Abstract
Overall effectiveness of infection in preventing reinfection with SARS-CoV-2 JN.1 variant was estimated at 1.8% (95% CI: −9.3to 12.6%), and demonstrated rapid decline over time since the previous infection, decreasing from 82.4% (95% CI: 40.9 to 94.7%) within 3 to less than 6 months, to a negligible level after one year.
Keywords: COVID-19, reinfection, case-control, test-negative, immunity, epidemiology
Evidence at the level of neutralizing antibodies suggests that the SARS-CoV-2 JN.1 variant demonstrates increased immune evasion compared to its parent lineage BA.2.86 and to recently circulating variants, such as XBB.1.5 and EG.5.1.1 JN.1 has also exhibited a growth advantage over other variants and triggered large SARS-CoV-2 waves in various countries,2 prompting the World Health Organization to classify it as a variant of interest on 19 December 2023.2 We estimated the effectiveness of natural infection in preventing reinfection with JN.1 during a large JN.1 wave in Qatar using the test-negative case-control study design.3,4
Qatar's national COVID-19 databases were analysed between 4 December 2023, when JN.1 dominated incidence (Fig. S1 of the Supplementary Appendix), and 12 February 2024. These databases encompass all laboratory and medically supervised SARS-CoV-2 testing, infection clinical outcomes, COVID-19 vaccination and demographic details within the country (Sections S1-S2).
Cases (SARS-CoV-2-positive tests) and controls (SARS-CoV-2-negative tests) were matched exactly one-to-two by factors that could influence the risk of infection, including sex, 10-year age group, nationality, number of coexisting conditions, number of vaccine doses, calendar week of the SARS-CoV-2 test, method of testing (polymerase chain reaction versus rapid antigen) and reason for testing (Section S3). Previous infection was defined as a SARS-CoV-2-positive test ≥90 days before the study test. Subgroup analyses estimating effectiveness against specifically symptomatic reinfection, and by vaccination status, were conducted.
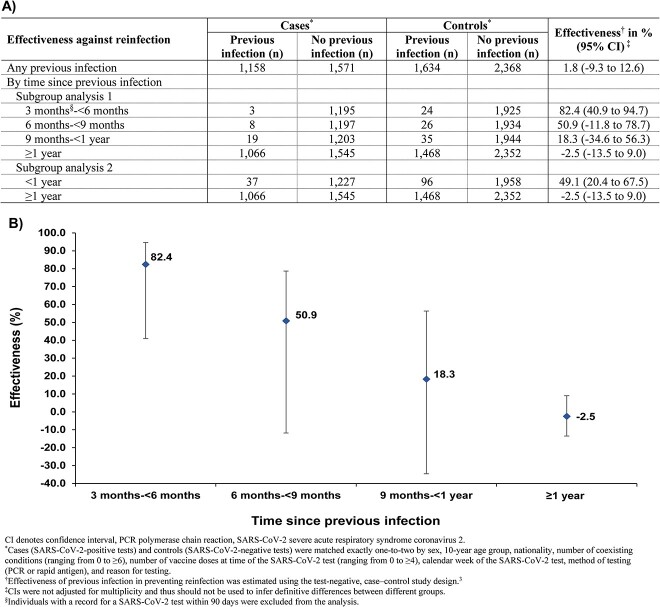
Figure S2 and Table S1, respectively, show the study population selection process and characteristics. The overall effectiveness of previous infection in preventing reinfection with JN.1, regardless of symptoms, was estimated at 1.8% (95% CI: −9.3 to 12.6%) (Fig. 1). This effectiveness demonstrated a rapid decline over time since the previous infection, decreasing from 82.4% (95% CI: 40.9 to 94.7%) within 3 to less than 6 months after the previous infection to 50.9% (95% CI: −11.8 to 78.7%) in the subsequent 3 months, and further dropping to 18.3% (95% CI: −34.6 to 56.3) in the subsequent 3 months. Ultimately, it reached a negligible level after one year. The effectiveness was estimated at 49.1% (95% CI: 20.4 to 67.5%) during the first year and at −2.5% (95% CI: −13.5 to 9.0%) thereafter.
Figure 1.
Protection against reinfection with JN.1, irrespective of symptoms, overall (A) and by time since previous infection (A and B)
The effectiveness against symptomatic reinfection with JN.1 demonstrated a similar pattern to that observed for any reinfection (Table S2). The overall effectiveness against symptomatic reinfection was −2.3% (95% CI: −14.4 to 10.3%). Subgroup analyses for unvaccinated and vaccinated individuals yielded results similar to those of the main analysis (Table S2). Limitations are discussed in Section S3. The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Table S3).
The protection of natural infection against reinfection was strong among those who were infected within the last 6 months, with variants such as XBB*. However, this protection waned rapidly and was entirely lost one year after the previous infection. These findings support a considerable immune evasion by JN.1, and that this immune evasion led to the observed rapid waning of the protection against JN.1 (Fig. 1), a pattern for the effect of immune evasion first characterized for SARS-CoV-2 following the omicron variant emergence at the end of 2021.4,5
Supplementary Material
Contributor Information
Hiam Chemaitelly, Weill Cornell Medicine-Qatar, Cornell University, Doha, Qatar.
Peter Coyle, Hamad Medical Corporation, Doha, Qatar.
Mohamed Ali Ben Kacem, Hamad Medical Corporation, Doha, Qatar.
Houssein H Ayoub, Department of Mathematics and Statistics and Department of Biomedical Science and Department of Public Health, Qatar University, Doha, Qatar.
Patrick Tang, Department of Pathology, Sidra Medicine, Doha, Qatar.
Mohammad R Hasan, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Canada.
Hadi M Yassine, Department of Mathematics and Statistics and Department of Biomedical Science and Department of Public Health, Qatar University, Doha, Qatar.
Asmaa A Al Thani, Department of Mathematics and Statistics and Department of Biomedical Science and Department of Public Health, Qatar University, Doha, Qatar.
Zaina Al-Kanaani, Hamad Medical Corporation, Doha, Qatar.
Einas Al-Kuwari, Hamad Medical Corporation, Doha, Qatar.
Andrew Jeremijenko, Hamad Medical Corporation, Doha, Qatar.
Anvar H Kaleeckal, Hamad Medical Corporation, Doha, Qatar.
Ali N Latif, Hamad Medical Corporation, Doha, Qatar.
Riyazuddin M Shaik, Hamad Medical Corporation, Doha, Qatar.
Hanan F Abdul-Rahim, Department of Mathematics and Statistics and Department of Biomedical Science and Department of Public Health, Qatar University, Doha, Qatar.
Gheyath K Nasrallah, Department of Mathematics and Statistics and Department of Biomedical Science and Department of Public Health, Qatar University, Doha, Qatar.
Mohamed Ghaith Al-Kuwari, Primary Health Care Corporation, Doha, Qatar.
Adeel A Butt, Hamad Medical Corporation, Doha, Qatar.
Hamad E Al-Romaihi, Ministry of Public Health, Doha, Qatar.
Mohamed H Al-Thani, Ministry of Public Health, Doha, Qatar.
Abdullatif Al-Khal, Hamad Medical Corporation, Doha, Qatar.
Roberto Bertollini, Ministry of Public Health, Doha, Qatar.
Laith J Abu-Raddad, Weill Cornell Medicine-Qatar, Cornell University, Doha, Qatar.
Funding
The authors are grateful for support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar, the Qatar Ministry of Public Health, Hamad Medical Corporation and Sidra Medicine. The authors are also grateful for the Qatar Genome Programme and Qatar University Biomedical Research Center for institutional support for the reagents needed for the viral genome sequencing.
Author contributions
Hiam Chemaitelly (Data curation [Equal], Formal analysis [Lead], Methodology [Equal], Validation [Equal], Writing—original draft [Equal], Writing—review & editing [Equal]), Peter Coyle (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Mohamed Ali Kacem (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Houssein Ayoub (Data curation [Equal], Writing—review & editing [Equal]), Patrick Tang (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Mohammad Hasan (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Hadi Yassine (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Asmaa Althani (Data curation [Equal], Investigation [Equal], Writing—review & editing [Equal]), Zaina Al Kanaani (Data curation [Equal], Writing—review & editing [Equal]), Einas Al Kuwari (Data curation [Equal], Writing—review & editing [Equal]), Andrew Jeremijenko (Data curation [Equal], Writing—review & editing [Equal]), Anvar Hassan Kaleeckal (Data curation [Equal], Writing—review & editing [Equal]), Ali Nizar Latif (Data curation [Equal], Writing—review & editing [Equal]), Riyazuddin Shaik (Data curation [Equal], Writing—review & editing [Equal]), Hanan Abdul Rahim (Data curation [Equal], Writing—review & editing [Equal]), Gheyath Nasrallah (Data curation [Equal], Writing—review & editing [Equal]), Mohamed Ghaith Al Kuwari (Data curation [Equal], Writing—review & editing [Equal]), Adeel Butt (Data curation [Equal], Writing—review & editing [Equal]), Hamad Al-Romaihi (Data curation [Equal], Writing—review & editing [Equal]), Mohammed H. Al Thani (Data curation [Equal], Writing—review & editing [Equal]), Abdul Latif Al-Khal (Data curation [Equal], Writing—review & editing [Equal]), Roberto Bertollini (Data curation [Equal], Writing—review & editing [Equal]), Laith Abu-Raddad (Conceptualization [Lead], Data curation [Equal], Formal analysis [Equal], Funding acquisition [Lead], Project administration [Lead], Supervision [Lead], Validation [Equal], Writing—original draft [Equal], Writing—review & editing [Equal]).
Data availability
The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Her Excellency the Minister of Public Health (https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-HealthCommunication-Center.aspx). The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the paper and its supplementary information.
References
- 1. Yang S, Yu Y, Xu Yet al. . Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect Dis 2024; 24:e70–2. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Excutive Summary Initial Risk Evaluation of JN.1. 2023. https://www.who.int/docs/default-source/coronaviruse/18122023_jn.1_ire_clean.pdf?sfvrsn=6103754a_3 (31 January 2024, date last accessed.
- 3. Ayoub HH, Tomy M, Chemaitelly Het al. . Estimating protection afforded by prior infection in preventing reinfection: applying the test-negative study design. Am J Epidemiol 2023;kwad239. 10.1093/aje/kwad239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chemaitelly H, Tang P, Coyle Pet al. . Protection against reinfection with the omicron BA.2.75 subvariant. N Engl J Med 2023; 388:665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chemaitelly H, Nagelkerke N, Ayoub HHet al. . Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J Travel Med 2022; 29:taac109. 10.1093/jtm/taac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Her Excellency the Minister of Public Health (https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-HealthCommunication-Center.aspx). The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the paper and its supplementary information.