Abstract
We present the case of a 62-year-old woman with probable behavioral variant of frontotemporal dementia (bvFTD) with cognitive/language deficits who demonstrated improved performance on cognitive/language testing and in functional tasks following long-term, home-based transcranial direct current stimulation (tDCS) coupled with computerized cognitive training (CCT). The patient underwent home-based tDCS (anode on the left prefrontal cortex and cathode on the right homologue) for 46 sessions over 10 weeks along with CCT. On post-treatment testing, the patient improved by 3 points on the Mini-Mental State Exam (MMSE) (23 to 26). She also showed improvement on several cognitive/language tasks, such as immediate recall of single words and word pairs, total accurate words in sentence repetition, delayed recall, semantic processing, and sentence level comprehension. There was no decline in several other cognitive and language tasks. Family members reported subjective improvements in expressiveness, communication, and interaction with others as well as increased attention to grooming and style which contrasted with her pre-treatment condition. This report suggests that home-based tDCS combined with CCT for an extended period may slow decline, and improve cognitive/language performance and everyday function in FTD.
Keywords: Frontotemporal dementia, behavioral variant frontotemporal dementia, transcranial direct current stimulation, case report
Plain Language Summary
Long-term, Home-based Transcranial Direct Current Stimulation Coupled with Computerized Cognitive Training in Frontotemporal Dementia: A Case Report: A 62-year-old woman with probable behavioral variant of frontotemporal dementia (bvFTD) improved on cognitive/language testing and in functional tasks following long-term, home-based transcranial direct current stimulation (tDCS) coupled with computerized cognitive training (CCT). The patient underwent home-based tDCS for 46 sessions over 10 weeks along with CCT. On post-treatment testing, the patient improved by three points on the Mini-Mental State Exam (MMSE) (23 to 26). She also improved immediate recall of single words and word pairs, total accurate words in sentence repetition, delayed recall, semantic processing, and sentence level comprehension. There was no decline in several other cognitive and language tasks. Family members described improvements in expressiveness, communication, and interaction with others and increased attention to grooming and style which was different from her pre-treatment condition. This case report suggests that home-based tDCS combined with CCT for an extended period may slow decline and improve cognitive/language performance and everyday function in FTD.
Introduction
Frontotemporal dementia (FTD) encompasses a group of neurodegenerative disorders characterized by a progressive decline in behavior, personality, executive function, language, and motor abilities 1 that typically manifest before age 652-7 (for review see 8 , 9 ). FTD includes 3 main clinical variants/subtypes: (1) behavioral-variant FTD (bvFTD)10,11; (2) semantic variant primary progressive aphasia (svPPA); and (3) nonfluent variant primary progressive aphasia (nfvPPA). 12 Behavioral-variant FTD, the focus of this case report, is characterized by progressive deterioration in behavior (disinhibition, apathy/inertia, decreased empathy/sympathy, perseverative/compulsive/ritualistic behavior, and hyperorality) and cognition/language (executive/generation dysfunction).11,13 According to international consensus criteria, 3 of the 6 clinical features must be present for a diagnosis of possible bvFTD. A diagnosis of probable bvFTD is supported by functional disability and characteristic neuroimaging with atrophy or hypoperfusion in the frontal and/or anterior temporal lobes. Histopathological confirmation or pathogenic mutation is required for a diagnosis of definite bvFTD. 11
While there are treatments to manage FTD symptoms, such as selective serotonin reuptake inhibitors to control disinhibition, irritability, depression, and compulsive behavior, 14 there is a dearth of treatments to slow neurodegeneration and illness progression.15-17 Approximately 20%–30% of individuals with FTD have familial FTD (fFTD) and carry a genetic mutation [eg, chromosome 9 open reading frame 72 (C9orf72), granulin (GRN), microtubule‐associated protein tau (MAPT)]. There has been encouraging progress in the development of disease-modifying therapy for specific forms of genetic FTD.9,18
Transcranial direct current stimulation (tDCS) has attracted attention in neurodegenerative disorders, and primary progressive aphasia (PPA) in particular, as a potential symptom-alleviating strategy. Studies of tDCS in PPA by different groups have focused on the treatment of language deficits in this clinical syndrome and have shown significant augmentative tDCS effects in several language functions by stimulating left hemisphere language regions.19-28
In 4 studies, the effect of tDCS in bvFTD was investigated, without, however, coupling it with any behavioral treatment. Agarwal et al 29 described improvement in language and behavior in a 45-year-old woman with bvFTD after 10 sessions of tDCS to the left dorsolateral prefrontal cortex (DLPFC). After tDCS intervention, this patient resumed responsibilities in the home setting (eg, cooking and laundry). The authors also reported improved speech output, although specifics of speech and language changes were not detailed. These improvements were sustained over 7 months. Cotelli et al 30 analyzed theory of mind ability, specifically communication intention processing, in 16 patients with mild bvFTD after a single session of tDCS stimulation over the left medial frontal cortex (MFC; Fpz site, with the cathode between Oz and Inion). Participants viewed pictured scenes and were required to attribute private (eg, hanging a picture on the wall) and communicative intentions (eg, asking another person to obtain a glass of water for them). They found that tDCS over the MFC selectively increased the accuracy of comprehension of communicative intentions. Pina et al 31 investigated the polarity-specific effects of 10 sessions of tDCS in Alzheimer’s disease (AD) and bvFTD. Participants received either anodal [target network: default mode network (DMN) in AD, salience network (SN) in bvFTD] or cathodal stimulation [target network: SN in AD, DMN in bvFTD). For the anode-DMN protocol, the anode was placed over the right inferior parietal DMN node (P4-P6 on the EEG 10/20 system); the cathode was placed over the contralateral supraorbital region. For the cathode-SN protocol, the cathode was placed over the right dorsolateral pre-frontal SN node (Fp2-AF4); the anode placed over the inion. The same tDCS configuration was used in bvFTD, but the polarity of the electrodes was reversed. In AD, there was improvement in composite memory and language test scores after anodal stimulation of the DMN (ie, posterior cingulate cortex, precuneus, medial prefrontal, and inferior parietal cortices), and improvement in composite memory test scores and ratings on the Neuropsychiatric Inventory (NPI) after cathodal stimulation of the SN (ie, anterior insula, anterior cingulate cortex, and ventral striatum). In bvFTD, there was a significant improvement in composite language test scores after anodal stimulation of the SN and improvement on the NPI after cathodal stimulation of the DMN. Benussi et al 32 reported short- and long-term improvement (3- and 6-months post-treatment) in cognitive symptoms and intracortical inhibitory and excitatory circuits in 25 individuals with symptomatic bvFTD, 30 individuals with PPA, and 15 presymptomatic carriers after 2 weeks of treatment with anodal left prefrontal tDCS. In the bvFTD group, there was a significant time × treatment interaction for Trail Making A and B and the modified Ekman emotion recognition test, but not for the Mini Mental State Examination (MMSE), phonemic verbal fluency, Stroop test, digit symbol substitution test, and Cambridge Behavior Inventory (CBI).
In sum, to date, the majority of tDCS studies have focused on PPA. In these studies, tDCS has been paired with behavioral intervention. The tDCS/behavioral intervention was of short duration, up to 3 weeks, and performed in a medical or research environment under the supervision of an experimenter. In bvFTD, tDCS has been delivered for brief periods as well, but without behavioral intervention. A potentially promising therapeutic intervention in bvFTD combines tDCS with behavioral intervention. The left DLPFC is a key region for language processing and executive functions, and is well-interconnected with other nodes and networks, including the perisylvian language network. 33 The left DLPFC is structurally connected to left supramarginal gyrus (an important node in the perisylvian language network supporting verbal short term memory and phonological processing) and the pars opercularis of the inferior frontal gyrus via parts of the superior longitudinal fasciculus III.34,35 The left DLPFC is thought to be a hub for recovery of language functions in post-stroke aphasia 36 and PPA37,38 with tDCS or TMS over the LDLPFC in PPA resulting in naming compensation.39,40 Thus, tDCS over the left DLPFC potentially can induce equivalent generalization to both executive and language-specific tasks.
Recent attention has been drawn to home-based tDCS (a pre-configured device designed for independent use in the home setting) and its feasibility and effectiveness since it allows for more extended periods of intervention. 41 However, there is no evidence of the feasibility or effectiveness of home-based neuromodulatory interventions for an extended period in neurodegenerative diseases. In the present case study, we describe the longest (10 weeks) home-based tDCS treatment with computerized cognitive training (CCT), to our knowledge, reported in FTD. We provide evidence of both the feasibility and effectiveness of long-term, home-based tDCS in FTD.
Case report
A 62-year-old, right-handed woman with 14 years of education, was diagnosed with probable bvFTD following a 5-year history of cognitive/language, behavioral, and functional decline.
Cognitive/Language Assessment and Neuroimaging
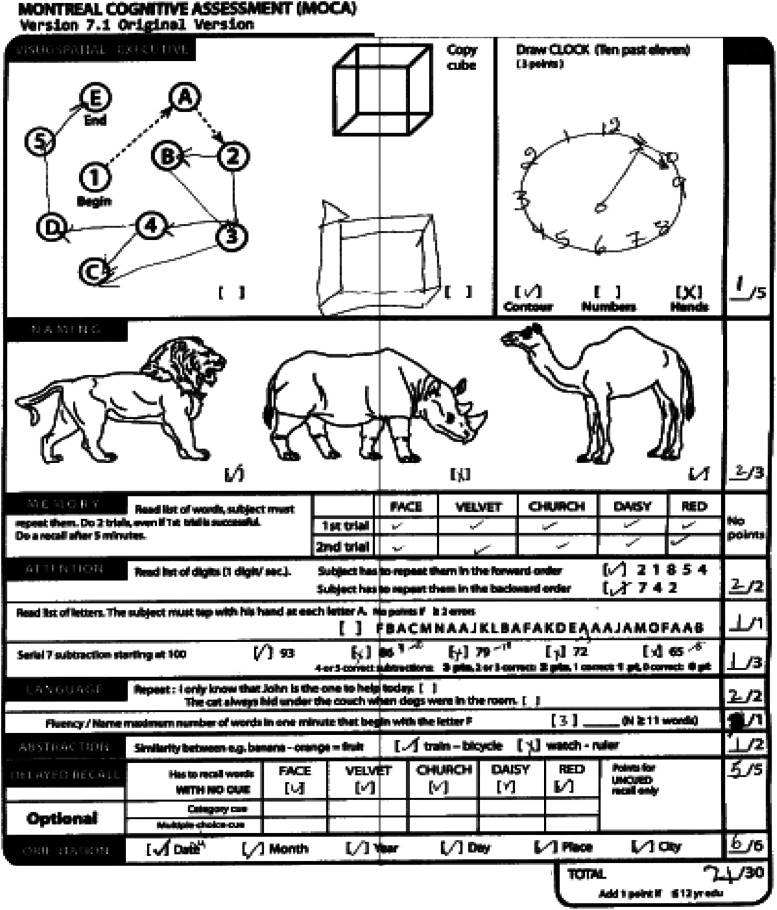
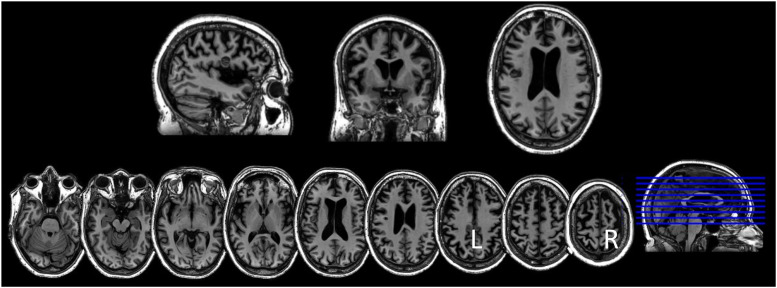
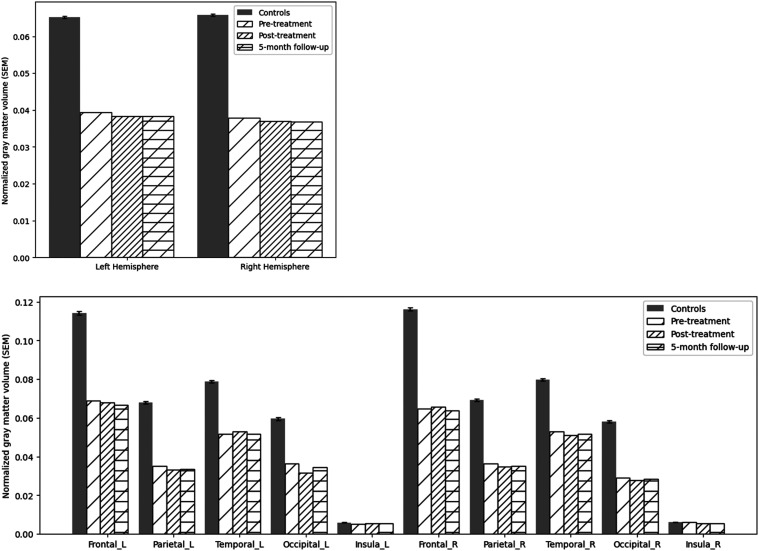
The family noticed that 5 to 6 years ago the patient had become less expressive, aloof, withdrawn, and preoccupied with TV. Two years before our assessment, she underwent a neurological evaluation at an outside hospital. Brain MRI showed modest diffuse, bilateral atrophy and a small left frontal cavernoma. FDG-PET showed reduced tracer uptake in both frontal medial and associative cortices, worse on the left. She subsequently was evaluated in the Frontotemporal Dementias and Young-Onset Dementias Clinic at the Johns Hopkins Hospital. During that evaluation (in March 2022), she was oriented to time, location, and context. Her speech was diminished in spontaneity; rate, fluency, and phrase length were within expected limits. She scored 21/30 on the Montreal Cognitive Assessment (MoCA) 42 at that visit, ie, 8 months pre-treatment [Figure 1; Mini Mental State Exam (MMSE) 43 was not administered at that time point]. Imaging obtained at our institution before and after treatment showed diffuse cortical atrophy, especially in the frontotemporal lobes (Figure 2). Quantitative assessment of cortical gray matter volume showed that, compared to a group of 30 older healthy controls (mean age = 61.46 years, SD 7.1 years), she presented widespread significant gray matter reduction, with the right hemisphere gray matter reduction being slightly more severe than the left (Crawford’s adjust t-values at pre-treatment, post-treatment, and 5-month follow-up: 13.9, 14.5, 14.5; right-hemisphere: 17.8, 18.3, 18.4, see Figure 3). Based on behavioral, cognitive/language, and imaging data, the patient was judged to fulfill the current criteria for probable bvFTD 11 by experts in neuro- and geriatric psychiatry (CO, CM).
Figure 1.
Baseline performance on the Montreal Cognitive Assessment.
Figure 2.
Saggital, coronal, and axial MRI showing diffuse, bilateral atrophy and a small left inferior frontal cavernoma.
Figure 3.
Cortical gray matter volumes of the whole brain (top) and by lobe (bottom) in the left and right hemispheres compared to healthy controls before and after tDCS with computerized cognitive training. Y-axis indicates the values of gray matter volume normalized by individuals’ intracranial volume. All of the patient’s values are significantly lower than the control group (p<1 × 10−4) except for the insula cortex bilaterally (ps > .1), evaluated by Crawford’t test.
Treatment Study
The patient was referred for experimental neuromodulatory treatment. CARE Guidelines were used in preparing this case report.44,45
The Brain HQ (Posit Science, Inc.) tasks that the patient practiced were Card Shark, Juggle Factor, Mind Bender, Mental Map, and Target Tracker, which are tasks targeting cognitive/language functions, like attention, cognitive control and decision-making. All tasks coupled with anodal tDCS (atDCS) from November 2022 to January 2023 (for a total of 46 sessions which were documented electronically after each Brain HQ task). She received atDCS over the DLPFC (anode in the left DLPFC and cathode in the right DLPFC) for 10 weeks (for a total of 46 sessions). The patient was instructed on how to use the home-based tDCS device and piloted it with the help of the experimenters in person before starting the remote therapy. She underwent cognitive/language evaluation at baseline, before, and after this treatment. There were no adverse or unanticipated events during the evaluation and treatment.
Cognitive/Language Test Results
The results of the evaluation at the 3 time points are presented in Table 1. The patient demonstrated a three-point gain (23 to 26 points) on the MMSE from pre-to post-treatment. Specifically, she gained 3 points for spelling the word “world” backward and for correctly following a three-step command. Before initiating treatment, performance on the MoCA decreased by nine points from March 2022 (baseline evaluation) to November 2022 (pre-treatment evaluation), ie, from 21 to 12 points. When evaluated in November 2022 the patient did not score any points on digit repetition, sentence repetition, and delayed recall tasks which she had completed correctly on the prior assessment. After treatment, there was stabilization of MoCA performance. Specifically, on both pre- (November 2022) and post-treatment (January 2023) evaluations the patient scored 12 points. There was a two-point gain on visuospatial tasks, but a loss of 1 point each on attention and orientation. With regard to other tasks, there was improvement in immediate recall (in RAVLT and WAIS paired associates), syntactic comprehension (SOAP task), and total accurate words in sentence repetition (NACC task). These test/re-test improvements were corroborated by family member observations of increased expressiveness, communication, and interaction with others as well as increased attention to personal hygiene which contrasted with her pre-treatment condition.
Table 1.
Baseline, pre- and post-treatment cognitive/language test scores.
| Baseline | Pre | Post | Normative Data | |
|---|---|---|---|---|
| Mar 2022 | Nov 2022 | Jan 2023 | ||
| Mini-Mental State (of 30) | 23 | 26 | Total for normal score >24 a ; mean (SD) for clinically cognitive normal adults 29.0 (1.3) b | |
| • Visuospatial/Executive | 0 | 0 | ||
| • Naming | 2 | 2 | ||
| • Attention | 3 | 5 | ||
| • Language | 3 | 4 | ||
| • Memory | 6 | 6 | ||
| • Orientation | 9 | 9 | ||
| Montreal Cognitive assessment (of 30) | 21 | 12 | 12 | Total for normal score >26 c |
| • Visuospatial/Executive | 1 | 1 | 3 | |
| • Naming | 2 | 2 | 2 | |
| • Attention | 4 | 2 | 1 | |
| • Language | 2 | 0 | 0 | |
| • Abstraction | 1 | 1 | 1 | |
| • Delayed recall | 5 | 0 | 0 | |
| • Orientation | 6 | 6 | 5 | |
| Rey Auditory Verbal Learning Test | Means (SD) for women age | |||
| • Sum 1-5 (of 75) | 44 | 26 | 60-69 years d , 49.0 (7.1) | |
| • Interference (of 15) | 2 | 2 | 5.3 (1.1) | |
| • Immediate recall (of 15) | 8 | 12 | 9.8 (1.6) | |
| • Delayed recall (of 15) | 10 | 11 | 10.3 (2.3) | |
| • Recognition (of 15) | 15 | 15 | 13.8 (1.1) | |
| Digit Span Forward (of 9; longest digit sequence) | 5 | 5 | Mean (SD) for clinically cognitive normal adults 6.7 (1.1) b | |
| Digit Span Backward (of 8; longest digit sequence) | 4 | 3 | Mean (SD) for clinically cognitive normal adults 5.0 (1.2) b | |
| Trail Making A | Mean (SD) for age 60-64 years e , 12+ years education, 31.32 (6.96) | |||
| • Duration (in seconds) | 50 | 58 | ||
| • Number of errors | 3 | 0 | ||
| Trail Making B (seconds) | Mean (SD) for age 60-64 years e , 12+ years education, 64.58 (18.59) | |||
| • Duration (in seconds) | >300 | 300 | ||
| • Number of errors | 6 | 12 | ||
| WAIS Digit Symbol (of 93) | 31 | 32 | Mean (SD) for clinically cognitive normal adults, 47.0 (12.5) b ; mean (SD) for age 69.3 (7.3) years f , 45.5 (17.9) | |
| Ravens Coloured Progressive Matrices (of 36) | 23 | 20 | 50th percentile for age 65 years g = 24 | |
| WMS Verbal Paired Associates | ||||
| • Immediate recall (of 24) | 12 | 17 | ||
| • Delayed recall (of 8) | 7 | 7 | ||
| Hopkins Assessment of Action Naming (of 30) | 12 | 13 | Mean (SD) for age 64.0 (12.05) years h , 23.92 (3.46) | |
| FAS | 4 | 3 | Mean (SD) for age 60-79 years i , 32.31 (12.70) | |
| Semantic fluency | 19 | 19 | ||
| SOAP (of 40) | ||||
| • percent correct | 25 (63%) | 29 (73%) | 90%–100% correct for unimpaired controls j | |
| S (of 10) | 8 | 7 | ||
| O (of 10) | 3 | 5 | ||
| A (of 10) | 9 | 9 | ||
| P (of 10) | 5 | 8 | ||
| Spelling to Dictation (of 30) | 25.77 | 25.87 | ||
| Pyramids and Palm Trees (of 14) | 11 | 12 | Mean (SD) for age 59.1 (9.48) years k , 13.9 (.24) | |
| Kissing and Dancing (of 15) | 9 | 7 | ||
| NACC Sentence Repetition | Mean (SD) for age <65 years l for accurate sentences,4.6 (.6) | |||
| • Total accurate sentences (of 5) | 5 | 4 | ||
| • Total accurate words (of 37) | 33 | 37 |
aDick JP, Guiloff RJ, Stewart A, Blackstock J, Bielawska C, Paul EA, Marsden CD. Mini-Mental State examination in neurological patients. J Neurol Neurosurg Psychiatry. 1984 May;47(5):496-9. doi: 10.1136/jnnp.47.5.496.
bWeintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009 Apr-Jun;23(2):91-101. doi: 10.1097/WAD.0b013e318191c7dd.
cNasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695-9. doi: 10.1111/j.1532-5415.2005.53221.x. Erratum in: J Am Geriatr Soc. 2019 Sep;67(9):1991.
dGeffen G, Moar KJ, O’hanlon AP, Clark CR, Geffen LB. Performance measures of 16- to 86-year-old males and females on the auditory verbal learning test. Clin Neuropsychol. 1990 Mar;4(1):45-63. doi: 10.1080/13854049008401496.
eTombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004 Mar;19(2):203-14. doi: 10.1016/S0887-6177(0300039-8).
fSemenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association between vestibular and cognitive function in U.S. adults: data from the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2016 Feb;71(2):243-50. doi: 10.1093/gerona/glv069. Epub 2015 Jul 28.
gRaven JC. Guide to Using the Coloured Progressive Matrices. London: Lewis and Company and Harrap and Company, 1956.
hBreining BL, Faria AV, Caffo B, Meier EL, Sheppard SM, Sebastian R, Tippett DC, Hillis AE. Neural regions underlying object and action naming: complementary evidence from acute stroke and primary progressive aphasia. Aphasiology. 2022;36(6):732-60. doi: 10.1080/02687038.2021.1907291.
iLoonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Appl Neuropsychol. 2001;8(3):161-6. doi:10.1207/S15324826AN0803_5.
jLove T, Oster E. On the categorization of aphasic typologies: the SOAP (a test of syntactic complexity). J Psycholinguist Res. 2002;31(5):503-29. doi:10.1023/a:1021208903394.
kBreining BL, LalaT, Cuitino MM, Manes F, Peristeri E, Tsapkini K, Faria AV, Hillis AE. A brief assessment of object semantics in primary progressive aphasia. Aphasiology. 2015;29(4):488-505. doi: 10.1080/02687038.2014.973360.
lThe NIA Alzheimer’s Disease Research Centers Program, National Alzheimer’s Coordinating Center, FTLD Module https://files.alz.washington.edu/documentation/ftld3-means.pdf (2012, accessed 23 October 2023).
Discussion
After home-based tDCS over 10 weeks along with computerized cognitive/language training, our patient demonstrated a three-point gain on the MMSE, a measure of global cognition, while her performance on the MoCA, another measure of global cognition, remained stable, in contrast to her previous decline. It should be noted that a three-point change (decline or improvement) on the MMSE is considered clinically significant. Similar findings have not been reported in association with any medication or therapeutic intervention in a neurodegenerative disorder to our knowledge. In addition, she showed improvement on several other language tasks such as syntactic comprehension, total accurate words in sentence repetition, semantic processing, and stable performance in verb naming and semantic fluency.
While the observed improvement in MMSE is impressive, 1 could attribute the gains to a possible learning from practice effect. Hensel et al 46 conducted repeated administrations of the MMSE in neurotypical older adults to investigate changes in test scores that reflect true clinical change rather than change due to other factors, such as practice effects or measurement error. They concluded that a 2 to four-point change in repeated assessments with 17-month intervals indicates reliable change at the 90% confidence level. Furthermore, in elderly individuals who participated in the Canadian Study of Health and Aging, reliable change was defined by 3 MMSE points for 3-month intervals and by 3 to 4 MMSE points for 5-year intervals as calculated by reliable change index-regression scores which control for practice effects, psychometric errors, and regression to the mean. 47 The reversal of expected decline in global cognition in a patient with a neurodegenerative disorder, instead of expected decline, constitutes a universal ‘first’ for tDCS effects in neurodegenerative disorders and highlight the potential impact of such a stimulation paradigm in neurodegeneration.
Here we will entertain some other possible explanations for these effects: (a) the DLPFC is a target for neuromodulatory treatments for depression and so the improvements reflect an overall mood improvement, 48 and (b) symptoms can fluctuate over time in FTD. The patient’s family did not report a history of these types of fluctuations but did comment upon behavioral changes after tDCS treatment to the DLPFC, including enhanced interest, expressiveness, communication, and interaction.
Limitations
Limitations of this case report include the open-label, single-subject design. Despite being very preliminary, this case warrants a randomized clinical trial to investigate long-term, home-based tDCS delivery and cognitive/language training. A question of interest that is currently under investigation is the role of behavioral therapy and whether the results would be different had the patient not been practicing with the computerized cognitive program.
Conclusions
Our study demonstrates the feasibility and potential effectiveness of long-term, home-based tDCS for the treatment of behavioral and cognitive/language symptoms in FTD. At a time when cognitive improvement is rarely seen in neurodegenerative disease, (including recent anti-amyloid treatments for Alzheimer’s disease in which the drug contributed a .5 difference in the adjusted mean change on the Clinical Dementia Rating Scale-Sum of Boxes between the treatment and placebo groups which was significantly different, although not conclusively clinically meaningful 49 ), this study provides a premise for the possible effectiveness of long-term, home-based tDCS in FTD. Rigorous evaluation of tDCS coupled with behavioral intervention in a randomized double-blind design is required to establish the efficacy and clinical utility of tDCS in treating cognitive and behavioral symptoms in FTD, and further investigation is necessary regarding the implementation of home-based tDCS. Importantly, home-based tDCS may offer access to neuromodulation for individuals for whom distance to a clinical setting is prohibitive and for whom travel to a clinical setting is challenging because of motor and cognitive/language deficits. In addition, home-based tDCS offers the opportunity to investigate dose/response relationships over the long term. Practitioners should be aware that home-based tDCS paired with CCT may be a new option in the future for individuals with neurodegenerative syndromes for which disease-modifying pharmacologic therapies are limited and often hard to access.
Acknowledgements
The authors thank the participant, her family, and referring physicians for their dedication and interest in this study.
Footnotes
Author Contributions: CRediT Taxonomy D.T.: Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing K.N.: Conceptualization, Writing – review & editing Y.T.: Visualization, Data curation, Writing – review & editing J.G.: Data curation, Investigation, Writing – review & editing C.M.: Conceptualization, Writing – review & editing C.O.: Conceptualization, Writing – review & editing K.T.: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Donna Tippett receives salary support from NIH NIA R01AG068881, R01AG075404, R01AG075111. Kyriaki Neophytou receives salary support from NIH NIA R01AG068881, R01AG075404, R01AG075111. Yuan Tao receives salary support from NIH NIA R01AG068881and R01AG075404. Jessica Gallegos receives salary support from NIH NIA R01AG068881, R01AG075404, R01AG075111. Christopher Morrow receives salary support from NIH KL2TR003099. Chiadi Onyike receives research funding from Alector Inc., Transposon Therapeutics, and Denali Therapeutics and is a consultant for Eisai, Otsuka, Reata.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by YBrain Inc with device and funding contributions. The authors declare that the funding body did not have any role in the design of the study or the analysis and interpretation of data.
Ethical Statement
Ethical Approval
The patient consented in writing to participate in a pilot study of remote, home-based tDCS (Ybrain Inc. YMS-201B+) coupled with cognitive/language tasks of Brain HQ (brainhq.com) which has Johns Hopkins University School of Medicine Institutional Review Board approval (IRB NA_0071337, initial approval date 04/19/2012).
Informed Consent
The patient signed a written authorization for the release of health information in a case report required by the Johns Hopkins Institutional Review Board.
ORCID iD
Donna C. Tippett https://orcid.org/0000-0001-9485-9745
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study. All test/re-test scores are reported in the manuscript.
References
- 1.Miller BL, Cummings JL, Villanueva-Meyer J, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology. 1991;41(9):1374-1382. doi: 10.1212/wnl.41.9.1374. [DOI] [PubMed] [Google Scholar]
- 2.Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386(10004):1672-1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyle-Gilchrist IT, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62(6):925-930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 5.Moheb N, Mendez MF, Kremen SA, Teng E. Executive dysfunction and behavioral symptoms are associated with deficits in instrumental activities of daily living in frontotemporal dementia. Dement Geriatr Cogn Disord. 2017;43(1-2):89-99. doi: 10.1159/000455119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130-137. doi: 10.3109/09540261.2013.776523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 8.Borghesani V, DeLeon J, Gorno-Tempini ML. Frontotemporal dementia: a unique window on the functional role of the temporal lobes. Handb Clin Neurol. 2022;187:429-448. doi: 10.1016/B978-0-12-823493-8.00011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman M, Seeley WW, Boxer AL, et al. Frontotemporal lobar degeneration. Nat Rev Dis Primers. 2023;9(1):40. doi: 10.1038/s41572-023-00447-0. [DOI] [PubMed] [Google Scholar]
- 10.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 11.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1114. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18(1):28-36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- 14.Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi: 10.1007/s11940-014-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopade P, Chopade N, Zhao Z, Mitragotri S, Liao R, Chandran Suja V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng Transl Med. 2022;8(1):e10367. doi: 10.1002/btm2.10367. Published 2022 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, Wang J, Zhao M, et al. Progress and challenges in targeted protein degradation for neurodegenerative disease therapy. J Med Chem. 2022;65(17):11454-11477. doi: 10.1021/acs.jmedchem.2c00844. [DOI] [PubMed] [Google Scholar]
- 17.Janicki Hsieh S, Alexopoulou Z, Mehrotra N, Struyk A, Stoch SA. Neurodegenerative diseases: the value of early predictive end points. Clin Pharmacol Ther. 2022;111(4):835-839. doi: 10.1002/cpt.2544. [DOI] [PubMed] [Google Scholar]
- 18.Benussi A, Borroni B. Advances in the treatment and management of frontotemporal dementia. Expert Rev Neurother. 2023;23(7):621-639. doi: 10.1080/14737175.2023.2228491. [DOI] [PubMed] [Google Scholar]
- 19.Cotelli M, Manenti R, Petesi M, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis. 2014;39(4):799-808. doi: 10.3233/JAD-131427. [DOI] [PubMed] [Google Scholar]
- 20.Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology. 2014;28(8-9):1112-1130. doi: 10.1080/02687038.2014.930410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotelli M, Manenti R, Paternicò D, et al. Grey matter density predicts the improvement of naming abilities after tDCS intervention in agrammatic variant of primary progressive aphasia. Brain Topogr. 2016;29(5):738-751. doi: 10.1007/s10548-016-0494-2. Epub 2016 May 19. [DOI] [PubMed] [Google Scholar]
- 22.Gervits F, Ash S, Coslett HB, Rascovsky K, Grossman M, Hamilton R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: an open-label pilot study. Brain Lang. 2016;162:35-41. doi: 10.1016/j.bandl.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung J, Bauer A, Grossman M, Hamilton RH, Coslett HB, Reilly J. Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Front Hum Neurosci. 2017;11:253. doi: 10.3389/fnhum.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ficek BN, Wang Z, Zhao Y, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. Neuroimage Clin. 2018;19:703-715. doi: 10.1016/j.nicl.2018.05.023. Erratum in: Neuroimage Clin. 2019;22:101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsapkini K, Webster KT, Ficek BN, et al. Electrical brain stimulation in different variants of primary progressive aphasia: a randomized clinical trial. Alzheimers Dement (N Y). 2018;4:461-472. doi: 10.1016/j.trci.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenner AS, Webster KT, Ficek BN, Frangakis CE, Tsapkini K. Written verb naming improves after tDCS over the left IFG in primary progressive aphasia. Front Psychol. 2019;10:1396. doi: 10.3389/fpsyg.2019.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheppard SM, Goldberg EB, Sebastian R, Walker A, Meier EL, Hillis AE. Transcranial direct current stimulation paired with verb network strengthening treatment improves verb naming in primary progressive aphasia: a case series. Am J Speech Lang Pathol. 2022;31(4):1736-1754. doi: 10.1044/2022_AJSLP-21-00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Ficek BN, Webster KT, et al. Specificity in generalization effects of transcranial direct current stimulation over the left inferior frontal gyrus in primary progressive aphasia. Neuromodulation. 2023;26(4):850-860. doi: 10.1016/j.neurom.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal SM, Rajur S, Bose A, et al. Use of transcranial direct current stimulation (tDCS) in a woman with behavioral variant fronto-temporal dementia. Asian J Psychiatr. 2016;21:31-32. doi: 10.1016/j.ajp.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Cotelli M, Adenzato M, Cantoni V, et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn Affect Behav Neurosci. 2018;18(6):1065-1075. doi: 10.3758/s13415-018-0622-4. [DOI] [PubMed] [Google Scholar]
- 31.Pini L, Pizzini FB, Boscolo-Galazzo I, et al. Brain network modulation in Alzheimer's and frontotemporal dementia with transcranial electrical stimulation. Neurobiol Aging. 2022;111:24-34. doi: 10.1016/j.neurobiolaging.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Benussi A, Dell'Era V, Cosseddu M, et al. Transcranial stimulation in frontotemporal dementia: a randomized, double-blind, sham-controlled trial. Alzheimers Dement (N Y). 2020;6(1):e12033. doi: 10.1002/trc2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertrich I, Dietrich S, Blum C, Ackermann H. The role of the dorsolateral prefrontal cortex for speech and language processing. Front Hum Neurosci. 2021;15:645209. doi: 10.3389/fnhum.2021.645209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrides M. Neuroanatomy of Language Regions of the Human Brain. New York, NY: Academic Press; 2014. [Google Scholar]
- 35.Petrides M. The ventrolateral frontal region. In: Neurobiology of Language. Cambridge: Academic Press, Elsevier; 2015. [Google Scholar]
- 36.Hartwigsen G. Flexible redistribution in cognitive networks. Trends Cogn Sci. 2018;22(8):687-698. doi: 10.1016/j.tics.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y, Ficek B, Rapp B, Tsapkini K. Different patterns of functional network reorganization across the variants of primary progressive aphasia: a graph-theoretic analysis. Neurobiol Aging. 2020;96:184-196. doi: 10.1016/j.neurobiolaging.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandelli ML, Welch AE, Vilaplana E, et al. Altered topology of the functional speech production network in non-fluent/agrammatic variant of PPA. Cortex. 2018;108:252-264. doi: 10.1016/j.cortex.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, Cappa SF. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in Primary Progressive Aphasia: a meta-analysis and systematic review. Neurosci Biobehav Rev. 2020;108:498-525. doi: 10.1016/j.neubiorev.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Manenti R, Cotelli M, Calabria M, Maioli C, Miniussi C. The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: a repetitive transcranial magnetic stimulation study. Neuroscience. 2010;166(2):501-507. doi: 10.1016/j.neuroscience.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 41.Cappon D, den Boer T, Yu W, et al. An educational program for remote training and supervision of home-based transcranial electrical stimulation: feasibility and preliminary effectiveness. Neuromodulation. 2023. 8:S1094-S7159. doi: 10.1016/j.neurom.2023.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick JP, Guiloff RJ, Stewart A, et al. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry. 1984;47(5):496-499. doi: 10.1136/jnnp.47.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x. Erratum in: J Am Geriatr Soc. 2019 Sep;67(9):1991. [DOI] [PubMed] [Google Scholar]
- 44.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38-43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218-235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298-1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20(4):485-503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Fu Y, Liu C, Meng Z. Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front Behav Neurosci. 2022;16:893955. doi: 10.3389/fnbeh.2022.893955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study. All test/re-test scores are reported in the manuscript.