Abstract
Background:
Cardiovascular disease (CVD) mortality is persistently higher in the Black population than in other racial/ethnic groups in the US.
Objective:
To examine the degree to which social, behavioral, and metabolic risk factors are associated with CVD mortality and the extent to which racial differences in CVD mortality persist after accounting for these factors.
Design:
A prospective cohort study.
Setting:
The National Health and Nutrition Examination Surveys (NHANES) 1999-2018.
Participants:
A nationally representative sample of 50,808 individuals aged ≥20 years.
Measurements:
Data on social, behavioral, and metabolic factors were collected in each NHANES survey using standard methods. CVD deaths were ascertained from linkage to the National Death Index with follow-up through 2019.
Results:
Over an average of 9.4 years of follow-up, 2,598 CVD deaths were confirmed. The age- and sex-standardized CVD mortality rate was 484.7/100,000 in Black, 384.5/100,000 in White, 292.4/100,00 in Hispanic, and 255.1/100,000 in Other race groups. In a multiple Cox regression analysis adjusted for all measured risk factors simultaneously, several social (unemployment status, low family income, food insecurity, lack of home ownership, unpartnered status), behavioral (current smoking, lack of leisure-time physical activity, sleep <6 or >8 hours/day), and metabolic risk factors (obesity, hypertension, diabetes) were associated with a significantly higher risk for CVD mortality. After adjusting for these metabolic, behavioral, and social risk factors, separately, hazard ratios (95% CI) of CVD mortality for Black compared to White individuals were attenuated from 1.54 (1.34-1.77) to 1.34 (1.16-1.55), 1.31 (1.15-1.50), and 1.04 (0.90-1.21), respectively.
Limitations:
Causal contributions of social, behavioral, and metabolic risk factors to racial/ethnic disparities in CVD mortality could not be established.
Conclusions:
The Black-White difference in CVD mortality diminished after adjusting for behavioral and metabolic risk factors and completely dissipated with adjustment for social determinants of health in the US population.
Funding Source:
National Institutes of Health
Keywords: behavioral risk factors, cardiovascular disease mortality, metabolic risk factors, racial disparity, social determinants of health
Introduction
Despite a substantial reduction in cardiovascular disease (CVD) mortality in the US general population, racial and ethnic disparities persist.(1-3) For example, age-adjusted CVD mortality was 32% higher for Black women and 33% higher for Black men compared with their respective White counterparts in 2019.(2) In the US, Black persons are at an increased risk for obesity, diabetes, hypertension, and other CVD risk factors compared with White persons.(4-8) Elevated CVD risk factors partially explain the increased risk of CVD among the Black population.(3, 9-11) In the Multiethnic Cohort Study, established lifestyle and clinical risk factors explained the majority of racial differences in CVD mortality between Black and White participants.(10)
Social determinants of health (SDOH) have been associated with CVD risk factors and CVD events and mortality in epidemiologic studies.(12-15) Several prospective cohort studies have also reported that socioeconomic status was associated with racial differences in CVD mortality in the US.(3, 9, 11) However, there are scarce data on the complex relationship of social, behavioral, and metabolic risk factors with racial differences in CVD mortality in a large nationally representative sample in the US population. Such information may be critical to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities in CVD mortality.
The present analysis aims to determine the degree to which social, behavioral, and metabolic risk factors are associated with CVD mortality in the US general population and the extent to which racial differences in CVD mortality persist after accounting for these factors. Data from the National Health and Nutrition Examination Surveys (NHANES) 1999-2018 mortality follow-up study were used to achieve these goals.
Methods
Study Participants
NHANES uses stratified, multistage probability sampling methods to select a series of nationally representative samples of noninstitutionalized US adults in 2-year cycles since 1999-2000.(16) We included 10 survey cycles conducted from 1999-2000 through 2017-2018 and excluded 122 individuals missing unique identifiers sufficient for linkage to the National Death Index (NDI). In addition, 1,468 pregnant participants were excluded. After these exclusions, a total of 50,808 individuals aged ≥20 years were included in the present analysis (eFigure 1). NHANES protocols were approved by the National Center for Health Statistics’ IRB, and each participant provided written informed consent.
Data Collection
In each 2-year survey, participants completed in-home interviews and visited a mobile examination center, where they responded to additional questionnaires and underwent a physical examination and blood sample collection.
Self-reported race in 6 categories (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Pacific Islander, White, or other), in addition to Hispanic origin, were collected using a standardized questionnaire. Because Asian race was not listed as a separate category in NHANES until 2011-2012, we grouped participants into 4 mutually exclusive racial and ethnic categories: non-Hispanic Black, Hispanic, non-Hispanic White, and Other.
We identified all SDOH variables across the five Healthy People 2030 domains: economic stability, education access and quality, health care access and quality, neighborhood and built environment, and social and community context by reviewing the NHANES questionnaires.(17) Eight SDOH, including employment status, family income-to-poverty ratio, food security, education level, health care access, health insurance status, home ownership, and marital status, were available in each NHANES cycle 1999-2018 and included in the analysis (eTable 1). Participants were asked what type of work they had done in the last week. Those not working were classified as unemployed, except students or retired persons who were grouped with those reporting employment. Ratio of family income-to-poverty was calculated by dividing family income by a family size-specific poverty threshold.(18) Household food insecurity was measured using the validated 10-item US Food Security Survey Module.(19) Food insecurity was defined as one or more affirmative responses.(20) Participants were also asked for the highest grade or level of school they completed. Access to healthcare was determined by asking participants if there was a place they usually go when sick or needing advice about health. If they answered “no” or “hospital emergency room” to this question, they were classified as no routine place for healthcare. Participants were also asked whether they were covered by health insurance and types of insurance. Housing ownership was determined by asking if the home they were living in was owned or being bought, rented, or occupied by some other arrangement. Participants were asked whether they were married, widowed, divorced, separated, never married, or living with a partner. Those reporting marriage or living with a partner were grouped together.
Behavioral and metabolic variables for CVD included American Heart Association Life's Essential 8: diet, physical activity, smoking, sleep, body weight, cholesterol, glucose, and BP.(21) Dietary intake was assessed by one or two 24-hour dietary recalls. Dietary data from the first recall was used to calculate the Healthy Eating Index-2015 (HEI).(22) The HEI scores range from 0 to 100 with a score of 100 reflecting that the set of foods ideally aligns with key recommendations from the 2015-2020 Dietary Guidelines for Americans. In NHANES 1999-2004, physical activity over the last 30 days preceding the examination accrued from leisure and household activities and transportation was assessed using an interviewer-administered questionnaire.(16) In NHANES 2005-2018, usual physical activity during work, transport, and leisure time in a typical week was evaluated using the Global Physical Activity Questionnaire.(23) In the current analysis, the duration of leisure-time physical activity was calculated as the minutes of moderate-intensity physical activity plus twice the minutes of vigorous-intensity physical activity.(24) The duration of sleep during the night on weekdays or workdays was assessed and classified as 6-8 hours per day or <6 hours or >8 hours per day.
During the physical examination, weight, height, and waist circumference were measured, and body-mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure (BP) was measured by trained staff using a mercury sphygmomanometer after the participant rested quietly in a seated position for at least 5 minutes.(25) Three BP measurements were obtained, and the mean of all measurements was used in analyses. Blood samples were collected at the mobile examination center, stored at −20°C, and sent to central laboratories for the determination of total cholesterol, HDL-cholesterol, and hemoglobin A1c using standard methods. Fasting blood samples were only available in a subsample of survey participants for measuring LDL-cholesterol and glucose. Obesity was defined as BMI ≥30 kg/m2, and central obesity was defined as waist circumference ≥102 cm in men and ≥88 cm in women.(26) Hypertension was defined as systolic BP ≥130 mmHg or diastolic BP≥80 mmHg or use of antihypertensive medications.(27) Diabetes was defined as fasting glucose ≥126 mg/dL or hemoglobin A1c ≥6.5% or diagnosed diabetes.(28) Hypercholesterolemia was defined as total-to-HDL cholesterol ratio ≥5.(29)
Ascertainment of Deaths
Deaths of NHANES participants were identified through linkage to the NDI by the National Center for Health Statistics using a probabilistic matching strategy. A complete description of the methodology is available elsewhere.(30) The Linked Mortality Files have been updated with mortality follow-up data through December 31, 2019. The underlying cause of death was identified according to ICD-10, and CVD mortality was defined as death due to heart disease (codes I00–I09, I11, I13, I20–I51) and stroke (codes I60–I69). NDI-derived CVD mortality had sensitivity of 73.4%, specificity of 84.5%, positive predictive value of 70.6%, and negative predictive value of 86.2% compared with expert adjudication.(31) Follow-up time for each person was calculated as the difference between the baseline examination date and the date of CVD death, the date of censoring due to non-CVD death, or the last known date alive (December 31, 2019). Participants not matched with a death record were considered alive throughout the entire follow-up period.
Statistical Analysis
In the primary analysis, multiple imputation by chained equation was applied to address partially missing data among individuals and systematically missing data for specific NHANES cycles (i.e., lack of sleep data in NHANES 1999-2004).(32) We assumed that data were missing at random.(33) The study outcome and all covariables, e.g., age, sex, race/ethnicity, social, behavioral, and metabolic risk factors, were included in the analytic model for multiple imputation. Ten imputed datasets were generated and analyzed using the statistical methods described below. The results from ten imputed datasets were combined for inference. In additional sensitivity analysis, a complete case analysis was performed. Social, behavioral, and metabolic risk factors were dichotomized based on conventional cut-points or the median if there was a linear association between exposure and CVD mortality.
The distributions of social, behavioral, and metabolic risk factors were compared among racial and ethnic groups using ANOVA for continuous variables and χ2 test for categorical variables. Age- and sex-standardized CVD mortality rates and their 95% CIs (per 100,000 person-years) by race/ethnicity were calculated according to 2010 US census data using direct standardization. Pairwise correlations among dichotomous risk factors were assessed using Cramér's V.(34)
We tested the independent associations of social, behavioral, and metabolic risk factors with CVD mortality by including these risk factors in a multivariable Cox regression model simultaneously, in addition to age, sex, and race/ethnicity. Furthermore, the dose-response association of the number of social, behavioral, and metabolic risk factors with CVD mortality was assessed. The proportional hazard assumption was verified using weighted Schoenfeld residuals. Given that baseline data collection extended over 20 years, all analyses were stratified by NHANES examination cycle to account for potential secular trends. In addition, we conducted a sensitivity analysis stratified by two 10-year survey periods (1999-2008 and 2009-2018) to check for consistency of associations of social, behavioral, and metabolic risk factors with CVD mortality over time. The associations of social, behavioral, and metabolic risk factors with racial differences in CVD mortality were investigated using multivariable Cox regression model. We hypothesized that racial/ethnic differences in CVD mortality was entirely associated with racial/ethnic differences in social, behavioral, and metabolic risk factors (eFigure 2). Because age- and sex-adjusted CVD mortality was not significantly different between White vs. Hispanic or Other Race groups, we focused on Black-White differences. Social, behavioral, and metabolic risk factors were adjusted in three separate models to assess the degree to which Black-White differences in CVD mortality persisted after adjustment for each of these risk factor domains.
All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc) and R software, version 4.2.2 (R Foundation) with survey analysis procedures to account for the complex sampling design. Survey examination weights were used for analysis to obtain nationally representative estimates. All statistical tests were 2-sided, and p<0.05 was considered statistically significant. Because of the potential for type I error due to the absence of adjustment for multiple comparisons, results should be interpreted as exploratory.
Results
Participant Characteristics
Characteristics of study participants are presented in Table 1. In general, Black, Hispanic, and Other racial/ethnic populations had higher proportions of social risk factors than White populations. Most social risk factors were moderately correlated with each other (eTable 2).
Table 1.
Social, Behavioral, and Metabolic Risk Factors among Study Participants by Race and Ethnicity, NHANES 1999-2018*
| Characteristics | No. of participants with measured risk factors† |
All participants (N =50808) |
Self-reported Race/Ethnicity | |||
|---|---|---|---|---|---|---|
| Black (N = 10796) |
Hispanic (N = 12959) |
White (N = 22350) |
Other‡ (N = 4703) |
|||
| Age, mean (SE), years | 50808 | 47.3 (0.2) | 44.9 (0.2) | 41.3 (0.3) | 49.2 (0.2) | 44.6 (0.4) |
| Male, n (%) | 50808 | 25143 (48.7) | 5275 (45.5) | 6277 (51.0) | 11255 (48.8) | 2336 (48.7) |
| Social determinants of health | ||||||
| Unemployed, n (%) | 50770 | 11496 (19.1) | 2732 (25.6) | 3512 (24.4) | 4233 (16.7) | 1019 (21.5) |
| Family income-to-poverty ratio <300%, n (%) | 46217 | 29121 (50.5) | 6619 (67.8) | 8841 (75.6) | 11365 (42.9) | 2296 (51.8) |
| Marginal or lower food security, n (%)§ | 49630 | 13921 (21.2) | 3530 (34.6) | 5278 (42.8) | 4064 (14.7) | 1049 (21.4) |
| Not owning a home, n (%) | 50013 | 18716 (32.0) | 5155 (51.5) | 5719 (49.7) | 5957 (24.6) | 1885 (39.9) |
| Less than high school education, n (%) | 50727 | 13895 (17.6) | 2862 (24.1) | 6580 (42.6) | 3674 (11.7) | 779 (15.3) |
| No regular health care access, n (%) | 50803 | 11045 (20.1) | 2359 (23.6) | 3970 (34.7) | 3538 (16.0) | 1178 (25.4) |
| No private health insurance, n (%) | 50276 | 23511 (36.6) | 5435 (50.1) | 7928 (59.6) | 8116 (29.4) | 2032 (40.9) |
| Not married nor living with partner, n (%) | 50340 | 20402 (36.8) | 6004 (56.9) | 4556 (36.5) | 8286 (33.8) | 1556 (34.5) |
| No. of unfavorable social determinants, n (%) | ||||||
| 0 | 6453 | 6453 (21.6) | 872 (9.0) | 755 (7.3) | 4171 (26.7) | 655 (17.1) |
| 1 | 7380 | 7380 (20.4) | 1203 (12.6) | 1060 (10.0) | 4356 (23.8) | 761 (18.5) |
| 2 | 7133 | 7133 (16.4) | 1388 (14.3) | 1349 (12.7) | 3673 (17.2) | 723 (18.2) |
| 3 | 6879 | 6879 (13.6) | 1484 (15.6) | 1743 (15.4) | 2997 (12.8) | 655 (15.8) |
| 4 | 6635 | 6635 (11.8) | 1635 (17.7) | 2011 (18.1) | 2353 (9.5) | 636 (14.0) |
| 5 or more | 10566 | 10566 (16.1) | 2856 (30.9) | 4124 (36.5) | 2878 (10.0) | 708 (16.5) |
| Behavioral risk factors | ||||||
| Current smoking, n (%) | 50757 | 10780 (21.7) | 2715 (25.5) | 2202 (18.6) | 5133 (22.0) | 730 (19.1) |
| Healthy eating index <52, n (%)¶ | 42649 | 20779 (50.1) | 4863 (56.9) | 4840 (48.9) | 9783 (49.8) | 1293 (43.8) |
| No leisure-time physical activity, n (%) | 50755 | 25430 (42.9) | 5742 (50.7) | 7435 (52.3) | 10146 (39.7) | 2107 (43.4) |
| Sleeping duration <6 or >8 hours/day, n (%) | 37272 | 10476 (25.4) | 2790 (34.5) | 2525 (26.0) | 4038 (23.7) | 1123 (26.0) |
| No. of behavioral risk factors, n (%) | ||||||
| 0 | 6269 | 6269 (24.4) | 1009 (15.4) | 1319 (19.0) | 3052 (26.5) | 889 (27.5) |
| 1 | 10220 | 10220 (33.9) | 2059 (31.3) | 2817 (37.6) | 4290 (33.5) | 1054 (34.9) |
| 2 | 8731 | 8731 (27.1) | 2132 (32.6) | 2195 (29.8) | 3674 (25.9) | 730 (25.6) |
| 3 | 4018 | 4018 (11.8) | 1077 (16.6) | 862 (11.7) | 1819 (11.3) | 260 (9.6) |
| 4 | 919 | 919 (2.8) | 271 (4.2) | 121 (1.9) | 469 (2.8) | 58 (2.4) |
| Metabolic risk factors ∥ | ||||||
| Obesity, n (%) | 49773 | 18115 (35.4) | 4778 (45.6) | 4975 (38.7) | 7429 (34.3) | 933 (23.3) |
| Central obesity, n (%) | 47877 | 26560 (54.3) | 5889 (57.7) | 7069 (53.4) | 12094 (55.6) | 1508 (37.9) |
| Hypertension, n (%) | 48964 | 25949 (47.6) | 6484 (56.6) | 5712 (37.0) | 11666 (48.6) | 2087 (44.7) |
| Diabetes, n (%) | 48560 | 8296 (12.3) | 2164 (17.5) | 2440 (13.8) | 2918 (10.9) | 774 (15.2) |
| Total-to-HDL cholesterol ratio ≥5, n (%) | 47686 | 9826 (20.6) | 1315 (13.4) | 3108 (24.7) | 4569 (20.8) | 834 (20.9) |
| No. of metabolic risk factors, n (%) | ||||||
| 0 | 9634 | 9634 (25.4) | 1554 (20.4) | 2361 (26.2) | 4421 (25.3) | 1298 (31.8) |
| 1 | 10020 | 10020 (22.8) | 1943 (21.6) | 2467 (21.9) | 4556 (22.7) | 1054 (26.4) |
| 2 | 10109 | 10109 (22.3) | 1959 (22.3) | 2739 (23.4) | 4592 (22.3) | 819 (19.5) |
| 3 | 8815 | 8815 (18.7) | 2067 (22.6) | 2314 (17.8) | 3913 (18.7) | 521 (13.9) |
| 4 | 4688 | 4688 (9.2) | 1155 (11.1) | 1278 (9.0) | 2013 (9.2) | 242 (6.9) |
| 5 | 878 | 878 (1.9) | 200 (2.2) | 260 (1.9) | 373 (1.9) | 45 (1.5) |
SE=Standard error; NHANES=National Health and Nutrition Examination Survey.
The sample sizes and actual frequency numbers are unweighted but means (standard errors) or percentages are weighted. See Supplementary eTable1 for definition of social, behavioral, and metabolic risk factors.
Number (percentage) of participants with missing data: employment 38 (0.1%), family income-to-poverty ratio 4591 (9.0%), food security 1178 (2.3%), home ownership 795 (1.6%), education 81 (0.2%), regular health care access 5 (<0.1%), private health insurance 532 (1.0%), marital status 468 (0.9%), cigarette smoking 51 (0.1%), healthy eating index 8159 (16.1%), leisure-time physical activity 53 (0.1%), sleeping duration 13536 (26.6%), obesity 1035 (2.0%), central obesity 2931 (5.8%), hypertension 1844 (3.6%), diabetes 2248 (4.4%), and total-to-HDL cholesterol ratio 3122 (6.1%). Sleep data were only available in NHANES 2005-2018.
Non-Hispanic Asian was listed as a separate race/ethnicity group starting in NHANES 2011-2012. American Indian or Alaska Native and Native Hawaiian or Pacific Islander were not listed as separate race/ethnicity groups in National Health and Nutrition Examination Survey (NHANES) datasets.
Food insecurity was assessed with the 18-item US Food Security Survey Module with zero affirmative responses indicating high food security and ≥1 affirmative responses indicating marginal food security or food insecurity.
The Healthy Eating Index (HEI) uses a scoring system to assess how well a set of foods aligns with key recommendations of the Dietary Guidelines for Americans. The scores range from 0 to 100 with a higher score indicating greater consistency of the diet with the Dietary Guidelines for Americans. Median HEI score was 52 among NHANES 1999-2018 participants.
Obesity was defined as body mass index ≥30 kg/m2; central obesity was defined as waist circumference ≥102 cm in men and ≥88 cm in women; hypertension was defined as systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥80 mmHg or use of antihypertensive medications; and diabetes was defined as fasting glucose ≥126 mg/dL or hemoglobin A1c ≥6.5% or diagnosed diabetes.
Race, Ethnicity and CVD Deaths
Over an average of 9.4 years of follow-up, 2,589 CVD deaths (537 in Black, 415 in Hispanic, 1563 in White, and 74 in Other individuals) were confirmed. The age- and sex-standardized CVD mortality rate in the overall population was 383.0 per 100,000 (95% CI 343.4-428.9). The age- and sex-standardized CVD mortality rate was highest among Black adults [484.7 (397.3-599.1) per 100,000] followed by White adults [384.5 (338.3-441.6)], Hispanic adults [292.4 (217.8-402.7)] and adults of other race/ethnicity [255.1 (143.0-510.2)].
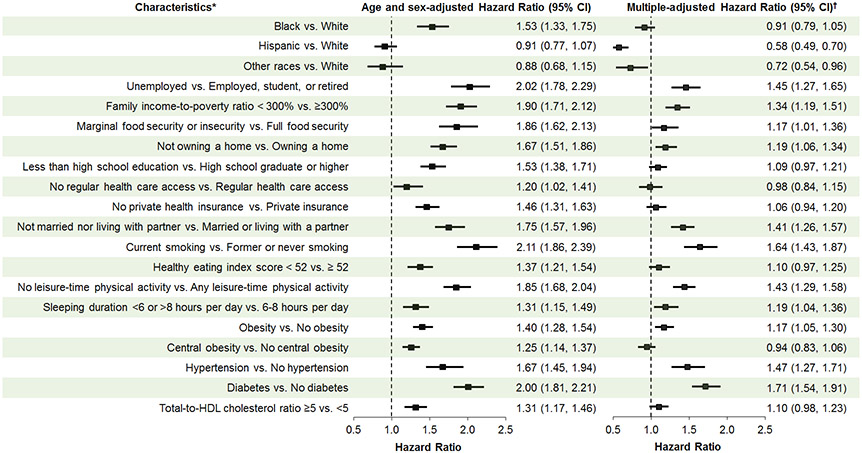
Figure 1 presents age- and sex-adjusted and fully adjusted associations between race and CVD mortality after accounting for measured social, behavioral and metabolic factors. In the age- and sex-adjusted analysis, Black individuals, but not Hispanic or Other race individuals had a higher risk of CVD mortality compared to White individuals.
Figure 1. Multiple-adjusted hazard ratios (95% CI) of race/ethnicity and social, behavioral, and metabolic risk factors associated with cardiovascular disease mortality in the US adults aged 20 and older, NHANES 1999-2018.
* Healthy eating index scores range from 0 to 100 with a higher score indicating greater consistency of the diet with the Dietary Guidelines for Americans. The median healthy eating index score was 52 among NHANES 1999-2018 participants. Obesity was defined as body mass index ≥30 kg/m2. Central obesity was defined as waist circumference ≥102 cm in men and ≥88 cm in women. Hypertension was defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg or use of antihypertensive medications. Diabetes was defined as fasting glucose ≥126mg/dL or hemoglobin A1c ≥6.5% or diagnosed diabetes. † Adjustment for age, sex, race/ethnicity, and all other social, behavioral, and metabolic risk factors listed in the figure.
Social, Behavioral, and Metabolic Risk Factors and Racial Differences in CVD Mortality
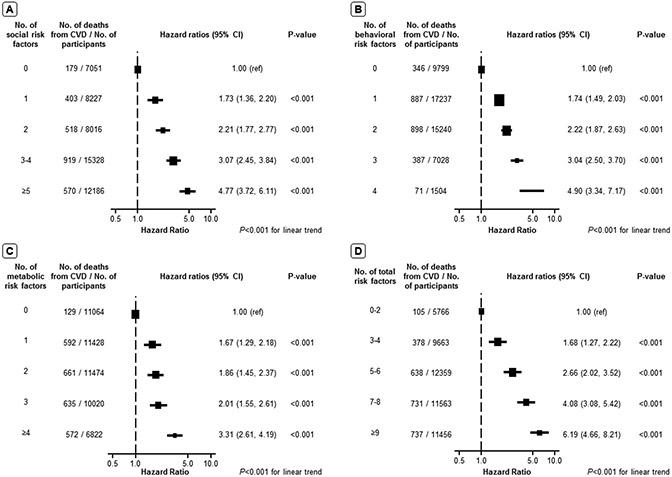
After adjustment, social risk factors – including unemployment, lower family income, food insecurity, not owning a home and not being married nor living with a partner – were independently associated with higher CVD mortality (Figure 1). Behavioral risk factors such as current smoking, physical inactivity, and sleep duration <6 or >8 hours/day were also independently associated with higher CVD mortality as was the metabolic risk factors – obesity, hypertension, and diabetes. A dose-response association was identified between the number of risk factors and the HR of CVD mortality after adjusting for age, sex, and race/ethnicity (Figure 2).
Figure 2. Hazard ratio of cardiovascular disease mortality associated with the number of social, behavioral, and metabolic risk factors in the US population.
(A). hazard ratio of cardiovascular disease mortality by the number of social risk factors; (B). hazard ratio of cardiovascular disease mortality by the number of behavioral risk factors; (C). hazard ratio of cardiovascular disease mortality by the number of metabolic risk factors; (D). hazard ratio of cardiovascular disease mortality by the number of overall social, behavioral, and metabolic risk factors. Error bars indicate 95% CIs. All hazard ratios were stratified by NHANES cycles and adjusted for age, sex, race and ethnicity.
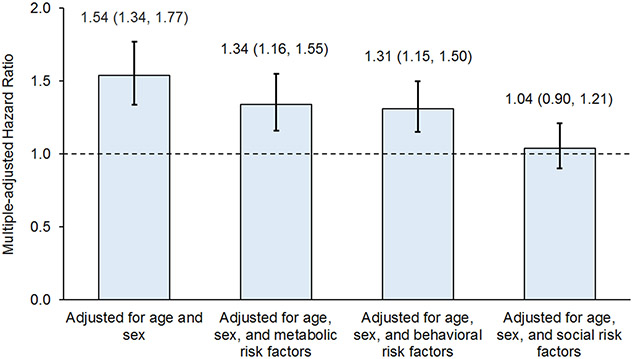
In the subset of Black and White adults, the age- and sex-adjusted HR (95% CI) of CVD mortality in Black compared to White individuals was 1.54 (1.34-1.77). After additionally adjusting for metabolic, behavioral, and social risk factors in three separate models, the HR for the Black-White difference decreased to 1.34 (1.16-1.55), 1.31 (1.15-1.50), and 1.04 (0.90-1.21), respectively (Figure 3). Similarly, the HR (95% CI) of CVD mortality for the Black-White difference in our primary analysis was also diminished from 1.53 (1.33, 1.75) after adjustment for age and sex to 0.91 (0.79, 1.05) after full adjustment for measured social, behavioral, and metabolic risk factors (Figure 1).
Figure 3. Hazard ratio of Black-White difference in cardiovascular mortality adjusting for metabolic, behavioral, and social risk factors in the US adults aged 20 and older, NHANES 1999-2018.
Metabolic risk factors included obesity, central obesity, hypertension, diabetes, and total-to-HDL cholesterol ratio ≥5. Behavioral risk factors included current smoking, healthy eating index score <52, no leisure-time physical activity, and sleep duration <6 or >8 hours per day. Social risk factors included unemployed, family income-to-poverty ratio <300%, marginal or low food security, not owning a home, less than high school education, no regular health care access, no private health insurance, and not married nor living with a partner.
Sensitivity analyses
Associations of social, behavioral, and metabolic risk factors using various cut-points with CVD mortality were consistent (eTable 3). In addition, multivariable-adjusted HRs of CVD mortality associated with social, behavioral, and metabolic risk factors were consistent between the NHANES 1999-2008 and NHANES 2009-2018 cohorts (eTable 4). Furthermore, associations of social, behavioral, and metabolic risk factors with CVD mortality, as well as the Black-White difference in CVD mortality, after adjusting for social, behavioral, and metabolic risk factors in the complete case analysis were largely consistent with primary analyses based on multiple-imputed datasets although the magnitude and precision around some estimate changed modestly (eTables 5 and eFigure 3).
Discussion
Our study found that age- and sex-adjusted CVD mortality was significantly higher in Black individuals compared to White individuals. There was no significant difference in age- and sex-adjusted CVD mortality between Hispanic, Other race, and White individuals. In addition to behavioral and metabolic risk factors, several social factors --including unemployment, lower family income, food insecurity, not owning a home, and not being married nor living with a partner -- were significantly associated with CVD mortality independent of established behavioral and metabolic risk factors. Black-White differences in CVD mortality were diminished after adjusting for behavioral and metabolic risk factors, and entirely dissipated after adjusting for social factors.
Previous prospective cohort studies showed that lower socioeconomic status was associated with a higher risk of CVD.(14, 15, 35) In an analysis of data from the UK Biobank, Zhang and colleagues reported that individuals with low socioeconomic status (household income, education level, and employment status) had a 2.25-fold higher risk of CVD mortality compared to those with high socioeconomic status.(15) In the Prospective Urban Rural Epidemiologic (PURE) study, low education was associated with an increased risk of major CVD, but household wealth, consistent with measures of income and expenditure, was not associated with CVD risk.(14) In our study, education level was not associated with CVD mortality after adjusting for other social, behavioral, and metabolic risk factors. However, employment, family income, and home ownership were independently associated with CVD mortality.
We found that individuals who were unmarried or living without a partner had a higher risk for CVD mortality. Being married or living with a partner may be a proxy for several important factors, including greater social support and a healthier lifestyle.(36) Future studies are needed to understand the mechanisms underlying the association between marital status and CVD risk in order to develop novel strategies for intervention. We also found that food insecurity was associated with a higher risk of CVD mortality. Previous studies showed that food insecurity was associated with poor nutrition and increased CVD risk factors.(37) Future studies are needed to identify the most vulnerable populations, enhance access to food assistance programs, and promote awareness and access to healthful foods to improve food security, nutrition, and cardiovascular health.(38)
Our findings are consistent with prior studies. In the Multiethnic Cohort Study, racial differences in mortality rates from myocardial infarction and other heart disease between Black and White participants disappeared in men but remained in women after adjusting for established CVD risk factors, including BMI, hypertension, diabetes, smoking, alcohol consumption, physical activity, educational level, and diet, and, for women, menopause and hormone replacement therapy use.(10) Except for education, other social risk factors were not included in that analysis. In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, socioeconomic status (education, income, and health insurance) explained 21.2% and 38.0% of the Black-White difference in CVD mortality among participants <65 years and ≥65 years, respectively.(3) In the Multi-Ethnic Study of Atherosclerosis (MESA), HRs for the Black-White difference in CVD mortality reduced from 1.72 to 0.95 after adjusting for socioeconomic status (neighborhood socioeconomic status, education, income, and health insurance), lifestyle and psychosocial factors, and clinical risk factors.(9) A recent analysis of NHANES data showed that SDOH, such as education, contributed to racial/ethnic differences in behavioral and metabolic risk factors for CVD.(12) In aggregate, available evidence indicates that SDOH explain most of the race-related differences in CVD mortality between Black and White populations in the US.
The strengths of NHANES include that it was conducted in a nationally representative sample of Black, Hispanic, White, and Other racial/ethnic populations; social, behavioral, and metabolic risk factors were systematically collected with standard methods and stringent quality control; and mortality data were matched with national death registration. However, there are some limitations of our study. First, social, behavioral, and metabolic risk factors were collected cross-sectionally in NHANES so causal ordering among these risk factors with CVD mortality could not be studied. Second, the number of deaths from heart disease and stroke was too small to conduct cause-specific analyses. Third, although NDI is accurate in ascertaining vital status, it only has modest accuracy in classifying CVD mortality.(31) Fourth, baseline data collection extended over 20 years from 1999 to 2018, so the effect of secular trends could be a concern. However, our primary models were stratified by two-year NHANES cycles, and sensitivity analysis by the first and second 10-year NHANES cohorts yielded similar findings. Fifth, some NHANES cycles did not collect data on sleep duration. Given the rich information on covariates, multiple imputation by the chained equation method should provide valid estimates. Furthermore, the results were consistent with those obtained in the complete case analysis. Sixth, data on the neighborhood and built environment were not collected and, therefore, could not be analyzed. In addition, fasting blood samples were only available in half of the sample, and glucose and LDL-cholesterol were not included in our analysis. However, hemoglobin A1c, total and HDL-cholesterol were included. Furthermore, data on incident CVD events were not collected in NHANES, so we focused on CVD mortality, which has direct public health implications.
The Black-White difference in CVD mortality diminished after adjusting for behavioral and metabolic risks factors and completely dissipated after adjusting for SDOH. Future research is warranted to understand the underlying mechanisms of SDOH on CVD mortality and develop novel interventions for reducing CVD mortality in populations, especially in Black individuals.
Supplementary Material
Acknowledgment
Funding sources: The research reported in this publication was supported by the National Heart, Lung, and Blood Institute under award numbers R01 HL133790 and UG3 HL151309, the National Institute of General Medical Sciences under award number P20 GM109036, and the National Institute of Minority Health and Health Disparity under award number R01 MD018193. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclosures: None.
Dr. He had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Statistical Code: Available to interested readers by contacting Dr. Jiang He at jhe@tulane.edu.
Data: The NHANES data are publicly available and listed on the Questionnaires, Datasets, and Related Documentation page by survey cycle. https://wwwn.cdc.gov/nchs/nhanes/
Contributor Information
Jiang He, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana; Department of Medicine, Tulane University School of Medicine, New Orleans, Louisiana.
Joshua D. Bundy, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Siyi Geng, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Ling Tian, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Hua He, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Xingyan Li, Department of Biostatistics and Data Science, School of Public Health, The University of Texas Health Science Center at Houston, Houston, Texas.
Keith C. Ferdinand, Tulane University Translational Science Institute, New Orleans, Louisiana; Department of Medicine, Tulane University School of Medicine, New Orleans, Louisiana.
Amanda H. Anderson, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Kirsten S. Dorans, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Ramachandran S. Vasan, University of Texas School of Public Health San Antonio, San Antonio, Texas.
Katherine T. Mills, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana.
Jing Chen, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Tulane University Translational Science Institute, New Orleans, Louisiana; Department of Medicine, Tulane University School of Medicine, New Orleans, Louisiana.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Kyalwazi AN, Loccoh EC, Brewer LC, Ofili EO, Xu J, Song Y, et al. Disparities in Cardiovascular Mortality Between Black and White Adults in the United States, 1999 to 2019. Circulation. 2022;146(3):211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajeu GS, Safford MM, Howard G, Howard VJ, Chen L, Long DL, et al. Black-White Differences in Cardiovascular Disease Mortality: A Prospective US Study, 2003-2017. Am J Public Health. 2020;110(5):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in Cardiovascular Risk Factors in US Adults by Race and Ethnicity and Socioeconomic Status, 1999-2018. Jama. 2021;326(13):1286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999-2000 to 2017-2018. Jama. 2020;324(12):1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. Trends in Obesity Prevalence by Race and Hispanic Origin-1999-2000 to 2017-2018. Jama. 2020;324(12):1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, et al. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. Jama. 2021;326(8):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Post WS, Watson KE, Hansen S, Folsom AR, Szklo M, Shea S, et al. Racial and Ethnic Differences in All-Cause and Cardiovascular Disease Mortality: The MESA Study. Circulation. 2022;146(3):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson SO, Haiman CA, Wilkens LR, Kolonel LN, Wan P, Pike MC. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS One. 2007;2(4):e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NS, Ning H, Petito LC, Kershaw KN, Bancks MP, Reis JP, et al. Associations of Clinical and Social Risk Factors With Racial Differences in Premature Cardiovascular Disease. Circulation. 2022;146(3):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah NS, Huang X, Petito LC, Bancks MP, Ning H, Cameron NA, et al. Social and Psychosocial Determinants of Racial and Ethnic Differences in Cardiovascular Health in the United States Population. Circulation. 2023;147(3):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, et al. Social Determinants of Cardiovascular Disease. Circ Res. 2022;130(5):782–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Health. 2019;7(6):e748–e60. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. Bmj. 2021;373:n604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhanes/index.htm.
- 17.US Department of Health and Human Services. Healthy People 2030: Social Determinants of Health. Accessed December 12, 2022. https://health.gov/healthypeople/objectives-and-data/social-determinants-health. .
- 18.US Census Bureau. How the Census Bureau Measures Poverty. Accessed January 11, 2023. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html.
- 19.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000, Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service; 2000. Accessed December 12, 2022. https://naldc.nal.usda.gov/download/38369/PDF. [Google Scholar]
- 20.Carlson SJ, Andrews MS, Bickel GW. Measuring food insecurity and hunger in the United States: development of a national benchmark measure and prevalence estimates. J Nutr. 1999;129(2S Suppl):510s–6s. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146(5):e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center for Nutrition Policy and Promotion, US Department of Agriculture. Healthy Eating Index. Accessed Decembee 14, 2022. https://www.fns.usda.gov/healthy-eating-index-hei.
- 23.Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health. 2006;14(2):66–70. [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7(2):102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 28.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–s40.36507649 [Google Scholar]
- 29.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama. 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. The Linkage of National Center for Health Statistics Survey Data to the National Death Index — 2019 Linked Mortality File (LMF): Linkage Methodology and Analytic Considerations, June 2022. Hyattsville, Maryland. Assessed July 7, 2022. https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm. . [Google Scholar]
- 31.Olubowale OT, Safford MM, Brown TM, Durant RW, Howard VJ, Gamboa C, et al. Comparison of Expert Adjudicated Coronary Heart Disease and Cardiovascular Disease Mortality With the National Death Index: Results From the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc. 2017;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resche-Rigon M, White IR. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat Methods Med Res. 2018;27(6):1634–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhaskaran K, Smeeth L. What is the difference between missing completely at random and missing at random? Int J Epidemiol. 2014;43(4):1336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 3rd Edition. 2003; New York: Chapman and Hall/CRC. 10.1201/9781420036268. [DOI] [Google Scholar]
- 35.Minhas AMK, Jain V, Li M, Ariss RW, Fudim M, Michos ED, et al. Family income and cardiovascular disease risk in American adults. Sci Rep. 2023;13(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robards J, Evandrou M, Falkingham J, Vlachantoni A. Marital status, health and mortality. Maturitas. 2012;73(4):295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt EJ, Mozaffarian D, Leung CW, Berkowitz SA, Murthy VL. Diet and Food and Nutrition Insecurity and Cardiometabolic Disease. Circ Res. 2023;132(12):1692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorndike AN, Gardner CD, Kendrick KB, Seligman HK, Yaroch AL, Gomes AV, et al. Strengthening US Food Policies and Programs to Promote Equity in Nutrition Security: A Policy Statement From the American Heart Association. Circulation. 2022;145(24):e1077–e93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.