Abstract
Lumbar disc herniation (LDH) is often managed surgically. Enzymatic chemonucleolysis emerged as a non-surgical alternative. This systematic review and meta-analysis aims to assess the efficacy and safety of chemonucleolytic enzymes for LDH. The primary objective is to evaluate efficacy through “treatment success” (i.e., pain reduction) and severe adverse events (SAEs) rates. Additionally, differences in efficacy and safety trends among chemonucleolytic enzymes are explored. Following our PROSPERO registered protocol (CRD42023451546) and PRISMA guidelines, a systematic search of PubMed and Web of Science databases was conducted up to July 18, 2023. Inclusion criteria involved human LDH treatment with enzymatic chemonucleolysis reagents, assessing pain alleviation, imaging changes, and reporting on SAEs, with focus on allergic reactions. Quality assessment employed the Cochrane Source of Bias and MINORS tools. Meta-analysis utilized odds ratios (OR) with 95% confidence intervals (CI). Among 62 included studies (12,368 patients), chemonucleolysis demonstrated an 79% treatment success rate and significantly outperformed placebo controls (OR 3.35, 95% CI 2.41–4.65) and scored similar to surgical interventions (OR 0.65, 95% CI 0.20–2.10). SAEs occurred in 1.4% of cases, with slightly higher rates in chymopapain cohorts. No significant differences in “proceeding to surgery” rates were observed between chemonucleolysis and control cohorts. Limitations include dated and heterogeneous studies, emphasizing the need for higher-quality trials. Further optimization through careful patient selection and advances in therapy implementation may further enhance outcomes. The observed benefits call for wider clinical exploration and adoption. No funding was received for this review.
Keywords: Lumbar disc herniation, Low back pain, Chemonucleolysis, Condoliase, Chymopapain, Collagenase
Subject terms: Outcomes research, Musculoskeletal system, Surgery
Introduction
Lumbar disc herniation (LDH) represents a pervasive spinal pathology characterized by rupture of the annulus fibrosus, resulting in the extrusion of the nucleus pulposus (NP)1. The consequent bulging or exposure of the NP tissue may exert physical compression or inflammation on adjacent nerve roots2–5, thereby inciting symptoms such as debilitating low back pain (LBP) and radiculopathy, with potential repercussions for the patient’s overall quality of life (Fig. 1A, B). The conventional management of LDH typically entails surgical interventions, often pursued following unsuccessful conservative treatments3. Nonetheless, surgical treatments for LDH may be accompanied by unsatisfactory outcomes in up to 15% of cases while exposing patients to not negligible risks, such as neurovascular injury and chronic post-operative LBP6.
Figure 1.
Representation of (A) lumbar disc herniation (LDH), (B) LDH types, and (C) representation of predicted targeted tissues for the different chemonucleolytic enzymes and their enzymatic reaction. Abbreviations: AF—annulus fibrosus, FC—facet joints, NP—nucleus pulposus, SC—spinal cord, and SN—spinal nerves.
In the historical context, chemonucleolysis emerged as a compelling alternative to surgical procedures, aiming to enzymatically degrade herniated disc material and assuage associated symptoms7. The inception of enzymatic chemonucleolysis can be attributed to chymopapain (Fig. 1C), which was introduced by Smith and Schwartz in 19647 as a less invasive technique for treating LDH. This general proteolytic enzyme demonstrated promising outcomes by mitigating complications in comparison to conventional discectomy8–10. Despite its initial success, safety concerns surfaced, encompassing adverse reactions such as anaphylaxis and nerve root disturbances11. Notwithstanding, the Food and Drug Administration (FDA) retained its approval; however, the primary manufacturer terminated its production for unspecified reasons, rendering it inactive in clinical applications12.
Contemporary research has borne witness to a potential renaissance in chemonucleolysis, with condoliase, a chondroitin sulfate ABC endolyase (also known as chondroitinase ABC), emerging as a novel and potentially more targeted alternative. Possessing substrate-specificity for chondroitin sulfate and hyaluronic acid, condoliase selectively degrades proteoglycan-rich tissues while preserving surrounding non-proteoglycan structures13,14 (Fig. 1C). Approved in Japan since 2018, condoliase has undergone extensive clinical trials affirming its safety and efficacy, positioning it as a prospective advancement in LDH treatment15,16. Alternatively, collagenase-based therapies were also explored in the 1990s but have recently garnered some new interest as a re-explored alternative LDH-targeted chemonucleolysis therapies17. The evolutionary trajectory of chemonucleolysis underscores the imperative to reassess its efficacy and safety, particularly within the realm of LDH treatment.
In navigating the comparative landscape of chemonucleolysis, this systematic review and meta-analysis endeavor to evaluate the general potential and safety of chemonucleolytic treatments as an alternative, nonsurgical LDH therapy, and specifically, to discern the clinical efficacy of distinct enzymatic products. The primary objective of this systematic review is to comprehensively investigate the outcomes and trends associated with the use of chemonucleolytic enzymes in the treatment of LDH. Specifically, we aim to analyze reported data on pain alleviation, occurrence of severe adverse events (SAE), and findings from various imaging modalities following chemonucleolytic enzyme interventions. In addition, through a careful meta-analysis, our secondary objectives encompass evaluating the overall rates of SAE occurrences, treatment success rates, and the frequency of patients opting for surgery due to unsatisfactory chemonucleolytic treatment, in comparison to sham and surgery cohorts.
Materials and methods
The study has been reported following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines18,19. The review protocol has been approved by the International Prospective Register of Systematic Reviews (PROSPERO) under the ID CRD4202345154620.
Systematic search strategy
The initial phase involved a comprehensive systematic search of the PubMed and Web of Science databases, conducted on July 18, 2023, using a predefined syntax involving intradiscal or epidural “chemonucleolysis,” inclusive of chymopapain, condoliase, and collagenase, in conjunction with human LDH. The detailed search syntax is available in Supplemental item 1. We included eligible articles that involved (i) the injection of an enzymatic chemonucleolysis reagent, (ii) targeted treatment for LDH, and (iii) included assessments of pain, disability, imaging changes, or reported on the number of SAEs and complications following injection, with a specific focus on allergic reactions. Studies including < 10 patients, reviews, meta-analyses, case reports, letters to the editor, cadaveric studies, technical notes, preclinical studies, editorials, commentaries, and articles written in languages other than English were also excluded from the analysis.
Study selection
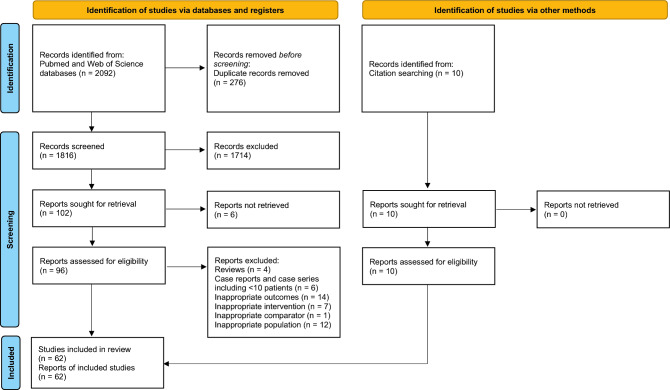
Article hits underwent automatic duplicate screening using the CADIMA software21. Subsequently, three researchers (JS, ST, and LA) independently screened the titles and abstracts of the identified articles against predetermined inclusion criteria. Articles suggested for inclusion by at least one of the three reviewers proceeded to the second round of full-text screening. In this stage, identical researchers conducted a comprehensive assessment to confirm adherence to the previously stated inclusion and exclusion criteria. Additionally, papers meeting the criteria were considered for inclusion in the meta-analysis if they incorporated a control group treated with (micro)discectomy, placebo or sham injection, or general conservative interventions. The article screening workflow has been reported in a PRISMA flow diagram (Fig. 2).
Figure 2.
Adjusted PRISMA flowchart presenting the total number of screened papers and subsequent papers included for the systematic review and meta-analysis.
Data extraction
General study characteristics extracted included: first author name, year of publication, country, funding source (if any), study design, sample size, age, sex, follow-up range (including individual timepoints, minimum, maximum, and mean, when reported), indication for chemonucleolysis, previous and concurrent treatments, technical description of the intervention (i.e., experimental product, enzyme source, injected volume and concentration, injection site, needle gauge, and number of discs injected). In comparative studies, characteristics of the control groups and respective treatments (i.e., sham injections or surgical discectomy) were recorded. Complications, success/failure rates, likelihood of undergoing further surgery, and SAEs were assessed in all included patients. Treatment success was defined as a good to excellent outcome as subjectively reported by included patients, whereas worsening or no improvement results were considered a treatment failure. An SAE was defined as a post-injection complication leading to or risking death, severely prolonging hospitalization, or causing lasting disability. Additionally, specific complications of anaphylactic shock, allergic reactions and infectious complications were separately recorded. Imaging changes (e.g., Pfirrmann classification22, T2-intensities at magnetic resonance imaging, disc height index, or LDH-volume/size) were also acquired. Data presented graphically were extracted using WebPlotDigitizer version 4.7 (https://automeris.io/WebPlotDigitizer, by A. Rohatgi) to approximate reported scores. Imaging outcomes were assessed at baseline and 3-, 6-, 12-, and 24-months post-transplantation, with average values, variability, and population size documented for each time point.
Risk of bias
The Cochrane Source of Bias tool23 was employed to assess the quality of randomized controlled trials, while the Methodological Index for Non-Randomized Studies (MINORS) scheme24 was utilized to assess the risk of bias in non-randomized clinical trials. To avoid imprecision, the included papers were rated independently by one reviewer, confirmed by a second reviewer, and eventually validated by a third reviewer.
Statistical analysis
Meta-analysis was performed using odds ratios (OR) with 95% confidence intervals (Cl) to describe dichotomous variables. The level of significance (p) was set at 0.05. Heterogeneity among comparisons was calculated according to the I2 test and was rated as “low” (I2 ≤ 25%), “moderate” (I2 = 26–74%), or “high” (I2 ≥ 75%). When I2 was ≤ 50%, a fixed-effect model was employed for analysis, whereas a random effect model was used in case of statistically significant values ≥ 50%. Pooled estimates were calculated with the Mantel–Haenszel model for treatment success rate (vs. discectomy) and rates of proceeding to surgery, while inverse variance was used for treatment success rate (vs. sham), SAE rates, and all the outcomes investigated in single-arm studies included for meta-analysis. Due to the presence of < 10 articles per each investigated outcome in comparative studies, publication bias was not evaluated. Meta-analysis was performed using the Review Manager software (v. 5.4, Cochrane Collaboration, UK) and the RStudio software (version 2023.12.1 + 402, Posit Software, MA, USA) via the metaprop package. All figures were meticulously generated using GraphPad Prism v10.0.2 (GraphPad Software LLC, USA) and Adobe Illustrator version 27.8.1 (Adobe Inc., USA).
Results
Literature screening
The initial literature search yielded a total of 2092 articles, resulting in 1816 articles for screening following duplicate removal (Fig. 2). Then, 1714 studies were excluded through title and abstract screening, and 6 reports could not be found, with 96 studies eventually considered for full text screening. Out of these studies, 44 were excluded (reviews, n = 4; case reports and case series including < 10 patients, n = 6; inappropriate outcomes, n = 14; inappropriate interventions, n = 7; inappropriate comparator, n = 1; inappropriate study population, n = 12). Furthermore, 10 additional papers were identified through hand citation searching and screened. Finally, 62 papers met the inclusion criteria (Table 1).
Table 1.
Tabular overview of all included articles and their trial design sorted on the enzyme type being examined.
| Article | Ref | Country | Trial type | FUmax (M) | Product | Funding | Patient (n) | Avg. Age (y) | Sex (M/F) | Control group (s) | Patient (n) | Avg. age (y) | Sex (M/F) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smith (1967) | 52 | USA | Retrospective | 3 | Chymopapain | ns | 75 | ns | ns | – | – | – | – |
| Nordby (1972) | 53 | USA | Retrospective | 12 | Chymopapain | ns | 100 | 44 | 64/36 | Surgery | 91 | 43 | 67/24 |
| Schwetschenau (1976) | 35 | USA | RCT | 11 | Chymopapain | ns | 31 | 38 | 19/12 | Placebo | 35 | 35 | 10/25 |
| Maroon (1976) | 36 | USA | Prospective | 14 | Chymopapain | None | 48 | ns | ns | – | – | – | – |
| Ravichandran (1980) | 37 | UK | Prospective | 9 | Chymopapain | ns | 26 | 39 | 19/7 | – | – | – | – |
| Javid (1983) | 25 | USA | RCT | 6 | Chymopapain | ns | 55 | 38 | 35/20 | Placebo | 53 | 40 | 28/25 |
| Hall (1983) | 54 | Canada | Retrospective | ns | Chymopapain | ns | 4282 | ns | ns | – | – | – | – |
| Ejeskar (1983) | 26 | Sweden | RCT | 12 | Chymopapain | Materials provided by manufacturer | 15 | 37 | 11/4 | Surgery | 14 | 42 | 11/3 |
| Parkinson (1983) | 55 | Canada | Retrospective | 6 | Chymopapain | None | 33 | ns | ns | – | – | – | – |
| Sutton (1985a) | 56 | Canada | Retrospective | 56 | Chymopapain | ns | 33 | ns | 23/10 | – | – | – | – |
| Sutton (1985b) | 57 | Canada | Retrospective | 134 | Chymopapain | ns | 189 | 39 | 107/82 | – | – | – | – |
| Dabezies (1985) | 58 | USA | Retrospective | 144 | Chymopapain | ns | 244 | ns | ns | – | – | – | – |
| Jabaay (1985) | 38 | USA | Prospective | 120 | Chymopapain | ns | 265 | 40 | 146/119 | – | – | – | – |
| McDermott (1985) | 39 | USA | Prospective | 6 | Chymopapain | ns | 1498 | 39 | 996/502 | – | – | – | – |
| Javid (1985) | 59 | USA | Retrospective | 144 | Chymopapain | ns | 105 | ns | 69/36 | – | – | – | – |
| Lorenz (1985) | 60 | Canada | Retrospective | ns | Chymopapain | ns | 55 | ns | 35/20 | – | – | – | – |
| Nordby (1986) | 82 | USA | Cross–sectional | 156 | Chymopapain | ns | 785 | ns | ns | – | – | – | – |
| Maciunas (1986) | 40 | USA | Prospective | 120 | Chymopapain | ns | 268 | 39 | 178/90 | – | – | – | – |
| Hill (1986) | 61 | USA | Retrospective | 2 | Chymopapain | ns | 335 | 44 | ns | – | – | – | – |
| Dabezies (1987) | 27 | USA | RCT | 6 | Chymopapain | ns | 78 | 37 | 57/25 | Placebo | 81 | 39 | 50/32 |
| Shields (1987) | 41 | USA | Prospective | 70 | Chymopapain | ns | 150 | 43 | 78/72 | – | – | – | – |
| Zeiger (1987) | 62 | USA | Retrospective | 46 | Chymopapain | ns | 45 | ns | ns | Surgery | 81 | ns | ns |
| Hofstra (1989) | 63 | Netherlands | Retrospective | 80 | Chymopapain | None | 16 | 43 | 11/5 | – | – | – | – |
| Alexander (1989) | 64 | USA | Retrospective | 35 | Chymopapain | ns | 51 | 33 | 46/5 | Surgery | 49 | 34 | 44/5 |
| Brown (1989) | 50 | USA | Prospective | 3 | Chymopapain * | ns | 51 | 38 | 37/14 | Surgery | 19 | 39 | 12/7 |
| Boccanera (1990) | 43 | Italy | Prospective | 36 | Chymopapain | ns | 60 | 34 | 40/20 | – | – | – | – |
| Gogan (1991) | 28 | USA | RCT | 120 | Chymopapain | None | 30 | 37 | 15/15 | Placebo | 30 | 37 | 24/6 |
| LeBlanc (1991) | 65,101 | Canada | Retrospective | 168 | Chymopapain | None | 50 | ns | ns | – | – | – | – |
| Javid (1992) | 44 | USA | Prospective | 48 | Chymopapain | ns | 106 | ns | 71/35 | Surgery | 72 | 41 | 42/30 |
| Kato (1992) | 45,66 | Japan | Prospective | 24 | Chymopapain | None | 26 | 28 | 22/4 | – | – | – | – |
| Benoist (1993) | 31 | France | RCT | 12 | Chymopapain (Low dose) | ns | 58 | 41 | 36/22 | – | – | – | – |
| 12 | Chymopapain (Standard dose) | ns | 60 | 38 | 44/16 | – | – | – | – | ||||
| Kato (1993) | 66 | Japan | Retrospective | 60 | Chymopapain | ns | 28 | 28 | 24/4 | – | – | – | – |
| Leonardi (1993) | 84 | Italy | Unclear | ns | Chymopapain | ns | 733 | ns | ns | – | – | – | – |
| Benoist (1993) | 46 | France | Prospective | 96 | Chymopapain | ns | 42 | 67 | 25/17 | – | – | – | – |
| Louwaege (1996) | 83 | Belgium | Case series | 3 | Chymopapain | None | 84 | ns | 46/38 | – | – | – | – |
| Leivseth (1999) | 67 | Norway | Retrospective | 81 | Chymopapain | ns | 51 | ns | ns | – | – | – | – |
| Wittenberg (2001) | 32 | Germany | RCT | 60 | Chymopapain * | ns | 50 | 36 | 32/18 | – | – | – | – |
| Wardlaw (2013a) | 29,30 | UK | RCT | 324 | Chymopapain | ns | 48 | ns | ns | Surgery | 52 | ns | ns |
| Wardlaw (2013b) | 30 | UK | RCT | 324 | Chymopapain | ns | 48 | ns | 27/21 | Surgery | 52 | ns | 33/19 |
| Chymopapain (total) | 10,307 | – | 2313/1269 | – | – | – | – | ||||||
| Matsuyama (2018) | 33 | Japan | RCT | 12 | Condoliase (Low dose) | Seikagaku Corp | 49 | 42 | 38/11 | Placebo | 47 | 34 | 31/16 |
| 12 | Condoliase (Medium dose) | Seikagaku Corp | 49 | 38 | 33/16 | Placebo | 47 | 34 | 31/16 | ||||
| 12 | Condoliase (High dose) | Seikagaku Corp | 49 | 36 | 34/15 | Placebo | 47 | 34 | 31/16 | ||||
| Chiba (2018) | 34 | Japan | RCT | 12 | Condoliase | Seikagaku Corp | 82 | 40 | 51/31 | Placebo | 81 | 39 | 48/33 |
| Ishibashi (2020) | 68 | Japan | Retrospective | 3 | Condoliase | Iwai Medical Foundation | 34 | 32 | 24/10 | – | – | – | – |
| Nakajima (2020) | 69 | Japan | Retrospective | 3 | Condoliase | ns | 42 | 46 | 29/13 | – | – | – | – |
| Okada (2020) | 15 | Japan | Retrospective | 12 | Condoliase | ns | 82 | 47 | 55/27 | – | – | – | – |
| Inoue (2021) | 47 | Japan | Prospective | 6 | Condoliase | None | 84 | 44 | 52/32 | – | – | – | – |
| Banno (2021) | 51 | Japan | Prospective | 3 | Condoliase | ns | 47 | 48 | 27/20 | – | – | – | – |
| Oshita (2022) | 16 | Japan | Retrospective | 3 | Condoliase | None | 71 | ns | 38/33 | – | – | – | – |
| Takeuchi (2022) | 71,102 | Japan | Retrospective | 3 | Condoliase | None | 101 | 53 | 80/21 | – | – | – | – |
| Kobayashi (2022) | 72 | Japan | Retrospective | 3 | Condoliase | Instituional sources | 127 | 47 | 88/39 | – | – | – | – |
| Hirai (2022) | 73 | Japan | Retrospective | 6 | Condoliase | None | 52 | 45 | 35/17 | – | – | – | – |
| Banno (2022) | 74 | Japan | Retrospective | 12 | Condoliase | ns | 60 | 45 | 37/23 | – | – | – | – |
| Matsuyama (2023) | 75 | Japan | Retrospective | 84 | Condoliase | Seikagaku Corp | 109 | 41 | 69/40 | Placebo | 70 | 40 | 37/33 |
| Banno (2023) | 76 | Japan | Retrospective | 33 | Condoliase | ns | 67 | 47 | 44/23 | – | – | – | – |
| Kagami (2023) | 77 | Japan | Retrospective | 12 | Condoliase (Lateral LDH) | None | 24 | 64 | 9/15 | – | – | – | – |
| 12 | Condoliase (Medial LDH) | None | 133 | 51 | 61/72 | – | – | – | – | ||||
| Ohtonari (2023) | 78 | Japan | Retrospective | 3 | Condoliase | ns | 47 | 40 | 31/16 | – | – | – | – |
| Kobayashi (2023) | 79 | Japan | Retrospective | 6 | Condoliase | None | 26 | 21 | 19/7 | – | – | – | – |
| Condoliase (total) | 1,417 | – | 909/508 | – | – | – | – | ||||||
| Sussman (1981) | 48 | USA | Prospective | 24 | Collagenase | ns | 82 | 37 | 54/28 | – | – | – | – |
| Bromley (1982) | 80 | USA | Retrospective | 11 | Collagenase | ns | 29 | 37 | 19/10 | – | – | – | – |
| Bromley (1983) | 49 | USA | Prospective | 30 | Collagenase | ns | 52 | 35 | 38/14 | – | – | – | – |
| Brown (1985) | 42 | USA | Prospective | 38 | Collagenase | ns | 54 | 36 | 36/18 | – | – | – | – |
| Brown (1989) | 50 | USA | Prospective | 3 | Collagenase* | ns | 15 | 35 | 10/5 | Surgery | 19 | 39 | 12/7 |
| Zhang (2015) | 81 | China | Retrospective | 3 | Collagenase | ns | 236 | 37 | 144/92 | – | – | – | – |
| Wang (2021) | 17 | China | Retrospective | 120 | Collagenase | National Natural Science Foundation of China | 126 | 44 | 84/42 | – | – | – | – |
| Wittenberg (2001) | 32 | Germany | RCT | 60 | Collagenase* | ns | 50 | 38 | 33/17 | – | 50 | 36 | 32/18 |
| Collagenase (total) | 644 | – | 418/226 | – | – | – | – | ||||||
| Chemonuclease (total) | 12,368 | 40.7 | 3640/2003 | – | – | – | – | ||||||
| Surgery (total) | 430 | – | 209/88 | ||||||||||
| Placebo (total) | 397 | – | 228/170 | ||||||||||
| Combined (total) | 827 | 38.6 | 437/258 | ||||||||||
Cohort summaries (cumulative totals) are in bold.
*Study involving a group of collagenase and a group of chymopapain injections.
Abbreviations: Avg.—Average, Corp—Corporation, FUmax—Maximum follow up time (indicated in months), ns—Not specified, RCT—Randomized controlled trial.
Study characteristics
Included studies consisted of 11 RCTs25–35, 16 prospective studies36–51, 32 retrospective studies15,17,52–81, 1 cross-sectional study82, 1 case series83, and 1 study with an unclear design84 (Table 1, Fig. 2, supplemental item 2). These reports were published between 196752 and 2023 from the USA25,27,28,35,36,38–42,44,48–50,52,53,58,61,62,64,80,82, UK29,30,37, Canada54–57,60,65, Sweden26, Netherlands63, Italy43,84, Japan15,33,34,45,47,51,66,68–79, France31,46, Belgium83, Norway67, Germany32, and China17,81. Within this set of articles, 39 (56.2%)25–32,35–41,43–46,50,52–67,82–84 involved the injection of chymopapain, 17 (26.6%)15,33,34,47,51,68–78,85 involved condoliase, and 8 (17.2%)17,32,42,48–50,80,81 involved collagenase (Table 1, supplemental item 3). Notably, the chymopapain studies were predominantly conducted in North America and Europe before 2000, whereas condoliase and collagenase studies were primarily performed in Asia, with condoliase being carried out mostly after 2015. A total of 12,368 patients with a mean age of 40.7 years were treated with chemonucleolysis. In comparative studies, the control groups consisted of 827 patients with a mean age of 38.6 years treated with either placebo, surgery, or another chemonucleolytic agent. Last follow-up ranged from a minimum of 261 to 324 months29,30. All included patients were diagnosed single- or multilevel LDH through a combination of clinical and imaging investigations and underwent chemonucleolysis at either one or multiple disc levels (Supplemental items 2 and 4).
In chymopapain studies, the active compound was administered under the commercial names Discase (Baxter-Travenor Laboratories Inc., USA; Boots Pharmaceuticals, UK), Chymodiactin (Smith Laboratories Inc., USA; Flint Laboratories/Boots Co., USA; Boots Pharmaceuticals) when disclosed (Supplemental item 3). Injected volumes and concentrations ranged between 1.0–2.5 mL and 1000–2000 UI/mL respectively. Concentration was also reported as 4 mg/mL or 2000 pkat/mL in some studies. The final product consisted of 2000–4000 UI, 4–8 mg, or 3000–4000 pkat injected intradiscally using needles sized between 18 and 27G in most studies (Table 3). In condoliase studies, the active compound was purchased from Seigaku Corp. (Japan) under the commercial name Hernicore in the majority of studies. Injected volumes and concentrations varied between 1.0–1.2 mL and 1.0–5.0 U/mL, totaling 1.0–5.0 U eventually administered. The drug was delivered intradiscally using 21–23G needles. In collagenase studies, the drug was purchased from Advance Biofactures Corp. (USA) under the name of Nucleolysin or from Liaoning Wei Bang (China, commercial name not reported). Administered volumes and concentrations varied between 0.1–1.0 mL and 125.0–2000.0 U/mL, resulting in 5.0–600.0 U injected intradiscally with 18–22G needles.
Table 3.
Tabular overview of reported adverse events and the rate of patients proceeding to surgery.
| Article | Refs | Patient with SAE (n/N [%]) | Infectious SAE (n/N [%]) | Allergy prevention | Allergic reaction (n/N [%]) | Anaphylactic shock (n/N [%]) | Control number of SAE (n/N [%]) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Chymopapain | Smith (1967) | 52 | 3/75 [4%] | 0/150 [0%] | ns | ns | 1/75 [1%] | – | ||
| Nordby (1972) | 53 | 0/100 [0%] | 1/45 [2%] | ns | ns | ns | 3/91 [3%] | |||
| Schwetschenau (1976) | 35 | 0/31 [0%] | 0/16 [0%] | H1 blocker, corticosteroid, antimuscarinic | 0/31 [0%] | 0/31 [0%] | 0/35 [0%] | |||
| Maroon (1976) | 36 | 4/48 [8%] | 2/51 [4%] | Corticosteroids | 2/48 [4%] | 0/48 [0%] | – | |||
| Ravichandran (1980) | 37 | 0/26 [0%] | ns | ns | 2/26 [8%] | 0/26 [0%] | – | |||
| Javid (1983) | 25 | 0/55 [0%] | 0/60 [0%] | ns | 0/55 [0%] | 0/55 [0%] | 1/53 [2%] | |||
| Hall (1983) | 54 | 15/4282 [0%] | 1/30 [3%] | ns | ns | 15/4282 [0%] | – | |||
| Ejeskar (1983) | 26 | ns | ns | ns | 0/15 [0%] | 0/15 [0%] | ns | |||
| Parkinson (1983) | 55 | 0/33 [0%] | 0/106 [0%] | None | 0/33 [0%] | 0/33 [0%] | – | |||
| Sutton (1985a) | 56 | 3/33 [9%] | ns | H1/H2 blockers | 2/33 [6%] | 1/33 [3%] | – | |||
| Sutton (1985b) | 57 | 17/189 [9%] | 0/58 [0%] | ns | 3/189 [2%] | 1/189 [1%] | – | |||
| Dabezies (1985) | 58 | ns | 0/60 [0%] | ns | ns | ns | – | |||
| Jabaay (1985) | 38 | ns | ns | ns | ns | ns | – | |||
| McDermott (1985) | 39 | 73/1498 [5%] | 1/733 [0%] | H1/H2 blockers | 55/1498 [4%] | 14/1498 [1%] | – | |||
| Javid (1985) | 59 | ns | 0/42 [0%] | ns | ns | ns | – | |||
| Lorenz (1985) | 60 | 3/55 [5%] | 1/84 [1%] | ns | 1/55 [2%] | 1/55 [2%] | – | |||
| Nordby (1986) | 82 | 3/748 [0%] | ns | ns | ns | 2/748 [0%] | – | |||
| Maciunas (1986) | 103 | 0/268 [0%] | 0/50 [0%] | ns | 0/268 [0%] | 0/268 [0%] | – | |||
| Hill (1986) | 61 | 2/335 [1%] | ns | H1/H2 blockers | ns | 1/335 [0%] | – | |||
| Dabezies (1987) | 27 | 1/78 [1%] | 0/48 [0%] | ns | 2/78 [3%] | 1/78 [1%] | 0/81 [0%] | |||
| Shields (1987) | 41 | 1/150 [1%] | 0/150 [0%] | H1/H2 blockers, corticosteroids (partly) | 5/150 [3%] | 0/150 [0%] | – | |||
| Zeiger (1987) | 62 | 1/45 [2%] | 1/45 [2%] | ns | 0/45 [0%] | 0/45 [0%] | 1/81 [1%] | |||
| Hofstra (1989) | 63 | 0/16 [0%] | 0/16 [0%] | Corticosteroid | 1/16 [6%] | 0/16 [0%] | – | |||
| Alexander (1989) | 64 | 2/51 [4%] | 2/51 [4%] | H1/H2 blockers | 0/51 [0%] | 0/51 [0%] | 5/49 [10%] | |||
| Brown (1989) * | 50 | ns | ns | ns | ns | ns | ns | |||
| Boccanera (1990) | 43 | 15/60 [25%] | 0/60 [0%] | H1/H2 blockers | 1/60 [2%] | 0/60 [0%] | – | |||
| Gogan (1991) | 28 | 2/30 [7%] | 1/30 [3%] | ns | 0/30 [0%] | 0/30 [0%] | 6/30 [20%] | |||
| LeBlanc (1991) | 65 | 1/50 [2%] | ns | H1/H2 blockers | 1/50 [2%] | 0/50 [0%] | – | |||
| Javid (1992) | 44 | 0/106 [0%] | 0/106 [0%] | H1 blocker | 1/106 [1%] | 0/106 [0%] | 0/72 [0%] | |||
| Kato (1992) | 45 | ns | ns | ns | ns | ns | – | |||
| Benoist (1993) (L) | 31 | 1/58 [2%] | 0/58 [0%] | H1 blocker, antimuscarinic | 2/58 [3%] | 1/58 [2%] | – | |||
| Benoist (1993) (M) | 0/60 [0%] | 0/60 [0%] | H1 blocker, antimuscarinic | 1/60 [2%] | 0/60 [0%] | – | ||||
| Kato (1993) | 66 | ns | ns | ns | ns | ns | – | |||
| Leonardi (1993) | 84 | 1/733 [0%] | 1/733 [0%] | H1/H2 blockers, complement blockers | 6/733 [1%] | 0/733 [0%] | – | |||
| Benoist (1993 | 46 | 0/42 [0%] | 0/42 [0%] | ns | 0/42 [0%] | 0/42 [0%] | – | |||
| Louwaege (1996 | 83 | 1/84 [1%] | 1/84 [1%] | H1/H2 blockers , corticosteroids | 0/84 [0%] | 0/84 [0%] | – | |||
| Leivseth (1999 | 67 | ns | ns | ns | ns | ns | – | |||
| Wittenberg (2001 * | 32 | 1/50 [2%] | 0/50 [0%] | H1/H2 blockers, corticosteroids | 6/50 [12%] | 0/50 [0%] | – | |||
| Wardlaw (2013 | 29 | ns | ns | ns | ns | ns | ns | |||
| Wardlaw (2013 | 30 | 1/48 [2%] | 0/48 [0%] | ns | ns | 0/48 [0%] | 1/52 [2%] | |||
| Chymopapain (total) | 151/9437 [1.6%] | 9/4508 [0.2%] | – | 91/3864 [2.4%] | 38/9352 [0.4%] | – | ||||
| Condoliase | Matsuyama (2018) (L) | 33 | 0/49 [0%] | 0/49 [0%] | ns | 2/49 [4%] | 0/49 [0%] | 2/47 [4%] | ||
| (M) | 0/49 [0%] | 0/49 [0%] | ns | 1/49 [2%] | 0/49 [0%] | – | ||||
| (H) | 0/49 [0%] | 0/49 [0%] | ns | 1/49 [2%] | 0/49 [0%] | – | ||||
| Chiba (2018) | 34 | 4/82 [5%] | 0/82 [0%] | ns | 4/82 [5%] | 0/82 [0%] | 6/81 [7%] | |||
| Ishibashi (2020) | 68 | ns | ns | ns | ns | ns | – | |||
| Nakajima (2020) | 69 | 0/42 [0%] | 0/42 [0%] | ns | ns | 0/42 [0%] | – | |||
| Okada (2020) | 15 | 0/82 [0%] | 0/82 [0%] | ns | 3/82 [4%] | 0/82 [0%] | – | |||
| Inoue (2021) | 47 | 2/84 [2%] | 0/84 [0%] | ns | 3/84 [4%] | 0/84 [0%] | – | |||
| Banno (2021) | 51 | 0/47 [0%] | 0/47 [0%] | ns | 1/47 [2%] | 0/47 [0%] | – | |||
| Oshita (2022) | 16 | ns | ns | ns | ns | ns | – | |||
| Takeuchi (2022) | 71 | 0/101 [0%] | 0/101 [0%] | ns | 5/101 [5%] | 0/101 [0%] | – | |||
| Kobayashi (2022) | 72 | 0/127 [0%] | 0/127 [0%] | ns | 3/127 [2%] | 0/127 [0%] | – | |||
| Hirai (2022) | 73 | 0/52 [0%] | 0/52 [0%] | ns | 1/52 [2%] | 0/52 [0%] | – | |||
| Banno (2022) | 74 | 0/60 [0%] | 0/60 [0%] | ns | 4/60 [7%] | 0/60 [0%] | – | |||
| Matsuyama (2023) | 75 | ns | ns | ns | ns | ns | ns | |||
| Banno (2023) | 76 | 0/67 [0%] | 0/67 [0%] | ns | 3/57 [5%] | 0/67 [0%] | – | |||
| Kagami (2023) | 77 | ns | ns | None | 11/157 [7%] | 0/157 [0%] | – | |||
| Ohtonari (2023) | 78 | ns | ns | ns | ns | ns | – | |||
| Kobayashi (2023) | 79 | 0/26 [0%] | 0/26 [0%] | ns | 0/26 [0%] | 0/26 [0%] | – | |||
| Condoliase (total) | 6/917 [0.7%] | 0/917 [0.0%] | – | 42/1022 [4.1%] | 0/1074 [0.0%] | – | ||||
| Collagenase | Sussman (1981) | 48 | 0/29 [0%] | 0/29 [0%] | ns | 0/29 [0%] | 0/29 [0%] | – | ||
| Bromley (1982) | 80 | 0/82 [0%] | 0/82 [0%] | ns | ns | 0/82 [0%] | – | |||
| Bromley (1983) | 49 | 0/52 [0%] | ns | ns | 0/52 [0%] | 0/52 [0%] | – | |||
| Brown (1985) | 42 | 1/54 [2%] | 1/54 [2%] | H1 blocker, corticosteroid | ns | 0/54 [0%] | – | |||
| Brown (1989) * | 50 | ns | ns | ns | ns | ns | ns | |||
| Zhang (2015) | 81 | 0/236 [0%] | 0/236 [0%] | ns | ns | 0/236 [0%] | – | |||
| Wang (2021) | 17 | 1/126 [1%] | 0/126 [0%] | ns | 0/126 [0%] | 0/126 [0%] | – | |||
| Wittenberg (2001) * | 32 | 0/50 [0%] | 0/50 [0%] | H1/H2 blockers, corticosteroids | 0/50 [0%] | 0/50 [0%] | – | |||
| Collagenase (total) | 2/629 [0.3%] | 1/577 [0.2%] | – | 0/257 [0.0%] | 0/629 [0.0%] | – | ||||
| Chemonucleolysis (total) | 159/10,983 [1.4%] | 10/6002 [0.2%] | – | 133/5143 [2.6%] | 38/11055[0.3%] | – | ||||
| Surgery (total) | 16/426 [3.8%] | |||||||||
| Placebo/Sham (total) | 9/246 [3.7%] | |||||||||
| Control (total) | 25/672 [3.7%] | |||||||||
Values are given as the number of events (n) within the patient population (N) and the corresponding percentage (%). Cohort summaries (cumulative totals) are in bold.
*Study involving a group of collagenase and a group of chymopapain injections.
Abbreviations: ns—not specified, SAE—Severe adverse events.
Risk of bias
The MINORS tool was employed to assess the quality of evidence of included nonrandomized clinical trials, with an average score of 8/16 for noncomparative studies and 16/24 for comparative studies, indicating a serious risk of bias. Likewise, according to the Cochrane Source of Bias tool, the risk of bias in included randomized controlled trials was also significant, with an average score of 18/26 (Supplemental item 5).
Treatment success rates
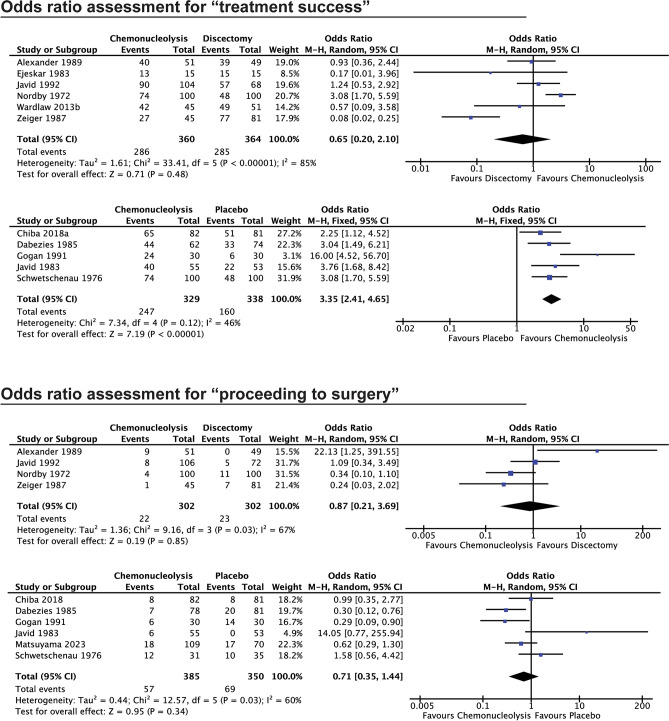
Treatment success, as defined by improvement in pain from baseline, was trackable in 56/62 reports, and 59/67 experimental cohorts (Table 2, supplemental item 6). Overall, 6030/7588 chemonucleolysis procedures were reported as successful, which equates to 79% of treated patients. Similar rates were seen for chymopapain, condoliase and collagenase specifically treated patients at 79%, 78%, and 82% respectively. Eight comparative studies26,29,42,44,50,53,62,64 compared chemonucleolysis with surgical decompression and 5 studies25,27,28,34,35 compared chemonucleolysis to placebo treatments. While no statistically significant intergroup difference was found among the former (OR: 0.65, 95% CI: 0.20–2.10, p = 0.16; Fig. 3), chemonucleolysis showed a significantly higher treatment success rate vs. placebo injections (OR: 3.55, 95% CI: 2.41–4.65, p = 0.003; Fig. 3).
Table 2.
Tabular overview of treatment success as indicated by improvement in pain outcomes. Values are given as the number of events (n) within the patient population (N) and the corresponding percentage (%).
| Article | Ref | Treatment success (n/N [%]) | Treatment failure (n/N [%]) | Proceeded to surgery (n/N [%]) | Control | Control success (n/N [%]) | Control failure (n/N [%]) | Control proceeded to surgery (n/N [%]) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymopapain | Smith (1967) | 52 | 68/75 [91%] | 7/75 [9%] | 2/75 [3%] | – | – | – | – | ||
| Nordby (1972) | 53 | 74/100 [74%] | 26/100 [26%] | 4/100 [4%] | Surgery | 48/100 [48%] | 52/100 [52%] | 11/100 [11%] | |||
| Schwetschenau (1976) | 35 | 15/31 [48%] | 16/31 [52%] | 12/31 [39%] | Placebo | 20/35 [57%] | 15/35 [43%] | 10/35 [29%] | |||
| Maroon (1976) | 36 | 39/48 [81%] | 9/48 [19%] | 9/48 [19%] | – | – | – | – | |||
| Ravichandran (1980) | 37 | 20/26 [77%] | 6/26 [23%] | 5/26 [19%] | – | – | – | – | |||
| Javid (1983) | 25 | 40/55 [73%] | 15/55 [27%] | 6/55 [11%] | Placebo | 22/53 [42%] | 31/53 [58%] | 0/53 [0%] | |||
| Hall (1983) | 54 | ns | ns | ns | – | – | – | – | |||
| Ejeskar (1983) | 26 | 13/15 [87%] | 2/15 [13%] | ns | Surgery | 15/15 [100%] | 0/15 [0%] | ns | |||
| Parkinson (1983) | 55 | 198/200 [99%] | 2/200 [1%] | ns | – | – | – | – | |||
| Sutton (1985a) | 56 | 24/33 [73%] | 9/33 [27%] | 5/33 [15%] | – | – | – | – | |||
| Sutton (1985b) | 57 | 147/189 [78%] | 42/189 [22%] | 24/189 [13%] | – | – | – | – | |||
| Dabezies (1985) | 58 | 272/382 [71%] | 110/382 [29%] | 36/244 [15%] | – | – | – | – | |||
| Jabaay (1985) | 38 | 221/263 [84%] | 42/263 [16%] | ns | – | – | – | – | |||
| McDermott (1985) | 39 | 1226/1402 [87%] | 176/1402 [13%] | 62/1498 [4%] | – | – | – | – | |||
| Javid (1985) | 59 | 85/105 [81%] | 20/105 [19%] | 17/105 [16%] | – | – | – | – | |||
| Lorenz (1985) | 60 | 44/55 [80%] | 11/55 [20%] | 11/55 [20%] | – | – | – | – | |||
| Nordby (1986) | 82 | 493/739 [67%] | 246/739 [33%] | 129/739 [17%] | – | – | – | – | |||
| Maciunas (1986) | 40 | 230/268 [86%] | 38/268 [14%] | 54/268 [20%] | – | – | – | – | |||
| Hill (1986) | 61 | 261/335 [78%] | 74/335 [22%] | 74/335 [22%] | – | – | – | – | |||
| Dabezies (1987) | 27 | 44/62 [71%] | 18/62 [29%] | 7/78 [9%] | Placebo | 33/74 [45%] | 41/74 [55%] | 20/81 [25%] | |||
| Shields (1987) | 41 | 50/126 [40%] | 76/126 [60%] | 23/126 [18%] | – | – | – | – | |||
| Zeiger (1987) | 62 | 27/45 [60%] | 18/45 [40%] | 1/45 [2%] | Surgery | 77/81 [95%] | 4/81 [5%] | 7/81 [9%] | |||
| Hofstra (1989) | 63 | 15/15 [100%] | 0/15 [0%] | 0/16 [0%] | – | – | – | – | |||
| Alexander (1989) | 64 | 40/51 [78%] | 11/51 [22%] | 9/51 [18%] | Surgery | 39/49 [80%] | 10/49 [20%] | 0/49 [0%] | |||
| Brown (1989) | 50 | 35/51 [68%] | 16/51 [32%] | 6/51 [12%] | Surgery ‹ | 18/19 [95%] | 1/19 [5%] | 0/19 [0%] | |||
| Boccanera (1990) | 43 | 54/60 [90%] | 6/60 [10%] | ns | – | – | – | – | |||
| Gogan (1991) | 28 | 24/30 [80%] | 6/30 [20%] | 6/30 [20%] | Placebo | 9/26 [35%] | 17/26 [65%] | 14/30 [47%] | |||
| LeBlanc (1991) | 65 | 37/50 [74%] # | 13/50 [26%] # | 13/50 [26%] | – | – | – | – | |||
| Javid (1992) | 44 | 90/104 [87%] | 14/104 [13%] | 8/106 [8%] | Surgery | 57/68 [84%] | 11/68 [16%] | 5/72 [7%] | |||
| Kato (1992) | 45 | ns | ns | ns | – | – | – | – | |||
| Benoist (1993) (L) | 31 | 36/41 [88%] | 5/41 [12%] | ns | – | – | – | – | |||
| Benoist (1993) (M) | 38/43 [88%] | 5/43 [12%] | ns | – | – | – | – | ||||
| Kato (1993) | 66 | 27/28 [96%] | 1/28 [4%] | 1/28 [4%] | – | – | – | – | |||
| Leonardi (1993) | 84 | 602/733 [82%] | 131/733 [18%] | ns | – | – | – | – | |||
| Benoist (1993) | 46 | 33/42 [79%] | 9/42 [21%] | 2/42 [5%] | – | – | – | – | |||
| Louwaege (1996) | 83 | 61/84 [72%] † | 23/84 [27%] † | 2/84 [2%] | – | – | – | – | |||
| Leivseth (1999) | 67 | 24/50 [48%] | 26/50 [52%] | ns | – | – | – | – | |||
| Wittenberg (2001) * | 32 | 36/50 [72%] | 14/50 [28%] | 9/50 [18%] | – | – | – | – | |||
| Wardlaw (2013a) | 29 | ns | ns | ns | – | – | – | – | |||
| Wardlaw (2013b) | 30 | 42/45 [93%] | 3/45 [7%] | 9/48 [19%] | Surgery | 49/51 [96%] | 2/51 [4%] | ns | |||
| Chymopapain (total) | 4785/6031 [79%] | 1246/6031 [21%] | 546/4606 [11.9%] | – | – | – | – | ||||
| Condoliase | Matsuyama (2018) (L) | 33 | ns | ns | ns | – | – | – | ns | ||
| Matsuyama (2018) (M) | ns | ns | ns | – | – | – | – | ||||
| Matsuyama (2018) (H) | ns | ns | ns | – | – | – | – | ||||
| Chiba (2018) | 34 | 65/82 [79%] | 17/82 [21%] | 8/81 [10%] | Placebo | 51/81 [63%] | 30/81 [37%] | 8/81 [10%] | |||
| Ishibashi (2020) | 68 | 21/34 [62%] | 13/34 [38%] | 6/34 [18%] | – | – | – | – | |||
| Nakajima (2020) | 69 | 32/42 [76%] ‡ | 10/42 [24%] ‡ | ns | – | – | – | – | |||
| Okada (2020) | 15 | 70/82 [85%] ‡ | 12/82 [15%] ‡ | 4/82 [5%] | – | – | – | – | |||
| Inoue (2021) | 47 | 65/84 [77%] ‡ | 19/84 [23%] ‡ | 11/84 [13%] | – | – | – | – | |||
| Banno (2021) | 51 | 33/46 [70%] ˆ | 14/47 [30%] ˆ | 11/47 [23%] | – | – | – | – | |||
| Oshita (2022) | 16 | 55/71 [77%] | 16/71 [23%] | ns | – | – | – | – | |||
| Takeuchi (2022) | 71 | 88/101 [87%] | 13/101 [13%] | 13/101 [13%] | – | – | – | – | |||
| Kobayashi (2022) | 72 | ns | ns | 16/127 [13%] | – | – | – | – | |||
| Hirai (2022) | 73 | 40/52 [77%] ‡ | 12/52 [23%] ‡ | 3/52 [6%] | – | – | – | – | |||
| Banno (2022) | 74 | 47/60 [78%] | 13/60 [22%] | 8/60 [13%] | – | – | – | – | |||
| Matsuyama (2023) | 75 | ns | ns | 18/109 [17%] | Placebo | ns | ns | 17/70 [24%] | |||
| Banno (2023) | 76 | 51/67 [76%] ‡ | 16/67 [24%] ‡ | 8/67 [12%] | – | – | – | – | |||
| Kagami (2023) | 77 | 121/157 [77%] ‡ | 36/157 [23%] ‡ | 29/157 [18%] | – | – | – | – | |||
| Ohtonari (2023) | 78 | 34/41 [83%] | 7/41 [17%] | ns | – | – | – | – | |||
| Kobayashi (2023) | 79 | ns | ns | ns | – | – | – | – | |||
| Condoliase (total) | 722/919 [78%] | 198/920 [22%] | 135/1001 [13.4%] | – | – | – | – | ||||
| Collagenase | Sussman (1981) | 48 | 25/29 [86%] | 4/29 [14%] | 4/29 [14%] | – | – | – | – | ||
| Bromley (1982) | 80 | 70/82 [85%] | 12/82 [15%] | 10/82 [12%] | – | – | – | – | |||
| Bromley (1983) | 49 | 40/46 [87%] | 6/46 [13%] | 6/46 [13%] | – | – | – | – | |||
| Brown (1985) | 42 | 34/54 [63%] | 20/54 [37%] | 13/54 [24%] | – | – | – | – | |||
| Brown (1989) * | 50 | 10/15 [67%] | 5/15 [33%] | 6/15 [40%] | Surgery ‹ | 18/19 [95%] | 1/19 [5%] | 0/19 [0%] | |||
| Zhang (2015) | 81 | 208/236 [88%] | 28/236 [12%] | ns | – | – | – | – | |||
| Wang (2021) | 17 | 110/126 [87%] † | 16/126 [13%] † | 9/126 [7%] | – | – | – | – | |||
| Wittenberg (2001) * | 32 | 26/50 [52%] | 24/50 [48%] | 14/50 [28%] | – | – | – | – | |||
| Collagenase (total) | 523/638 [82%] | 115/638 [18%] | 62/402 [15.4%] | – | – | – | – | ||||
| Chemonucleolysis (total) | 6030/7588 [79%] | 1559/7589 [21%] | 743/6009 [12.4%] | – | – | – | – | ||||
| Surgery (total) | 303/383 [79%] | 80/383 [21%] | 31/402 [7.7%] | ||||||||
| Placebo (total) | 135/269 [50%] | 134/269 [50%] | 61/269 [22.7%] | ||||||||
| Combined (total) | 438/652 [67%] | 214/652 [33%] | 92/671 [13.7%] | ||||||||
Cohort summaries (cumulative totals) are in bold.
*Study involving a group of collagenase and a group of chymopapain-treated patients , #self-declared improvement, †as defined by Modified MacNab criteria, ‡Based on > 50% pain score (leg or back) improvement compared to baseline, ˆBased on > 20 mm VAS change, ‹Surgery-cohort applied in both collagenase and chymopapain from Brown et al. (1989).
Abbreviations: (L)—Low dose, (M)—Medium dose, (H)—High dose, ns—not specified.
Figure 3.
Meta-analysis forest plots depicting the assessment of (in order) the rate of treatment success of chemonucleolysis treated patients compared to surgical discectomy and placebo-treated patients, followed by plots demonstrating the rate of proceeding to surgery compared to surgical discectomy and placebo-treated patients.
Risk of proceeding to surgery
From 46 articles including 6009 chemonucleolysis-treated LDH patients, 743 (12.4%) were reported to proceed to surgical alternatives after a variable period of time due to unsolved or recurring symptoms (Table 3, supplementary item 7). Rates were 11.9% and 13.4% for chymopapain and condoliase respectively, and slightly higher at 15.4% for collagenase-treated cohorts. In 6 studies42,44,50,53,62,64 comparing chemonucleolysis vs. surgical decompression, the pooled analysis of patients requiring further surgery due to failure of the index procedure did not show any significant difference (OR: 0.87, 95% CI: 0.42–7.41, p = 0.44; Fig. 3). Similar results were found following meta-analysis of the 6 studies25,27,28,34,35,75 comparing chemonucleolysis vs. placebo procedures (OR: 0.71, 95% CI: 0.35–1.44, p = 0.34; Fig. 3).
Occurrence of SAEs
The rate of SAE was reported in 48 studies and showed 159/10,983 (1.4%) treated patients with one or more SAEs (Table 3, supplemental item 8). Notably, rates were slightly higher in chymopapain cohorts (1.6%) than in condoliase- (0.7%) and collagenase- (0.3%) treated cohorts. Five included studies29,44,53,62,64 examined the rate of SAEs in patients treated with chemonucleolysis vs. surgical decompression and 6 studies25,27,28,34,35,75 compared chemonucleolysis with placebo injections. In both cases, chemonucleolysis was not associated with a significantly higher rate of SAEs compared to both surgery (OR: 0.44, 95% CI: 0.15–1.34, p = 0.15; supplemental item 9) and placebo treatments (OR: 0.49, 95% CI: 0.21–1.15, p = 0.10; supplemental item 10).
SAEs were predominantly categorized as infectious, anaphylactic, or involving a severe worsening of LBP or disc features. Notably, infectious SAEs were almost exclusively observed in chymopapain-treated cohorts, accounting for 0.2% of cases, while only a single infection was reported in collagenase-treated patients (Table 3). Similarly, the occurrence of anaphylactic shock was solely documented in chymopapain-treated cohorts, affecting 38/9352 treated patients (0.4%; Table 3, supplemental item 11).
Interestingly, the prevalence of allergic reactions varied among different chemonucleolytic agents. Condoliase-treated cohorts exhibited a slightly higher incidence of allergic reactions, such as rashes, affecting 4.1% of patients, in contrast to 2.4% in chymopapain-treated cohorts and 0.0% in collagenase-treated cohorts (Table 3, supplementary item 11). Notably, it is important to highlight the absence of detailed specifications regarding preventative allergy suppressant medication in the condoliase group, while most studies in the chymopapain cohorts clearly implemented such preventative measures.
Discussion
Chemonucleolysis has a historical context rooted in the pursuit of alternatives to conventional surgical interventions for LDH, with chymopapain being an early but subsequently discontinued option due to safety concerns11. Our findings shed light on the evolving landscape of chemonucleolysis, encompassing newer agents like condoliase and re-explored interest in collagenase-based therapies. Here our meta-analysis showed that chemonucleolysis significantly outperforms placebo in terms of treatment success, demonstrating its efficacy in alleviating LDH-associated radiculopathy and disability. The comparable rates of treatment success between chemonucleolysis and surgical intervention suggests that chemonucleolysis may be an effective standalone intervention that can be considered as an alternative to surgical interventions.
Concerns about SAEs (particularly anaphylactic shock) associated with chemonucleolysis were addressed in our analysis. While chymopapain exhibited a higher rate of severe anaphylactic reactions and infectious SAEs, condoliase and collagenase treatments demonstrated safer profiles, with no reported anaphylactic shocks and lower overall SAE rates. No prophylactic allergy treatments are provided with condoliase administration in Japan and none of the included studies provided detailed specifications regarding allergy suppressant medication. The potential benefit of such regimen is a possible avenue of research to potentially further enhance its safety profile. Condoliase, with its substrate-specificity for chondroitin sulfate and hyaluronic acid, presents a more targeted enzymatic alternative, potentially minimizing off-target effects observed with earlier chemonucleolytic agents. The exceptional specificity of condoliase for chondroitin sulfate, renders it an exceptionally selective agent within the intervertebral disc space13,14. This specificity stands in contrast to chymopapain or collagenase, as illustrated in Fig. 1C, emphasizing the expected superior safety profile associated with condoliase. Furthermore, condoliase may have additional implications, as it may simultaneously aid in nerve and spinal cord repair. Chondroitin sulfate is produced in response to damage in nerve tissue and hinders nerve growth and axon proliferation. Notably, chondroitinase ABC, a product of condoliase, has demonstrated efficacy in promoting functional recovery following spinal cord injury86. This dual capability of selectively targeting LDH-related pathology while potentially supporting neural repair underlines its potential therapeutic scope.
Despite promising observations, it is imperative to acknowledge the limitations of the current body of evidence, warranting a call for further comprehensive studies. The controlled studies included in this review, while providing valuable insights, exhibit certain drawbacks. The majority, especially those pertaining to chymopapain, are dated, with a considerable proportion conducted before the 2000s. Furthermore, a prevalent retrospective nature, relatively small cohort sizes, and high-risk of bias scores contribute to the overall limitations. Additionally, the heterogeneity in outcome measures and patient indications across studies, particularly in comparisons involving various chemonucleolytic agents, further underscores the need for caution in drawing definitive conclusions. To advance our understanding of the potential of chemonucleolytic enzymes, future research endeavors should prioritize the design and implementation of higher-quality trials. Large randomized controlled trials, characterized by rigorous methodologies and standardized reporting, hold the potential to offer more robust and generalizable insights into the efficacy and safety of these enzymatic chemonucleolytic interventions for LDH.
Moreover, further optimizations of chemonucleolysis techniques and study designs are warranted. For example, optimal patient selection criteria, considering factors like age, Pfirrmann grade22, and type of LDH have been suggested to enhance treatment outcomes13,15,16,69. Other aspects such as the type of contrast agent used, have also been shown to potentially influence enzymatic activity87. In addition, there is very little literature reporting long-term imaging evaluations of discs treated with chemonucleolysis (Supplemental item 4), and long-term clinical outcomes and more objective assessments remain largely undetermined88. Therefore, future work may consider revising the enzymatic destruction of herniated disc tissue through supplementation with regenerative treatments16,89,90 e.g., co-injection of cells91,92, biomaterials93,94, extracellular vesicles95,96, or platelet-rich plasma (PRP)97 to support the restoration of the disc over time, particularly regarding younger patients16,79. For example, previous animal studies have shown the capacity of PRP products to reverse condoliase-mediated disc deterioration98. Here, longer follow-ups are critical to fully grasp the impact of the enzymatic digestion of disc tissue on long-term spinal health16. Alternative chemonucleolysis methods, not involving enzymatic digestion, such as ethanol99 or ozone100 are also being explored and future reviews should seek to compare efficacy and safety outcomes of these approaches to enzymatic chemonucleolysis-treated LDH.
Conclusion
Our comprehensive analysis underscores the evolving landscape of chemonucleolysis as a viable non-surgical intervention for LDH. With newer agents like condoliase exhibiting enhanced safety profiles and promising efficacy, the reevaluation of chemonucleolytic enzymes offers a valuable therapeutic avenue. Further research, particularly large randomized controlled trials, is imperative to solidify the evidence base and refine the clinical application of these enzymatic interventions, fostering their broader adoption in LDH management. We advocate for wider adoption and thorough evaluation of these techniques in clinical settings, particularly in Europe and the USA, to validate and refine their potential and determine the place of chemonucleolysis in the clinical toolbox to mend LDH.
Supplementary Information
Author contributions
J. S., L. A., and S. T. contributed equally to this work. Conceptualization: J. S.; search and screening: J. S., L. A., S. T.; data curation: J. S., L. A., S. T.; methodology: J. S., L. A., S. T.; illustration: J. S.; meta-analysis: L. A.; funding acquisition: D. S.; validation: J. S., L. A., S. T., K. J., C. R. F., A. N., D. S., writing—original draft: J. S., L. A., S. T.; writing—review & editing: J. S., L. A., S. T., K. J., C. R. F., A. N., D. S. All authors have read and approved the final version of the submitted manuscript.
Data availability
All data produced as part of this review are available in the main text or as a supplementary file. Additional requests can be made to the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jordy Schol, Luca Ambrosio and Shota Tamagawa.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-62792-8.
References
- 1.Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: A review of the literature. Neurosurg. Focus. 2002;13:E1. doi: 10.3171/foc.2002.13.2.2. [DOI] [PubMed] [Google Scholar]
- 2.Ye F, Lyu FJ, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine. 2022;5:e1196. doi: 10.1002/jsp2.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haro H, et al. Japanese orthopaedic association (JOA) clinical practice guidelines on the management of lumbar disc herniation, third edition-secondary publication. J. Orthop. Sci. 2022;27:31–78. doi: 10.1016/j.jos.2021.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. J. Neurosurg. 1964;21:74–81. doi: 10.3171/jns.1964.21.1.0074. [DOI] [Google Scholar]
- 5.Zhou M, Theologis AA, O’Connell GD. Understanding the etiopathogenesis of lumbar intervertebral disc herniation: From clinical evidence to basic scientific research. JOR Spine. 2024;7:e1289. doi: 10.1002/jsp2.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad JN, Moskovich R. Lumbar disc herniations. Clin. Orthop. Relat. Res. 2006;443:183–197. doi: 10.1097/01.blo.0000198724.54891.3a. [DOI] [PubMed] [Google Scholar]
- 7.Smith L. Enzyme dissolution of the nucleus pulposus in humans. Jama. 1964;187:137–140. doi: 10.1001/jama.1964.03060150061016. [DOI] [PubMed] [Google Scholar]
- 8.Couto JM, Castilho EA, Menezes PR. Chemonucleolysis in lumbar disc herniation: A meta-analysis. Clinics (Sao Paulo) 2007;62:175–180. doi: 10.1590/s1807-59322007000200013. [DOI] [PubMed] [Google Scholar]
- 9.Simmons JW, Nordby EJ, Hadjipavlou AG. Chemonucleolysis: The state of the art. Eur. Spine J. 2001;10:192–202. doi: 10.1007/s005860000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardlaw D. Sciatica caused by disc herniation: Why is chymopapain chemonucleolysis denied to our patients? Int. J. Spine Surg. 2016;10:44. doi: 10.14444/3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordby EJ, Wright PH, Schofield SR. Safety of chemonucleolysis adverse effects reported in the United States, 1982–1991. Clin. Orthop. Relat. Res. 1993;293:122–134. doi: 10.1097/00003086-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Simmons, J. W. & Fraser, R. D. in Arthroscopic and Endoscopic Spinal Surgery (ed Parviz Kambin) Ch. 18, 351–358 (Humana Press, 2005).
- 13.Minamisawa, Y., Shirogane, T., Watanabe, I. & Dezawa, A. Histological analysis of nucleus pulposus tissue from patients with lumbar disc herniation after condoliase administration. JOR Spine7(2), e1328. https://onlinelibrary.wiley.com/doi/full/10.1002/jsp2.1328 (2024). [DOI] [PMC free article] [PubMed]
- 14.Hamai A, et al. Two distinct chondroitin sulfate ABC lyases. An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J. Biol. Chem. 1997;272:9123–9130. doi: 10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- 15.Okada E, et al. The effectiveness of chemonucleolysis with condoliase for treatment of painful lumbar disc herniation. J. Orthop. Sci. 2021;26:548–554. doi: 10.1016/j.jos.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Oshita Y, et al. Multicenter retrospective analysis of intradiscal condoliase injection therapy for lumbar disc herniation. Medicina (Kaunas) 2022;58:1284. doi: 10.3390/medicina58091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, et al. Low-dose collagenase chemonucleolysis combined with radiofrequency in the treatment of lumbar disc herniation: A 10-year retrospective study. Evid. Based Complement. Alternat. Med. 2021;2021:8234558. doi: 10.1155/2021/8234558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schol, J., Ambrosio, L. & Tamagawa, S. Systematic review on the efficacy, safety, and development of chemonucleolysis for intervertebral disc herniation. PROSPERO, CRD42023451546 (2023).
- 21.Kohl C, et al. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: A case study on CADIMA and review of existing tools. Environ. Evid. 2018;7:8. doi: 10.1186/s13750-018-0115-5. [DOI] [Google Scholar]
- 22.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Furlan AD, et al. 2015 updated method guideline for systematic reviews in the cochrane back and neck group. Spine (Phila Pa 1976) 2015;40:1660–1673. doi: 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 24.Slim K, et al. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 25.Javid MJ, et al. Safety and efficacy of chymopapain (Chymodiactin) in herniated nucleus pulposus with sciatica. Results of a randomized, double-blind study. Jama. 1983;249:2489–2494. doi: 10.1001/jama.1983.03330420035030. [DOI] [PubMed] [Google Scholar]
- 26.Ejeskar A, et al. Surgery versus chemonucleolysis for herniated lumbar discs. A prospective study with random assignment. Clin. Orthop. Relat. Res. 1983;174:236–242. [PubMed] [Google Scholar]
- 27.Dabezies EJ, Langford K, Morris J, Shields CB, Wilkinson HA. Safety and efficacy of chymopapain (Discase) in the treatment of sciatica due to a herniated nucleus pulposus. Results of a randomized, double-blind study. Spine (Phila Pa 1976) 1988;13:561–565. doi: 10.1097/00007632-198805000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Gogan WJ, Fraser RD. Chymopapain. A 10-year, double-blind study. Spine (Phila Pa 1976) 1992;17:388–394. doi: 10.1097/00007632-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Wardlaw D, Rithchie IK, Sabboubeh AF, Vavdha M, Eastmond CJ. Prospective randomized trial of chemonucleolysis compared with surgery for soft disc herniation with 1-year, intermediate, and long-term outcome: Part I: The clinical outcome. Spine (Phila Pa 1976) 2013;38:E1051–E1057. doi: 10.1097/BRS.0b013e31829729b3. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw D, et al. Prospective randomized trial of chemonucleolysis compared with surgery for soft disc herniation with 1-year, intermediate, and long-term outcome: Part II: The radiological outcome. Spine (Phila Pa 1976) 2013;38:E1058–E1064. doi: 10.1097/BRS.0b013e3182996301. [DOI] [PubMed] [Google Scholar]
- 31.Benoist M, et al. A randomized, double-blind study to compare low-dose with standard-dose chymopapain in the treatment of herniated lumbar intervertebral discs. Spine (Phila Pa 1976) 1993;18:28–34. doi: 10.1097/00007632-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Wittenberg RH, Oppel S, Rubenthaler FA, Steffen R. Five-year results from chemonucleolysis with chymopapain or collagenase: A prospective randomized study. Spine (Phila Pa 1976) 2001;26:1835–1841. doi: 10.1097/00007632-200109010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama Y, Chiba K, Iwata H, Seo T, Toyama Y. A multicenter, randomized, double-blind, dose-finding study of condoliase in patients with lumbar disc herniation. J. Neurosurg. Spine. 2018;28:499–511. doi: 10.3171/2017.7.SPINE161327. [DOI] [PubMed] [Google Scholar]
- 34.Chiba K, Matsuyama Y, Seo T, Toyama Y. Condoliase for the treatment of lumbar disc herniation: A randomized controlled trial. Spine (Phila Pa 1976) 2018;43:E869–E876. doi: 10.1097/BRS.0000000000002528. [DOI] [PubMed] [Google Scholar]
- 35.Schwetschenau PR, Ramirez A, Johnston J, Wiggs C, Martins AN. Double-blind evaluation of intradiscal chymopapain for herniated lumbar discs. Early results. J. Neurosurg. 1976;45:622–627. doi: 10.3171/jns.1976.45.6.0622. [DOI] [PubMed] [Google Scholar]
- 36.Maroon JC, Holst RA, Osgood CP. Chymopapain in the treatment of ruptured lumbar discs. Preliminary experience in 48 patients. J. Neurol. Neurosurg. Psychiatry. 1976;39:508–513. doi: 10.1136/jnnp.39.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravichandran G, Mulholland RC. Chymopapain chemonucleolysis. A preliminary report. Spine (Phila Pa 1976) 1980;5:380–384. doi: 10.1097/00007632-198007000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Jabaay GA. Chemonucleolysis. Eight- to ten-year follow-up evaluation. Clin. Orthop. Relat. Res. 1986;206:24–31. doi: 10.1097/00003086-198605000-00006. [DOI] [PubMed] [Google Scholar]
- 39.McDermott DJ, et al. Chymodiactin in patients with herniated lumbar intervertebral disc(s). An open-label, multicenter study. Spine (Phila Pa 1976) 1985;10:242–249. doi: 10.1097/00007632-198504000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Maciunas RJ, Onofrio BM. The long-term results of chymopapain chemonucleolysis for lumbar disc disease. Ten-year follow-up results in 268 patients injected at the Mayo Clinic. J. Neurosurg. 1986;65:1–8. doi: 10.3171/jns.1986.65.1.0001. [DOI] [PubMed] [Google Scholar]
- 41.Shields CB, Reiss SJ, Garretson HD. Chemonucleolysis with chymopapain: Results in 150 patients. J. Neurosurg. 1987;67:187–191. doi: 10.3171/jns.1987.67.2.0187. [DOI] [PubMed] [Google Scholar]
- 42.Brown MD, Tompkins JS. Chemonucleolysis (discolysis) with collagenase. Spine (Phila Pa 1976) 1986;11:123–130. doi: 10.1097/00007632-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Boccanera L, Laus M. Chemonucleolysis: Advantages and disadvantages. Chir. Organi. Mov. 1990;75:25–32. [PubMed] [Google Scholar]
- 44.Javid MJ. A 1- to 4-year follow-up review of treatment of sciatica using chemonucleolysis or laminectomy. J. Neurosurg. 1992;76:184–190. doi: 10.3171/jns.1992.76.2.0184. [DOI] [PubMed] [Google Scholar]
- 45.Kato F, Mimatsu K, Kawakami N, Miura T. Changes seen on magnetic resonance imaging in the intervertebral disc space after chemonucleolysis: A hypothesis concerning regeneration of the disc after chemonucleolysis. Neuroradiology. 1992;34:267–270. doi: 10.1007/BF00588178. [DOI] [PubMed] [Google Scholar]
- 46.Benoist M, Parent H, Nizard M, Lassale B, Deburge A. Lumbar discal herniation in the elderly: Long-term results of chymopapain chemonucleolysis. Eur. Spine J. 1993;2:149–152. doi: 10.1007/BF00301413. [DOI] [PubMed] [Google Scholar]
- 47.Inoue M, et al. Efficacy and safety of condoliase disc administration as a new treatment for lumbar disc herniation. Spine Surg. Relat. Res. 2022;6:31–37. doi: 10.22603/ssrr.2021-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussman BJ, Bromley JW, Gomez JC. Injection of collagenase in the treatment of herniated lumbar disk. Initial clinical report. Jama. 1981;245:730–732. doi: 10.1001/jama.1981.03310320052026. [DOI] [PubMed] [Google Scholar]
- 49.Bromley JW, et al. Double-blind evaluation of collagenase injections for herniated lumbar discs. Spine (Phila Pa 1976) 1984;9:486–488. doi: 10.1097/00007632-198407000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Brown MD, Tompkins JS. Pain response post-chemonucleolysis or disc excision. Spine (Phila Pa 1976) 1989;14:321–326. doi: 10.1097/00007632-198903000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Banno T, et al. Clinical outcome of condoliase injection treatment for lumbar disc herniation: Indications for condoliase therapy. J. Orthop. Sci. 2021;26:79–85. doi: 10.1016/j.jos.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Smith L, Brown JE. Treatment of lumbar intervertebral disc lesions by direct injection of chymopapain. J. Bone Joint Surg. Br. Vol. 1967;49:502–519. doi: 10.1302/0301-620X.49B3.502. [DOI] [PubMed] [Google Scholar]
- 53.Nordby EJ, Lucas GL. A comparative analysis of lumbar disk disease treated by laminectomy or chemonucleolysis. Clin. Orthop. Relat. Res. 1973;90:119–129 . [PubMed] [Google Scholar]
- 54.Hall BB, McCulloch JA. Anaphylactic reactions following the intradiscal injection of chymopapain under local anesthesia. J. Bone Joint Surg. Am. Vol. 1983;65:1215–1219. doi: 10.2106/00004623-198365090-00001. [DOI] [PubMed] [Google Scholar]
- 55.Parkinson D. Late results of treatment of intervertebral disc disease with chymopapain. J. Neurosurg. 1983;59:990–993. doi: 10.3171/jns.1983.59.6.0990. [DOI] [PubMed] [Google Scholar]
- 56.Sutton JC., Jr Repeat chemonucleolysis. Clin. Orthop. Relat. Res. 1986;206:45–49. doi: 10.1097/00003086-198605000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Sutton JC., Jr Chemonucleolysis in the management of lumbar disc disease. A minimum six-year follow-up evaluation. Clin. Orthop. Relat. Res. 1986;206:56–60. doi: 10.1097/00003086-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Dabezies EJ, Beck C, Shoji H. Chymopapain in perspective. Clin. Orthop. Relat. Res. 1986;206:10–14. doi: 10.1097/00003086-198605000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Javid MJ. Efficacy of chymopapain chemonucleolysis. A long-term review of 105 patients. J. Neurosurg. 1985;62:662–666. doi: 10.3171/jns.1985.62.5.0662. [DOI] [PubMed] [Google Scholar]
- 60.Lorenz M, McCulloch J. Chemonucleolysis for herniated nucleus pulposus in adolescents. J. Bone Joint Surg. Am. Vol. 1985;67:1402–1404. doi: 10.2106/00004623-198567090-00016. [DOI] [PubMed] [Google Scholar]
- 61.Hill GM, Ellis EA. Chemonucleolysis as an alternative to laminectomy for the herniated lumbar disc. Experience with patients in a private orthopedic practice. Clin. Orthop. Relat. Res. 1987;225:229–233. doi: 10.1097/00003086-198712000-00019. [DOI] [PubMed] [Google Scholar]
- 62.Zeiger HE., Jr Comparison of chemonucleolysis and microsurgical discectomy for the treatment of herniated lumbar disc. Spine (Phila Pa 1976) 1987;12:796–799. doi: 10.1097/00007632-198710000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Hofstra L, van Woerden HH, Deutman R. Chemonucleolysis in the herniated L3–L4 disk. Clin. Orthop. Relat. Res. 1991;269:151–156. doi: 10.1097/00003086-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 64.Alexander AH, Burkus JK, Mitchell JB, Ayers WV. Chymopapain chemonucleolysis versus surgical discectomy in a military population. Clin. Orthop. Relat. Res. 1989;244:158–165. doi: 10.1097/00003086-198907000-00013. [DOI] [PubMed] [Google Scholar]
- 65.LeBlanc FE. Sciatica management: Chemonucleolysis vs. surgical discectomy. Todays OR Nurse. 1991;13:18–23. [PubMed] [Google Scholar]
- 66.Kato F, Ando T, Kawakami N, Mimatsu K, Iwata H. The increased signal intensity at the vertebral body endplates after chemonucleolysis demonstrated by magnetic resonance imaging. Spine (Phila Pa 1976) 1993;18:2276–2281. doi: 10.1097/00007632-199311000-00023. [DOI] [PubMed] [Google Scholar]
- 67.Leivseth G, Salvesen R, Hemminghytt S, Brinckmann P, Frobin W. Do human lumbar discs reconstitute after chemonucleolysis? A 7-year follow-up study. Spine (Phila Pa 1976) 1999;24:342–347. doi: 10.1097/00007632-199902150-00008. [DOI] [PubMed] [Google Scholar]
- 68.Ishibashi K, et al. Chemonucleolysis with chondroitin sulfate ABC endolyase for treating lumbar disc herniation: Exploration of prognostic factors for good or poor clinical outcomes. Medicina (Kaunas) 2020;56:627. doi: 10.3390/medicina56110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakajima H, et al. Short-term outcome and predictors of therapeutic effects of intradiscal condoliase injection for patients with lumbar disc herniation. Spine Surg. Relat. Res. 2021;5:264–271. doi: 10.22603/ssrr.2020-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oshita Y, et al. Multicenter retrospective analysis of intradiscal condoliase injection therapy for lumbar disc herniation. Medicina. 2022;58:1284. doi: 10.3390/medicina58091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeuchi S, et al. Predictive factors for poor outcome following chemonucleolysis with condoliase in lumbar disc herniation. Medicina (Kaunas) 2022;58:1868. doi: 10.3390/medicina58121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi K, Sato K, Ando T. Factors associated with disc degeneration based on Pfirrmann criteria after condoliase treatment for lumbar disc herniation. J. Orthop. Sci. 2023;28:976–983. doi: 10.1016/j.jos.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Hirai T, et al. Intradiscal injection with condoliase (chondroitin sulfate ABC endolyase) for painful radiculopathy caused by lumbar disc herniation. Spine Surg. Relat. Res. 2022;6:252–260. doi: 10.22603/ssrr.2021-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banno T, et al. Disc degeneration could be recovered after chemonucleolysis with condoliase-1 year clinical outcome of condoliase therapy. J. Orthop. Sci. 2022;27:767–773. doi: 10.1016/j.jos.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Matsuyama Y, Seo T, Chiba K. Condoliase chemonucleolysis for lumbar disc herniation: A post-hoc follow-up study of patients in previous clinical trials. J. Orthop. Sci. 2023;28:724–732. doi: 10.1016/j.jos.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Banno T, et al. Condoliase therapy for lumbar disc herniation -2 year clinical outcome. J. Orthop. Sci. 2024;29:64–70. doi: 10.1016/j.jos.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Kagami Y, Nakashima H, Segi N, Shinjo R, Imagama S. Clinical outcomes of condoliase injection therapy for lateral lumbar disc herniation. Spine Surg. Relat. Res. 2023;7:363–370. doi: 10.22603/ssrr.2022-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohtonari T, Torii R, Noguchi S, Kitagawa T, Nishihara N. Short-term clinical and radiographic outcomes of chemonucleolysis with condoliase for painful lumbar disc herniation and analysis regarding intradiscal injection area. Neurosurg. Rev. 2023;46:59. doi: 10.1007/s10143-023-01966-w. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi K, Sato K, Ando T, Ando K. MRI characteristics of disc degeneration after condoliase injection in young patients: A consecutive case series. J. Orthop. Sci. 2023 doi: 10.1016/j.jos.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 80.Bromley JW. Intervertebral discolysis with collagenase. Arzneimittelforschung. 1982;32:1405–1408. [PubMed] [Google Scholar]
- 81.Zhang D, Zhang Y, Wang Z, Zhang X, Sheng M. Target radiofrequency combined with collagenase chemonucleolysis in the treatment of lumbar intervertebral disc herniation. Int. J. Clin. Exp. Med. 2015;8:526–532. [PMC free article] [PubMed] [Google Scholar]
- 82.Nordby EJ. Eight- to 13-year follow-up evaluation of chemonucleolysis patients. Clin. Orthop. Relat. Res. 1986;206:18–23. doi: 10.1097/00003086-198605000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Louwaege A, et al. Efficiency of discography followed by chemonucleolysis in the treatment of sciatica. J. Belge Radiol. 1996;79:68–71. [PubMed] [Google Scholar]
- 84.Leonardi M, Fabris G, Lavaroni A. Percutaneous treatment of lumbar disc herniation. Ann. Chir. Gynaecol. 1993;82:141–148. [PubMed] [Google Scholar]
- 85.Kobayashi K, Sato K, Ando T, Ando K. MRI characteristics of disc degeneration after condoliase injection in young patients: A consecutive case series. J. Orthop. Sci. 2024;29:494–501. doi: 10.1016/j.jos.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe I, Shirogane T, Matsuyama Y, Chiba K. Effect of contrast media on the enzyme activity of condoliase: In vitro assessment. JOR Spine. 2022;5:e1221. doi: 10.1002/jsp2.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamagawa S, et al. Imaging evaluation of intervertebral disc degeneration and painful discs—advances and challenges in quantitative MRI. Diagnostics. 2022;12:707. doi: 10.3390/diagnostics12030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peredo AP, Gullbrand SE, Mauck RL, Smith HE. A challenging playing field: Identifying the endogenous impediments to annulus fibrosus repair. JOR Spine. 2021;4:e1133. doi: 10.1002/jsp2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang F, et al. Intradiscal injection for the management of low back pain. JOR Spine. 2022;5:e1186. doi: 10.1002/jsp2.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schol J, Sakai D. Comprehensive narrative review on the analysis of outcomes from cell transplantation clinical trials for discogenic low back pain. N. Am. Spine Soc. J. 2023;13:100195. doi: 10.1016/j.xnsj.2022.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schol J, et al. Homing of vertebral-delivered mesenchymal stromal cells for degenerative intervertebral discs repair—an in vivo proof-of-concept study. JOR Spine. 2023;6:e1228. doi: 10.1002/jsp2.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexeev D, Tschopp M, Helgason B, Ferguson SJ. Electrospun biodegradable poly(epsilon-caprolactone) membranes for annulus fibrosus repair: Long-term material stability and mechanical competence. JOR Spine. 2021;4:e1130. doi: 10.1002/jsp2.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Croft AS, et al. Biomedical applications of silk and its role for intervertebral disc repair. JOR Spine. 2022;5:e1225. doi: 10.1002/jsp2.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ambrosio L, et al. ISSLS PRIZE in basic science 2024: Superiority of nucleus pulposus cell-versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur. Spine J. 2024 doi: 10.1007/s00586-024-08163-3. [DOI] [PubMed] [Google Scholar]
- 96.Tilotta V, et al. Wharton’s Jelly mesenchymal stromal cell-derived extracellular vesicles promote nucleus pulposus cell anabolism in an in vitro 3D alginate-bead culture model. JOR Spine. 2024;7(1):e1274. doi: 10.1002/jsp2.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akeda K, et al. Platelet-rich plasma releasate versus corticosteroid for the treatment of discogenic low back pain: A double-blind randomized controlled trial. J. Clin. Med. 2022;11:304. doi: 10.3390/jcm11020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hasegawa T, et al. Regenerative effects of platelet-rich plasma releasate injection in rabbit discs degenerated by intradiscal injection of condoliase. Arthritis Res. Ther. 2023;25:216. doi: 10.1186/s13075-023-03200-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marcia S, et al. Efficacy of an ethyl alcohol gel in symptomatic disc herniation. Eur. J. Radiol. 2018;109:101–107. doi: 10.1016/j.ejrad.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 100.Ercalik T, Kilic M. Efficacy of intradiscal ozone therapy with or without periforaminal steroid injection on lumbar disc herniation: A double-blinded controlled study. Pain Phys. 2020;23:477–484. doi: 10.36076/ppj.2020/23/477. [DOI] [PubMed] [Google Scholar]
- 101.LeBlanc FE. Sciatica: Management by chemonucleolysis vs. surgical discectomy. Orthopedics. 1991;14:429–433. doi: 10.3928/0147-7447-19910401-06. [DOI] [PubMed] [Google Scholar]
- 102.Takeuchi R, Katagiri W, Endo S, Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One. 2019;14:e0225472. doi: 10.1371/journal.pone.0225472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maciunas RJ, Onofrio BM. The long-term results of chymopapain. Ten-year follow-up of 268 patients after chemonucleolysis. Clin. Orthop. Relat. Res. 1986;206:37–41. doi: 10.1097/00003086-198605000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data produced as part of this review are available in the main text or as a supplementary file. Additional requests can be made to the corresponding author upon reasonable request.