Highlights
-
•
Patients with systolic heart failure admitted for pneumonia had worse in-hospital outcomes compared to patients with diastolic heart failure.
-
•
There were no substantial differences in cost or length of stay between systolic and diastolic HF patients admitted for pneumonia.
-
•
Patients with systolic heart failure were frequently not prescribed heart failure treatments during hospitalization, even when they became hemodynamically stable enough to discharge home.
-
•
Among patients hospitalized for pneumonia, there is a need to optimize guideline-directed medications in systolic HF prior to discharge.
Keywords: Systolic heart failure, Diastolic heart failure, Pneumonia
Abstract
Background
Patients admitted with pneumonia and heart failure (HF) have increased mortality and cost compared to those without HF, but it is not known whether outcomes differ between systolic and diastolic HF. Management of concomitant pneumonia and HF is complicated because HF treatments can worsen complications of pneumonia.
Methods
This is a retrospective cohort study from the Premier Database among patients admitted with pneumonia between 2010 and 2015. Patients were categorized based on systolic, diastolic, and combined HF using ICD-9 codes. The primary outcome was in-hospital mortality. Secondary outcomes included use of HF medications, length of stay, cost, intensive care unit (ICU) admission, as well as use of invasive mechanical ventilation (IMV), vasopressors and inotropes. Multivariable logistic regression was used to describe associations of these outcomes with type of HF.
Results
Of 123,211 patients with pneumonia and HF, 41,196 (33.4%) had systolic HF, 69,982 (56.8%) diastolic HF, and 12,033 (9.8%) had combined HF. Compared to patients with diastolic HF, after multivariable adjustment systolic HF was associated with higher in-hospital mortality (OR 1.15; 95% CI:1.11–1.20), ICU admission, and use of IMV and vasoactive agents, but not with increased length of stay or cost. Among patients with systolic HF, 80% received a loop diuretic, 72% a beta blocker, 48% angiotensin converting enzyme inhibitor or angiotensin receptor blocker, and 12.5% a mineralocorticoid receptor antagonist.
Conclusion
Systolic HF is associated with added risk in pneumonia compared to diastolic HF. There may also be an opportunity to optimize medications in systolic HF prior to discharge.
Introduction
Pneumonia and heart failure (HF) are two of the most common reasons for hospital admission. They often co-exist—more than 25% of patients hospitalized for pneumonia also have concomitant HF.1 Among patients hospitalized for pneumonia, mortality ranges from 10 to 15% and one study found that concomitant HF increased mortality by 50%.2,3 Furthermore, the management of pneumonia and HF can be challenging given that treatments for HF can worsen commonly encountered complications of pneumonia such as hypotension and kidney injury. These competing priorities—maintaining blood pressure while avoiding fluid overload—complicate treatment decisions.
It was previously thought that outcomes were worse with systolic HF than diastolic HF,4,5 but more recent literature suggests they are comparable.6 The presence of HF appears to increase mortality from pneumonia,7 but previous studies did not evaluate outcomes of hospitalized pneumonia patients according to the subtypes of HF. In addition, withholding HF medications from outpatients with pneumonia is associated with poorer outcomes.8 Although it is appropriate to temporarily withhold guideline directed medical therapy (GDMT) due to acute kidney injury or hypotension, it is important to reassess appropriateness prior to discharge to ensure that these essential medications are not inadvertently discontinued.
In this retrospective cohort study, we aimed to describe the prevalence, characteristics, cost, treatment, and outcomes of systolic and diastolic HF among patients hospitalized with pneumonia.
Materials and methods
Data source
We conducted a retrospective cohort study of patients admitted to a Premier Database Hospital for pneumonia from July 1, 2010 to June 30, 2015. The database contains information derived from the uniform billing 04 (UB-04) form such as age, gender, International Classification of Diseases 9th version (ICD-9), and hospital and physician information as well as all items and services charged to the patient or their insurer, including medications, procedures, and laboratory tests. Data are collected electronically from participating hospitals and audited regularly to ensure data completeness. The database represents approximately 25% of all annual US hospital admissions9 and has been extensively used in clinical, epidemiologic, and outcomes research.10, 11, 12
Patients
We identified all adult patients, age ≥18 years, that were admitted with a principal ICD-9 code of pneumonia (481-488 or 507.0) or a secondary diagnosis code of pneumonia paired with a principal diagnosis of respiratory failure (518.81 and 518.84), acute respiratory distress syndrome (769), respiratory arrest (799.1), sepsis (995.91, 995.92) or influenza (487, 488). Moreover, each patient had to undergo a chest radiograph and receive antimicrobials by the second hospital day. We excluded any patient who was transferred to or from other acute care facilities because we could not assess their treatment prior to admission or outcomes. Finally, to assure a cohort most consistent with moderate to severe community-acquired pneumonia, we also excluded patients with a length of stay of less than 2 days, patients with cystic fibrosis, ventilator-associated pneumonia, those with a Medicare Severity Diagnosis-Related Group inconsistent with pneumonia or its sequelae, and those with a present-on-admission modifier code indicating that the pneumonia was hospital-acquired. Details of inclusion and exclusion criteria are found in Fig. 1.
Fig. 1.
Patient flowchart.
We then identified patients with a secondary diagnosis of HF (ICD-9 codes: 428.xx and 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.9) and either systolic (ICD-9 code: 428.20 -428.23), diastolic (ICD-9 code: 428.30 - 428.33) or combined systolic and diastolic HF (ICD-9 code: 428.40 - 428.43 or any combination of a systolic and diastolic HF code). Individuals that did not have an indication for being systolic, diastolic, or both (i.e., unknown) were excluded from the analyses.
Patient and hospital information
For each patient, in addition to age, sex, and race, we recorded the presence of several comorbidities. We used the Elixhauser Comorbidity Software from AHRQ, which has been used to predict in-hospital cost and mortality, to combine ICD-9 codes into larger diagnostic groups.13,14,15. We also recorded data on the use of HF medications including furosemide, thiazide, mineralocorticoid receptor antagonists, inotropic agents, afterload reducing agents (angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB), hydralazine, isosorbide dinitrate) and beta-blockers (carvedilol, metoprolol, and bisoprolol). In addition, we recorded key hospital characteristics including number of beds, geographic region and urban/rural location. Because the Premier database does not contain identifiable patient information, the Institutional Review Board at Cleveland Clinic determined that this study did not constitute human subject research.
Outcomes
Our primary outcome was inpatient mortality. Secondary outcomes included length of stay, cost, patterns of HF medication use, intensive care unit (ICU) transfer, acute kidney injury (AKI), use of mechanical ventilation, vasopressors and inotropes. Costs were inflation-adjusted to 2015 dollars by the medical care component of the Consumer Price Index by multiplying the cost by the ratio of this CPI component in 2015 to its value in the year in which the patient was treated.
Statistical analyses
We compared patients with and without heart failure and patients with systolic, diastolic and combined systolic and diastolic HF using frequencies, proportions, and chi-square tests for categorical data and medians, interquartile ranges, and Kruskal-Wallis rank analysis of variance tests for continuous variables. Mixed logistic regression analyses were used to examine the associations of the type of HF with dichotomous outcomes (in hospital mortality, acute kidney injury, use of ICU, IMV, Vasopressor and Inotropes/vasodilators) and log-link gamma generalized linear mixed models were used for associations with skewed continuous outcomes (length of stay and cost). All models included random hospital effects to account for patient clustering within hospital and were adjusted for hospital characteristics (Census Bureau region, number of beds, urban or rural, teaching or non-teaching), and patient baseline demographics, insurance status, a subset of Elixhauser comorbidities, and whether patients were receiving anticoagulant/antithrombotic therapy at baseline. All analyses were conducted using SAS version 9.4 (SAS Corporation, Cary, NC).
Results
Patient characteristics
Of 783,702 patients who met our inclusion criteria, 123,211 (17%) had a secondary diagnosis of HF; 88,992 (11%) were excluded for not having a specific type of HF, leaving 123,211 patients for our analysis. Of these, 41,196 (33.4%) had systolic HF, 69,982 (56.8%) had diastolic HF, and 12,033 (9.8%) had combined diastolic and systolic HF. The median age was 79 (95% CI, 68–86), 52% were women, 76.7% white, and Medicare was the most common primary health insurance. Overall, 60% of patients were in non-teaching hospitals, 84% were in urban hospitals, and 41% in hospitals with >400 beds.
Table 1 shows the characteristics of the sample stratified by HF type. Compared to patients with systolic HF, those with diastolic HF were older (79 vs. 77); they had similar combined comorbidity scores,16 but specific comorbidities differed by type of HF. Individuals with diastolic heart failure were more likely to have chronic pulmonary disease (55.4 vs 48.5%), hypertension (78.0 vs. 73.7%), obesity (21.3 vs 13.1%), pulmonary circulation disease (21.2 vs. 14.2%), and obstructive sleep apnea (15.7 vs. 10.3%). On the other hand, patients with systolic heart failure were more likely to have coronary artery disease (31.8 vs. 11.0%) and acute coronary syndrome (10.2 vs. 4.8%). Both were similarly likely to have valvular diseases, cardiac dysrhythmias, and atrial fibrillation, and a similar proportion of each had a DNR order. Patients with combined heart failure most closely resembled those with systolic heart failure. The hospital characteristics were very similar between systolic and diastolic HF with additional information provided in Table 2.
Table 1.
Baseline characteristics of the study participants.1
| Number (%) of patients |
||||
|---|---|---|---|---|
| Total (N = 123,211) | Systolic HF (n = 41,196) | Diastolic HF (n = 69,982) | Combined (n = 12,033) | |
| Age, Median (IQR), years | 79.0 (68.0 – 86.0) | 77.0 (67.0 – 85.0) | 79.0 (69.0– 87.0) | 78.0 (68.0– 86.0) |
| Gender, Female | 64,040 (52.0) | 16,112 (39.1) | 42,636 (60.9) | 5,292(44.0) |
| Race/Ethnicity | ||||
| White | 94,536 (76.7) | 30,952 (75.1) | 54,388 (77.7) | 9,196 (76.4) |
| Black | 12,843 (10.4) | 4,624 (11.2) | 6,955 (9.9) | 1,264 (10.5) |
| Hispanic | 548 (0.44) | 160 (0.39) | 326 (0.47) | 62 (0.52) |
| Others | 15,206 (12.3) | 5,433 (13.2) | 8,274 (11.8) | 1,499 (12.5) |
| Unknown | 78 (0.06) | 27 (0.07) | 39 (0.06) | 12 (0.10) |
| Insurance status | ||||
| Medicare | 104,228 (84.6) | 33,801 (82.0) | 60,250 (86.1) | 10,177 (84.6) |
| Medicaid | 6,605 (5.4) | 2,613 (6.3) | 3,354 (4.8) | 638 (5.3) |
| Managed Care | 6,615 (5.4) | 2,463 (6.0) | 3,532 (5.0) | 620 (5.2) |
| Commercial Indemnity | 2,085 (1.7) | 801 (1.9) | 1,099 (1.6) | 185 (1.5) |
| Others | 3,678 (3.0) | 1,518 (3.7) | 1,747 (2.5) | 413 (3.4) |
| Comorbidities | ||||
| Combined comorbidity scores, Median (IQR) | 5.0 (4.0 – 7.0) | 5.0 (4.0 – 7.0) | 5.0 (4.0 – 7.0) | 6.0 (4.0 – 7.0) |
| Combined comorbidity scores, Mean ± SD | 5.5 ± 2.4 | 5.5 ± 2.4 | 5.4 ± 2.3 | 5.9 ± 2.4 |
| Anemia | 46,039 (37.4) | 14,421(35.0) | 26,917 (38.5) | 4,701 (39.1) |
| Chronic pulmonary disease | 65,130 (52.9) | 19,985 (48.5) | 38,767 (55.4) | 6,378 (53.0) |
| Coagulopathy | 10,842 (8.8) | 4,017 (9.8) | 5,593 (8.0) | 1,232 (10.2) |
| Depression | 17,949 (14.6) | 5,193 (12.6) | 11,113 (15.9) | 1,643 (13.7) |
| Diabetes | 23,231 (18.9) | 7,072 (17.2) | 13,762 (19.7) | 2,397 (19.9) |
| Hypertension | 94,413 (76.6) | 30,380 (73.7) | 54,609 (78.0) | 9,424 (78.3) |
| Hypothyroidism | 6,822 (5.5) | 1,935 (4.7) | 4,232 (6.0) | 655 (5.4) |
| Liver disease | 3,470 (2.8) | 1,124 (2.7) | 1,993 (2.8) | 353 (2.9) |
| Obesity | 22,242 (18.1) | 5,396 (13.1) | 14,877 (21.3) | 1,969 (16.4) |
| Peripheral vascular disease | 16,994 (13.8) | 6,125 (14.9) | 8,854 (12.7) | 2,015 (16.7) |
| Pulmonary circulation disease | 23,217 (18.8) | 5,860 (14.2) | 14,836 (21.2) | 2,521 (21.0) |
| Valvular disease | 27,964 (22.7) | 9,437 (22.9) | 15,332 (21.9) | 3,195 (26.6) |
| CKD | 24,303 (19.7) | 8,537 (20.7) | 13,084 (18.7) | 2,682 (22.3) |
| Cardiac dysrhythmia | 242 (0.20) | 87 (0.21) | 131 (0.19) | 24 (0.20) |
| Atrial fibrillation | 51,914 (42.1) | 17,625 (42.8) | 28,949 (41.4) | 5,340 (44.4) |
| Coronary artery disease | 24,187 (19.6) | 13,108 (31.8) | 7,720 (11.0) | 3,359 (27.9) |
| Acute coronary syndrome | 8,834 (7.2) | 4,198 (10.2) | 3,360 (4.8) | 1,276 (10.6) |
| Mitral stenosis or insufficiency | 10,062 (8.2) | 3,894 (9.5) | 4,922 (7.0) | 1,246 (10.4) |
| Aortic stenosis or insufficiency | 8,151 (6.6) | 2,305 (5.6) | 4,979 (7.1) | 867 (7.2) |
| Obstructive sleep apnea | 16,818 (13.6) | 4,244 (10.3) | 11,021 (15.7) | 1,553 (12.9) |
| Drug abuse | 2,612 (2.1) | 1,087 (2.6) | 1,211 (1.7) | 314 (2.6) |
| Alcohol abuse | 3,492 (2.8) | 1,435 (3.5) | 1,645 (2.4) | 412 (3.4) |
| DNR Status | 21,360(17.3) | 6,855(16.6) | 12,434(17.8) | 2,071(17.2) |
p ≤ 0.001 for tests of homogeneity of the three groups (Pearson's chi-square for dichotomous or other categorical variables and Kruskal-Wallis rank analysis of variance for continuous variables) for each tabulated variable.
Table 2.
Medical Management during hospital stay of patients admitted with pneumonia according to systolic and diastolic heart failure.1
| Number (%) of patients |
|||||
|---|---|---|---|---|---|
| Treatment (any day) | Total (N = 123,211) | Systolic HF (n = 41,196) | Diastolic HF (n = 69,982) | Combined (n = 12,033) | P-value |
| ACEi or ARB | 50,915 (41.3) | 19,844 (48.2) | 25,652 (36.7) | 5,419 (45.0) | <0.001 |
| Loop Diuretics | <0.001 | ||||
| Oral | 62,384 (50.6) | 21,051 (51.1) | 34,989 (50.0) | 6,344 (52.7) | |
| Intravenous | 80,275 (65.2) | 26,588 (64.5) | 45,514 (65.0) | 8,173 (67.9) | |
| Any loop diuretic | 99,738 (80.9) | 33,081 (80.3) | 56,665 (81.0) | 9,992 (83.0) | |
| Mineralocorticoid receptor antagonist | 10,859 (8.8) | 5,144 (12.5) | 4,295 (6.1) | 1,420 (11.8) | <0.001 |
| Thiazide | 5,671 (4.6) | 1,892 (4.6) | 3,020 (4.3) | 759 (6.3) | <0.001 |
| Calcium channel blocker | 46,915 (38.1) | 11,529 (28.0) | 31,373 (44.8) | 4,013 (33.3) | <0.001 |
| Isosorbide Dinitrate | 14,354 (11.6) | 5,358 (13.0) | 7,226 (10.3) | 1,770 (14.7) | <0.001 |
| Beta blocker | 77,503 (62.9) | 29,585 (71.8) | 39,424 (56.3) | 8,494 (70.6) | <0.001 |
| Hydralazine | 18,725 (15.2) | 5,142 (12.5) | 11,695 (16.7) | 1,888 (15.7) | <0.001 |
| Digoxin | 22,619 (18.4) | 9,211 (22.4) | 10,843 (15.5) | 2,565 (21.3) | <0.001 |
| Antiarrhythmics | 18,231 (14.8) | 7,645 (18.6) | 8,493 (12.1) | 2,093 (17.4) | <0.001 |
| Antiplatelets | 73,783 (59.9) | 26,469 (64.3) | 39,604 (56.6) | 7,710 (64.1) | <0.001 |
| Anticoagulants | 53,307 (43.3) | 17,554 (42.6) | 30,650 (43.8) | 5,103 (42.4) | <0.001 |
| Fluid Administration | <0.001 | ||||
| Isotonic | 67,500 (54.8) | 22,829 (55.4) | 38,049 (54.4) | 6,622 (55.0) | |
| Hypertonic | 137 (0.11) | 50 (0.12) | 70 (0.10) | 17 (0.14) | |
| Hypotonic | 18,551 (15.1) | 6,175 (15.0) | 10,471 (15.0) | 1,905 (15.8) | |
| Any fluid | 72,654 (59.0) | 24,470 (59.4) | 41,028 (58.6) | 7,156 (59.5) | |
p ≤ 0.001 for tests of homogeneity of the three groups (Pearson's chi-square for dichotomous or other categorical variables and Kruskal-Wallis rank analysis of variance for continuous variables) for each tabulated variable.
ACEi, Angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Medical management
Table 2 shows the medications received by patients stratified by type of HF. Patients with systolic HF were more likely to receive ACEi, ARBs, mineralocorticoid receptor antagonist, beta blockers, digoxin, and antiplatelet therapy. Diastolic HF patients were more likely to receive calcium channel blockers and hydralazine. Both were equally likely to receive intravenous (IV) fluids, oral and intravenous loop diuretics, and thiazides. During admission, 80% of patients with systolic HF received a loop diuretic (IV and oral), 72% received a beta blocker, 48% received ACEi/ARB, and 12.5% received mineralocorticoid receptor antagonist; rates of treatment were lower on hospital day one, when only 41% received loop diuretics, 31.5% beta-blockers, 13.5% ACEi/ARB, and 3% mineralocorticoid receptor agonists. Furthermore, 59% received IV fluids, and 35% received both fluids and loop diuretics on the same day. Similarly, in patients with diastolic HF, 81% received a loop diuretic (IV and oral), 56% received a beta blocker, 36.7% received an ACEi/ARB, and 6% received mineralocorticoid receptor antagonist; 59% received intravenous fluids and 34% received fluids and diuretics on the same day. Similar to systolic HF, combined HF were more likely to receive ACEi or ARBs, mineralocorticoid receptor antagonist, beta blockers, digoxin, and antiplatelet therapy, but they received more calcium channel blockers and hydralazine.
Patient outcomes
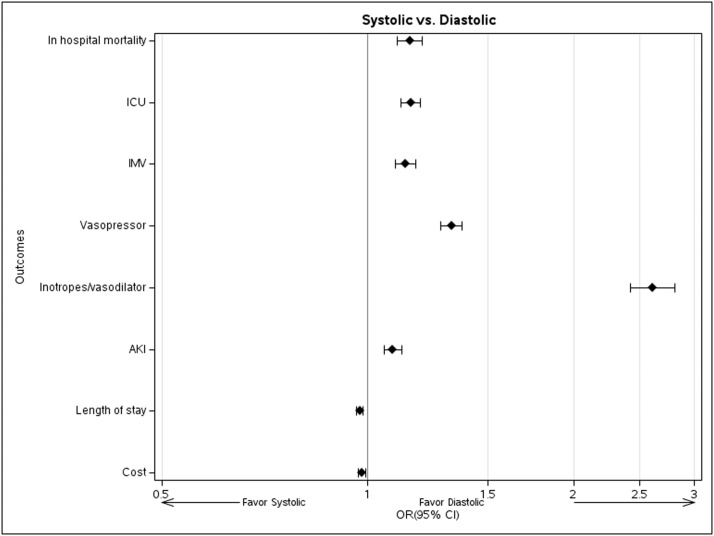
Compared to patients with diastolic HF, those with systolic HF had higher inpatient mortality (12.7 vs. 10.5%), higher rates of acute kidney injury (37.2 vs 34.0%), ICU transfer (27.4 vs. 23.5%), vasopressor use (21.4 vs. 14.8%), inotrope use (6.1 vs. 1.9%) and mechanical ventilation (23.2 vs. 19.8%); however, they had comparable median hospital costs ($11,836 vs. $12,017) and similar length of stay (Median of 6 days, IQR 4 – 10, Table 3). Adjustment for demographics, comorbidities and hospital characteristics had little impact on the relationships: the adjusted OR of systolic relative to diastolic HF for in-hospital mortality was 1.15 (95% CI, 1.11 –1.20). ICU transfer (OR 1.16, 1.12-1.19), mechanical ventilation (OR 1.13, 1.10-1.17), vasopressor use (OR 1.32, 1.28-1.38), and acute kidney injury (OR 1.09, 1.06-1.12) were also modestly to moderately increased among systolic relative to diastolic HF patients, and inotrope/vasodilator use (OR 2.60, 2.42-2.81) more so (Fig. 2). Length of stay (multiplier 0.97, 0.96-0.98) and costs (multiplier 0.98, 0.97-0.99) did not, however, increase. P-values for all associations above were <0.001 except for cost, with p = 0.003. Individuals with combined HF had outcomes similar to systolic HF patients, except for increased length of stay and cost.
Table 3.
Unadjusted study outcomes among patients admitted with pneumonia according to systolic and diastolic heart failure.
| Number (%) of patients |
||||
|---|---|---|---|---|
| Total (N = 123,211) | Systolic HF (n = 41,196) | Diastolic HF (n = 69,982) | Combined (n = 12,033) | |
| Length of stay, Median (IQR) | 6.0 (4.0 – 10.0) | 6.0 (4.0 – 10.0) | 6.0 (4.0 – 10.0) | 6.0 (4.0 – 10.0) |
| Cost, Median dollar, (IQR) | 12,059 (7,278 – 21,154) |
11,836 (7,000 – 21,295) |
12,017 (7,370 – 20,732) |
13,147 (7,800 – 23,176) |
| In hospital mortality | 14,117 (11.5) | 5,247(12.7) | 7,336 (10.5) | 1,534 (12.7) |
| ICU Admission | 31,049 (25.2) | 11,270(27.4) | 16,450 (23.5) | 3,329 (27.7) |
| IMV | 26,263 (21.3) | 9,542(23.2) | 13,866 (19.8) | 2,855 (23.7) |
| Vasopressor | 21,683 (17.6) | 8,798(21.4) | 10,387 (14.8) | 2,498 (20.8) |
| Inotropes/vasodilators | 4,481 (3.6) | 2,518(6.1) | 1,325 (1.9) | 638 (5.3) |
| AKI | 43,949 (35.7) | 15,343 (37.2) | 23,824 (34.0) | 4,782 (39.7) |
ICU, Intensive Care Unit; IMV, Invasive Mechanical Ventilation; AKI, Acute Kidney Injury.
Fig. 2.
Forest plot for multivariable regression models adjusted for age, gender, race, marital status, insurance status, hospital characteristics, DNR status, and comorbidities. The effects plotted are estimated odds ratios from mixed logistic regression models for dichotomous outcomes, and estimated multipliers of median length of stay and cost from gamma log link generalized linear mixed models, with respective 95% confidence intervals.
Discussion
In this retrospective cohort study of 783,702 patients admitted with community acquired pneumonia from 653 US hospitals, 123,211 patients had concomitant pneumonia and heart failure with more than half of them being classified as diastolic HF. Although patients with diastolic HF had a higher prevalence of certain chronic conditions, including pulmonary disease, hypertension, obesity, and obstructive sleep apnea, patients with systolic HF had significantly worse outcomes and in-hospital mortality, which was little changed by adjustment for demographics, comorbidities, DNR status and hospital characteristics. There were no substantial differences in cost or length of stay between systolic and diastolic HF patients.
To our knowledge, this is the first study to compare these two forms of HF among pneumonia patients, and also the first to assess inpatient HF treatment patterns. Others have also found that patients with diastolic HF were more likely to have certain comorbidities such as hypertension, but were less likely to have coronary artery disease,17 but unlike our study, they found that the outcomes of both conditions were comparable.6,18 Supporting our findings, a recent post-hoc analysis7 of the prospective PARADIGM-HF and PARAGON-HF trials found a higher incidence of pneumonia among patients with HF with preserved ejection fraction. Moreover, the risk of mortality was higher in patients who developed pneumonia from the PARADIGM-HF trial (with systolic HF) than in patients who developed pneumonia from the PARAGON-HF trial (with diastolic HF).
Determining volume status can be challenging in this population. In our study, we noted that 59% of patients with HF received intravenous fluids, often on the same day that they received diuretics. This highlights the diagnostic uncertainty created when heart failure and pneumonia coexist. This is a problem because for patients with very low ejection fractions who are experiencing a severe infection, receipt of intravenous fluids may lead to accumulation of alveolar fluid, worsening hypoxia, impaired bacterial clearance and disruption of the local defense against infection.19,20 These may manifest as respiratory failure requiring admission to the ICU and use of supportive measures including IMV, inotropes and vasopressors. They may also contribute to the higher mortality we noted in systolic HF compared to diastolic HF. Alternatively, if patients are volume depleted, administering diuretics may worsen acute kidney injury or hypotension.
We also describe the in-hospital medication management of patients with pneumonia and systolic HF. ACEi or ARB (and more recently with angiotensin-receptor blocker/neprilysn inhibitor), beta blockers, and mineralocorticoid antagonists are currently considered the mainstay treatment of individuals with outpatient systolic HF.21,22 We found that compared to patients with diastolic HF, patients with systolic HF were more likely to receive these medications and less likely to receive calcium channel blockers and hydralazine; there was no difference in fluid intake and loop diuretics. However, less than 50% of individuals with systolic HF in our study received ACEi or ARBs and less than 15% received mineralocorticoid receptor antagonists during their admission. In one study that examined patients hospitalized for HF as a principal diagnosis, 75% continued ACE or ARB, 87% continued beta blockers and 8% received mineralocorticoid antagonists. They also found that their discontinuation was associated with increased mortality,23 and several clinical trials have demonstrated that inpatient continuation of guideline-directed medical therapy (GDMT) is safe in hemodynamically stable individuals.24 The low rate of these medications in our study compared to outpatient GDMT use, which ranges from 54.8% to 78%,25, 26, 27 likely represents discontinuation of outpatient medications in acutely ill individuals, perhaps due to hypotension or acute kidney injury, although it could also indicate suboptimal care prior to admission.28 At least some patients likely had their medications appropriately withheld but not resumed during the hospitalization. Although we do not have access to orders placed at discharge, it seems probable that many of these patients did not have their medications resumed immediately. Others have found that medications discontinued in the ICU often are not reinstated for at least 90 days, and that such discontinuation was associated with increased mortality at one year.29 This finding highlights the importance of resuming GDMT when patients become hemodynamically stable and return to their baseline to avoid the possibility of life-saving medications being inadvertently discontinued more permanently.
This study has several strengths. It is the first study to examine detailed clinical characteristics and outcomes of systolic versus diastolic HF among patients admitted for pneumonia. The inclusion of a very large number of patients from more than 600 hospitals supports the generalizability of the findings and increases the external validity. It also allowed us to adjust comparisons of outcomes for many comorbid conditions and investigate a number of outcomes that characterize the severity of patients’ hospital course (AKI, ICU stay with use of inotropes, vasopressors, mechanical ventilation). There are also limitations. In retrospective analysis of such a hospital discharge database, there is always the possibility of residual confounding due to clinical variables or other variables either not measured or not included. We also used ICD-9 codes assigned for billing purposes to identify cases of pneumonia, HF, and other comorbidities, which risks ascertainment bias that could undermine the validity of our findings. Our results are also susceptible to inaccurate coding of systolic and diastolic HF, however, several prior studies have used billing data for similar retrospective observational studies.30 Another limitation is the lack of data about New York Heart Association (NYHA) Functional Classification or echocardiogram results; hence we could not account for the severity of heart failure or associated echocardiographic findings of prognostic utility. Furthermore, our data are from 2011 to 2015 and may not reflect current heart failure management, which could limit applicability to current practice. In addition, some sociodemographic variables (e.g., income, residential location, education) are also potentially important with regards to our outcomes, but our data source is a de-identified hospital discharge database that did not include such other variables.
In conclusion, our study showed that pneumonia patients with systolic HF had worse outcomes characterized by higher in-hospital mortality and use of intensive care compared to patients with diastolic HF. However, these differences did not translate into substantially higher in-hospital cost and length of stay. We also found that individuals with systolic heart failure were frequently not prescribed HF treatments during hospitalization, even when they became hemodynamically stable enough to discharge home. This is concerning as many times these essential medications may be overlooked upon discharge, potentially leading to unintentional morbidity and mortality. Incorporating medical reconciliation at discharge for these patients should be advocated to ensure GDMT continuity and patient safety. Future studies are needed to focus on the management of these patients including optimizing the use of fluids, diuretics, and GDMT.
Disclosures
This study was supported by a grant from the Agency for Healthcare Research and Quality (AHRQ) (R01HS024277). Dr. Pack was supported by a grant from the National Heart, Lung and Blood Institute of the National Institutes of Health (1K23HL135440).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix
Covariates included in models for outcomes:
Demographics: age, sex (female, male), race (Black, Hispanic, White, Other, Unknown), marital status (married, single, other, unknown), insurance type (Medicare, Medicaid, managed care, Commercial indemnity, Other).
Hospital characteristics: bed size (≤ 200, 201-399, ≥ 400), US Census Bureau region (Northeast, Midwest, South, West), location population density (rural, urban), teaching status (non-teaching, teaching).
Baseline (present on admission) comorbidities: alcohol abuse disorder, drug abuse disorder, anemia, rheumatoid arthritis/collagen vascular disorder, chronic lung disease, coagulation disorder, diabetes, hypertension, hypothyroidism, liver disease, lymphoma, solid tumor without metastases, metastatic cancer, fluid or electrolyte disorder, psychoses, other neurological disorder, weight loss, obesity, obstructive sleep apnea, parathyroid disease, peripheral vascular disease, pulmonary circulation disorder, valvular disease, chronic kidney disease, chronic liver disease, cardiac dysrhythmia, atrial fibrillation, coronary artery disease, acute coronary syndrome, thyrotoxicosis, mitral stenosis, aortic stenosis, urinary tract infection.
Baseline (present on admission) medical management: Current long-term use of anticoagulant, antiplatelet/antithrombotic, or aspirin; do not resuscitate order on admission.
References
- 1.Ramirez J.A., Wiemken T.L., Peyrani P., et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan V., Angus D.C., Griffin M.F., Clermont G., Scott Watson R., Linde-Zwirble W.T. Hospitalized community-acquired pneumonia in the elderly: age-and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 3.Fine M.J., Smith M.A., Carson C.A., et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 4.Kontogeorgos S., Thunström E., Johansson M.C., Fu M. Heart failure with preserved ejection fraction has a better long-term prognosis than heart failure with reduced ejection fraction in old patients in a 5-year follow-up retrospective study. Int J Cardiol. 2017;232:86–92. doi: 10.1016/j.ijcard.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Ghali J.K., Kadakia S., Bhatt A., Cooper R., Liao Y. Survival of heart failure patients with preserved versus impaired systolic function: the prognostic implication of blood pressure. Am Heart J. 1992;123:993–997. doi: 10.1016/0002-8703(92)90709-5. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F., Weston S.A., Redfield M.M., et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 7.Shen L., Jhund P.S., Anand I.S., et al. Incidence and outcomes of pneumonia in patients with heart failure. J Am Coll Cardiol. 2021;77:1961–1973. doi: 10.1016/j.jacc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen E.M., Coley C.M., Singer D.E., et al. Causes of death for patients with community-acquired pneumonia: results from the pneumonia patient outcomes research team cohort study. Arch Internl Med. 2002;162:1059–1064. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 9.Premier Healthcare Database 2020. (Accessed September 21 2021, at https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf.)
- 10.Rothberg M.B., Haessler S., Lagu T., et al. Outcomes of patients with healthcare-associated pneumonia: worse disease or sicker patients? Infect Control Hosp Epidemiol. 2014;35:S107–SS15. doi: 10.1086/677829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenauer P.K., Stefan M.S., Johnson K.G., Priya A., Pekow P.S., Rothberg M.B. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145:1032–1038. doi: 10.1378/chest.13-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenauer P.K., Pekow P.S., Lahti M.C., Lee Y., Benjamin E.M., Rothberg M.B. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality (AHRQ) (Accessed 11/9/21, 2021.
- 14.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Thompson N.R., Fan Y., Dalton J.E., et al. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care. 2015;53:374–379. doi: 10.1097/MLR.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagne J.J., Glynn R.J., Avorn J., Levin R., Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonarow G.C. The Acute Decompensated Heart Failure National Registry (ADHERETM): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4:21–30. [PubMed] [Google Scholar]
- 18.Smith G.L., Masoudi F.A., Vaccarino V., Radford M.J., Krumholz H.M. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–1518. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 19.Harford C., Hara M. Proceedings [of the] Annual Meeting Central Society for Clinical Research (US) 1947. Effect of pulmonary edema on susceptibility of mice to pneumococcal pneumonia; p. 15. [PubMed] [Google Scholar]
- 20.Laforce F.M., Mullane J.F., Boehme R.F., Kelly W.J., Huber G.L. The effect of pulmonary edema on antibacterial defenses of the lung. J Lab Clin Med. 1973;82:634–648. [PubMed] [Google Scholar]
- 21.Ponikowski P., Voors A.A., Anker S.D., et al. ESC scientific document group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 22.Tomasoni D., Adamo M., Lombardi C.M., Metra M. Highlights in heart failure. ESC Heart Fail. 2019;6:1105–1127. doi: 10.1002/ehf2.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava P.K., DeVore A.D., Hellkamp A.S., et al. Heart failure hospitalization and guideline-directed prescribing patterns among heart failure with reduced ejection fraction patients. Heart Fail. 2021;9:28–38. doi: 10.1016/j.jchf.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Bhagat A.A., Greene S.J., Vaduganathan M., Fonarow G.C., Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7:1–12. doi: 10.1016/j.jchf.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownell N.K., Ziaeian B., Fonarow G.C. The gap to fill: rationale for rapid initiation and optimal titration of comprehensive disease-modifying medical therapy for heart failure with reduced ejection fraction. Card Fail Rev. 2021;7:e18. doi: 10.15420/cfr.2021.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddox T.M., Song Y., Allen J., et al. Trends in US ambulatory cardiovascular care 2013 to 2017: JACC review topic of the week. J Am Coll Cardiol. 2020;75:93–112. doi: 10.1016/j.jacc.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Greene S.J., Butler J., Albert N.M., et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 28.Mor A., Thomsen R.W., Ulrichsen S.P., Sørensen H.T. Chronic heart failure and risk of hospitalization with pneumonia: a population-based study. Eur J Intern med. 2013;24:349–353. doi: 10.1016/j.ejim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Bell C.M., Brener S.S., Gunraj N., et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 30.Bratzler D.W., Normand S.L.T., Wang Y., et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PloS One. 2011;6:e17401. doi: 10.1371/journal.pone.0017401. [DOI] [PMC free article] [PubMed] [Google Scholar]