Abstract
Background
Dengue is a significant mosquito-borne disease. Several studies have utilized estimates from the Global Burden of Disease (GBD) study to assess the global, regional or national burden of dengue over time. However, our recent investigation suggests that GBD’s estimates for dengue cases in Taiwan are unrealistically high. The current study extends the scope to compare reported dengue cases with GBD estimates across 30 high-burden countries and territories, aiming to assess the accuracy and interpretability of the GBD’s dengue estimates.
Methods
Data for this study were sourced from the GBD 2019 study and various national and international databases documenting reported dengue cases. The analysis targeted the top 30 countries and territories with the highest 10-year average of reported cases from 2010 to 2019. Discrepancies were quantified by computing absolute differences and ratios between the 10-year average of reported cases and GBD estimates. Coefficients of variation (CV) and estimated annual percentage changes (EAPCs) were calculated to assess variations and trends in the two data sources.
Results
Significant discrepancies were noted between reported data and GBD estimates in the number of dengue cases, incidence rates, and EAPCs. GBD estimates were substantially higher than reported cases for many entities, with the most notable differences found in China (570.0-fold), India (303.0-fold), Bangladesh (115.4-fold), Taiwan (85.5-fold) and Indonesia (23.2-fold). Furthermore, the GBD’s estimates did not accurately reflect the extensive yearly fluctuations in dengue outbreaks, particularly in non-endemic regions such as Taiwan, China and Argentina, as evidenced by high CVs.
Conclusions
This study reveals substantial discrepancies between GBD estimates and reported dengue cases, underscoring the imperative for comprehensive analysis in areas with pronounced disparities. The failure of GBD estimates to represent the considerable annual fluctuations in dengue outbreaks highlights the critical need for improvement in disease burden estimation methodologies for dengue.
Keywords: Dengue, dengue virus, global burden of disease study, reported data, incidence
Introduction
Dengue is a significant mosquito-borne disease caused by the dengue virus (DENV). Infections resulting from DENV display a broad spectrum of clinical manifestations, ranging from asymptomatic conditions and mild flu-like symptoms to severe dengue.1 Over the past two decades, the number of dengue cases reported to the World Health Organization (WHO) has surged dramatically, from 505 430 in 2000 to 5.2 million in 2019.2 Moreover, the geographical distribution of DENV has expanded into regions previously unaffected, likely attributable to a confluence of factors, including global climate change, the increase in international travel and rapid urbanization.3,4 It is estimated that annually 390 million DENV infections occur globally, resulting in ~96 million symptomatic cases and 40 000 deaths.3,5
Evaluating disease burden is crucial for informing health policy and strategic planning on national and international levels. Despite dengue being a notifiable disease in many regions, the reported incidence significantly underestimates its actual burden. This discrepancy primarily arises due to dengue’s symptom overlap with other febrile illnesses, combined with the tendency of patients with milder symptoms to forego seeking healthcare, leading to under-recognition and under-reporting of symptomatic dengue.6 Initiated in 1991, the Global Burden of Disease (GBD) Study is a comprehensive effort to quantify burdens from communicable and non-communicable diseases, injuries, and various risk factors, providing regularly updated burden estimates.7 The significance of the GBD’s estimates in both academic research and policy formulation to assess temporal changes and inter-country progress has grown over time. However, there have been growing debates regarding the credibility of its disease-specific estimates.8,9 A number of studies have employed GBD estimates to assess the global, regional, or national burden of dengue over time.6,10–15 Recently, we compared the GBD’s annual estimates of dengue cases in Taiwan with the figures reported by the Taiwan Centers for Disease Control (Taiwan CDC). Our findings suggest that the GBD’s estimates were implausibly high and failed to capture the substantial annual fluctuations in Taiwan’s dengue epidemics.16 The aim of this study was to compare the annual number of reported dengue cases between 2010 and 2019 with GBD estimates in countries and territories mostly affected by dengue, thereby offering insights into the accuracy and interpretation of the GBD’s estimates of the dengue burden.
Methods
Data source
Estimates of annual dengue cases, along with their 95% uncertainty intervals, from the GBD 2019 study were acquired from the Global Health Data Exchange, which is coordinated by the Institute for Health Metrics and Evaluation at the University of Washington (https://vizhub.healthdata.org/gbd-results/). The methodologies used for estimating dengue incidence by the GBD study have been described in prior literature.17 Briefly, the incidence models incorporated reported cases from various sources, including scholarly articles, official reports and data from organizations such as the WHO and the Pan American Health Organization. To adjust for under-reporting, a comprehensive literature review was conducted to compare incidence rates from active and passive surveillance. Subsequently, a meta-regression-Bayesian approach was implemented, incorporating the socio-demographic index (SDI) and reported incidence rates. After adjusting for under-reporting, a hybrid approach was employed to produce estimates of incidence using a space–time Gaussian process regression and a negative binomial regression using fixed effects to model overall incidence.10,17 The latest GBD 2019 study provides estimates of annual dengue case numbers between 1990 and 2019 for a total of 204 countries and territories.
Separate from the GBD estimates, the annual reported numbers of dengue cases for various countries and territories were sourced from two main online databases. The first source, the PLISA Health Information Platform for the Americas, offers data from 1980 to 2023 for 51 countries and territories.18 Second, the WHO Dengue Explore provides annual reported case counts from 123 countries and territories but only covers the years from 1990 to 2014.19 To fill in data gaps for the period from 2015 to 2019, additional sources were consulted, including official governmental websites, academic publications, news reports, the WHO’s Dengue Situation Updates, and reports from the European Centre for Disease Prevention and Control. Data specific to Taiwan were sourced directly from the Taiwan CDC.20 Upon examination of the overlap between the two data sources, dengue case estimates are available for 101 countries and territories from both the GBD 2019 study and the reported data. Consequently, our analysis focused on these 101 entities, enabling a direct comparison between the two data sources.
Given that all data utilized in this study were sourced from publicly available repositories and did not contain any personal information, an exemption from full review was granted by the Institutional Review Board of National Cheng Kung University Hospital (reference number: A-EX-112-037).
Statistical analysis
In this study, an initial calculation was performed to determine the 10-year average number of reported cases from 2010 to 2019 for the 101 countries and territories. Our analysis specifically focused on the top 30 countries and territories with the highest average annual counts of reported dengue cases. This selection criterion was implemented to concentrate on the regions with the most significant disease burden and presumably more effective surveillance systems. Correspondingly, the 10-year average annual case numbers as estimated by the GBD study for these selected 30 locations were also calculated. Additionally, the coefficients of variation (CV) were determined to assess the variability in the two data sources. To quantify the discrepancies, the absolute differences and ratios between the 10-year average reported cases and the GBD estimates were computed. Furthermore, the estimated annual percentage change (EAPC) from 2010 to 2019 was determined by fitting a regression line to the natural logarithm of the annual case numbers, represented as ln(y) = α + βx + ε, where ‘y’ denotes the annual case number and ‘x’ represents the calendar year. The EAPC was then calculated using the formula 100 ×(eβ—1).21 Ultimately, the countries were ranked based on their 10-year average numbers, differences, ratios, and EAPCs as derived from the two data sources.
In addition to annual case numbers, we extended our analysis to compute the crude incidence rates by dividing the case numbers by the total population for each country or territory, using the population estimates provided by the United Nations Population Division.22 Age-standardized rates could not be calculated because the age of reported dengue cases was not available.
Results
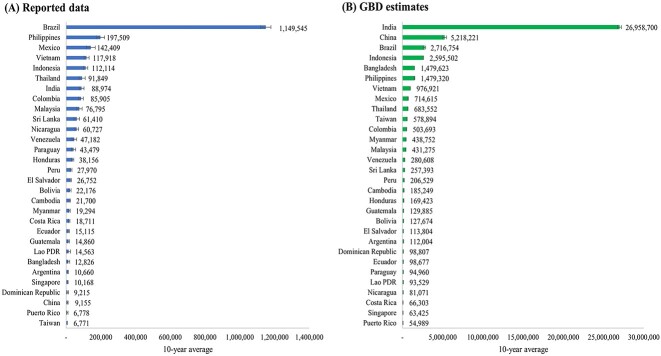
The top 30 countries and territories with the highest 10-year average annual counts of reported dengue cases, as selected for our study, are detailed in Supplementary Table S1 available as Supplementary data at JTM online. This table presents the annual counts of reported dengue cases for each country and territory from 2010 to 2019. It also includes references indicating the sources of these reported data, along with the GBD estimates. Supplementary Table S2 available as Supplementary data at JTM online showcases the 10-year averages of dengue cases, the CVs, and the estimated EAPCs for both reported data and GBD estimates during the corresponding period. The 10-year average of reported dengue cases was highest in Brazil (1 149 545), followed by the Philippines (197 509), Mexico (142 409), Vietnam (117 918) and Indonesia (112 114), as displayed in Supplementary Table S2available as Supplementary data at JTM online and Figure 1. Conversely, the GBD 2019 estimates indicated that the 10-year average of dengue cases was highest in India (26 958 700), followed by China (5 218 221), Brazil (2 716 754), Indonesia (2 595 502) and Bangladesh (1 479 623).
Figure 1.
Ten-year averages of annual dengue case number for 30 selected countries and territories according to (A) reported data (B) GBD estimates (2010–2019).
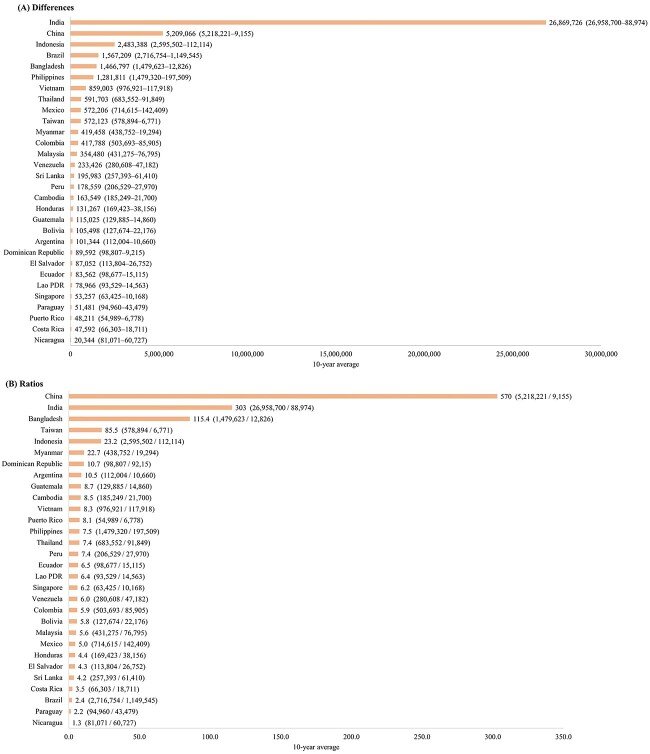
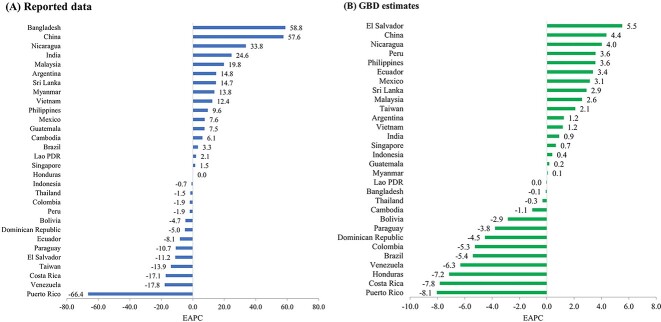
The discrepancies between the case numbers estimated by the GBD study and the actual reported cases are depicted in Figure 2. The most substantial differences were observed in India (26 869 726), China (5 209 066), Indonesia (2 483 388), Brazil (1567 209) and Bangladesh (1 466 797). The ‘GBD-to-reported case ratio’ is calculated by dividing the GBD estimates by the reported case numbers. The examination of these ratios revealed the largest disparities in China (570.0-fold), India (303.0-fold), Bangladesh (115.4-fold), Taiwan (85.5-fold) and Indonesia (23.2-fold). On the other hand, the lowest ratios were identified in Nicaragua (1.3-fold), Paraguay (2.2-fold), Brazil (2.4-fold), Costa Rica (3.5-fold) and Sri Lanka (4.2-fold). In terms of the EAPCs, the highest figures based on reported data were noted in Bangladesh (58.8), China (57.6), Nicaragua (33.8), India (24.6) and Malaysia (19.8). Conversely, when analysing the GBD estimates, the largest EAPCs were recorded in El Salvador (5.5), China (4.4), Nicaragua (4.0), Peru (3.6) and the Philippines (3.6) (Figure 3).
Figure 2.
Discrepancies in 10-year average case numbers between reported data and GBD estimates in 30 selected countries and territories (2010–2019): (A) absolute differences and (B) GBD-to-reported case ratios.
Figure 3.
The Estimated Annual Percent Change (EAPC) of annual dengue cases (2010–2019) in 30 selected countries or territories according to (A) reported data and (B) GBD estimates.
Furthermore, the CVs for reported dengue case numbers, ranging from 0.41 to 2.44, were considerably higher than those for GBD estimates, which spanned from 0.01 to 0.31 (Supplementary Table S2 available as Supplementary data at JTM online). This indicates that the yearly variation in reported data was much greater than that in the GBD estimates. Countries and territories such as Argentina, Bangladesh, China, Honduras, Laos, Puerto Rico and Taiwan exhibited very high yearly variation in reported dengue case numbers, as evidenced by CVs > 1. The annual counts of reported dengue cases in the 30 countries and territories compared with corresponding GBD estimates are shown in Supplementary Figure S1 available as Supplementary data at JTM online.
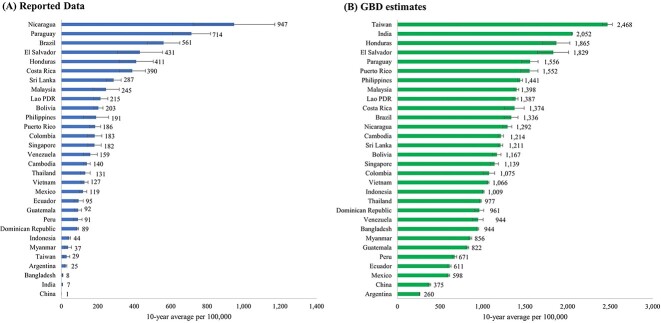
Shifting focus from the average annual case numbers, the following section delves into the average incidence rates. Supplementary Table S3, available as Supplementary data at JTM online, presents the 10-year average incidence rates of dengue cases, along with the CVs and EAPCs, based on both reported data and GBD estimates for the period 2010–2019. The countries with the highest 10-year average incidence rates per 100 000 based on reported data were Nicaragua (947), Paraguay (714), Brazil (561), El Salvador (431) and Honduras (411) (Table S3 available as Supplementary data at JTM online, Figure 4). In contrast, the GBD 2019 estimates revealed that the countries with the highest average rates per 100 000 were Taiwan (2468), India (2052), Honduras (1865), El Salvador (1829) and Paraguay (1556). Figure 5 illustrates the disparities between GBD study estimates and reported dengue incidence rates, emphasizing substantial differences per 100 000 in Taiwan (2439), India (2045), Honduras (1454), El Salvador (1398) and Puerto Rico (1366) (Figure 5A). The GBD-to-reported ratios based on average rates (Figure 5B) are very close to those calculated using average numbers (Figure 2B). This resemblance occurs because rates are calculated by dividing cases by the population for each year and then averaging. Since year-to-year population changes are generally marginal, they minimally impact the ratio comparison. Similarly, EAPCs calculated using incidence rates closely align with those derived from case numbers (Supplementary Table S2 and S3 available as Supplementary data at JTM online).
Figure 4.
Ten-year averages of dengue incidence rate per 100 000 population for 30 selected countries and territories according to (A) reported data (B) GBD estimates (2010–2019).
Figure 5.

Discrepancies in 10-year average incidence rate between reported data and GBD study in 30 selected countries and territories (2010–2019): (A) absolute differences per 100 000 population (B) GBD-to-reported case ratios.
Discussion
In this comparative analysis, significant discrepancies were noted between the reported data and the GBD estimates regarding the number of dengue cases, as well as in the calculations of CVs and EAPCs between 2010 and 2019. The most substantial GBD-to-reported case ratios were observed in China, India, Bangladesh, Taiwan and Indonesia. Moreover, the GBD 2019 study pinpointed Taiwan as having the highest 10-year average incidence rate of dengue among the assessed regions.
In our prior study on Taiwan, we showed GBD’s dengue estimates were implausibly high for this non-endemic region, overlooking the yearly variation and the significant reduction in underreporting due to widespread use of rapid diagnostic tests for the non-structural protein 1 (NS1) antigen since 2015. Consequently, the GBD’s estimate that Taiwan has the world’s highest dengue incidence is highly improbable.16 Therefore, we recommend that entities with notable discrepancies between reported cases and GBD estimates critically assess their dengue epidemiology and surveillance systems to evaluate if the GBD study might also overestimate their dengue burden. For example, dengue is also not considered endemic in China,23 suggesting that the GBD estimates may significantly overstate its burden. Conversely, in India, characterized by a less robust surveillance system for symptomatic dengue and a high force of infection—as indicated by serosurvey data24,25—the discrepancy between GBD estimates and reported data might not necessarily imply an overestimation by the GBD.
Among the 30 entities analysed, Singapore, with the highest SDI, likely has the best dengue surveillance system, integrating case, vector, virus and environmental aspects.26 The GBD-to-reported ratio for Singapore, based on the 10-year average case number, stands at 6.2. This figure raises questions about the potential underestimation of dengue cases by the GBD in countries with ratios < 6.2, as it’s improbable that their surveillance systems surpass Singapore’s in efficacy. For instance, Nicaragua exhibited the lowest GBD-to-reported ratio of 1.3, suggesting minimal under-reporting, a scenario that seems unlikely given the global surveillance system discrepancies. Furthermore, Singapore noted a mismatch between the increase in reported dengue cases and the decrease in seroprevalence, likely due to enhanced case detection rates resulting from advancements in diagnostics, particularly after the introduction of the NS1 rapid test in 2008.26
Several limitations of the GBD methodology that may explain the significant discrepancies between GBD estimates and reported data include the following. Firstly, a major issue with the GBD team’s methodology for estimating dengue incidence lies in its correction factor for under-reporting. This crucial component is based on a systematic review comparing passive and active case detection rates.17 This review identified 17 sources, resulting in 34 comparisons,17 which we scrutinized through an advanced search on the Global Health Data Exchange website (https://ghdx.healthdata.org/) using ‘dengue’ as the keyword. Our findings indicate that the majority of these studies are outdated, primarily conducted before 2010. Consequently, this correction factor might not adequately reflect recent decreases in under-reporting, especially due to the widespread adoption of NS1 rapid tests in regions like Taiwan and Singapore.
Additionally, another significant limitation of the GBD estimates is their failure to capture the marked yearly variation in reported dengue cases. Dengue, an acute infectious disease, involves complex interactions among humans, vectors and environmental factors. Typically, its transmission follows seasonal patterns linked to warmer, rainy periods and exhibits cyclical trends, with major outbreaks occurring every 3 to 5 years.27 The GBD’s model, which includes a systematic analytical framework with a smoothing technique, might be more suitable for chronic diseases but seems less capable of reflecting the substantial fluctuations inherent in acute infectious diseases like dengue.17 Particularly in non-endemic regions such as Taiwan,28 China23 and Argentina 29 reported dengue cases exhibit more noticeable annual variations with occasional large outbreaks,30 as reflected by high CVs, highlighting the sporadic nature of the outbreaks.
Furthermore, the GBD’s methodology lacks detailed quality assessment of its data sources. Dengue surveillance systems vary across locations and evolve over time. Criteria for case definition, including the necessity for laboratory confirmation and the specific laboratory criteria for confirmation, differ. Moreover, some surveillance systems cover only hospitals but exclude clinics. We selected 30 entities with the highest 10-year average case numbers based on the most recent decade of available GBD estimates, prioritizing the analysis of higher-quality reported data. Low case numbers often suggest a less robust surveillance system, making the comparison less meaningful. Despite the GBD’s comprehensive integration of surveillance data into its estimation process, it is necessary to provide detailed information on the quality assessment of these data sources. This may include details such as reporting standards, testing methods, disease fatality rates, and other relevant parameters. Such transparency regarding data sources and quality assessment is essential for constructing a more suitable estimation model in the future.
Dengue has emerged as the primary cause of febrile illness among travellers from all continents, excluding Africa.31 The risk of infection increases with longer stays in endemic regions.32 Surveillance data from international travellers returning from dengue-endemic regions have been used to detect ongoing outbreaks in countries with inadequate surveillance systems, as seen in Cuba.33 These data could also help assess dengue activities in such countries and verify their national reported data. However, this type of information is currently not included in the GBD study. GeoSentinel, a global network for travellers and migrants, could provide valuable data that the GBD team might use to refine or verify future dengue models, particularly for countries with less effective surveillance systems.34
Climate change has significantly expanded the geographic distribution of mosquito vectors like Aedes albopictus, a secondary but highly invasive vector now found on every continent except Antarctica.3,4,35 Importantly, the importation of dengue by travellers can trigger local outbreaks in non-endemic areas or regions previously unaffected, provided that competent vectors are present, as observed in countries like Australia, Portugal and France.36–39 Modelling the disease burden in locales with minimal transmission activity poses additional challenges. Nonetheless, it would be advantageous for the GBD team to refine their estimates by distinctly differentiating between travel-associated and locally-acquired cases in future assessments.
Estimates from the GBD have been compared with those generated by other groups across various health metrics, including maternal mortality,40 cause of child death,41 tuberculosis mortality,42 malaria mortality43 seasonal influenza mortality44 and prevalence of chronic hepatitis B virus infection.45 Discrepancies in estimates from various groups are frequently observed; however, determining which estimates are more accurate than others poses a significant challenge. Considering the increasing significance of global health estimates in worldwide monitoring and policymaking, it is crucial to devise methods for validating these estimates. This study compared the GBD’s dengue estimates with reported data from 30 selected locations, aiming to evaluate the credibility of the GBD’s estimates on the dengue burden and to assist in interpreting these estimates. Nonetheless, a significant limitation of our study is the inherent underestimation of the dengue burden by reported data. Consequently, large discrepancies between reported data and GBD estimates do not necessarily invalidate the GBD’s estimates.
Conclusion
This study reveals notable discrepancies between GBD estimates and reported dengue cases from 2010 to 2019, underscoring the need for in-depth analysis in regions with the largest disparities to assess the plausibility of the GBD’s estimates. Furthermore, the inability of the GBD’s estimates to reflect the extensive yearly fluctuations in dengue outbreaks accentuates the necessity of enhancing disease burden estimation methods for dengue but potentially for other acute infectious diseases as well to ensure credible global health estimates for informed decision-making and policy formulation.
Funding
This study was supported by National Cheng Kung University Hospital (grant number NCKUH-11303004).
Author contributions
Sin Yee Lee (Data curation, Formal analysis, Software, Visualization, Writing—original draft), Hsin-I Shih (Formal analysis, Investigation, Methodology, Writing—review & editing), Wei-Cheng Lo (Methodology, Validation, Writing—review & editing), Tsung-Hsueh Lu (Conceptualization, Methodology, Resources, Visualization, Writing—review & editing) and Yu-Wen Chien (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—original draft).
Conflict of interest: The authors have declared no conflicts of interest.
Data availability
All the data used in this study are provided in Supplementary Table S1.
Editorial assistance
The authors would like to acknowledge the use of ChatGPT, an AI language model developed by OpenAI, for providing editorial assistance in improving the English language of this manuscript. It is important to note that the assistance provided by ChatGPT was limited to language editing, while all original content, ideas, data analysis, results, and discussions were solely generated by the authors. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Supplementary Material
Contributor Information
Sin Yee Lee, Department of Public Health, College of Medicine, National Cheng Kung University, No. 1, University Road, Tainan 701, Taiwan.
Hsin-I Shih, Department of Emergency Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, No. 138, Sheng Li Road, Tainan 704, Taiwan; School of Medicine, College of Medicine, National Cheng Kung University, No. 1, University Road, Tainan 701, Taiwan.
Wei-Cheng Lo, Master Program in Applied Epidemiology, College of Public Health, Taipei Medical University, No. 301, Yuantong Road, Zhonghe District, New Taipei City 235, Taiwan.
Tsung-Hsueh Lu, Department of Public Health, College of Medicine, National Cheng Kung University, No. 1, University Road, Tainan 701, Taiwan.
Yu-Wen Chien, Department of Public Health, College of Medicine, National Cheng Kung University, No. 1, University Road, Tainan 701, Taiwan; Department of Occupational and Environmental Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, No. 138, Sheng Li Road, Tainan 704, Taiwan.
References
- 1. Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet 2019; 393:350–63. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Dengue and Severe Dengue. Fact sheet, 23 April 2024. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (9 May 2024, date last accessed).
- 3. Bhatt S, Gething PW, Brady OJet al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013; 5:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Vector-borne diseases. Fact Sheet, 2 March 2020. Available from: http://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (5 February 2024, date last accessed). [Google Scholar]
- 6. Stanaway JD, Shepard DS, Undurraga EAet al. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis 2016; 16:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathers CD. History of global burden of disease assessment at the World Health Organization. Arch Public Health 2020; 78:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byass P. In retrospect: global health estimated over two decades. Nature 2017; 545:421–2. [DOI] [PubMed] [Google Scholar]
- 9. AbouZahr C, Boerma T, Hogan D. Global estimates of country health indicators: useful, unnecessary, inevitable? Glob Health Action 2017; 10:1290370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du M, Jing W, Liu M, Liu J. The global trends and regional differences in incidence of dengue infection from 1990 to 2019: an analysis from the global burden of disease study 2019. Infect Dis Ther 2021; 10:1625–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng Z, Zhan J, Chen L, Chen H, Cheng S. Global, regional, and national dengue burden from 1990 to 2017: a systematic analysis based on the global burden of disease study 2017. EClinicalMedicine 2021; 32:100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Quam MBM, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med 2021; 28:taab146. [DOI] [PubMed] [Google Scholar]
- 13. Dutta O, Prasanth A, Kumari A, Akanksha K, Deeba F, Salam N. Burden of dengue, leishmaniasis and lymphatic filariasis in India and its states from 1990-2019: analysis from the global burden of disease study (gbd 2019). PloS One 2023; 18:e0292723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martins-Melo FR, Carneiro M, Ramos AN Jr, Heukelbach J, Ribeiro ALP, Werneck GL. The burden of neglected tropical diseases in Brazil, 1990-2016: a subnational analysis from the global burden of disease study 2016. PLoS Negl Trop Dis 2018; 12:e0006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Y, Fang K, Zheng Y, Wang HL, Wu J. Global burden and trends of neglected tropical diseases from 1990 to 2019. J Travel Med 2022; 29:taac031. [DOI] [PubMed] [Google Scholar]
- 16. Lee SY, Shih HI, King CC, Lu TH, Chien YW. Substantial discrepancies in dengue case estimates between the global burden of disease study and Taiwan centers for disease control. J Travel Med 2024; 31:taae009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan American Health Organization . PLISA health information platform for the Americas - dengue cases. Available from: https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html (5 November 2023, date last accessed).
- 19. World Health Organization . Dengue explorer. 2017. Available from: https://ntdhq.shinyapps.io/dengue5/ (5 November 2023, date last accessed).
- 20. Taiwan Centers for Disease Control . Taiwan National Infectious Disease Statistics System. Available from: https://nidss.cdc.gov.tw/ (30 Auguest 2023, date last accessed).
- 21. Liu Z, Jiang Y, Yuan Het al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol 2019; 70:674–83. [DOI] [PubMed] [Google Scholar]
- 22. United Nations , Department of Economic and Social Affairs, Population Division. World Population Prospects: 2022, Online Edition: https://population.un.org/wpp/. [Google Scholar]
- 23. Yue Y, Liu X, Ren D, Wu H, Liu Q. Spatial dynamics of dengue fever in mainland China, 2019. Int J Environ Res Public Health 2021; 18:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilder-Smith A, Rupali P. Estimating the dengue burden in India. Lancet Glob Health 2019; 7:e988–9. [DOI] [PubMed] [Google Scholar]
- 25. Murhekar MV, Kamaraj P, Kumar MSet al. Burden of dengue infection in India, 2017: a cross-sectional population based serosurvey. Lancet Glob Health 2019; 7:e1065–73. [DOI] [PubMed] [Google Scholar]
- 26. Ho SH, Lim JT, Ong J, Hapuarachchi HC, Sim S, Ng LC. Singapore’s 5 decades of dengue prevention and control—implications for global dengue control. PLoS Negl Trop Dis 2023; 17:e0011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization . Dengue: guidelines for diagnosis, treatment, prevention and control, new edn. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 28. Chien YW, Shu YC, Chuang KTet al. High estimated prevalence of asymptomatic dengue viremia in blood donors during a dengue epidemic in southern Taiwan, 2015. Transfusion 2017; 57:2649–56. [DOI] [PubMed] [Google Scholar]
- 29. Masuh HM. Re-emergence of dengue in Argentina : historical development and future challenges. Dengue Bull 2008; 32:44–54. [Google Scholar]
- 30. Chiu NC, Chi H, Weng SL, Lin CY. Unprecedented dengue outbreak in Taiwan following COVID-19. J Travel Med 2024; 31:taae015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz E, Weld LH, Wilder-Smith Aet al. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997-2006. Emerg Infect Dis 2008; 14:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitro A, Imad HA, Pisutsan Pet al. Seroprevalence of dengue, Japanese encephalitis and Zika among long-term expatriates in Thailand. J Travel Med 2024; 31:taae022. [DOI] [PubMed] [Google Scholar]
- 33. Díaz-Menéndez M, Angelo KM, Miguel BRet al. Dengue outbreak amongst travellers returning from Cuba—GeoSentinel surveillance network, January–September 2022. J Travel Med 2023; 30:taac139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Osman S, Preet R. Dengue, chikungunya and Zika in GeoSentinel surveillance of international travellers: a literature review from 1995 to 2020. J Travel Med 2020; 27:taaa222. [DOI] [PubMed] [Google Scholar]
- 35. Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 2013; 29:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sohail A, Anders KL, McGuinness SL, Leder K. The epidemiology of imported and locally acquired dengue in Australia, 2012–2022. J Travel Med 2024; 31:taae014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilder-Smith A, Quam M, Sessions Oet al. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill 2014; 19:20718. [DOI] [PubMed] [Google Scholar]
- 38. Chen LH, Marti C, Diaz Perez C, Jackson BM, Simon AM, Lu M. Epidemiology and burden of dengue fever in the United States: a systematic review. J Travel Med 2023; 30:taad127. [DOI] [PubMed] [Google Scholar]
- 39. Cochet A, Calba C, Jourdain Fet al. Autochthonous dengue in mainland France, 2022: geographical extension and incidence increase. Euro Surveill 2022; 27:2200818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kassebaum NJ, Lopez AD, Murray CJ, Lozano R. A comparison of maternal mortality estimates from GBD 2013 and WHO. Lancet 2014; 384:2209–10. [DOI] [PubMed] [Google Scholar]
- 41. Liu L, Black RE, Cousens S, Mathers C, Lawn JE, Hogan DR. Causes of child death: comparison of MCEE and GBD 2013 estimates. Lancet 2015; 385:2461–2. [DOI] [PubMed] [Google Scholar]
- 42. García-Basteiro AL, Brew J, Williams B, Borgdorff M, Cobelens F. What is the true tuberculosis mortality burden? Differences in estimates by the world health organization and the global burden of disease study. Int J Epidemiol 2018; 47:1549–60. [DOI] [PubMed] [Google Scholar]
- 43. Ye Y, Kyobutungi C, Ogutu Bet al. Malaria mortality estimates: need for agreeable approach. Trop Med Int Health 2013; 18:219–21. [DOI] [PubMed] [Google Scholar]
- 44. Cozza V, Campbell H, Chang HHet al. Global seasonal influenza mortality estimates: a comparison of 3 different approaches. Am J Epidemiol 2021; 190:718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmit N, Nayagam S, Thursz MR, Hallett TB. The global burden of chronic hepatitis b virus infection: comparison of country-level prevalence estimates from four research groups. Int J Epidemiol 2021; 50:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used in this study are provided in Supplementary Table S1.