Abstract
Background:
Previous studies of association between exposure to poly- and perfluoroalkyl substances (PFAS) and gestational hypertension (GH) and preeclampsia (PE) have shown conflicting results, but most dichotomized outcome and did not study continuous blood pressure (BP) changes.
Objectives:
To study the association between PFAS exposure in early pregnancy and maternal BP trajectories in pregnancy, gestational hypertension and preeclampsia.
Methods:
1436 women were enrolled in the Odense Child Cohort in early pregnancy and had a serum sample drawn, from which perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) were measured using LC–MS/MS. Repeated BP measurements through pregnancy and information on PE were obtained from hospital files. Adjusted linear mixed models were used to investigate association between PFAS exposure and BP trajectory. Associations between PFAS and PE and GH were assessed by Cox proportional hazards model.
Results:
All women had measurable concentrations of PFAS. In all of many comparisons higher PFAS exposure (apart from PFHxS) was associated with higher systolic (SBP) and diastolic (DBP) blood pressures, although not all were significant, which is unlikely to be due to chance. After adjustment, each doubling in PFOS or PFOA exposure was associated with 0.47 mmHg (95% CI: −0.13; 1.08) and 0.36 mmHg (−0.19; 0.92) higher SBP; and 0.58 mmHg (0.13; 1.04) and 0.37 mmHg (−0.05; 0.79) higher DBP. No clear associations between PFAS exposure and PE or GH were found.
Discussion:
The magnitude of the association between PFAS exposure and BP might appear small, statistically non-significant and the possible clinical importance low. However, at a population level this may slightly shift the distribution of BP towards an increased incidence of GH. If BP increases in pregnancy, it may have long-term impact on health not only of the pregnant woman but also of her offspring.
Keywords: Perfluoroalkyl substances (PFAS), Pregnancy, Environmental chemicals, Preeclampsia, Blood pressure
1. Introduction
Pregnancy-induced hypertension (PIH) is a common and severe pregnancy complication, encompassing gestational hypertension (GH) and preeclampsia (PE). GH is characterized by hypertension (blood pressure ≥140/90 mmHg) that occurs after 20 weeks of gestation. The new International Society for the Study of Hypertension in Pregnancy guidelines define PE as GH accompanied by proteinuria, intrauterine growth restriction or other maternal end-organ damage (Brown et al., 2018). PIH affects 5–10 percent of all pregnancies and is one of the most common causes of perinatal and maternal morbidity and mortality (Sibai et al., 2005). PIH increases the risk of long-term maternal and fetal morbidity, particularly from cardiovascular disease (Wilson et al., 2003). Risk factors for GH and PE include high maternal age, obesity, nulliparity, multiple pregnancy, history of PE or hypertension and use of assisted reproductive technology, whereas smoking seems protective (Bartsch et al., 2016; Stone et al., 2007). However, hypertensive disorders of pregnancy are heterogenous and the pathogenesis is not fully elucidated, although for PE it is suggested that insufficient trophoblastic invasion of the uterine spiral arteries plays a role, as do other potential intermediate factors like maternal inflammation, oxidative stress and endothelial dysfunction (Steegers et al., 2010).
The role of environmental contaminants in PIH is attracting scientific attention. Exposure to perfluoroalkyl substances (PFAS) may increase susceptibility to PIH (Blake and Fenton 2020). PFAS are persistent chemicals with endocrine disrupting properties and long elimination half-lives of 4 to 8 years depending on their chemical structure (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). They are widely used in the industrial and commercial production of water-resistant or repellent fabrics, non-stick coatings, grease proof materials and as flame retardants (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). Humans are exposed to these substances in everyday life through ingestion of contaminated drinking water and food and inhalation of indoor air particles (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). PFAS are detectable in almost all human serum-samples (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020), especially perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) used for decades. They are being phased out from most industries (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). However, the use of newer PFAS including perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) are increasingly leading to continued or even increased human exposure (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020).
Although some previous studies have detected associations between PFAS exposure and GH, the results have been conflicting (Avanasi et al., 2016; Borghese et al., 2020; Darrow et al., 2013; Huang et al., 2019; Huo et al., 2020; Nolan et al., 2010; Rylander et al., 2020; Savitz et al., 2012; Starling et al., 2014; Stein et al., 2009; Wikstrom et al., 2019). Part of the apparent discrepancy is due to differences in exposure levels, different mixtures, and different study designs, with exposure imprecision. A recent Canadian birth cohort study found no association between PFOA or PFOS exposure and GH or PE, however, PFHxS exposure was associated with PE, and the effect was more pronounced among women carrying a female fetus, whereas women carrying a male fetus had higher risk of GH (Borghese et al., 2020). In addition, higher concentrations of PFAS were associated with increases in blood pressure in each trimester. Most other previous studies, although prospective, only recorded BP and diagnosed PE once during pregnancy and dichotomized outcome and did not study continuous BP changes, which may result in loss of power (Huo et al., 2020; Rylander et al., 2020; Starling et al., 2014; Wikstrom et al., 2019).
In the Odense Child Cohort, we had access to data on repeated blood pressure (BP) measures and clinical information on the onset of PE, as well as measurements of PFAS in early pregnancy among 1436 women. We therefore studied the association between PFAS exposure in early pregnancy and maternal blood pressure trajectories in pregnancy, gestational hypertension and preeclampsia. Finally, we investigated whether these effects were modified by pre-pregnancy maternal weight, parity and fetal sex.
2. Materials and methods
2.1. Study population
Data from the Odense Child Cohort, a prospective population-based cohort from the municipality of Odense, Southern Denmark was used. Eligible women were residing in the municipality of Odense, Region of Southern Denmark and were recruited at the first antenatal visit (between 8 and 16 completed gestational weeks (GW)) between 2010 and 2012. Study aims, design and participants have been described in detail previously (Kyhl et al., 2015). Anthropometric data (age, height and pre-pregnancy weight and body mass index (BMI)), parity and smoking status were derived from hospital records. Educational level (high school or less, high school + 1–4 years, high school+ >4 years) was extracted from questionnaires, and missing data were retrieved from hospital records.
The present study included all singleton pregnancies with at least one BP measurement in pregnancy and PFAS concentrations measured in 1st trimester, resulting in 1436 women eligible for the analyses (Fig. 1, supplemental Table 1). Maternal BP in pregnancy was measured as clinical routine, using appropriate cuff size by a general practitioner or a midwife. Maternal pregnancy complications were defined as follows: Gestational hypertension (GH) was characterized as de novo BP > 140/90 mm Hg after 20 weeks of gestation on two or more episodes with at least 4 h in between or significant aggravation of pre-existing hypertension, preeclampsia (PE) as GH with proteinuria (>0.3 g/24 h or at least + 1 on sterile urine dipstick) according to the Danish Society of Obstetrics and Gynecology diagnostic criteria of hypertension and preeclampsia 2007–2012 (Luef et al., 2016). PIH encompassed GH and PE. Diagnoses of GH and PE were validated by a person reviewing all hospital files and all BP measurements in pregnancy collected by a retrospective evaluation of the electronic patient files and hand-written maternity health charts (Birukov et al., 2020; Luef et al., 2016).
Fig. 1.
Flow chart for the included 1436 women from the Odense Child Cohort.
2.2. PFAS measurements
Serum samples obtained at inclusion at GA 8–16 weeks from 1699 women were analyzed for concentrations PFHxS, PFOS, PFOA, PFNA and PFDA using online solid phase extraction followed by liquid chromatography and triple quadropole mass spectrometry (LC–MS/MS) at Department of Environmental Medicine, University of Southern Denmark (Vorkamp et al., 2014). Of the samples, 199 were analyzed in 2011, further 330 were analyzed in 2013, 191 in 2014 and the remaining 979 in 2019. The within-batch coefficients of variation (CVs) were <3% and the between batch CVs, for all sets analyzed were <10.5% (Beck et al., 2019). The limit of quantification (LOQ) was 0.03 ng/ml for all compounds. PFOS, PFOA, PFNA and PFDA were 100% quantifiable, whereas PFHxS were quantifiable in 98% of samples.
2.3. Ethical approval
All participating women provided written informed consent. The study has been approved by the Regional Scientific Ethical Committees for Southern Denmark (no. S-20090130) and the Data Protection Agency (j.no. 2008–58-0035).
2.4. Statistical analysis
Normally distributed data were reported as means with corresponding standard deviation, SDs, non-normally distributed data as medians with interquartile ranges. PFAS were not normally distributed and were log2-transformed to allow assessment of the relative change in the outcome parameters for a doubling of exposure. We used descriptive statistics to inspect distributions of explanatory variables for both GH and PE. The maternal BP trajectory consisted of longitudinally collected BP data, which was structured in four-week intervals. Crude and adjusted linear mixed models based on random intercept with variance components covariance structure were used to investigate prospective relationships between maternal 1st trimester PFAS exposure (log2-transformed) and BP trajectory as continuous outcome as previously described (Birukov et al., 2020). In brief, linear mixed models (also called hierarchical linear models or multilevel models) account for intra-individual autocorrelations or missing data resulting from longitudinal repeated measurements on the same subjects. Fixed effects in the adjusted linear models included log-transformed PFAS, pre-pregnancy BMI, maternal age, smoking status and parity. We also stratified the analyses for BP and PE by fetal sex, parity and BMI as these parameters may modify the associations (Jensen et al., 2018). Effect modification was evaluated by construction of interaction terms on multiplicative scale and evaluation of significance level. In addition, we performed a sensitivity analysis in women with at least three available BP measurements. Associations between PFAS and the incidence of PIH, GH and PE were assessed by Cox proportional hazards model. The same confounder structure as in linear mixed models was used in the Cox models, as these factors can modify the a-priori risk for PE and were associated with PFAS exposure (Table 1) (Bartsch et al., 2016).
Table 1.
Characteristics of the 1436 pregnant women in the Odense Child Cohort according to gestational hypertension (GH) and preeclampsia (PE) status.
| Maternal characteristics | Total N = 1436 (100%) | No PE or GH N = 1283 (89.3%) | GH N = 49 (3.4%) | PE N = 104 (7.2%) |
|---|---|---|---|---|
| Age (years) | 30.3 (4.45) | 30.3 (4.45) | 30.9 (3.89) | 30.2 (4.74) |
| <25 | 318 (19.7) | 130 (10.1) | 1 (2.0) | 11 (10.6) |
| 25–34 | 1031 (64.0) | 920 (71.7) | 40 (81.6) | 71 (68.3) |
| >34 | 263 (16.3) | 233 (18.2) | 8 (16.3) | 22 (21.2) |
| Prepregnancy BMI (kg/m2) | 24.3 (4.38) | 24.0 (4.02) | 26.1 (5.96) | 26.9 (6.33) |
| <20 | 339 (21.0) | 150 (11.7) | 6 (12.2) | 7 (6.7) |
| 20–25 | 787 (48.8) | 726 (56.6) | 18 (36.7) | 43 (41.4) |
| >25 | 486 (30.2) | 407 (31.7) | 25 (51.0) | 54 (51.9) |
| Maternal education | ||||
| High school or less | 413 (29.1) | 362 (28.8) | 14 (28.6) | 36 (34.6) |
| High school + 1–4 years | 712 (50.2) | 636 (50.5) | 22 (44.9) | 52 (50.0) |
| High school + > 4 years | 292 (20.6) | 261 (20.7) | 13 (26.5) | 16 (15.4) |
| Parity | ||||
| Nulliparous | 808 (56.3) | 693 (54.0) | 36 (73.5) | 79 (76.0) |
| Multiparous | 628 (43.7) | 590 (46.0) | 13 (26.5) | 25 (24.0) |
| Smoking | ||||
| No | 1364 (95.0) | 1221 (95.2) | 46 (93.9) | 97 (93.3) |
| Yes | 72 (5.0) | 62 (4.8) | 3 (6.1) | 7 (6.7) |
| Mean systolic blood pressure (SD) (mmHg) throughout pregnancy | 118.9 (8.60) | 117.7 (7.83) | 130.7 (6.58) | 127.9 (9.05) |
| First trimester | 116.4 (10.8) | 115.7 (10.2) | 124.6 (12.4) | 121.4 (13.6) |
| Second trimester | 116.0 (10.1) | 114.8 (9.43) | 126.9 (11.8) | 123.8 (10.5) |
| Third trimester | 121.0 (9.21) | 119.5 (8.24) | 133.7 (7.55) | 131.8 (9.35) |
| Mean diastolic blood pressure (SD) (mmHg) throughout pregnancy | 73.9 (6.61) | 73.0 (5.94) | 82.7 (5.30) | 81.4 (7.20) |
| First trimester | 71.4 (8.69) | 70.8 (8.37) | 77.9 (8.36) | 75.9 (10.1) |
| Second trimester | 70.8 (7.67) | 70.0 (7.13) | 78.2 (8.15) | 76.7 (8.89) |
| Third trimester | 76.0 (7.01) | 74.9 (6.22) | 84.9 (5.20) | 85.5 (6.58) |
| Median and 25–75 percentile (ng/ml) | ||||
| PFHxS | 0.36 (0.25–0.50) | 0.35 (0.24–0.50) | 0.36 (0.27–0.50) | 0.37 (0.28–0.52) |
| PFOS | 7.50 (5.51–10.24) | 7.52 (5.49–10.20) | 7.49 (5.35–10.21) | 7.57 (6.07–10.98) |
| PFOA | 1.68 (1.12–2.38) | 1.66 (1.10–2.36) | 1.92 (1.18–2.62) | 1.78 (1.16–2.51) |
| PFNA | 0.64 (0.47–0.86) | 0.64 (0.64–0.86) | 0.66 (0.48–0.87) | 0.64 (0.48–0.87) |
| PFDA | 0.29 (0.22–0.40) | 0.29 (0.22–0.40) | 0.29 (0.22–0.41) | 0.26 (0.21–0.38) |
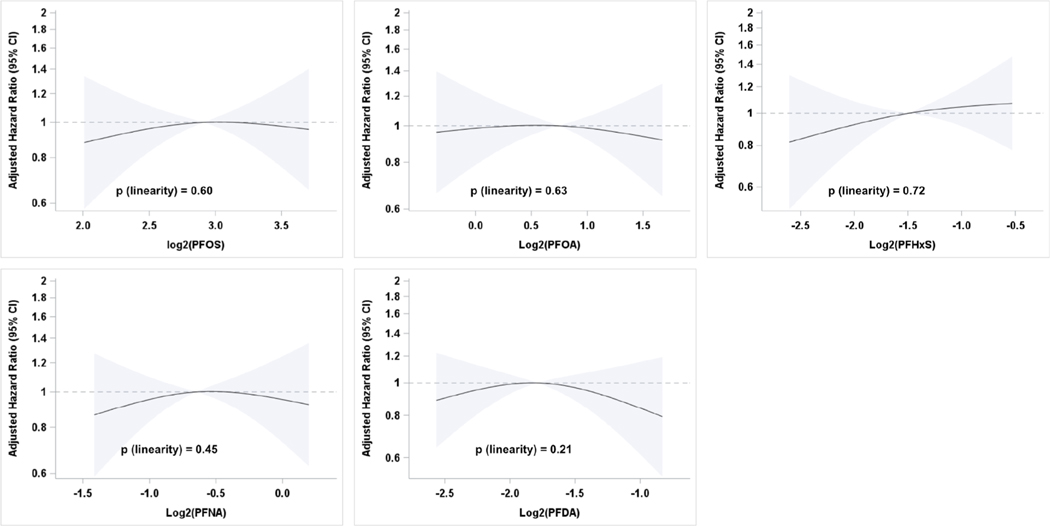
In the Cox analyses, follow-up of pregnant women began at the date of 1st trimester blood sampling and ended with censoring at the date of an event (PE, GH) or uneventful delivery. Restricted cubic splines were used to assess potential non-linearity in the relationships between PFAS concentration and PE incidence with median PFAS values as reference and knots at the 5th, 50th and 95th percentiles.
All statistical analyses were carried out in R version 4.0.2 and SAS (version 9.4, Enterprise Guide 7.1, SAS Institute Inc., Cary, NC, USA). A two-sided p-value <0.05 was considered statistically significant.
3. Results
A total of 1436 women were included (Fig. 1). The vast majority were Caucasian (96.1%), their mean age was 30.3 years (4.5), and their mean BMI was 24.3 kg/m2 (4.4). Most of the participants had 1 to 4 years post high-school educational background (50.2%), 5.0% of the women were smokers before pregnancy (Table 1). The total prevalence of GH and PE was 3.4% (N = 49/1436) and 7.2% (N = 104/1436) respectively. Mean SBP and DBP in the first, second and third trimesters were 116.4 (10.8), 116.0 (10.1), 121.0 (9.21) mmHg for SBP and 71.4 (8.69), 70.8 (7.67), 76.0 (7.01) mmHg for DBP (Table 1). Women with PE and GH had higher pre-pregnancy BMI and were more often nulliparous (Table 1). The blood pressure trajectory of women with PE and GH was elevated throughout pregnancy, starting as early as the 1st trimester as shown in previously published data from the Odense Child Cohort (Birukov et al., 2020). A total of 152 women (10.6%) had <3 BP measurements, 1021 (71.1%) between 3 and 6, and 263 (18.3%) >6 BP measurements during pregnancy (Suppl Table 1).
Median concentrations of PFHxS, PFOS, PFOA, PFNA, PFDA and PFOS were 0.4, 7.5, 1.7, 0.6 and 0.3 ng/ml, respectively (Table 1). PFAS were moderately to strongly intercorrelated (Spearman correlation coefficients between 0.30 and 0.67). Younger, smoking, normal-weight, nulliparous women had higher median serum concentrations of PFAS (Jensen et al., 2018).
Women with higher PFOS, PFOA, PFNA, PFDA exposure generally had higher SBP and DBP both in the unadjusted and adjusted models (Table 2), whereas PFHxS exposure was associated with reduced blood pressure. These associations were statistically significant for PFOS exposure and DBP and PFHxS exposure and SBP. After adjustment for age, pre-pregnancy BMI, parity and smoking status, each doubling in PFOS exposure was associated with 0.47 mmHg (95% CI: −0.13; 1.08) higher SBP and 0.58 mmHg (0.13; 1.04) higher DBP. Each doubling of PFHxS exposure was associated with a significant reduction of −0.51 mmHg (−0.97; −0.04) in SBP and −0.30 mmHg (−0.65; 0.06) in DBP. Generally, no clear associations were found between PFAS exposure and PE, GH or PIH (Table 3) in Cox regressions, and no non-linear associations between exposure to PFAS and PE could be detected after inspection of restricted cubic splines (Fig. 2). Sensitivity analyses among women with at least three available BP measurements showed associations within the same direction and magnitude (data not shown).
Table 2.
Unadjusted and adjusted* β coefficients and 95% confidence intervals (95% CI) from linear mixed models for 1st trimester PFAS in regard to gestational systolic and diastolic blood pressures (SBP and DBP) trajectories among 1436 women. Associations are shown as change in mm Hg per doubling in PFAS concentrations.
| Doubling of PFAS (ng/ml) | Unadjusted β coefficients (95% CI) |
Adjusted* β coefficients (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| SBP trajectory (mmHg) | p-value | DBP trajectory (mmHg) | p-value | SBP trajectory (mmHg) | p-value | DBP trajectory (mmHg) | p-value | |
| PFHxS | −0.17 (−0.65; 0.30) | 0.48 | −0.08 (−0.44; 0.29) | 0.69 | −0.51 (−0.97; −0.04) | 0.03 | −0.30 (−0.65; 0.06) | 0.10 |
| PFOS | 0.72 (0.09; 1.34) | 0.02 | 0.73 (0.24; 1.21) | <0.01 | 0.47 (−0.13; 1.08) | 0.13 | 0.58 (0.13; 1.04) | 0.01 |
| PFOA | 0.78 (0.25; 1.31) | <0.01 | 0.61 (0.20; 1.02) | <0.01 | 0.36 (−0.19; 0.92) | 0.20 | 0.37 (−0.05; 0.79) | 0.08 |
| PFNA | 0.32 (−0.33; 0.97) | 0.34 | 0.23 (−0.28; 0.73) | 0.38 | 0.19 (−0.44; 0.82) | 0.55 | 0.18 (−0.29; 0.66) | 0.45 |
| PFDA | −0.22 (−0.79; 0.36) | 0.46 | −0.19 (−0.64; 0.25) | 0.39 | 0.07 (−0.48; 0.61) | 0.80 | 0.08 (−0.33; 0.49) | 0.70 |
Adjusted for maternal age, pre-pregnancy BMI, parity and smoking status.
Table 3.
Adjusted* hazard ratios and 95% confidence intervals (95% CI) between 1st trimester PFAS and pregnancy induced hypertension (PIH), gestational hypertension (GH) and preeclampsia (PE). Associations are shown as HR per doubling in PFAS concentrations.
| Doubling of PFAS (ng/ml) | Adjusted* hazard ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|
| PIH N = 153 | p-value | GH N = 49 | p-value | PE N = 104 | p-value | |
| PFHxS | 1.10 (0.91; 1.34) | 0.32 | 0.97 (0.66; 1.43) | 0.87 | 1.14 (0.91; 1.42) | 0.26 |
| PFOS | 0.99 (0.77; 1.27) | 0.92 | 0.77 (0.47; 1.27) | 0.31 | 1.05 (0.79; 1.40) | 0.72 |
| PFOA | 1.03 (0.82; 1.28) | 0.82 | 1.25 (0.78; 1.99) | 0.36 | 0.97 (0.75; 1.24) | 0.79 |
| PFNA | 1.05 (0.80; 1.36) | 0.74 | 1.07 (0.61; 1.87) | 0.81 | 1.01 (0.76; 1.36) | 0.94 |
| PFDA | 1.04 (0.82; 1.31) | 0.76 | 1.35 (0.86; 2.11) | 0.19 | 0.93 (0.71; 1.22) | 0.61 |
Adjusted for maternal age, pre-pregnancy BMI, parity and smoking.
Fig. 2.
Restricted cubic splines assessing potential non-linearity in the relationships between preeclampsia and PFAS.
No clear differences in blood pressure were found between women carrying a male or a female fetus and being exposed to PFAS (Suppl Table 2), and no significant interaction with fetal sex for any PFAS (all p-values for interaction >0.05) was seen. However, there was a significant interaction between PFOA and maternal overweight status (p-interaction with SBP p = 0.02 and with DBP p = 0.04), PFOA associated with higher BP only in normal-weight women, (suppl Table 3), while in overweight women the tendency went in the opposite direction. Likewise, PFDA and PFNA exposure was associated with higher BP in normal weight women compared to overweight women, whereas PFOS exposure had higher impact in overweight women (Suppl Table 3). No clear difference in BP and PE among nulli- and multiparous women exposed to PFAS was found, though PFOS significantly and positively associated with SBP and DBP trajectories in nulliparous women (p-interaction for SBP and DBP p = 0.02) (Suppl Table 4).
4. Discussion
In this large prospective cohort study, we found that first trimester exposure to PFAS was associated with slightly higher blood pressure in pregnancy. Exposure to four out of five PFAS was associated with higher BP in all analyses, though not all were statistically significant, and we therefore consider it unlikely that the findings are due to chance. PFHxS exposure was associated with a reduction in blood pressure, and since we have no biological explanation for this, we consider this a chance finding. We did not find any significant association between PFAS exposure and preeclampsia nor with GH or PIH.
The magnitude of the observed effects might appear small at first glance, and a doubling of exposure seems considerable, however, the variation in PFAS exposure was large, with >6 times higher exposure among high compared to low exposed women. The clinical significance of these findings might not impact the anticipated pregnancy outcome of the individual women. Yet, at a population level, this tendency could slightly shift the distribution of blood pressure towards an increased incidence of gestational hypertension. In addition, higher blood pressure in pregnancy is associated with poorer long-term cardiovascular health of both the mother and child (Birukov et al., 2020), and a recent prospective study suggested that pregnancy concentrations of PFAS were associated with adverse postpartum cardiometabolic markers including blood pressure three years postpartum (Mitro et al., 2020). Our findings highlight the importance of using continuous outcome measures in healthy participants instead of dichotomizing into healthy versus non-healthy participants. By dichotomizing BP measurements, the study power is reduced, the extent of variation in outcome measures is underestimated between healthy and diseased groups, and the impact of the findings is limited to identifying risk factors for diseases.
Only one previous study used continuous blood pressure measures, like we did, and generally found that PFOS, PFOA and PFHxS exposure was associated with increase in DBP and for PFOA and PFHxS also for SBP in the same magnitudes as ours, though not all were statistically significant (Borghese et al., 2020). We found lower blood pressure among PFHxS exposed women, discrepancies may be due to the fact that PFHxS exposure was much higher among Canadian compared to Danish women. The Canadian study found higher risk of PE among women exposed to PFAS and carrying a female fetus, findings which we could not confirm. They did not measure PFNA or PFDA and only measured blood pressure three times during pregnancy, while we included up to 9 measures per woman, after averaging blood pressure measurements in 4 weeks intervals.
The epidemiological evidence for the associations between PFAS and GH and PE relies on different study designs and possible variability in both exposure assessment and the outcome assessment, possibly therefore findings are mostly null and weak positive findings (Avanasi et al., 2016; Borghese et al., 2020; Darrow et al., 2013; Huang et al., 2019; Huo et al., 2020; Nolan et al., 2010; Rylander et al., 2020; Savitz et al., 2012; Starling et al., 2014; Stein et al., 2009; Wikstrom et al., 2019). Most studies examined PE only, and few studied GH. A population of pregnant women from the Mid-Ohio Valley region has been studied, they were exposed to high levels of PFOA through contaminated drinking water (Darrow et al., 2013; Nolan et al., 2010; Savitz et al., 2012; Stein et al., 2009). They found both positive and negative associations, however, findings may not be generalizable to other populations. Three cohort studies of general obstetric populations have demonstrated conflicting results between PFAS exposure and PE (Borghese et al., 2020; Huo et al., 2020; Wikstrom et al., 2019). Differences in timing of PFAS measurement as well as exposure levels and definition of PE limits comparability. Two case-control studies (Rylander et al., 2020; Starling et al., 2014) found no association between PFAS exposure and PE, however, PE diagnoses were obtained from registers. Likewise, a Chinese study found no association between cord blood PFAS concentrations and PE, however cord blood may not be a good marker of early pregnancy exposure (Huang et al., 2019). Two studies have examined the impact of PFAS exposure on GH and found no clear associations, which is in accordance with our findings (Borghese et al., 2020; Huo et al., 2020). Overall, our study suggests that PFAS exposure in pregnancy may increase blood pressure, whereas the effect on PE is limited.
Mechanisms behind an association between PFAS exposure and blood pressure are yet to be resolved, but the concerns about immunologic effects from PFAS may be considered (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). The vascular disturbances, which play a key role in PE development, include endothelial dysfunction that is in turn associated with oxidative stress and increased inflammatory response (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). Oxidative stress is the key mechanism of arterial damage and endothelial dysfunction, which promote increases in blood pressure, vascular disease and arterial stiffness. Given that PFOS have been shown to induce oxidative stress in human endothelial cells (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020) and the support for immune system changes to PFOS exposure, we can only speculate on pathways involving oxidative stress and inflammation behind the present associations. There is some toxicological evidence to support the potential for fetal-sex specific effects of prenatal exposure to PFAS through the inhibition of aromatase activity within human placental trophoblastic cells (Gorrochategui et al., 2014), but further toxicological evidence is required to better understand any potential fetal sex-specific effects.
A major strength of our study is the population-based design in a low-risk setting of Danish women. However, only 42% of the eligible pregnant women participated in the Odense Child Cohort, and they were more likely to be non-smokers, older and nulliparous than non-participants (Kyhl et al., 2015). Diagnoses of GH and PE (3.4% and 7.2%) were higher than in the general Danish population probably partly explained by the few smokers and the fact that diagnoses were validated by medical chart review for all participants (Luef et al., 2016). A population-based US cohort study found that the discharge diagnoses of PE reported to the national patient registry substantially underestimated the true prevalence as detected by retrospective patient chart review (Geller et al., 2004). In addition, the women were unaware of their PFAS exposure, so this is unlikely to have affected their participation. Since we compared women across PFAS exposure, whether they represent the general population is of less importance. Further strengths are multiple BP measurements over the course of entire pregnancy, prospective study design, extensive adjustments for confounders and effect modification analyses. Moreover, we measured several PFAS in one setting, which allowed us to perform comparative analyses of exposure to several PFAS.
Limitations include the observational nature of associations. We did not have information about women with hypertension before pregnancy, but we expect the number to be low and possibly dilute our findings toward an underestimation of the risk. Furthermore, our study data are derived from BP measurements in pregnancy collected at routine visits with a midwife or general practitioner and therefore not standardized according to protocol e.g. same equipment, rest periods or three independent measures. However, we would expect that the measurement error would be random, and thus could potentially dilute our findings rather than introducing a systematic bias. In addition, serum concentrations of PFAS were measured in early pregnancy before the outcomes, as they are known to decrease throughout pregnancy, likely due both to foetal transfer and to dilution as a result of increased maternal blood volume during pregnancy (Bach et al., 2015). Still, PFAS measurements at different timepoints during pregnancy are highly intercorrelated, and the major compounds have long half-lives of 4–5 years (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020). The exposure level reflected by the serum concentration was therefore believed to be representative of the exposure during the entire pregnancy. PFOA and PFOS exposure had the highest impact on BP and PE in pregnancy but also appeared in the highest concentrations. To what extent other PFAS contribute to this tendency is difficult to determine due to the intercorrelations between the substances, and we did not study mixture effects and cannot decipher the individual contribution of single PFAS.
A recent publication from the European Food Safety Authority (EFSA) has highlighted the lack of data on PFAS and blood pressure not only in pregnancy but across the whole life perspective (EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk 2020), and some studies in non-pregnant individuals have suggested association between PFAS exposure and blood pressure (Lin et al., 2020; Min et al., 2012). Our findings support that even in this relatively low-level exposed population of pregnant women, exposure to PFAS may increase blood pressure and possibly impact future health of both mother and child (Birukov et al., 2020; Wilson et al., 2003).
In conclusion, in this large prospective cohort study, we found associations between first-trimester exposure to four out of five PFAS and modest increases in blood pressure in pregnancy in all different analyses although only significant for PFOS. No clear association between PFAS exposure and preeclampsia was found. At population level, this tendency could slightly shift the distribution of blood pressure towards an increased incidence of gestational hypertension and therefore highlight the importance of using continuous outcome measures instead of formal diagnoses. Our findings are of general public interest, as all pregnant women were exposed to these chemicals, and if blood pressures increase in pregnancy, it may have long-term impact on health not only for the women but also for their children.
Supplementary Material
Acknowledgement
The families in OCC are acknowledged for their participation and commitment to the study. The midwives at the Department of Obstetrics and Gynecology, technicians at Hans Christian Andersen’s Children’s Hospital and at the Department of Environmental Medicine are acknowledged for their careful examination of the participants and analysis of PFAS in serum samples. This work was supported by the Danish Foundation for Scientific Innovation and Technology (09-067180), Innovation Fund Denmark, Odense University Hospital, the Region of Southern Denmark, the Municipality of Odense, the Mental Health Service of the Region of Southern Denmark, The European Union, Human Biomonitorying for EU (HBM4EU), Odense University Hospital Research Foundation, Odense Patient data Exploratory Network (OPEN), Novo Nordisk Foundation (grant nr. NNF19OC0058266 and NNF17OC0029404), the Danish Council for Independent Research (4004-00352B_FSS), The Foundation for research collaboration between Rigshospitalet and Odense University Hospital, and the Health Foundation (Helsefonden). PG is supported by the National Institute of Environmental Health Sciences, NIH (P42ES027706).
Footnotes
CRediT authorship contribution statement
Anna Birukov: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing - review & editing, Investigation. Louise Bjørkholt Andersen: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing - review & editing, Investigation. Marianne Skovsager Andersen: Writing - review & editing. Julie H. Nielsen: Validation, Investigation, Writing-review & editing. Flemming Nielsen: Validation, Writing - review & editing. Henriette Boye Kyhl: Validation, Investigation, Writing - review & editing. Jan Stener Jørgensen: Conceptualization, Resources, Writing - review & editing, Funding acquisition. Philippe Grandjean: Writing - review & editing. Ralf Dechend: Conceptualization, Methodology, Resources, Writing - review & editing, Funding acquisition. Tina Kold Jensen: Conceptualization, Methodology, Resources, Writing - original draft, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106442.
References
- Avanasi R, Shin HM, Vieira VM, Bartell SM, 2016. Impacts of geocoding uncertainty on reconstructed pfoa exposures and their epidemiological association with preeclampsia. Environ. Res 151, 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB, 2015. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit. Rev. Toxicol 45, 53–67. [DOI] [PubMed] [Google Scholar]
- Bartsch E, Medcalf KE, Park AL, Ray JG, 2016. High Risk of Pre-eclampsia Identification G. 2016 Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353, i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck IH, Timmermann CAG, Nielsen F, Schoeters G, Johnk C, Kyhl HB, et al. , 2019. Association between prenatal exposure to perfluoroalkyl substances and asthma in 5-year-old children in the odense child cohort. Environ. Health: Glob. Access Sci. Source 18, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov A, Herse F, Nielsen JH, Kyhl HB, Golic M, Kraker K, et al. , 2020. Blood pressure and angiogenic markers in pregnancy: contributors to pregnancy-induced hypertension and offspring cardiovascular risk. Hypertension 76, 901–909. [DOI] [PubMed] [Google Scholar]
- Blake BE, Fenton SE, 2020. Early life exposure to per- and polyfluoroalkyl substances (pfas) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443, 152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese MM, Walker M, Helewa ME, Fraser WD, Arbuckle TE, 2020. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the mirec study. Environ. Int 141, 105789. [DOI] [PubMed] [Google Scholar]
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. , 2018. The hypertensive disorders of pregnancy: isshp classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 13, 291–310. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Stein CR, Steenland K, 2013. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the mid-ohio valley, 2005–2010. Environ. Health Perspect 121, 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Dieter Schrenk MB, Bodin Laurent, Chipman James Kevin, Mazo Jesús Del, Grasl-Kraupp Bettina, Hogstrand Christer, Hoogenboom Laurentius Ron, Leblanc Jean-Charles, Nebbia Carlo Stefano, Nielsen Elsa, Ntzani Evangelia, Petersen Annette, Sand Salomon, Vleminckx Christiane, Wallace Heather, Barregård Lars, Ceccatelli Sandra, Cravedi Jean-Pierre, Halldorsson Thorhallur Ingi, Haug Line Småstuen, Johansson Niklas, Knutsen Helle Katrine, Rose Martin, Roudot Alain-Claude, Van Loveren Henk, Vollmer Günter, Mackay Karen, Riolo Francesca, Schwerdtle Tanja. 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ, 2004. International classification of diseases-9th revision coding for preeclampsia: how accurate is it? Am. J. Obstet. Gynecol 190, 1629–1633 discussion 1633–1624. [DOI] [PubMed] [Google Scholar]
- Gorrochategui E, Perez-Albaladejo E, Casas J, Lacorte S, Porte C, 2014. Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol 277, 124–130. [DOI] [PubMed] [Google Scholar]
- Huang R, Chen Q, Zhang L, Luo K, Chen L, Zhao S, et al. , 2019. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health: Global Access Sci. Source 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Huang R, Gan Y, Luo K, Aimuzi R, Nian M, et al. , 2020. Perfluoroalkyl substances in early pregnancy and risk of hypertensive disorders of pregnancy: a prospective cohort study. Environ. Int 138, 105656. [DOI] [PubMed] [Google Scholar]
- Jensen RC, Glintborg D, Timmermann CAG, Nielsen F, Kyhl HB, Andersen HR, et al. , 2018. Perfluoroalkyl substances and glycemic status in pregnant danish women: the odense child cohort. Environ. Int 116, 101–107. [DOI] [PubMed] [Google Scholar]
- Kyhl HB, Jensen TK, Barington T, Buhl S, Norberg LA, Jorgensen JS, et al. , 2015. The odense child cohort: aims, design, and cohort profile. Paediatr. Perinat. Epidemiol 29, 250–258. [DOI] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. , 2020. Per- and polyfluoroalkyl substances and blood pressure in pre-diabetic adults-cross-sectional and longitudinal analyses of the diabetes prevention program outcomes study. Environ. Int 137, 105573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luef BM, Andersen LB, Renault KM, Nohr EA, Jorgensen JS, Christesen HT, 2016. Validation of hospital discharge diagnoses for hypertensive disorders of pregnancy. Acta Obstet. Gynecol. Scand 95, 1288–1294. [DOI] [PubMed] [Google Scholar]
- Min JY, Lee KJ, Park JB, Min KB, 2012. Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in us adults. Occup. Environ. Med 69, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Sagiv SK, Fleisch AF, Jaacks LM, Williams PL, Rifas-Shiman SL, et al. , 2020. Pregnancy per- and polyfluoroalkyl substance concentrations and postpartum health in project viva, a prospective cohort. J. Clin. Endocrinol. Metab [DOI] [PMC free article] [PubMed]
- Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA, 2010. Congenital anomalies, labor/delivery complications, maternal risk factors and their relationship with perfluorooctanoic acid (pfoa)-contaminated public drinking water. Reprod. Toxicol 29, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander L, Lindh CH, Hansson SR, Broberg K, Kallen K, 2020. Per- and polyfluoroalkyl substances in early pregnancy and risk for preeclampsia: a case-control study in southern sweden. Toxics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Stein CR, Elston B, Wellenius GA, Bartell SM, Shin HM, et al. , 2012. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the mid-ohio valley. Environ. Health Perspect 120, 1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M, 2005. Pre-eclampsia. Lancet 365, 785–799. [DOI] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Richardson DB, Baird DD, Haug LS, Stuebe AM, et al. , 2014. Perfluoroalkyl substances during pregnancy and validated preeclampsia among nulliparous women in the norwegian mother and child cohort study. Am. J. Epidemiol 179, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R, 2010. Pre-eclampsia. Lancet 376, 631–644. [DOI] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Dougan M, 2009. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol 170, 837–846. [DOI] [PubMed] [Google Scholar]
- Stone CD, Diallo O, Shyken J, Leet T, 2007. The combined effect of maternal smoking and obesity on the risk of preeclampsia. J. Perinat. Med 35, 28–31. [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Nielsen F, Kyhl HB, Husby S, Nielsen LB, Barington T, et al. , 2014. Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: levels, correlations, and potential health implications. Arch. Environ. Contam. Toxicol 67, 9–20. [DOI] [PubMed] [Google Scholar]
- Wikstrom S, Lindh CH, Shu H, Bornehag CG, 2019. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in swedish women. Sci. Rep 9, 9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. , 2003. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 326, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.