Abstract
Background
In host erythrocytes, the malaria parasite must contend with ion and drug transport across three membranes; its own plasma membrane, the parasitophorous membrane and the host plasma membrane. Isolation of pure and intact Plasmodium falciparum plasma membrane would provide a suitable model to elucidate the possible role played by the parasite plasma membrane in ion balance and drug transport.
Results
This study describes a procedure for isolating parasite plasma membrane from P. falciparum-infected erythrocytes. With this method, the trophozoites released by saponin treatment were cleansed of erythrocyte membranes using anti-erythrocyte antibodies fixed to polystyrene beads. These trophozoites were then biotinylated and the parasite plasma membrane was disrupted by nitrogen cavitation. This process allows the membranes to reform into vesicles. The magnetic streptavidin beads bind specifically to the biotinylated parasite plasma membrane vesicles facilitating their recovery with a magnet. These vesicles can then be easily released from the magnetic beads by treatment with dithiotreithol. The parasite plasma membrane showed optimal ATPase activity at 2 mM ATP and 2 mM Mg2+. It was also found that Ca2+ could not substitute for Mg2+ ATPase activity in parasite plasma membranes whereas activity was completely preserved when Mn2+ was used instead of Mg2+. Other nucleoside triphosphates tested were hydrolysed as efficiently as ATP, while the nucleoside monophosphate AMP was not.
Conclusions
We have described the successful isolation of intact P. falciparum plasma membrane vesicles free of contaminating organelles and determined the experimental conditions for optimum ATPase activity.
Background
P. falciparum can be obtained by continuous culture of parasitised human erythrocytes [1]. Although extracellular, axenic development of the erythrocyte cycle of P. falciparum has been obtained [2-4], the number of merozoites completing the cycle is not sufficient to permit continuous extracellular culture. Under the best conditions, only 1% of the merozoites further develops into trophozoites. Therefore, the isolation of trophozoites still relies on their liberation from the host cells.
Several methods for the isolation of trophozoites have been developed [5] including: agglutination and lysis by passage through a series of filters [6], glycerol-enhanced haemolysis [7] and sorbitol lysis [8]. However, to prepare intact trophozoites in high yield and of high purity remains technically difficult. These methods either affect the integrity of the parasites or fail to remove host materials from the parasites. The most widely used method for freeing parasites from their host cells is saponin lysis [9], but electron microscopy studies have shown that these parasites are still trapped within the erythrocyte plasma membrane. Released parasites need to be further cleansed of host cell materials, in particular erythrocytes and their ghosts, as well as unlysed infected erythrocytes.
Collecting released parasites is only part of the preparation process. The next step involves obtaining plasmodial constituents from isolated trophozoites. While methods to isolate host cell ghosts from parasitised erythrocytes have been developed [10,8], there is no published method to isolate the parasite plasma membrane. The isolation of the parasite plasma membrane presents technical difficulties due to the presence of three closely related membranes (1) the erythrocyte plasma membrane (2) the parasitophorous vacuolar membrane and (3) the parasite plasma membrane, as well as the food vacuole membrane. An attempt has been made to prepare membrane vesicles from parasitised erythrocytes [11]. Unfortunately these preparations were found to contain membrane elements of both parasites and host cells.
Although the P. falciparum vacuolar ATPase activity have been characterised [12], the ATPase activity of the parasite plasma membrane has not yet been investigated. To date, investigation of the ATPase activity of the plasma membrane has been hampered by the presence of contaminants in the membrane preparations. Preparation of uncontaminated parasite plasma membranes would represent a major experimental advance for approaching this problem. For this reason, a method was developed to prepare pure and intact parasite plasma membranes from P. falciparum infected-erythrocytes. The dependence of P. falciparum plasma membrane ATPase activity on ATP and other nucleotides, divalent cations, time, ATP and Mg2+ concentrations was also evaluated.
Results
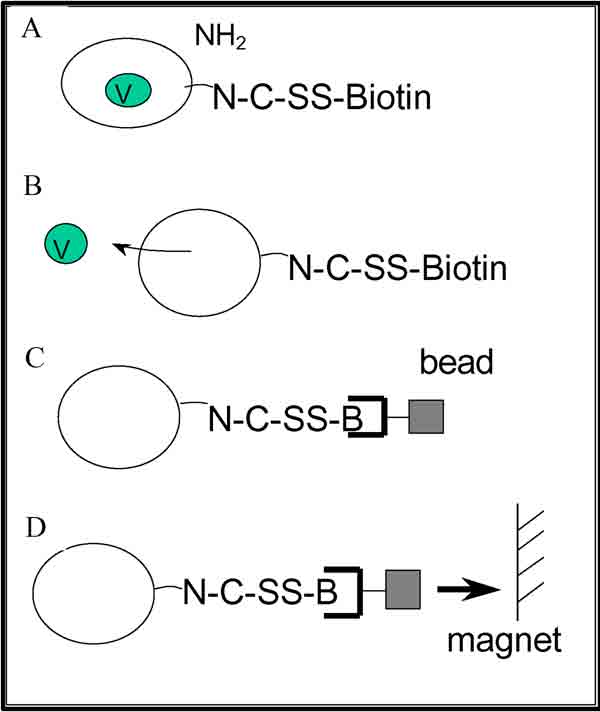
Parasites synchronized in the late trophozoite stage were released from erythrocytes by saponin lysis. Removal of the erythrocyte membranes was obtained by immunoaffinity. For this purpose, polystyrene beads coated with anti-erythrocyte antibodies were incubated with the trophozoite preparation. Trophozoites were then biotinylated with NHS-SS-biotin prior to nitrogen cavitation in the presence of protease inhibitors and DnaseI. The resulting biotinylated membranes bound to streptavidin-magnetic beads and were separated from the lysate using a magnet (Figure 1). Membrane vesicle preparations contained 8.2% of the total isolated-trophozoite protein.
Figure 1.
Isolation of P. falciparum plasma membrane vesicles. (A) Biotinylation of the trophozoite membrane with a reversible agent. (B) Disruption of the P. falciparum plasma membrane by nitrogen decompression. (C) Binding of the magnetic streptavidin beads to the biotinylated plasma membrane. (D) Recovery of the parasite plasma membrane using a magnet.
The integrity of purified trophozoites was monitored by measuring the uptake of the Trypan blue dye into the parasite cytoplasm. It was shown that 98% of the trophozoites released by saponin lysis did not concentrate the dye, indicating an intact plasma membrane.
Purified P. falciparum plasma membranes examined using transmission electron microscopy were observed to form intact vesicles, although some open membranes could be seen. These vesicles were found to have an average diameter of 500 nm. No visible contamination by other membranes or organelles was observed, although some liberated hemozoin crystal could be seen (results not shown).
The purity of the parasite plasma membrane preparations was further assayed by the use of enzyme markers of P. falciparum cytosol, the parasite lactate dehydrogenase (pLDH) and of human erythrocyte membranes, the acetylcholine esterase (AchE). There were no detectable AchE or pLDH activities (less than 0.01 μmol/min/mg protein) in the parasite plasma membrane preparations (Table 1). By comparison, the specific activity of AchE was 2.30 ± 0.15 and 0.05 ± 0.03 μmol/min/mg protein in erythrocyte ghosts and isolated trophozoites (n = 3) respectively, while the specific pLDH activity in isolated trophozoites was 0.14 ± 0.01 μmol/min/mg protein (n = 3).
Table 1.
Marker enzyme activities of isolated erythrocyte ghosts, trophozoites and parasite plasma membranes.
| Samples | AchE specific activities (μmol/min/mg protein) | pLDH specific activities (μmol/min/mg protein) |
| Erythrocyte ghosts | 2.30 ± 0.15 | N.D. |
| Trophozoites | 0.05 ± 0.03 | 0.14 ± 0.01 |
| Parasite plasma membranes | No activity | No activity |
Acetylcholine esterase (AchE) and parasite lactate dehydrogenase (pLDH) activities of isolated trophozoites, parasite plasma membranes and/or erythrocyte ghosts were determined as described under materials and methods section. Values are means ± standard deviation of three separate experiments from separate parasite plasma isolations, trophozoite isolations or erythrocyte membrane preparations. N.D.: not determined.
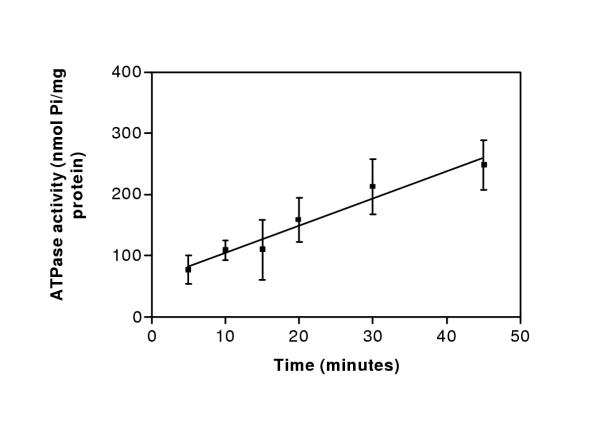
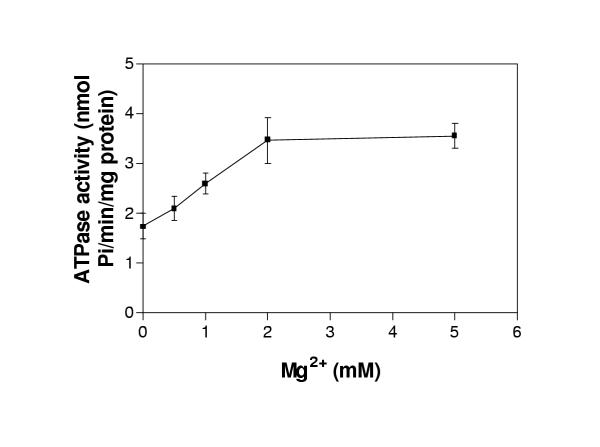
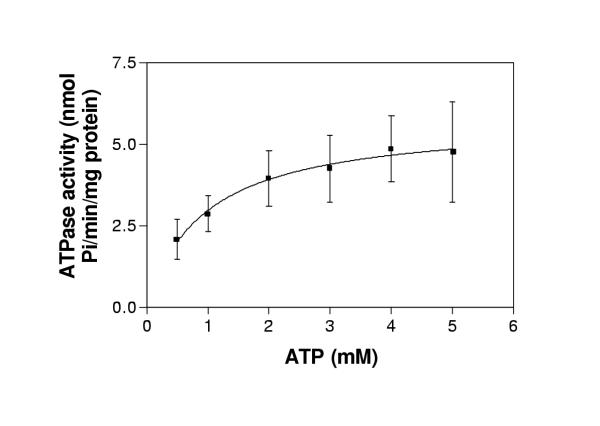
Time dependence of the ATPase activity of plasma membranes isolated from the P. falciparum strain D10 was evaluated over a time period of 45 minutes. A linear relationship of time versus ATPase activity was found with a correlation coefficient of 0.717 (n = 3) (Figure 2). ATPase in plasma membrane preparations required the presence of divalent cations to maintain its activity. ATPase activity was maximal with 2 mM Mg2+ in the presence of 2 mM ATP (Figures 3,4). The ability of 2 mM concentration of Mg2+ and the other divalent cations Mn2+ and Ca2+ to support the ATPase activity of purified plasma membranes isolated from the P. falciparum strain D10 was evaluated, along with no divalent cation addition as a control (Table 2). Maximal ATPase activity was obtained in presence of 2 mM Mg2+. A significant activity (88 ± 33%) was measured when Mg2+ was replaced with Mn2+. When Ca2+ was substituted for Mg2+, only 21 ± 19% of the ATPase activity was retained. Plasma membranes were also capable of hydrolysing other nucleoside triphosphates as effectively as ATP (Table 3). Only adenosine monophosphate (AMP) was not hydrolysed by the D10 plasma membranes. Purified parasite plasma membranes isolated from the sensitive strains D10 displayed an ATPase activity of 3.2 ± 1.7 nmol Pi/min/mg protein.
Figure 2.
Time course of D10 plasma membranes ATPase activity. ATPase activity (nmol Pi/mg protein) of D10 plasma membranes was determined over a time period of 45 minutes and the activity of the membranes tested without ATP was subtracted. Error bars represent standard deviations from means of three separate experiments, each performed in quadruplicate.
Figure 3.
Dependence of ATPase activity on Mg2+ concentration. ATPase activity of freshly purified D10 plasma membranes expressed as nmol Pi/min/mg protein was measured over Mg2+ concentrations ranging from 0 to 5 mM. ATPase activity of parasite plasma membranes tested without ATP and absorbance due to ATP hydrolysis was subtracted. Error bars represent standard deviations from means of three separate experiments, each performed in quadruplicate.
Figure 4.
Dependence of ATPase activity on ATP concentration. ATPase activity of freshly purified D10 plasma membranes expressed as nmol Pi/min/mg protein was measured over ATP concentrations ranging from 0.5 to 5 mM. ATPase activity of parasite plasma membranes tested without ATP and absorbance due to ATP hydrolysis were subtracted. Error bars represent standard deviations from means of three separate experiments, each performed in quadruplicate.
Table 2.
Effect of divalent cations on purified D10 plasma membranes ATPase activity.
| Cations | ATPase activity (% of control) |
| Mg2+ (control) | 100 |
| Mn2+ | 87.8 ± 33.1 |
| Ca2+ | 20.9 ± 19.2 |
ATPase activity was determined using 2 mM concentration of Mg2+, Mn2+ and Ca2+ and the ATPase activity in the absence of divalent cation was subtracted. Values are means of three separate experiments, each performed in quadruplicate ± standard deviations and are expressed as % of control in presence of Mg2+.
Table 3.
ATPase activity in the presence of 2 mM concentrations of various nucleotides.
| ATPase activity (% of control) | |||
| Nucleotide | Triphosphate | Diphosphate | Monophosphate |
| Adenosine | 100 | 72.6 ± 16.7 | -9.2 ± 9.3 |
| Guanosine | 120.8 ± 19.6 | 55.4 ± 17.1 | N.D. |
| Cytosine | 64.2 ± 7.6 | N.D. | N.D. |
| Uridine | 101.4 ± 55.0 | N.D. | N.D. |
ATPase activity of isolated D10 plasma membranes tested without nucleotide and absorbance due to the nucleotide hydrolysis was subtracted. Values are means of three separate experiments, each performed in quadruplicate ± standard deviations and are expressed as % of control in presence of ATP. N.D.: not determined.
Discussion
A procedure for isolating parasite plasma membrane from P. falciparum-infected erythrocytes was developed. In this method, trophozoites were released from the erythrocytes by saponin lysis, the remaining erythrocytes membranes being removed by immunoaffinity using anti-erythrocyte antibodies. Previously, anti-erythrocyte monoclonal antibodies have been used to remove unlysed erythrocytes and ghost membranes after sorbitol treatment [8]. However, in order to remove most of the erythrocyte ghosts by centrifugation, all the erythrocytes must be lysed by the detergent, thus allowing isolation of the trophozoites on a larger scale. Sorbitol lyses only the parasitized erythrocytes, leaving uninfected erythrocytes intact. Saponin, on the other hand lysed uninfected and infected erythrocytes. For this reason, sorbitol lysis of the trophozoites was not retained in this study. However, because saponin-treatment of parasitized erythrocytes has been shown to permeabilise both the erythrocyte plasma membrane and the parasitophorous membrane [13,14] The exposure of the trophozoites to saponin was very brief to minimize membrane damage. Contamination of the plasma membrane vesicle preparations by the parasitophorous membrane, however, could not be discounted since, to our knowledge, there is no specific marker for this membrane.
A number of criteria have to be met when isolating subcellular fractions for biochemical experiments. The subcellular constituents have to be pure, intact, isolated in high yield and retain their normal physiological capabilities. Evidence for the membrane integrity of saponin-freed parasites came from studies measuring the rate of incorporation of [14C]-isoleucine into protein [15] and the rate of phosphorylation of the pantothenic acid [16]. In this study, experiments with the trypan blue showed that 98% of the trophozoites released by saponin lysis were capable of maintaining an intact plasma membrane.
Nitrogen decompression has been previously shown to be effective for the disruption of cells [17] and was used in this study to disrupt the parasite plasma membranes. A cocktail of protease inhibitors (aprotinin, leupeptin and phenylmethylsulfonyl fluoride) was used to prevent the degradation of membrane proteins by the proteases released during the nitrogen cavitation step. The presence of DNA presents an additional problem, as the parasites adhere to the sticky DNA liberated during parasite lysis. Dnase I was, therefore, included in the isolation process to eliminate the DNA liberated during the nitrogen cavitation step. The recovery yield of the parasite plasma membranes, 8.2% of the total isolated-trophozoite protein, is consistent with the percent protein yield (8–10%) obtained for membrane vesicles prepared from cancer cells [17].
Purified P. falciparum examined using transmission electron microscopy were observed to be intact and free of contaminating membranes and organelles, even though a small amount of liberated haemozoin could occasionally be seen in certain fields. Contamination of the plasma membrane vesicles with fragments of food vacuole membrane could not be ruled out since nitrogen cavitation is likely to disrupt the food vacuole membrane as well as the plasma membrane. It would therefore had been interesting to investigate whether the parasite plasma membrane preparations were contaminated by food vacuole membrane components using antibodies to PfCRT. The absence of parasite and erythrocyte protein in the parasite plasma membrane preparation was confirmed by non-detectable parasite lactate dehydrogenase and erythrocyte acetylcholine esterase activities. The fact that the membranes were bound to a support facilitated their purification from contaminants and their recovery.
The ATPase activity of parasite plasma membranes (3.2 ± 1.7 nmol Pi/min/mg protein) obtained in this study was found to be of similar magnitude to that of digestive vacuoles isolated from P. falciparum[18]. For comparison, the specific ATPase activity of plasma membranes prepared from multidrug-resistant cancer cells is 1.15 μmol/min/mg membrane protein[19].
Conditions required for optimal P. falciparum plasma membrane ATPase activity were determined using the chloroquine-sensitive strain D10. Parasite plasma membranes showed optimal ATPase activity at 2 mM Mg2+ and 2 mM ATP. Other divalent cations can be substituted for Mg2+ and maintain the function of a variety of different ATPases [20]. In this study, it was found that Ca2+ could not be substituted for Mg2+ to sustain ATPase activity in parasite plasma membranes, whereas activity was preserved when Mg2+ was replaced by Mn2+, indicating that the parasite plasma membrane ATPase activity was similar to the vacuolar membrane ATPase activity [18]. However, this contradicts the Choi and Mego [19] study which showed that Ca2+ could support the P. falciparum vacuolar ATPase activity in the absence of Mg2+. Other nucleoside triphosphates and diphosphates tested were hydrolysed as effectively as ATP. However, the nucleoside monophosphate AMP was not hydrolysed, suggesting that the parasite plasma membrane ATPase activity is distinguishable from that of membrane-associated alkaline and acid phosphatases [21].
Since the parasite plasma membranes were isolated in the form of intact vesicles the right-side-out, the ATP hydrolysing regions of the ATPase proteins should be enclosed within the vesicles. This raises the question of whether all of the compounds tested are gaining access to the relevant part of the protein, and whether the differences between the ATPase activities measured in the presence of the different agents might reflect, at least to some extent, the different ability of these agents to gain access to the interior of the vesicles. Therefore, it could not be excluded that the saturable dependence of ATPase activity on the concentration of ATP in the medium could reflect the saturable transport of ATP into the vesicles, rather than the saturation of the ATPase itself. Similarly, the differential ability of divalent cations to support ATPase activity could be the result of a differential transport of these cations into the vesicles.
Conclusions
In conclusion, a method for the isolation and purification of the P. falciparum plasma membrane was developed and optimal conditions for P. falciparum plasma membrane ATPase activity were determined. These purified membranes were intact and isolated in a high enough yield to enable a characterisation of their ATPase activity. Thus, plasma membranes cleansed of P. falciparum can be used as a model to establish the role played by ATPases in ion transport. The availability of purified P. falciparum plasma membrane will be of value not only to investigate processes such as nutrient uptake/efflux, pH regulation and ion balance but also in the understanding of drug transport in this organelle.
Materials and Methods
Cell cultures
P. falciparum strain D10 was cultured essentially as described by Trager and Jensen [1]. Chloroquine-sensitive D10 was cloned from the Papua New Guinean isolate FCQ-27 [22]. Parasites were maintained in continuous culture at a 5% haematocrit in RPMI-1640 with glutamine (Sigma) supplemented with hypoxanthine (44 mg/l), Hepes (6 g/l), glucose (4 g/l), NaHCO3 (2.1 g/l), gentamycin (50 mg/l), and 10% A+ human serum (Western Province Blood transfusion Service and Haematology Department, Groote Schuur Hospital, Cape Town, South Africa). Parasites were grown in O-positive red cells and the parasitemia was kept between 10–15 %. Parasite cultures were incubated at 37°C in dessicator cabinets under an atmosphere of 93% nitrogen, 4% carbon dioxide, 3% oxygen. Cultures were synchronized by treatment with sorbitol [23].
Trophozoite isolation
Infected erythrocytes were washed twice in 10 volumes of phosphate buffered saline (PBS). The pellet was suspended in 10 volumes of PBS containing 0.05 % (w/v) saponin in order to lyse the erythrocytes. It was then incubated for 2–3 min at room temperature and centrifuged at 1,500 g for 10 min. The resulting pellet was washed twice in PBS and resuspended in 5 volumes of PBS. The protease inhibitors aprotinin, leupeptin and phenylmethylsulfonylfluoride (Boehringer Mannheim) were added to final concentration of 10 μg/ml, 10 μg/ml and 0.1 mM, respectively. Unlysed erythrocytes and erythrocyte membranes contained in the trophozoite preparation were adsorbed by immuno-affinity. Anti-human erythrocytes (polyvalent immunoglobulin (G,A,M) rabbit, Sigma) were diluted 1: 1000 in PBS. 5 g of polystyrene beads (Pierce) were incubated in 20 ml of the above solution for two hours at room temperature with gentle agitation. The beads were washed in PBS and further incubated for one hour with 20 ml 1 % bovine serum albumin in PBS. The beads were then washed again in PBS and incubated with the trophozoite preparation for 30 min. The purified trophozoites were collected and centrifuged at 1,500 g for 10 min.
Trypan blue uptake
The ability of purified trophozoites to exclude trypan blue was monitored by adding 10 μl of Trypan blue (10 mg/ml in distilled water) to 100 μl of trophozoite suspension in PBS (phosphate buffered saline). After five minutes incubation at room temperature, the proportion of parasites showing trypan blue accumulation was evaluated using a phase contrast microscope.
Plasma membrane isolation
The biotylination reaction was performed by incubating 250 μl of 1 mg/ml NHS-SS-Biotin (sulfosuccinimidyl 2-[biotin-amido] ethyl-1,3-dithiopropionate) (Pierce) with 500 μl of trophozoites (2 mg of protein) for 30 min at room temperature. The resulting biotinylated trophozoites were washed once in PBS, then twice in Vesicle buffer I (0.25 M sucrose, 1 mM E.D.T.A., 10 mM Tris-HCl pH 7.4). Vesicles were prepared by nitrogen cavitation according to the method of Lever et al.[24]. The cells were resuspended in 500 μl vesicle buffer I containing aprotinin, leupeptin and phenylmethylsulfonylfluoride (10 μg/ml, 10 μg/ml and 0.1 mM, respectively) and equilibrated at 4°C under nitrogen pressure at 800 psi for 30 min. The homogenate was then incubated for five min at 37°C with Dnase I (50 μg/ml). The biotinylated vesicles were incubated with 100 μl of streptavidin immobilized on iron oxide (Sigma) previously washed with PBS for 15 min at room temperature and then separated using a magnet (Magnetic Cell Sorting, Myltenyi Biotec). The magnetic particles were bound to the side of the tube and washed with 1 ml of 0.25 M sucrose, 10 mM Tris-HCl pH 7.4. The key steps in the purification procedure are shown in Figure 1.
Electron microscopy analysis
Parasite plasma membrane vesicles were fixed with glutaraldehyde (0.8%)/formaldehyde (8%) in PBS for one hour at room temperature and washed in PBS, followed by addition of 50 mM NH4Cl for 30 minutes and two washes in PBS. Samples were dehydrated in 70% ethanol and embedded in LR White resin (Polyscience Inc.) which was then polymerised at 60°C for 28 hours. Then 1 μm-thick sections were cut using a reichert Ultracut S ultramicrotome. Sections were mounted on copper grids and stained with 2% uranyl acetate and lead acetate. Samples were viewed on a JEM 200CX transmission electron microscope.
Acetylcholine esterase activity
The acetylcholine esterase (AchE) activity was determined by a modification of the method of Ellman et al. [25]. Enzyme assays were carried out with 200 μl of freshly prepared 0.1 M sodium phosphate pH 7.5, 0.5 mM 2,2'-dinitro-5,5'-dithiobenzoic acid, 0.6 mM S-acetylthiocholine iodide to which was added 50 μl of a suspension of erythrocyte ghost, trophozoites or parasite plasma membrane, in microtitre 96-well plates. 10 μg of erythrocyte ghost protein, 3–5 μg of trophozoites protein or 20 μg of parasite plasma membrane protein were used to measure an initial rate. 50 μl of PBS or vesicle buffer was added to the control wells. Wells containing 200 μl H2O and 50 μl of erythrocyte ghosts/ trophozoites/ vesicles suspensions were included to subtract any absorbance due to the cells. Changes in absorbance at 405 nm were measured for at least 20 min, using a 7520 Microplate Reader (Cambridge Technology, Inc).
Parasite lactate dehydrogenase activity
Measurements of parasite lactate dehydrogenase (pLDH) activity were carried out essentially as described by Makler and Hinrichs [26]. For these measurements, 5 μg of trophozoite protein or membrane vesicle protein suspended in 20 μl PBS or vesicle buffer were added to 100 μl Malstat™ reagent (Flow, inc.) and 25 μl 0.24 mM phenazine ethosulfate (PES)/1.96 mM nitro blue tetrazolium (NBT) in microtitre 96-well plates. 20 μl of H2O was added to the control wells. Wells containing 20 μl trophozoites/ membrane vesicles and 125 μl H2O were included to subtract any absorbance due to the cells. Changes in absorbance at 620 nm were measured for at least 20 min, using a 7520 Microplate Reader (Cambridge Technology, Inc).
Protein determinations
Protein determinations were performed by the method of Lowry et al. [27].
ATPase activity
The ATPase activity was determined using a colorimetric assay adapted from those of Chifflet et al.[28] and Doige et al.[20]. Enzyme assays were carried out with 50 μg of freshly isolated P. falciparum plasma membranes suspended in 450 μl of reaction buffer (50 mM Tris-HCl, 0.15 M NH4Cl, 2 mM MgCl2, 0.02% NaN3, pH 7.4). To initiate the reaction 50 μl of ATP or the appropriate nucleotide tested in the reaction buffer was added, giving a final concentration of 2 mM ATP or the appropriate nucleotide. A control containing 50 μg of parasite plasma membranes suspended in 500 μl of reaction buffer was included to subtract any absorbance due to the membranes. Another control consisting of 500 μl reaction buffer containing 2 mM ATP was added to evaluate the ATP hydrolysis. After 45 min. at 37°C, the suspensions were centrifuged at 13,000 rpm for three minutes to pellet the membranes. Aliquots of 100 μl of the resulting supernatant were transferred to the wells of a 96-well microtitre plate. The reaction was stopped by addition of 100 μl of fresly prepared solution A (6% SDS, 3% L-ascorbic acid, 0.5% ammonium molybdate in 0.25 M sulfuric acid). After 15 minutes, the phosphoammoniummolybdate complex formed was stabilised by the addition of 100 μl of solution B (2% sodium citrate, 2% sodium arsenite, 2% acetic acid). After 15 minutes, the absorbance at 710 nm was measured using a 7520 Microplate Reader (Cambridge Technology, Inc).
Acknowledgments
Acknowledgements
This work was supported by the South African Medical Research Council.
Contributor Information
Laurence M Elandalloussi, Email: lelanda@ualg.pt.
Pete J Smith, Email: psmith@uctgsh1.uct.ac.za.
References
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W, Williams J, Gill GS. Extracellular development, in vitro, of the erythrocytic cycle of Plasmodium falciparum. Parasitology today. 1992;8:384–387. doi: 10.1016/0169-4758(92)90177-4. [DOI] [PubMed] [Google Scholar]
- Williams JH, Gill GS, Trager W. Effect of erythrocyte membrane on extracellular development of the erythrocytic cycle of Plasmodium falciparum. Proc Natl Acad Sci USA. 1995;92:566–568. doi: 10.1073/pnas.92.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Williams J, Gill GS. Extracellular development of erythrocytic stages of Plasmodium falciparum. Methods in Cell Science. 1996;18:219–227. [Google Scholar]
- Hamburger J, Kreier JP. Isolation of malarial parasites and their constituents. In Malaria Immunology and Immunization, New York: Academic Press. 1980;3:1–65. [Google Scholar]
- Heidrich HG, Leutner G. Two types of vesicles from the erythrocyte-ghost membrane differing in surface charge. Separation and characterization by preparative free-flow electrophoresis. Eur J Biochem. 1974;41:37–43. doi: 10.1111/j.1432-1033.1974.tb03241.x. [DOI] [PubMed] [Google Scholar]
- Wunderlich F, Helwig M, Schillinger G, Vial H, Philippot J, Speth V. Isolation and characterization of parasites and host cell ghosts from erythrocytes infected with Plasmodium chabaudi. Mol Biochem Parasitol. 1987;23:103–115. doi: 10.1016/0166-6851(87)90145-9. [DOI] [PubMed] [Google Scholar]
- Hoppe HC, Coetzee J, Louw AI. Plasmodium falciparum : isolation of intact and erythrocyte-free trophozoites from sorbitol lysates. Parasitology. 1992;104:379–385. doi: 10.1017/s0031182000063629. [DOI] [PubMed] [Google Scholar]
- Christopher SR, Fulton JD. Experiments with isolated malaria parasites (Plasmodium knowlesi) free from red cells. Ann Trop Med Parasitol. 1939;33:161–170. [Google Scholar]
- Gruenberg J, Sherman IW. Isolation and characterization of the plasma membrane of human erythrocytes infected with the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1983;80:1087–1091. doi: 10.1073/pnas.80.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwaldt BL, Schlesinger PH, Krogstad DJ. Accumulation of chloroquine by membrane preparations from Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:257–268. doi: 10.1016/0166-6851(90)90169-M. [DOI] [PubMed] [Google Scholar]
- Choi I, Mego JL. Purification of Plasmodium falciparum digestive vacuoles and partial characterisation of the vacuolar membrane ATPase. Mol Biochem Parasitol. 1988;31:71–78. doi: 10.1016/0166-6851(88)90146-6. [DOI] [PubMed] [Google Scholar]
- Ansorge I, Benting J, Bhakdi S, Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem J. 1996;315:307–314. doi: 10.1042/bj3150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge I, Paprotka K, Bhakdi S, Lingelbach K. Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol Biochem Parasitol. 1997;84:259–261. doi: 10.1016/S0166-6851(96)02806-X. [DOI] [PubMed] [Google Scholar]
- Saliba KJ, Horner HA, Kirk K. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem. 1998;273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- Saliba KJ, Kirk K. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H(+) extrusion via a V-type H(+)-ATPase. J Biol Chem. 1999;274:33213–33219. doi: 10.1074/jbc.274.47.33213. [DOI] [PubMed] [Google Scholar]
- Cornwell MM, Safa AR, Felsted RI, Gottesman MM, Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150 to 170 kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci USA. 1986;83:3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B. An investigation of P-type and V-type ATPase components and chloroquine sensitivity of digestive vacuoles purified from Plasmodium falciparum. Thesis submitted for doctoral degree at the University of Cape Town, South Africa. 1999.
- Rebbeor JF, Senior AE. Effects of cardiovascular drugs on ATPase activity of P-glycoprotein in plasma membranes and in purified reconstituted form. Biochim Biophys Acta. 1998;1369:85–93. doi: 10.1016/S0005-2736(97)00185-5. [DOI] [PubMed] [Google Scholar]
- Doige CA, Yu X, Sharom FJ. ATPase activity of partially purified P-glycoprotein from multidrug-resistant chinese hamster ovary cells. Biochim Biophys Acta. 1992;1109:149–160. doi: 10.1016/0005-2736(92)90078-Z. [DOI] [PubMed] [Google Scholar]
- Ketcham CM, Baumbach GA, Bazer FW, Roberts RM. The type 5, acid phosphatase from spleen of humans with hairy cell leukemia. Purification, properties, immunological characterization, and comparison with porcine uteroferrin. J Biol Chem. 1985;260:5768–5776. [PubMed] [Google Scholar]
- Ekong RM, Robson KJ, Baker DA, Warhurst DC. Transcripts of the multidrug resistance genes in chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Parasitology. 1993;106:107–115. doi: 10.1017/s0031182000074904. [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Lever JE. Active amino acid transport in plasma membrane vesicles from Simian virus 40-transformed mouse fibroblasts. J Biochem. 1977;252:1990–1997. [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Makler MT, Hinrich DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]