Abstract
Introduction
A class of disorders known as inborn errors of immunity (IEI) is defined by a compromised or missing immune response, which increases the vulnerability to infections, immunological dysregulation, and cancer. Severe combined immunodeficiencies (SCIDs), affecting both T and B-cell function are rare but often severe diseases. In this report, we describe a 10-month-old SCID patient from Sudan with disseminated BCG infection.
Case Presentation
A 10-month-old boy whose parents were first degree relatives, presented with a six-month history of repeated chest infections and fever. Physical examination revealed a very ill-looking boy with respiratory distress dependent on oxygen, had slight abdominal distention and hepatomegaly. Investigations revealed positive polymerase chain reaction (PCR) for M. tuberculosis complex infection and low CD4+ and CD8+ cells. Genetic testing showed compound heterozygosity in trans for two variants in the Zeta-chain Associated Protein Kinase 70 (ZAP70) gene associated with autosomal recessive SCID. The patient was started on BCG-related infection treatment, intravenous immunoglobulin (IVIG) replacement and trimethoprim/sulfamethoxazole prophylaxis with an excellent response and the patient responded well to the treatment.
Conclusion
SCIDs are rare, and early management is crucial. In this case, a diagnosis of ZAP70 deficiency was based on next-generation sequencing and inhouse bioinformatic computational analysis of the ZAP70 gene, highlighting the importance of genetic testing in the workup of immunodeficiencies in low resource settings.
Keywords: Inborn errors of immunity, SCID, disseminated BCG infection, ZAP70 deficiency, pediatric immunology
Introduction
A group of about 500 uncommon genetic diseases known as inborn errors of immunity (IEI) are defined with impaired immune systems. These conditions lead to higher vulnerability to infections and other immune-related problems.1 Among these is severe combined immunodeficiency (SCID) caused by an autosomal recessively inherited ZAP70 (ζ-chain associated protein kinase 70) deficiency, usually leading to death from severe sepsis by the age of two years.2 ZAP70 protein is a cytoplasmic tyrosine kinase which plays an important roles in T-cell receptor (TCR) signaling and function. ZAP70 deficiency caused by biallelic variants in the ZAP70 gene can lead to a rare form of SCID which is characterized by anergic CD4+ T cells that are not reactive to mitogens and CD8+ T-cell lymphopenia; however, the clinical presentation may vary3 with recurrent bacterial infections and autoimmune manifestations.
In this paper, we present a rare case of Sudanese male patient with a novel compound heterozygous genotype resulting in ZAP70 deficiency with a SCID phenotype. He presented with disseminated mycobacterial infection caused by Mycobacterium bovis from the BCG vaccine.
Case Presentation
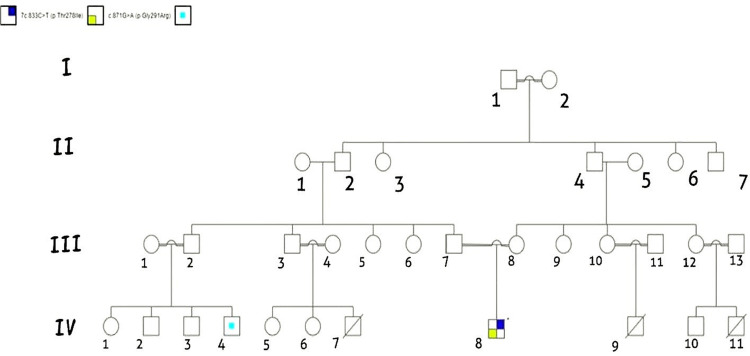
A 10-month-old Sudanese male infant (Figure 1; the proband, IV-8) was brought to our pediatric immunology department with a history of fever and recurrent chest infections for the past six months. His parents (Figure 1; III-7, III-8) are 1st degree consanguineous. There is a family history of recurrent chest infections requiring hospitalizations and intravenous antibiotics, along with recurrent oral thrush. The family reported three infantile deaths due to undiagnosed febrile illness (Figure 1; IV-7, IV-9, IV-11) as well as recurrent febrile illness with convulsions (Figure 1; IV-4). The proband had received all vaccinations recommended for his age. On physical examination, he appeared very ill with respiratory distress, and he required oxygen therapy at five liters/minute via face mask to maintain normal oxygen saturation. The patient’s weight was below the 3rd percentile, while his height and head circumference were on the 10th and 25th percentiles, respectively. His abdomen was slightly distended, and hepatomegaly was noted, with a palpable liver at two centimeters below the costal margin. Bilateral wheezing with poor air entry was noted during chest examination. Examination of the BCG vaccine site revealed a discharging sinus with an enlarged axillary lymph node on the same side, measuring 3 × 2.5 cm; chest X-ray showed bilateral pneumonic infiltrates, while abdominal ultrasound was normal apart from an enlarged liver.
Figure 1.
This pedigree displays four generations of relations to the patient who is indicated with the black arrow in IV-8. The family reported three infantile deaths due to undiagnosed febrile illness (IV-7, IV-9, IV-11), IV-4 has recurrent febrile illness with seizures. The pedigree shows six consanguineous marriages one in the first generation (I-1 and I-2), and five in the third generation (III-1 and III-2), (III-3 and III-4), (III-7 and III-8), (III-10 and III-11), and (III-12 and III-13). This pedigree is created using (https://progenygenetics.com) website.
Investigations
The possibility of disseminated BCG infection with probable immunodeficiency was discussed as a potential diagnosis for this patient. Smears from gastric lavage fluid were collected over two consecutive days, were negative for acid-fast bacilli. However, the fine needle aspiration cytology (FNAC) tissue (lymph-node) displayed caseating granuloma, and both blood and sputum polymerase chain reaction (PCR) using two primers (IS6110 & mtp40) were positive to M. tuberculosis complex, most likely M. bovis based on the patient’s history and examination findings. Further workup revealed a normocytic-normochromic red blood cell count of 8.9 g/dL, and Immunophenotyping analysis is shown in (Table 1).
Table 1.
This Tables Shows the Hematological and Immunophenotyping Results for the Patient
| Test | Patient Result | Normal Range |
|---|---|---|
| Hematological results | ||
| White blood cells | 6.8 | 4.0–7.0 (103/uL) |
| Hemoglobin | 8.9 | 11.3–14.1 g/dl |
| Absolute neutrophils count | 2.2 | 1.8–7.6 (103/uL) |
| Monocytes count | 1.65 | 0.33–1.21 (103/uL) |
| Absolute lymphocyte count | 2900 | 4000–10,500 mm3 |
| Platelets | 822 | 150–450 (103/uL) |
| Flow cytometry results | ||
| Total CD3 | 1400 | 1900–5900 cells/cmm |
| CD4 | 1000 | 1400–4300 cells/cmm |
| CD8 | 375 | 500–1700 cells/cmm |
| CD19 | 671 | 600–2600 cells/cmm |
| CD16 | 110 | 160–950 cells/cmm |
| CD4-ve/CD8-ve % | 3 | N/A |
Materials and Methods
A blood sample from the patient was sent for the INVITAE platform to test for 429 genes in the primary immunodeficiency (PID) panel using Illumina technology. This comprehensive genetic testing panel was used to identify various genetic variants associated with SCIDs. Protein functions were predicted in response to missense variants using the SIFT, PolyPhen-2, and Align-GVGD algorithms. After the result emerged, we mutated the normal-human ZAP70 protein obtained from uniport (https://www.uniprot.org/uniprotkb/P43403/entry) with the ZAP70 variants identified in the patient’s result, here, we created a comparative bioinformatic analysis, and 3D homology modeling of ZAP70 protein structures using SWISS-MODEL (https://swissmodel.expasy.org), while Chimera 1.16 software was used to visualize the protein and its affected domain, and the mutated protein sequence was illustrated and compared to wild type using Jalview 2.11 software.
Genetic Results
The panel test result reported 17 gene variants of uncertain significance (VUS), including compound heterozygous (trans) missense variants in exons 7 and 8 of ZAP70 (c.833C > T, p.Thr278Ile, and c.871G > A, p.Gly291Arg, respectively), both are affecting interdomain B region of the protein (Figures 2 and 3). These ZAP70 variants are on opposite chromatids and are associated with autosomal recessive SCID (MedGen UID: 376544). Accordingly, we proposed a diagnosis of ZAP70 deficiency based on compound biallelic variants affecting the ZAP70 gene, which was confirmed by platform-targeted exome sequencing and computational analysis of the gene.
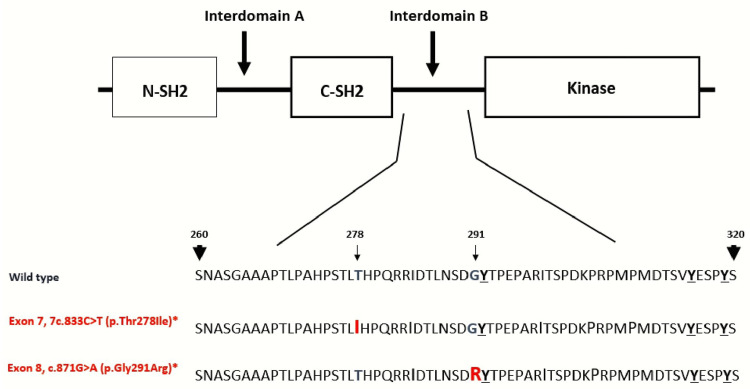
Figure 2.
This is a schematic structure of the affected sites of ZAP70 protein. ZAP70 protein domains are the amino-terminal SH2 domain (N-SH2), interdomain A, carboxy-terminal SH2 domain (C-SH2), interdomain B and lastly the kinase domain of the protein. The tandem SH2 domains interact with the doubly phosphorylated tyrosine-based activation motif of CD247/CD3Z. Also, both mutations in this case occur in the interdomain B. The interdomain B region contains three tyrosine (Tyr-292, Tyr-315, Tyr-319) that are phosphorylated after T cell receptor activation. Interestingly, (p.Gly291Arg) mutation is immediately next to Tyr-292 and it may impact the ZAP70 protein as resulted in this case, notable (p.Thr278Ile) and (p.Gly291Arg) mutations are on opposite chromosomes.
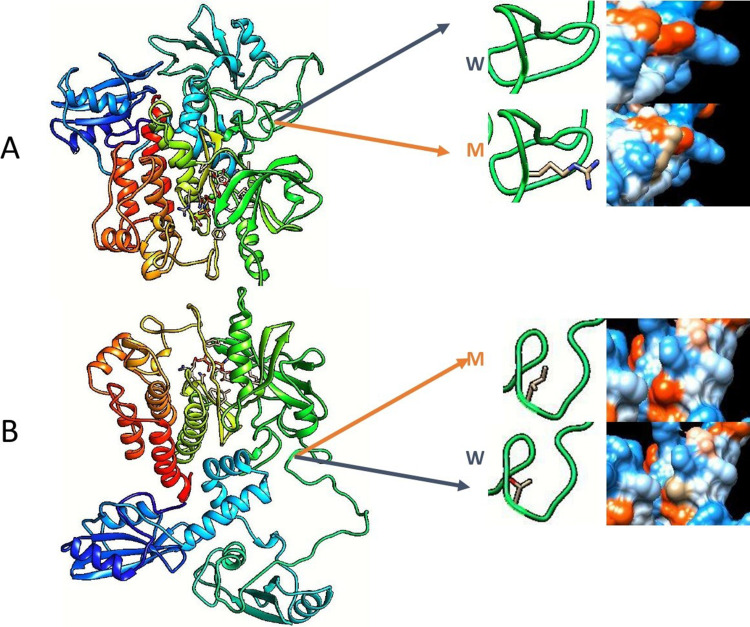
Figure 3.
This is a molecular visualizations of ZAP70 protein implicated in this case, (A) shows the c.871G>A (p.Gly291Arg) mutation. (B) shows 7c.833C>T (p.Thr278Ile) mutation. The (W) letter represent the wild type while (M) represent mutant type in both figures. Due to mutations, amino acid hydrophobicity may changed in the ZAP70 protein, and it is shown next to the amino acid in both figures. Notably, from SIFT, Align-GVGD, and PolyPhen-2 algorithms, only the later predicted that (p.Gly291Arg) mutation is “Possibly Damaging”, and also predicted that other mutation is “Benign”. We created this illustration using Swiss-MODEL (https://swissmodel.expasy.org) and Chimera 1.16 software after mutating the ZAP70 with the patient’s ZAP70 mutations.
The first sequence change replaces threonine, which is a small polar, neutral side chain, with isoleucine: a non-polar, large, aliphatic, and hydrophobic amino acid (p.Thr278Ile). This variant is exceedingly rare in population databases (Figures 2 and 3A). The second variant causes a replacement of glycine, which is a non-polar tiny amino acid with no side chain, with arginine: a basic, polar, large amino acid with a positively charged side chain, at codon 291 of the ZAP70 protein. This variant is unavailable in population databases (gnomAD AF 0) and may disrupt the consensus splice site (Figures 2 and 3B).
The American College of Medical Genetics (ACMG) verdicts for both variants are uncertain significance, since no ZAP70 deficiency patients have been reported with them, and there is no functional evidence of pathogenicity; however, the patient’s clinical phenotype is consistent with ZAP70 deficiency. Moreover, the panel identified other heterozygous genetic mutations in several genes including C8B, CD59, CR2, CTC1, DEF6, DOCK8, DUOX2, LYST, MCM4, PARN, UNC13D, and ZNF341, also, the patient has hemizygous G6PD and TLR7 variants. However, aside from the ZAP70 variants, the remaining genetic variations are VUS and do not align with the patient’s clinical phenotypes.
Treatment
The patient was initiated on a combination therapy of Isoniazid, Rifampicin, and Ethambutol with Levofloxacin, as well as on regular intravenous immunoglobulin (IVIG) replacement and Trimethoprim/sulfamethoxazole prophylaxis, which led to excellent response and good weight gain. The patient now is free from infections and is planned for 36 months of BCG-related infection treatments.
Discussion
In this work, we describe a case of an infant male, with exceedingly rare biallelic missense variants in the ZAP70 gene, consistent with an early history of BCG-osis and CD8+ lymphopenia. The tyrosine kinase enzyme ZAP70 has a crucial role in TCR signaling. Upon TCR activation, ZAP70 is recruited to the TCR complex and phosphorylates several downstream signaling molecules, including the adaptor protein LAT (linker for activation of T cells),4 which recruits and activates other downstream signaling molecules such as PLC-gamma-1 and SLP-76. These signaling pathways ultimately lead to the stimulation of transcription factors such as NFAT and AP-1, which regulate the expression of genes involved in T-cell activities.
The function of ZAP70 in T-cells is critical, studies have shown that mice deficient in ZAP70 have severely impaired T-cell function, leading to immunodeficiency.5,6 In humans, mutations in the ZAP70 gene have been related to SCID.7 Firstly, identified in Mennonite families, ZAP70 pathogenic mutations later emerged in individuals from Portuguese, Japanese, Hispanic, and Turkish origins.6–11 Moreover, the ZAP70 protein has three domains and two interdomains shown in Figure 2, according to a recent meta-analysis, most of the ZAP70 gene mutations occur in the kinase domain but without clear hotspot through the gene.12 Here, we report the first Sudanese patient with two mutations on opposite chromosomes both are affecting interdomain B region of the ZAP70 protein. This domain is critical as it contains three tyrosine residues (Tyr-319, Tyr-315, and Tyr-292) (Figure 2) that are phosphorylated upon T-cell activation by an antigen. These sites are also involved in binding to the other signaling molecules like VAV1 or CBL, thus the ZAP70 protein functions as a scaffold by calling and recruiting more factors for T-cell receptor stimulation.13
The clinical presentation of ZAP70 deficiency varies considerably depending on the underlying genetic defect, its degree of penetrance, and the severity of immunodeficiency. In low-income countries (LICs) such as Sudan, diagnosing ZAP70 deficiency is challenging. The initial steps in the diagnosis of IEI involve assessing a complete blood count, serum immunoglobulin levels, flow cytometric, analysis of peripheral blood lymphocytes, and T-cell responses to mitogens in vitro.14,15 Low CD8 T-cells in the bloodstream, despite normal or slightly reduced levels of total T-cells, along with a healthy count of peripheral blood lymphocytes, may indicate a diagnosis of ZAP-70 deficiency. Still, genetic testing is required to confirm the genetic defect.14
Additionally, analyzing the levels of T-cell receptor excision circles (TRECs) can be insightful, as these levels tend to decrease over the first year of life in individuals with this condition.8,16 The absence of T-cell proliferation in response to TCR-mediated stimuli, including phytohemagglutinin (PHA), CD3 monoclonal antibody, or alloantigen in mixed lymphocyte culture, is indicative of a cellular immunodeficiency and can be used to confirm the diagnosis. IgM and IgA levels remain normal, whereas serum IgG may either be normal or decreased in patients with ZAP-70 deficiency.6–8,17 Here, we report similar findings as IgM and IgG levels were normal in this patient. Also, ZAP70 protein expression by immunoblot is useful for diagnosing this condition. cDNA or genomic DNA sequencing can identify pathogenic variants in the ZAP70 gene and further validate the diagnosis,18 because, most of these diagnostic tests are unavailable in Sudan, a collaborative approach is imperative by establishing partnerships between high-income countries with advanced genomic facilities is a feasible solution in the future. Lastly, in infants with a family history of SCID, BCG vaccination can cause disseminated disease; therefore, studies recommend newborn screening, and delaying BCG vaccination until SCID is ruled out.19–22 The limitation of this report, in addition to being a single case, is a lack of functional validation assays, unavailability of ZAP70 protein expression tests and lack of parent genetic testing.
Conclusion
The risks and benefits of BCG vaccination should be evaluated in patients with suspected SCIDs. Genetic diagnosis is important for IEI patients in LICs, particularly for major decision such as bone marrow transplant in such patient(s) and for family counseling and early screening or diagnosis.
Acknowledgment
We acknowledge Dr Saúl O. Lugo-Reyes for helping us to improve our work quality throughout his constructive comments and feedbacks.
Data Sharing Statement
Data that support this case presentation are available upon contacting the corresponding author.
Consent for Publication
After obtaining the patient’s parents provided written informed consent for the case details to be published, the Tropical Disease Teaching Hospital ethical committee approved this study on 4/December/2022 serial number [TDTH/B/231/12].
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. 2022;2022:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder M, Triggs-Raine B, Zelinski T. Genotyping an immunodeficiency causing C. 1624–11g> a Zap70 mutation in Canadian Mennonites. BMC Med Genet. 2016;17:1–4. doi: 10.1186/s12881-016-0312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esenboga S, Ayvaz DC, Cetinkaya PG, van der Burg M, Tezcan İ. An infant with Zap-70 deficiency with disseminated mycobacterial disease. J Clin Immunol. 2016;36:103–106. doi: 10.1007/s10875-015-0229-2 [DOI] [PubMed] [Google Scholar]
- 4.Courtney AH, Lo WL, Weiss A. Tcr signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43(2):108–123. doi: 10.1016/j.tibs.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negishi I, Motoyama N, Nakayama K, et al. Essential role for Zap-70 in both positive and negative selection of thymocytes. Nature. 1995;376(6539):435–438. doi: 10.1038/376435a0 [DOI] [PubMed] [Google Scholar]
- 6.Arpaia E, Shahar M, Dadi H, Cohen A, Rolfman CM. Defective T Cell Receptor Signaling and Cd8+ Thymic Selection in Humans Lacking Zap-70 Kinase. Cell. 1994;76(5):947–958. doi: 10.1016/0092-8674(94)90368-9 [DOI] [PubMed] [Google Scholar]
- 7.Elder ME, Lin D, Clever J, et al. Human Severe Combined Immunodeficiency Due to a Defect in Zap-70, a T Cell Tyrosine Kinase. Science. 1994;264(5165):1596–1599. doi: 10.1126/science.8202712 [DOI] [PubMed] [Google Scholar]
- 8.Roifman CM, Hummel D, Martinez-Valdez H, et al. Depletion of Cd8+ cells in human thymic medulla results in selective immune deficiency. J Exp Med. 1989;170(6):2177–2182. doi: 10.1084/jem.170.6.2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katamura K, Tai G, Tachibana T, et al. Existence of activated and memory Cd4+ T cells in peripheral blood and their skin infiltration in Cd8 deficiency. Clin Exp Immunol. 1999;115(1):124–130. doi: 10.1046/j.1365-2249.1999.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turul T, Tezcan I, Artac H, et al. Clinical heterogeneity can hamper the diagnosis of patients with Zap70 deficiency. Eur J Pediatr. 2009;168(1):87–93. doi: 10.1007/s00431-008-0718-x [DOI] [PubMed] [Google Scholar]
- 11.Fagioli F, Biasin E, Berger M, et al. Successful unrelated cord blood transplantation in two children with severe combined immunodeficiency syndrome. Bone Marr Transpl. 2003;31(2):133–136. doi: 10.1038/sj.bmt.1703800 [DOI] [PubMed] [Google Scholar]
- 12.Sharifinejad N, Jamee M, Zaki-Dizaji M, et al. Clinical, immunological, and genetic features in 49 patients with Zap-70 deficiency: a systematic review. Front Immunol. 2020;11:831. doi: 10.3389/fimmu.2020.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A, Picard C, Chemin K, et al. Zap70: a master regulator of adaptive immunity. Semin Immunopathol. 2010;32:107–116. doi: 10.1007/s00281-010-0196-x [DOI] [PubMed] [Google Scholar]
- 14.Grumach AS, Goudouris ES. Inborn errors of immunity: how to diagnose them? J Pediatr. 2021;97(Suppl 1):S84–s90. doi: 10.1016/j.jped.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roifman CM. Primary T-Cell Immunodeficiencies. Clin Immunol. 2019;2019:489–508. e1. [Google Scholar]
- 16.Suresh S, Dadi H, Reid B, Vong L, Bulman DE, Roifman CM. Time-dependent decline of T-cell receptor excision circle levels in zap-70 deficiency. J Allergy Clin Immunol Pract. 2020;8(2):806–8.e2. doi: 10.1016/j.jaip.2019.08.018 [DOI] [PubMed] [Google Scholar]
- 17.Chan AC, Kadlecek TA, Elder ME, et al. Zap-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264(5165):1599–1601. doi: 10.1126/science.8202713 [DOI] [PubMed] [Google Scholar]
- 18.Seleman M, Hoyos-Bachiloglu R, Geha RS, Chou J. Uses of next-generation sequencing technologies for the diagnosis of primary immunodeficiencies. Front Immunol. 2017;8:847. doi: 10.3389/fimmu.2017.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Herz W, Husain EH, Adeli M, et al. Bcg vaccine–associated complications in a large cohort of children with combined immunodeficiencies affecting cellular and humoral immunity. Pediatr Infect Dis J. 2022;41(11):933–937. doi: 10.1097/INF.0000000000003678 [DOI] [PubMed] [Google Scholar]
- 20.Marciano BE, Huang C-Y, Joshi G, et al. Bcg Vaccination in Patients with Severe Combined Immunodeficiency: complications, Risks, and Vaccination Policies. J Allergy Clin Immunol. 2014;133(4):1134–1141. doi: 10.1016/j.jaci.2014.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fekrvand S, Yazdani R, Olbrich P, et al. Primary Immunodeficiency diseases and bacillus Calmette-Guérin (Bcg)-vaccine–derived complications: a systematic review. J All Clin Immunol Pract. 2020;8(4):1371–1386. doi: 10.1016/j.jaip.2020.01.038 [DOI] [PubMed] [Google Scholar]
- 22.Mazzucchelli J, Bonfim C, Castro G, et al. Severe combined immunodeficiency in brazil: management, prognosis, and bcg-associated complications. J Invest Allergol Clin Immunol. 2014;24:184–191. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support this case presentation are available upon contacting the corresponding author.