Abstract
Background.
Point-of-care (POC) viral load (VL) tests provide results within hours, enabling same-day treatment interventions. We assessed treatment outcomes with POC vs standard-of-care (SOC) VL monitoring.
Methods.
We implemented a randomized controlled trial at an urban and rural hospital in Nigeria. Participants initiating antiretroviral therapy (ART) were randomized 1:1 for monitoring via the POC Cepheid Xpert or SOC Roche COBAS (v2.0) HIV-1 VL assays. Viral suppression (VS) and retention in care at 12 months were compared via intention-to-treat (ITT) and per-protocol (PP) analyses. Post-trial surveys for POC patients and healthcare workers (HCWs) evaluated acceptability.
Results.
During April 2018–October 2019, 268 SOC and 273 POC patients enrolled in the trial. Viral suppression at <1000 copies/mL at 12 months was 59.3% (162/273) for POC and 52.2% (140/268) for SOC (P = .096) in ITT analysis and 77.1% (158/205) for POC and 65.9% (137/208) for SOC (P = .012) in PP analysis. Retention was not significantly different in ITT analysis but was 85.9% for POC and 76.9% for SOC (P = .02) in PP analysis. The increased VS in the POC arm was attributable to improved retention and documentation of VL results. POC monitoring was preferred over SOC by 90.2% (147/163) of patients and 100% (15/15) of HCWs thought it facilitated patient care.
Conclusions.
POC VL monitoring did not improve 12-month VS among those with results but did improve retention and VS documentation and was preferred by most patients and HCWs. Further research can inform best POC implementation conditions and approaches to optimize patient care.
Clinical Trials Registration.
Keywords: point-of-care, viral load, randomized controlled trial, outcomes, HIV
Human immunodeficiency virus (HIV) viral load (VL) testing is essential for monitoring antiretroviral therapy (ART). Prompt results are critical as continuing ART with suboptimal adherence or a failing regimen increases risks of viremia, transmission, drug-resistance mutations, and clinical decline [1, 2].
Challenges to standard-of-care (SOC) VL testing include its requirements for laboratory infrastructure, skilled personnel, specimen handling, and processing time. In resource-limited settings (RLSs), some clinics lack access or rely on central laboratories, contributing to backlogs and long turnaround times.
Recently developed point-of-care (POC) VL assays simplify testing and training requirements [3, 4]. The Cepheid Xpert HIV-1 VL assay (Sunnyvale, CA) provides results within 90 minutes, after 20 minutes for plasma preparation, demonstrating high concordance with standard assays [5, 6]. Rapid testing enables same-day results-based adherence counseling—reinforcement or enhanced support—potentially leading to prompter improvement in adherence and viral suppression (VS) [7]. Point-of-care results also enable rapid transition of stable patients to differentiated services, freeing resources for high-risk patients, and switching of patients failing ART to second-line regimens and genotype testing, where available.
World Health Organization (WHO) 2021 guidelines recommend POC VL monitoring [8, 9]. Prioritization is recommended for high-risk populations who require rapid results, including infants, children, adolescents, pregnant/breastfeeding women, and people with advanced disease or suspected treatment failure.
Observational studies have demonstrated the feasibility and utility of POC VL testing for routine monitoring or targeted use [5, 10–14]. However, implementation gaps included staff training and commitment, documentation, patient follow-up, and timely communication and clinical use of results, which, if not optimized, nullify POC benefits. Successful POC implementation relies on efficient systems and workflows.
Data comparing clinical outcomes for POC versus SOC testing and on patient and healthcare worker (HCW) perspectives are limited. The Simplifying HIV TREAtment and Monitoring (STREAM) trial (South Africa) found that 90% of patients monitored with POC VL versus 76% of SOC patients had the composite outcome of 12-month VS and retention [15]. The intervention combined POC testing with task shifting to enrolled nurses, so the extent each played in improved outcomes is uncertain.
Our trial compared clinical outcomes of adult and pediatric patients routinely monitored with POC versus SOC VL assays without additional interventions. We hypothesized that POC monitoring could improve 12-month VS and retention by enabling same-day counseling and treatment optimization.
METHODS
Study Design
Jos University Teaching Hospital (JUTH) is an urban tertiary hospital serving greater Jos, Nigeria. Its satellite facility, Comprehensive Health Centre Zamko (CHCZ), is a rural secondary hospital 200 km southeast of Jos. JUTH’s HIV clinic laboratory performs VL testing for 168 health facilities; CHCZ ships VL samples to JUTH weekly.
We implemented an unblinded 2-arm randomized controlled trial (RCT) at JUTH and CHCZ (Clinical Trials Identifier NCT03533868). The study protocol was previously published [16]. Patients with newly diagnosed HIV were randomized to receive either POC or SOC VL testing, routinely 6 and 12 months after ART initiation. Clinical outcomes were monitored up to 15 months after ART initiation to allow for delays in the 12-month visit.
The research was approved by JUTH’s and Harvard’s institutional review boards. The US Centers for Disease Control and Prevention (CDC) determined that its involvement did not constitute engagement in human subject research.
Participants
Adult (aged >15 years) and pediatric (aged 0–15 years) patients, newly initiating ART at JUTH or CHCZ between April 2018 and October 2019, were eligible for the trial. Patients with prior ART experience or current pregnancy were excluded. All participants provided written informed consent. For children younger than 18 years, written parental permission was obtained, with assent from children aged 7–17 years.
All POC patients were eligible for a post-trial survey. All HCWs directly involved in patient care or testing for the trial were eligible for a post-trial HCW survey and provided written consent.
The sample size of 538 could detect a 10% increase in VS (from 80%) with a power of 0.8 and ɑ of 0.05, estimating 26% loss to follow-up (LTFU) by 15 months. Enrollment was summed weekly and concluded at the end of the week that the sample size was reached at 541.
Randomization
At ART initiation, enrolling participants were randomized 1:1 to the SOC or POC VL monitoring arm. Four separate lists for the JUTH and CHCZ adult and pediatric clinics were computer-generated before enrollment commenced using permuted block randomization, determining the trial assignment sequence.
Standard-of-Care
Based on Nigeria’s 2016 National ART guidelines, patients with newly diagnosed HIV initiated ART after clinical assessment [17]. Patients picked up ART monthly at the pharmacy, with clinical visits at 3 months, 6 months, and every 6 months thereafter for adults, and monthly to every 2 months for children. Samples were collected for VL testing by Roche COBAS AmpliPrep/TaqMan HIV-1 Test, v2.0 (Indianapolis, IN), at the JUTH laboratory at the 6- and 12-month clinical visits and every 12 months thereafter. If virally suppressed, patients received their results with standard counseling at their next scheduled clinical visit. If virally unsuppressed (≥1000 copies/mL), the patient was referred at their next ART pick-up (ie, 7–9 months on ART) for 3 monthly enhanced adherence counseling (EAC) sessions and a post-EAC VL test 3 months later (ie, 10–12 months on ART). Patients returned for clinical review 4 weeks later (ie, 11–13 months on ART), when, if unsuppressed, their regimen was switched to include a second-line protease inhibitor (PI) (Supplementary Table 1).
Nonroutine baseline VL tests were performed for both trial arms to understand pre-ART differences.
Point-of-Care
Point-of-care patients followed the SOC clinical visit algorithm. At the start of the 6- and 12-month clinical visits, samples were collected for Cepheid Xpert testing and patients waited to receive their results with same-day counseling. If virally unsuppressed, patients received 3 monthly EAC sessions and a post-EAC Cepheid VL test after 3 months. If again unsuppressed, patients switched to a second-line PI regimen the same day (Supplementary Table 1). All other aspects of care followed standard clinic procedures.
Outcomes
The primary outcome was the proportion of patients in each trial arm with VS at 12 months, defined as a documented 12-month VL of less than 1000 copies/mL, the threshold used to define treatment failure by WHO [9]. In sensitivity analyses, we also evaluated VS at 200 copies/mL or less and 50 copies/mL or less (undetectable). Secondary outcomes included 12-month retention in care (proportion with a 12-month clinical visit), ART adherence, and LTFU from ART. We added documentation of 12-month VL results as a secondary outcome to determine its impact on VS differences.
ART adherence was measured using the medication possession ratio based on electronic pharmacy refill data [18]. Adherence was calculated as number of days supplied with ART over total number of days in the 0–12- and 6–12-month time intervals, using patients’ actual clinical visit dates. If a patient missed a visit, the interval used a proxy endpoint 6 months after the prior visit. Failure to pick up medication for any reason (default, LTFU, death) resulted in reduced adherence during the time interval.
Loss-to-follow-up was defined as having run out of ART, based on pharmacy dispensing records, at least 60 days before data were censored (15 months after ART initiation).
Post-Trial Surveys
To assess perceptions of POC VL monitoring, post-trial surveys were administered in person by adherence counselors to retained POC patients at the end of their 12-month visit. Counselors read questions together with patients and circled the patients’ responses. Eligible HCWs self-completed a written post-trial staff survey. Survey responses were rated on a Likert scale, and the proportion of respondents who agreed or strongly agreed with the given statements was calculated.
Data Collection
Study enrollment, visit, and survey data were entered in trial forms and corresponding databases. Routine clinical data, including VL results, were entered into patient medical records charts and the electronic medical record (EMR) (Claris FileMaker Pro), consisting of networked clinical, laboratory, and pharmacy databases. Relevant EMR data were extracted for analyses.
Statistical Analysis
For baseline patient demographic and clinical characteristics, site differences were assessed using chi-square or Fisher’s exact tests.
Outcomes were compared between trial arms using risk ratios and differences. The primary outcome of 12-month VS was analyzed 3 ways. In intention-to-treat (ITT), all patients were included in the denominator and a missing 12-month VL was treated as a failure. In complete case (CC), patients missing a 12-month VL result were excluded. In per-protocol (PP) analysis, a missing 12-month VL was treated as a failure and patients who did not make a 6-month visit (did not receive the intervention), withdrew from the trial, or switched to second-line ART due to virologic failure (unable to receive a 12-month VL by testing algorithm) were excluded. Sensitivity analyses compared patients included and excluded from the PP analysis using chi-square tests. Secondary outcomes were analyzed using ITT and PP only, as all patients were assessed.
Bivariate associations between documented VS and patient characteristics were examined using chi-square tests (PP analysis). Variables with P < .25 were included in a multiple logistic regression model; the adjusted model retained variables with P < .05. Analyses were performed using Stata 15 (StataCorp, College Station, TX).
RESULTS
Total trial enrollment was 541 patients: 293 JUTH adults, 203 CHCZ adults, 28 JUTH children, and 17 CHCZ children (Tables 1 and 2).
Table 1.
Baseline Demographic and Clinical Characteristics of Adult Trial Patients, Jos University Teaching Hospital and Comprehensive Health Centre Zamko
| JUTH Adult |
CHCZ Adult |
Total Adult |
Pa: JUTH vs CHCZ | ||||
|---|---|---|---|---|---|---|---|
| POC (n = 148) | SOC (n = 145) | POC (n = 102) | SOC (n = 101) | POC (n = 250) | SOC (n = 246) | ||
|
| |||||||
| Age, median (IQR) | 38 (31,45) | 37 (30, 42) | 33 (29, 40) | 35 (30, 43) | 36 (30, 43) | 36 (30, 42) | |
| 18–29 years | 28 (18.9) | 31 (21.4) | 28 (27.5) | 24 (23.8) | 56 (22.4) | 55 (22.4) | .079 |
| 30–35 years | 35 (23.6) | 35 (24.1) | 30 (29.4) | 31 (30.7) | 65 (26.0) | 66 (26.8) | |
| 36–42 years | 34 (23.0) | 44 (30.3) | 26 (25.5) | 20 (19.8) | 60 (24.0) | 64 (26.0) | |
| >42 years | 51 (34.5) | 35 (24.1) | 18 (17.6) | 26 (25.7) | 69 (27.6) | 61 (24.8) | |
| Female sex (vs male) | 88 (59.5) | 92 (63.4) | 76 (74.5) | 70 (69.3) | 164 (65.6) | 162 (65.9) | .016 |
| Education | |||||||
| None | 14 (9.5) | 10 (6.9) | 55 (53.9) | 57 (56.4) | 69 (27.6) | 67 (27.2) | <.001* |
| Primary | 43 (29.1) | 33 (22.8) | 22 (21.6) | 21 (20.8) | 65 (26.0) | 54 (22.0) | |
| Secondary | 43 (29.1) | 43 (29.7) | 23 (22.5) | 20 (19.8) | 66 (26.4) | 63 (25.6) | |
| Tertiary | 48 (32.4) | 59 (40.7) | 2 (2.0) | 3 (3.0) | 50 (20.0) | 62 (25.2) | |
| Employment | |||||||
| Non-income-generating | 34 (23.0) | 31 (21.4) | 12 (14.3) | 13 (15.1) | 46 (19.8) | 44 (19.1) | <.001* |
| Labor/service/trade | 87 (58.8) | 86 (59.3) | 69 (82.1) | 72 (83.7) | 156 (67.2) | 158 (68.4) | |
| Prof essiona l/ma nageria 1 | 27 (18.2) | 28 (19.3) | 3 (3.6) | 1 (1.2) | 30 (12.9) | 29 (12.6) | |
| Marital status | |||||||
| Single | 44 (29.7) | 43 (29.7) | 18 (17.6) | 14 (13.9) | 62 (24.8) | 57 (23.2) | .001 |
| Married | 77 (52.0) | 69 (47.6) | 61 (59.8) | 57 (56.4) | 138 (55.2) | 126 (51.2) | |
| Divorced/sepa rated | 13 (8.8) | 18 (12.4) | 16 (15.7) | 20 (19.8) | 29(11.6) | 38 (15.5) | |
| Widowed | 14 (9.5) | 15 (10.3) | 7 (6.9) | 10 (9.9) | 21 (8.4) | 25 (10.2) | |
| WHO clinical stage | |||||||
| 1 | 91 (61.9) | 97 (67.4) | 73 (71.6) | 69 (68.3) | 164 (65.9) | 166 (67.8) | .017* |
| 2 | 28 (19.0) | 24 (16.7) | 19 (18.6) | 23 (22.8) | 47 (18.9) | 47 (19.2) | |
| 3 | 27 (18.4) | 21 (14.6) | 8 (7.8) | 7 (6.9) | 35 (14.1) | 28 (11.4) | |
| 4 | 1 (0.7) | 2 (1.4) | 2 (2.0) | 2 (2.0) | 3 (1.2) | 4 (1.6) | |
| TB coinfection | 14 (9.5) | 10 (6.9) | 3 (2.9) | 0 (0.0) | 17 (6.8) | 10 (4.1) | .001* |
| BMI (kg/m2), median (IQR) | 21.2 (19.3, 24.2) | 21.0 (18.7, 25.2) | 20.4 (18.3, 23.2) | 20.4 (18.0, 23.4) | 21.0 (18.8, 23.7) | 20.9 (18.5, 24.0) | |
| Underweight (<18.5) | 25 (17.7) | 31 (22.6) | 24 (27.3) | 23 (28.4) | 49 (21.4) | 54 (24.8) | .003* |
| Normal (18.5–24.9) | 92 (65.2) | 71 (51.8) | 58 (65.9) | 47 (58.0) | 150 (65.5) | 118 (54.1) | |
| Overweight (25.0–29.9) | 18 (12.8) | 26 (19.0) | 5 (5.7) | 11 (13.6) | 23 (10.0) | 37 (17.0) | |
| Obese (≥30.0) | 6 (4.3) | 9 (6.6) | 1 (1.1) | 0 (0.0) | 7(3.1) | 9 (4.1) | |
| Viral load (copies/mL), median (IQR) | 95 922(17 748–301 519) | 121 850(20 919–362 895) | 113 416(17 369–453 136) | 80 322(22 839–417 132) | 106347(17,748, 342,093) | 99836(21 810, 394 839) | |
| <10000 | 29 (19.6) | 26 (18.2) | 21 (21.0) | 17 (18.5) | 50 (20.2) | 43 (18.3) | .855 |
| 10000–99999 | 45 (30.4) | 42 (29.4) | 27 (27.0) | 33 (35.9) | 72 (29.0) | 75 (31.9) | |
| 100 000–999999 | 64 (43.2) | 59 (41.3) | 41 (41.0) | 33 (35.9) | 105 (42.3) | 92 (39.2) | |
| ≥1 000000 | 10 (6.8) | 16(11.2) | 11 (11.0) | 9 (9.8) | 21 (8.5) | 25 (10.6) | |
| Initial ART regimen | |||||||
| ABC-3TC-DTG | 1 (0.7) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) | .009* |
| ABC-3TC-EFV | 2 (1.4) | 4 (2.8) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 4 (1.6) | |
| AZT-3TC-NVP | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| TDF-3TC-DTG | 46 (31.1) | 47 (32.4) | 26 (25.5) | 22 (21.8) | 72 (28.8) | 69 (28.1) | |
| TDF-3TC-EFV | 99 (66.9) | 93 (64.1) | 75 (73.5) | 79 (78.2) | 174 (69.6) | 172 (69.9) | |
Values shown are n (%) unless otherwise indicated; % is of those with recorded value.
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; BMI, body mass index; CHCZ, Comprehensive Health Centre Zamko; DTG, dolutegravir; EFV, efavirenz; IQR, interquartile range; JUTH, Jos University Teaching Hospital; NVP, nevirapine; POC, point-of-care; SOC, standard-of-care; TB, tuberculosis; TDF, tenofovir; WHO, World Health Organization; 3TC, lamivudine.
Used chi-square test, or Fisher’s exact test for frequencies ≤5 (asterisked “*”) to obtain A values for comparisons between the JUTH and CHCZ clinics.
Table 2.
Baseline Demographic and Clinical Characteristics of Pediatric Trial Patients, Jos University Teaching Hospital and Comprehensive Health Centre Zamko
| JUTH Pediatric |
CHCZ Pediatric |
All Pediatric |
Pa: JUTH vs CHCZ | ||||
|---|---|---|---|---|---|---|---|
| POC (n = 14) | SOC (n = 14) | POC (n = 9) | SOC (n = 8) | POC (n = 23) | SOC (n = 22) | ||
|
| |||||||
| Age | |||||||
| <5 years | 4 (28.6) | 4 (28.6) | 2 (22.2) | 6 (75.0) | 6 (26.1) | 10 (45.5) | .077 |
| 5–9 years | 5 (35.7) | 5 (35.7) | 6 (66.7) | 2 (25.0) | 11 (47.8) | 7 (31.8) | |
| 10–17 years | 5 (35.7) | 5 (35.7) | 1 (11.1) | 0 (0.0) | 6 (26.1) | 5 (22.7) | |
| Female sex (vs male) | 8 (57.1) | 8 (57.1) | 6 (66.7) | 1 (12.5) | 14 (60.9) | 9 (40.9) | .365 |
| WHO clinical stage | |||||||
| 1 | 7 (70.0) | 6 (54.5) | 4 (50.0) | 3 (37.5) | 11 (61.1) | 9 (47.4) | .545 |
| 2 | 3 (30.0) | 2 (18.2) | 4 (50.0) | 2 (25.0) | 7 (38.9) | 4(21.1) | |
| 3 | 0 (0.0) | 3 (27.3) | 0 (0.0) | 2 (25.0) | 0 (0.0) | 5 (26.3) | |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (5.3) | |
| TB coinfection | 0 (0.0) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (9.1) | .519 |
| Weight-for-age (z score) | |||||||
| Wasted: < −2 SDs | 3 (21.4) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 3 (20.0) | 1 (6.3) | 1 |
| Normal: −2 SDs to +2 SDs (<5 years); −2 SDs to +1 SD (≥5 years) | 9 (64.3) | 12 (85.7) | 1 (100) | 2 (100) | 10 (66.7) | 14 (87.5) | |
| Overweight: > +2 SDs (<5 years); > +1 SD (≥5 years) | 2 (14.3) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 2 (13.3) | 1 (6.3) | |
| Viral load (copies/mL) | |||||||
| <10000 | 2 (14.3) | 2 (14.3) | 2 (33.3) | 2 (28.6) | 4 (20.0) | 4 (19.1) | .039 |
| 10001–99999 | 6 (42.9) | 4 (28.6) | 2 (33.3) | 3 (42.9) | 8 (40.0) | 7 (33.3) | |
| 100000–999999 | 6 (42.9) | 8 (57.1) | 1 (16.7) | 1 (14.3) | 7 (35.0) | 9(42.9) | |
| ≥1 000 000 | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (14.3) | 1 (5.0) | 1 (4.8) | |
| Initial ART regimen | |||||||
| ABC-3TC-EFV | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.6) | <.001 |
| ABC-3TC-LPV/r | 2 (14.3) | 3 (21.4) | 2 (22.2) | 0 (0.0) | 4 (17.4) | 3 (13.6) | |
| AZT-3TC-EFV | 5 (35.7) | 7 (50.0) | 0 (0.0) | 1 (12.5) | 5 (21.7) | 8 (36.4) | |
| AZT-3TC-LPV/r | 0 (0.0) | 0 (0.0) | 3 (33.3) | 2 (25.0) | 3 (13.0) | 2 (9.1) | |
| AZT-3TC-NVP | 3 (21.4) | 1 (7.1) | 4 (44.4) | 5 (62.5) | 7 (30.4) | 6 (27.3) | |
| TDF-3TC-DTG | 2 (14.3) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 2 (9.1) | |
| TDF-3TC-EFV | 2 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 0 (0.0) | |
Values shown are n (%) unless otherwise indicated; % is of those with recorded value.
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; CHCZ, Comprehensive Health Centre Zamko; DTG, dolutegravir; EFV, efavirenz; JUTH, Jos University Teaching Hospital; LPV/r, lopinavir/ritonavir; NVP, nevirapine; POC, point-of-care; SD, standard deviation; SOC, standard-of-care; TB, tuberculosis; TDF, tenofovir; WHO, World Health Organization; 3TC, lamivudine.
Used Fisher’s exact test to obtain P values for comparisons between the JUTH and CHCZ clinics.
Among all adults, the median age was 36 years (interquartile range [IQR]: 30–43 years), and 65.7% (326/496) were female. Compared with JUTH, a significantly higher proportion of CHCZ adults were female, less educated, and in labor/service versus professional/managerial occupations, and a lower proportion were single and had advanced WHO stage, tuberculosis coinfections, high body mass index, and dolutegravir (DTG)-based initial ART regimens (P < .05) (Table 1). Among all 45 pediatric patients, 16 (35.6%) were younger than 5 years, 18 (40.0%) were aged 5–9 years, 11 (24.4%) were aged 10–17 years, and 23 (51.1%) were female (Table 2).
At enrollment, 273 patients were allocated to the POC arm and 268 to the SOC arm (Figure 1). At month 6, 79.9% (218/273) of POC and 80.2% (215/268) of SOC patients made their clinical visit, receiving the intervention (see also Supplementary Table 2). At month 12, 66.7% (182/273) of POC and 62.3% (167/268) of SOC patients made their clinical visit; 33.3% (91/273) of POC patients and 37.7% (101/268) of SOC patients discontinued the trial due to transfer out, withdrawal, death, or LTFU.
Figure 1.
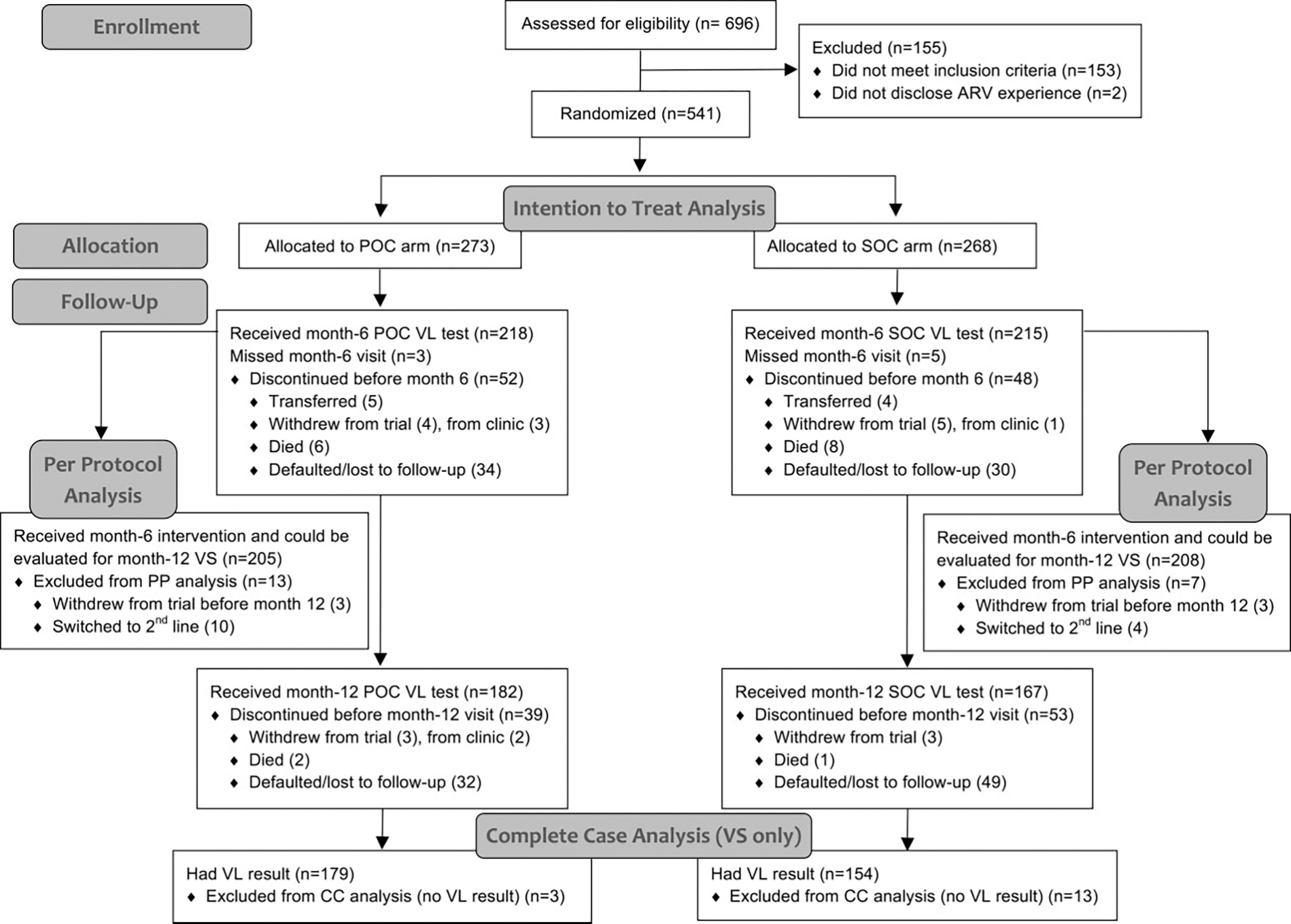
Trial flow diagram. Flow diagram adapted from Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines [19]. Abbreviations: ARV, antiretroviral; CC, complete case; POC, point-of-care; PP, per protocol; SOC, standard-of-care; VL, viral load; VS, viral suppression.
In sensitivity analyses, no difference was detected in the proportion of POC patients (68/273, 24.9%) versus SOC patients (60/208, 22.4%) excluded from the PP analysis (P = .490). Patients included in the PP analysis did not differ in baseline characteristics by trial arm (data not shown).
Primary Outcome
In ITT analysis, 59.3% (162/273) of POC patients versus 52.2% (140/268) of SOC patients had documented 12-month VS at less than 1000 copies/mL (P = .096) (Table 3). No difference was detected in CC analysis: 90.5% (162/179) of POC patients and 90.9% (140/154) of SOC patients had 12-month VS (P = .899). In PP analysis, 77.1% (158/205) of POC patients had 12-month VS compared with 65.9% (137/208) of SOC patients, with an 11.2% risk difference (P = .012). Comparisons were similar at VS thresholds of 200 copies/mL or less and 50 copies/mL or less, except at 200 copies/mL or less the 9.0% difference was significant (P = .012) in ITT analysis.
Table 3.
Comparison of Primary and Secondary Outcomes by Trial Assignment, Adult and Pediatric Patients at Jos University Teaching Hospital and Comprehensive Health Centre Zamko
| Number (%) |
Risk Ratio (95% CI) | Risk Difference, % (95% CI) | Chi-square P Value | ||
|---|---|---|---|---|---|
| POC (%) | SOC (%) | ||||
|
| |||||
| Primary outcomes | |||||
| Viral suppression (<1000 copies/mL) at 12 months | |||||
| Intention-to-treata,b | 162/273 (59.3) | 140/268 (52.2) | 1.14 (.98–1.32) | 7.1 (−1.2–15.5) | .096 |
| Complete casec | 162/179 (90.5) | 140/154 (90.9) | 1.00 (.93–1.07) | .4 (−6.7–5.8) | .899 |
| Per protocolb,d | 158/205 (77.1) | 137/208 (65.9) | 1.17 (1.03–1.32) | 11.2 (2.6–19.8) | .012 |
| Viral suppression (≤200 copies/mL) at 12 months | |||||
| Intention-to-treat | 159/273 (58.2) | 132/268 (49.3) | 1.18(1.01–1.38) | 9.0 (.6–17.4) | .036 |
| Complete case | 159/179 (88.8) | 132/154 (85.7) | 1.04 (.95–1.13) | 3.1 (−4.1–10.3) | .394 |
| Per protocol | 155/205 (75.6) | 129/208 (62.0) | 1.22 (1.07–1.39) | 13.6 (4.8–22.4) | .003 |
| Viral suppression (≤50 copies/mL) at 12 months | |||||
| Intention-to-treat | 136/273 (49.8) | 114/268 (42.5) | 1.17 (.98–1.41) | 7.3 (−1.1–15.7) | .090 |
| Complete case | 136/179 (76.0) | 114/154 (74.0) | 1.03 (.91–1.16) | 2.0 (−7.4–11.3) | .681 |
| Per protocol | 134/205 (65.4) | 112/208 (53.9) | 1.21 (1.03–1.43) | 11.5 (2.1–20.9) | .017 |
| Secondary outcomes | |||||
| Documented month-12 viral load result | |||||
| Intention-to-treat | 179/273 (65.6) | 154/268 (57.5) | 1.14(1.00–1.30) | 8.1 (−.1–16.3) | .053 |
| Per protocol | 173/205 (84.4) | 148/208 (71.2) | 1.19(1.07–1.32) | 13.2 (5.3–21.1) | .001 |
| Retention in care at 12 months | |||||
| Intention-to-treat | 182/273 (66.7) | 167/268 (62.3) | 1.07 (.94–1.21) | 4.4 (−3.7–12.4) | .29 |
| Per protocol | 176/205 (85.9) | 160/208 (76.9) | 1.12 (1.02–1.22) | 8.9 (1.5–16.4) | .02 |
| ART adherence, 0–12 months | |||||
| MPR ≥95%, intention-to-treat | 119/273 (43.6) | 118/268 (44.0) | .99 (.82–1.20) | −.4 (−8.8–7.9) | .918 |
| MPR ≥95%, per protocol | 112/205 (54.6) | 114/208 (54.8) | 1.00 (.84–1.19) | −.2 (−9.8–9.4) | .972 |
| ART adherence, 6–12 months | |||||
| MPR ≥95%, intention-to-treat | 131/273 (48.0) | 119/268 (44.4) | 1.08 (.90–1.30) | 3.6 (−4.8–12.0) | .403 |
| MPR ≥95%, per protocol | 122/205 (59.5) | 113/208 (54.3) | 1.10 (.93–1.30) | 5.2 (−4.4–14.7) | .287 |
| Lost to follow-up from ART at 15 months | |||||
| Intention-to-treat | 53/273(19.4) | 54/268 (20.2) | .96 (.69–1.35) | −.7 (−7.4–6.0) | .83 |
| Per protocol | 21/205(10.2) | 26/208(12.5) | .82 (.48–1.41) | −2.3 (−8.4–3.9) | .47 |
Abbreviations: ART, antiretroviral therapy; CHCZ, Comprehensive Health Centre Zamko; CI, confidence interval; DTG, dolutegravir; JUTH, Jos University Teaching Hospital; MPR, medication possession ratio; NNRTI, non-nucleoside reverse transcriptase inhibitor; POC, point-of-care; PP, per protocol; SOC, standard-of-care; WHO, World Health Organization.
Intention-to-treat: no patients excluded from analysis/denominator; missing 12-month viral load = failure.
Patients excluded from the PP analysis differed from those included: 62.7% (74/118) of excluded patients vs 47.9% (181/378) of included patients had no/primary education (P= .005); 20.6% (26/126) of excluded patients vs 12.4% (50/405) of included patients were in WHO clinical stage 3/4 (P= .02); and 79.2% (99/125) of excluded patients vs 70.1% (283/404) of included patients were initiated on an NNRTI (vs DTG) (P= .046).
Complete case: patients missing 12-month viral load excluded from analysis.
Per protocol: patients who did not make a 6-month visit (did not have intervention), who transferred out of the program or withdrew from the trial (could not be evaluated for 12-month viral load), and who switched to second-line ART due to virologic failure (did not have time to get 12-month viral load during trial) were excluded from analysis; missing 12-month viral load = failure.
Secondary Outcomes
In exploring the differences in VS in ITT and PP but not in CC analysis, we found that the POC arm had more documented VL results than the SOC arm, a difference of 8.1% (P = .053) in ITT and 13.2% (P = .001) in PP analysis (Table 3).
For 12-month retention in care, ITT analysis detected no significant difference between trial arms. However, 85.9% (176/205) of POC patients versus 76.9% (160/208) of SOC patients made a 12-month clinical visit (P = .02) in PP analysis.
For adherence, ITT analysis detected no difference between trial arms. In PP analysis, a marginally higher proportion of POC patients had month 6–12 adherence of 95% or greater (5.2% difference) compared with SOC patients, although this was not significant. No difference was detected between arms in LTFU from ART.
Predictors of Documented Viral Suppression
After adjusting for demographic and clinical characteristics, those with POC trial assignment (odds ratio [OR]: 1.95), age 18 years and older (OR: 3.86–6.36), professional/managerial occupation (OR: 4.85), and 80% or greater adherence (OR: 4.03–10.52) had significantly higher odds of documented VS compared with their reference groups (Table 4) in PP analysis. Patients with baseline VL of 100 000 copies/mL or more versus less than 100 000 copies/mL had lower odds (OR: .58) of VS.
Table 4.
Predictors of Documented Viral Suppression <1000 Copies/mLat 12 Months After ART Initiation in Per-Protocol Bivariate and Multiple Logistic Regression Analysis
| Bivariate Analysis |
Logistic Regression Analysis |
||||||
|---|---|---|---|---|---|---|---|
| VL ≥1000 Copies/mL or Missing, (%)a | VL <1000 n copies/mL, n (%) | Chi-square P Value | Unadjusted OR (95% CI)b | Chi-square P Value | Adjusted OR (95% CI)c | Chi-square P Value | |
|
| |||||||
| Trial assignment | |||||||
| SOC | 71 (34.1) | 137 (65.9) | .012 | Ref | Ref | ||
| POC | 47 (22.9) | 158 (77.1) | 1.74 (.98–3.09) | .058 | 1.95(1.19–3.19) | .008 | |
| Site | |||||||
| JUTH | 75 (30.0) | 175 (70.0) | .426 | Ref | … | ||
| CHCZ | 43 (26.4) | 120 (73.6) | 1.2 (.77–1.86) | .426 | … | ||
| Clinic | |||||||
| Adult | 105 (27.8) | 273 (72.2) | .241 | Ref | … | ||
| Pediatric | 13 (37.1) | 22 (62.9) | .65 (.23–1.85) | .419 | … | ||
| Enrollment year | |||||||
| 2018 | 41 (22.3) | 143 (77.7) | .011 | Ref | … | ||
| 2019 | 77 (33.6) | 152 (66.4) | .57 (.24–1.35) | .200 | … | ||
| Sex | |||||||
| Male | 45 (29.4) | 108 (70.6) | .772 | Ref | … | ||
| Female | 73 (28.1) | 187 (71.9) | 1.07 (.83–1.38) | .620 | … | ||
| Age | |||||||
| <18 years | 13 (37.1) | 22 (62.9) | .060 | Ref | Ref | ||
| 18–29 years | 31 (38.3) | 50 (61.7) | .95 (.26–3.55) | .943 | 3.99 (2.54–6.27) | <.001 | |
| 30–39 years | 37 (23.3) | 122 (76.7) | 1.95 (.79–4.79) | .146 | 6.36 (5.06–8.00) | <.001 | |
| ≥40 years | 37 (26.8) | 101 (73.2) | 1.61 (.56–4.63) | .374 | 3.86 (2.38–6.27) | <.001 | |
| Education | |||||||
| None/primary | 55 (30.4) | 126 (69.6) | .282 | Ref | … | ||
| Secondary/tertiary | 50 (25.4) | 147 (74.6) | 1.28 (.72–2.27) | .393 | … | ||
| Missing | 13 (37.1) | 22 (62.9) | .74 (.18–3.10) | .679 | … | ||
| Employment | |||||||
| Student | 10(37.0) | 17 (63.0) | .096 | Ref | Ref | ||
| Unemployed/housewife | 13 (28.9) | 32 (71.1) | 1.45 (1.28–1.64) | <.001 | 2.10 (.98–4.49) | .057 | |
| Labor/service/trade | 71 (30.5) | 162 (69.5) | 1.34 (.71–2.52) | .360 | 1.61 (.54–4.77) | .392 | |
| Professional/managerial | 6 (12.2) | 43 (87.8) | 4.22 (2.73–6.51) | <.001 | 4.85 (2.67–8.82) | <.001 | |
| Missing | 18 (30.5) | 41 (69.5) | 1.34 (1.12–1.61) | .002 | 5.14 (4.79–5.52) | <.001 | |
| Marital status | |||||||
| Single/separated | 45 (30.4) | 103 (69.6) | .172 | Ref | … | ||
| Married | 48 (24.1) | 151 (75.9) | 1.37 (1.25–1.51) | <.001 | … | ||
| Widowed | 12 (38.7) | 19 (61.3) | .69 (.29–1.63) | .398 | … | ||
| Missing | 13 (37.1) | 22 (62.9) | .74 (.26–2.11) | .572 | … | ||
| WHO clinical stage | |||||||
| 1/2 | 95 (26.8) | 260 (73.2) | .103 | Ref | … | ||
| 3/4 | 19 (38.0) | 31 (62.0) | .60 (.27–1.30) | .192 | … | ||
| Missing | 4 (50.0) | 4 (50.0) | .37 (.29−.45) | <.001 | … | ||
| BMI at enrollment | |||||||
| Normal/overweight | 70 (25.7) | 202 (74.3) | .208 | Ref | … | ||
| Underweight | 27 (34.2) | 52 (65.8) | .67 (.62−.72) | <.001 | … | ||
| Missing/pediatric | 21 (33.9) | 41 (66.1) | .68 (.36–1.26) | .216 | … | ||
| VL at enrollment | |||||||
| <100000 copies/mL | 52 (25.7) | 150 (74.3) | .195 | Ref | Ref | ||
| ≥100000 copies/mL | 64 (32.3) | 134 (67.7) | .73 (.68−.77) | <.001 | .58 (.55−.60) | <.001 | |
| Missing | 2 (15.4) | 11 (84.6) | 1.91 (.84–4.34) | .124 | 3.58(1.12–11.44) | .031 | |
| Last ART regimen before final study visit | |||||||
| NNRTI-based | 46 (28.9) | 113(71.1) | .690 | Ref | … | ||
| DTG-based | 65 (27.7) | 170 (72.3) | 1.06 (.59–1.93) | .836 | … | ||
| PI-based | 7 (36.8) | 12 (63.2) | .70 (.57−.85) | <.001 | … | ||
| % MPR, 0–12 months | |||||||
| <80% | 49 (61.3) | 31 (38.8) | <.001 | Ref | Ref | ||
| 80–94.9% | 33 (30.8) | 74 (69.2) | 3.54 (1.55–8.09) | .003 | 4.03 (1.50–10.81) | .006 | |
| ≥95% | 36(15.9) | 190 (84.1) | 8.34 (4.15–16.77) | <.001 | 10.52 (5.40–20.53) | <.001 | |
N = 413.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CHCZ, Comprehensive Health Centre Zamko; DTG, dolutegravir; JUTH, Jos University Teaching Hospital; MPR, medication possession ratio; NNRTI, non-nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor; POC, point-of-care; Ref, reference; SOC, standard-of-care; VL, viral load; WHO, World Health Organization.
Percentages shown are row percentages (% with/without viral suppression in each category).
For logistic regression, used the site as a cluster variable.
Did not include clinic (adult/pediatric) in multiple logistic regression model because of collinearity with age.
Acceptability of Point-of-Care Viral Load Testing
Of the 273 enrolled POC patients, 182 (66.7%) had a 12-month clinical visit and 163 (89.6%) of those completed a post-trial survey (Figure 2). Among respondents, 90.8% (148/163) did not mind waiting for results, 96.3% (156/162) believed same-day results encouraged ART adherence, 85.0% (136/160) believed the POC VL assay is reliable, and 90.2% (147/163) preferred POC over SOC testing. At CHCZ, 95.9% (71/74) of patients preferred POC VL testing versus 85.4% (76/89) at JUTH (P = .033).
Figure 2.
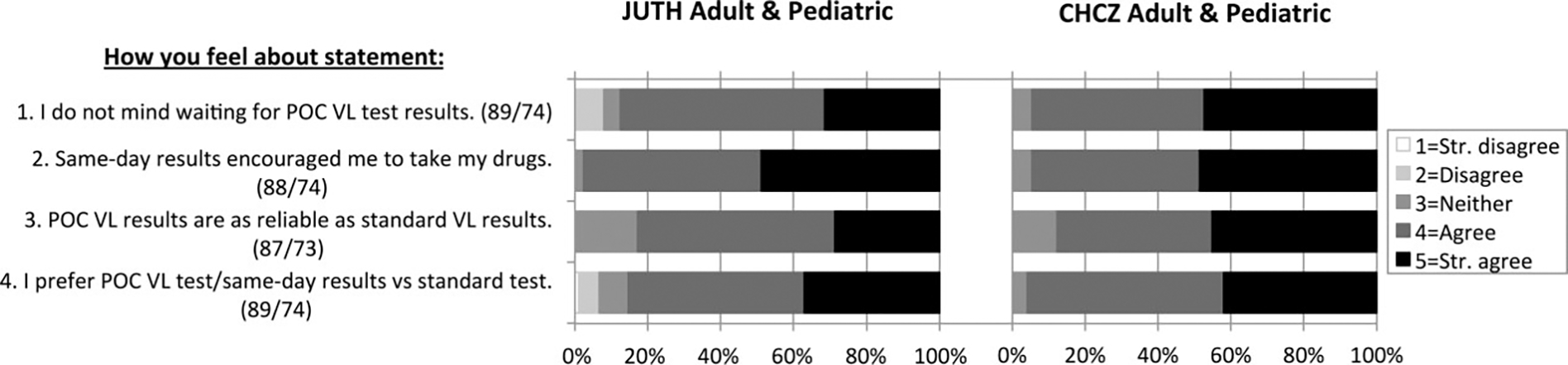
Post-trial point-of-care patient survey results. Surveys were completed by 89 adult and pediatric patients at JUTH, and 74 adult and pediatric patients at CHCZ. Values in parentheses after each survey question represent the numbers of patients who responded to the given question at JUTH and CHCZ, respectively. Abbreviations: CHCZ, Comprehensive Health Centre Zamko; JUTH, Jos University Teaching Hospital; POC, point-of-care; Str., strongly; VL, viral load.
All 15 eligible HCWs—10 JUTH and 5 CHCZ—completed the post-trial HCW survey (Figure 3). All (100%) HCWs felt confident in the Cepheid results (15/15) and thought that POC VL results arrived in patients’ EMR more quickly (15/15), helped patient care (15/15), and aided in timely treatment decisions (14/14). Additionally, 93.3% (14/15) of HCWs believed that POC VL benefitted patients. However, 43% (6/14) of HCWs noted Cepheid test supply problems, and 33.3% (5/15) thought POC VL required more nurse/counseling time.
Figure 3.
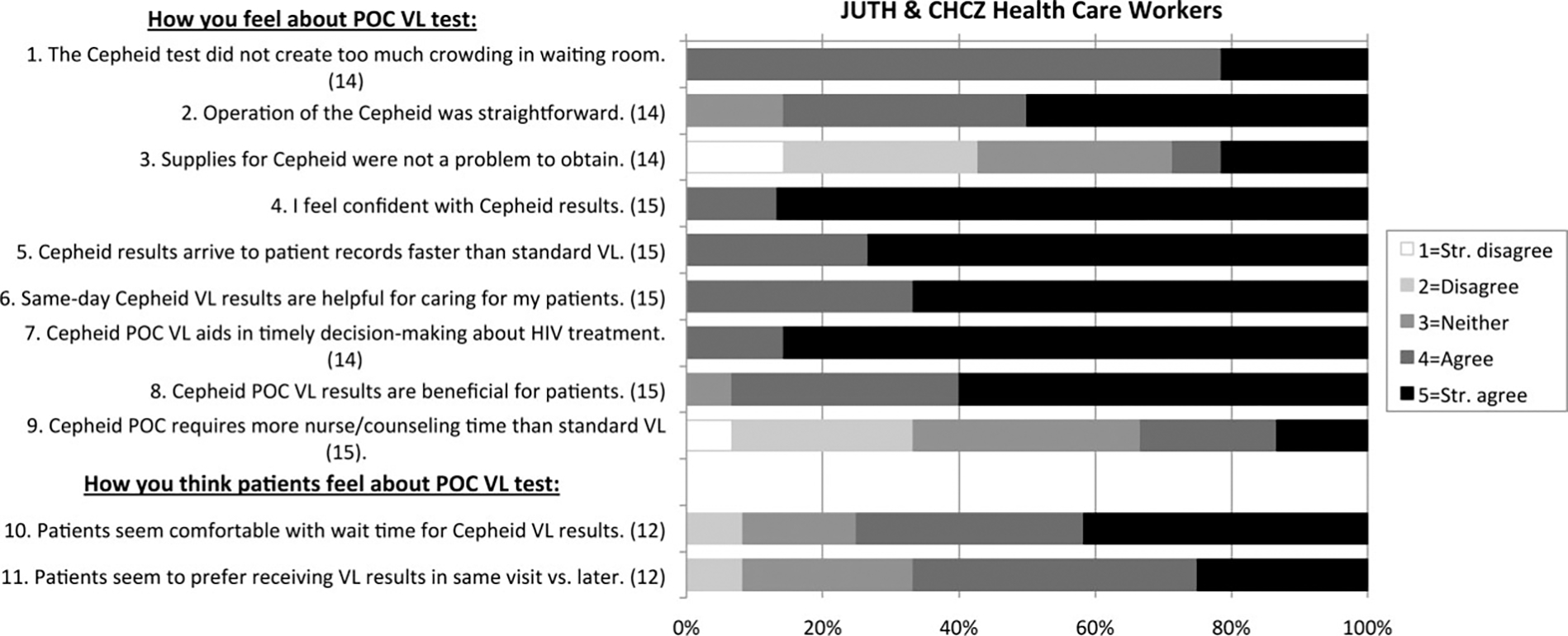
Post-trial HCW survey results. Surveys were completed by 10 HCWs at JUTH and 5 HCWs at CHCZ. Survey participants included 6 physicians, 2 nurses, 3 phlebotomists, and 4 laboratory technicians. Values in parentheses after each survey question represent the number of HCWs who responded to the given question (excluded if not applicable). Abbreviations: CHCZ, Comprehensive Health Centre Zamko; HCW, healthcare worker; JUTH, Jos University Teaching Hospital; POC, point-of-care; VL, viral load; Str., strongly.
DISCUSSION
This RCT at an urban hospital and affiliated rural facility in Nigeria showed POC VL monitoring modestly improved 12-month documented VS (11.2%) and retention (8.9%) (PP analysis). Point-of-care monitoring was preferred over SOC by the majority (90.2%) of patients and acceptable to HCWs.
Because of high patient LTFU before receiving the intervention at 6 months in both arms, inclusion of these patients in ITT analysis diluted the effect estimates. As there was no evidence of attrition bias and included patients remained comparable between trial arms, the PP analysis better estimates the actual intervention effects. We did not detect differences in VS in CC analysis, or in adherence or LTFU from ART. However, documented VS was significantly greater in the POC arm at all VL thresholds in PP analysis, and at 200 copies/mL or less in ITT analysis. The mechanisms by which POC monitoring improves documented VS in PP analysis appear to be by increasing 12-month retention and improving documentation of VL results. Point-of-care monitoring may increase retention by improving engagement in care, particularly patients who might have been LTFU before receiving their result in SOC care. Point-of-care testing may intrinsically improve documentation by reducing SOC barriers of specimen storage and transport, batched and delayed laboratory testing, and possible specimen labeling and data issues.
In addition to POC trial assignment, other predictors of documented VS included having 95% or greater adherence, age 18 years and older, and non-student status. Lower VS rates among children and adolescents versus adults have been documented [20–22]. A Haiti trial comparing POC versus SOC VL monitoring among 150 ART-experienced youth aged 10–24 years did not detect a difference in VS or adherence 6 months later [23]. Combined age-appropriate interventions for youth to address their unique social-behavioral challenges, retention and adherence issues, higher drug resistance, and caregiver burdens may be needed to observe POC benefits.
Acceptability is important in determining the utility of POC VL monitoring. Point-of-care patients spent a median of 4.5 hours (IQR: 3.3, 5.2 hours) in the clinic at their follow-up visits compared with 3.2 hours (IQR: 2.3, 4.1 hours) for SOC patients, partially accounted for by same-day counseling time. Nevertheless, 90.2% of surveyed patients preferred POC VL, and 100% of 15 HCWs believed POC VL helped patient care. The STREAM trial also found high patient and nurse acceptability [24]. The higher preference for POC VL monitoring among CHCZ (95.9%) versus JUTH (85.4%) patients might reflect longer hospital commutes in rural settings and busyness of urban professionals making them less amenable to waiting for results.
Our study had a number of limitations. First, Cepheid’s plasma separation requirement necessitated installing a safety hood in the clinics. A true POC assay utilizing whole blood may address this barrier and decrease waiting time. Second, it is possible that study team bias might have bolstered the intervention. Alternatively, HCWs needed to learn and follow nonroutine POC procedures, potentially impairing the intervention. Third, a number of unanticipated factors impacted our trial. Nigeria’s adult HIV prevalence decreased to 1.4%, as published in 2019 (previously estimated at 2.8%), impacting trial enrollment [25]. With lower enrollment, our trial was also not powered to detect differences by site, which would have provided important information comparing urban versus rural settings. In 2019, DTG, a highly effective antiretroviral with high resistance barrier, was rolled out in Nigeria, which likely affected VS at both the 6-month and 12-month time points in both trial arms. During the course of the trial, Jos was affected by HCW strikes, national protests, and, most significantly, the global coronavirus disease 2019 (COVID-19) pandemic, resulting in numerous lockdowns beginning March 2020, disrupting patient care and visits. Multi-month dispensing to ensure ART continuity altered visit scheduling and may have reduced the utility of medication possession ratio in measuring adherence. Fourth, our trial enrolled only patients initiating ART, and may not be generalizable for all patient populations and clinics. Finally, we measured 12-month VS, after having the intervention once. Multiple tests over a longer follow-up may demonstrate different effects.
Nevertheless, this pragmatic RCT contributes to the limited literature on clinical outcomes and utility of POC VL in both urban and rural RLSs. We compared a POC VL strategy aimed at utilizing the rapid results to unmodified SOC procedures without changing other aspects of care, enabling assessment of the POC intervention alone. Further, our trial design included a qualitative patient and HCW acceptability assessment to contextualize the quantitative results. Our trial found that POC monitoring significantly improved timeliness of results and time to second-line switch for virologic failure [26].
In conclusion, our trial found POC VL monitoring improved 12-month documented VS and retention in care, was feasible in urban and rural clinics, and was highly acceptable to most patients and HCWs. Further operational research could inform the characteristics of the clinics, patient populations, and implementation approaches that will determine where POC VL monitoring will most benefit patient care.
Supplementary Material
Acknowledgments.
The authors thank Laura Smeaton and Harry Reyes Nieva for their significant contributions in providing statistical review and expertise. They are also grateful to all the patients who participated in this trial to advance knowledge, and to the clinical, laboratory, and data staff in the JUTH and CHCZ APIN Public Health Initiatives and President’s Emergency Plan for AIDS Relief (PEPFAR) programs whose efforts enabled this work.
Financial support.
This work was supported by the US Centers for Disease Control and Prevention (CDC; grant number GH-16–005). Funders provided technical support in study design, oversight, monitoring of study activities and data analyses, and manuscript preparation. The JUTH and CHCZ APIN Public Health Initiatives HIV programs have been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the CDC (cooperative agreement NU2GGH002098).
Footnotes
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts the editors consider relevant to the manuscript’s content have been disclosed.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the respective funding agencies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Raffa JD, Tossonian HK, Grebely J, Petkau AJ, DeVlaming S, Conway B. Intermediate highly active antiretroviral therapy adherence thresholds and empirical models for the development of drug resistance mutations. J Acquir Immune Defic Syndr 2008; 47:397–9. [DOI] [PubMed] [Google Scholar]
- 2.D’Ettorre G, Forcina G, Ceccarelli G, et al. Adherence and genotypic drug resistance mutations in HIV-1-infected patients failing current antiretroviral therapy. J Chemother 2011; 23:24–7. [DOI] [PubMed] [Google Scholar]
- 3.Drain PK, Dorward J, Bender A, et al. Point-of-care HIV viral load testing: an essential tool for a sustainable global HIV/AIDS response. Clin Microbiol Rev 2019; 32:e00097–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook G, Stepchenkova T, Ali IM, Chipuka S, Goel N, Lee H. Study to evaluate the performance of a point-of-care whole-blood HIV viral load test (SAMBA II HIV-1 semi-Q whole blood). J Clin Microbiol 2021; 59:e02555–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyo S, Mohammed T, Wirth KE, et al. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol 2016; 54:3050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash M, Huddart S, Badar S, Baliga S, Saravu K, Pai M. Performance of the Xpert HIV-1 viral load assay: a systematic review and meta-analysis. J Clin Microbiol 2018; 56:e01673–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson D, Keiluhu AK, Kogrum S, et al. HIV-1 viral load monitoring: an opportunity to reinforce treatment adherence in a resource-limited setting in Thailand. Trans R Soc Trop Med Hyg 2009; 103:601–6. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
- 10.Nicholas S, Poulet E, Wolters L, et al. Point-of-care viral load monitoring: outcomes from a decentralized HIV programme in Malawi. J Int AIDS Soc 2019; 22:e25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kufa T, Mazanderani AH, Sherman GG, et al. Point-of-care HIV maternal viral load and early infant diagnosis testing around time of delivery at tertiary obstetric units in South Africa: a prospective study of coverage, results return and turnaround times. J Int AIDS Soc 2020; 23:e25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeke CE, Joseph J, Atem C, et al. Evaluation of near point-of-care viral load implementation in public health facilities across seven countries in sub-Saharan Africa. J Int AIDS Soc 2021; 24:e25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh P, Heller T, Chione B, et al. Near point-of-care HIV viral load: targeted testing at large facilities. J Acquir Immune Defic Syndr 2021; 86:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meggi B, Bollinger T, Zitha A, et al. Performance of a true point-of-care assay for HIV-1/2 viral load measurement at antenatal and postpartum services. J Acquir Immune Defic Syndr 2021; 87:693–9. [DOI] [PubMed] [Google Scholar]
- 15.Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV 2020; 7:e229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meloni ST, Agbaji O, Chang CA, et al. The role of point-of-care viral load monitoring in achieving the target of 90% suppression in HIV-infected patients in Nigeria: study protocol for a randomized controlled trial. BMC Infect Dis 2019; 19:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National AIDS and STI’s Control Programme Federal Ministry of Health. National guidelines for HIV prevention treatment and care. Abuja, Nigeria: Federal Ministry of Health, 2016. [Google Scholar]
- 18.Grossberg R, Gross R. Use of pharmacy refill data as a measure of antiretroviral adherence. Curr HIV/AIDS Rep 2007; 4:187–91. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiamsakul A, Kariminia A, Althoff KN, et al. HIV viral load suppression in adults and children receiving antiretroviral therapy-results from the IeDEA collaboration. J Acquir Immune Defic Syndr 2017; 76:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fokam J, Sosso SM, Yagai B, et al. Viral suppression in adults, adolescents and children receiving antiretroviral therapy in Cameroon: adolescents at high risk of virological failure in the era of “test and treat”. AIDS Res Ther 2019; 16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sovershaeva E, Shamu T, Wilsgaard T, et al. Patterns of detectable viraemia among children and adults with HIV infection taking antiretroviral therapy in Zimbabwe. Int J Infect Dis 2019; 78:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reif LK, Belizaire ME, Rouzier V, et al. Point-of-care viral load testing among adolescents and young adults living with HIV in Haiti: a randomized control trial. AIDS Care 2021; 34:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Msimango L, Gibbs A, Shozi H, et al. Acceptability of point-of-care viral load testing to facilitate differentiated care: a qualitative assessment of people living with HIV and nurses in South Africa. BMC Health Serv Res 2020; 20:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federal Ministry of Health Nigeria. Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: technical report. Abuja, Nigeria: Federal Ministry of Health, 2019. [Google Scholar]
- 26.Chaplin B, Agbaji O, Nieva HR, et al. The timeliness of point of care viral load results improves HIV monitoring in Nigeria. Clin Infect Dis 2022:ciac609. doi: 10.1093/cid/ciac609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


