Abstract
Background
There is an ongoing debate as to whether sex could be associated with immune checkpoint inhibitor (ICI) benefit. Existing literature data reveal contradictory results, and data on first-line immune combinations are lacking.
Method
This was a real-world, multicenter, international, observational study to determine the sex effects on the clinical outcomes in metastatic renal cell carcinoma (mRCC) patients treated with immuno-oncology combinations as first-line therapy.
Results
A total of 1827 mRCC patients from 71 cancer centers in 21 countries were included. The median OS was 38.7 months (95% CI 32.7–44.2) in the overall study population: 40.0 months (95% CI 32.7–51.6) in males and 38.7 months (95% CI 26.4–41.0) in females (p = 0.202). The median OS was higher in males vs. females in patients aged 18-49y (36.9 months, 95% CI 29.0–51.6, vs. 24.8 months, 95% CI 16.8–40.4, p = 0.426, with + 19% of 2y-OS rate, 72% vs. 53%, p = 0.006), in the clear cell histology subgroup (44.2 months, 95% CI 35.8–55.7, vs. 38.7 months, 95% CI 26.0–41.0, p = 0.047), and in patients with sarcomatoid differentiation (34.4 months, 95% CI 26.4–59.0, vs. 15.3 months, 95% CI 8.9–41.0, p < 0.001). Sex female was an independent negative prognostic factor in the sarcomatoid population (HR 1.72, 95% CI 1.15 − 2.57, p = 0.008).
Conclusions
Although the female’s innate and adaptive immunity has been observed to be more active than the male’s, women in the subgroup of clear cell histology, sarcomatoid differentiation, and those under 50 years of age showed shorter OS than males.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03719-0.
Keywords: ARON-1 study, Gender differences, Immunotherapy, Immune-based combinations, NCT05287464, Renal cell carcinoma
Introduction
Immunogenicity and vascularization are the main features of renal cell carcinoma (RCC) [1]. Immune-based combinations, including two immune checkpoint inhibitors (ICIs), or ICI and a tyrosine kinase inhibitor (TKI) with anti-angiogenic activity, have emerged as the standard first-line treatment [2–7].
Although immune-based therapies have clearly extended patient survival, the immunotherapy benefit and response rate are variable, and extensive efforts have been undertaken to identify robust biomarkers or clinical factors for optimal patient selection [8]. Biological factors, specific for certain individuals, have a clear effect on the variation in immunotherapy response.
Currently, there is an ongoing debate as to whether sex could be associated with ICI benefit [9]. Sex-based differences are involved in immune profiles [10], but it is unclear whether these differences play a role in immunotherapy benefit. Existing literature data reveal contradictory results. Conforti et al. reported in a meta-analysis of randomized clinical trials that male cancer patients treated with immune checkpoint inhibitors (ICI) derived greater efficacy than female patients [11]. Conversely, others following data found no difference in overall survival (OS) from ICIs when comparing the efficacy of these treatments between males and females [12, 13]. A retrospective analysis performed in patients with metastatic RCC (mRCC) revealed no difference between nivolumab and everolimus among the two sexes, although the small sample size limits clear conclusions [14]. The CheckMate-214 trial on nivolumab plus ipilimumab versus sunitinib in the first-line treatment of intermediate/poor-risk mRCC patients showed a wider OS advantage from ICI-combination among females (HR, 0.52; 95% CI, 0.34–0.78) compared to males (HR, 0.71; 95% CI, 0.55–0.92) [2], while inconsistent conclusions derived in mRCC population from a following meta-analysis [15].
Nevertheless, the existence of sexual dimorphism in immunological responses is supported by several observations, from the evidence of sex-associated molecular mechanisms to the reports on specific immune features potentially altering treatment responsiveness [16, 17]. In a comprehensive analysis of molecular biomarkers from The Cancer Genome Atlas (TCGA), sex-associated divergent patterns were observed in RCC. The higher mutation rate for the PBRM1 gene was in male clear cell RCC (ccRCC) patients and higher tumor mutation burden (TMB) and cytolytic activity (CYT) of T cells in males with renal papillary cell carcinoma. In the same research, sex-based differences were also in multiple immune features, including immune checkpoints (e.g., CD28 and CD86) and immune cell populations (e.g., active CD4 + T cells) [17].
In addition to these elements, which highlight the importance of molecular profiling and immune components for the ICI response, sex imbalance includes predominance and age selectivity for women in Xp11.2 translocation RCC [18] as well as the known differences in environmental stressors and lifestyle or behavior, disparities in body mass index (BMI) and comorbidities, sex hormone modulation with updated data on hormone changes during immunotherapy, and an increased emphasis on epigenetic influence [19–22].
The ARON-1 study (ClinicalTrials.gov identifier NCT05287464) was a multicenter, international, retrospective study to collect real-world data on clinical outcomes of metastatic RCC patients treated with immuno-oncology combinations as first-line therapy [23, 24]. In the current manuscript, we present the results from the analysis focused on the impact of sex on immune-based combinations effectiveness, stratified by ICI plus ICI, or ICI plus anti-angiogenic agent.
Patients and methods
Study design and population
The study population included patients diagnosed at age ≥ 18 years with RCC and radiologically confirmed metastatic disease, treated from January 1st, 2016, to October 1st, 2023, in 71 cancer centers in 21 countries (Table S1). All included patients had known data on age, sex, tumor histology, prognostic risk group according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria, previous nephrectomy, sites of metastases, type of immuno-combination, durations and response to therapy according to Response Evaluation Criteria In Solid Tumors 1.1 (RECIST 1.1) measured by investigators in each center. Pre-treatment neutrophil-to-lymphocyte ratio (NLR) and body mass index (BMI) were also collected. The NLR was recorded from the routinely performed blood cell count, as the absolute count of neutrophils divided by the absolute count of lymphocytes from peripheral blood samples collected at baseline. BMI was calculated as weight in kilograms divided by height in meters squared. Normal weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25–29.9 kg/m2), and obesity (BMI ≥ 30 kg/m2) were classified based on the World Health Organization (WHO) recommendations.
Clinical data were retrospectively and locally extracted, at each participating center, from the patients' medical records. The pathological information was abstracted from pathology reports for clinical use. First-line therapy was continued until the evidence of clinical and/or radiological tumor progression, unacceptable toxicities as per clinical local practice, or death. Computed tomography (CT), magnetic resonance imaging (MRI) scans, and laboratory tests were performed following standard local procedures.
Patients with lacking the above-mentioned information were excluded from the ARON-1 study.
The study was conducted according to Good Clinical Practice (GCP) and has been designed with the ethical principles laid down in the Declaration of Helsinki on human experimentation. The study protocol was approved by the Ethical Committee of the coordinating center (Marche Region-2021-492, Study Protocol “ARON 1 Project”) and by the Institutional Review Boards of international participating centers.
Study objectives
Our primary objective was to assess OS of metastatic patients treated with first-line immune combinations according to the patient's sex. Secondary objectives were the comparison of the tumor response [progression disease (PD), stable disease (SD), partial response (PR), complete response (CR)], objective response rate (ORR), to first-line treatment, between female and male patients. OS was calculated from the start of treatment to death for any cause. Patients without a tumor progression to the following line of treatment or death or lost at follow-up at the time of analysis were censored at their last follow-up date. Adverse events, dose reductions, and treatment interruptions were also collected.
Statistical considerations
The comparison between subgroups was performed with the chi-square test. The best cut-off for the number of metastatic sites was calculated by ROC curve and resulted > 2. For BMI and NLR, the best cut-offs calculated by ROC curves were ≥ 25 and ≥ 4.
OS was calculated from the time of the start of first-line therapy until death. Progression-free survival (PFS) was calculated as the time from the start of first-line therapy to documented disease progression or death from any cause, whichever occurred first. Patients without disease progression or death or lost at follow-up at the time of the analysis were censored at the last follow-up visit. The analysis of OS between groups was compared using the Kaplan–Meier method and log-rank test. To identify independent prognostic factors for OS, univariate and multivariate Cox proportional hazard regression models were performed. The list of variables included sex, age, BMI, nephrectomy, sarcomatoid differentiation, IMDC group, number and type of metastatic sites.
The chi-square test was used to compare groups for categorical variables. P values < 0.05 were considered statistically significant. Statistical analyses were conducted using MedCalc version 19.6.4 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium).
Results
Patients population
We included 1827 patients treated with immune combinations from the ARON-1 dataset; 1352 (74%) were males and 475 (26%) were females, with 30% of patients aged ≥ 70y. Clear cell histology was predominant (1578 patients, 86%); non-clear cell histology was in 249 patients (14%), including 97 papillary (5%), 36 chromophobe (2%), and 116 other histology subtypes (6%). In the group of non-clear cell histology, 11 patients presented Xp11.2 translocation (male-to-female ratio 4:7). Among the patients with clear cell histology, 274 patients (15%) reported sarcomatoid differentiation, [180 females (65.7%) and 94 (34.3%) males]. Lung (68%), lymph nodes (34%) and bone (33%) were the most frequent metastatic sites. The majority of patients (65%) underwent nephrectomy. Favorable, intermediate, and poor IMDC features were present in 14%, 62%, and 24% of all cases, respectively. The complete list of patients' characteristics is reported in Table 1.
Table 1.
Patient characteristics
| Characteristics | Overall No. (%) | Males No. (%) | Females No. (%) | p value |
|---|---|---|---|---|
| Total patients | 1827 (100) | 1352 (100) | 475 (100) | – |
| Age (years) | ||||
| 18–49 | 240 (13) | 180 (13) | 60 (13) | 0.999 |
| 50–69 | 1046 (57) | 780 (58) | 266 (56) | |
| ≥ 70 | 541 (30) | 392 (29) | 149 (31) | |
| Clear cell histology | 1578 (86) | 1170 (87) | 408 (86) | 0.837 |
| Non-clear cell histology | 249 (14) | 182 (13) | 67 (14) | 0.837 |
| Papillary | 97 (5) | 73 (5) | 24 (5) | 1.000 |
| Chromophobe | 36 (2) | 25 (2) | 11 (2) | 1.000 |
| Other | 116 (6) | 84 (6) | 32 (7) | 0.775 |
| Sarcomatoid differentiation | 274 (15) | 180 (13) | 94 (20) | 0.184 |
| Metastatic at diagnosis | 1034 (57) | 758 (56) | 276 (58) | 0.776 |
| Previous nephrectomy | 1179 (65) | 881 (65) | 298 (63) | 0.769 |
| No. of metastatic sites > 2 | 532 (29) | 396 (29) | 136 (29) | 1.000 |
| Site of metastasis, individual | ||||
| Lung | 1251 (68) | 947 (70) | 304 (64) | 0.368 |
| Lymph node | 614 (34) | 445 (33) | 169 (36) | 0.656 |
| Liver | 323 (18) | 225 (17) | 98 (21) | 0.472 |
| Bone | 603 (33) | 451 (34) | 152 (32) | 0.764 |
| Brain | 129 (7) | 88 (7) | 41 (9) | 0.603 |
| IMDC Prognostic Risk Group | ||||
| Favorable | 259 (14) | 194 (14) | 65 (14) | 1.000 |
| Intermediate | 1139 (62) | 852 (63) | 287 (60) | 0.664 |
| Poor | 429 (24) | 306 (23) | 123 (26) | 0.623 |
| NLR ≥ 4 | 533 (29) | 390 (29) | 143 (30) | 0.877 |
| BMI ≥ 25 | 1154 (63) | 899 (66) | 255 (54) | 0.084 |
BMI, Body Mass Index; IQR, interquartile range; NLR, Neutrophil-to-Lymphocyte Ratio
Survival analysis and response to first-line therapy
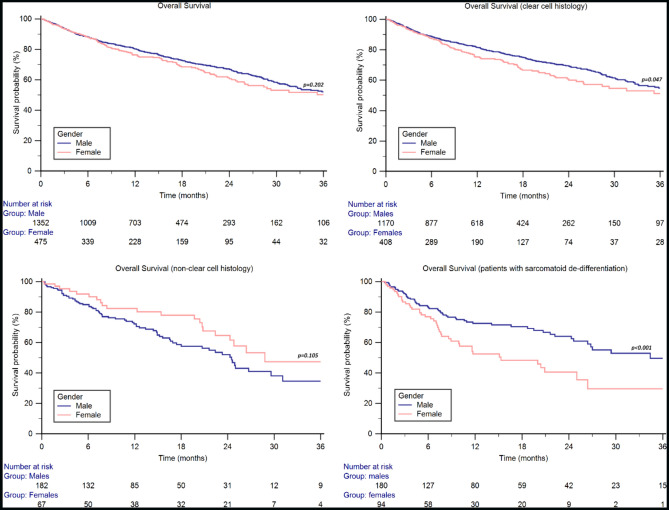
The median OS was 38.7 months (95% CI 32.7–44.2) in the overall study population and was 40.0 months (95% CI 32.7–51.6) in males and 38.7 months (95% CI 26.4–41.0) in females (p = 0.202, Fig. 1). The 2y-OS rate was 67% in males and 61% in females. On the other hand, no statistically significant differences were found in terms of median PFS, which was 15.7 months (95% CI 14.3–17.6) in males and 15.7 months (95% CI 12.0–19.2, p = 0.259) in females (Figure S1).
Fig. 1.
Overall Survival by gender in the ARON-1 study population and stratified by tumor histology and presence of sarcomatoid differentiation
In patients aged 18-49y, the median OS was 36.9 months (95% CI 29.0–51.6) in males and 24.8 months (95% CI 16.8–40.4, p = 0.426) in females, with + 19% of 2y-OS rate (72% vs. 53%, p = 0.006).
In patients aged 50-69y, the median OS was 44.2 months (95% CI 35.3–61.8) in males and NR (95% CI NR–NR, p = 0.533) in females, with a 2y-OS rate or 68% vs. 67%, respectively (p = 0.880).
In the elderly population (≥ 70y), the median OS was 31.4 months (95% CI 26.4–49.2) in males and 26.0 (95% CI 20.7–39.9, p = 0.525) in females, with a 2y-OS rate or 62% vs. 57%, respectively (p = 0.473).
The median OS was longer in males vs. females in the clear cell histology subgroup (44.2 months, 95% CI 35.8–55.7, vs. 38.7 months, 95% CI 26.0–41.0, p = 0.047, Fig. 1), while in the 251 patients with non-clear cell histology the median OS was higher in females, although the difference was not statistically significant (males: 24.6 months, 95% CI 20.7–31.1; females: 28.8 months, 95% CI 22.4–40.4; p = 0.105, Fig. 1).
In patients with sarcomatoid differentiation, the median OS was significantly longer in males (34.4 months, 95% CI 26.4–59.0 vs. 15.3 months, 95% CI 8.9–41.0, p < 0.001, Fig. 1), with + 20% of 1y-OS rate (71% vs. 51%) and + 24% of 2y-OS rate (64% vs. 40%).
No significant differences were found between males and females in patients with favorable (51.6 months, 95% CI 36.5–51.6 vs. NR, 95% CI NR–NR, p = 0.423), intermediate (40.3 months, 95% CI 31.7–55.7 vs. 35.2 months, 95% CI 26.0–44.4, p = 0.226) or poor-risk features (22.1 months, 95% CI 13.6–29.7, vs. 15.5 months, 95% CI 11.7–29.3, p = 0.730).
Furthermore, no significant differences in terms of median OS were found between males and females stratified by site of metastasis (lung: 38.9 months, 95% CI 31.7–52.2 vs. 31.6 months, 95% CI 22.4–41.0, p = 0.098; lymph nodes: 33.1 months, 95% CI 27.5–40.0 vs. 26.4 months, 95% CI 20.9–41.0, p = 0.565; bone: 26.8 months, 95% CI 22.2–30.4 vs. 26.0 months, 95% CI 17.7–29.3, p = 0.591; liver: 24.5 months, 95% CI 19.5–32.7 vs. 25.9 months, 95% CI 16.8–39.9, p = 0.746; brain: 23.6 months, 95% CI 15.4–28.2 vs. 16.8 months, 95% CI 11.7–41.0, p = 0.853).
Overall, 1295 patients (71%) presented 1 or 2 metastatic sites, while 532 patients (29%) reported > 2 metastatic sites. The difference between males and females was not statistically significant for both patients with 1–2 (44.2 months, 95% CI 35.3–59.0 vs. 40.2 months, 95% CI 25.9–41.0, p = 0.163) or with > 2 metastatic sites (29.0 months, 95% CI 25.0–40.0 vs. 28.4 months, 95% CI 24.0–39.9, p = 0.780).
No statistically significant differences were observed between males and females in both patients with BMI ≥ 25 kg/m2 (44.2 months, 95% CI 34.3–55.7 vs. NR, 95% CI NR–NR, p = 0.171) and BMI < 25 kg/m2 (31.4 months, 95% CI 27.3–40.8 vs. 31.6 months, 95% CI 25.9–40.2, p = 0.917) as well as in patients with NLR ≥ 4 (26.4 months, 95% CI 19.7–29.6, vs. 22.1 months, 95% CI 16.1–25.9, p = 0.649) or < 4 (49.2 months, 95% CI 36.9–59.6, vs. NR, 95% CI NR–NR, p = 0.688). The difference was slightly different when we considered patients with BMI > 30 kg/m2 (males: NR, 95% CI NR–NR vs. 28.8 months, 21.6–28.8, p = 0.081).
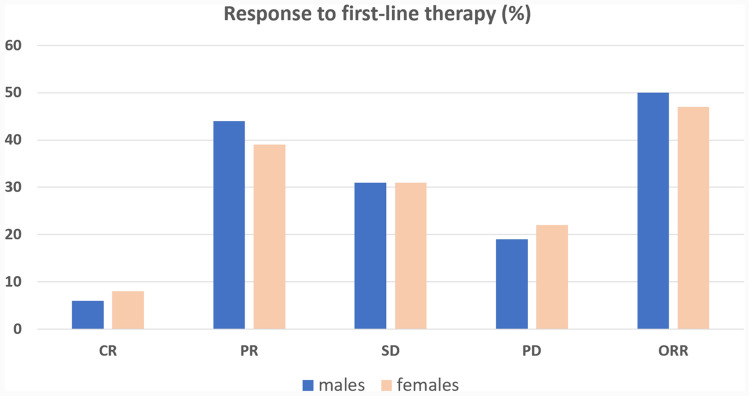
Male patients showed 6% CR, 44% PR, 31% SD and 19% PD, while females reported 8% CR, 39% PR, 31% SD and 22% PD (Fig. 2). By chi-square test, no differences in terms of ORR were found in patients with clear cell (males: 52% vs. females: 49%, p = 0.672) or non-clear cell histology (males: 40% vs. females: 41%, p = 0.886).
Fig. 2.
Response to therapy stratified by gender in the ARON-1 study population
IO + IO vs. IO + TKI
IO + IO combination was the first-line therapy chosen by clinicians in 40% of males and 39% of females. Among the IO + TKI combinations, pembrolizumab plus axitinib was the most frequent, representing 45% and 44% of first-line therapies in males and females, respectively (Table 2).
Table 2.
Overall Study Population stratified by type of first-line immune combination and gender. The percentages refer to the total number of males (1352) and females (475) included in this study
| Characteristics | IO + IO | p value | IO + TKI | p value | ||
|---|---|---|---|---|---|---|
| Males No. (%) |
Females No. (%) |
Males No. (%) |
Females No. (%) |
|||
| Total patients | 538 (40) | 184 (39) | 0.885 | 814 (60) | 291 (61) | 0.885 |
| Age < 50y | 65 (5) | 26 (5) | 1.000 | 115 (9) | 34 (7) | 0.603 |
| Age > 70y | 156 (12) | 64 (13) | 0.831 | 236 (17) | 85 (18) | 0.853 |
| Clear cell histology | 480 (36) | 161 (34) | 0.767 | 690 (51) | 247 (52) | 0.888 |
| Sarcomatoid differentiation | 101 (7) | 45 (9) | 0.969 | 79 (6) | 49 (10) | 0.298 |
| Metastatic at diagnosis | 333 (25) | 118 (25) | 1.000 | 425 (31) | 158 (33) | 0.762 |
| Previous nephrectomy | 345 (26) | 114 (24) | 0.744 | 536 (40) | 184 (39) | 0.885 |
|
No. of metastatic sites > 2 |
154 (11) | 48 (10) | 0.818 | 242 (18) | 88 (19) | 0.856 |
| Site of metastasis, individual | ||||||
| Lung | 403 (30) | 120 (25) | 0.430 | 544 (40) | 184 (39) | 0.885 |
| Lymph node | 181 (13) | 65 (14) | 0.837 | 264 (20) | 104 (22) | 0.729 |
| Liver | 95 (7) | 35 (7) | 1.000 | 130 (10) | 63 (13) | 0.507 |
| Bone | 177 (13) | 52 (11) | 0.664 | 274 (20) | 100 (21) | 0.861 |
| Brain | 39 (3) | 17 (4) | 0.701 | 49 (4) | 24 (5) | 0.734 |
| IMDC Prognostic Risk Group | ||||||
| Favorable | 0 (0) | 0 (0) | 1.000 | 194 (14) | 65 (14) | 1.000 |
| Intermediate | 390 (29) | 126 (27) | 0.732 | 462 (34) | 161 (34) | 1.000 |
| Poor | 148 (11) | 58 (12) | 0.825 | 158 (12) | 65 (13) | 0.831 |
| NLR ≥ 4 | 176 (13) | 55 (12) | 0.831 | 214 (16) | 88 (19) | 0.578 |
| BMI ≥ 25 | 374 (28) | 102 (21) | 0.251 | 525 (39) | 153 (32) | 0.302 |
|
First-line therapy Nivolumab plus ipilimumab Pembrolizumab plus axitinib Nivolumab plus Cabozantinib Pembrolizumab plus lenvatinib |
538 (40) – – – |
183 (39) – – – |
0.885 – – – |
– 601 (45) 153 (11) 60 (4) |
– 208 (44) 53 (11) 31 (7) |
– 0.887 1.000 0.353 |
|
Second-line therapy Cabozantinib Sunitinib Lenvatinib plus Everolimus Nivolumab Everolimus Clinical trials |
123 (9) 57 (4) 5 (< 1) 3 (< 1) 0 (0) 18 (1) |
39 (9) 22 (5) 1 (< 1) 2 (< 1) 2 (< 1) 5 (1) |
- |
148 (11) 39 (3) 10 (< 1) 3 (< 1) 4 (< 1) 28 (2) |
67 (14) 11 (2) 0 (0) 2 (< 1) 1 (< 1) 12 (3) |
– |
BMI, Body Mass Index; NLR, Neutrophil-to-Lymphocyte Ratio
In the IO + IO subgroup, the median OS was 30.1 months in males (95% CI 26.7–59.0) and 26.0 months in females (95% CI 20.1–40.2, p = 0.325). Males showed 7% of CR, 36% PR, 35% SD, and 26% PD, while females reported 11% CR, 35% PR, 26% SD, and 28% PD.
In the IO + TKI subgroup, the median OS was 40.5 months in males (95% CI 34.3–59.6) and 40.4 months in females (95% CI 29.3–40.4, p = 0.402). Males showed 6% of CR, 49% PR, 32% SD, and 13% PD, while females reported 7% CR, 42% PR, 34% SD, and 17% PD.
Six hundred and two patients (33%) received second-line therapies, 32% of males and 35% of females, respectively. The complete list of second-line treatments is reported in Table 2.
Severe adverse events, dose reductions, and treatment interruptions
Data on severe adverse events (SAEs) (Grade 3–4) were available for 1493 patients from the ARON-1 dataset and are illustrated in Table S6; SAEs were reported in 32% of males and 35% of females (Table S6).
The proportion of adverse events was higher in females than in males with BMI < 25 kg/m2 (female-to-male ratio, FMR: 1.6). The FMR was 3.0 for hypothyroidism and 1.5 for diarrhea.
In patients treated with the IO + IO combination, the FMR was 1.1, being 1.9 in the BMI < 25 kg/m2 subgroup and 1.6 for ICI interruptions. On the other hand, patients treated with IO + TKI combinations showed a FMR of 1.2. The proportion of hypothyroidism (FMR = 2.5), diarrhea (FMR = 2.0), and hypertension (FMR = 1.5) were higher in females, who were also characterized by a higher proportion of TKI interruptions (FMR = 1.2).
Univariate and multivariate analyses
In patients with sarcomatoid differentiation, at univariate analysis, sex, age, nephrectomy status, IMDC group, bone and liver metastases were significantly associated with OS. In multivariate analysis, only the nephrectomy status did not confirm their prognostic role (Table 3).
Table 3.
Univariate and multivariate analysis in the population of patients with sarcomatoid differentiation
| Overall survival (Overall population) | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI ) | p value | HR (95% CI ) | p value | |
| Sex (females vs. males) | 1.95 (1.31 − 2.90) | 0.001 | 1.72 (1.15 − 2.57) | 0.008 |
| Age (≥ 70y vs. < 70y) | 1.52 (1.01 − 2.28) | 0.045 | 1.64 (1.07 − 2.51) | 0.022 |
| BMI (> 25 vs. ≤ 25) | 0.93 (0.63 − 1.38) | 0.732 | ||
| Nephrectomy (yes vs. no) | 0.48 (0.31 − 0.75) | 0.001 | 0.74 (0.47 − 1.17) | 0.199 |
| Histology (nccRCC vs. ccRCC) | 1.04 (0.81 − 1.32) | 0.773 | ||
| IMDC group (poor vs. intermediate) | 2.13 (1.46 − 3.10) | < 0.001 | 2.13 (1.42 − 3.19) | < 0.001 |
| Number of metastatic sites (> 2 vs ≤ 2) | 1.19 (0.80 − 1.78) | 0.382 | ||
| Lung metastases (yes vs. no) | 0.87 (0.57 − 1.33) | 0.519 | ||
| Lymph node metastases (yes vs. no) | 1.09 (0.84 − 1.41) | 0.505 | ||
| Bone metastases (yes vs. no) | 1.75 (1.18–2.60) | 0.005 | 1.62 (1.08–2.43) | 0.021 |
| Liver metastases (yes vs. no) | 1.82 (1.18 − 2.80) | 0.006 | 1.78 (1.15–2.75) | 0.010 |
| Brain metastases (yes vs. no) | 1.61 (0.91 − 2.83) | 0.101 | ||
BMI = Body Mass Index; ccRCC = clear cell Renal Cell Carcinoma; IMDC = International Metastatic RCC Database Consortium; nccRCC = non-clear cell Renal Cell Carcinoma
In the clear cell histology subgroup, at univariate analysis, sex, age, BMI, nephrectomy status, sarcomatoid differentiation, IMDC group, number of metastatic sites, lymph node, bone, liver and brain metastases were significantly associated with OS. In multivariate analysis, age, nephrectomy status, sarcomatoid differentiation, IMDC group, bone, liver and brain metastases confirm their prognostic role (Table S2).
In the non-clear cell histology subgroup, at univariate analysis, sex, age, BMI, nephrectomy status, sarcomatoid differentiation, IMDC group, number of metastatic sites, lymph node, bone, liver and brain metastases were significantly associated with OS. In multivariate analysis, age, nephrectomy status, sarcomatoid differentiation, IMDC group, bone, liver and brain metastases confirm their prognostic role (Table S3).
Univariate and multivariate analyses in the overall population and patients under 50 years of age are presented in Tables S4 and S5, respectively.
Discussion
Sex is a known factor that influences cancer occurrence, progression, and prognosis [25, 26]. Sex differences are observed in the innate and adaptive immune escape mechanisms. Female seems to have a more efficient and stronger immune response than males, representing most cases of autoimmune disease, just as women infected with HIV have a lower viral load than men [27, 28]. The underlying biological, hormonal, and metabolic mechanisms explaining these findings might be also involved in sex-specific differences in immunotherapy response.
Previous research showed that mRCC female patients treated with ICI in the US in 2015–2016, i.e., before approval of immune-based combinations, had an increased risk of death than male patients (HR: 1.12, p = 0.004) [29]. Otherwise, a meta-analysis that included three trials with immune checkpoint inhibitor combination therapies in the first-line setting and one trial with ICI in the second-line did not demonstrate any difference in survival between males and females [30]. To date, real-world data on the possible association between sex and oncological outcomes in patients treated with first-line immune-based combinations are lacking. With this goal, we performed a sub-analysis of the international, real-world, ARON-1 study, which included 1827 patients treated with IO + IO vs. IO + TKI in 71 cancer centers in 21 countries.
For the entire cohort, we did not demonstrate the difference in OS and ORR between the sexes. Interestingly, we observed that among patients with clear cell histology, sarcomatoid differentiation, or < 50 years of age, the females showed lower OS than males. Specifically, the 2y-OS rate was 19% higher for male patients aged 18-49y (males vs. females, 72% vs. 53%, p = 0.006). Our results do not confirm previous data on localized disease showing a reduced risk of death in female RCC patients with < 59 years [31]. Recent OS results from the third prespecified interim analysis of Phase 3 KEYNOTE-564 study showed OS benefit for the adjuvant pembrolizumab vs. placebo for the treatment of clear cell RCC. Interestingly, greater OS benefit was for males [HR (95% CI), female vs. male: HR 1.08 (0.57–2.04) vs. 0.50 (0.33–0.75), respectively] and patients under 65 years of age [32].
At multivariate analyses, the negative impact of female sex on OS was maintained only in patients with sarcomatoid histology. These data are consistent with our previous work including patients with sarcomatoid histology treated with cabozantinib in advanced treatment lines [33] and suggest sex female as a prognostic negative factor irrespective to treatment administered.
One reason for these findings could be the sex hormone-associated immunomodulation of the RCC tumor microenvironment (TME) [34, 35]. Recent research using single-cell RNA sequencing data showed that RCC TME of males had a higher level of CD8 + T-cell infiltration than females, but were mostly exhausted CD8 + T-cells, where the exhausted state was induced by androgen [36]. This androgen-mediated immune dysfunction in male RCC patients could play a key role in the ICI response; the PD-1/PD-L1 inhibitors, through the hormonal effects on the PD-1/PD-L1 pathway and the reinvigoration of T-cell activities, could make the tumors markedly more responsive to immunotherapy [36].
Sex hormones can also shape the interaction between the immune system and genes, extending their influence at the epigenetic level [37]. In RCC, sex-specific mutation spectra emerged and involved mutations of X chromosome encoded genes, such as KDM5C, and chromatin remodeling genes with epigenetic effects, such as BAP1. A mutation sequencing study on clear cell RCC identified a significantly increased mutation rate of KDM5C in male and BAP1 in female patients [38]. Interestingly, mutations of BAP1 gene were previously associated with poorer prognosis in RCC, highlighting potential implications of genetic and epigenetic events in the sex-related disparities of the clinical outcome [30].
Sex hormones, in particular androgens, seem to have a critical influence also on the gut microbiota composition [16]. In mouse model, the sex differences in gut microbiota started with puberty, and upon castration, the gut microbiota of the male was similar to the female [39]. Early life microbial exposures determined sex hormone levels and the transfer of gut microbiota from male to female resulted in increased female testosterone, reduced autoimmunity, and metabolic variations [40]. Although hormones and genes are the most well-characterized factors mediating the sex differences in immune responses, lifestyle and other environmental variables can also contribute to differential modulation of the immune system between the sexes. A sex dimorphism is reported in many health-related behaviors, such as diet, physical activities, or alcohol consumption. These factors, which further affect the patient’s immune status and immune surveillance, are extremely difficult to measure and reflect the complex interactions among environmental, biological and immunological elements, that require a deeper understanding and future efforts to explain sex and gender disparities in anti-cancer immune response and cancer immunotherapy efficacy [41]. The type of systemic treatment in our study cohort follows the available standard of care for mRCC, with the majority of patients having received IO + TKI equally between the genders. There was no difference in OS according to the type of immune-based combination; however, numerically, the ORR was higher in female patients treated with IO + IO and in male patients treated with IO + TKI.
According to previous data on different cancer types treated with ICI, showing that female patients have an increased risk of severe adverse events than male patients [42], the proportion of severe adverse events such as hypothyroidism, diarrhea, and hypertension, as well as treatment interruptions, were higher in women treated with IO + TKI. The greater risk for autoimmunity and severe side effects appear paradoxically conflicting with the less favorable immunotherapeutic response. The highest incidence in females of autoimmune diseases and SAEs may be partly explained by the localization of specific genes and micro-RNAs to the X-chromosome and by escape from physiological X-chromosome inactivation in immune cells in women [43]. At the same time, sex hormone-dependent differences affect the immune cell numbers, composition and ratio. Thus, younger females, compared to older females and males, tend to have cold and immune-infiltrated tumors, but with higher expression of inhibitory immune checkpoints, higher density of immune-suppressive cells including regulatory T cells (T-Reg), and tumor neoantigens less visible to the immune system, ultimately less responsive when stimulated by ICI [16, 44, 45].
It is important to underline that, in our study, only 26% of patients were female. This imbalance in gender representation is similar to that reported by the Checkmate-214 trial and it is in line with less than 30% of female patients included in the Keynote-426, Checkmate-9ER, and CLEAR trials [5–7]. Despite the incidence ratio of RCC in males to females of 2:1 [46] and the implemented cancer clinical trials in the last 20 years, the issue of under-representation of female patients remains to be addressed [47]. Considering the gender differences in the immune system, behaviors, and lifestyle, as well as the heterogeneity of efficacy and outcomes with ICI treatment [48], specific measures such as stratifying patients by gender should be promptly applied in clinical trials [49].
The current study has several strengths, including the large sample size. To our knowledge, it is the first real-world study that investigates the influence of sex on first-line immune combinations and that showed the female sex as a negative prognostic factor in patients with sarcomatoid histology.
We acknowledge some limitations of our study. First, the retrospective data analysis and, consequently, the lack of a central radiological review to confirm response and/or progression, and to assess the ORR. Second, data on other environmental factors or molecular and epigenetic events, still not fully understood, could act as modifiers on TME, further influencing the response to immune-based combinations. A further understanding of sex differences in epidemiology, biology, and treatment outcomes will help to personalize the therapeutic choices for the mRCC patients.
Conclusion
Although the female’s innate and adaptive immunity has been observed to be more active than the male’s, our real-world evidence on mRCC patients treated with immune-based combinations in first-line setting did not demonstrate a difference in OS and ORR in the overall population. However, women in the subgroup of clear cell histology, sarcomatoid differentiation, and those under 50 years of age showed shorter OS than males. Female sex was an independent negative prognostic factor in the sarcomatoid histology population. A further understanding is required to better clinically address these differences.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization and formal analysis and data curation were done by LI and MS; Methodology was done by LI, FSMM, SB, and MS; Investigation was done by LI, FSMM, FM, PS, GR, OF, MZ, JK, JMC, DS, TB, AP, JK, AZ, MP, TB, RP, GF, APF, AS, RM, LI, ZK, MTB, DB, JA, RK, EG, SB, and MS; Writing—Original Draft was done by LI, FSMM, and MS; Supervision was done by SB and MS. All authors reviewed the results and approved the final version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Declarations
Conflict of interest
Dr. Buchler reported receiving personal fees from Roche, Bristol Myers Squibb, Merck Sharp Dohme, Merck, Ipsen, Eisai, Novartis and AstraZeneca outside the submitted work; Dr. Buti reported receiving personal fees from Bristol Myers Squibb, Pfizer, MSD, Ipsen, Roche, Eli Lilly, AstraZeneca, Pierre-Fabre, Novartis, Merck, Gentili, Astellas outside the submitted work; Dr. Incorvaia reported receiving personal fees from Bristol Myers Squibb and IPSEN outside the submitted work; Dr. Manneh reported receiving personal fees from AstraZeneca, Amgen, EliLilly, IPSEN, Novartis, Pfizer, Tecnofarma, Bayer, Janssen, Bristol Myers Squibb, Astellas, MSD, Merck Serono, Roche outside the submitted work; Dr. Massari reported receiving personal fees from Astellas, BMS, Janssen, Ipsen, MSD, and Pfizer outside the submitted work; Dr. Molina-Cerrillo reported receiving personal fees from IPSEN, Roche, Pfizer, Sanofi, Janssen, and BMS outside the submitted work; Dr. Fiala reported receiving personal fees from Novartis, Janssen, Merck and Pfizer outside the submitted work; Dr. Kucharz reported receiving personal fees from Angelini, Astellas, Astra Zeneca, Bayer, Bristol Myers Squibb, IPSEN, Janssen, Merck MSD, Novartis, Pfizer, outside the submitted work; Dr. M. Monteiro reported receiving personal fees from Janssen, Ipsen, Bristol Myers Squibb, and Merck Sharp Dome outside the submitted work; A. Poprach reported receiving personal fees from Roche, Bristol Myers Squibb, Merck KGaA, MSD, Novartis, Astellas, Janssen, and Sanofi/Aventis, Ipsen, and Pfizer outside the submitted work; Dr. Zeppellini reported receiving personal fees from J&J and Bristol Myers Squibb outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorena Incorvaia and Fernando Sabino Marques Monteiro have contributed equally.
Sebastiano Buti and Matteo Santoni are the Senior authors.
Contributor Information
Lorena Incorvaia, Email: lorena.incorvaia@unipa.it.
Fernando Sabino Marques Monteiro, Email: fsabinocba@gmail.com.
References
- 1.Hirsch L, Flippot R, Escudier B, Albiges L (2020) Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs 80(12):1169–1181. 10.1007/s40265-020-01327-7. (PMID: 32601914) 10.1007/s40265-020-01327-7 [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Meli- char B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed]
- 3.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C et al (2019) Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393(10189):2404–2415 10.1016/S0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 6.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300 10.1056/NEJMoa2035716 [DOI] [PubMed] [Google Scholar]
- 7.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B et al (2021) Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384(9):829–841 10.1056/NEJMoa2026982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Montero CM, Rini BI, Finke JH (2020) The immunology of renal cell carcinoma. Nat Rev Nephrol 16(12):721–735. 10.1038/s41581-020-0316-3. (Epub 2020 Jul 30 PMID: 32733094) 10.1038/s41581-020-0316-3 [DOI] [PubMed] [Google Scholar]
- 9.Wang PF, Song HF, Zhang Q, Yan CX (2020) Pan-cancer immunogenomic analyses reveal sex disparity in the efficacy of cancer immunotherapy. Eur J Cancer 126:136–138. 10.1016/j.ejca.2019.12.008. (Epub 2020 Jan 9 PMID: 31927214) 10.1016/j.ejca.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 10.Oertelt-Prigione S (2012) The influence of sex and gender on the immune response. Autoimmun Rev 11(6–7):A479–A485. 10.1016/j.autrev.2011.11.022. (Epub 2011 Dec 3 PMID: 22155201) 10.1016/j.autrev.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 11.Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G et al (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 19(6):737–746. 10.1016/S1470-2045(18)30261-4. (Epub 2018 May 16 PMID: 29778737) 10.1016/S1470-2045(18)30261-4 [DOI] [PubMed] [Google Scholar]
- 12.Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O et al (2019) Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol 5(4):529–536. 10.1001/jamaoncol.2018.5904 10.1001/jamaoncol.2018.5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L et al (2022) The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol 170:103596. 10.1016/j.critrevonc.2022.103596. (Epub 2022 Jan 12 PMID: 35031442) 10.1016/j.critrevonc.2022.103596 [DOI] [PubMed] [Google Scholar]
- 14.Graham J, Abdel-Rahman O, Choueiri TK, Heng DYC (2018) International mRCC Database Consortium. Re: Fabio Conforti, Laura Pala, Vincenzo Bagnardi, et al. Cancer Immunotherapy Efficacy and Patients' Sex: A Systematic Review and Meta-analysis. Lancet Oncol 19:737–46: Outcomes of Metastatic Renal Cell Carcinoma by Gender: Contrasting Results from the International mRCC Database Consortium. Eur Urol. 74(6):e139-e140. 10.1016/j.eururo.2018.07.004 [DOI] [PubMed]
- 15.Yanagisawa T, Kawada T, Quhal F, Bekku K, Laukhtina E, Rajwa P et al (2023) Impact of sex on the efficacy of immune checkpoint inhibitors in kidney and urothelial cancers: a systematic review and meta-analysis. World J Urol 41(7):1763–1774. 10.1007/s00345-023-04412-0 10.1007/s00345-023-04412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner AD, Oertelt-Prigione S, Adjei A, Buclin T, Cristina V, Csajka C et al (2019) Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol 30(12):1914–1924. 10.1093/annonc/mdz414. (PMID: 31613312) 10.1093/annonc/mdz414 [DOI] [PubMed] [Google Scholar]
- 17.Ye Y, Jing Y, Li L, Mills GB, Diao L, Liu H et al (2020) Sex-associated molecular differences for cancer immunotherapy. Nat Commun 11(1):1779. 10.1038/s41467-020-15679-x 10.1038/s41467-020-15679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Zhu Y, Ma W, Liu N, Dong X, Shi Q et al (2023) Estrogen associates with female predominance in Xp112 translocation renal cell carcinoma. Sci Rep 13:6141 10.1038/s41598-023-33363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoni M, Massari F, Myint ZW, Iacovelli R, Pichler M, Basso U et al (2023) Clinico-pathological features influencing the prognostic role of body mass index in patients with advanced renal cell carcinoma treated by immuno-oncology combinations (ARON-1). Clin Genitourin Cancer 21(5):e309–e319. 10.1016/j.clgc.2023.03.006 10.1016/j.clgc.2023.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Santoni M, Molina-Cerrillo J, Myint ZW, Massari F, Buchler T, Buti S et al (2022) Concomitant use of statins, metformin, or proton pump inhibitors in patients with advanced renal cell carcinoma treated with first-line combination therapies. Target Oncol 17(5):571–581. 10.1007/s11523-022-00907-9. (Epub 2022 Aug 10 PMID: 35947324) 10.1007/s11523-022-00907-9 [DOI] [PubMed] [Google Scholar]
- 21.Santoni M, Massari F, Matrana MR, Basso U, De Giorgi U, Aurilio G et al (2022) Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur J Cancer 172:191–198. 10.1016/j.ejca.2022.04.035. (Epub 2022 Jun 30 PMID: 35780525) 10.1016/j.ejca.2022.04.035 [DOI] [PubMed] [Google Scholar]
- 22.Tulchiner G, Pichler R, Ulmer H, Staudacher N, Lindner AK, Brunner A et al (2021) Sex-specific hormone changes during immunotherapy and its influence on survival in metastatic renal cell carcinoma. Cancer Immunol Immunother 70(10):2805–2817. 10.1007/s00262-021-02882-y 10.1007/s00262-021-02882-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoni M, Massari F, Myint ZW, Iacovelli R, Pichler M, Basso U et al (2023) Global real-world outcomes of patients receiving immuno-oncology combinations for advanced renal cell carcinoma: the ARON-1 study. Target Oncol 18(4):559–570. 10.1007/s11523-023-00978-2. (Epub 2023 Jun 27 PMID: 37369815) 10.1007/s11523-023-00978-2 [DOI] [PubMed] [Google Scholar]
- 24.Porta C, Bamias A, Zakopoulou R, Myint ZW, Cavasin N, Iacovelli R et al (2023) Geographical differences in the management of metastatic de novo renal cell carcinoma in the era of immune-combinations. Minerva Urol Nephrol 75(4):460–470. 10.23736/S2724-6051.23.05369-7. (PMID: 37530662) 10.23736/S2724-6051.23.05369-7 [DOI] [PubMed] [Google Scholar]
- 25.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM et al (2009) Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev 18(4):1174–1182. 10.1158/1055-9965.EPI-08-1118. (Epub 2009 Mar 17 PMID: 19293308) 10.1158/1055-9965.EPI-08-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF (2011) Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 20(8):1629–1637. 10.1158/1055-9965.EPI-11-0246. (Epub 2011 Jul 12 PMID: 21750167) 10.1158/1055-9965.EPI-11-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson DL, Gange SJ, Rose NR, Graham NM (1997) Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84(3):223–243. 10.1006/clin.1997.4412. (PMID: 9281381) 10.1006/clin.1997.4412 [DOI] [PubMed] [Google Scholar]
- 28.Griesbeck M, Scully E, Altfeld M (2016) Sex and gender differences in HIV-1 infection. Clin Sci 130(16):1435–1451. 10.1042/CS20160112. (PMID: 27389589) 10.1042/CS20160112 [DOI] [PubMed] [Google Scholar]
- 29.Grigg C, Trufan S, Clark PE, Riggs SB, Zhu J, Justin T et al (2021) Survival trends of men and women with metastatic clear cell renal cell carcinoma. J Clin Oncol. 10.1200/JCO.2021.39.15_suppl.4566 10.1200/JCO.2021.39.15_suppl.4566 [DOI] [Google Scholar]
- 30.Hassler MR, Abufaraj M, Kimura S, Stangl-Kremser J, Gust K, Glybochko PV et al (2020) Impact of patients’ gender on efficacy of immunotherapy in patients with metastatic kidney cancer: a systematic review and meta-analysis. Clin Genitourin Cancer 18(2):88-94.e2. 10.1016/j.clgc.2019.09.004. (Epub 2019 Sep 27 PMID: 31668768) 10.1016/j.clgc.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Marchioni M, Martel T, Bandini M, Pompe RS, Tian Z, Kapoor A et al (2017) Marital status and gender affect stage, tumor grade, treatment type and cancer specific mortality in T1–2 N0 M0 renal cell carcinoma. World J Urol 35(12):1899–1905. 10.1007/s00345-017-2082-9. (Epub 2017 Aug 28 PMID: 28849260) 10.1007/s00345-017-2082-9 [DOI] [PubMed] [Google Scholar]
- 32.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN (2024) Overall survival results from the phase 3 KEYNOTE-564 study of adjuvant pembrolizumab versus placebo for the treatment of clear cell renal cell carcinoma (ccRCC). J Clin Oncol. 10.1200/JCO.2024.42.4_suppl.LBA35938701382 10.1200/JCO.2024.42.4_suppl.LBA359 [DOI] [Google Scholar]
- 33.Santoni M, Massari F, Grande E, Procopio G, Matrana MR, Rizzo M et al (2021) Cabozantinib in pretreated patients with metastatic renal cell carcinoma with sarcomatoid differentiation: a real-world study. Target Oncol 16(5):625–632. 10.1007/s11523-021-00828-z. (Epub 2021 Aug 2 PMID: 34338966) 10.1007/s11523-021-00828-z [DOI] [PubMed] [Google Scholar]
- 34.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H (2006) Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 84(2):370–378. 10.1002/jnr.20881. (PMID: 16676326) 10.1002/jnr.20881 [DOI] [PubMed] [Google Scholar]
- 35.Ding J, Yeh CR, Sun Y, Lin C, Chou J, Ou Z, Chang C, Qi J, Yeh S (2018) Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene 37(37):5037–5053. 10.1038/s41388-018-0175-6. (Epub 2018 May 23 PMID: 29789714) 10.1038/s41388-018-0175-6 [DOI] [PubMed] [Google Scholar]
- 36.Ning K, Peng Y, Jiang Y, Li Z, Luo X, Lin L et al (2023) Sex differences in renal cell carcinoma: a single-cell analysis reveals exhausted CD8+ T-cells highly infiltrated in males. Biol Sex Differ 14(1):58. 10.1186/s13293-023-00540-9 10.1186/s13293-023-00540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, Yao Y, Tian Y, Chen K, Liu B (2022) Advances in sex disparities for cancer immunotherapy: unveiling the dilemma of Yin and Yang. Biol Sex Differ 13(1):58. 10.1186/s13293-022-00469-5. (PMID: 36273184) 10.1186/s13293-022-00469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricketts CJ, Linehan WM (2015) Gender specific mutation incidence and survival associations in clear cell renal cell carcinoma (CCRCC). PLoS ONE 10(10):e0140257. 10.1371/journal.pone.0140257. (PMID: 26484545) 10.1371/journal.pone.0140257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YS, Unno T, Kim BY, Park MS (2020) Sex differences in gut microbiota. World J Mens Health 38(1):48–60. 10.5534/wjmh.190009. (Epub 2019 Mar 25 PMID: 30929328) 10.5534/wjmh.190009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U et al (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339(6123):1084–1088. 10.1126/science.1233521 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- 41.Irelli A, Sirufo MM, D’Ugo C, Ginaldi L, De Martinis M (2020) Sex and gender influences on cancer immunotherapy response. Biomedicines 8(7):232. 10.3390/biomedicines8070232. (PMID: 32708265) 10.3390/biomedicines8070232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unger JM, Vaidya R, Albain KS, LeBlanc M, Minasian LM, Gotay CC et al (2022) Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J Clin Oncol 40(13):1474–1486. 10.1200/JCO.21.02377 10.1200/JCO.21.02377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libert C, Dejager L, Pinheiro I (2010) The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10(8):594–604 10.1038/nri2815 [DOI] [PubMed] [Google Scholar]
- 44.Conforti F, Pala L, Pagan E, Bagnardi V, De Pas T, Queirolo P et al (2021) Sex-based dimorphism of anticancer immune response and molecular mechanisms of immune evasion. Clin Cancer Res 27(15):4311–4324. 10.1158/1078-0432.CCR-21-0136 10.1158/1078-0432.CCR-21-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X, Li J, Zou J, Feng X, Zhang C, Zheng R et al (2019) Association of germline variants in natural killer cells with tumor immune microenvironment subtypes, tumor-infiltrating lymphocytes, immunotherapy response, clinical outcomes, and cancer risk. JAMA Netw Open 2(9):e199292. 10.1001/jamanetworkopen.2019.9292. (Erratum.In:JAMANetwOpen.2020Apr1;3(4):e206708 PMID: 31483464) 10.1001/jamanetworkopen.2019.9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scelo G, Li P, Chanudet E, Muller DC (2018) Variability of sex disparities in cancer incidence over 30 years: the striking case of kidney cancer. Eur Urol Focus 4(4):586–590. 10.1016/j.euf.2017.01.006. (Epub 2017 Jan 31 PMID: 28753845) 10.1016/j.euf.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 47.Perera ND, Bellomo TR, Schmidt WM, Litt HK, Shyu M, Stavins MA et al (2023) Analysis of female participant representation in registered oncology clinical trials in the united states from 2008 to 2020. Oncologist 28(6):510–519. 10.1093/oncolo/oyad009 10.1093/oncolo/oyad009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2(5):e192535. 10.1001/jamanetworkopen.2019.2535 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner AD, Oertelt-Prigione S, Adjei A, Buclin T, Cristina V, Csajka C et al (2019) Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol 30(12):1914–1924. 10.1093/annonc/mdz414. (PMID: 31613312) 10.1093/annonc/mdz414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.