Abstract
Infection and chronic post-traumatic osteomyelitis of the tibia after open fracture are complex problems that cause significant morbidity and threaten the viability of a limb. Therefore, it is of utmost importance for the orthopaedic surgeon to understand both patient and treatment factors that modify the risk of developing these disastrous complications. Infection risk is largely based on severity of open injury in addition to inherent patient factors. Orthopaedic surgeons can work to mitigate this risk with prompt antibiotic administration, thorough and complete debridement, expedient fracture stabilization, and early wound closure. In the case osteomyelitis does occur, the surgeon should use a systematic multidisciplinary approach for eradication.
Keywords: open tibia, osteomyelitis, infection
1. Introduction
Open tibia fractures can result in significant patient morbidity with relatively high rates of postoperative complications. Largely because of the lack of a robust soft-tissue envelope around the tibia and high contamination risk at the time of injury, infection remains among the most common of these complications. Open tibia fractures carry a 4.5%–20% reported incidence of post-traumatic osteomyelitis.1 Osteomyelitis can cause a significant reduction in a patient's quality of life. In addition to chronic pain, loss of function, and emotional and financial burden, osteomyelitis of the tibia can pose difficult surgical challenges because patients are prone to suffer disastrous complications such as pathologic fracture, delayed healing or nonunion, or amputation.2
Once established, post-traumatic osteomyelitis is extremely difficult to treat successfully, with reported recurrence rates of 20%–30%.3 In addition, the prevalence of osteomyelitis in the United States has continually increased over the past half century, and because overall medical advancement, patients with osteomyelitis are living longer and requiring more treatment requiring orthopaedic intervention.4 Therefore, the prudent orthopaedic surgeon will understand both the unmodifiable and modifiable risk factors of developing infection and osteomyelitis in patients with open tibia fractures. This understanding will lead to effectively tailored treatment plans with the intention to best prevent this complication. In addition, in the case that post-traumatic osteomyelitis does occur after an open tibia fracture, the surgeon should understand the treatment options available.
2. Osteomyelitis
Osteomyelitis can be defined as an infection involving bone characterized by progressive inflammatory destruction and the apposition of new bone. It is caused by hematogenous spread, contiguous spread, or due to complications such as ulceration from more systemic problems such as vascular insufficiency or diabetes. The most common causes of post-traumatic osteomyelitis of the tibia are retained necrotic and infected bone, retained infected implant, avascular or infected scar, dead space and inadequate skin cover, and chronic granulation tissue in the medullary canal.5,6
This infection can be defined as either acute or chronic. Acute osteomyelitis is that of a short duration characterized by suppuration, which occurs in a few days or weeks after trauma. In acute osteomyelitis, systemic symptoms such as fevers, chills, nausea, and fatigue are common. Chronic osteomyelitis is an infection of the bone that is long-standing, from weeks to years after the initial insult. There is no universally accepted cutoff separating acute from chronic infection. Systemic symptoms are not as common in chronic osteomyelitis; however, local symptoms such as redness, pain, swelling, and drainage may persist with varying severity. Chronic osteomyelitis is often characterized by necrotic bone that develops a nidus for infection called a sequestrum. New bone forms around the necrotic bone called an involucrum. In the chronic setting, bacteria develop a biofilm, in which bacterial cells remain hidden and dormant in a hydrophobic matrix, making it difficult for antibiotics to be very effective.7
3. Osteomyelitis Risk Factors at the Time of Trauma
At the time of trauma of an open tibial fracture, there are certain risk factors of developing osteomyelitis that the orthopaedic surgeon should be aware of. These risk factors can be categorized into 2 groups: those pertaining to the trauma and those related to the patient's general health and lifestyle. When the risk factors pertaining to the trauma itself are considered, the most important risk factor is the severity of the injury. Since 1976, the Gustilo-Anderson classification system has been used successfully to describe the severity of open fractures, and it has been shown that increasing injury severity is directly correlated with increased risk of infection and osteomyelitis.1 Type III open fractures have a higher risk of infection (up to 50%) compared with type II (2%–10%) and type I (2%) injuries.8 The Tscherne classification for open fractures, which was developed in 1984, is likewise prognostic of infection. Studies have shown that Tscherne type IV and type III open fractures carry a higher risk of infection (up to 66% and 50%, respectively) while type I and II type open fractures carry a lower risk (up to 19% and 25%, respectively).9,10 Vascularity is also an important consideration, and studies have shown that arterial disruption during open fractures increases the risk of soft-tissue complications and infection.11,12 In addition, the amount and type of wound contamination certainly affect infection risk, with dirtier wounds logically being more at risk of more severe infection.
Alternatively, host-specific risk factors of infection and osteomyelitis are those related to the patient's overall health. It is well known that patients who are smokers, diabetic, obese, neuropathic, and immunocompromised are at a significantly higher risk of infection.8,13 Other risk factors, which may not be as easily quantifiable, but still should be taken into account by the treatment team, are the patient's prior and current access to health care, social support system, and overall health care literacy.
4. Prevention: A Systematic Approach
A large focus of research over the past several decades related to treating open tibia fractures has been on the prevention of post-traumatic infection and osteomyelitis. Advances in fracture fixation, soft-tissue management, and antimicrobial therapy have significantly improved treatment options for patients.14 Institutional algorithms and protocols for treating open tibia fractures, and open fractures in general, should be developed on evidence-based recommendations. We aim to briefly summarize the key, evidence-based interventions, which improve outcomes and prevent infection and osteomyelitis after open tibia fractures.
4.1. Intravenous Antibiotic Treatment
One of the most important interventions to prevent infection for any open fracture is prompt antibiotic administration. In their early work on 1025 open long-bone fractures, Gustilo and Anderson found that 70% of open wounds were contaminated with bacteria. They argued that the routine use of antibiotics was in fact therapeutic, rather than prophylactic.15 Patzakis and Wilkins16 looked at 1104 open fractures and found an infection rate of 4.7% when antibiotics were administered within 3 hours of injury, compared with 7.4% in the cohort that did not receive timely antibiotics. In addition, a more recent study by Lack et al8 looked at 137 type III open tibia fractures and found that antibiotic administration beyond 66 minutes from injury was a predictor for deep infection within 90 days. Therefore, institutions are urged to promote antibiotic administration as soon as possible for open tibia fractures after injury. It has been proposed that prehospital diagnosis of open fracture and antibiotic administration by emergency medical services may be beneficial for mitigating infection risk.8
According to the 2011 Eastern Association for the Surgery of Trauma guidelines, antibiotic choice and duration should be guided by the injury type and severity based on the Gustilo-Anderson classification. For type I and II fractures, a first-generation cephalosporin is advised. This should be continued for 24 hours. For type III fractures, a combination of a first-generation cephalosporin and aminoglycoside is suggested, with the addition of penicillin if there is a risk of fecal or clostridial contamination. These should be continued for 72 hours after injury or 24 hours after soft-tissue coverage.17 An alternative to the common combination of cefazolin and gentamicin for type III fractures is piperacillin/tazobactam. This alternative offers a safer profile and better bone distribution, can be used as the only antibiotic, and is equally effective as the cefazolin and gentamicin combination.18 In addition, for patients who are not up-to-date with their vaccination status, tetanus prophylaxis should be administered.17
4.2. Irrigation and Debridement
After emergent antibiotic administration and expedient trauma survey and patient stabilization, the orthopaedic specialist should have a plan of action for the management of the open tibia fracture. While there is no evidence advocating bedside irrigation and debridement of wounds, removing immediately visible contaminates such as leaves and torn clothes may be beneficial. Such foreign materials can cause infection if they are pushed deeper into the soft tissues after preliminary fracture reduction.
Once preliminary fracture reduction and stabilization is complete, the question that arises is when to take the patient to the operating room for formal irrigation and debridement? Historically, the answer to this question was within 6 hours of injury based on the early works of Gustilo and Anderson,15 but more recent evidence shows otherwise. In one level II study of 315 patients with high-energy open fractures found that time to debridement was not an independent predictor of the risk of infection within the first 3 months after surgery. However, most of these patients were taken to the operating room within 24 hours of injury.19 Similarly, another prospective study of 315 patients with open fractures (70.2% lower extremity fractures and 47.9% type III fractures) concluded that provided irrigation and debridement was performed within the first 24 hours after emergency department admission, time to irrigation and debridement did not affect early or late infection rates.20 When looking specifically at open tibia fractures, one study of 106 fractures found no significant increase in infection rates in patients treated within or after 6 hours. In fact, the study concluded that increasing Gustilo-Anderson grade was a more prognostic indicator for infection.21 In this light, orthopaedic surgeons are more likely to take patients with contaminated wounds and higher degree of injury to the operating room more emergently.22 However, in less severe cases, treating these fractures in normal daylight hours with regular, experienced teams may be acceptable.
The aim of irrigation and debridement of an open tibia fracture is to prevent infection and promote healing. An adequate debridement effectively removes all contaminated and nonviable tissue including skin, subcutaneous fat, muscle, and bone. The wound should be longitudinally extended if required, the bone ends should be delivered, and the medullary canal should be thoroughly cleaned. Bone and skin viability can be assessed by its capacity to bleed. Muscle viability can be assessed by its color, contractility, consistency, and capacity to bleed. Depending on the energy of the initial trauma and the extent of soft-tissue injury, multiple debridement procedures may be required.23
Based on recent research, the type of irrigation that should be used is very low-pressure gravity irrigation with normal saline.24 The volume of irrigation remains more controversial, with no clinical studies to support any recommendation. As a general rule of thumb, a surgeon may use 3 L of irrigation for a type I fracture, 6 L for a type 2 fracture, and 9 L for a type III fracture.25
4.3. Fracture Stabilization
During the initial irrigation and debridement procedure, it is not only important to evaluate and clean the wound but also to provide bony stabilization. Fracture stabilization is essential because aside from pain relief, it prevents further soft-tissue injury, restores soft-tissue tension, allows for decreased swelling, and improves circulation. This improved circulation is vital for a contaminated wound because it can facilitate the much needed immune response.26 There are a variety of options for fracture stabilization of the tibia. Splinting, external fixation, intramedullary nailing, and plate fixation are all viable options used in different scenarios depending on fracture pattern, location, and extent of soft-tissue injury. In open tibia fractures with severe soft-tissue defects that require temporization before definitive reconstruction, external fixation remains the gold standard. In a prospective randomized study by Tornetta et al27 involving 29 patients with type IIIB open tibial fractures randomized to external fixation or unreamed nailing, there was no significant difference in healing times, range of motion, and infection rates between the 2 groups. In addition, the SPRINT trial concluded that there is no difference in reoperation rates due to infection in patients who undergo reamed nailing compared with patients who undergo unreamed nailing for open tibia fractures.28
4.4. Management of the Soft-Tissue Defect
Owing to the relative lack of soft-tissue envelope around the tibia, operative management of soft-tissue defects for open tibia fractures can be challenging and require a multidisciplinary approach between orthopaedics and plastic surgery. The approach should be systematic and methodical, and the surgeons should be aware that fracture stabilization methods can affect ultimate wound management treatment plans. First, cleanliness of the wound should be assessed. A surgeon should ensure before closure or coverage that all necrotic, contaminated tissue has been removed and the wound bed is clean. If there is any doubt about this, then serial debridement may be required. This is especially true in wounds contaminated with feces, dirt, stagnant water, farm-related injuries, and freshwater boating accidents.26
In larger contaminated defects in which serial debridement is planned, antibiotic-impregnated beads or, in some cases, cement spacers can be used for local antibiotic delivery as adjuncts to systemic antibiotics. One retrospective study of 1085 open fractures investigated the effectiveness of antibiotic beads. Two hundred forty patients received only systemic antibiotics, and 845 received patients received systemic antibiotics in addition to tobramycin-impregnated polymethylmethacrylate (PMMA) beads. A significant reduction in acute infection rates in type IIIB and C fractures was found in the antibiotic bead group. In addition, the incidence of osteomyelitis was significantly lower in type II and IIIB open fractures.29
The next step after ensuring that there is a clean wound bed is planning for closure. Type I, type II, and type IIIA open tibia fractures can generally be closed primarily. Recent evidence shows that primary closure of open fractures significantly lowers infection and nonunion rates.30 However, type IIIB tibia fractures will require a combined “orthoplastic” reconstructive approach. Often, coverage is staged, and interim wound coverage is needed between serial debridement or between initial debridement and final coverage. One option for interim wound coverage is negative-pressure wound therapy (NPWT). This provides vacuum-assisted temporary soft-tissue coverage that reduces edema, increases vascularity, and increases granulation tissue formation. A retrospective study looking at 229 open tibia fractures in which patients either received conventional dressings (63 patients) or NPWT (166 patients) found a decreased rate of deep infection of close to 80% in the NPWT group.31 However, large randomized prospective trials are still lacking in the literature, and debate does exist about the true efficacy of NPWT.
For type IIIB fractures, 2 important interdisciplinary decisions should be made. The first is type of soft-tissue coverage, and the second is time to soft-tissue coverage. Type of soft-tissue flap coverage is a complex topic that deals with the vascularity, size, and location of the defect. This decision should be made in conjunction with a plastic surgeon if the orthopaedist is not comfortable with soft-tissue coverage procedures. The second decision is time to coverage. Evidence is emerging that this should be performed as early as possible. Gopal et al32 advocated for early internal fixation and soft-tissue reconstruction within 72 hours of injury. In their study of 84 open tibia fractures, they found a higher rate of infection in patients with delayed coverage, although this was not significant. In addition, D'Alleyrand et al33 found, in a retrospective series of 69 patients with open tibia fractures, that the odds of infection increased by 16% for each day that flap coverage was delayed past 7 days.
4.5. Monitoring and Follow-up
With a significant reported risk of up to 20% of open tibia fractures developing osteomyelitis, postoperative surveillance is vital.1 The orthopaedic surgeon should have in place regular planned follow-up with patients with open tibia fractures who have been treated. Physicians should be aware and suspicious of osteomyelitis if patients present with common symptoms such as pain, leg swelling, wound drainage, erythema, fevers, and nausea.
5. Eradication: Be Methodical
The eradication of tibial post-traumatic osteomyelitis is a vast topic with a large body of literature. A thorough and detailed discussion is certainly beyond the scope of this review; however, when addressing tibial post-traumatic osteomyelitis, it is important to be systematic and methodical, just as when initially approaching an open tibia fracture. This discussion on eradication will briefly focus on the salient points of this methodical approach.
5.1. Staging Determines Treatment Options
Once a diagnosis of osteomyelitis has been made, the first step in a methodical treatment plan is staging. Staging is important because it grades not only the severity of the infection but also guides treatment options. For osteomyelitis, the Cierny-Mader classification is used.34 This classification system takes into account 2 vital factors: the extent of bony necrosis and the physiologic condition of the host. There are 4 anatomic types and 3 physiologic host classes (Fig. 1). The physiologic host class is an important distinction as it dictates whether a patient is optimized for any surgical treatment. The anatomic type is important because it dictates type of bony surgical intervention. For example, a type I, or medullary, disease of the tibia is generally caused by an infected intramedullary nail or direct hematogenous spread. Treatment can consist of removal of the infected implant and isolated intramedullary debridement. This can be performed with reaming and/or irrigation of the intramedullary canal. If there is concern of bony instability, an antibiotic nail may be used. On the contrary, the fourth anatomic type is diffuse, meaning that an entire segmental portion of bone is infected. This is due to major devascularization and colonization of the bone. Treatment involves resection of the entire segment of bone with some sort of reconstructive procedure such as a Masquelet reconstruction or bone transport (Fig. 2).
Figure 1.
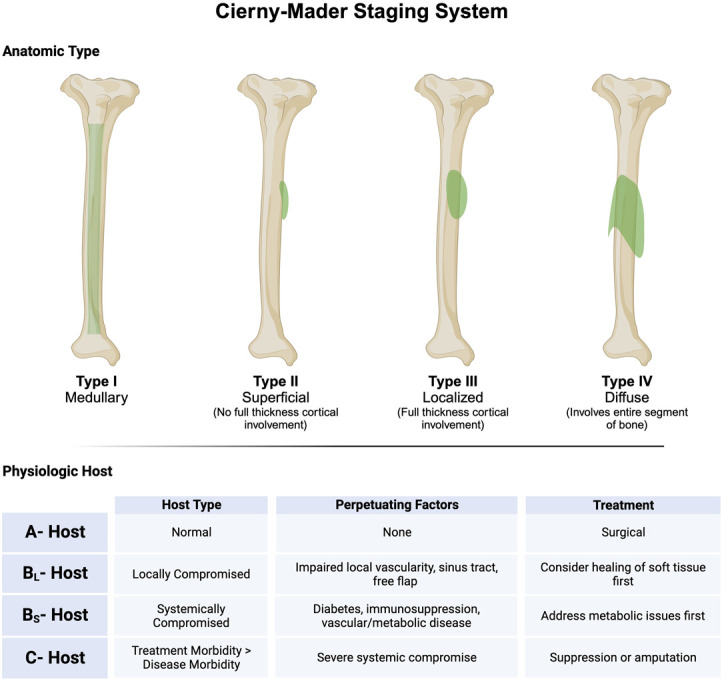
The Cierny-Mader classification of osteomyelitis created with BioRender.com.
Figure 2.
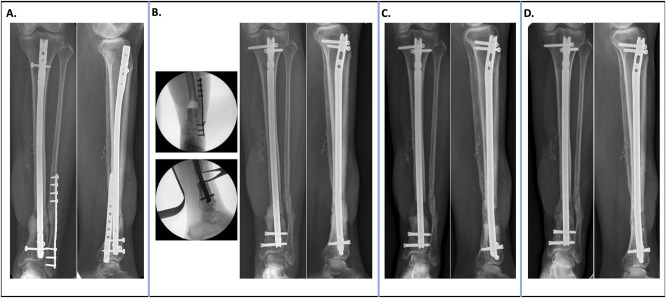
Case of a 53-year-old patient who sustained a Gustilo IIIB open tibia fracture 2 years before presentation. The patient developed osteomyelitis of the distal third tibia. A, Initial radiographs. B, The entire infected segment of bone was removed, the prior hardware was removed, and exchange nailing was performed. The critical defect was filled with a PMMA antibiotic spacer. C, Six weeks later, the spacer was removed and filled with distal femur metaphyseal cancellous autograft mixed with allograft. D, Radiographs 6 months postoperatively show bony healing and consolidation. PMMA = polymethylmethacrylate.
5.2. Sequence of Events
After staging, if a patient is deemed a surgical candidate for limb salvage, then the surgeon, in conjunction with an interdisciplinary team should develop a management plan based on detailed evaluation of the patient, the involved bone and soft tissues, degree of associated lower extremity injury, and type of bacterial pathogens based on biopsy. Patzakis described surgical treatment of tibial osteomyelitis in 3 stages. The first stage involves thorough debridement of bone and soft tissue, skeletal stabilization (if required), and antibiotic therapy. If present, the dead space resulting from debridement can temporarily be filled with microbial-specific PMMA antibiotic beads for local delivery of antibiotics. Systemic antibiotic therapy should be used if deemed safe and necessary under consultation of an infectious disease specialist if available. The second stage is wound management, which involves deciding whether primary or delayed closure with soft-tissue coverage in the form of a graft or flap is required. This should be performed in conjunction with a surgeon comfortable with plastic surgery techniques. Coverage is generally performed 3–7 days after initial debridement. The final stage, if required, such as in Cierny-Mader type IV disease, is bony reconstruction. There are numerous options for approaching critical sized defects of the tibia; a discussion is outside the scope of this review.7,35,36 Finally, just as in acute open fracture treatment, surveillance of the patient is vital, especially with such a high recurrence of osteomyelitis.
6. Conclusion
Open tibia fractures are devastating injuries that carry a high risk of infection and osteomyelitis. Osteomyelitis is an extremely difficult problem for physicians to treat, especially because there is a high recurrence rate despite the best surgical efforts. As a result, orthopaedic surgeons should understand the modifiable and non-modifiable factors for infection when treating open tibia fractures. They should also have a methodical plan when treating open fractures. Ultimately, if osteomyelitis does develop, surgeons should have a systematic way to approach treatment.
Footnotes
No authors have any conflicts of interest pertinent to this study to report.
Source of Funding: Nil.
The study was deemed exempt from Institutional Review Board and Animal Use Committee Review.
References
- 1.Campbell R, Berry MG, Deva A, et al. Aggressive management of tibial osteomyelitis shows good functional outcomes. Eplasty. 2011;11:e3. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou C-H, Ren Y, Ali A, et al. Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: a retrospective study of 42 patients. J Orthop Surg Res. 2020;15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tulner SA, Schaap GR, Strackee SD, et al. Long-term results of multiple-stage treatment for posttraumatic osteomyelitis of the tibia. J Trauma. 2004;56:633–642. [DOI] [PubMed] [Google Scholar]
- 4.Kremers HM, Nwojo ME, Ransom JE, et al. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am. 2015;97:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ger R, Efron G. New operative approach in the treatment of chronic osteomyelitis of the tibial diaphysis. A preliminary report. Clin Orthop Relat Res. 1970;70:165–169. [PubMed] [Google Scholar]
- 6.Mathes SJ, Alpert BS, Chang N. Use of the muscle flap in chronic osteomyelitis: experimental and clinical correlation. Plast Reconstr Surg. 1982;69:815–829. [DOI] [PubMed] [Google Scholar]
- 7.Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13:417–427. [DOI] [PubMed] [Google Scholar]
- 8.Lack WD, Karunakar MA, Angerame MR, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma. 2015;29:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira RV, Cruz LP, Matos MA. Comparative accuracy assessment of the Gustilo and Tscherne classification systems as predictors of infection in open fractures. Rev Bras Ortop. 2018;53:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matos MA, Lima LG, de Oliveira LA. Predisposing factors for early infection in patients with open fractures and proposal for a risk score. J Orthop Traumatol. 2015;16:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi AS, Fisher ND, Parola R, et al. Arterial injury portends worse soft tissue outcomes and delayed coverage in open tibial fractures. J Orthop Trauma. 2022;36:535–543. [DOI] [PubMed] [Google Scholar]
- 12.Seligson D, Ostermann PA, Henry SL, et al. The management of open fractures associated with arterial injury requiring vascular repair. J Trauma. 1994;37:938–940. [DOI] [PubMed] [Google Scholar]
- 13.Lu V, Zhang J, Patel R, et al. Fracture related infections and their risk factors for treatment failure—a major trauma centre perspective. Diagnostics. 2022;12:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo IA, Heiner JA, Meremikwu RI, et al. Where are we in 2022? A summary of 11,000 open tibia fractures over 4 decades. J Orthop Trauma. 2023;37:e326–e334. [DOI] [PubMed] [Google Scholar]
- 15.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 16.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;243:36–40. [PubMed] [Google Scholar]
- 17.Hoff WS, Bonadies JA, Cachecho R, et al. East Practice Management Guidelines Work Group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70:751–754. [DOI] [PubMed] [Google Scholar]
- 18.Redfern J, Wasilko SM, Groth ME, et al. Surgical site infections in patients with type 3 open fractures: comparing antibiotic prophylaxis with cefazolin plus gentamicin versus piperacillin/tazobactam. J Orthop Trauma. 2016;30:415–419. [DOI] [PubMed] [Google Scholar]
- 19.Pollak AN, Jones AL, Castillo RC, et al. The relationship between time to surgical debridement and incidence of infection after open high-energy lower extremity trauma. J Bone Joint Surg Am. 2010;92:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srour M, Inaba K, Okoye O, et al. Prospective evaluation of treatment of open fractures: effect of time to irrigation and debridement. JAMA Surg. 2015;150:332–336. [DOI] [PubMed] [Google Scholar]
- 21.Khatod M, Botte MJ, Hoyt DB, et al. Outcomes in open tibia fractures: relationship between delay in treatment and infection. J Trauma. 2003;55:949–954. [DOI] [PubMed] [Google Scholar]
- 22.Obremskey W, Molina C, Collinge C, et al. Current practice in the management of open fractures among orthopaedic trauma surgeons. Part A: initial management. A survey of orthopaedic trauma surgeons. J Orthop Trauma. 2014;28:e198–e202. [DOI] [PubMed] [Google Scholar]
- 23.Halawi MJ, Morwood MP. Acute management of open fractures: an evidence-based review. Orthopedics. 2015;38:e1025–e1033. [DOI] [PubMed] [Google Scholar]
- 24.Investigators F, Bhandari M, Jeray KJ, et al. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med. 2015;373:2629–2641. [DOI] [PubMed] [Google Scholar]
- 25.Anglen JO. Wound irrigation in musculoskeletal injury. J Am Acad Orthop Surg. 2001;9:219–226. [DOI] [PubMed] [Google Scholar]
- 26.You DZ, Schneider PS. Surgical timing for open fractures: middle of the night or the light of day, which fractures, what time? OTA Int. 2020;3:e067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tornetta P, III, Bergman M, Watnik N, et al. Treatment of grade-IIIb open tibial fractures. A prospective randomised comparison of external fixation and non-reamed locked nailing. J Bone Joint Surg Br. 1994;76:13–19. [PubMed] [Google Scholar]
- 28.Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures I, Bhandari M, Guyatt G, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am 2008;90:2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br. 1995;77:93–97. [PubMed] [Google Scholar]
- 30.Scharfenberger AV, Alabassi K, Smith S, et al. Primary wound closure after open fracture: a prospective cohort study examining nonunion and deep infection. J Orthop Trauma. 2017;31:121–126. [DOI] [PubMed] [Google Scholar]
- 31.Blum ML, Esser M, Richardson M, et al. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma. 2012;26:499–505. [DOI] [PubMed] [Google Scholar]
- 32.Gopal S, Majumder S, Batchelor AG, et al. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82:959–966. [DOI] [PubMed] [Google Scholar]
- 33.D'Alleyrand JC, Manson TT, Dancy L, et al. Is time to flap coverage of open tibial fractures an independent predictor of flap-related complications? J Orthop Trauma. 2014;28:288–293. [DOI] [PubMed] [Google Scholar]
- 34.Cierny G, III, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. [DOI] [PubMed] [Google Scholar]
- 35.Gayle LB, Lineaweaver WC, Oliva A, et al. Treatment of chronic osteomyelitis of the lower extremities with debridement and microvascular muscle transfer. Clin Plast Surg. 1992;19:895–903. [PubMed] [Google Scholar]
- 36.Siegel HJ, Patzakis MJ, Holtom PD, et al. Limb salvage for chronic tibial osteomyelitis: an outcomes study. J Trauma. 2000;48:484–489. [DOI] [PubMed] [Google Scholar]