up-regulation of the Cancer #2 gene signature (Figure 5H, p < 0.001) and induced activation of all nine top transcription factors activated in the Cancer #2 subpopulation (Figures S2C and S6D). Collectively, phenotypic alteration of cancer spheroids induced by co-cultivation with CAFs largely simulated the gene expression profiles of the Cancer #2 subpopulation.
We also compared gene expression profiles of CAFs under co-culture and monoculture conditions by ssGSEA. The EMT pathway, which was strongly upregulated in CAFs in chemoresistant OCCC (Figure 4B), was induced under co-culture conditions (Figure S6E). In addition, we found that transforming growth factor β (TGF-β) signaling was strongly induced after co-cultivation (Figure S6E), suggesting that TGF-β signaling, which is a well-known signaling pathway that drives CAF generation,2,23 may account for the EMT phenotype of the CAF induced under co-culture conditions. Furthermore, co-cultivation upregulated the myCAF signature (Figure 5I, p < 0.001) as well as genes representative of myCAF, including FAP, THBS2, and TPM1 (Figure S6F), induction of which was also observed in chemoresistant OCCC (Figure 4E). Thus, both cancer spheroids and CAFs, when incubated together, undergo phenotypic alterations associated with chemoresistant OCCC. These data strongly suggest that interactions between cancer cells and CAFs lead to formation of a chemoresistant niche in OCCC.
CAF activation by cancer-derived PDGF mediates chemoresistance and activates HIF-1α in cancer cells
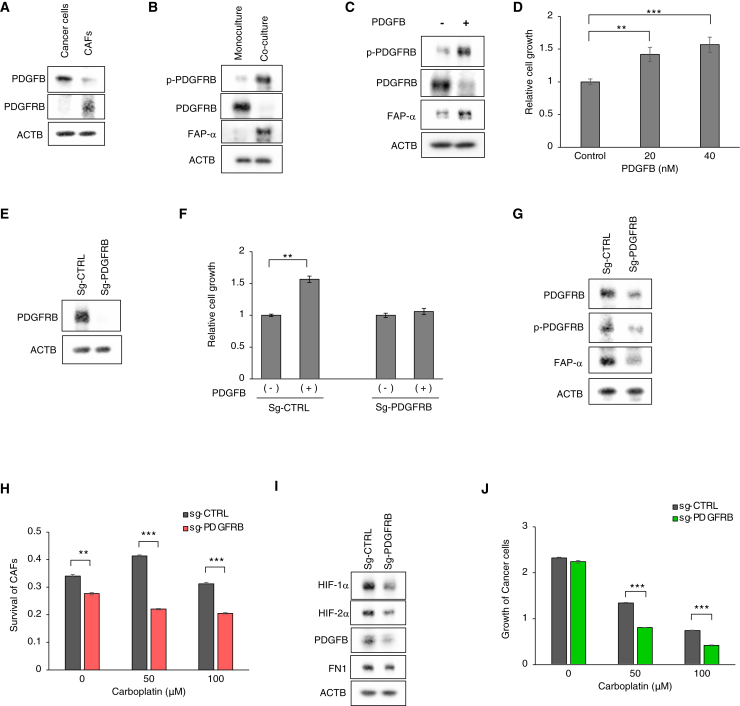
To better understand the molecular mechanism underlying chemoresistance mediated by the cancer-CAF interaction, we used NicheNet24 to examine potential ligand-receptor interactions between these cells (Figure S7A). When we examined the snRNA-seq data, we identified 12 ligand-coding genes that were highly expressed in the Cancer #2 subpopulation (NAMPT, EFNA5, PDGFB, C3, ANXA1, SPP1, FN1, ITGB1, LAMC2, LAMB1, LAMA1, and RELN) (Figure S7A). Receptor-ligand analyses revealed that interaction between PDGFB (the β subunit of PDGF) and PDGFRB (the β subunit of PDGF receptor) was a strong candidate mediator of cell-cell signaling between cancer cells and CAFs (Figure S7A). In support of this, PDGFB was preferentially expressed in the Cancer #2 subpopulation from chemoresistant tumors (Figure S7B), and immunostaining of chemoresistant tumor showed activating phosphorylation of PDGFRB in α-SMA-positive CAFs that resided near KRT7-positive cancer cells (Figure S7C). Activation of CAF by PDGF signaling has also been reported in other types of cancer.2,3
Therefore, we next examined the functional significance of the PDGF-PDGFR interaction in the co-culture system. As expected, PDGFB and PDGFRB were expressed at high levels by cancer spheroid cells and CAFs, respectively (Figure 6A). Co-culture led to activating phosphorylation of PDGFR (p-PDGFRB) and expression of a myCAF marker (FAP-α) in CAFs (Figure 6B). Indeed, treatment of CAFs with a purified PDGFB ligand induced phosphorylation of PDGFRB and expression of FAP-α (Figure 6C) and increased proliferation of CAFs (Figure 6D), thereby phenocopying the effects of co-culture with cancer spheroids. Conversely, CRISPR-mediated knockout of PDGFRB in CAFs abolished PDGF-mediated proliferation (Figures 6E and 6F) and FAP-α expression (Figure 6G), indicating that the PDGFB-induced proliferation and expression of the myCAF-like phenotype were mediated by activation of the PDGF receptor in CAFs. In accordance, immunostaining of chemoresistant OCCC showed activating phosphorylation of PDGFRB in α-SMA-positive CAFs that resided near KRT7-positive cancer cells (Figure S7C).
Figure 6.
CAF activation by cancer-derived PDGF mediates chemoresistance and HIF-1α activation of cancer cells
(A) Western blot analysis of GFP-labeled cancer cells (OVN-48) and mCherry-labeled CAFs with the indicated antibodies.
(B) Western blot analysis of CAFs, grown under monoculture or co-culture conditions for 3 days with the indicated antibodies. Note that a PDGFRB level was reduced under co-culture conditions, presumably via negative feedback regulation.25
(C) Western blot analyses of CAFs treated with 40 nM PDGFB for 3 days.
(D) Relative growth of CAFs treated with different concentrations of PDGFB for 7 days. The data are presented as mean ± SD (n = 3). p values were determined by Student’s t test. ∗∗p < 0.01, ∗∗∗p < 0.001.
(E) Western blot analyses of CAFs subjected to Cas9/CRIPSR-mediated knockout with the indicated sgRNAs.
(F) Relative growth of CAFs transduced with the indicated sgRNA and then treated with 20 nM of PDGFB for 7 days. The data are presented as mean ± SD (n = 3). p values were determined by Student’s t test. Statistically significant differences are indicated. ∗∗p < 0.01.
(G) Western blot analyses of control and PDGFRB-deficient CAFs that were FACS-sorted on mCherry after incubation with cancer cells for 3 days.
(H) Survival of control and PDGFRB-deficient CAFs that were incubated with cancer cells for 7 days (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001.
(I) Western blot analysis of cancer cells that were FACS-sorted on GFP after incubation for 3 days with control or PDGFRB-deficient CAFs. p values were determined by Student’s t test. Statistically significant differences are indicated: ∗∗p < 0.01, ∗∗∗p < 0.001.
(J) Proliferation of cancer cells cultured for 7 days with control or PDGFRB-deficient CAFs in the presence of the indicated concentrations of carboplatin (n = 3). ∗∗∗p < 0.001.
Next, we examined the functional role of PDGFR signaling in cancer chemoresistance. PDGFR knockout in CAFs reduced its viability (Figure 6H). Remarkably, the PDGFRB knockout in CAFs inhibited expression of HIF-1α, HIF-2α, and PDGFB in cancer cells (Figure 6I). Moreover, the knockout reduced cancer chemoresistance to carboplatin (Figure 6J). Taken together, these data indicate the presence of a positive feedback loop between cancer cells and CAFs: PDGF expressed by OCCC induces PDGFR-mediated activation and survival of CAFs, which, in turn, augments HIF activation, PDGF expression, and chemoresistance in cancer cells.
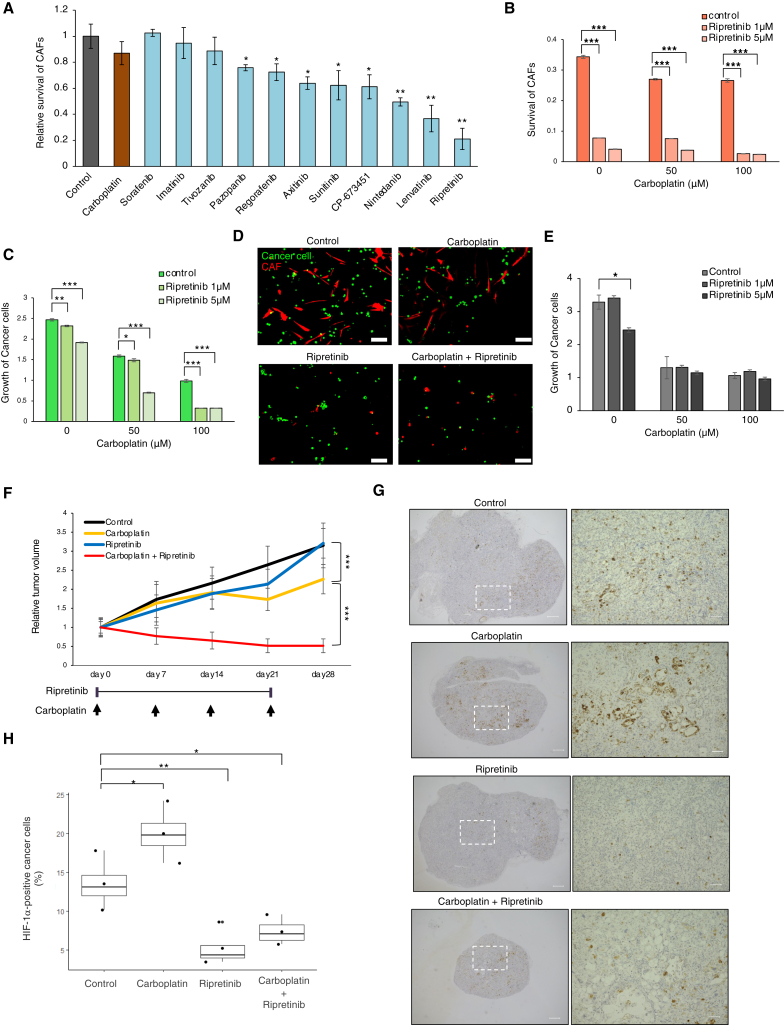
CAF inhibition by ripretinib in combination with carboplatin inhibits growth of OCCC
The crucial role of the PDGF-PDGFR signaling axis in CAF-mediated chemoresistance tempted us to devise an effective therapy targeting PDGFR signaling in CAFs. To this end, we initially searched for tyrosine kinase inhibitors (TKIs) that inhibit CAFs. Carboplatin did not have any significant effect on CAF growth (Figure 7A). TKIs showed varying levels of growth inhibition, with ripretinib showing decent efficacy (Figures 7A and S8A). In fact, 1 μM ripretinib inhibited activating phosphorylation of PDGFRB and expression of FAP-α within 24 h (Figure S8B). Subsequently, we examined the inhibitory effects of ripretinib in the presence or absence of carboplatin in the in vitro co-culture system. As expected, ripretinib at 1–5 μM effectively reduced the viability of CAFs even in the absence of carboplatin (Figure 7B). Importantly, inhibition of cancer cell proliferation by carboplatin was markedly enhanced by ripretinib (Figures 7C and 7D). By contrast, ripretinib did not show significant enhancement of carboplatin-mediated inhibition upon monoculture of cancer cells (Figure 7E), indicating that ripretinib inhibits OCCC growth via CAF suppression.
Figure 7.
CAF inhibition by ripretinib blocks growth of OCCC in combination with carboplatin
(A) Inhibition of CAFs cultured for 7 days grown on attachment culture conditions in the presence of the indicated TKIs or carboplatin (1 μM). The data are presented as mean ± SD (n = 3). p values were determined by Student’s t test. Statistically significant differences are indicated: ∗p < 0.05, ∗∗p < 0.01.
(B) Inhibition of CAFs by ripretinib. CAFs co-cultured with cancer cells under co-culture conditions were treated with the indicated concentrations of ripretinib and carboplatin for 7 days (n = 3). ∗∗∗p < 0.001.
(C) Co-operative inhibition of the growth of co-cultivated cancer cells (OVN-48) by ripretinib and carboplatin. Cancer cells co-cultured with CAFs were treated for 7 days with the indicated concentrations of ripretinib and carboplatin.
(D) Fluorescence images of cancer cells and CAFs co-cultured for 7 days in the presence or absence of 100 μM carboplatin and/or 5 μM ripretinib. Scale bars, 100 μm.
(E) Cancer cells grown under monoculture conditions were treated with the indicated concentrations of ripretinib and carboplatin for 7 days (n = 3). ∗p < 0.05.
(F) Xenografted tumors (OVN-48, 49 days after co-transplantation of cancer cells and CAFs) were treated with the indicated combinations of carboplatin and/or ripretinib, and tumor volume (mean ± standard error of the mean) was measured weekly (n = 8). ∗∗∗p < 0.001.
(G) Immunostaining of xenograft tumors (78 days post transplantation) with HIF-1α. Magnified images are shown on the right. Scale bars, 500 μm (right) and 100 μm (left).
(H) Boxplots showing the percentage fraction of HIF-1α-positive cancer cells in the tumor tissues shown in (G). Average values ± SEM are shown. p values were determined by Student’s t test. Statistically significant differences are indicated: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Finally, we examined the effect of combined treatment with carboplatin and ripretinib on OCCC xenografts. The cancer spheroids and CAFs used in the co-culture assays were mixed at a 1:1 ratio and transplanted subcutaneously into immunocompromised NOG mice. Notably, ripretinib in combination with carboplatin led to marked inhibition of tumor growth (Figures 7F, S8C, and S8D).
According to our model, ripretinib inhibits growth of chemoresistant OCCC by inhibiting CAF. Therefore, we predicted that a fraction of the HIF-1α-positive cancer cells would be reduced in the presence of ripretinib. Indeed, we observed a marked reduction in a fraction of HIF-1α-positive cancer cells (Figures 7G and 7H) as well as α-SMA-positive CAFs (Figure S8E) after treatment.
We could generate xenografted tumors when only the cancer spheroids were used for the transplantation. We observed extensive infiltration of α-SMA-positive cells in the generated tumors (Figure S8F), suggesting that host-derived CAFs were also capable of interacting with xenografted cancer cells. Indeed, ripretinib enhanced the inhibition of tumor growth by carboplain (Figure S8G). Collectively, these data indicate that CAF inhibition is an effective treatment that eliminates chemoresistant OCCC when combined with standard chemotherapy agents.
Discussion
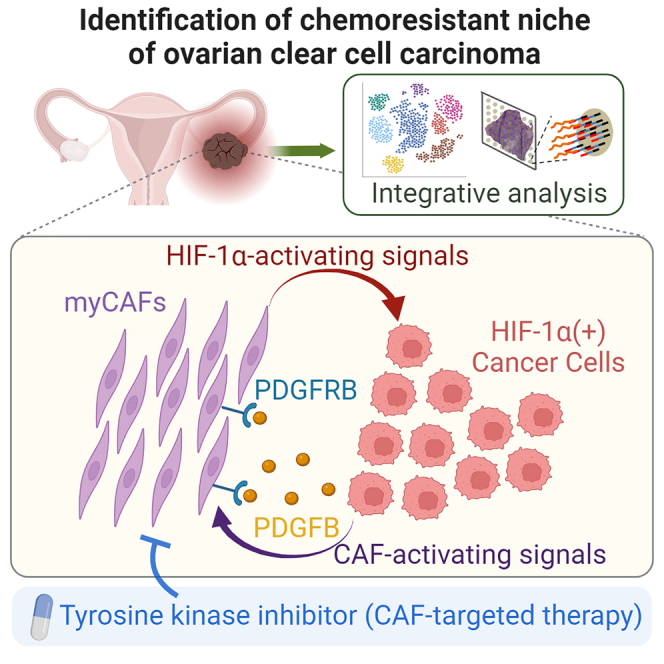
Recent progress in spatial transcriptomics and other spatial omics technologies has enabled visualization of the molecular architecture of cancer tissues in histological samples.7,9 The spatial location of cell populations of interest can be inferred by integrating spatial transcriptomics with single-cell gene expression analyses.10,26,27 Here, we exploited the power of this integrative approach to determine the spatial localization of regions associated with OCCC chemoresistance. These studies, in combination with multicolor immunostaining, revealed that chemoresistance is associated with regions in which HIF-1α-activated cancer cells co-localize with myCAFs and that expansion of these cells profoundly affects the prognosis of OCCC patients.
Integrative analyses of snRNA-seq and the spatial transcriptome are descriptive in nature; therefore, we used an in vitro co-culture system to functionally validate the importance of interactions between cancer cells and CAFs. Previously, we established spheroid cultures from clinical specimens of HGSOC and colorectal cancer and investigated their biological characteristics.28,29,30,31 By applying a similar method, we established spheroid cultures from surgical specimens of OCCC and showed that OCCC-derived CAFs confer chemoresistance in vitro. Further investigations at the molecular level revealed that cancer-derived PDGF activates CAFs, which, in turn, induce HIF-1α signaling and chemoresistance in OCCC.
CAFs are involved in cancer progression through interaction with cancer cells and other cell types in the tumor microenvironment.2,3 Although CAFs are regarded as potential therapeutic targets due to their cancer-promoting roles, effective cancer therapy targeting them is not yet established.2 This may be due to the heterogeneity of CAFs; there are cancer-promoting and -inhibiting populations, and the biological characteristics of a targetable cancer-promoting CAF subpopulation are not well defined.2,32
In this study, we demonstrate that CAF inhibition by ripretinib acts co-operatively with standard chemotherapy to inhibit OCCC tumor growth both in in vitro co-culture assays and in vivo mouse xenografts. It is noteworthy that, because of frequent oncogenic mutations in PIK3CA and other genes affecting phosphatidylinositol 3-kinase-AKT signaling in OCCC, clinical trials have been conducted to examine the efficacy of some TKI inhibitors.12,33 However, the TKIs used in these trials are intended to directly inhibit proliferation of cancer cells. On the other hand, our data indicate that ripretinib is capable of inhibiting PDGFR activation and survival of CAFs, although the compound may also affect OCCC cells. Considering that the presence of CAFs confers chemoresistance, TKI-mediated inhibition of CAFs may be exploited to overcome OCCC chemoresistance.
It is not clear why ripretinib was better at inhibiting CAFs than other TKIs, but it may be attributed to extensive suppression of PDGFR isoforms and/or related kinases. Indeed, ripretinib blocks PDGFRA (the subunit α isoform), KIT, and BRAF in addition to PDGFRB (the subunit β isoform).34 The effectiveness of CAF-targeting therapy may also reflect the dominance of cancer-promoting populations within OCCC. In any case, our data suggest that CAF-targeting cancer therapy may be applicable to other types of cancers in which CAFs play a growth-promoting role.
Another salient finding of our study is that CAF activates HIF-1α in a neighboring chemoresistant subpopulation of OCCC. HIF-1α is activated under hypoxic conditions,35,36 after which its plays an essential role in progression and chemoresistance of a variety of cancers, including EOC.37,38 By contrast, our data indicate that HIF-1α is induced in OCCC only when cultivated together with CAFs, suggesting that diffusible signals from activated CAFs, not hypoxia itself, induce HIF-1α in OCCC. For example, the induction of HIF-1α may be attributable to cytokines, exosomes, or metabolic products expressed in activated CAFs. Alternatively, it may be attributable to metabolic products. Importantly, HIF-1α is capable of transactivating PDGFB,39 suggesting that activation of HIF-1α leads to formation of a positive feedback loop between HIF-1α-activated OCCC and CAFs in which PDGFR signaling is activated. HIF-1α induction is associated with chemoresistance from various cancers,38 and our data suggest that the feedback loop formation between cancer cells and CAFs mediates chemoresistance via HIF-1α induction.
In summary, we show that cancer cell-CAF interactions constitute a chemoresistant niche in the tumor microenvironment of OCCC. Although we focused on CAF functions in this paper, our data do not exclude the possibility that other non-tumor cells, such as macrophages or tumor-infiltrating lymphocytes, also participate in cancer chemoresistance. Comprehensive analyses of the tumor microenvironment, such as neighborhood analyses using multiplexed imaging,40 will be needed to fully map the complex cell-cell interactions within the tumor microenvironment.
Limitations of the study
To fully establish the functional importance of the interaction between cancer cells and CAFs, a comprehensive co-culture study using a variety of patient-derived cancer spheroids and CAFs is required. In this report, only one CAF and two cancer spheroids were used, mainly due to the difficulty of establishing and expanding patient-derived cells from OCCC. In future, it would be desirable to (1) develop methods to efficiently establish and expand patient-derived cancer spheroids and CAFs from OCCC, (2) firmly establish the functional significance of the cancer cell-CAF interaction, and (3) exploit the established co-culture system to elucidate the molecular basis of the interaction.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-human PAX8 | Proteintech | Cat# 10336-1-AP, RRID: AB_2236705 |
| Mouse anti-human Cytokeratin 7 | Dako | Cat# M7018, RRID: AB_2134589 |
| Mouse anti-human α-SMA | Abcam | Cat# ab7817, RRID: AB_262054 |
| Rabbit anti-human Collagen I | Abcam | Cat# ab138492, RRID: AB_2861258 |
| Mouse anti-human β-Actin | Sigma-Aldrich | Cat# A5316, RRID: AB_476743 |
| Rabbit anti-human HIF-1α | Abcam | Cat# ab51608, RRID: AB_880418 |
| Rabbit anti-human HIF-2α | Abcam | Cat# ab199, RRID: AB_302739 |
| Rabbit anti-human PDGFB | Abcam | Cat# ab23914, RRID: AB_2162180 |
| Rabbit anti-human Fibronectin | Abcam | Cat# ab268020, RRID: AB_2941028 |
| Rabbit anti-human PDGFRβ | Cell Signaling Technology | Cat# 3169, RRID: AB_2162497 |
| Rabbit anti-human PDGFRβ (phospho Y1021) | Abcam | Cat# ab16868, RRID: AB_302554 |
| Rabbit anti-human FAP-α | Abcam | Cat# ab53066, RRID: AB_880077 |
| Goat Anti-Rabbit IgG Antibody (H + L), Biotinylated | Vector Laboratories | Cat# BA-1000, RRID: AB_2313606 |
| Donkey anti-rabbit IgG Alexa Fluor 750 | Abcam | Cat# ab175728, RRID: AB_2924801 |
| Goat anti-mouse IgG Alexa Fluor 555 | Thermo Fisher Scientific | Cat# A-21424, RRID: AB_141780 |
| Rabbit anti-human FAP-α | Abcam | Cat# ab227703 |
| Biological samples | ||
| Ovarian clear cell carcinoma tissue samples | National Cancer Center Japan, Niigata University | N/A |
| Mouse xenograft tumors | National Cancer Center Japan | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| ProLongTM Diamond Antifade Mountant with DAPI | Thermo Fisher Scientific | Cat# P36971 |
| 3,3′-diaminobenzidine | Sigma-Aldrich | Cat# D12384 |
| Nuclei EZ Lysis buffer | Sigma-Aldrich | Cat# NUC-101 |
| Recombinant RNase Inhibitor | Clontech/TaKaRa | Cat# 2313A |
| Collagenase/hyaluronidase | Stemcell Technologies | Cat# 7912 |
| Histodenz | Sigma-Aldrich | Cat# D2158 |
| ACK Lysing Buffer | Thermo Fisher Scientific | Cat# A1049201 |
| STEMPRO hESC SFM | Thermo Fisher Scientific | Cat# A1000701 |
| basic fibroblast growth factor | Thermo Fisher Scientific | Cat# AA10-155 |
| Accumax | Innovative Cell Technologies | Cat# AM105 |
| MEM-α | Thermo Fisher Scientific | Cat# 12561-5 |
| Fetal Bovine Serum | Thermo Fisher Scientific | Cat# 10270106 |
| TripLE Express Enzyme | Thermo Fisher Scientific | Cat# 12604013 |
| DMEM/F12-GlutaMAX | Thermo Fisher Scientific | Cat# 10565-042, |
| HEPES | Thermo Fisher Scientific | Cat# 15630106 |
| N-2 Supplement | Thermo Fisher Scientific | Cat# 17502-001 |
| B-27 supplement | Thermo Fisher Scientific | Cat# 17504-001 |
| Human EGF | Thermo Fisher Scientific | Cat# PHG0313 |
| N-acetylcysteine | Sigma-Aldrich | Cat# A7250 |
| Growth Factor Reduced Matrigel | Corning | Cat# 356231 |
| Cell Recovery Solution | Corning | Cat# 354253 |
| Human Recombinant PDGFB | Fuji Film Wako | Cat# 160-24033 |
| Carboplatin | Selleck Chemicals | Cat# S1215 |
| CP-673451 | Selleck Chemicals | Cat# S1536 |
| Sorafenib | Selleck Chemicals | Cat# S7397 |
| Imatinib | Selleck Chemicals | Cat# S2475 |
| Regorafenib | Selleck Chemicals | Cat# S1178 |
| Tivozanib | Selleck Chemicals | Cat# S1207 |
| Pazopanib | Selleck Chemicals | Cat# S3012 |
| Axitinib | Selleck Chemicals | Cat# S1005 |
| Sunitinib | Selleck Chemicals | Cat# S7781 |
| Nintedanib | Selleck Chemicals | Cat# S1010 |
| Lenvatinib | Selleck Chemicals | Cat# S1164 |
| Ripretinib | Selleck Chemicals | Cat# S8757 |
| Alexa Fluor™ 488 Tyramide Reagent | Thermo Fisher Scientific | Cat# B40953 |
| ProLong Glass Antifade Mountant | Thermo Fisher Scientific | Cat# P36984 |
| Critical commercial assays | ||
| Vectastain Elite ABC detection kit | Vector Laboratories | Cat# PK-6100 |
| Chromium Single Cell 3′ Reagent Kit v3 | 10x Genomics | Cat# PN-1000075 |
| Visium Spatial Tissue Optimization Kit | 10x Genomics | Cat# PN-1000193 |
| 3′ CellPlex Kit Set A | 10x Genomics | Cat# PN-1000261 |
| DNeasy Blood & Tissue kit | Qiagen | Cat# 69504 |
| SureSelectXT reagent kit | Agilent | Cat# G9611A |
| SureSelect NCC Oncopanel | Agilent | Cat# 931195 |
| Dual Luciferase Reporter Assay System | Promega | Cat# E1960 |
| CellTiter-Glo Luminescent Cell Viability Assays | Promega | Cat# G7571 |
| Opal 6-Plex Detection kit | Akoya Biosciences | Cat# NEL871001KT |
| Deposited data | ||
| Ovarian clear cell carcinoma in vitro scRNA-seq data | Gene Expression Omnibus | GSE224333 |
| Ovarian clear cell carcinoma snRNA-seq data | Gene Expression Omnibus | GSE224334 |
| Ovarian clear cell carcinoma visium data | Gene Expression Omnibus | GSE224335 |
| Experimental models: Organisms/strains | ||
| NOD/Shi-scid IL-2Rγnull mice | Japan Crea | N/A |
| Oligonucleotides | ||
| Edit-R Synthetic sgRNA Non-targeting Control #1 | Dharmacon-Horizon Discovery | Cat# U-009501-01-001p |
| Edit-R Human Synthetic sgRNA pool for PDGFRB | Dharmacon-Horizon Discovery | Cat# SQ-003163-01-0002 |
| Recombinant DNA | ||
| pCDH-CMV-MCS-EF1α-copGFP | System biosciences | Cat# CD511B-1 |
| pGL4.51[luc2/CMV/Neo] | Promega | Cat# E1320 |
| pcDNA5-MTS-TagBFP-P2AT2A-EGFP-NLS-P2AT2A-mCherry-PTS1 | Addgene | Cat# 87829 |
| pGL4.74[hRluc/TK] | Promega | Cat# E6921 |
| Software and algorithms | ||
| CellRanger version 3.0.2 | 10x Genomics | https://www.10xgenomics.com |
| SpaceRanger version 1.1.0 | 10x Genomics | https://www.10xgenomics.com |
| cisCall | Kato et al.41 | https://www.ciscall.org/ |
| CellRanger version 6.1.2 | 10x Genomics | https://www.10xgenomics.com |
| Seurat version 3.2.2 | Butler et al.42; Stuart et al.18 | https://github.com/satijalab/seurat |
| InferCNV version 1.5.0 | N/A | https://github.com/broadinstitute/inferCNV |
| escape version 1.8.0 | N/A | https://github.com/ncborcherding/escape |
| clusterProfiler | Yu et al.43 | https://github.com/YuLab-SMU/clusterProfiler |
| VIPER version 1.30.0 | Alvarez et al.44 | https://www.bioconductor.org/packages/release/bioc/html/viper.html |
| DoRothEA version 1.6.0 | Garcia-Alonso et al.45 | https://github.com/saezlab/DoRothEA |
| survival | Therneau and Grambsch46 | https://cran.r-project.org/web/packages/survival/index.html |
| igraph | N/A | https://CRAN.R-project.org/package=igraph |
| NicheNet | Browaeys et al.47 | https://github.com/saeyslab/nichenetr |
| QuPath | Bankhead et al.48 | https://qupath.github.io/ |
| StarDist | Schmidt et al.49 | https://github.com/stardist/stardist |
| CytoMAP | Stoltzfus et al.50 | https://gitlab.com/gernerlab/cytomap |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Koji Okamoto (okamoto.kouji.dm@teikyo-u.ac.jp).
Materials availability
Plasmid vectors generated in this study are available from the lead contact upon request with a materials transfer agreement.
Data and code availability
Accession numbers of RNA-seq data (GSE224333, GSE224334, GSE224335) are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Clinical samples
All procedures were performed using protocols reviewed and approved by the Ethics Committee of Teikyo University, Niigata University, and the National Cancer Center. All participants provided informed consent. For the snRNA-seq and spatial transcriptomics, surgical specimens were obtained from patients between the ages of 44–72 (median age: 56), and their baseline characteristics was provided in Table S1. For the Vectra multi-color analyses, surgical specimens were obtained from patients between the ages of 25–78 (median age: 55). Isolated surgical specimens were stored in at −80°C, subjected to formalin-fixed paraffin-embedded (FFPE) analyses, or used for establishment of cancer spheroids or CAFs.
Establishment of tumor-derived spheroids and CAF
To establish patient-derived cancer spheroids (OVN-48 and OVN-43), OCCC tissues obtained by surgical excision were washed immediately with PBS, cut into ∼10 mm3 pieces using a scalpel, and dissociated with collagenase/hyaluronidase (#7912, Stem Cell Technologies) for 2 h at 37°C. Dissociated cells were filtered sequentially through 100- and 70-μm cell strainers (352350, BD Falcon) and isolated by density gradient centrifugal purification in PBS containing Histodenz (D2158, Sigma).29 After lysis of red blood cells with ACK Lysing Buffer (A1049201, Thermo Fisher Scientific), the isolated cells were used to establish cancer spheroids by cultivating them on ultra-low-attachment culture dishes (#3471 or #3262, Corning) in STEMPRO hESC SFM (A1000701, Thermo Fisher Scientific) supplemented with 8 ng/mL basic fibroblast growth factor (#AA10-155, Thermo Fisher Scientific) and penicillin/streptomycin (37°C, 5% CO2). Serial passage of formed cancer spheroids was performed every 2 weeks by dissociating the spheroids with Accumax (AM105, Innovative Cell Technologies). To establish CAF cultures, the red blood cell-removed Histodenz-purified cells were grown on attachment culture dishes (#35003, Corning) in MEM-α (#12561-05, Thermo Fisher Scientific) containing 10% FBS (#10270106, Thermo Fisher Scientific) and penicillin/streptomycin (37°C, 5% CO2). For serial passage of established CAFs, attached cells were dissociated with TripLE Express Enzyme (#12604013, Thermo Fisher Scientific) every 2 weeks.
Animal experiments
All procedures of mouse experiments were performed using protocols reviewed and approved by the Institutional Animal Care and Use Committee of Teikyo University and the National Cancer Center. To examine the synergistic effects of Carboplatin and Ripretinib on xenograft tumors, Luc2-GFP-labeled cancer spheroid cells and HRluc-mCherry-labeled CAFs were dissociated and mixed at a 1:1 ratio. Next, 1 × 105 mixed cells were suspended in 100 μL of E medium containing 50% GFR Matrigel, and injected subcutaneously into the flanks of NOG (NOD/Shi-scid IL-2Rγnull) mice (Japan Crea). At 49 days post-transplantation (tumor volume: ∼100 mm3), mice were randomized into four groups and with/without Carboplatin (40 mg/kg per week, intraperitoneal injection) and/or Ripretinib (50 mg/kg/day, oral administration) for an additional 28 days. Tumor volume was calculated weekly using the standard formula: length × width × height × π/6). Evaluation of tumor volume according to luciferase activity was performed using the IVIS Spectrum imaging system (Caliper Life Science), which measured total luminescence emitted from the area of the mouse abdomen (photons/sec/cm2/sr) at 10 min post-intraperitoneal administration of 15 mg/mL D-luciferin potassium salt (10 μL per 1 g body weight, Wako). The data were analyzed using Living Image software (v. 4.2; Caliper Life Science).
Method details
Nucleus isolation
Frozen OCCC samples were homogenized in 500 μL of ice-cold Nuclei EZ Lysis buffer (NUC-101, Sigma-Aldrich) using a KIMBLE Dounce tissue grinder (D8938, Sigma-Aldrich), and incubated on ice for 5 min with an additional 1 mL of the lysis buffer. The homogenate was filtered through a 70-μm cell strainer (#352350, Corning) and then centrifuged at 500×g for 1 min at 4°C. The pellet was resuspended, washed with 1 mL of Lysis buffer, and incubated on ice for 5 min. After another cycle of washing with Lysis buffer, the pellet was washed twice in 1 mL of Nuclei Suspension Buffer (1× PBS, 1% BSA, and 0.2% RNase inhibitor (2313A, Clontech/TaKaRa)). The nuclear pellet was resuspended in 1 mL of Nuclei Suspension Buffer and filtered twice through a 35-μm cell strainer (#352235, Corning).
Single-nucleus RNA-seq (snRNA-seq)
For snRNA-seq of OCCC tissues, cDNA libraries were prepared from isolated nuclei (4000–8000 nuclei) on Chromium controller (10X Genomics) using the Single Cell 3′ Reagent Kit v3 (PN-1000075, 10X Genomics). Next-generation sequencing of the cDNA libraries was performed on the HiSeq 2500 (Illumina) platform at a median depth of 65,124 reads/cell. Fastq files of the sequencing data were processed by the cellranger pipeline (version 3.0.2, 10X Genomics) and mapped to the GRCh38 (version 3.0.0 for premRNA) reference genome to generate matrices of unique molecular identifier (UMI) and cell-associated barcodes.
Spatial transcriptomics
Frozen OCCC samples were embedded in pre-chilled OCT compound (#25608-930, Sakura Finetek Japan Co., Ltd.), re-frozen on dry ice, and then stored at −80°C. Preparation of cDNA libraries from the tissue sections was performed using a Visium Spatial Gene Expression kit (10X Genomics) according to the manufacturer’s instructions. Optimal parameters for permeabilization of OCCC tissue were determined using the Visium Spatial Tissue Optimization Kit (PN-1000193; 10x Genomics). Subsequently, 10 μm sections cut from OCT-embedded samples were subjected to H&E staining and cDNA library preparation from barcoded Visium spots after permeabilization for 20 min. Next-generation sequencing was performed on a HiSeq 2500 (Illumina) platform. Fastq files of the sequencing data were processed by the spaceranger pipeline (version 1.1.0., 10X Genomics) and mapped to the GRCh38 reference genome to generate matrices of UMI and spot-associated barcodes.
Targeted genome sequencing
Genomic DNA was extracted from frozen OCCC tissues using a DNeasy Blood & Tissue kit (#69504, Qiagen) prior to selection of targeted sequences using the SureSelect NCC Oncopanel (v.4.0; Agilent Technologies). The captured sequences were subsequently used to construct libraries using a SureSelectXT reagent kit (Agilent Technologies). Paired-end sequencing (2 × 150 bp) was performed by using NextSeq 500 (Illumina). Mutations (single-nucleotide variations, and short insertions, and deletions), gene amplifications, and gene fusions, were detected using the cisCall system.41
Bulk RNA-seq analysis
Total RNA extraction and library preparation were performed as previously reported.51 Briefly, total RNA was extracted from frozen samples of OCCC (30 cases) using TRIzol (#15596026, Invitrogen), and then subjected to cDNA library preparation by using a TruSeq Stranded mRNA Library Prep Kit (RS-20020595, Illumina), according to the manufacturer’s instructions. Subsequently, cDNA libraries were sequenced on the Illumina HiSeq 2500 platform with the 2 × 100-bp paired-end read module. Sequenced reads were mapped onto the human genome reference sequence (UCSU hg19) using Basespace (Illumina).
Plasmid construction
To generate the pCDH-Luc2-T2A-copGFP plasmid, the Luc2-T2A-copGFP cassette was initially generated by ligating a synthesized T2A sequence to Luc2 (PCR-amplified from pGL4.51[Luc2/CMV/Neo] (Promega, E1320)) and copGFP (PCR-amplified from pCDH-CMV-MCS-EF1α-copGFP (System biosciences, CD511B-1)). Subsequently, the Luc2-T2A-TagBFP sequence in pCDH-Luc2-T2A-TagBFP28 was substituted with the Luc2-T2A-copGFP cassette via the EcoRI and SalI sites to generate pCDH-Luc2-T2A-copGFP. To generate the pCDH-hRluc-T2A-mCherry plasmid, the hRluc-T2A-mCherry cassette was first generated by ligating the synthesized T2A sequence with hRluc (PCR-amplified from pGL4.74[hRluc/TK] (Promega, E6921)) and mCherry (PCR-amplified from pcDNA5-MTS-TagBFP-P2AT2A-EGFP-NLS-P2AT2A-mCherry-PTS1 (Addgene, #87829)). Subsequently, pCDH-hRluc-T2A-mCherry was generated from pCDH-Luc2-T2A-TagBFP using a similar construction strategy. The pCDH-Luc2-T2A-copGFP and pCDH-hRluc-T2A-mCherry plasmids were used to generate lentiviruses for gene transduction into cancer cells and CAF, respectively.
In vitro co-culture assay
OCCC spheroid cells and CAF were infected by lentiviruses expressing Luc2 and GFP (pCDH-Luc2-T2A-copGFP), and lentiviruses expressing hRLuc and mCherry (pCDH-hRLuc-T2A-mCherry), respectively. For co-culture, the infected spheroid cells and CAF were mixed at 1:1 ratio. Subsequently, monocultured cancer cells, monocultured CAF, or co-cultured cells were plated into a 96-well plate (1 × 104 cells/well) layered with growth factor-reduced (GFR) Matrigel (#356231, Corning), and then incubated for 6 h with MEM-α supplemented with 10% FBS. After removing floating dead cells, the remaining cells were overlaid with GFR Matrigel and, subsequently, with E medium (DMEM/F12-GlutaMAX (#10565-042, Thermo Fisher Scientific)) supplemented with penicillin-streptomycin, 10 mM HEPES (#15630106, Thermo Fisher Scientific), N-2 Supplement (#17502-001, Thermo Fisher Scientific), B-27 supplement (#17504-001, Thermo Fisher Scientific), 1 mM N-acetylcysteine (A7250, Sigma-Aldrich), and 50 ng/mL human EGF (PHG0313, Thermo Fisher Scientific)). For the chemosensitivity assay, monocultured or co-cultured cells were treated with Carboplatin (S1215, Selleck Chemicals). Cell growth was evaluated using a dual luciferase reporter kit (E1960, Promega). For western blot analysis, cultivated cells were harvested with Cell Recovery Solution (#354253, Corning) and GFP-expressing cancer cells and mCherry-expressing CAFs were selected by flow cytometry (FACS Aria III, Beckton Dickinson, Franklin Lakes, New Jersey).
Single-cell RNA-seq (scRNA-seq) of in vitro-cultivated cells
Monoculture and co-culture of cancer cells and CAF (3 days after incubation) were used to prepare single-cell cDNA libraries. For this, 3000–6000 cells were applied to a Chromium controller (10X Genomics). Library construction was performed using the Single Cell 3′ Reagent Kit v3 and 3′ CellPlex Kit Set A (10X Genomics), according to the manufacturer’s instructions. Next-generation sequencing of the cDNA libraries was performed using a HiSeq 2500 (Illumina). Fastq files of the sequencing data were processed by the cellranger pipeline (version 6.1.2, 10X Genomics) using the command “cellranger multi”, and then mapped onto the GRCh38 reference genome to generate matrices of UMI and cell-associated barcodes.
In vitro proliferation assays of CAF
To examine the chemosensitivity of CAF, in vitro-cultivated CAFs at Day 7–10 post-passage were enzymatically dissociated and used for chemosensitivity assays. The following TKIs were purchased from Selleck Chemicals and used for the assay: CP-673451 (S1536), Sorafenib (S7397), Imatinib (S2475), Regorafenib (S1178), Tivozanib (S1207), Pazopanib (S3012), Axitinib (S1005), Sunitinib (S7781), Nintedanib (S1010), Lenvatinib (S1164), and Ripretinib (S8757). Human recombinant PDGFB (160–24033, Fuji Film Wako) was used to examine the effects of PDGF signaling on CAF. The effects of TKIs or PDGFB on cell growth were quantified by measuring luciferase activity in a CellTiter-Glo Luminescent Cell Viability Assay (G7571, Promega) according to the manufacturer’s instructions.
CRISPR/Cas9-mediated gene knockout
CAFs grown for 7–10 days after passaging were enzymatically dissociated and subjected to Cas9-mediated gene knockout using the Neon Transfection System (Thermo Fisher Scientific), according to the manufacturer’s instructions. The sgRNA/Cas9 complex formed after mixing Cas9 protein (Invitrogen) with Edit-R Human Synthetic sgRNA pool for PDGFRB (SQ-003163-01-0002, Dharmacon-Horizon Discovery) or Edit-R Synthetic sgRNA Non-targeting Control #1 (U-009501-01-001p, Dharmacon-Horizon Discovery) was used for electroporation (1600 V, 10 ms, two pulses).
Western blot analyses
Western blot analyses were performed as previously described.30 Antibodies specific for the following markers were purchased from the indicated suppliers: PAX8 (10336-1-AP, Proteintech; dilution, 1:2000), Cytokeratin 7 (M7018, Dako; 1:1000), α-SMA (ab7817, abcam; 1:3000), Collagen I (ab138492, abcam; 1:1000), β-Actin (A5316, Sigma-Aldrich; 1:1000), HIF-1α (ab51608, abcam; 1:1000), HIF-2α (ab199, abcam; 1:1000), PDGFB (ab23914, abcam; 1:1000), Fibronectin (ab268020, abcam; 1:1000), PDGFRB (#3169, Cell Signaling Technology; 1:1000), PDGFRB (phospho Y1021; ab16868, abcam; 1:1000), FAP-α (ab53066, abcam; 1:1000).
Immunofluorescence analysis of clinical specimens
To immunostain clinical samples of OCCC, surgical specimens were fixed in 10% formaldehyde, embedded in paraffin, and sliced into 4-μm sections. For histological examination, sections were stained with H&E. For immunofluorescence analyses, sections were subjected to antigen retrieval with 10 mM citric acid buffer (pH 6.0), followed by blocking of endogenous peroxidase activity with 0.3% hydrogen peroxide. For co-staining with PAX8, HIF-1α, and α-SMA, slides were stained sequentially with a rabbit anti-PAX8 antibody (1:1000; Proteintech, 10336-1-AP), biotinylated-goat anti-rabbit IgG (1:500; Vector Laboratories, BA-1000), the Vectastain Elite ABC detection kit (Vector Laboratories, PK-6100), and Alexa Fluor 488 Tyramide Reagent (Invitrogen, B40953). For consecutive staining with anti-HIF-1α and anti-α-SMA antibodies, slides were boiled in 10 mM citric acid buffer (pH 6.0) for >15 min to remove the PAX8-secondary antibody complex. Slides were then stained with a rabbit anti-HIF-1α antibody (1:100; abcam, ab51608) and a mouse anti-α-SMA antibody (1:600; abcam, ab7817), followed by donkey anti-rabbit IgG AlexaFluor 750-conjugated (1:1000; abcam, ab175728) or goat anti-mouse IgG- AlexaFluor 555-conjugated (1:1000; Invitrogen, A21424) secondary antibodies. The immunostained slides were then mounted using ProLong Diamond Antifade Mountant containing DAPI (Invitrogen, P36971). The same procedure was used for immunostaining with anti-KRT7, anti-α-SMA, and anti-PDGFRB (phospho Y1021) antibodies (Figure S7B). Antibodies specific for the following markers were purchased from the listed suppliers: cytokeratin 7 (M7018, Dako, 1:100), α-SMA (ab7817, abcam, 1:600), and PDGFRB (phospho Y1021) (ab16868, abcam, 1:100). Immunofluorescence images were evaluated by Vectra Polaris (Akoya Biosciences).
Immunofluorescence analysis of in vitro-cultivated cells
Cancer cells under monoculture conditions or under co-culture conditions with CAF were plated on GFR Matrigel-coated glass-bottom dishes (D11140H, Matsunami Glass Ind.), fixed with cold methanol, and permeabilized with 0.1% Triton X-(Sigma-Aldrich). After blocking with 5% BSA, fixed cells were incubated with rabbit anti-HIF-1α (1:100; abcam, ab51608) and mouse anti-α-SMA (1:600; abcam, ab7817) antibodies, followed by donkey anti-rabbit IgG AlexaFluor 750-conjugated (1:1000; abcam, ab175728) or goat anti-mouse IgG AlexaFluor 555-conjugated (1:1000; Invitrogen, A21424). Subsequently, cells were mounted in ProLong Diamond Antifade Mountant with DAPI (Invitrogen, P36971). Fluorescence images were taken under a Keyence BZ-800 Microscope (Keyence).
Immunohistochemical staining
Clinical tumor samples and mouse xenograft tumors were fixed in neutral formalin and embedded in paraffin. Immunohistochemical staining was performed as previously described.30 Briefly, sections were used for H&E staining or immunostaining with primary anti-FAP-α (ab53066, abcam, 1:100), anti-HIF-1α (ab51608, abcam, 1:100) or anti-α-SMA (ab7817, abcam, 1:500) antibodies, followed by staining with biotinylated secondary antibodies (Vector Laboratories) and incubation with The Vector stain ABC kit (PK6100, Vector Laboratories) and 3,3′-diaminobenzidine (D12384, Sigma). For evaluation of HIF-1α staining, positive cells in four representative areas were counted using Hybrid Cell Count software (Keyence).
Multi-color immunostaining by Vectra Polaris
For multi-colored immunofluorescence, formalin fixed paraffin-embedded sections (4-μm) were stained on a BondRX automated stainer (Leica) with Opal 6-Plex Detection kit (Akoya Biosciences, NEL871001KT) according to the manufacturers’ instructions. After deparaffinization, slides were subjected to epitope retrieval with Epitope Retrieval solution 2 (Leica Biosystems, AR9640), incubation with primary and secondary antibodies (Opal polymer HRP mouse plus rabbit, Akoya Biosciences, NEL871001KT), and tyramide signal amplification with Opal fluorophores (Table S4). Primary and secondary antibodies were removed during sequential epitope retrieval steps. Subsequently, slides were counterstained with spectral DAPI and mounted with ProLong Glass Antifade Mountant (Invitrogen, P36984). Whole slide scanning was performed on a Vectra Polaris multispectral imaging system.
86 cases of OCCC were classified according to the percent of cancer cells expressing HIF-1α and CAFs expressing FAP-α. Areas to be analyzed were selected based upon extensive presence of both PAX8-positive cancer cells and α-SMA-positive CAFs, and ≧3 areas were chosen to calculate the average value of fraction of HIF-1α-positive cells in PAX8 positive cancer cells and FAP-α-positive cells in α-SMA-positive CAFs. Samples with high level (>10%) or low level (<10%) of HIF-1α-positive fraction of cancer cells were categorized as HIF-1α high or HIF-1α low, respectively. Samples with high level (>80%) or low level (<80%) of FAP-α-positive fraction of CAFs were categorized as FAP-α high or FAP-α low, respectively.
Quantification and statistical analysis
Processing of snRNA-seq data
The gene count matrices were analyzed by Seurat software v3.2.2 running on R v3.6.0. The following cells were removed from the dataset: cells with >1% mitochondrial gene counts; cells with >6,000 unique featureCounts; and cells with <400 unique featureCounts. The gene-barcode matrix of the filtered cells was normalized using ‘LogNormalize’. The top 2,000 variable genes were then identified using the ‘vst’ method in the Seurat FindVariableFeatures function. All cells from ten OCCC samples were integrated using the FindIntegrationAnchors and the IntegrateData functions of Seurat. After filtering of cells and data integration, a total of 62,673 cells were subjected to scaling using the Seurat ScaleData function. Subsequently, the scaled data were analyzed by PCA using the Seurat RunPCA function, with npcs = 30 parameters. UMAP plots were generated by the Seurat RunUMAP function, with dims = 1:30.
Annotation of cell populations in OCCC
To stratify cell populations using the integrated snRNA-seq data, low-resolution clustering was performed using the FindClusters function, with a resolution of 0.2. To annotate the five classified cell populations, specific marker genes were used to identify cell populations corresponding to epithelial cell and non-tumor cell populations (Figure S1C). Annotation of these cell populations was confirmed by examining expression of various marker genes (Figure S1D).52,53
Copy number inference from sequencing data
InferCNV (https://github.com/broadinstitute/inferCNV) was used to analyze large-scale chromosome copy number alterations based on the single-cell sequencing data. The InferCNV pattern in each chromosome was examined in epithelial cells, using non-tumor cells (CAFs and endothelial cells) as a reference.
Enrichment analysis
To perform ssGSEA of cancer subpopulations, measurement of the signature scores for HALLMARK gene sets54 expressed by each cancer subpopulation was based upon ssGSEA of the single-nucleus RNA-seq data. ssGSEA was performed using escape (v1.8.0, http://www.bioconductor.org/packages/release/bioc/vignettes/escape/inst/doc/vignette.html) running on R v4.2.1. To perform GO enrichment analyses, differentially expressed genes (DEGs) were selected using the FindAllMarkers function in Seurat. Subsequently, clusterProfiler55 (v4.2.2) used the DEGs in each subpopulation to identify the top 10 most significant GO terms in biological processes (BP) categories.
Quantification of transcription factor activity
The activity of major transcription factors in each cell was inferred by VIPER (Virtual Inference of Protein-activity by Enriched Regulon analysis) v1.30.0 running on R v4.2.1. Transcription factor-target interactions classified as confidence level A (DoRothEA v1.6.045) were used exclusively to calculate VIPER scores. VIPER scores were visualized in violin plots and heatmaps. Transcription factor-target interactions of the Cancer #2 cluster (shown in Figure S2D) were depicted by the igraph package in R.
Prognosis analysis
The top 20 DEGs that were selected using the FindAllMarkers function of Seurat were defined as signature genes for each cancer subpopulation. Surgical specimens of thirty advanced stage OCCC (Stages II-IV) were subjected to bulk RNA-seq analyses, and the patients were classified into two groups based upon average expression of the signature genes. Kaplan–Meier analysis was performed using the ‘Survival’ package in R to evaluate the prognostic value of cancer cell clusters. p values for overall survival and progression-free survival were evaluated using a stratified log rank test.
Ligand-receptor interaction analysis
Ligand-receptor interaction analysis based on snRNA-seq data was performed by NicheNet.24 Ligands and receptors were selected from the DEGs of each cell population using the FindAllMarkers function in Seurat. The selected ligands form the Cancer #2 subpopulation were used to identify paired receptors from the DEGs of non-tumor cells based on the NicheNet ligand-receptor network. The Seurat AverageExpression function was used to visualize average expression of the ligands and receptors of each population in the heatmap. Subsequently, the interaction potential between the selected ligand-receptor pairs was estimated using NicheNet weighted integrated networks.
Spatial transcriptomics data processing
The Visium spot-gene expression matrices and spatial information of spatial transcriptomics data were imported into Seurat v3.2.0 for downstream analysis. The Sctransform function of Seurat was used to normalize the UMI count in each spot.56 The object was run through PCA using the Seurat RunPCA function, with npcs = 20 parameters, and UMAP plots were generated by the Seurat RunUMAP function, with dims = 1:20. Clustering of the Visium spots was performed using the FindClusters function with resolution = 20.
Integration of snRNA-seq and spatial transcriptomics data
An anchor-based integration method in Seurat v3.2.018 was used to integrate snRNA-seq and Visium data. Transfer anchors were detected using the Seurat FindTransferAnchors function, setting the combined snRNA-seq datasets as a reference and one of the Visium datasets as a query. Following integration, the cluster labels of snRNA-seq datasets were transferred to the spatial dataset using the Seurat TransferData function, thereby providing a prediction score for each snRNA-seq cluster per spot.
Processing of scRNA-seq data
The gene count matrices were imported to Seurat software v3.2.2 running on R v3.6.0. The following cells were removed from the dataset: cells with >10% mitochondrial gene counts; cells with >6,000 unique featureCounts; and cells with <200 unique featureCounts. The filtered gene-barcode matrix was normalized using ‘LogNormalize’ in Seurat. Subsequently, the top 2,000 variable genes were then identified using the ‘vst’ method in the Seurat FindVariableFeatures function. Data obtained from co-cultured and monocultured cells were merged using the Seurat merge function. After filtering and merging, a total of 8,208 cells were processed for the following analysis: The merged object was scaled via the Seurat ScaleData function and run through PCA using the Seurat RunPCA function, with npcs = 30. UMAP plots were generated by the Seurat RunUMAP function, with dims = 1:30. ssGSEA and VIPER analyses were performed as described above.
Image analysis of multiplexed immunofluorescence
QuPath48 (version 0.2.1) was used to measure immunofluorescence intensity in tumor and non-tumor cells. After loading whole images, cells were segmented using StarDist,57,58 and the fluorescence intensity of each cell was measured. Subsequently, cancer cells and CAFs were identified based on expression of PAX8 and α-SMA, respectively. An identical threshold intensity for immunofluorescence signals was applied for all samples. The centroid distances between α-SMA (+) cells and PAX8 (+)/HIF-1α (+) cells were estimated using the “Detect centroid distance 2D″ command. After annotation of each cell, data were exported into CytoMAP50 (version 1.4.21) for nearest neighbor analysis.
Statistical analyses of multi-color immunostaining data
Kaplan–Meier analysis was performed by ‘Survival’ package in R to evaluate the prognostic value of HIF-1α-expressing cancer cell and FAP-α-expressing CAFs. p-values of overall survival and progression free survival were evaluated by performing a stratified log rank test. Univariate and multivariate survival analysis was carried out using the Cox proportional hazards regression model. The average distance from FAP-α-positive cell to nearest HIF-1α -positive/negative cell was calculated as described above.
Acknowledgments
This research was supported by Grant-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (JSPS) under grants JP21H03081 (to K.O.), 18K07283 (to D.S.), and 20K22826 (to Y.M.); the Japan Agency for Medical Research and Development (AMED) under grants JP19cm0106563h0001 and JP21cm0106183h0001 (to K.O.); the Japan Science and Technology Agency (JST) for CREST (Core Research for Evolutional Science and Technology) under grant 21-211035300 (to K.O.); the Japan Health Research Promotion Bureau (JH) under grant 2020-B-03 (to K.O.); JSPS KAKENHI grant 22H04925 (PAGS); and The Vehicle Racing Commemorative Foundation (to K.O.).
Author contributions
K.O. conceived the project. Y.M. oversaw the project. K.O. wrote the initial manuscript, and Y.M. edited the manuscript. Y.M. designed and performed the experiments. H.S. and Y.O. assisted with the in vivo experiments. Y.K., D.S., and H.O. assisted with in vitro culture. H.U., M.S. and A.H. supported QuPath analyses. H.Y. and Y.Y. performed pathological analyses. T.S., R.T., K. Yamawaki, T.I., T.K., K. Yoshihara, and T.E. provided surgical specimens and clinical information. K.O. and Y.M. provided grant support.
Declaration of interests
Teikyo University has filed for a patent application on CAF inhibition by tyrosine kinase inhibitors, and K.O. and Y.M. are named as inventors on this patent.
Published: April 25, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101532.
Supplemental information
References
- 1.Marusyk A., Janiszewska M., Polyak K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell. 2020;37:471–484. doi: 10.1016/j.ccell.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., McAndrews K.M., Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim B., Lin Y., Navin N. Advancing Cancer Research and Medicine with Single-Cell Genomics. Cancer Cell. 2020;37:456–470. doi: 10.1016/j.ccell.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenblatt-Rosen O., Regev A., Oberdoerffer P., Nawy T., Hupalowska A., Rood J.E., Ashenberg O., Cerami E., Coffey R.J., Demir E., et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell. 2020;181:236–249. doi: 10.1016/j.cell.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slyper M., Porter C.B.M., Ashenberg O., Waldman J., Drokhlyansky E., Wakiro I., Smillie C., Smith-Rosario G., Wu J., Dionne D., et al. A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat. Med. 2020;26:792–802. doi: 10.1038/s41591-020-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao A., Barkley D., França G.S., Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature. 2021;596:211–220. doi: 10.1038/s41586-021-03634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asp M., Giacomello S., Larsson L., Wu C., Fürth D., Qian X., Wärdell E., Custodio J., Reimegård J., Salmén F., et al. A Spatiotemporal Organ-Wide Gene Expression and Cell Atlas of the Developing Human Heart. Cell. 2019;179:1647–1660.e19. doi: 10.1016/j.cell.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Akhoundova D., Rubin M.A. Clinical application of advanced multi-omics tumor profiling: Shaping precision oncology of the future. Cancer Cell. 2022;40:920–938. doi: 10.1016/j.ccell.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Moncada R., Barkley D., Wagner F., Chiodin M., Devlin J.C., Baron M., Hajdu C.H., Simeone D.M., Yanai I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020;38:333–342. doi: 10.1038/s41587-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 11.Lheureux S., Gourley C., Vergote I., Oza A.M. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 12.Shoji T., Tatsuki S., Abe M., Tomabechi H., Takatori E., Kaido Y., Nagasawa T., Kagabu M., Baba T., Itamochi H. Novel Therapeutic Strategies for Refractory Ovarian Cancers: Clear Cell and Mucinous Carcinomas. Cancers. 2021;13 doi: 10.3390/cancers13236120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Leo A., Santini D., Ceccarelli C., Santandrea G., Palicelli A., Acquaviva G., Chiarucci F., Rosini F., Ravegnini G., Pession A., et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics. 2021;11 doi: 10.3390/diagnostics11040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadducci A., Multinu F., Cosio S., Carinelli S., Ghioni M., Aletti G.D. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol. Oncol. 2021;162:741–750. doi: 10.1016/j.ygyno.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Izar B., Tirosh I., Stover E.H., Wakiro I., Cuoco M.S., Alter I., Rodman C., Leeson R., Su M.J., Shah P., et al. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 2020;26:1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stur E., Corvigno S., Xu M., Chen K., Tan Y., Lee S., Liu J., Ricco E., Kraushaar D., Castro P., et al. Spatially resolved transcriptomics of high-grade serous ovarian carcinoma. iScience. 2022;25 doi: 10.1016/j.isci.2022.103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L., Hernandez M.O., Zhao Y., Mehta M., Tran B., Kelly M., Rae Z., Hernandez J.M., Davis J.L., Martin S.P., et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell. 2019;36:418–430.e6. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummins E.P., Taylor C.T. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 21.Lavie D., Ben-Shmuel A., Erez N., Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer. 2022;3:793–807. doi: 10.1038/s43018-022-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A., Teinor J.A., Belleau P., Biffi G., Lucito M.S., et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilchez Mercedes S.A., Bocci F., Levine H., Onuchic J.N., Jolly M.K., Wong P.K. Decoding leader cells in collective cancer invasion. Nat. Rev. Cancer. 2021;21:592–604. doi: 10.1038/s41568-021-00376-8. [DOI] [PubMed] [Google Scholar]
- 24.Browaeys R., Saelens W., Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 25.Heldin C.H., Ostman A., Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 26.Yerly L., Pich-Bavastro C., Di Domizio J., Wyss T., Tissot-Renaud S., Cangkrama M., Gilliet M., Werner S., Kuonen F. Integrated multi-omics reveals cellular and molecular interactions governing the invasive niche of basal cell carcinoma. Nat. Commun. 2022;13:4897. doi: 10.1038/s41467-022-32670-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiokawa D., Sakai H., Ohata H., Miyazaki T., Kanda Y., Sekine S., Narushima D., Hosokawa M., Kato M., Suzuki Y., et al. Slow-Cycling Cancer Stem Cells Regulate Progression and Chemoresistance in Colon Cancer. Cancer Res. 2020;80:4451–4464. doi: 10.1158/0008-5472.CAN-20-0378. [DOI] [PubMed] [Google Scholar]
- 29.Ohata H., Shiokawa D., Obata Y., Sato A., Sakai H., Fukami M., Hara W., Taniguchi H., Ono M., Nakagama H., Okamoto K. NOX1-Dependent mTORC1 Activation via S100A9 Oxidation in Cancer Stem-like Cells Leads to Colon Cancer Progression. Cell Rep. 2019;28:1282–1295.e8. doi: 10.1016/j.celrep.2019.06.085. [DOI] [PubMed] [Google Scholar]
- 30.Ishiguro T., Sato A., Ohata H., Ikarashi Y., Takahashi R.U., Ochiya T., Yoshida M., Tsuda H., Onda T., Kato T., et al. Establishment and Characterization of an In Vitro Model of Ovarian Cancer Stem-like Cells with an Enhanced Proliferative Capacity. Cancer Res. 2016;76:150–160. doi: 10.1158/0008-5472.CAN-15-0361. [DOI] [PubMed] [Google Scholar]
- 31.Ohata H., Ishiguro T., Aihara Y., Sato A., Sakai H., Sekine S., Taniguchi H., Akasu T., Fujita S., Nakagama H., Okamoto K. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72:5101–5110. doi: 10.1158/0008-5472.CAN-11-3812. [DOI] [PubMed] [Google Scholar]
- 32.Biffi G., Tuveson D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katopodis P., Chudasama D., Wander G., Sales L., Kumar J., Pandhal M., Anikin V., Chatterjee J., Hall M., Karteris E. Kinase Inhibitors and Ovarian Cancer. Cancers. 2019;11 doi: 10.3390/cancers11091357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon S. Ripretinib: First Approval. Drugs. 2020;80:1133–1138. doi: 10.1007/s40265-020-01348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Du Z.W., Xu T.M., Wang X.J., Li W., Gao J.L., Li J., Zhu H. HIF-1alpha Is a Rational Target for Future Ovarian Cancer Therapies. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doktorova H., Hrabeta J., Khalil M.A., Eckschlager T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2015;159:166–177. doi: 10.5507/bp.2015.025. [DOI] [PubMed] [Google Scholar]
- 39.Schito L., Rey S., Tafani M., Zhang H., Wong C.C.L., Russo A., Russo M.A., Semenza G.L. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2012;109:E2707–E2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schürch C.M., Bhate S.S., Barlow G.L., Phillips D.J., Noti L., Zlobec I., Chu P., Black S., Demeter J., McIlwain D.R., et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell. 2020;182:1341–1359.e19. doi: 10.1016/j.cell.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato M., Nakamura H., Nagai M., Kubo T., Elzawahry A., Totoki Y., Tanabe Y., Furukawa E., Miyamoto J., Sakamoto H., et al. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med. 2018;10:44. doi: 10.1186/s13073-018-0547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez M.J., Shen Y., Giorgi F.M., Lachmann A., Ding B.B., Ye B.H., Califano A. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat Genet. 2016;48:838–847. doi: 10.1038/ng.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Alonso L., Holland C.H., Ibrahim M.M., Turei D., Saez-Rodriguez J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019;29:1363–1375. doi: 10.1101/gr.240663.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Therneau T.M., Grambsch P.M. Estimating the Survival and Hazard Functions. In Modeling Survival Data:Extending the Cox Model. Springer; New York: 2000. pp. 7–37. [DOI] [Google Scholar]
- 47.Browaeys R., Saelens W., Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 48.Bankhead P., Loughrey M.B., Fernández J.A., Dombrowski Y., McArt D.G., Dunne P.D., McQuaid S., Gray R.T., Murray L.J., Coleman H.G., et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt, U., Weigert, M., Broaddus, C., Myers, G., (2018). Cell Detection with Star-Convex Polygons. In Lecture Notes in Computer Science, (Springer International Publishing), pp. 265–273. 10.1007/978-3-030-00934-2_30. [DOI]
- 50.Stoltzfus C.R., Filipek J., Gern B.H., Olin B.E., Leal J.M., Wu Y., Lyons-Cohen M.R., Huang J.Y., Paz-Stoltzfus C.L., Plumlee C.R., et al. CytoMAP: A Spatial Analysis Toolbox Reveals Features of Myeloid Cell Organization in Lymphoid Tissues. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamura R., Yoshihara K., Matsuo K., Yachida N., Miyoshi A., Takahashi K., Sugino K., Yamaguchi M., Mori Y., Suda K., et al. Proposing a molecular classification associated with hypercoagulation in ovarian clear cell carcinoma. Gynecol. Oncol. 2021;163:327–333. doi: 10.1016/j.ygyno.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Hu Z., Artibani M., Alsaadi A., Wietek N., Morotti M., Shi T., Zhong Z., Santana Gonzalez L., El-Sahhar S., Carrami E.M., et al. The Repertoire of Serous Ovarian Cancer Non-genetic Heterogeneity Revealed by Single-Cell Sequencing of Normal Fallopian Tube Epithelial Cells. Cancer Cell. 2020;37:226–242.e7. doi: 10.1016/j.ccell.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Luo H., Xia X., Huang L.B., An H., Cao M., Kim G.D., Chen H.N., Zhang W.H., Shu Y., Kong X., et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022;13:6619. doi: 10.1038/s41467-022-34395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hafemeister C., Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296. doi: 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens M., Nanou A., Terstappen L.W.M.M., Driemel C., Stoecklein N.H., Coumans F.A.W. StarDist Image Segmentation Improves Circulating Tumor Cell Detection. Cancers. 2022;14 doi: 10.3390/cancers14122916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uwe Schmidt M.W., Coleman B., Myers G. International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI) 2018. Cell Detection with Star-convex Polygons. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession numbers of RNA-seq data (GSE224333, GSE224334, GSE224335) are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.