Abstract
This paper reviews the ecosystem of GATE, an open-source Monte Carlo toolkit for medical physics. Based on the shoulders of Geant4, the principal modules (geometry, physics, scorers) are described with brief descriptions of some key concepts (Volume, Actors, Digitizer). The main source code repositories are detailed together with the automated compilation and tests processes (Continuous Integration). We then described how the OpenGATE collaboration managed the collaborative development of about one hundred developers during almost 20 years. The impact of GATE on medical physics and cancer research is then summarized, and examples of a few key applications are given. Finally, future development perspectives are indicated.
1. Motivation and significance
Monte Carlo (MC) simulations are a group of computational methods that can be used for approximating real phenomena by sampling the known physical laws of nature encoded in a computer by models. MC simulations are an indispensable tool for performing virtual experiments involving ionizing irradiation and radiation transport phenomena. As such, these tools have been widely used for investigating radiation processes in medical imaging technologies and radiotherapy, assessing the efficiency and accuracy of imaging and treatment devices used in medicine. This is particularly true for the diagnosis and management of cancer where some of the most often used imaging (e.g. computer tomography (CT), single photon emission computer tomography (SPECT) and positron emission tomography (PET)) and therapy modalities (e.g. external beam radiation therapy (EBRT), Radiopharmaceutical therapy (RPT), trans-arterial radioembolization (TARE) and brachytherapy) use radiation for cancer characterization and management. In this paper, we describe the ecosystem and general structure of the GATE Monte Carlo toolkit (GEANT4 Application for Tomographic Emission) which was originally developed as a tool for simulating emission tomography applications, but was later expanded to simulate a large range of imaging and therapy approaches for cancer management. The GATE toolkit has been in the research landscape for almost 20 years [62].
This open-source software is designed to help researchers and engineers to perform a large range of Monte Carlo simulations in the medical physics field. Typical applications are generally split into two sub-domains: imaging and dosimetry. The first one includes simulation of radiation-based imaging systems such as: PET, SPECT, Compton Camera, CT, CBCT (Cone-Beam CT), etc. Such types of simulation represents a keystone to design or improve imaging systems, to optimize acquisition parameters, and to develop advanced image processing algorithms (reconstruction, segmentation, denoising, image corrections etc). The second domain refers to several types of radiation therapies such as external beam radiotherapy (including proton and carbon ion therapy). This also concerns absorbed dose assessment and energy deposition in imaging, such as CT or interventional radiology. Among numerous other applications, the most prominent examples contain beam characterization of various beam delivery systems or dosimetry studies.
Several review articles have already been published, accompanying the major evolution of the code. From a historical perspective, we can mention: the first publication on PET and SPECT developments in 2004 [62], the evolution towards radiation therapy in 2011 [63], some extensions to other dosimetry applications in 2014 [131] and, more recently in 2021, a specific topic for emission tomography imaging [132]. Even if the major novelties and applications have been already described, no description of the architecture and/or about the organization of the collaborative source code development have been published. Moreover, in addition to the core software, satellite projects have been conducted and are currently used by the community while having never been presented. The goal of this paper is thus to describe the GATE ecosystem and the technical organization of the whole project. In the conclusion, we describe the work in progress and the projects aiming to reshape the future of GATE.
2. GATE ecosystem
2.1. On the shoulders of Geant4
The underlying pillar of GATE is the Geant4 toolkit [1; 6; 7], developed at CERN about 25 years ago (first release in 1998). This open-source toolkit offers C++ classes and functions allowing users to build complex Monte Carlo simulations tracking particles through matter. A large number of experiments in high energy physics, astrophysics, space science, medical physics, and radiation protection are using Geant4, having more than 34k citations on google scholar.
GATE is a regular Geant4 application, written in C++, focused on the medical physics and radiation protection field, like other Geant4 applications such as (among others): Gamos [11], Topas [107] or PTSIM [14]. Consequently, the core Monte Carlo particle tracking engine, all physics databases and models are originating from Geant4. The main principle of GATE is to facilitate the modeling of complex medical physics simulations such that the end-user only needs to write simple text files containing high-level commands in a so-called “macro” language. Advanced users and GATE developers collaboratively contribute to the same C++ source code, which is open (see next section), providing brick classes and specific algorithms that are accessible through macros. Once installed, GATE provides one command line executable taking as input a file of macro commands describing the simulation to run. In addition to user-friendly access to Geant4 simulations, GATE also offers the user a large number of tools, to both speed-up simulations (variance reduction techniques, parallel execution with split and merge tools, source geometries and moving volumes, interface to medical imaging standards like DICOM, etc) and to facilitate the extraction of input data and exploitation of simulation results. In this regard, GATE provides built-in support of additional toolkits (ROOT, ITK-RTK, and PyTorch).
2.2. Regular releases
Because Geant4 regularly evolves, with about one major version per year (often in December), GATE follows the same pace. It has been decided to provide a new GATE version per year, compatible with the latest Geant4 release. Every GATE version is thus associated and tested with one single Geant4 version. In practice, however, an advanced user may compile GATE against some older versions of Geant4, although this is not officially supported. Latest versions of GATE are presented in table 1.
Table 1.
Latest GATE releases and their corresponding Geant4 version.
| GATE | Geant4 | Year |
|---|---|---|
|
| ||
| 9.2 | 11.0 | 2022 |
| 9.1 | 10.7 | 2021 |
| 9.0 | 10.6 | 2020 |
| 8.2 | 10.5 | 2019 |
| 8.1 | 10.4 | 2018 |
| 8.0 | 10.3 | 2017 |
| 7.2 | 10.2 | 2016 |
In addition to the source code, GATE can be installed from Virtual Machine system (the so-called vGATE) or with a Docker version that can easily be deployed for example on clusters.
Openness and users contributions.
The philosophy of GATE is that everyone can contribute to the source code, from simple typo corrections in the documentation up to the addition of new features. Indeed, when users want to propose additional code, they contact the collaboration. They can either directly propose their own code on the platform or discuss with the developers about its integration. In practice, all developers’ contributions are managed via the GitHub PullRequest (PR) mechanism. Once the new source code is proposed, it is discussed and integrated in the main source code by maintainers. Thanks to the GitHub Actions mechanism, every time a modification is proposed in any part of the source code, the whole code is recompiled and tested on several architectures (Linux, MacOS), with different options. In principle, any new contribution should be proposed via PR with 1) source code, 2) documentation and 3) test case (or benchmark). Once a year, all changes are integrated into the new release.
2.3. The GATE ecosystem and open organisation
Through the years, the GATE ecosystem evolved a lot and is now - in 2022 - the following. All source files are stored in Git repositories, currently hosted on the GitHub platform, in one single organization named OpenGATE. The Git system is the cornerstone of the GATE software development as it allows to host the source code, to manage all user proposed modifications (enhancement or bug correction), as well as the documentation, benchmarks, software tests, example simulations, and related tools. Note that, even if everything is currently hosted on the GitHub platform, the intrinsic decentralized nature of Git would allow to easily move to alternative hosting systems. Four main repositories are included under OpenGATE organization name: the main source code is in Gate, the benchmarks are gathered in GateBenchmarks, GateContrib regroups some user contributions and GateTools propose additional convenient Python tools. They are detailed below.
Gate repository.
This repository contains all the C++ source files of the main executable. It is composed of approximately 600 classes, in 3200 files and 780,000 lines of code. The source code is divided into folders grouping classes of related functionalities. Figure 1 shows a description of the main classes in GATE. The main folders are:
Figure 1.
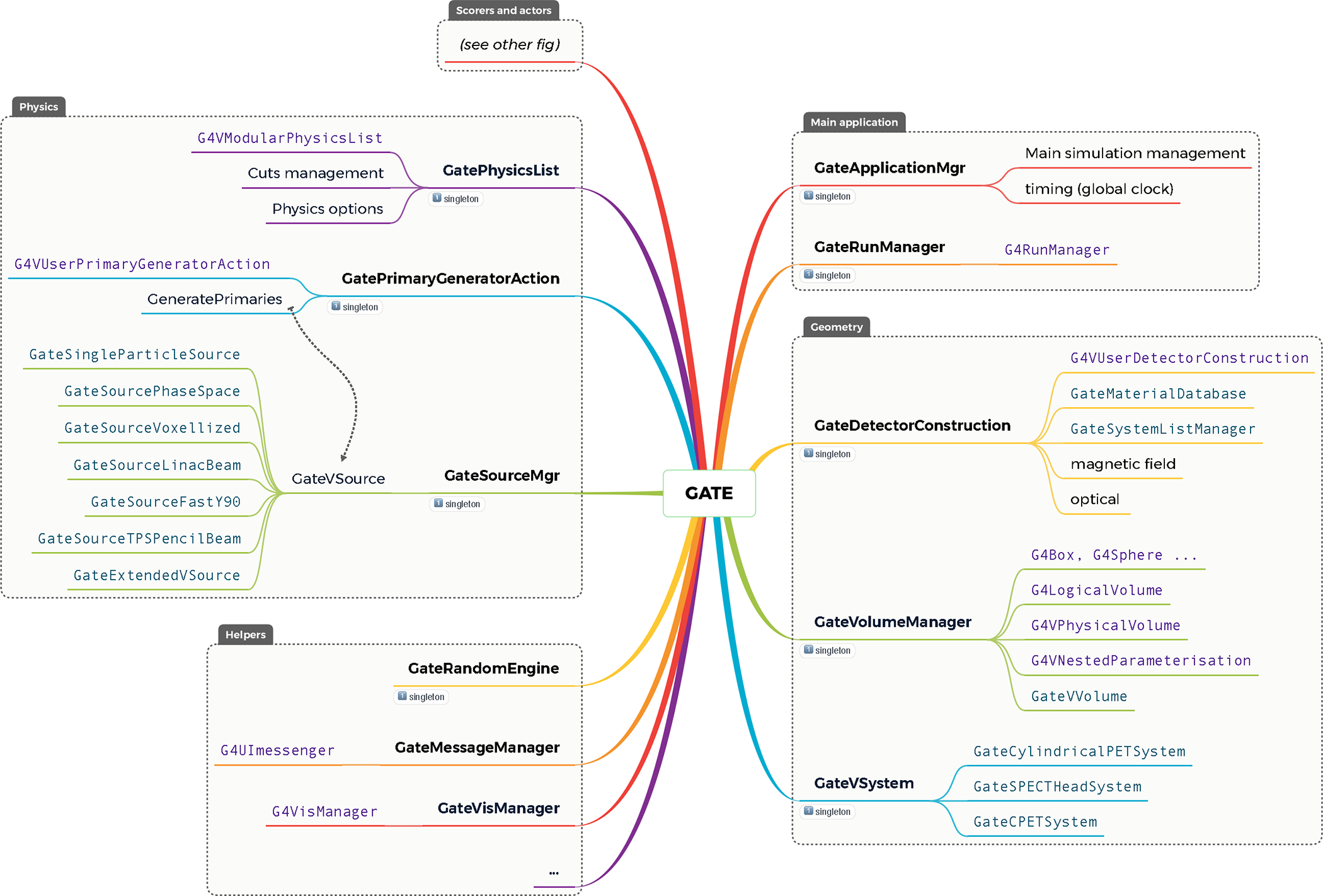
Main GATE classes architecture and link with Geant4 classes. Singleton classes are depicted with a small “1” icon.
geometry. Contains classes related to volume definition, shape, material, position etc, including management of voxelized volumes;
physics. Contains classes related to physics lists, cuts management and all types of particle sources (such as voxelized source or source dedicated to pencil beam scanning in hadrontherapy);
digits_hits. Contains classes related to all scoring methods (see section 2.4), including all actors and management of digitization process of detectors (such as coincidence sorter for PET systems);
general. Contains generic tools and classes used to manage a complete simulation;
externals. Contains external open source code developed by other organizations, such as some ITK methods used to read and write image.
In this repository, several Git branches are used. The main ones are the following: 1) the develop branch containing the last work in progress version of the source code. 2) the branch GateRTIon containing the specific version for GATE-RTion (see section 2.6). Every released version has its own tag (v9.1, v9.0, etc.) making it easy to retrieve previous stable versions.
GateBenchmarks repository.
This repository contains a set of test cases. They are used to check and evaluate every new change in the source code. Every time a modification is proposed in the source code, the whole list of tests is executed and analyzed. Every test is composed of a (ideally simple and short) GATE simulation, described by macro files and is associated with a Python script comparing the simulation output with a reference output. If differences exceed a given test-specific threshold, a flag is raised requiring further investigation. There are currently more than 20 different benchmarks, each of them evaluating a specific feature, such as energy deposition or dose scoring in voxelized geometries or quantification of NECR (Noise Equivalent Count Rate) in PET systems.
GateContrib repository.
Examples of GATE simulations provided by users that can be used as reference or template, are provided in this repository. Note that these simulations are not evaluated and remain at the responsibility of the users to be tested. These simulations mainly serve as examples and show-how for the different applications of GATE.
GateTools repository.
This repository contains a companion project aiming at providing Python functions and command-line tools to help create or analyse outputs from GATE simulations. Provided functions deal with various tasks such as images’ management (conversion from and to DICOM format, resampling, etc), DVH (Dose Volume Histogram), gamma index, phase-space analysis, etc. It heavily relies on the ITK toolkit‡.
2.4. Focus on three key concepts: Volumes, Actors, Digitizer
In this section, three key concepts of the GATE architecture are highlighted: “Volumes” the handling of the geometry, “Actors” that serve several scoring purposes, and the “Digitizer” specific for imaging systems.
Volumes.
In Geant4, all geometrical elements that compose a simulation are described with three concepts. LogicalVolumes represent the properties (e.g. material composition), PhysicalVolumes manage the spatial positioning, and Solids define the shape of a geometrical element. In GATE, the three concepts are encapsulated into a single GateVolume that abstracts those notions. The user only manipulates Volumes without the burden to manage the three different concepts. This allows to simplify the simulation description, but some advanced Geant4 capabilities are not available within GATE (e.g. “parallel world”).
Actors.
The Actors are scorers that encapsulate several Geant4 concepts. They are used as a callback from the Geant4 engine to score information or modify the default behavior of particles during a simulation. An Actor combines the Geant4 SensitiveDetector and Actions callbacks within a single class that can perform tasks each time a Run, Event, Track or Step starts or ends in a given volume. Actors are mainly used to record parameters or information of interest calculated during the simulation, but they can also be used to act on the current particle, for example to stop tracking it. About 30 Actors are currently available. Among the most used are:
the GateDoseActor: recording of the absorbed dose (or energy deposited) in a volume according to a 3D matrix whose dimensions are defined by the user. The Actor also records the uncertainty on the calculated dose value in each voxel;
the GatePhaseSpaceActor: recording of the characteristics of the radiation (type of particles, energy, direction, creation process, etc.) passing through a volume;
the GateKillActor: stop tracking a particle if it reaches the volume, in order to save computation time. It is the responsibility of the user to ensure that the physics is correct (it is always recommended to the users, to validate their results to justify the appropriate use of the Actors).
It is also possible to associate each Actor with one or more filters allowing to select a type of particle, an energy or even a specific direction. Figure 2 depicts the structure of the Scorers and Actors in GATE.
Figure 2.
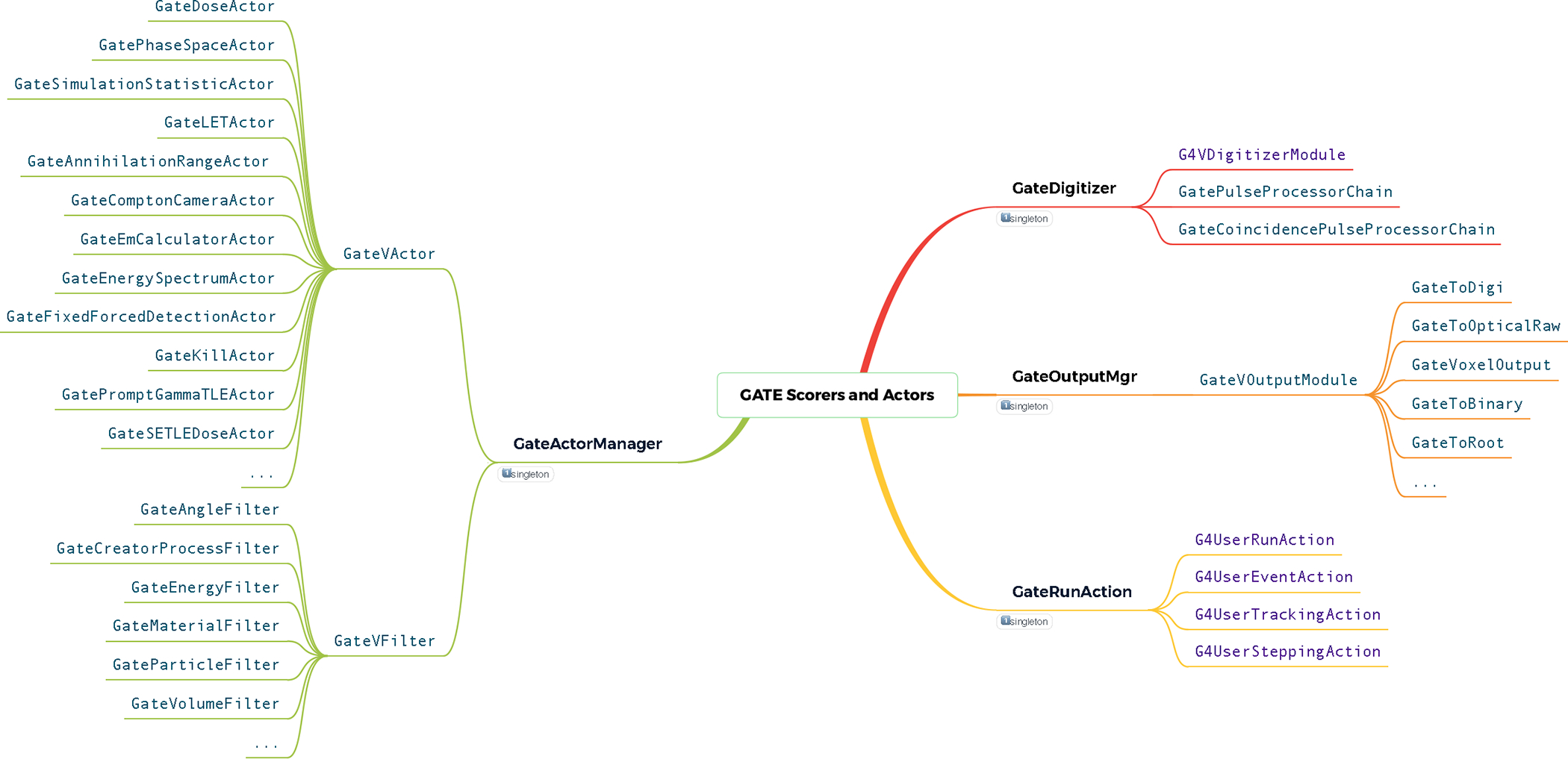
Classes architecture of the GATE scorers and actors module.
Digitizer.
The response of the photodetection components, for example in PET, SPECT or Compton Camera detectors modeling, is managed by a suite of analytical models that form a Digitizer (Figure 3). This module is based on Geant4 virtual methods dedicated for such functionality. It takes as input a list of interaction events, the Hits gathered in a HitsCollection, within a crystal or detector element (Sensitive Detector), and generates digital pulses, called Digits, with associated information (energy, position, time, etc) gathered in DigiCollection. In a digitization, a digit represents a detector output, such as an ADC/TDC count or a trigger signal. The first step of the Digitizer is to construct Single digits, each of them mimics one specific hardware response with their associated uncertainty. Currently, there are 37 signal processing modules available in GATE that can be enabled by users to represent hardware output (see examples in Figure 3 and in [62], [37]). In the case of PET imaging, a specific Digitizer part for constructing coincidences exists. It passes, firstly, by a CoincidenceSorter where coincidence candidates are pre-selected. Then, they can be processed by one of the 5 dedicated Coincidence Digitizer Modules. A Digitizer chain may also be separated from the main Monte Carlo simulation (offline): the simulation stores hits and/or singles in a large root file, and the digitization can then be performed with this file as input, allowing to compare several digitization chains with the same hits.
Figure 3.
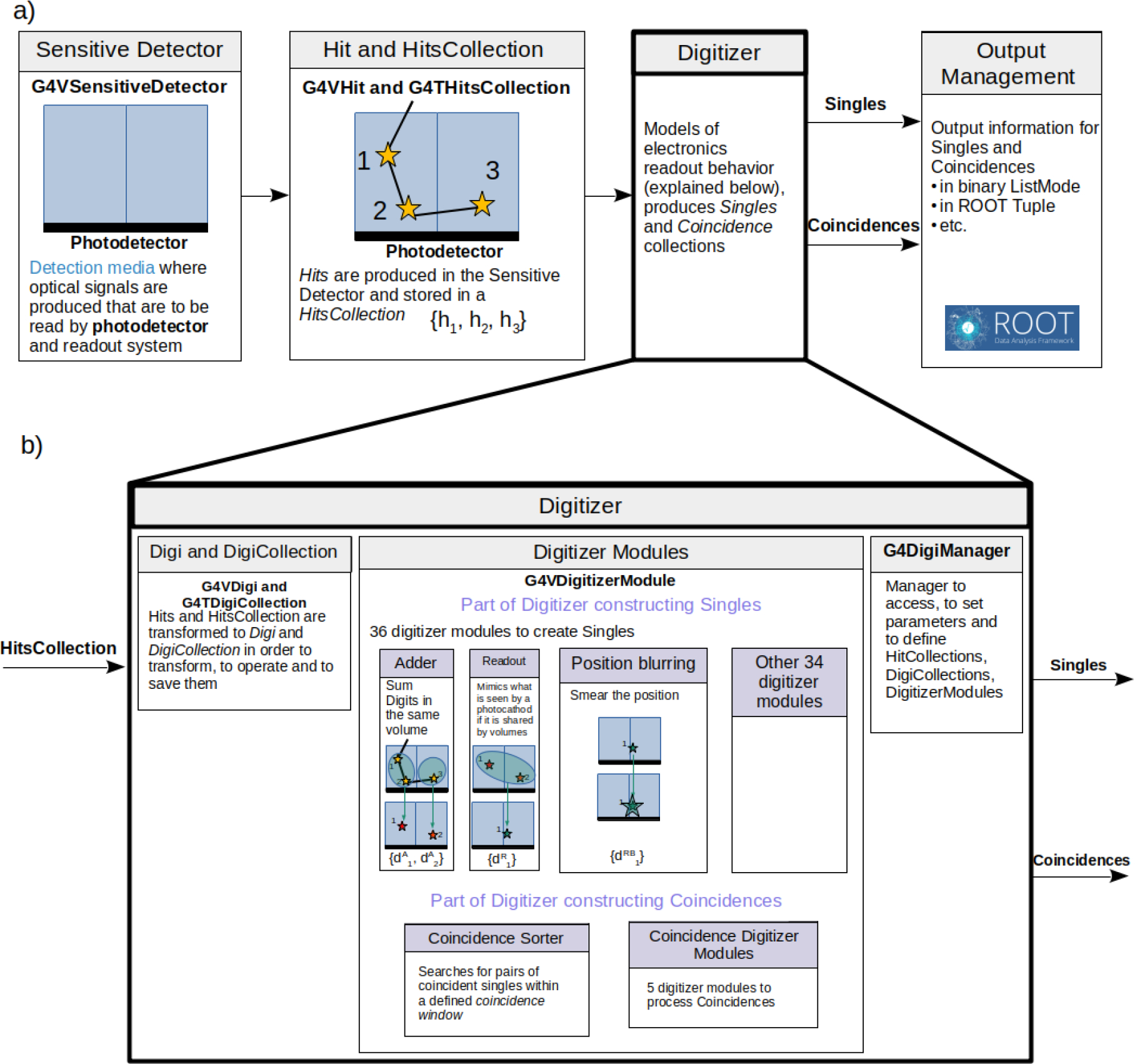
A schematic of the GATE digitizer main workflow and elements: a) digitizer module within the GATE environment and its interaction with other modules; b) digitizer module. An example of a scintillation detector containing two scintillation crystals and a photocathode is illustrated.
2.5. Collaboration with other toolkits
In addition to Geant4 which GATE is built on, several toolkits cooperate with GATE. The ROOT [25] data analysis framework is heavily used throughout the hits analysis to store and organize all physical information during a simulation. Note that, since version 9.0, an alternative NumPy [55] output can also be used. The Python module UpRoot is used within the GateTool to efficiently process ROOT files [110]. Also in GateTools, all 2D and 3D images are managed with ITK [89]. SpekCalc [112] can also be used to define the X-ray beam source characteristics for X-ray based imaging devices. Image reconstruction from simulated list-mode or projections may be performed with other open-source toolkits such as STIR [141], CASToR [94] or RTK [117]. Indeed, STIR implement analytical and iterative reconstruction methods for PET [71] and SPECT [67] imaging devices using GATE’s output as input. CASToR also proposed iterative PET reconstruction algorithms and can conveniently read GATE generated ROOT output [64]. RTK [117] performs tomographic reconstructions from GATE generated projection images. While initially designed for CBCT images, it can now also be used for SPECT reconstruction (OSEM, with attenuation, scatter and PSF corrections) [120; 121] and contains specific features for motion compensated reconstruction. It is also used internally within the GATE code to perform variance reduction algorithms (see section 3.1). All those toolkits helped GATE to build this ecosystem on medical physics.
2.6. Slow pace development: GATE-RTion
GATE was historically the first Geant4-based application for medical physics. Over the past 15 years, its usage and interest in the field of light ion beam therapy has been growing considerably, with the first modeling of an IBA pencil beam scanning system [50] and several subsequent applications related to beam modeling [8; 35; 45], dosimetry [22; 44; 51; 52; 114; 128] and associated imaging applications [93; 122; 123]. The idea of the Gate-RTion project was initiated in 2017, with the purpose of easing the implementation of GATE in clinical centers [53]. The first Gate-RTion version was released in 2018. It provides a stable and long term GATE release, including validation tests (available in the GateContrib repository) for dosimetric applications in light ion beam therapy (mainly scanned proton and carbon ion beams). It provides a collection of tools necessary for the clinical users to interface GATE with the clinical environment (available in the GateTools repository). Gate-RTion establishes a bridge between researchers and clinical users, to facilitate the transfer of research applications into the clinics and allows users to establish more standardized guidelines [152]. The next version of Gate-RTion is currently planned for 2023.
2.7. The OpenGATE collaboration
The OpenGATE scientific collaboration currently brings together 25 public and private institutions (list of partners available on www.opengatecollaboration.org) committed to develop, maintain, and promote the GATE software by respecting the rules set by the steering committee. The collaboration is based on a Gentleman agreement and is open to every group willing to contribute. The collaboration is represented by a spokesperson and its developments are supervised by a scientific coordinator. Twice a year, the collaboration organizes a scientific workshop during which current developments and the latest validations are presented. During these workshops, the limits and priorities for each development are identified. In addition, a group of developers meets regularly with the scientific coordinator to solve technical challenges. The group is open to everyone who wants to contribute. The current GATE mailing list contains more than 2000 registered people, it may give an indication on the number of users.
Twice a year, the collaboration organizes training for beginners as well as training to help advanced users in their analyzes of simulation data with the Python language. These courses are open to researchers from the academic and private sectors. The collaboration supports any initiative to use the platform in the university environment for teaching. As an example, all medical physics students that are following the DQPRM diploma in France (required to be certified as medical physicist) follow a GATE initiation course. In addition, several schools are set up internationally to train master’s level students. These training courses are organized mainly remotely, participants can practice exercises under the supervision of expert collaborators by accessing a complete IT environment on a dedicated remote server. For example, the OpenGATE collaboration in association with IEEE Nuclear and Plasma Sciences society are organizing two schools per year in different parts of the world by adapting the scientific content to the needs of the students in collaboration with the academics of each country (schools have been organized in Asia and Africa, and will be extended to North and South America).
2.8. Limitations
The collaboration does not manage money (except from a small amount dedicated to the organisation of one-time events or to pay GitHub servers) and the collaboration does not employ engineers and developers directly. All contributions are performed by researchers according to their own projects and budgets, inherently limited in duration. It is indeed a very flexible organisation but depends on the success of the members to grant applications. Hence, the long-term management and maintenance of the software is challenging as authors of some specific features may leave. The number of active developers is also relatively small: around 20 people contributed to the source code in the last 3 years.
3. Impact on cancer research and medical physics
This section briefly reviews the impact of GATE regarding cancer-related medical physics applications. A representative part of the available applications is presented here focusing to the most recent developments. For clarity, the section is split into two main parts dedicated to imaging and dosimetry. Previous papers are referred, wherever appropriate, for illustration of the ecosystem’s applications. Figure 4 depicts an estimation of the number of publications throughout the lifetime of GATE, covering all the available applications in medical physics (with almost 900 publications from 2004 until 2021). The graph is provided by the Scopus platform (www.scopus.com) and incorporates the different publications in the fields of medical imaging, dosimetry and radiotherapy. Figure 5 summarizes the main applications of GATE that can be found in the literature, also separated into two main fields, imaging and dosimetry.
Figure 4.
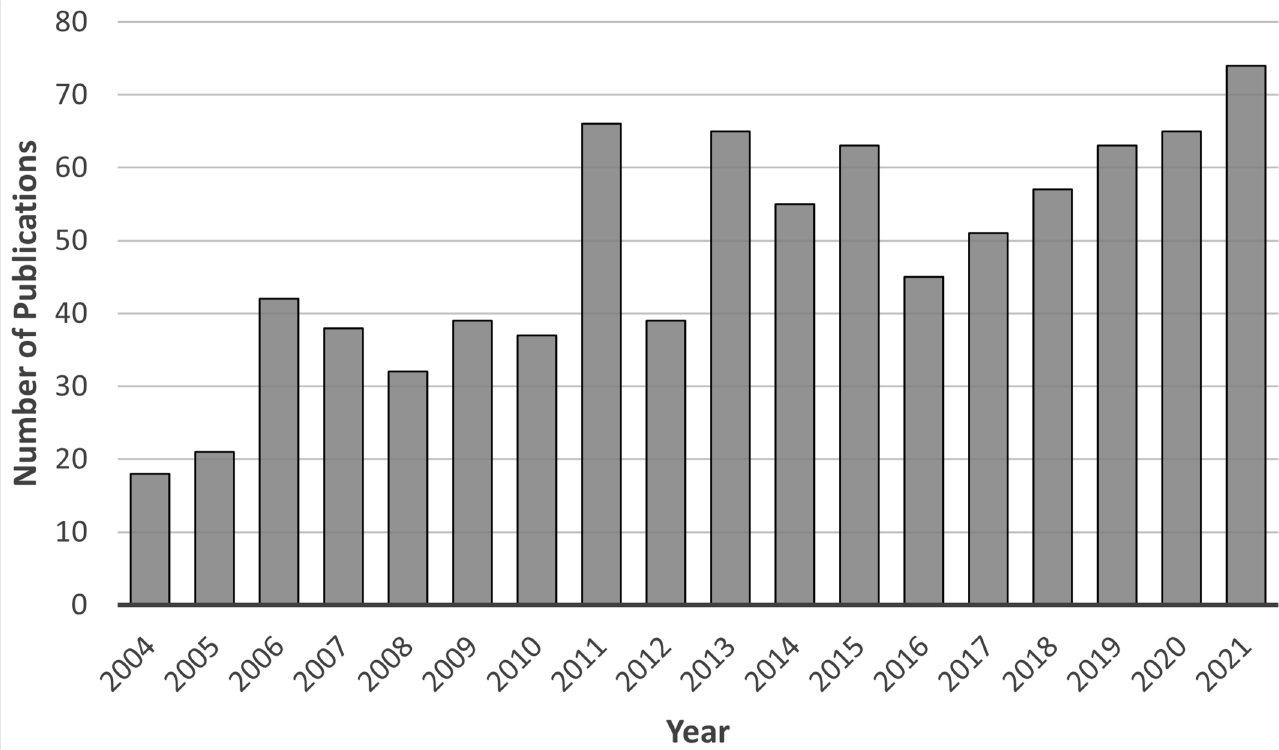
Estimated number of publications of GATE from 2004 to 2021 regarding imaging, dosimetry and radiotherapy applications.
Figure 5.
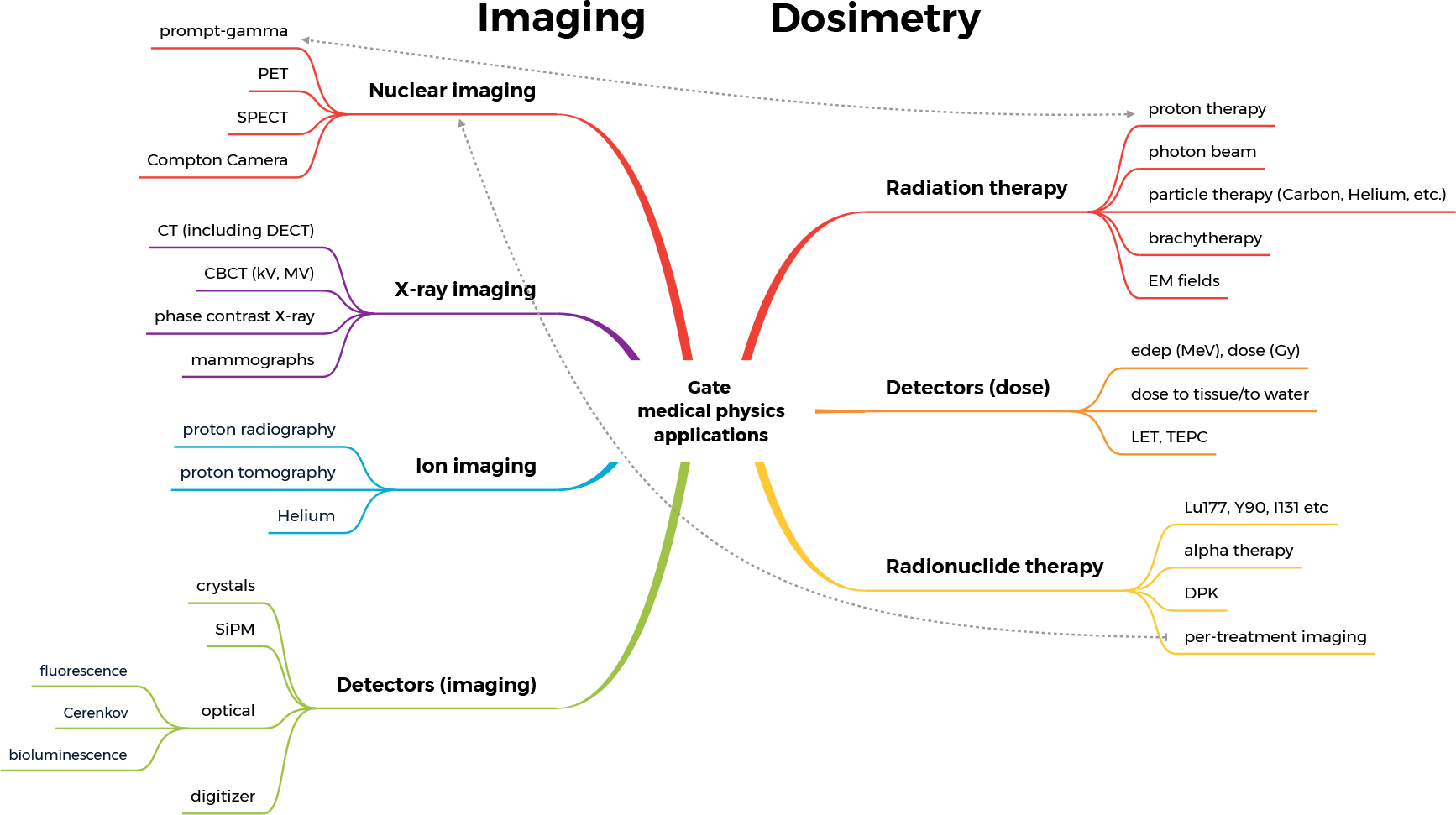
Main applications of GATE in medical physics.
3.1. Imaging-related applications
Nuclear medicine imaging.
In the last few decades there has been a dramatic evolution of nuclear medicine imaging, which provided methods for earlier and more accurate cancer diagnosis, therapy and therapy response assessment. This period coincided with the development of GATE, which since its first release in 2004, has been used to advance nuclear imaging capabilities and accuracy, particularly in PET and SPECT imaging [16; 146]. GATE has been extensively used and is a well-validated tool for: i) the design and the development of new imaging systems , ii) the evaluation and the optimization of image processing algorithms, iii) the development of new radiotracers. Recent developments of GATE MC simulations for emission tomography were presented in a topical review [132].
For more than 20 years, imaging systems like CT, PET and SPECT modalities have become essential tools in cancer diagnosis and for therapeutic efficiency evaluation. GATE is widely used to characterize and investigate/optimize image quality of scanners produced by most main manufacturers (e.g. Siemens [4], General Electric [134] or Philips [147]) as well as to design, optimize and validate new systems that will be put on the market. GATE is for example used to optimize the choice of the detection medium (crystal or liquid scintillator, semi-conductor, etc.), the light collection system (PMT, APD, PSPMT, SiPM, etc.), the geometry of the scanner (dead zone reducing, packing fraction optimization, etc.), to improve the spatial and temporal resolution, the sensitivity and the image contrast recovery. All these parameters are critical in image quantification for oncology applications. GATE is also widely used for prototype detector developments and the next imaging system generations integrating concepts like heterostructured scintillators for ultra-fast TOF-PET scanners [76], total body acquisition [98; 146], or Compton Camera systems [40; 41; 43; 101; 102] with a dedicated module [37]. GATE has been also used to optimize PET detector design for proton therapy range monitoring that is particularly challenged by low signal-to-noise ratio with respect to the diagnostic PET scanners (see section 3.1). In addition, GATE is also used in the field of data correction and tomographic reconstruction where the production of simulated data is crucial to validate the interest of new algorithms to improve quantitative analysis.
Optical imaging
Optical imaging techniques such as bioluminescence imaging [116], fluorescence imaging [82], and Cerenkov luminescence imaging [124] are essential for translational cancer research. GATE can simulate optical imaging techniques by modeling the optical photon transport inside biological tissues [32] as validated against MCML optical simulation software [150]. The optical properties of biological tissues such as refractive index, absorption and scattering coefficients can be defined as a function of wavelength thereby allowing realistic simulations optical imaging and near-infrared thermal therapy [33]. In addition, an optical lens was implemented into GATE for more realistic optical imaging simulations of bioluminescence and near infrared fluorescence (NIRF) imaging [66]. Moreover, GATE also allows the Cerenkov luminescence simulations (see examples in Figure 6) that can be used as a basic research tool for both nuclear medicine [30] and radiotherapy [68; 140].
Figure 6.
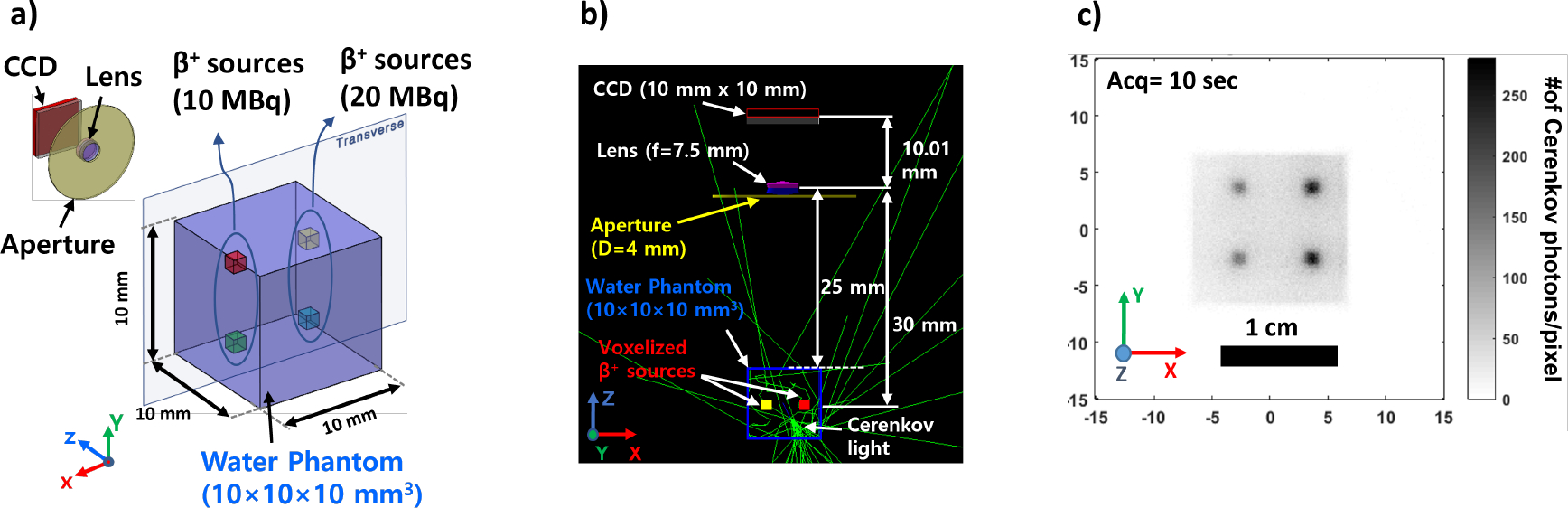
a) GATE Cerenkov luminescence imaging setup with optical imaging system and positron sources inside a water phantom, b) GATE Cerenkov luminescence imaging simulation geometry, c) the CCD image that collected Cerenkov luminescence light for 10 seconds.
Positronium imaging.
The GATE platform can be used as a tool to develop novel PET imaging methods beyond the conventional two-photons tomography. In particular, GATE/Geant4 is capable to simulate positronium decay, a bound atomic state of an electron and a positron, that is formed before annihilation occurs. The measurement of positronium properties, e.g. its mean lifetime, can provide supplementary information about the metabolic processes in the patient’s body [99]. In tissues, the positronium properties are influenced by the size of inter- and intramolecular voids and the concentration of molecules such as molecular oxygen in them, and the extraction of this information may provide insight into disease progression [98]. This approach opens a new field of applications in cancer diagnosis [97; 135]. The positronium tomography technique is especially suitable for large field-of-view high-sensitivity scanners. Positronium imaging performance studies for several large-field-of-view plastic-based scanner prototypes has been described in [100].
X-ray imaging.
GATE is also used for X-ray medical applications, either for imaging or/and dosimetry, as in CT [18; 56; 73; 103], dual energy CT (DECT) [77], Cone-Beam CT (CBCT) with kV beams [13; 26; 57; 86; 149; 153; 154] and MV beams [19; 20], or mammography [38; 38; 39]. Imaging systems were simulated either to assist image improvement methods (such as scatter estimation), or to estimate the dose to the patient (such as organ dose calculations). SpekCalc [112] can be used to define the X-ray beam source characteristics. GATE also proposes various variance reduction techniques dedicated to X-ray imaging, like the Fixed-Forced Detection (FFD) that makes use of the fast ray-tracing capabilities of the RTK library [27; 154]. More recently, a module was added to GATE in order to perform X-ray phase-contrast imaging [36], as well as processes for the refraction and total reflection of X-rays and an analytical wave optics algorithm for generating Fresnel diffraction patterns. All these tools specific to X-ray imaging techniques were successfully validated against data.
Ion imaging.
Patient imaging using particles like protons or ions is also a promising research field and several studies have been done with GATE for simulating ion beams in complex configurations (voxelized volumes, detectors, etc.). Using GATE capabilities for both imaging and dosimetry, these works mainly investigate potential benefits of proton radiography and tomography techniques while optimizing reconstruction algorithms [9; 72; 75; 113; 118; 137]. Imaging with uncommon beams such as with muon [2] or with Helium [108] was also proposed.
Proton range monitoring.
Among the applications of GATE for radiation therapy, a newly implemented multi-stage GATE framework, ProTheRaMon [23], joins and extends GATE capability to simulate proton therapy treatments, secondary radiation induction (Sec. 3.2), and PET imaging including reconstruction with CASTOR package (see Sec. 3.1). ProTheRaMon was developed to be executed on a computational cluster with the aim to simplify multi-parameter simulation studies exploiting large number of patient CT images and treatment plans, all needed to design, test and compare PET detector designs and precision of proton therapy range monitoring in clinical environment [74; 85]. ProTheRaMon was developed in the frame of a research project aiming at designing and optimizing a range monitoring detector based on J-PET technology [96–98; 100] at CCB Krakow proton therapy centre (Poland) [61; 127]. It is an open-source GATE repository available on ProTheRaMon. Currently, ProTheRaMon is also used by researchers from Paul Scherrer Institute (PSI, Switzerland) within the scope of the PETITION project (Swiss National Science Foundation, Grant CRSII5_189969), a collaboration between ETH Zurich, Le Centre hospitalier universitaire vaudois (CHUV, Lausanne) and PSI [119], which aims to develop a dedicated proton therapy PET scanner for brain and head-and-neck tumours, aiming at hypoxia guided proton therapy [84] and proton therapy range monitoring [92]. Others online proton range monitoring methods are also studied with GATE, such as line-cone reconstruction with Compton Camera [80].
3.2. Dose-related applications
External beam radiation therapy.
GATE has been used to provide answers to complex issues that cannot be resolved with clinical software available for photon radiotherapy. These are, for example, dose calculations with large heterogeneities [17], skin dose for breast cancer treatments [10], or double calculation of radiotherapy treatment plans [79]. However, GATE is less used for external photon beam therapy than, for example, EGSnrc [70]. GATE has a stronger impact in light ion beam therapy centers supporting research and development, as well as clinical applications derived from the Gate-RTion release in particular. AUTOMC was the first Gate-RTion-based proton Independent Dose Calculation (IDC) system. It has been set-up in clinical operation since the start of the Christie NHS Foundation Trust, a proton therapy facility that opened in December 2018 in Manchester (UK) [3]. It is used to support the Patient Specific Quality Assurance (PSQA) Process. AUTOMC/Gate-RTion was successfully validated against more than 730 clinical plans, for which physical QA measurements were performed with a 2D detector array and a thimble ionization chamber. AUTOMC is a stand-alone software directly providing the IDC output in terms of gamma pass rate [81]. IDEAL (Independent DosE cAlculation for Light ion beam therapy) is an alternative Gate-RTion-based IDC system for light ions. IDEAL was designed in a DICOM-in/DICOM-out fashion, in order to be compatible with standard DICOM interfaces available at light ion beam therapy centers. IDEAL is a proton and carbon ion IDC system and the first version was released in March 2021 [54] (available on the Git repository of OpenGATE). It is being used at the MedAustron ion therapy center in Austria, mainly for carbon ion IDC. Current hot topics in the field of proton and carbon ion therapy, such as variable proton Relative Biological Effectiveness (RBE) depending on Linear Energy Transfer (LET) [136] (see next sections), the influence of fragmentation spectra on carbon ion RBE [115], influence of new stopping power tables (e.g. ICRU90) for 3D dosimetric applications with protons and carbon ions [22] are fully supported by the Gate-RTion release.
Radionuclide therapy.
The impact of GATE on cancer research significantly increased after the addition of radiation therapy and dosimetry applications in 2014 [33; 54; 131], MC simulations serve as gold standard for dosimetry calculations and can now be conveniently used for combined imaging and therapy applications in GATE, which is of high importance for theranostics [95; 145]. Emulated by Geant4 to model the energy deposition in biological media, GATE allows dosimetry through full Monte Carlo, dose point kernels (DPKs), or more recently deep learning (DL) for fast dose computation [42; 47; 48; 78; 106]. GATE has been used for image-based dosimetry in research and clinical practice [10; 20; 24; 34; 125]. Specific to nuclear medicine, high-precision dosimetry becomes an essential tool for targeted radionuclide therapy with 177Lu or 90Y [111; 125], and is poised to continue growing in importance with the development of short range alpha emitters [143]. In Targeted Alpha Therapy (TAT), the understanding of the dose deposition in tissue such as the bone marrow close to bony metastases is essential to develop efficient and safe therapeutics. Very recently, GATE was extensively validated for brachytherapy applications incorporating both high dose rate (HDR) and low dose rate (LDR) sources with 192Ir and 125I respectively [29]. The GATE platform combining high precision dosimetry and well-validated image modeling has the potential to become a tool of choice for investigating and developing novel approaches using a plethora of radionuclides.
Radiobiology.
GATE is becoming extensively used to predict the effect of radiation at micro and nano scales, from preclinical cell irradiations to advanced hadrontherapy treatments [87; 88; 105; 109; 126; 136; 138; 139; 148]. The platform is the preferred tool to simulate preclinical or clinical beam characteristics that are detailed in a phase-space file before being combined to Geant4-DNA simulations [21; 58–60] to assess radiobiological effects. To date, GATE is not able to fully handle multi-scale simulations from the calculation of dose to organs to an accurate identification and understanding of cellular and molecular damage. Upcoming developments will favor the integration of relevant features that have been tested so far through Geant4-DNA simulations. As example, we can mention the integration of various cell population models, specifically 3D cell populations, from the C++ Cell POPulation modeler (CPOP) already compliant with the Geant4 toolkit [83]. We will propose the same integration for the IDDRRA platform to score radiation induced DNA damage with a user-friendly simulation of different DNA molecules [28]. Recently, specific actors are being developed for proton and carbon ion therapy to calculate LET or biological dose-based clinical treatment planning. Linear energy transfer (LET), especially dose-averaged LET (LETd), either as a physics component of variable RBE models [91] or alone, as a physical treatment planning parameter [90; 144], is increasingly considered in clinical practise. The recent literature indicates that the LETd alone may not be predictive of biological effectiveness in proton radiotherapy [15; 46; 65; 104]. The GATE framework offers access to information on single particle interactions, thus, enabling calculation of single particle LET in a voxelized patient geometry. Figure 7 shows dose and LETd distributions, as well as LET spectra for 150 MeV therapeutic proton pencil beam at the Bragg peak depth computed with GATE and measured experimentally with a TimePix detector [49]. In addition, the estimation of the biological dose will be proposed through the BioDoseActor [5]. The input data of this new actor are pre-calculated α and β cell survival parameters for Human Salivary Gland (HSG) cell line produced with mMKM [69] and NanOx [31] biophysical models for monoenergetic ions (hydrogen, carbon, helium, and oxygen) and energies ranging from 0.1 to 400 MeV/n. Some biophysical models, like the NanOx model, also consider the physicochemical and chemical consequences of radiation at nano scales by evaluating the oxidative stress undergone by cells during water radiolysis. In the future, one can expect ongoing developments concerning the simulation of chemical species reactions during water radiolysis for different dose rates [142] will be gathered in a new open-source release and could help to better estimate treatment outcomes.
Figure 7.
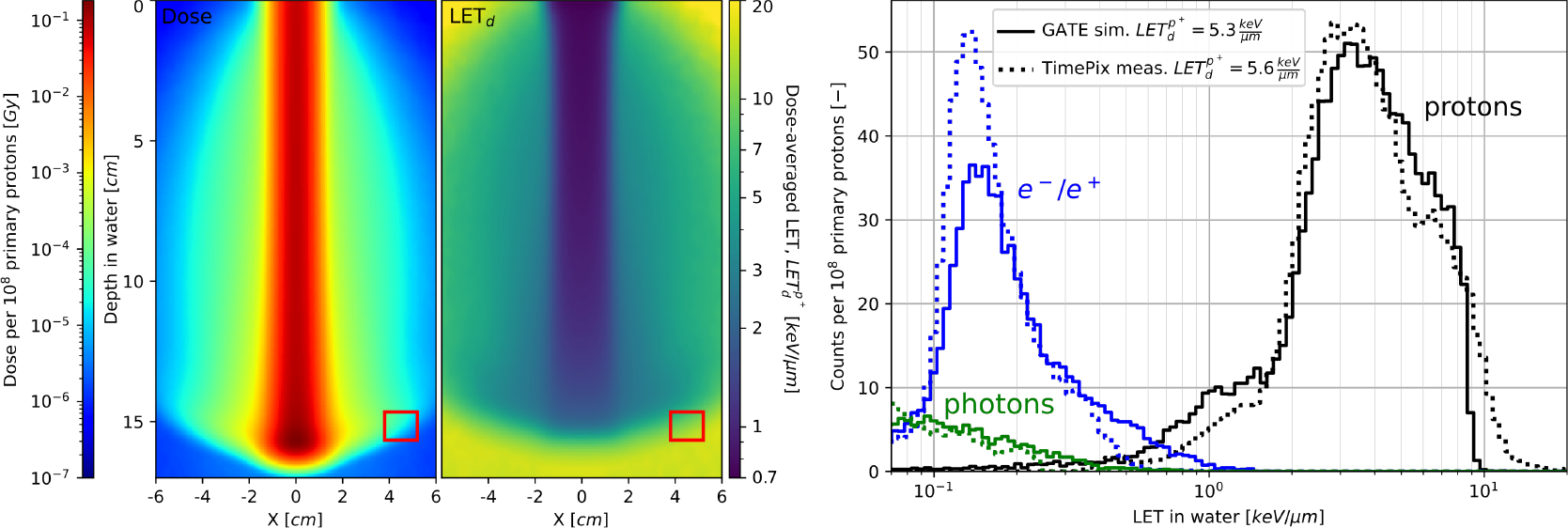
2D dose (left panel) and LETd of protons (middle panel) distributions in water for therapeutic pencil proton beam at 150 MeV and the corresponding LET spectrum (right panel) for the measurement point at the Bragg peak depth, 45 mm from the beam axis, indicated with red squares on left and middle panels. Proton, electron and photon contributions to the LET spectrum are calculated with GATE and compared to the measurement with a TimePix detector. Wide range of proton LET values is approximated by the averaged LETd given in the legend.
3.3. General considerations
There are several advantages of GATE for medical physics research. First, GATE offers Monte Carlo simulation environments for medical physics with user-friendly macro command lines as well as interactive visualization capability thereby allowing users to conveniently develop and debug their own simulation. Second, various pre-defined medical imaging systems such as PET, SPECT, Compton camera, X-ray CT, and optical imaging are readily available thus researchers can implement new studies based on those systems. In addition, the radiotherapy and internal dosimetry simulations are available with well-validated physics models thereby allowing researchers to investigate the new cancer therapy strategies and concepts in conjunction with the radiation exposure to patients. One drawback of GATE is that Geant4 multi-threading is not available. GATE provides however job splitting tools in which the simulation tasks can be separated and assigned to multiple CPU cores thereby reducing the overall simulation time substantially. Another drawback of GATE is that the users are limited to the pre-defined imaging systems with their fixed system hierarchy.
GATE is one among several other Monte Carlo codes which have been and are in use in Medical physics applications. These codes include GEANT4 itself, EGSnrc [70], MCNP [151], TOPAS [107], GAMOS [11; 12] and each of them has its own advantages and limitations. The collaborative approach which is a feature of both GATE and GEANT4 provides both advantages (broad and powerful feature enrichment) and limitations (code coherence and efficiency). While other code systems have focused mostly on accuracy (EGSnrc, MCNP), efficiency (EGSnrc) or a particular application (TOPAS), GATE is fully open-source and would be competitive in the realms of detailed modeling of the imaging and supporting instrumentation process (e.g. signal digitization by electronic modules), obtaining results for both imaging and dosimetric interpretation in a single simulation, ease of use, original AI-based methods (see next section), and broad international support and education program.
4. Future directions
The GATE software is constantly evolving, trying to propose new features and new improvements from release to release.
Digitizer.
The aim of the first ongoing collaborative project is the restructuring of the “digitizer module”. As described in previous sections, this module simulates the response of the photodetection components in several imaging systems (PET, SPECT, Compton Camera etc). This central module has evolved a lot since the beginning of the project and has become complex and hard to maintain with multiple layers of interdependencies. The main goals of the current development are: to preserve the current functionalities, to modularize the code, to keep it as close as possible to the Geant4 framework. The main basic components of this module will be based on the notion of HitsCollections, DigiCollections and DigitizerModule. The first manages a list of “hits” corresponding to some interactions in given volumes, described by several attributes (energy, position etc.) that may be selected dynamically according to user’s needs. The second notion, DigitizerModule manages procedures that will take as input some HitsCollection and DigiCollection and create another ones. At the end of a complete digitization task, composed of several chained DigitizerModules, the user will get the simulation of the photodetector response.
Artificial Intelligence.
Another ongoing experimental project aims to investigate the potential interest of AI, and more specifically GANs (Generative Adversarial Networks), for the modelling of phase-space or activity sources [130; 132; 133]. The main idea is to build a neural network model of a given probability distribution of particles such that they can be quickly generated, avoiding the tracking phase. Proofs of concept have been published but there are still a lot of unknowns and uncertainties in those approaches that remain to be studied. There is, in particular, some ongoing work on conditional GANs that may allow to model a family of GANs, and to avoid time-consuming re-training [129].
Python.
Finally, a third long-term project has started, aiming to completely rethink the way the simulations are described by the user. Indeed, the Geant4 messenger system, based on a text file of so-called “macro commands”, has been used for years. This is a powerful system, but has some limitations and is not always very user-friendly. For example, while feasible, it is not really convenient to create complex simulation structures involving loops, variables or computations. By acknowledging the fact that the Python language and its associated very large environment is de-facto a standard for data analysis, it has been decided to investigate whether simulations can be directly described in Python instead of macro files. The interests of such a mechanism are: a simulation can be described with regular Python scripts with loops or any type of variables, part of the simulation can be modularized and easily reused (with import), users do not have to learn a new language. A first prototype is currently showing the feasibility of such an approach. The mechanism is based on the Geant4 python binding thanks to pybind11§ that exposes to Python a fraction of the Geant4 API. The GATE engine is hence split into two main parts, one directly in Python that takes care of everything related to the simulation initialization part, and the second part, in C++, in charge of tasks occurring during a simulation run. Communication between Python and C++ is designed such, that only the computing-intensive parts of the simulation are still described in C++, leaving all other management to Python for simpler development. With this approach, there is no computation time penalty due to the use of Python, the core engine remaining Geant4. Multithreading capabilities of Geant4 are also maintained (thanks to the possibility to release the Python Global Interpreter Lock before going multi-thread). A potential drawback of this approach is the need to use two different coding languages to develop and add functionalities. However, by separating more clearly what is performed during initialization from what is done during run-time, and by moving to Python all high-level tasks, it is hoped that maintenance and development will be simplified. The first public experimental version of this approach, that may become the future GATE 10.x series, is planned in 2023.
5. Conclusion
GATE is a living open-source project run by the OpenGATE collaboration, contributing since around 20 years to medical physics cancer research. The associated community is the real strength of this initiative gathering people from different fields (radiology, nuclear medicine, radiotherapy, radiation protection, etc.) with complementary backgrounds (researchers, engineers, teachers, medical physicists, physicians, etc.).
Acknowledgments
The OpenGATE collaboration would like to warmly thanks the Geant4 collaboration for their great support and fruitful discussions. This work was performed within the framework of the SIRIC LYriCAN Grant INCa-INSERM-DGOS-12563, the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the ANR, and the POPEYE ERA PerMed 2019 project (ANR-19-PERM-0007-04). Optical modeling developments were supported by NIH grants R03 EB020097 and R01 EB027130. The contribution of Jan Gajewski was partially supported by the National Center for Research and Development (NCBiR) (grant No. LIDER/43/0222/L-12/20/NCBR/2021).
Footnotes
References
- [1].Agostinelli S, Allison J, Amako K, Apostolakis J, Araujo H, Arce P, Asai M, Axen D, Banerjee S, Barrand G, Behner F, Bellagamba L, Boudreau J, Broglia L, Brunengo A, Burkhardt H, Chauvie S, Chuma J, Chytracek R, Cooperman G, Cosmo G, Degtyarenko P, Dell’Acqua A, Depaola G, Dietrich D, Enami R, Feliciello A, Ferguson C, Fesefeldt H, Folger G, Foppiano F, Forti A, Garelli S, Giani S, Giannitrapani R, Gibin D, Gómez Cadenas JJ, González I, Gracia Abril G, Greeniaus G, Greiner W, Grichine V, Grossheim A, Guatelli S, Gumplinger P, Hamatsu R, Hashimoto K, Hasui H, Heikkinen A, Howard A, Ivanchenko V, Johnson A, Jones FW, Kallenbach J, Kanaya N, Kawabata M, Kawabata Y, Kawaguti M, Kelner S, Kent P, Kimura A, Kodama T, Kokoulin R, Kossov M, Kurashige H, Lamanna E, Lampén T, Lara V, Lefebure V, Lei F, Liendl M, Lockman W, Longo F, Magni S, Maire M, Medernach E, Minamimoto K, Mora de Freitas P, Morita Y, Murakami K, Nagamatu M, Nartallo R, Nieminen P, Nishimura T, Ohtsubo K, Okamura M, O’Neale S, Oohata Y, Paech K, Perl J, Pfeiffer A, Pia MG, Ranjard F, Rybin A, Sadilov S, Di Salvo E, Santin G, Sasaki T, Savvas N, Sawada Y, Scherer S, Sei S, Sirotenko V, Smith D, Starkov N, Stoecker H, Sulkimo J, Takahata M, Tanaka S, Tcherniaev E, Safai Tehrani E, Tropeano M, Truscott P, Uno H, Urban L, Urban P, Verderi M, Walkden A, Wander W, Weber H, Wellisch JP, Wenaus T, Williams DC, Wright D, Yamada T, Yoshida H, and Zschiesche D. Geant4 - a simulation toolkit. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 506(3):250–303, July 2003. ISSN 01689002. doi: 10/czv4cm. [Google Scholar]
- [2].Aguiar P, Casarejos E, Vilan JA, and Iglesias A. Geant4-GATE simulation of a large plastic scintillator for muon radiography. In 2013 3rd International Conference on Advancements in Nuclear Instrumentation, Measurement Methods and Their Applications (ANIMMA), pages 1–5, June 2013. doi: 10.1109/ANIMMA.2013.6728082. [Google Scholar]
- [3].Aitkenhead Adam H., Sitch Peter, Richardson Jenny C., Winterhalter Carla, Patel Imran, and Mackay Ranald I.. Automated Monte-Carlo re-calculation of proton therapy plans using Geant4/Gate: Implementation and comparison to plan-specific quality assurance measurements. The British Journal of Radiology, 93(1114): 20200228, October 2020. ISSN 1748–880X. doi: 10.1259/bjr.20200228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aklan B, Jakoby BW, Watson CC, Braun H, Ritt P, and Quick HH. GATE Monte Carlo simulations for variations of an integrated PET/MR hybrid imaging system based on the Biograph mMR model. Physics in Medicine and Biology, 60 (12):4731–4752, June 2015. ISSN 0031–9155. doi: 10/ghck7g. [DOI] [PubMed] [Google Scholar]
- [5].Ali Yasmine, Monini Caterina, Russeil Etienne, Jean Michel Létang Etienne Testa, Maigne Lydia, and Beuve Michael. Estimate of the Biological Dose in Hadrontherapy Using GATE. Cancers, 14(7):1667, March 2022. ISSN 2072–6694. doi: 10.3390/cancers14071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Allison J, Amako K, Apostolakis J, Araujo H, Arce Dubois P, Asai M, Barrand G, Capra R, Chauvie S, Chytracek R, P Cirrone GA, Cooperman G, Cosmo G, Cuttone G, G Daquino G, Donszelmann M, Dressel M, Folger G, Foppiano F, Generowicz J, Grichine V, Guatelli S, Gumplinger P, Heikkinen A, Hrivnacova I, Howard A, Incerti S, Ivanchenko V, Johnson T, Jones F, Koi T, Kokoulin R, Kossov M, Kurashige H, Lara V, Larsson S, Lei F, Link O, Longo F, Maire M, Mantero A, Mascialino B, McLaren I, Mendez Lorenzo P, Minamimoto K, Murakami K, Nieminen P, Pandola L, Parlati S, Peralta L, Perl J, Pfeiffer A, G Pia M, Ribon A, Rodrigues P, Russo G, Sadilov S, Santin G, Sasaki T, Smith D, Starkov N, Tanaka S, Tcherniaev E, Tome B, Trindade A, Truscott P, Urban L, Verderi M, Walkden A, P Wellisch J, C Williams D, Wright D, and Yoshida H. Geant4 developments and applications. IEEE Transactions on Nuclear Science, 53(1):270—278, February 2006. doi: 10.1109/TNS.2006.869826. [DOI] [Google Scholar]
- [7].Allison J, Amako K, Apostolakis J, Arce P, Asai M, Aso T, Bagli E, Bagulya A, Banerjee S, Barrand G, Beck BR, Bogdanov AG, Brandt D, Brown JMC, Burkhardt H, Canal Ph, Ott D, Chauvie S, Cho K, and Yoshida H. Recent developments in GEANT4. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 835: 186–225, 2016. [Google Scholar]
- [8].Almhagen E, Boersma DJ, Nyström H, and Ahnesjö A. A beam model for focused proton pencil beams. Physica Medica, 52:27–32, August 2018. ISSN 1120–1797. doi: 10.1016/j.ejmp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- [9].N Arbor D, Dauvergne G Dedes, J M Létang K, Parodi C T Quiñones, Testa E, and Rit S. Monte Carlo comparison of x-ray and proton CT for range calculations of proton therapy beams. Physics in Medicine and Biology, 60(19):7585–7599, October 2015. ISSN 0031–9155, 1361–6560. doi: 10/gf85ft. [DOI] [PubMed] [Google Scholar]
- [10].Arbor Nicolas, Gasteuil Jean, Noblet Caroline, Moreau Matthieu, and Meyer Philippe. A GATE/Geant4 Monte Carlo toolkit for surface dose calculation in VMAT breast cancer radiotherapy. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB), 61:112–117, May 2019. ISSN 1724–191X. doi: 10.1016/j.ejmp.2019.04.012. [DOI] [PubMed] [Google Scholar]
- [11].Arce Pedro, Rato Pedro, Canadas Mario, and Lagares Juan Ignacio . GAMOS: A Geant4-based easy and flexible framework for nuclear medicine applications. In 2008 IEEE Nuclear Science Symposium Conference Record, pages 3162–3168, October 2008. doi: 10.1109/NSSMIC.2008.4775023. [Google Scholar]
- [12].Arce Pedro, Juan Ignacio Lagares Laura Harkness, Daniel Pérez-Astudillo Mario Cañadas, Rato Pedro, María de Prado Yamiel Abreu, Gianluca de Lorenzo Machiel Kolstein, et al. Gamos: A framework to do Geant4 simulations in different physics fields with an user-friendly interface. NIMA, 735:304–313, 2014. [Google Scholar]
- [13].Ardenfors Oscar, Henry Thomas, Gudowska Irena, Poludniowski Gavin, and Dasu Alexandru. Organ doses from a proton gantry-mounted cone-beam computed tomography system characterized with MCNP6 and GATE. Physica Medica: European Journal of Medical Physics, 53:56–61, September 2018. ISSN 1120–1797. doi: 10.1016/j.ejmp.2018.08.011. [DOI] [PubMed] [Google Scholar]
- [14].Aso T, Mastushita K, Nishio T, Kabuki S, and Sasaki T. Extending Geant4 based particle therapy system simulation framework to medical imaging applications. In 2015 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), pages 1–4, October 2015. doi: 10.1109/NSSMIC.2015.7581922. [Google Scholar]
- [15].Bahn Emanuel, Bauer Julia, Harrabi Semi, Herfarth Klaus, Debus Jürgen, and Alber Markus. Late Contrast Enhancing Brain Lesions in Proton-Treated Patients With Low-Grade Glioma: Clinical Evidence for Increased Periventricular Sensitivity and Variable RBE. International Journal of Radiation Oncology, Biology, Physics, 107(3):571–578, July 2020. ISSN 1879–355X. doi: 10.1016/j.ijrobp.2020.03.013. [DOI] [PubMed] [Google Scholar]
- [16].Bailey Dale L. and Willowson Kathy P.. An Evidence-Based Review of Quantitative SPECT Imaging and Potential Clinical Applications. Journal of Nuclear Medicine, 54(1):83–89, January 2013. ISSN 0161–5505, 2159–662X. doi: 10.2967/jnumed.112.111476. [DOI] [PubMed] [Google Scholar]
- [17].Beilla S, Younes T, Vieillevigne L, Bardies M, Franceries X, and Simon L. Monte Carlo dose calculation in presence of low-density media: Application to lung SBRT treated during DIBH. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB), 41:46–52, September 2017. ISSN 1724–191X. doi: 10.1016/j.ejmp.2017.04.009. [DOI] [PubMed] [Google Scholar]
- [18].Benameur Y, Tahiri M, Mkimel M, El Baydaoui R, Mesradi MR, and El Hariri B. Fetal organ dose assessment during pelvic CT examination using Monte Carlo/GATE simulation and pregnancy voxelized phantom Katja. E3S Web of Conferences, 351:01072, 2022. ISSN 2267–1242. doi: 10.1051/e3sconf/202235101072. [DOI] [Google Scholar]
- [19].Benhalouche S, Bert J, Autret A, Visvikis D, Pradier O, and Boussion N. Imaging and radiation therapy: GATE Monte Carlo simulation of a Megavolt Cone Beam CT. In 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), pages 1–4, October 2013. doi: 10.1109/NSSMIC. 2013.6829050. [Google Scholar]
- [20].Benhalouche Saadia, Bert Julien, Boussion Nicolas, Autret Awen, Pradier Olivier, and Visvikis Dimitris. GATE Monte-Carlo Simulation of an MV-CBCT Flat Panel for Synergistic Imaging and Dosimetric Applications in Radiotherapy. IEEE Transactions on Radiation and Plasma Medical Sciences, 1(5):444–451, September 2017. ISSN 2469–7303. doi: 10.1109/TRPMS.2017.2718545. [DOI] [Google Scholar]
- [21].Bernal MA, Bordage MC, Brown JMC, Davídková M, Delage E, El Bitar Z, Enger SA, Francis Z, Guatelli S, Ivanchenko VN, Karamitros M, Kyriakou I, Maigne L, Meylan S, Murakami K, Okada S, Payno H, Perrot Y, Petrovic I, Pham QT, Ristic-Fira A, Sasaki T, Štěpán V, Tran HN, Villagrasa C, and Incerti S. Track structure modeling in liquid water: A review of the Geant4-DNA very low energy extension of the Geant4 Monte Carlo simulation toolkit. Physica Medica : European Journal of Medical Physics, 31(8):861–874, December 2015. ISSN 1120–1797. doi: 10.1016/j.ejmp.2015.10.087. [DOI] [PubMed] [Google Scholar]
- [22].Marta Bolsa-Ferruz Hugo Palmans, Boersma David, Stock Markus, and Grevillot Loïc. Monte Carlo computation of 3D distributions of stopping power ratios in Light Ion Beam Therapy using GATE-RTion. Medical Physics, 48(5), 2021. ISSN 2473–4209. doi: 10/ghzjbb. [DOI] [PubMed] [Google Scholar]
- [23].Borys D and et al. ProTheRaMon - A GATE simulation framework for proton therapy range monitoring using PET imaging. submitted to Physics in Medicine & Biology, under review. [DOI] [PubMed] [Google Scholar]
- [24].Bouzid D, Bert J, Dupré P-F, Benhalouche S, Visvikis D, Pradier O, and Boussion N. Monte Carlo simulation of the Intrabeam® system with the platform gate for breast cancer. Physica Medica: European Journal of Medical Physics, 29: e19–e20, June 2013. ISSN 1120–1797. doi: 10.1016/j.ejmp.2013.08.064. [DOI] [Google Scholar]
- [25].Brun Rene and Rademakers Fons. ROOT — An object oriented data analysis framework. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 389(1):81–86, April 1997. ISSN 0168–9002. doi: 10.1016/S0168-9002(97)00048-X. [DOI] [Google Scholar]
- [26].Cabanas Ana M., Mauricio Arriagada-Benítez Carlos Ubeda, Meseguer-Ruiz Oliver, and Arce Pedro. Dose Estimation by Geant4-Based Simulations for Cone-Beam CT Applications: A Systematic Review. Applied Sciences, 11(13):6136, July 2021. ISSN 2076–3417. doi: 10.3390/app11136136. [DOI] [Google Scholar]
- [27].Cajgfinger Thomas, Rit Simon, Jean Michel Létang Adrien Halty, and Sarrut David. Fixed forced detection for fast SPECT Monte-Carlo simulation. Physics in Medicine & Biology, 63(5):055011, 2018. doi: 10.1088/1361-6560/aa9e32. [DOI] [PubMed] [Google Scholar]
- [28].Chatzipapas Konstantinos P., Papadimitroulas Panagiotis, Loudos George, Papanikolaou Niko, and Kagadis George C.. IDDRRA: A novel platform, based on Geant4-DNA to quantify DNA damage by ionizing radiation. Medical Physics, 48 (5):2624–2636, May 2021. ISSN 2473–4209. doi: 10.1002/mp.14817. [DOI] [PubMed] [Google Scholar]
- [29].Chatzipapas Konstantinos P., Plachouris Dimitris, Papadimitroulas Panagiotis, Mountris Konstantinos A., Bert Julien, Visvikis Dimitris, Mihailidis Dimitris, and Kagadis George C.. Standardization and Validation of Brachytherapy Seeds’ Modelling Using GATE and GGEMS Monte Carlo Toolkits. Cancers, 13(21):5315, October 2021. ISSN 2072–6694. doi: 10.3390/cancers13215315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho Jennifer S., Taschereau Richard, Olma Sebastian, Liu Kan, Chen Yi-Chun, Shen Clifton K.-F, van Dam R. Michael, and Chatziioannou Arion F. Cerenkov radiation imaging as a method for quantitative measurements of beta particles in a microfluidic chip. Physics in Medicine and Biology, 54(22):6757–6771, November 2009. ISSN 1361–6560. doi: 10.1088/0031-9155/54/22/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cunha M, Monini C, Testa E, and Beuve M. NanOx, a new model to predict cell survival in the context of particle therapy. Physics in Medicine and Biology, 62(4):1248–1268, February 2017. ISSN 1361–6560. doi: 10.1088/1361-6560/aa54c9. [DOI] [PubMed] [Google Scholar]
- [32].Cuplov Vesna, Buvat Irene, Pain Frederic, and Jan Sebastien. Extension of the GATE Monte-Carlo simulation package to model bioluminescence and fluorescence imaging. Journal of Biomedical Optics, 19(2):026004, February 2014. ISSN 1083–3668, 1560–2281. doi: 10.1117/1.JBO.19.2.026004. [DOI] [PubMed] [Google Scholar]
- [33].Cuplov Vesna, Pain Frédéric, and Jan Sébastien. Simulation of nanoparticle-mediated near-infrared thermal therapy using GATE. Biomedical Optics Express, 8(3):1665–1681, March 2017. ISSN 2156–7085. doi: 10.1364/BOE.8.001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elazhar H, Deschler T, Létang JM, Nourreddine A, and Arbor N. Neutron track length estimator for GATE Monte Carlo dose calculation in radiotherapy. Physics in Medicine and Biology, 63(12):125018, June 2018. ISSN 1361–6560. doi: 10.1088/1361-6560/aac768. [DOI] [PubMed] [Google Scholar]
- [35].Elia Alessio, Andreas Franz Resch Antonio Carlino, Till Tobias Böhlen Hermann Fuchs, Palmans Hugo, Letellier Virgile, Dreindl Ralf, Osorio Jhonnatan, Stock Markus, Sarrut David, and Grevillot Loïc. A GATE/Geant4 beam model for the MedAustron non-isocentric proton treatment plans quality assurance. Physica Medica, 71:115–123, March 2020. ISSN 11201797. doi: 10/ggw5vd. [DOI] [PubMed] [Google Scholar]
- [36].Endrizzi Marco. X-ray phase-contrast imaging. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 878:88–98, January 2018. ISSN 0168–9002. doi: 10.1016/j.nima.2017.07.036. [DOI] [Google Scholar]
- [37].Etxebeste A, Dauvergne D, Fontana M, Létang JM, Llosá G, Munoz E, Oliver JF, Testa É, and Sarrut D. CCMod: A GATE module for Compton camera imaging simulation. Physics in Medicine and Biology, 65(5):055004, February 2020. ISSN 1361–6560. doi: 10/ggw5t8. [DOI] [PubMed] [Google Scholar]
- [38].Fathi I, Mkimel M, Halimi A, EL baydaoui R, Mesradi MR, Krim M, El rhazouani O, Saad E, and Hilali A. Experimental characterization and Monte Carlo simulation of radiation dose in mammography by AGMS-DM+ and OSL nanoDot™ detectors. In 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), pages 1–5, June 2019. doi: 10.1109/MeMeA.2019.8802135. [Google Scholar]
- [39].Fathi I, Mesradi MR, Krim M, El Baydaoui R, Mkimel M, El Rhazouani O, and Saad E. Validation of GATE Monte Carlo Simulation and Al2O3:C OSL Nanodots for Entrance Surface Dose Estimation in Mammography. Moscow University Physics Bulletin, 76(1):S80–S87, December 2021. ISSN 1934–8460. doi: 10.3103/S0027134922010167. [DOI] [Google Scholar]
- [40].Feng Yuemeng, Etxebeste Ane, Sarrut David, Létang Jean Michel, and Maxim Voichiţa. 3-D Reconstruction Benchmark of a Compton Camera Against a Parallel-Hole Gamma Camera on Ideal Data. IEEE Transactions on Radiation and Plasma Medical Sciences, 4(4):479–488, July 2020. ISSN 2469–7303. doi: 10/ggw5vc. [Google Scholar]
- [41].Feng Yuemeng, Jean Michel Létang David Sarrut, and Maxim Voichia. Influence of Doppler broadening model accuracy in Compton camera list-mode MLEM reconstruction. Inverse Problems in Science and Engineering, 29(13):3509–3529, December 2021. ISSN 1741–5977, 1741–5985. doi: 10.1080/17415977.2021.2011863. [DOI] [Google Scholar]
- [42].Finocchiaro Domenico, Berenato Salvatore, Bertolini Valentina, Castellani Gastone, Lanconelli Nico, Versari Annibale, Spezi Emiliano, Iori Mauro, Fioroni Federica, and Grassi Elisa. Comparison of different calculation techniques for absorbed dose assessment in patient specific peptide receptor radionuclide therapy. PLoS ONE, 15(8):e0236466, August 2020. ISSN 1932–6203. doi: 10.1371/journal.pone.0236466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].M Fontana Dauvergne D, Létang JM, Ley J-L, and Testa É. Compton camera study for high efficiency SPECT and benchmark with Anger system. Physics in Medicine & Biology, 62(23):8794–8812, November 2017. ISSN 1361–6560. doi: 10/ggjtkf. [DOI] [PubMed] [Google Scholar]
- [44].Fuchs Hermann, Vatnitsky Stanislav, Stock Markus, Georg Dietmar, and Grevillot Loïc. Evaluation of GATE/Geant4 multiple Coulomb scattering algorithms for a 160MeV proton beam. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 410:122–126, November 2017. ISSN 0168–583X. doi: 10.1016/j.nimb.2017.08.006. [DOI] [Google Scholar]
- [45].Fuchs Hermann, Elia Alessio, Resch Andreas F., Kuess Peter, Armin Lühr Marie Vidal, Grevillot Loïc, and Georg Dietmar. Computer-assisted beam modeling for particle therapy. Medical Physics, 48(2):841–851, 2021. ISSN 2473–4209. doi: 10/gh588h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garbacz Magdalena, Francesco Giuseppe Cordoni Marco Durante, Gajewski Jan, Kisielewicz Kamil, Krah Nils, Renata Kopeć Paweł Olko, Patera Vincenzo, Rinaldi Ilaria, Rydygier Marzena, Schiavi Angelo, Scifoni Emanuele, Tomasz Skóra Francesco Tommasino, and Rucinski Antoni. Study of relationship between dose, LET and the risk of brain necrosis after proton therapy for skull base tumors. Radiotherapy and Oncology, 163:143–149, October 2021. ISSN 0167–8140. doi: 10.1016/j.radonc.2021.08.015. [DOI] [PubMed] [Google Scholar]
- [47].Götz Th. I, Lang EW, Schmidkonz C, Kuwert T, and Ludwig B. Dose voxel kernel prediction with neural networks for radiation dose estimation. Zeitschrift für Medizinische Physik, October 2020. ISSN 0939–3889. doi: 10/ghzh94. [DOI] [PubMed] [Google Scholar]
- [48].Götz Th I. , Schmidkonz C, Chen S, Al-Baddai S, Kuwert T, and Lang EW. A deep learning approach to radiation dose estimation. Physics in Medicine and Biology, 65(3):035007, February 2020. ISSN 1361–6560. doi: 10.1088/1361-6560/ab65dc. [DOI] [PubMed] [Google Scholar]
- [49].Granja Carlos, Oancea Cristina, Jakubek Jan, Marek Lukas, Benton Eric, Kodaira Satoshi, Miller Jack, Rucinski Antoni, Gajewski Jan, Stasica Paulina, Zach Vaclav, Stursa Jan, Chvatil David, and Krist Pavel. Wide-range tracking and LET-spectra of energetic light and heavy charged particles. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 988:164901, February 2021. ISSN 0168–9002. doi: 10.1016/j.nima.2020.164901. [DOI] [Google Scholar]
- [50].L Grevillot, Bertrand D, Dessy F, Freud N, and Sarrut D. A Monte Carlo pencil beam scanning model for proton treatment plan simulation using GATE/GEANT4. Physics in Medicine and Biology, 56(16):5203–5219, August 2011. ISSN 0031–9155, 1361–6560. doi: 10/b5nhwv. [DOI] [PubMed] [Google Scholar]
- [51].Grevillot L, Bertrand D, Dessy F, Freud N, and Sarrut D. GATE as a GEANT4-based Monte Carlo platform for the evaluation of proton pencil beam scanning treatment plans. Phys Med Biol, 57(13):4223–4244, July 2012. doi: 10.1088/0031-9155/57/13/4223. [DOI] [PubMed] [Google Scholar]
- [52].Grevillot L, Stock M, and Vatnitsky S. Evaluation of beam delivery and ripple filter design for non-isocentric proton and carbon ion therapy. Physics in Medicine and Biology, 60(20):7985–8005, September 2015. ISSN 0031–9155. doi: 10.1088/0031-9155/60/20/7985. [DOI] [PubMed] [Google Scholar]
- [53].Grevillot L, Boersma DJ, Fuchs H, Aitkenhead A, Elia A, Bolsa M, Winterhalter C, Vidal M, Jan S, Pietrzyk U, Maigne L, and Sarrut D. GATE-RTion: A GATE/Geant4 release for clinical applications in scanned ion beam therapy. Medical Physics, 47(8), 2020. ISSN 2473–4209. doi: 10/ghbjz6. [DOI] [PubMed] [Google Scholar]
- [54].Grevillot L, Boersma DJ, Fuchs H, Bolsa-Ferruz M, Scheuchenpflug L, Georg D, Kronreif G, and Stock M. The GATE-RTion/IDEAL Independent Dose Calculation System for Light Ion Beam Therapy. Frontiers in Physics, 9, 2021. ISSN 2296–424X. doi: 10.3389/fphy.2021.704760. [DOI] [Google Scholar]
- [55].Harris Charles R., Jarrod Millman K, van der Walt Stéfan J. , Gommers Ralf, Virtanen Pauli, Cournapeau David, Wieser Eric, Taylor Julian, Berg Sebastian, Smith Nathaniel J., Kern Robert, Picus Matti, Hoyer Stephan, van Kerkwijk Marten H, Brett Matthew, Haldane Allan, Del Río Jaime Fernández, Wiebe Mark, Peterson Pearu, Gérard-Marchant Pierre, Sheppard Kevin, Reddy Tyler, Weckesser Warren, Abbasi Hameer, Gohlke Christoph, and Oliphant Travis E. Array programming with NumPy. Nature, 585(7825):357–362, September 2020. ISSN 1476–4687. doi: 10/ghbzf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hossein Mohammadi, Reza Shojaei Mohammad, and Jamshid Soltani-Nabipour. Image quality and dose assessment of collimator slit width effect in SLOT-SCAN X-ray imaging system. Applied Radiation and Isotopes, 171:109642, May 2021. ISSN 0969–8043. doi: 10.1016/j.apradiso.2021.109642. [DOI] [PubMed] [Google Scholar]
- [57].Hugo de las Heras Gala, Alberto Torresin, Alexandru Dasu, Osvaldo Rampado, Harry Delis, Hernández Girón Irene Theodorakou Chrysoula, Jonas Andersson, John Holroyd, Mats Nilsson, Sue Edyvean, Vesna Gershan, Lama Hadid-Beurrier, Christopher Hoog, Gregory Delpon, Sancho Kolster Ismael Peterlin Primož, Garayoa Roca Julia Caprile Paola, and Costas Zervides. QUALITY CONTROL IN CONE-BEAM COMPUTED TOMOGRAPHY (CBCT) EFOMP-ESTRO-IAEA PROTOCOL. European Federation of Organisations for Medical Physics, GB, first edition, June 2017. [Google Scholar]
- [58].Incerti S, Baldacchino G, Bernal M, Capra R, Champion C, Francis Z, Guèye P, Mantero A, Mascialino B, Moretto P, Nieminen P, Villagrasa C, and Zacharatou C. The geant4-dna project. International Journal of Modeling, Simulation, and Scientific Computing, 01(02):157–178, June 2010. ISSN 1793–9623. doi: 10.1142/S1793962310000122. [DOI] [Google Scholar]
- [59].Incerti S, Ivanchenko A, Karamitros M, Mantero A, Moretto P, Tran HN, Mascialino B, Champion C, Ivanchenko VN, Bernal MA, Francis Z, Villagrasa C, Baldacchino G, Guèye P, Capra R, Nieminen P, and Zacharatou C. Comparison of GEANT4 very low energy cross section models with experimental data in water. Medical Physics, 37(9):4692–4708, August 2010. ISSN 00942405. doi: 10/d8w37d. [DOI] [PubMed] [Google Scholar]
- [60].Incerti S, Kyriakou I, Bernal MA, Bordage MC, Francis Z, Guatelli S, Ivanchenko V, Karamitros M, Lampe N, Lee SB, Meylan S, Min CH, Shin WG, Nieminen P, Sakata D, Tang N, Villagrasa C, Tran HN, and Brown JMC. Geant4-DNA example applications for track structure simulations in liquid water: A report from the Geant4-DNA Project. Medical Physics, June 2018. ISSN 2473–4209. doi: 10.1002/mp.13048. [DOI] [PubMed] [Google Scholar]
- [61].Jakub Baran, Jan Gajewski, Monika Pawlik-Niedźwiecka, Moskal Paweł, and Ruciński Antonion. Studies of J-PET detector to monitor range uncertainty in proton therapy. In 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), pages 1–4, October 2019. doi: 10.1109/NSS/MIC42101.2019.9059793. [Google Scholar]
- [62].S Jan G, Santin D Strul, S Staelens K, Autret Assié, D, Avner S, R Barbier, Bardiès M, Bloomfield PM, Brasse D, Breton V, Bruyndonckx P, Buvat, Chatziioannou AF, Choi Y, Chung YH, Comtat C, Donnarieix D, Ferrer L, Glick SJ, Groiselle CJ, Guez D, Honore P-F, Kerhoas-Cavata S, Kirov AS, Kohli V, Koole M, Krieguer M, van der Laan DJ, Lamare F, Largeron G, Lartizien C, Lazaro D, Maas MC, Maigne L, Mayet F, Melot F, Merheb C, Pennacchio E, Perez J, Pietrzyk U, Rannou FR, Rey M, Schaart DR, Schmidtlein CR, Simon L, Song TY, Vieira J-M, Visvikis D, Van de Walle R, Wieërs E, and Morel C. GATE: A simulation toolkit for PET and SPECT. Physics in Medicine and Biology, 49 (19):4543–4561, October 2004. ISSN 0031–9155, 1361–6560. doi: 10/dz8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jan S, Benoit D, Becheva E, Carlier T, Cassol F, Descourt P, Frisson T, Grevillot L, Guigues L, Maigne L, Morel C, Perrot Y, Rehfeld N, Sarrut D, Schaart DR, Stute S, Pietrzyk U, Visvikis D, Zahra N, and Buvat I. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys Med Biol, 56(4):881–901, February 2011. doi: 10.1088/0031-9155/56/4/001. [DOI] [PubMed] [Google Scholar]
- [64].Kalaitzidis Philip, Gustafsson Johan, Hindorf Cecilia, and Ljungberg Michael. Validation of a computational chain from PET Monte Carlo simulations to reconstructed images. Heliyon, 8(4):e09316, April 2022. ISSN 2405–8440. doi: 10.1016/j.heliyon.2022.e09316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kalholm Fredrik, Grzanka Leszek, Traneus Erik, and Bassler Niels. A systematic review on the usage of averaged LET in radiation biology for particle therapy. Radiotherapy and Oncology, 161:211–221, August 2021. ISSN 0167–8140. doi: 10.1016/j.radonc.2021.04.007. [DOI] [PubMed] [Google Scholar]
- [66].Han Gyu Kang Swong Hyun Song, Young Been Han Kyeong Min Kim, and Hong Seong Jong. Lens implementation on the GATE Monte Carlo toolkit for optical imaging simulation. Journal of Biomedical Optics, 23(2):026003, February 2018. ISSN 1083–3668, 1560–2281. doi: 10.1117/1.JBO.23.2.026003. [DOI] [PubMed] [Google Scholar]
- [67].Han Gyu Kang Hideaki Tashima, Hong Seong Jong, and Yamaya Taiga. Optimization of a High Resolution Small Animal SPECT System using GATE and STIR Software. In 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), pages 1–3, November 2018. doi: 10/ghbjxm. [Google Scholar]
- [68].Han Gyu Kang Seiichi Yamamoto, Takyu Sodai, Nishikido Fumihiko, Mohammadi Akram, Horita Ryo, Sato Shinji, and Yamaya Taiga. Optical imaging for the characterization of radioactive carbon and oxygen ion beams. Physics in Medicine and Biology, 64(11):115009, May 2019. ISSN 1361–6560. doi: 10.1088/1361-6560/ab1ccf. [DOI] [PubMed] [Google Scholar]
- [69].Kase Yuki, Kanai Tatsuaki, Matsumoto Yoshitaka, Furusawa Yoshiya, Okamoto Hiroyuki, Asaba Toru, Sakama Makoto, and Shinoda Hiroshi. Microdosimetric measurements and estimation of human cell survival for heavy-ion beams. Radiation Research, 166(4):629–638, October 2006. ISSN 0033–7587. doi: 10.1667/RR0536.1. [DOI] [PubMed] [Google Scholar]
- [70].Kawrakow I. The EGSnrc Code System, Monte Carlo Simulation of Electron and photon Transport. NRCC Report PIRS-701, 2001. [Google Scholar]
- [71].Khateri Parisa, Fischer Jannis, Lustermann Werner, Tsoumpas Charalampos, and Dissertori Günther. Implementation of cylindrical PET scanners with block detector geometry in STIR. EJNMMI Physics, 6(1):15, December 2019. ISSN 2197–7364. doi: 10/gf685k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Khellaf Feriel, Krah Nils, Jean Michel Létang Charles-Antoine Collins-Fekete, and Rit Simon. A comparison of direct reconstruction algorithms in proton computed tomography. Physics in Medicine &$\mathsemicolon$ Biology, 65(10):105010, June 2020. ISSN 0031–9155. doi: 10.1088/1361-6560/ab7d53. [DOI] [PubMed] [Google Scholar]
- [73].Kim Dohyeon, Jo Byungdu, Lee Youngjin, Park Su-Jin, Lee Dong-Hoon, and Kim Hee-Joung. Evaluation of effective dose with chest digital tomosynthesis system using Monte Carlo simulation. In Medical Imaging 2015: Physics of Medical Imaging, volume 9412, pages 1319–1324. SPIE, March 2015. doi: 10.1117/12.2081778. [DOI] [Google Scholar]
- [74].Knopf Antje-Christin and Lomax Antony. In Vivo proton range verification: A review. Physics in Medicine and Biology, 58(15):R131–R160, August 2013. ISSN 0031–9155, 1361–6560. doi: 10/ggjtjn. [DOI] [PubMed] [Google Scholar]
- [75].Krah N, Quiñones CT, Létang JM, and Rit S. Scattering proton CT. Physics in Medicine and Biology, 65(22):225015, November 2020. ISSN 1361–6560. doi: 10.1088/1361-6560/abbd18. [DOI] [PubMed] [Google Scholar]
- [76].Lecoq Paul, Morel Christian, Prior John O., Visvikis Dimitris, Gundacker Stefan, Auffray Etiennette, Peter Križan Rosana Martinez Turtos, Thers Dominique, Charbon Edoardo, Varela Joao, de La Taille Christophe, Rivetti Angelo, Breton Dominique, Pratte Jean-François, Nuyts Johan, Surti Suleman, Vandenberghe Stefaan, Marsden Paul, Parodi Katia, Benlloch Jose Maria, and Benoit Mathieu. Roadmap toward the 10 ps time-of-flight PET challenge. Physics in Medicine and Biology, 65(21):21RM01, October 2020. ISSN 0031–9155. doi: 10/ghkfw8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lee D, s Kim Y, Choi S, Lee H, Choi S, Jo BD, Jeon P-H, Kim H, Kim D, Kim H, and Kim H-J. Improvement of material decomposition and image quality in dual-energy radiography by reducing image noise. Journal of Instrumentation, 11(08):T08003–T08003, August 2016. ISSN 1748–0221. doi: 10.1088/1748-0221/11/08/T08003. [DOI] [Google Scholar]
- [78].Min Sun Lee Donghwi Hwang, Kim Joong Hyun, and Lee Jae Sung. Deep-dose: A voxel dose estimation method using deep convolutional neural network for personalized internal dosimetry. Scientific Reports, 9(1):10308, December 2019. ISSN 2045–2322. doi: 10/gf684s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Leste Jeremy, Younes Tony, Chauvin Maxime, Franceries Xavier, Delbaere Alexia, Vieillevigne Laure, Ferrand Regis, Bardies Manuel, and Simon Luc. Technical note: GAMMORA, a free, open-source, and validated GATE-based model for Monte-Carlo simulations of the Varian TrueBeam. Physica Medica, 89:211–218, September 2021. ISSN 1120–1797. doi: 10.1016/j.ejmp.2021.07.037. [DOI] [PubMed] [Google Scholar]
- [80].Livingstone Jayde, Dauvergne Denis, Etxebeste Ane, Fontana Mattia, Marie-Laure Gallin-Martel Brent Huisman, Jean Michel Létang Sara Marcatili, Sarrut David, and Testa Etienne. Influence of sub-nanosecond time of flight resolution for online range verification in proton therapy using the line-cone reconstruction in Compton imaging. Physics in Medicine & Biology, 2021. ISSN 0031–9155. doi: 10.1088/1361-6560/ac03cb. [DOI] [PubMed] [Google Scholar]
- [81].Low Daniel A., Harms William B., Mutic Sasa, and Purdy James A.. A technique for the quantitative evaluation of dose distributions. Medical Physics, 25(5):656–661, May 1998. ISSN 00942405. doi: 10/fvvpnh. [DOI] [PubMed] [Google Scholar]
- [82].Luker Gary D. and Luker Kathryn E.. Optical imaging: Current applications and future directions. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine, 49(1):1–4, January 2008. ISSN 0161–5505. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- [83].Maigne L, Delsol A, Fois G, Debiton E, Degoul F, and Payno H. CPOP: An open source C++ cell POPulation modeler for radiation biology applications. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB), 89:41–50, September 2021. ISSN 1724–191X. doi: 10.1016/j.ejmp.2021.07.016. [DOI] [PubMed] [Google Scholar]
- [84].Makkar Shubhangi, Bogaert Anne-Sophie, Bachtiary Barbara, Zegers Catharina M.L, Roelofs Erik, McNamara Keegan, Hrbacek Jan, Weber Damien C, Lomax Antony, and Winterhalter Carla. Hypoxia based IMPT dose escalation using HX4 PET images in HNSCC patients. In 2022 PTCOG workshop 60, 2022. [Google Scholar]
- [85].Marafini M, Paramatti R, Pinci D, Battistoni G, Collamati F, De Lucia E, Faccini R, Frallicciardi PM, Mancini-Terracciano C, Mattei I, Muraro S, Piersanti L, Rovituso M, Rucinski A, Russomando A, Sarti A, Sciubba A, Solfaroli Camillocci E, Toppi M, Traini G, Voena C, and Patera V. Secondary radiation measurements for particle therapy applications: Nuclear fragmentation produced by 4He ion beams in a PMMA target. Physics in Medicine and Biology, 62(4):1291–1309, January 2017. ISSN 0031–9155. doi: 10.1088/1361-6560/aa5307. [DOI] [PubMed] [Google Scholar]
- [86].Marchant TE and Joshi KD. Comprehensive Monte Carlo study of patient doses from cone-beam CT imaging in radiotherapy. Journal of Radiological Protection, 37(1):13–30, December 2016. ISSN 0952–4746. doi: 10.1088/1361-6498/37/1/13. [DOI] [PubMed] [Google Scholar]
- [87].Marsolat F, De Marzi L, Pouzoulet F, and Mazal A. Analytical linear energy transfer model including secondary particles: Calculations along the central axis of the proton pencil beam. Physics in Medicine and Biology, 61(2):740–757, January 2016. ISSN 1361–6560. doi: 10.1088/0031-9155/61/2/740. [DOI] [PubMed] [Google Scholar]
- [88].Martínez-Rovira I and Prezado Y. Evaluation of the local dose enhancement in the combination of proton therapy and nanoparticles. Medical Physics, 42(11):6703–6710, November 2015. ISSN 2473–4209. doi: 10.1118/1.4934370. [DOI] [PubMed] [Google Scholar]
- [89].Matthew McCormick Xiaoxiao Liu, Ibanez Luis, Jomier Julien, and Marion Charles. ITK: Enabling reproducible research and open science. Frontiers in Neuroinformatics, 8, 2014. ISSN 1662–5196. [Google Scholar]
- [90].McMahon Stephen J. , Paganetti Harald, and Prise Kevin M. LET-weighted doses effectively reduce biological variability in proton radiotherapy planning. Physics in Medicine and Biology, 63(22):225009, November 2018. ISSN 0031–9155. doi: 10.1088/1361-6560/aae8a5. [DOI] [PubMed] [Google Scholar]
- [91].McNamara Aimee L., José Ramos-Méndez Joseph Perl, Held Kathryn, Dominguez Naoki, Moreno Eduardo, Henthorn Nicholas T., Kirkby Karen J., Meylan Sylvain, Villagrasa Carmen, Incerti Sebastien, Faddegon Bruce, Paganetti Harald, and Schuemann Jan. Geometrical structures for radiation biology research as implemented in the TOPAS-nBio toolkit. Physics in Medicine and Biology, 63(17):175018, September 2018. ISSN 0031–9155. doi: 10.1088/1361-6560/aad8eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Keegan McNamara Angelo Schiavi, Borys Damian, Brzezinski Karol, Gajewski Jan, Renata Kopeć Antoni Rucinski, Tomasz Skóra Shubhangi Makkar, Hrbacek Jan, Weber Damien C., Lomax Antony, and Winterhalter Carla. GPU accelerated Monte Carlo scoring of positron emitting isotopes produced during proton therapy for PET verification. Phys Med Biol, 2022. [DOI] [PubMed] [Google Scholar]
- [93].Heide Meißner Hermann Fuchs, Hirtl Albert, Reschl Christian, and Stock Markus. Towards offline PET monitoring of proton therapy at MedAustron. Zeitschrift Fur Medizinische Physik, 29(1):59–65, February 2019. ISSN 1876–4436. doi: 10.1016/j.zemedi.2018.05.003. [DOI] [PubMed] [Google Scholar]
- [94].Merlin Thibaut, Stute Simon, Benoit Didier, Bert Julien, Carlier Thomas, Comtat Claude, Filipovic Marina, Lamare Frédéric, and Visvikis Dimitris. CASToR: A generic data organization and processing code framework for multi-modal and multi-dimensional tomographic reconstruction. Physics in Medicine and Biology, 63(18):185005, September 2018. ISSN 1361–6560. doi: 10/gg2nwz. [DOI] [PubMed] [Google Scholar]
- [95].Miller Cassandra, Rousseau Julie, Ramogida Caterina F., Celler Anna, Rahmim Arman, and Uribe Carlos F.. Implications of physics, chemistry and biology for dosimetry calculations using theranostic pairs. Theranostics, 12(1):232–259, 2022. ISSN 1838–7640. doi: 10.7150/thno.62851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Moskal P, Kisielewska D, Curceanu C, Czerwiński E, Dulski K, Gajos A, Gorgol M, Hiesmayr B, Jasińska B, Kacprzak K, Kaplon L, Korcyl G , Kowalski P, Krzemień W, Kozik T, Kubicz E, Mohammed M, Niedźwiecki Sz, Palka M , Pawlik-Niedźwiecka M, Raczyński, Raj, Sharma S, Shopa Shivani, R. Y., Silarski M, Skurzok M, Stepień E, Wiślicki W, and Zgardzińska B. Feasibility study of the positronium imaging with the J-PET tomograph. Physics in Medicine and Biology, 64(5):055017, March 2019. ISSN 0031–9155. doi: 10/ghbjzz. [DOI] [PubMed] [Google Scholar]
- [97].Moskal P, Kowalski P, Shopa RY, Raczyński L, Baran J, Chug N, Curceanu C, Czerwiński E, Dadgar M, Dulski K, Gajos A, Hiesmayr BC, Kacprzak K, Kapłon Ł, Kisielewska D , Klimaszewski K, Kopka P, Korcyl G, Krawczyk N, Krzemień W, Kubicz E, Niedźwiecki Sz, Parzych Sz, Raj J, Sharma S, Shivani S, Stepień E, Tayefi F, and Wiślicki W. Simulating NEMA characteristics of the modular total-body J-PET scanner-an economic total-body PET from plastic scintillators. Physics in Medicine and Biology, 66(17), September 2021. ISSN 1361–6560. doi: 10.1088/1361-6560/ac16bd. [DOI] [PubMed] [Google Scholar]
- [98].Moskal Paweł and Stepień Ewa Ł. Prospects and Clinical Perspectives of Total-Body PET Imaging Using Plastic Scintillators. PET clinics, 15(4):439–452, October 2020. ISSN 1879–9809. doi: 10/ghdrd4. [DOI] [PubMed] [Google Scholar]
- [99].Moskal Paweł, Bożena Jasińska, Ewa Ł Stepień, and Steven D. Bass. Positronium in medicine and biology. Nature Reviews Physics, 1(9):527–529, September 2019. ISSN 2522–5820. doi: 10/ghdrd8. [Google Scholar]
- [100].Moskal Paweł, Dulski Kamil, Chug Neha, Curceanu Catalina, Czerwiński Eryk, Dadgar Meysam, Gajewski Jan, Gajos Aleksander, Grudzień Grzegorz, Hiesmayr Beatrix C., Kacprzak Krzysztof, Kapłon Łukasz, Karimi Hanieh, Klimaszewski Konrad, Korcyl Grzegorz, Kowalski Paweł, Kozik Tomasz, Krawczyk Nikodem, Krzemień Wojciech, Kubicz Ewelina, Małczak Piotr, Niedźwiecki Szymon, Pawlik-Niedźwiecka Monika, Pedziwiatr Michał, Raczyński Lech, Raj Juhi, Ruciński Antoni, Shivani Sushil Sharma, , Shopa Roman Y, Silarski Michał, Skurzok Magdalena, Stepień Ewa Ł, Szczepanek Monika, Tayefi Faranak, and Wiślicki Wojciech. Positronium imaging with the novel multiphoton PET scanner. Science Advances, 7(42):eabh4394, 2021. doi: 10.1126/sciadv.abh4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Enrique Muñoz John Barrio, Etxebeste Ane, Pablo G Ortega Carlos Lacasta, Josep F Oliver Carles Solaz, and Llosá Gabriela. Performance evaluation of MACACO: A multilayer Compton camera. Physics in Medicine & Biology, 62(18):7321–7341, August 2017. ISSN 1361–6560. doi: 10/gf685p. [DOI] [PubMed] [Google Scholar]
- [102].Enrique Muñoz John Barrio, José Bernabéu Ane Etxebeste, Lacasta Carlos, Gabriela Llosá Ana Ros, Roser Jorge, and Oliver Josep F.. Study and comparison of different sensitivity models for a two-plane Compton camera. Physics in Medicine & Biology, 63(13):135004, June 2018. ISSN 0031–9155. doi: 10/ghj5vt. [DOI] [PubMed] [Google Scholar]
- [103].Najafi Darmian A, Ay MR, Pouladian M, Shirazi A, Ghadiri H, Akbarzadeh A, Ghafarian P, and Zaidi H. Characterization of scattered radiation profile in volumetric 64 slice CT scanner: Monte Carlo study using GATE. In 2011 IEEE Nuclear Science Symposium Conference Record, pages 2692–2696, October 2011. doi: 10.1109/NSSMIC.2011.6152951. [DOI] [Google Scholar]
- [104].Niemierko Andrzej, Schuemann Jan, Niyazi Maximilian, Giantsoudi Drosoula, Maquilan Genevieve, Shih Helen A., and Paganetti Harald. Brain Necrosis in Adult Patients After Proton Therapy: Is There Evidence for Dependency on Linear Energy Transfer? International Journal of Radiation Oncology, Biology, Physics, 109(1):109–119, January 2021. ISSN 0360–3016. doi: 10.1016/j.ijrobp.2020.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ou Haifeng, Zhang Bin, and Zhao Shujun. Monte Carlo simulation of the relative biological effectiveness and DNA damage from a 400MeV/u carbon ion beam in water. Applied Radiation and Isotopes: Including Data, Instrumentation and Methods for Use in Agriculture, Industry and Medicine, 136:1–9, June 2018. ISSN 1872–9800. doi: 10.1016/j.apradiso.2018.01.038. [DOI] [PubMed] [Google Scholar]
- [106].Papadimitroulas Panagiotis, Loudos George, Nikiforidis George C., and Kagadis George C.. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: Comparison with other Monte Carlo codes. Med Phys, 39(8):5238–5247, August 2012. doi: 10.1118/1.4737096. [DOI] [PubMed] [Google Scholar]
- [107].Perl J, Shin J, Schümann J, Faddegon B, and Paganetti H. TOPAS: An innovative proton Monte Carlo platform for research and clinical applications: TOPAS: An innovative proton Monte Carlo platform. Medical Physics, 39(11):6818–6837, October 2012. ISSN 00942405. doi: 10/ggjtfw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pettersen Helge Egil Seime, Volz Lennart, Jarle Rambo Sølie Johan Alme, Gergely Gábor Barnaföldi Rene Barthel, van den Brink Anthony, Borshchov Vyacheslav, Chaar Mamdouh, Eikeland Viljar, Genov Georgi, Ola Grøttvik Håvard Helstrup, Keidel Ralf, Kobdaj Chinorat, van der Kolk Naomi, Mehendale Shruti, Meric Ilker, Odd Harald Odland Gábor Papp, Peitzmann Thomas, Piersimoni Pierluigi, Protsenko Maksym, Attiq Ur Rehman Matthias Richter, Andreas Tefre Samnøy Joao Seco, Shafiee Hesam, Songmoolnak Arnon, Tambave Ganesh, Tymchuk Ihor, Ullaland Kjetil, Monika Varga-Kofarago Boris Wagner, Xiao RenZheng, Yang Shiming, Yokoyama Hiroki, and Röhrich Dieter. Helium radiography with a digital tracking calorimeter—a Monte Carlo study for secondary track rejection. Physics in Medicine &$\mathsemicolon$ Biology, 66(3):035004, January 2021. ISSN 0031–9155. doi: 10.1088/1361-6560/abca03. [DOI] [PubMed] [Google Scholar]
- [109].Pinto Gabriella M., Bonifacio Daniel A. B., de Sá Lidia V, Lima Luis Felipe C. , Vieira Igor F, and Lopes Ricardo T. A cell-based dosimetry model for radium-223 dichloride therapy using bone micro-CT images and GATE simulations. Physics in Medicine and Biology, 65(4):045010, February 2020. ISSN 1361–6560. doi: 10.1088/1361-6560/ab6b42. [DOI] [PubMed] [Google Scholar]
- [110].Pivarski Jim, Elmer Peter, and Lange David. Awkward Arrays in Python, C++, and Numba. EPJ Web of Conferences, 245:05023, 2020. ISSN 2100–014X. doi: 10.1051/epjconf/202024505023. [DOI] [Google Scholar]
- [111].Plachouris Dimitris, Mountris Konstantinos A., Papadimitroulas Panagiotis, Spyridonidis Trifon, Katsanos Konstantinos, Apostolopoulos Dimitris, Papathanasiou Nikolaos, Hazle John D., Visvikis Dimitris, and Kagadis George C.. Clinical Evaluation of a Three-Dimensional Internal Dosimetry Technique for Liver Radioembolization with 90Y Microspheres Using Dose Voxel Kernels. Cancer Biotherapy & Radiopharmaceuticals, 36(10):809–819, December 2021. ISSN 1557–8852. doi: 10.1089/cbr.2020.4554. [DOI] [PubMed] [Google Scholar]
- [112].Poludniowski G, Landry G, DeBlois F, Evans PM, and Verhaegen F. SpekCalc : A program to calculate photon spectra from tungsten anode x-ray tubes. Physics in Medicine and Biology, 54(19):N433–N438, October 2009. ISSN 0031–9155, 1361–6560. doi: 10/czmdt5. [DOI] [PubMed] [Google Scholar]
- [113].Quiñones CT, Létang JM, and Rit S. Filtered back-projection reconstruction for attenuation proton CT along most likely paths. Physics in Medicine and Biology, 61(9):3258–3278, May 2016. ISSN 1361–6560. doi: 10.1088/0031-9155/61/9/3258. [DOI] [PubMed] [Google Scholar]
- [114].Resch Andreas F., Elia Alessio, Fuchs Hermann, Carlino Antonio, Palmans Hugo, Stock Markus, Georg Dietmar, and Grevillot Loïc. Evaluation of electromagnetic and nuclear scattering models in GATE/Geant4 for proton therapy. Medical Physics, 46(5):2444–2456, May 2019. ISSN 0094–2405. doi: 10.1002/mp.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Andreas Franz Resch Mansure Schafasand, Lackner Niklas, Niessen Tom, Beck Staffan, Elia Alessio, Boersma David, Grevillot Loïc, Fossati Piero, Glimelius Lars, Stock Markus, Georg Dietmar, and Carlino Antonio. Impact of beamline-specific particle energy spectra on clinical plans in carbon ion beam therapy. Medical Physics, April 2022. ISSN 2473–4209. doi: 10.1002/mp.15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rice BW, Cable MD, and Nelson MB. In vivo imaging of light-emitting probes. Journal of Biomedical Optics, 6(4):432–440, October 2001. ISSN 1083–3668. doi: 10.1117/1.1413210. [DOI] [PubMed] [Google Scholar]
- [117].Rit S, Vila Oliva M, Brousmiche S, Labarbe R, Sarrut D, and Sharp GC. The Reconstruction Toolkit (RTK), an open-source cone-beam CT reconstruction toolkit based on the Insight Toolkit (ITK). Journal of Physics: Conference Series, 489:012079, March 2014. ISSN 1742–6596. doi: 10/ggjtjg. [Google Scholar]
- [118].Rit Simon, Dedes George, Freud Nicolas, Sarrut David, and Létang Jean Michel. Filtered backprojection proton CT reconstruction along most likely paths. Med Phys, 40(3):031103, March 2013. doi: 10.1118/1.4789589. [DOI] [PubMed] [Google Scholar]
- [119].Ritzer C, Béguin M, Commichau V, Flock J, Fuentes C, Lomax A, Makkar S, McNamara K, Oddo M, Prior J, Weber DC, Winterhalter C, and Dissertori G. The Petition Project - Initial Monte-Carlo Simulations. In 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2021. [Google Scholar]
- [120].Robert Antoine, Rit Simon, Baudier Thomas, Jomier Julien, and Sarrut David. Data-Driven Respiration-Gated SPECT for Liver Radioembolization. IEEE Transactions on Radiation and Plasma Medical Sciences, pages 1–1, 2021. ISSN 2469–7303. doi: 10.1109/TRPMS.2021.3137990. [DOI] [Google Scholar]
- [121].Robert Antoine, Rit Simon, Jomier Julien, and Sarrut David. Data-driven motion compensated SPECT reconstruction for liver radioembolization. In Fully 3D Image Reconstruction in Radiology and Nuclear Medicine, pages 81–84, Leuven, Belgium, July 2021. [Google Scholar]
- [122].Robert C, Dedes G, Battistoni G, Böhlen TT, Buvat I, Cerutti F, Chin MPW , Ferrari, Gueth P, Kurz C, Lestand, Mairani, Montarou, Nicolini, Ortega, Parodi, Prezado, Sala, Sarrut, and Testa. Distributions of secondary particles in proton and carbon-ion therapy: A comparison between GATE/Geant4 and FLUKA Monte Carlo codes. Phys Med Biol, 58(9):2879–2899, May 2013. doi: 10.1088/0031-9155/58/9/2879. [DOI] [PubMed] [Google Scholar]
- [123].Robert Charlotte, Fourrier Nicolas, Sarrut David, Stute Simon, Gueth Pierre, Grevillot Loïc, and Buvat Irène. PET-based dose delivery verification in proton therapy: A GATE based simulation study of five PET system designs in clinical conditions. Physics in Medicine and Biology, 58(19):6867–6885, October 2013. ISSN 0031–9155, 1361–6560. doi: 10/ggjtb4. [DOI] [PubMed] [Google Scholar]
- [124].R Robertson M, Germanos S, Li C, Mitchell GS, Cherry SR, and Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Physics in medicine and biology, 54(16):N355–N365, August 2009. ISSN 0031–9155. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Roncali Emilie, Taebi Amirtahà, Foster Cameron, and Catherine Tram Vu. Personalized Dosimetry for Liver Cancer Y-90 Radioembolization Using Computational Fluid Dynamics and Monte Carlo Simulation. Annals of Biomedical Engineering, 48(5):1499–1510, May 2020. ISSN 1573–9686. doi: 10/ghn45r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rouchota Maritina, Loudos George, and Kagadis George C.. An in-silico method to predict and quantify the effect of gold nanoparticles in X-ray imaging. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB), 89:160–168, September 2021. ISSN 1724–191X. doi: 10.1016/j.ejmp.2021.07.033. [DOI] [PubMed] [Google Scholar]
- [127].Rucinski Antoni, Baran Jakub, Garbacz Magdalena, Pawlik-Niedzwiecka Monika, and Moskal Pawel. Plastic scintillator based PET detector technique for proton therapy range monitoring: A Monte Carlo study. In 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), pages 1–4, November 2018. doi: 10.1109/NSSMIC.2018.8824654. [Google Scholar]
- [128].Saini Jatinder, Maes Dominic, Egan Alexander, Bowen Stephen R., Sara St James Martin Janson, Wong Tony, and Bloch Charles. Dosimetric evaluation of a commercial proton spot scanning Monte-Carlo dose algorithm: Comparisons against measurements and simulations. Physics in Medicine and Biology, 62(19):7659–7681, September 2017. ISSN 1361–6560. doi: 10.1088/1361-6560/aa82a5. [DOI] [PubMed] [Google Scholar]
- [129].Saporta Albert, Etxebeste A, Krah Nils, Létang Jean Michel, and Sarrut David . Conditional GAN for Monte Carlo SPECT simulation. In International Conference on Monte Carlo Techniques for Medical Applications (MCMA 2022), Antwerpen, Belgium, April 2022. [Google Scholar]
- [130].Sarrut D, Krah N, and Létang JM. Generative adversarial networks (GAN) for compact beam source modelling in Monte Carlo simulations. Physics in Medicine and Biology, 64(21):215004, October 2019. ISSN 0031–9155. doi: 10/gf82xv. [DOI] [PubMed] [Google Scholar]
- [131].Sarrut David, Manuel Bardiès Nicolas Boussion, Freud Nicolas, Jan Sébastien, Jean-Michel Létang George Loudos, Maigne Lydia, Marcatili Sara, Mauxion Thibault, et al. A review of the use and potential of the GATE Monte Carlo simulation code for radiation therapy and dosimetry applications. Medical physics, 41(6Part1):064301, June 2014. doi: 10.1118/1.4871617. [DOI] [PubMed] [Google Scholar]
- [132].Sarrut David, Bala Mateusz, Manuel Bardiès Julien Bert, Chauvin Maxime, Chatzipapas Konstantinos, Dupont Mathieu, Etxebeste Ane, Fanchon Louise M., Jan Sébastien, Kayal Gunjan, Kirov Assen S., Kowalski Pawel, Krzemien Wojciech, Labour Joey, Lenz Mirjam, Loudos George, Mehadji Brahim, Laurent Ménard Christian Morel, Papadimitroulas Panagiotis, Rafecas Magdalena, Salvadori Julien, Seiter Daniel, Stockhoff Mariele, Testa Etienne, Trigila Carlotta, Pietrzyk Uwe, Vandenberghe Stefaan, Verdier Marc-Antoine, Visvikis Dimitris, Ziemons Karl, Zvolský Milan, and Roncali Emilie. Advanced Monte Carlo simulations of emission tomography imaging systems with GATE. Physics in Medicine and Biology, 66 (10):10TR03, May 2021. ISSN 0031–9155. doi: 10/gj6rkh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sarrut David, Etxebeste Ane, Krah Nils, and Létang Jean-Michel. Modeling complex particles phase space with GAN for Monte Carlo SPECT simulations: A proof of concept. Physics in Medicine and Biology, 2021. ISSN 0031–9155. doi: 10.1088/1361-6560/abde9a. [DOI] [PubMed] [Google Scholar]
- [134].Ross Schmidtlein C, Kirov Assen S. , Nehmeh Sadek A, Erdi Yusuf E, Humm John L, Amols Howard I, Bidaut Luc M, Ganin Alex, Stearns Charles W, McDaniel David L, and Hamacher Klaus A. Validation of GATE Monte Carlo simulations of the GE Advance/Discovery LS PET scanners. Medical Physics, 33(1):198–208, January 2006. ISSN 0094–2405. doi: 10/cgngq8. [DOI] [PubMed] [Google Scholar]
- [135].Shibuya Kengo, Saito Haruo, Nishikido Fumihiko, Takahashi Miwako, and Yamaya Taiga. Oxygen sensing ability of positronium atom for tumor hypoxia imaging. Communications Physics, 3(1):1–8, October 2020. ISSN 2399–3650. doi: 10/ghf3zq. [Google Scholar]
- [136].Smith Edward A. K., Winterhalter Carla, Underwood Tracy S. A., Aitkenhead Adam H., Richardson Jenny C., Merchant Michael J., Kirkby Norman F., Kirby Karen J., and Mackay Ranald I.. A Monte Carlo study of different LET definitions and calculation parameters for proton beam therapy. Biomedical Physics & Engineering Express, 8(1), December 2021. ISSN 2057–1976. doi: 10.1088/2057-1976/ac3f50. [DOI] [PubMed] [Google Scholar]
- [137].Jarle Rambo Sølie Lennart Volz, Pettersen Helge Egil Seime, Piersimoni Pierluigi, Odland Odd Harald, Röhrich Dieter, Helstrup Håvard, Peitzmann Thomas, Ullaland Kjetil, Varga-Kofarago Monika, Mehendale Shruti, Grøttvik Ola Slettevoll, Eikeland Viljar Nilsen, Meric Ilker, and Seco Joao. Image quality of list-mode proton imaging without front trackers. Phys Med Biol, 65(13):135012, July 2020. ISSN 0031–9155. doi: 10.1088/1361-6560/ab8ddb. [DOI] [PubMed] [Google Scholar]
- [138].Tamborino Giulia, Nonnekens Julie, Marijke De Saint-Hubert Lara Struelens, Feijtel Danny, de Jong Marion, and Konijnenberg Mark W.. Dosimetric Evaluation of the Effect of Receptor Heterogeneity on the Therapeutic Efficacy of Peptide Receptor Radionuclide Therapy: Correlation with DNA Damage Induction and In Vivo Survival. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine, 63(1):100–107, January 2022. ISSN 1535–5667. doi: 10.2967/jnumed.121.262122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Tang Wei, Tang Bo, Li Xiang, Wang Yidi, Li Zhanpeng, Gao Yunan, Gao Han, Yan Congchong, and Sun Liang. Cellular S-value evaluation based on real human cell models using the GATE MC package. Applied Radiation and Isotopes: Including Data, Instrumentation and Methods for Use in Agriculture, Industry and Medicine, 168:109509, February 2021. ISSN 1872–9800. doi: 10.1016/j.apradiso.2020.109509. [DOI] [PubMed] [Google Scholar]
- [140].Tanha Kaveh, Pashazadeh Ali Mahmoud, and Pogue Brian W. Review of biomedical Čerenkov luminescence imaging applications. Biomedical Optics Express, 6(8):3053–3065, August 2015. ISSN 2156–7085. doi: 10.1364/BOE.6.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Thielemans Kris, Tsoumpas Charalampos, Mustafovic Sanida, Beisel Tobias, Aguiar Pablo, Dikaios Nikolaos, and Jacobson Matthew W.. STIR: Software for tomographic image reconstruction release 2. Physics in Medicine and Biology, 57(4):867–883, January 2012. ISSN 0031–9155. doi: 10/fx689h. [DOI] [PubMed] [Google Scholar]
- [142].Hoang Ngoc Tran Flore Chappuis, Incerti Sébastien, Bochud Francois, and Desorgher Laurent. Geant4-DNA Modeling of Water Radiolysis beyond the Microsecond: An On-Lattice Stochastic Approach. International Journal of Molecular Sciences, 22(11):6023, June 2021. ISSN 1422–0067. doi: 10.3390/ijms22116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tranel Jonathan, Feng Felix Y., Sara St James, and Thomas A. Hope. Effect of microdistribution of alpha and beta-emitters in targeted radionuclide therapies on delivered absorbed dose in a GATE model of bone marrow. Physics in Medicine and Biology, 66(3):035016, January 2021. ISSN 0031–9155. doi: 10/gh7zmc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Unkelbach Jan and Paganetti Harald. Robust Proton Treatment Planning: Physical and Biological Optimization. Seminars in Radiation Oncology, 28(2):88–96, April 2018. ISSN 1532–9461. doi: 10.1016/j.semradonc.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].van Herk Marcel. Different styles of image-guided radiotherapy. Seminars in Radiation Oncology, 17(4):258–267, October 2007. ISSN 1053–4296. doi: 10.1016/j.semradonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [146].Vandenberghe Stefaan, Moskal Pawel, and Karp Joel S.. State of the art in total body PET. EJNMMI Physics, 7(1):35, May 2020. ISSN 2197–7364. doi: 10/ghbjxj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Viswanath Varsha, Daube-Witherspoon Margaret E, Werner Matthew E, Surti Suleman, Trindade Andreia, Rodrigues Pedro, Pantel Austin R., Perkins Amy E, and Karp Joel S. GATE simulations to study extended axial FOVs for the PennPET Explorer scanner. In 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), pages 1–5, October 2017. doi: 10.1109/NSSMIC.2017.8532747. [Google Scholar]
- [148].Vlastou Elena, Pantelis Evaggelos, Efstathopoulos Efstathios P., Karaiskos Pantelis, Kouloulias Vasileios, and Platoni Kalliopi. Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy. Cancers, 14(9):2167, April 2022. ISSN 2072–6694. doi: 10.3390/cancers14092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang Hui, Nie Ke, Chang Jacqueline, and Kuang Yu. A Monte Carlo study to investigate the feasibility of an on-board SPECT/spectral-CT/CBCT imager for medical linear accelerator. Medical Physics, 47(10):5112–5122, October 2020. ISSN 2473–4209. doi: 10.1002/mp.14398. [DOI] [PubMed] [Google Scholar]
- [150].Wang Lihong, Jacques Steven L., and Zheng Liqiong. MCML—Monte Carlo modeling of light transport in multi-layered tissues. Computer Methods and Programs in Biomedicine, 47(2):131–146, July 1995. ISSN 0169–2607. doi: 10.1016/0169-2607(95)01640-F. [DOI] [PubMed] [Google Scholar]
- [151].Werner Christopher J.. MCNP users manual-code version 6.2. Los Alamos national laboratory, 2017. [Google Scholar]
- [152].Winterhalter Carla, Taylor Michael, Boersma David, Elia Alessio, Guatelli Susanna, Mackay Ranald, Kirkby Karen, Maigne Lydia, Ivanchenko Vladimir, Resch Andreas F., Sarrut David, Sitch Peter, Vidal Marie, Grevillot Loïc, and Aitkenhead Adam. Evaluation of GATE-RTion (GATE/Geant4) Monte Carlo simulation settings for proton pencil beam scanning quality assurance. Medical Physics, 47 (11):5817–5828, November 2020. ISSN 2473–4209. doi: 10.1002/mp.14481. [DOI] [PubMed] [Google Scholar]
- [153].Zhao Wei, Vernekohl Don, Zhu Jun, Wang Luyao, and Xing Lei. A model-based scatter artifacts correction for cone beam CT. Medical Physics, 43(4):1736, April 2016. ISSN 2473–4209. doi: 10.1118/1.4943796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Christoph Zöllner Simon Rit, Kurz Christopher, Gloria Vilches-Freixas Florian Kamp, Dedes George, Belka Claus, Parodi Katia, and Landry Guillaume. Decomposing a prior-CT-based cone-beam CT projection correction algorithm into scatter and beam hardening components. Physics and Imaging in Radiation Oncology, 3:49–52, July 2017. ISSN 24056316. doi: 10/gf684f. [Google Scholar]


