Abstract
Open tibia fractures are the most common open long bone injury. Most of these injuries involve a high-energy mechanism. Many standards for management have been created to provide guidance and a baseline for quality. There are several factors that must be considered when determining the timing of coverage for an open fracture with soft tissue compromise. Understanding the available options for soft tissue coverage, including local/rotational flaps and free tissue transfer, will allow for a tailored approach based on the personality of the injury. The aim of this review was to characterize the critical window of treatment based on the current literature and to provide a review of the available soft tissue coverage options.
Keywords: tibia, fracture, coverage, free flap
1. Introduction
Open tibia fractures are the most common open long bone injury. There is an incidence of 16.9/100,000 with a bimodal distribution with peaks at ages 20 and 50 years.1 Most of these injuries involve a high-energy mechanism, such as traffic accidents and sports injuries; however, lower-energy mechanisms have also been characterized. Approximately one-fourth of tibia fractures are open, of which 60% are classified as Gustilo type III.2 These statistics shed light on the high-energy nature of these injuries which contributes to the complexity of their management.
Many standards for management have been created to provide guidance and a baseline for quality. The basic principles and tenants of open fracture management have remained consistent since the seminal works of Gustilo and Anderson as well as Patzakis, among others, in the 1970s. These standards are centered around early antibiotic administration and a thorough surgical debridement. In addition, in the setting of a Gustilo and Anderson type IIIB injury where soft tissue coverage is required, an equally important tenet of care is appropriately addressing the soft tissue envelope. The most concerning sequelae of an inadequate soft tissue envelope is deep infection and osteomyelitis. A staged approach is often considered the standard of care. This involves an initial thorough debridement and provisional stabilization followed by definitive fixation and soft tissue coverage. This approach requires a coordinated multidisciplinary approach to address both the bony and soft tissue components of the injury.
The modifiable factors pertaining to the soft tissue component of these injuries are (1) the thoroughness of the debridement, (2) the timing of soft tissue coverage, and (3) the techniques surrounding the soft tissue coverage. The general consensus is that the earlier the wound is covered, the better to mitigate the risk of infection. However, when coordinating a multidisciplinary approach, it is imperative to understand the window for treatment such that the outcome is not compromised. In addition, understanding the available options for soft tissue coverage, including local/rotational flaps and free tissue transfer, will allow for a tailored approach based on the personality of the injury. The aim of this review was to characterize the critical window of treatment based on the current literature and to provide a review of the available soft tissue coverage options.
2. Timing of Coverage
There are several factors that must be considered when determining the timing of coverage for an open fracture with soft tissue compromise. Early administration of antibiotics, properly defining the injury/wound, and an early and sufficient debridement all set the stage for a successful coverage plan.
2.1. Timing of Antibiotics
One of the most critical components of open fracture treatment is the timely administration of antibiotics. Patzakis et al reviewed 1104 open fractures and found that the single most important factor to reduce the risk of infection was early administration of antibiotics. They found that when antibiotics were administered within 3 hours of injury, the infection rate was 4.7%, compared with 7.4% when antibiotics were administered after 3 hours from the time of injury.3,4 More recent literature, specifically examining type III open tibia fractures, found that antibiotics administration greater than 66 minutes from the time of injury was an independent predictor of infection.5 Unfortunately, time from injury to presentation to the hospital is a variable that is not in the control of the treating physician. The consensus is that antibiotics be administered “as soon as possible.” Based on the current literature, the American College of Surgeons Trauma Quality Improvement Program recommends antibiotic administration within 60 minutes of presentation to the hospital.6
2.2. Defining the Zone of Injury & Timing of Surgical Debridement
Characterization of the injury is of utmost importance in determining the appropriate treatment. A clear understanding of the zone of injury is one of the most important factors that will allow for informed shared decision making to guide each step of care. There are several classifications that have been established to help categorize these injuries and guide care. The most well-known classification system regarding open fractures was initially described by Gustilo and Anderson in 1976 and subsequently modified to further subdivide type III open fractures.7,8 Another commonly used classification is the Orthopaedic Trauma Association Open Fracture Classification (OTA-OFC).9 However, the reliability of agreement of classification systems is not without limitations in part because of the lack of a consistent systematic approach to defining the zone of injury.10,11
Grading of the open fractures should occur only after a thorough debridement to avoid underestimation of the extent of soft tissue injury. The misclassification of a type IIIB open fracture as a type IIIA open fracture will result in a poor soft tissue envelope, increasing the risk of infection/osteomyelitis. For this reason, understanding how to assess the zone of injury is of utmost importance and critical to allowing the treatment of the injury to move forward and minimizing future sequelae. The initial debridement should be directed by the most senior surgeon and performed in a systematic fashion. The goal is the removal of all foreign material/contamination and nonviable tissue to provide a clean wound. Attention should not be given to the reconstructive strategy until a thorough debridement has been performed.
The zone of injury can be defined by maintaining a systematic approach by assessing the wound from superficial to deep. This requires extension of the wound to allow for examination of the entire zone of injury. Longitudinal incisions should be made with incorporation of traumatic lacerations. Care should be taken to avoid the medial subcutaneous border of the tibia as this will increase the potential are increasing the area requiring coverage.12 Soft tissue debridement should be performed without a tourniquet to allow for assessment of viability. Skin and subcutaneous tissue should be debrided circumferentially until there is punctate bleeding. Muscle viability can be assessed by using the 4 C's: contractility on being pinched, consistency (not waxy or “stewy”), capacity to bleed on being cut, and color (red, not pale or brown). If there is any concern for viability, the muscle should be excised to avoid a nidus for bacterial growth. Assessing bone is the most difficult aspect of the debridement. It is critical to examine bone for punctate bleeding. Bone without evidence of bleeding and devoid of soft tissue attachments should be removed. Articular fragments are the exception to the rule. These fragments should be retained. All efforts should be made to perform a thorough debridement during the index procedure to allow for expeditious skeletal stabilization and soft tissue coverage. Where tissue viability is questionable, a repeat debridement can be performed before reconstruction. However, the goal should be for an initial debridement that only leaves viable tissue.13 Under most conditions, if subsequent debridements are necessary, it is because of inadequate previous debridements. Furthermore, it is important to recognize that the zone of injury is dynamic; it can decrease in size as traumatized tissue recovers, but it can also increase especially in circumstances of infected nonviable tissue.
The timing of debridement is another factor that should be considered with these injuries. The urgency of surgical debridement remains controversial. The origins of the “6-hour rule” are thought to be based on experiments on guinea pigs in 1898 by a German military surgeon, Friedrich.14 The conclusions of this study were that beyond 6 hours, contaminated soft tissues showed exponential growth of bacteria increasing the risk of infection. Multiple studies have failed to corroborate the 6-hour rule showing no significant difference in the infection rate when the debridement of the open fracture was performed with 12 or 24 hours.5,15–18 Much of the initial surgical treatment of these injuries is dictated by the clinical decision making of the surgeon and individualized based on injury-specific factors such as open fracture type/degree of injury, level of contamination, and severity of soft tissue injury. It is our belief that the quality of debridement is a more important factor than timing of debridement.
2.3. Timing of Soft Tissue Coverage
Each of the factors discussed (appropriately defining the injury/wound, early antibiotic administration, and a thorough systematic debridement) has a unified goal of preventing a deep infection. Similarly, and of equal importance, in the setting of type IIIB open tibia fractures is wound coverage. Soft tissue coverage should be performed as soon as possible. However, there are multiple components to consider when planning for soft tissue coverage. These include having an amenable wound, fixation strategy, and coordination of the multidisciplinary team. Understanding the critical time point in which the rate of infection rises is important when coordinating care. Maintaining a sense of urgency is critical when planning each component of care.
There are several guidelines that have been established for the soft tissue coverage of open fractures. The American College of Surgeons Trauma Quality Improvement Program states that “soft tissue coverage should be completed within 7 days of injury for open fractures associated with wounds requiring skin grafting or soft tissue transfers.”6 The British Orthopaedic Association's Standards for Trauma Number 4 (BOAST-4), which was updated in 2017, states that “definitive soft tissue closure or coverage should be achieved within 72 hours of injury if it cannot be performed at the time of debridement.”19 The lack of a unified clear recommendation is indicative of the lack of strength of the literature.
Historically, early wound coverage/closure was believed to be a risk factor for infection due to the retained bacterial colonization.20,21 This treatment strategy prevailed in a time where antibiotics were not available and the debridement strategies were rudimentary. With advances in open fracture management and a more in depth understanding of antimicrobial treatment, concerns with acutely addressing the soft tissue component of open fractures diminished.22–24
Marko Godina, a pioneer of reconstructive microsurgery, was one of the initial advocates for aggressive debridement and early coverage. In his series of 532 patients undergoing reconstructive microsurgery after trauma, he compared the results in patients who underwent free-flap transfer within 72 hours, between 72 hours and 3 months, and beyond 3 months. He found that flap failure, postoperative infection, time to bony healing, average length of hospitalization, and average number of operations was most favorable in the patients who underwent free-flap transfer within 72 hours.25 Lack et al evaluated 137 patients with type III open tibia fractures to determine factors associated with deep infection. The authors found that time from injury to antibiotics and to wound coverage were independent predictors of infection. Specifically, a delay in definitive wound closure/coverage more than 5 days was associated with deep infection. Subgroup analysis of specifically type IIIB open tibia fractures showed that a delay of coverage beyond 3 days was predictive of deep infection.5 Gopal et al examined the collaborative “fix and flap” approach compared with the standard approach of fixation followed by soft tissue coverage. Overall, there was a flap failure of 3.5% with a deep infection rate of 9.5%. Outcome trends were most favorable in the single-stage procedure with an infection rate of 3%. A delay in coverage beyond 72 hours was associated with an infection rate of 19%.26 Haykal et al27 performed a meta-analysis further examining Godina's principle of early reconstruction. The authors included 43 articles in their comparison of early versus delayed versus late reconstruction. Early free-flap reconstruction performed within 72 hours was associated with a decrease in flap failure, infection rate, and number of additional procedures. Finally, Pinkus et al28 examined 672 patients at 140 levels I and II trauma centers comparing early (<7 days) and delayed (>7 days) coverage. The authors found that delayed coverage was associated with increased risk of deep infection and osteomyelitis. Each additional week of delay increased the adjusted risk of these complications by 40%. The authors concluded that their findings were consistent with the recommendations by the American College of Surgeons Trauma Quality Improvement Program. A limitation of this study is that periods shorter than 1 week were not examined. Therefore, conclusions cannot be made based on soft tissue coverage performed more acutely, that is, within 72 hours, as was examined by the other studies. Similarly, studies defining early flap coverage as <72 hours did not provide a comparison group of 72 hours to 7 days. The delayed group in most studies was defined as reconstruction between 72 hours and 3 months. The single study comparing <72 hours to 3–7 days did not find any significant differences in outcomes. Another timeframe to consider is the time between definitive fixation and coverage. Kuripala et al29 retrospectively examined 296 patients with type III open tibia fractures requiring soft tissue coverage. In their multivariate regression, the time from definitive fixation to flap coverage was the most predictive of wound infection. The time from injury to flap coverage and time from debridement to flap coverage were not found to be statistically significant risk factors for infection. Patients who developed an infection had an average time between definitive fixation and coverage of 7.30 days (±9.23) compared with 4.87 days (±6.60) for those who did not develop an infection.
Despite the variability and limitations associated with the current literature, striving for early flap coverage remains the standard of care. Based on this variability, each institution can make a determination of whether “early coverage” is defined as <72 hours or <7 days but should continue to re-examine the literature and adjust guidelines as indicated. As it stands, with the current guidelines, most of these studies found that most patients did not obtain soft tissue coverage within the recommended timeframe. Pincus et al28 found that more than 60% of patients in North America did not receive flap coverage with the recommended 7 days. The complexity of these injuries creates hurdles to care that are difficult to navigate, given the necessity for a multidisciplinary approach. A continued effort to establish initiatives is necessary to improve the care of patients with these injuries. It is our belief that an open wound is highly inflammatory and greater degrees of inflammation in the leg are directly correlated with factors such as difficulty of identifying and safely exposing the recipient vessels, unreliability of the recipient vessels in regards to vasospasm and thrombosis, and lowering the threshold of infection. Thus, minimizing the time of an open wound is critical to success.
3. Coverage Options
A general understanding of reconstructive options is imperative when planning the definitive management of type IIIB open tibia fractures. The reconstructive ladder serves as a guide when considering coverage options and adheres to the principle of using the simplest option that will achieve an optimal functional outcome.30 The key wording here is “optimal functional outcome” and is the basis of our general algorithm: (1) use the simplest option to achieve optimal function, (2) choose a complex reconstructive method if it will provide a meaningfully better long-term outcome, (3) replace like with like, and (4) anticipate the long-term reconstructive needs of the patient. Numerous factors need to be considered when determining the appropriate coverage options. These include size of defect, location of defect, zone of injury, type of donor tissue required, pedicle length, required durability, contour of the limb, functional potential of the patient, donor site morbidity, recipient vessels, and other orthopaedic injuries. More practically, there are a number of local flap and free-flap options for coverage of the open tibia fractures, each with advantages and limitations.
The location of the soft tissue defect plays a major role in dictating coverage options. The leg is divided into 3 zones: proximal third, middle third, and distal third. Soft tissue coverage in the proximal and middle thirds can often be achieved with local flaps. The medial head gastrocnemius rotational flap has served as work horse for coverage over the proximal third of the leg including the knee. The gastrocnemius rotational flap is based on the medial sural artery, which is a branch of the popliteal artery. Although this flap does not require a microvascular anastomosis, this can also be a limitation in its ability to cover more distant defects. Modifications have been made to the dissection of this flap to address these limitations; however, this requires a greater technical skillset and comfort with pedicle dissection in the popliteal fossa. Similarly, the hemisoleus pedicled muscle flap is a reliable option for coverage of the middle third of the tibia. The blood supply to the soleus is more variable with perforators from the popliteal trunk, posterior tibial artery, and peroneal artery. For larger defects, the medial soleus and gastrocnemius can be used simultaneously for coverage. After gastrocnemius or soleus rotational flaps, patients do not seem to have donor site morbidity based on gait analysis. However, there may be limitations with more demanding tasks such as fast walking or uphill walking.31
When considering coverage options for distal third tibia fractures, this territory is often considered to require free tissue transfer. However, there are local options that should be considered depending on the size and location of the defect. The peroneus brevis flap, based on perforators from the peroneal vessels, is a viable option for small defects in the distal leg and ankle. Another local flap is the reverse sural artery flap, which is a fasciocutaneous flap based on an anastomosis between the medial superficial sural artery and the peroneal artery (Fig. 1). Both of these local flaps are based on relatively short pedicles and rely on the length of the flap to provide distant coverage. This often requires significant rotation of the flap, which often results in bulk and concern for the patency of the vessels. Consequently, these flaps are fraught with complication.32 The primary issue with these flaps is a common phenomenon seen in soft tissue coverage where partial flap loss occurs in critical areas, most often at the distal extent of the flap, requiring further flap coverage. Given these inherent limitations and the significant morbidity associated with flap failure, free tissue transfer is often the more reliable option for critical wounds in the distal third of the leg.
Figure 1.
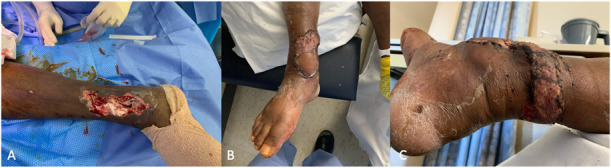
A, A medial wound over the distal tibia/ankle with exposed bone and hardware. B, A reverse sural artery flap was used to cover the defect. C, The rotational point of the flap requiring split-thickness skin graft.
Free flaps used for tibia coverage in the acute setting are often fasciocutaneous or muscular. Myofasciocutaneous flaps and those which include bone flaps are described, however, less common in the acute coverage setting. Multiple retrospective studies have compared fasciocutaneous flaps and muscle flaps with comparable results regarding complication rate and functional outcomes.33–36 Each flap type has utility, and selection should be individualized based on patient and injury factors.
Fasciocutaneous flaps have the benefit of improved postoperative monitoring, improved esthetic outcome, and easier elevation for staged bone grafting due to the neovascularization at the flap perimeter. The most common fasciocutaneous flap used for the coverage of type IIIB tibia fractures is the anterolateral thigh flap (Fig. 2). This flap is based on the descending branch of the lateral circumflex femoral artery. It has the ability to be harvested with a long pedicle to allow for distant anastomosis out of the zone of injury. In addition, the anterolateral thigh flap can be used for the coverage of large defects (8 cm × 25 cm) while also allowing for primary closure of the donor site. The most common donor site complications associated with this flap are paresthesias, wound dehiscence, contour deformity, and seroma.37 Depending on the body habitus of the individual, the thickness of this flap can often times be cosmetically unappealing requiring flap thinning/secondary contouring. Other commonly used flaps for smaller defects include the radial forearm free flap and medial sural artery perforator flap (Fig. 3).
Figure 2.

A, An open distal tibia fracture along the distal medial aspect of the leg complicated by infection resulting in nonunion and requiring multiple debridements. B, Anterolateral thigh flap. Not the length of the pedicle, which allows for anastomosis out of the zone of injury. C and D, Inset of the anterolateral thigh flap.
Figure 3.
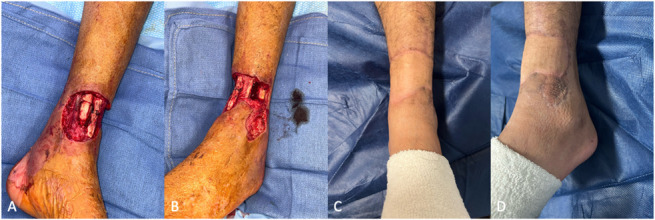
A and B, An open distal tibia fracture with a soft tissue defect involving with exposed bone, tendon, and neurovascular structures. C and D, A radial forearm free flap was used to cover the defect with anastomosis to the anterior tibial artery. The relatively thin nature of this fasciocutaneous flap allows for excellent contour that does not limit shoe wear.
Muscle flaps, unlike fasciocutaneous flaps, have the benefit of atrophying over time resulting in improved cosmesis. In addition, muscle flaps have the improved ability to contour to complex three-dimensional defects. Common muscle flaps include the latissimus dorsi muscle flap and the gracilis muscle flap (Fig. 4). The latissimus dorsi is particularly useful in the setting of large defects involving a majority of the leg due to its ability to cover defects >400 cm2.38 These flaps can be taken as a myofasciocutaneous flaps with a paddle to allow for both flap monitoring and wound closure; however, depending on the defect size/depth, this can often add unwanted bulk. Secondary bone grafting is often more difficult after muscle flaps due to the fibrosis and atrophy of the flap as well as the permanent reliance of the flap on the pedicle without significant peripheral collateral neovascularization.
Figure 4.

A, An open tibia fracture with a significant soft tissue defect involving skin, subcutaneous tissue, and muscle as well as a large bony defect with exposed bone, tendon, and neurovascular structures. B, Latissimus dorsi free flap with a paddle for monitoring. C, After inset and anastomosis of the flap, the latissimus dorsi can provide coverage to relatively large defects.
4. Summary
Open tibia fractures are inherently complex injuries that require a multidisciplinary approach. These injuries often involve a high-energy mechanism with significant soft tissue compromise making them highly susceptible to wound complications, infection, nonunion, and prolonged/permanent functional deficits. There must a continued vigilance in advancing care for these injuries regarding the timing of antibiotic administration, thoroughness of initial debridement, and coordination of timely fracture fixation and soft tissue coverage. As it stands, these injuries often do not undergo soft tissue coverage within the timeframe established by expert panels. This is evidence of the continued work that needs to be accomplished to develop multidisciplinary teams that are able to provide expedited coordinate care for these complex injuries to ensure the best possible patient outcome.
Footnotes
Source of funding: Nil.
The authors declare that there is no conflict of interest.
The study was deemed exempt from Institutional Review Board and Animal Use Committee Review.
References
- 1.Anandasivam NS, Russo GS, Swallow MS, et al. Tibial shaft fracture: a large-scale study defining the injured population and associated injuries. J Clin Orthop Trauma. 2017;8:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77:417–421. [PubMed] [Google Scholar]
- 3.Patzakis MJ, Wilkins J, Moore TM. Use of antibiotics in open tibial fractures. Clin Orthop Relat Res. 1983;178:31–35. [PubMed] [Google Scholar]
- 4.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;243:36–40. [PubMed] [Google Scholar]
- 5.Lack WD, Karunakar MA, Angerame MR, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma. 2015;29:1–6. [DOI] [PubMed] [Google Scholar]
- 6.American College of Surgeons trauma quality improvement program. Best Pract Manage Orthop Trauma. 2015:1–38. [Google Scholar]
- 7.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 8.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742–746. [DOI] [PubMed] [Google Scholar]
- 9.Orthopaedic Trauma Association: Open Fracture Study Group. A new classification scheme for open fractures. J Orthop Trauma. 2010;24:457–464. [DOI] [PubMed] [Google Scholar]
- 10.Horn BD, Rettig ME. Interobserver reliability in the Gustilo and Anderson classification of open fractures. J Orthop Trauma. 1993;7:357–360. [DOI] [PubMed] [Google Scholar]
- 11.Brumback RJ, Jones AL. Interobserver agreement in the classification of open fractures of the tibia. The results of a survey of two hundred and forty-five orthopaedic surgeons. J Bone Joint Surg Am. 1994;76:1162–1166. [DOI] [PubMed] [Google Scholar]
- 12.Marecek GS, Nicholson LT, Auran RT, et al. Use of a defined surgical approach for the debridement of open tibia fractures. J Orthop Trauma. 2018;32:e1. [DOI] [PubMed] [Google Scholar]
- 13.Sacks H, Hu J, Devendra A, et al. Relationship between number of debridements and clinical outcomes in open tibia fractures requiring free flap coverage: a retrospective cohort study. Orthoplastic Surg. 2023;14:9–14. [Google Scholar]
- 14.Friedrich PL, Die aseptische versorgung frischer wundern. Langenbecks Arch fur Klin Chir. 1898;57:288–310. [Google Scholar]
- 15.Crowley DJ, Kanakaris NK, Giannoudis PV. Debridement and wound closure of open fractures: the impact of the time factor on infection rates. Injury. 2007;38:879–889. [DOI] [PubMed] [Google Scholar]
- 16.Al-Arabi YB, Nader M, Hamidian-Jahromi AR, et al. The effect of the timing of antibiotics and surgical treatment on infection rates in open long-bone fractures: a 9-year prospective study from a district general hospital. Injury. 2007;38:900–905. [DOI] [PubMed] [Google Scholar]
- 17.Skaggs DL, Friend L, Alman B, et al. The effect of surgical delay on acute infection following 554 open fractures in children. J Bone Joint Surg Am. 2005;87:8–12. [DOI] [PubMed] [Google Scholar]
- 18.Srour M, Inaba K, Okoye O, et al. Prospective evaluation of treatment of open fractures: effect of time to irrigation and debridement. JAMA Surg. 2015;150:332–336. [DOI] [PubMed] [Google Scholar]
- 19.Boast 4: The Management of Severe Open Lower Limb Fractures. British Orthopaedic Association, 2017. Available at: https://www.boa.ac.uk/resource/boast-4-pdf.html. Accessed October 1, 2023. [Google Scholar]
- 20.Patzakis MJ, Dorr LD, Hammond W, et al. The effect of antibiotics, primary and secondary closure on clostridial contaminated open fracture wounds in rats. J Trauma. 1978;18:34–37. [DOI] [PubMed] [Google Scholar]
- 21.Davis AG, Erie MD. Primary closure of compound fracture wounds. J Bone Joint Surg Am. 1948;30:405–415. [PubMed] [Google Scholar]
- 22.Weitz-Marshall AD, Bosse MJ. Timing of closure of open fractures. J Am Acad Orthop Surg. 2002;10:379–384. [DOI] [PubMed] [Google Scholar]
- 23.Zalavras CG, Patzakis MJ. Open fractures: evaluation and management. J Am Acad Orthop Surg. 2003;11:212–219. [DOI] [PubMed] [Google Scholar]
- 24.Scharfenberger AV, Alabassi K, Smith S, et al. Primary wound closure after open fracture: a prospective cohort study examining nonunion and deep infection. J Orthop Trauma. 2017;31:121–126. [DOI] [PubMed] [Google Scholar]
- 25.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–292. [DOI] [PubMed] [Google Scholar]
- 26.Gopal S, Majumder S, Batchelor AG, et al. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82:959–966. [DOI] [PubMed] [Google Scholar]
- 27.Haykal S, Roy M, Patel A. Meta-analysis of timing for microsurgical free-flap reconstruction for lower limb injury: evaluation of the Godina principles. J Reconstr Microsurg. 2018;34:277–292. [DOI] [PubMed] [Google Scholar]
- 28.Pincus D, Byrne JP, Nathens AB, et al. Delay in flap coverage past 7 Days increases complications for open tibia fractures: a cohort study of 140 North American Trauma Centers. J Orthop Trauma. 2019;33:161–168. [DOI] [PubMed] [Google Scholar]
- 29.Kuripla C, Tornetta P, Foote CJ, et al. Timing of flap coverage with respect to definitive fixation in open tibia fractures. J Orthop Trauma. 2021;35:430–436. [DOI] [PubMed] [Google Scholar]
- 30.Shafiq B, Hacquebord J, Wright DJ, et al. Modern principles in the acute surgical management of open distal tibial fractures. J Am Acad Orthop Surg. 2021;29:e536. [DOI] [PubMed] [Google Scholar]
- 31.Kramers-de Quervain IA, Läuffer JM, Käch K, et al. Functional donor-site morbidity during level and uphill gait after a gastrocnemius or soleus muscle-flap procedure. J Bone Joint Surg Am. 2001;83:239–246. [DOI] [PubMed] [Google Scholar]
- 32.Parrett BM, Pribaz JJ, Matros E, et al. Risk analysis for the reverse sural fasciocutaneous flap in distal leg reconstruction. Plast Reconstr Surg. 2009;123:1499–1504. [DOI] [PubMed] [Google Scholar]
- 33.Fox CM, Beem HM, Wiper J, et al. Muscle versus fasciocutaneous free flaps in heel reconstruction: systematic review and meta-analysis. J Reconstr Microsurg. 2015;31:59–66. [DOI] [PubMed] [Google Scholar]
- 34.Sabino J, Polfer E, Tintle S, et al. A decade of conflict: flap coverage options and outcomes in traumatic war-related extremity reconstruction. Plast Reconstr Surg. 2015;135:895–902. [DOI] [PubMed] [Google Scholar]
- 35.Cho EH, Shammas RL, Carney MJ, et al. Muscle versus fasciocutaneous free flaps in lower extremity traumatic reconstruction: a multicenter outcomes analysis. Plast Reconstr Surg. 2018;141:191–199. [DOI] [PubMed] [Google Scholar]
- 36.Lee ZH, Abdou SA, Daar DA, et al. Comparing outcomes for fasciocutaneous versus muscle flaps in foot and ankle free flap reconstruction. J Reconstr Microsurg. 2019;35:646–651. [DOI] [PubMed] [Google Scholar]
- 37.Lakhiani C, DeFazio MV, Han K, et al. Donor-site morbidity following free tissue harvest from the thigh: a systematic review and pooled analysis of complications. J Reconstr Microsurg. 2016;32:342–357. [DOI] [PubMed] [Google Scholar]
- 38.Hacquebord JH, Hanel DP, Friedrich JB. The pedicled latissimus dorsi flap provides effective coverage for large and complex soft tissue injuries around the elbow. Hand (N Y). 2018;13:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]


