Sir,
A 48-year-old woman presented to the dermatology outpatient department with a 4-day history of a skin rash over the bilateral upper and lower limbs and trunk. She received the COVID vaccination’s booster dose (“Covaxin®,” a whole-virion inactivated SARS-CoV-2 antigen, strain: NIV-2020-770) 1 day before the onset of the rash. It was associated with an episode of fever and loose stool at the onset of the rash. There was no history of drug intake of any form or infection before skin eruption. Her previous medical history was unremarkable. Cutaneous examination revealed sharply marginated erythematous and edematous plaques on the bilateral upper limbs, lower limbs and trunk [Figure 1]. Other mucocutaneous, general, and systemic examinations were within normal limits. Investigations such as complete hemogram and liver and renal function tests were within normal limits. Histology showed a normal-looking epidermis, marked subepidermal edema and mild perivascular lymphocytic infiltration. The edematous area did not have any inflammatory infiltrate [Figure 2]. Special stains for bacteria and fungus were negative. A diagnosis of erysipelas-like eruption due to COVID-19 vaccination was made, and the patient was started with oral prednisolone 30 mg once daily for 5 days. The rash had completely resolved during the follow-up.
Figure 1.
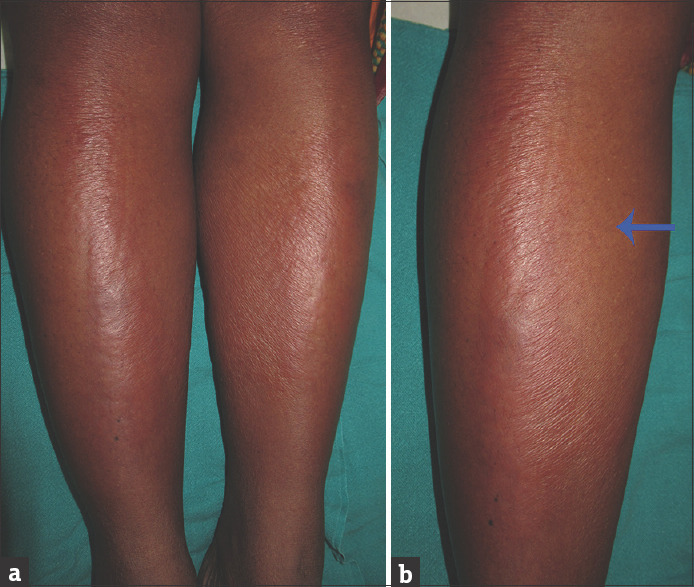
(a) Sharply marginated erythematous, edematous plaques on the bilateral lower limbs. (b) The arrow points to the surrounding normal-looking skin
Figure 2.
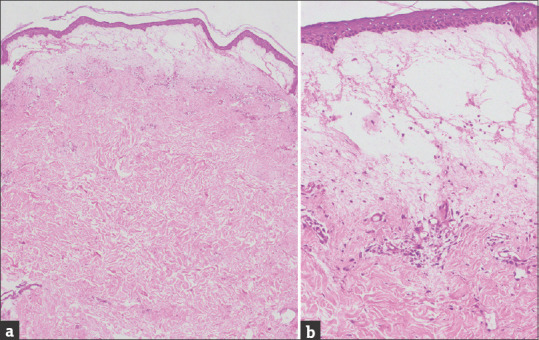
(a) Histology shows a normal-looking epidermis, marked subepidermal edema and mild perivascular lymphocytic infiltration (H and E, ×40). (b) The edematous area has no inflammatory infiltration (H and E, ×400)
COVID-19 vaccine-induced reactive dermatoses are being increasingly reported due to mass immunisation by different types of vaccines. A delayed large local reaction, characterised by erythematous and edematous rash, is the most commonly described vaccine-associated rash, which develops a minimum of 4 days after the vaccination. Morbiliform and maculopapular rash begin 2–3 days after the vaccination and persist for a week. However, a case that lasted for 1 month has been described.[1] Other cutaneous side-effects described are pernio-like lesions, urticaria, urticarial vasculitis, early-onset local injection site reactions, erythema multiforme (EMF), EMF-like and pityriasis rosea-like reactions, lichen planus and petechial and purpuric rash. In addition, reactivation of varicella–zoster and herpes simplex have been described.[1,2]
To our knowledge, an erysipelas-like eruption following the COVID-19 vaccination has not been reported. Uncontrolled activation of the immune system, especially the innate immune system and increased cytokine expression, is possibly responsible for extensive dermal edema.
Erysipelas-like eruption needs to be distinguished from erysipelas. In contrast to our case, erysipelas are marked by localised distribution, persistent fever, associated pain, lymphadenopathy and lymphangitis. In addition, histology will show moderate to dense neutrophilic infiltration and bacilli, besides marked dermal edema in the latter case. Other conditions where an erysipelas-like rash can be observed are the following: erysipelas-like erythema has been described in familial Mediterranean fever; migrating erysipelas-like erythemas due to nephrotic crisis; erysipelas-like eruption of mercury exanthem, histoplasmosis, Wells’ syndrome and metastatic Crohn’s disease; erysipelas-like dermatophytid; erysipelas-like erythema as a paraneoplastic eruption of renal cell carcinoma, erysipelas carcinomatosum and erysipelas melanomatosum are due to infiltration and inflammation of dermal lymphatics by adenocarcinoma and melanoma cells, respectively; and gemcitabine-induced erysipeloid rash.[3,4,5]
In conclusion, we report a rare erysipelas-like rash following COVID-19 vaccination. Physicians should be aware of this rare reactive dermatosis during a time of mass vaccination against COVID-19 and treat it accordingly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1.Sun Q, Fathy R, McMahon DE, Freeman EE. COVID-19 vaccines and the skin: The landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653–73. doi: 10.1016/j.det.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowe PA, Behera B, Sethy M, Viswan P. Erythema multiforme-like reaction following COVID-19 vaccination. Indian J Dermatol Venereol Leprol. 2022;1:1–3. doi: 10.25259/IJDVL_791_2021. [DOI] [PubMed] [Google Scholar]
- 3.Lidar M, Doron A, Barzilai A, Feld O, Zaks N, Livneh A, et al. Erysipelas-like erythema as the presenting feature of familial mediterranean fever. J Eur Acad Dermatol Venereol. 2013;27:912–5. doi: 10.1111/j.1468-3083.2011.04442.x. [DOI] [PubMed] [Google Scholar]
- 4.Belfeki N, Gharbi E, Flateau C, Diamantis S. Erysipelas-like presentation of Wells'syndrome (eosinophilic cellulitis) Reumatismo. 2020;71:226–9. doi: 10.4081/reumatismo.2019.1252. [DOI] [PubMed] [Google Scholar]
- 5.Kuku I, Kaya E, Sevinc A, Aydogdu I. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271–2. doi: 10.1046/j.1468-3083.2002.00485.x. [DOI] [PubMed] [Google Scholar]


