Abstract
In uninfected cells the G2/M transition is regulated by cyclin kinase complex containing cdc2 and, initially, cyclin A, followed by cyclin B. cdc2 is downregulated through phosphorylation by wee-1 and myt-1 and upregulated by cdc-25C phosphatase. We have examined the accumulation and activities of these proteins in cells infected with wild type and mutants of herpes simplex virus 1. The results were as follows. (i) Cyclin A and B levels were reduced beginning 4 h after infection and were undetectable at 12 to 16 h after infection. (ii) cdc2 protein also decreased in amount but was detectable at all times after infection. In addition, a fraction of cdc2 protein from infected cells exhibited altered electrophoretic mobility in denaturing gels. (iii) The levels of cdk7 or myt-1 proteins remained relatively constant throughout infection, whereas the level of wee-1 was significantly decreased. (iv) cdc-25C formed novel bands characterized by slower electrophoretic mobility that disappeared after treatment with phosphatase. In addition, one phosphatase-sensitive band reacted with MPM-2 antibody that recognizes a phosphoepitope phosphorylated exclusively in M phase. (v) cdc2 accumulating in infected cells exhibited kinase activity. The activity of cdc2 was higher in infected cell lysates than those of corresponding proteins present in lysates of mock-infected cells even though cyclins A and B were not detectable in lysates of infected cells. (vi) The decrease in the levels of cyclins A and B, the increase in activity of cdc2, and the hyperphosphorylation of cdc-25C were mediated by UL13 and α22/US1.5 gene products. In light of its normal functions, the activated cdc2 kinase may play a role in the changes in the morphology of the infected cell. These results are consistent with the accruing evidence that herpes simplex virus scavenges the cell for useful cell cycle proteins and subverts them for its own use.
The studies described in this report stemmed from the observation that the infected cell protein No.0 (ICP0) of herpes simplex virus 1 (HSV-1) binds to and stabilizes cyclin D3 (18). Further studies led to the observation that ICP0 and cyclin D3 colocalize in the infected cell nuclei and that ICP0 does not interfere with the phosphorylation of retinoblastoma protein (pRb) by cyclin D3-cdk4 complex. A role for cyclin D3 in the biology of HSV-1 emerged from mapping studies (44). Thus, substitution of aspartic acid 199 with alanine in ICP0 abolished stabilization of cyclin D3, reduced the yields of virus from resting cells, and reduced the capacity of the virus to invade the mouse central nervous system from a peripheral site. These studies demonstrated that HSV requires the participation of cell cycle proteins in the course of its replication even though the virus replicates efficiently in both resting and dividing cells. This conclusion is also supported by other observations, although in most instances a direct link to viral proteins is not yet available. Thus, pRb and p53 have been detected in the replication compartment of HSV (45). E2F DNA binding activity has been reported to be induced by HSV infection (14). ICP22 interacts with a novel cell cycle-regulated protein, p78 (5). The cellular protein, HCF, required for transactivation of viral α genes is a cell cycle regulator (12, 30). HSV-2 was reported to selectively activate cdk2 activity after infection (16). Inhibitors known to block the activity of cell cycle kinases cdk2 and cdc2 have been reported to reduce both α and β gene transcription, as well as reduce viral yields (41, 42).
In most of the instances described above, the cell cycle proteins associated with viral functions are involved in G1-to-S-phase transition. To further define the cellular environment in which optimal viral replication takes place, we initiated studies on the effects of HSV-1 infection on cell cycle proteins involved in the G2/M transition and in particular on cdc2 and its regulatory cyclins A and B.
The following two findings are relevant to this report. (i) Several small DNA viruses have been shown to be dependent on the phase of the cell cycle for optimal viral replication. Parvoviruses replicate their genome only when infected cells have progressed to S phase (3), while polyomaviruses (6) and papillomaviruses (17) induce cells to progress into the S phase. The requirement for S phase in infection by DNA viruses suggests that the replication of DNA viruses is dependent on cellular factors that are active for cellular DNA replication and that DNA viruses scavenge such factors to replicate viral DNA. Although HSV encodes proteins required for the synthesis of its DNA (reviewed in reference 39), cell cycle regulators may play a significant role in establishing a more efficient environment for viral gene expression.
(ii) Coordinate, rigid regulation of cyclin-dependent kinase (cdk) activity by appropriate cyclins and other regulatory proteins plays a central role in the transition of the cell from one phase of the cell cycle to the next (28). cdk4 and cdk6 regulated by D-type cyclins are active during early G1, whereas cdk2 regulated by cyclins E and A is active during late G1 and S phases (13, 43). The G2/M transition is regulated by cdc2 (cdk1) (8–10, 21, 26). This kinase is present throughout the cell cycle but is active in conjunction with the newly synthesized cyclins A and B. The activity of cdc2 is tightly regulated: it requires the interaction of newly synthesized cyclins A and B, and it is inactivated by phosphorylation of thr14 and thr15 by the kinases wee-1 and myt-1 (4, 24, 31, 32). The cdc2 kinase is activated by the removal of thr14 and thr15 phosphates by the cdc-25C phosphatase and by phosphorylation of thr161 by cyclin-activating kinase complex (8–10, 23, 46). cdc-25C, a key player in the activation of cdc2, must itself be activated by phosphorylation (21).
We report that although cyclins A and B were no longer detected and the cdc2 protein level decreased at 8 h after infection, cdc2 activity increased late in infection. Consistent with the activation of cdc2 activity, the level of wee-1 inhibitory kinase decreased, whereas the activating phosphatase cdc-25C was hyperphosphorylated and active, as evidenced by its recognition by MPM-2 antibody. The increases in activity of cdc2 were not observed in cells infected with HSV-1 mutants lacking the UL13 protein kinase or the α22/US1.5 genes. The results suggest that HSV-1 actively and specifically maintains cdc2 activity to foster its own needs.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were initially obtained from the American Type Culture Collection and maintained in Dulbecco modified Eagle medium (DMEM) with 10% serum. Rabbit skin cells were originally obtained from John McClaren. HSV-1(F) is the prototype wild-type HSV-1 strain used in our laboratory (11). R7356 (UL13−), R7358 (UL13R), R7802 (α22−/US1.5−), and R7804 (α22R/US1.5R) have been previously described (29, 35, 36).
Cell infection.
Confluent 150-cm2 flasks of HeLa cells were harvested and reseeded to 25-cm2 flasks. Cells were allowed to adhere for 1 h, after which unattached cells were aspirated and then exposed to 2 × 107 PFU of appropriate virus in 1 ml of 199V (mixture 199 supplemented with 1% calf serum) on a rotary shaker at 37°C. After 2 h, the inoculum was replaced with 5 ml of fresh DMEM medium with 10% newborn calf serum and gassed with CO2. Flasks were incubated at 37°C until the cells were harvested.
PAA treatment.
The procedure was as described above except that during the adherence time period phosphonoacetic acid (PAA; 300 μg/ml; a gift of Abbott Laboratories) was added at the time of seeding. PAA was present throughout the infection to prevent viral DNA replication.
Immunoblotting.
The cells contained in the 25-cm2 flasks were harvested at the times indicated in the results as follows. The medium was removed, and the cells were rinsed in phosphate-buffered saline (PBS), scraped into 5 ml of PBS(A), pelleted by centrifugation, and solubilized in 200 μl of disruption buffer (2% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 7.2], 2.75% sucrose, 5% β-mercaptoethanol, and bromophenol blue). The extract was sonicated, boiled for 2 min, subjected to electrophoresis on 10% bisacrylamide gels, transferred to nitrocellulose membranes, blocked for 2 h with 5% nonfat dry milk, and reacted with the appropriate antibody. Antibodies against cdc2, cyclin A, and cyclin B (Santa Cruz) were diluted 1:100 in PBS. Antibody to MPM-2 (Upstate Biotechnology) was diluted 1:500. Primary antibodies were reacted for 2 h with blots. Secondary antibody diluted 1:1,000 (goat anti-mouse antibody conjugated to peroxidase; Sigma) was applied for 1 h. Blots were developed by enhanced chemiluminescence (ECL) according to the instructions supplied by the manufacturer (Pierce). Antibodies to cdc-25C, cdk7, wee(hu)-1, and myt-1 (Santa Cruz) were diluted 1:500 in PBS(A) with 0.05% Tween 20 and 1% bovine serum albumin and exposed to the blots for 1 h. Secondary antibody diluted 1:3,000 (alkaline phosphatase [AP] conjugated; Bio-Rad) was exposed to the blots for 1 h. Blots were incubated in AP buffer (100 mM Tris, pH 9.5; 100 mM NaCl; 5 mM MgCl2), followed by AP buffer containing BCIP and nitroblue tetrazolium. The reaction was stopped with solution containing 100 mM Tris (pH 7.6) and 10 mM EDTA. All rinses were done with PBS containing 0.05% Tween 20. The anti-US11 monoclonal antibody described elsewhere (40) was used in the same fashion as that described above for cdc-25C except for the omission of Tween 20.
Phosphatase treatment.
Aliquots of extracts from uninfected and infected cells were incubated with 10 U of AP for 30 min at 34°C. Reactions were stopped by the addition of gel loading buffer containing SDS.
Histone H1 kinase assay.
Cells were infected and maintained as described above, suspended in lysis buffer (20 mM Tris, pH 8.0; 1 mM EDTA; 0.5% NP-40; 400 mM NaCl; 0.1 mM Na orthovanadate; 10 mM NaF; 2 mM dithiothreitol [DTT], tolylsulfonyl phenylalanyl chloromethyl ketone [TPCK], Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK], and phenylmethylsulfonyl fluoride) and maintained for 1 h on ice; the insoluble material was then pelleted by centrifugation (16). The supernatant fractions were precleared as described above and reacted with anti-cyclin B or anti-cdc2 antibody for 18 h at 4°C. The immunoprecipitate cyclin B or cdc2 was recovered by the addition of 20 μl of 50% protein A slurry for 1 h, rinsed twice with lysis buffer, rinsed twice with low-salt lysis buffer (20 mM Tris, pH 8.0; 1 mM EDTA; 0.5% NP-40; 1 mM NaCl; 2 mM DTT), and rinsed twice with incomplete kinase buffer (50 mM Tris, pH 7.4; 10 mM MgCl2; 5 mM DTT). Then, 40 μl of complete kinase buffer was added to each sample (2 μg of histone H1 [Boehringer Mannheim], 10 μM ATP, and 20 μCi of [γ-32P]ATP in incomplete kinase buffer), and samples were incubated at 30°C for 20 min. The reaction was stopped with 13 μl of 4× loading buffer containing SDS and heated for 5 min at 95°C, and the reaction mixtures were subjected to electrophoresis in 10% bispolyacrylamide gels, transferred to nitrocellulose membrane, and subjected to autoradiography.
RESULTS
The levels of cyclin A, cyclin B, and cdc2 decrease in HSV-1-infected cells relative to those of mock-infected cells.
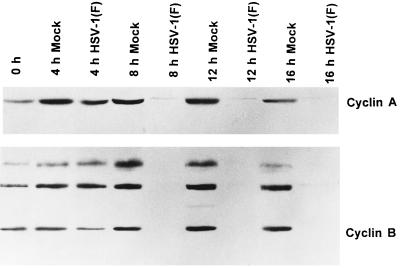
The purpose of this series of experiments was to determine the levels of cyclin A and B protein accumulations in the course of infection of cells with HSV-1(F). Figure 1 shows an immunoblot of both cyclin A and cyclin B accumulation in infected cells compared to those of uninfected cells over a 16-h interval. In this experiment HeLa cells were harvested every 4 h after seeding and assayed for the presence of the cyclins by immunoblotting electrophoretically separated proteins with appropriate antibodies as described in Materials and Methods. In uninfected cells, peak levels of cyclin A and cyclin B protein were observed at the 12-h time point consistent with expected progression of the uninfected cells across the G2/M interphase. In HSV-1-infected cells, no accumulation of cyclin A or B was observed. By 8 h after infection, there was a significant decrease in cyclins A and B compared to those of uninfected cells.
FIG. 1.
Photograph of an immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cyclin A or cyclin B and then with goat anti-mouse antibody and visualized by ECL as described in Materials and Methods. The cells were infected at time zero and harvested at the indicated times after infection. Cyclin B formed three bands on electrophoresis in denaturing 10% polyacrylamide gels.
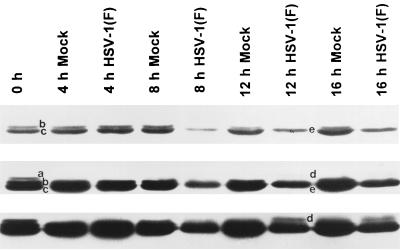
cdc2 protein levels are relatively constant throughout the cell cycle, and the activity of cdc2 is regulated both by the nature of the protein with which they are associated and by the site of phosphorylation. cdc2 is inhibited by phosphorylation at thr14 and tyr15 by the kinases wee-1 and myt-1 (8, 9). The inhibitory phosphates are removed by cdc-25C phosphatase. In addition, cdc2 is activated by phosphorylation of thr161 by the cyclin-activating kinase complex. The immunoblots shown in Fig. 2 were photographed at different exposures to resolve the three cdc2 bands usually present in uninfected cells. The fastest-migrating band (c) represents unphosphorylated cdc2 and cdc2 phosphorylated at thr161 (1). The two slower-migrating bands (a and b) represent cdc2 phosphorylated at either thr14 or tyr15 or at both sites. All three bands were detected in uninfected cell lysates (1).
FIG. 2.
Photograph showing three different exposures of one immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cdc2 kinase. The procedures were as described in the legend to Fig. 1 and in Materials and Methods. The three exposures were designed to show faint bands visible at low or intermediate exposures but which become fused with the larger bands on longer exposures. Bands in mock-infected lanes are labeled a, b, and c. Bands a and b represent inhibitory phosphorylation of cdc2 in its kinase domain. cdc2 reacting bands present solely in lysates of infected cells were labeled d and e. Overexposure of the blot (lowermost strip) showed that band e accumulated in infected cells.
In contrast, infected cells showed loss of the two slower-migrating bands of cdc2 but retention of the fastest-migrating band (Fig. 2, band c) by 8 h after infection. Interestingly, two new bands were detected in cells harvested at 12 and 16 h after infection. Thus, band e migrated more slowly than band c but faster than bands a and b. Band d migrated at a rate slightly slower than that for band a and was observed on overexposed ECL blots.
We conclude that the levels of cyclins A and B and of the cdc2 protein are reduced in infected cells. The reduction takes place between 4 and 8 h after infection.
The effects of HSV-1 infection upon regulators of cdc2 kinase.
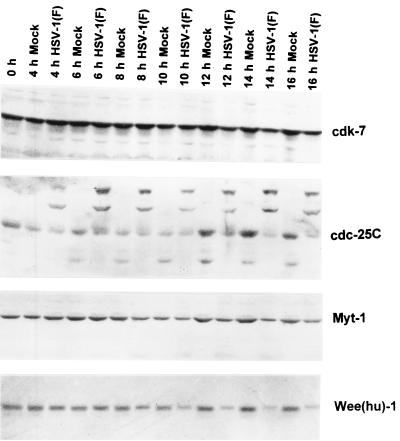
The objective of the experiments described in this section was to follow up the observation that the levels of cdc2 in protein bands containing the isoforms phosphorylated at thr14 and tyr15 were reduced in infected cells examined at 8 h and later times after infection. In the first series of experiments, we examined the effect of infection on the inhibitory kinases wee-1 and myt-1. Figure 3 shows that the levels of myt-1 in infected cells and uninfected cells were similar between 4 and 10 h after infection. At the 12- and 16-h time points, the levels of myt-1 were slightly higher in mock-infected than in uninfected cells. The levels of wee-1 in infected and uninfected cells were similar between 4 and 8 h after infection. At later time points the levels of wee-1 in infected cells decreased and, moreover, the infected cell protein migrated more slowly than that of uninfected cells.
FIG. 3.
Photograph of an immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cdk7, cdc-25C, myt-1, and human wee-1 [Wee(hu)-1]. The procedures were as described above and in Materials and Methods.
As noted above, cdc2 is activated by dephosphorylation of thr14 and tyr15 by the cdc-25C phosphatase. cdc-25C is optimally activated by phosphorylation (8–10, 15, 25). In HeLa cells, phosphorylated cdc-25C exhibits an Mr 5,000 shift from Mr 55,000 to 60,000 (15, 25). In our studies, the cdc-25C from uninfected HeLa cells formed a prominent doublet band that decreased in intensity sometime between 4 and 8 h after infection. Instead, the infected cells exhibited at least two new slower-migrating bands reacting with the anti-cdc-25C antibody (Fig. 3).
cdk7, a component of the cyclin-activating kinase complex for cdc2 (21), remained fairly constant in infected compared to uninfected cells and served as a protein loading control (Fig. 3).
The characteristics of cdc-25C accumulating in infected cells.
As shown above, the cdc-25C accumulating in infected cells formed several bands differing in electrophoretic mobilities in denaturing polyacrylamide gels. To identify and characterize the proteins contained in these bands, several experiments were done.
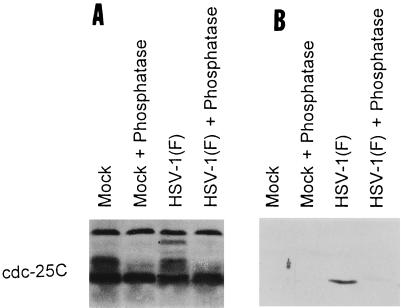
The objective of the first series was to determine whether the proteins contained in these bands were phosphorylated. Mock-infected or HSV-1(F)-infected HeLa cell extracts were treated with AP and then solubilized, electrophoretically separated in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the anti-cdc-25C antibody. The antibody reacted with three protein bands marked by the dots in Fig. 4A. The middle of the three bands was seen in both uninfected and infected cells and was eliminated by phosphatase treatment. The upper band was seen only in infected cell extract and was also phosphorylated, since it disappeared after phosphatase treatment. The fast-migrating, slightly convex marked band of cdc-25C of infected untreated cells differed in electrophoretic mobility from that of uninfected cells. After treatment with phosphatase, this band could not be differentiated from that formed by cdc-25C present in either treated or untreated lysates of uninfected cells. We conclude from these studies that the novel forms of cdc-25C detected in lysates of infected cells were phosphorylated.
FIG. 4.
Photograph of an immunoblot of lysates of uninfected or wild-type-virus-infected HeLa cells treated with AP, electrophoretically separated on denaturing polyacrylamide gels, and reacted to antibodies to cdc-25C (A) or MPM-2 (B). The cells were harvested 13 h after infection. The dots mark three distinct bands of proteins reacting with antibody to cdc-25C that were no longer detected after treatment with the phosphatase. The distinct curved band (lowest dot) in infected cells reacted with antibodies to both cdc-25C and MPM-2.
MPM-2 (mitotic protein monoclonal antibody-2) antibody was generated by using M-phase HeLa cells as an immunogen (7). Extensive characterization of the antibody led to the identification of the epitope recognized by MPM-2 as a phosphoepitope specifically phosphorylated during M phase. This epitope is present in several M-phase proteins, including cdc-25C (2, 22). As shown in Fig. 4B, the antibody reacted only with the slightly convex, rapidly migrating form of cdc-25C present in lysates of infected cells. The antibody no longer reacted with the protein after phosphatase treatment. We conclude from this experiment that at least one form of cdc-25C contained the MPM-2 phosphoepitope characteristic of cells in M phase.
The changes in cyclins A and B and in cdc2 and associated proteins are independent of the onset of viral DNA synthesis.
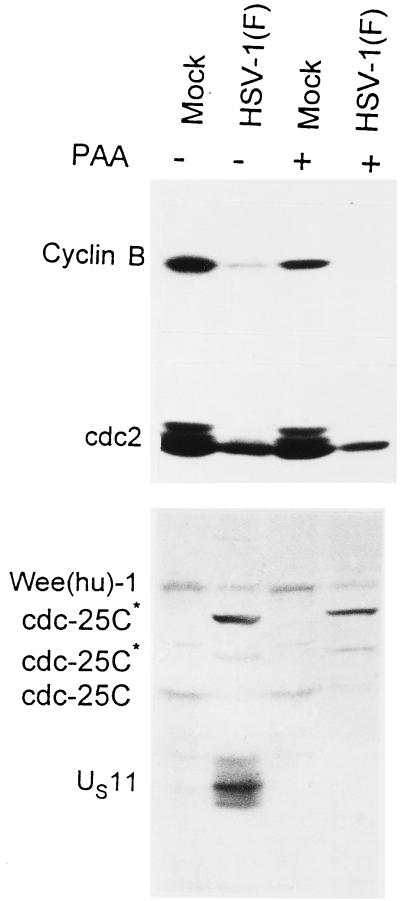
In this series of experiments the cells were infected and maintained in medium containing sufficient PAA to totally block viral DNA synthesis and preclude the expression of γ2 genes. The cells were harvested at 16 h after infection and processed as described in Materials and Methods. The results, shown in Fig. 5, were as follows. (i) Cyclin B was present in reduced amounts in mock-infected cells treated with PAA compared with untreated mock-infected cells. (ii) Cyclin B was not detected in either untreated or PAA-treated infected cells. (iii) Only cdc2 band e was detected in both treated and untreated infected cells. The cdc2 levels were too low to detect the d band. (iv) PAA had no effect on the levels of wee(hu)-1 or the modification of electrophoretic mobility of cdc-25C observed in infected cells. (v) As expected, inasmuch as the expression of the US11 gene is dependent on the onset of viral DNA synthesis, the HSV-1 γ2 protein US11 was detected in untreated, but not in PAA-treated, infected cells. We conclude from these studies that the viral function involved in the reduction in the levels of cyclin B and wee(hu)-1 and the modification of cdc-25C was expressed early in infection and did not require the onset and maintenance of viral DNA synthesis. These results are consistent with the observations noted earlier in the text that the decrease in the levels of cyclin B cdc2 took place between 4 and 8 h after infection.
FIG. 5.
Photograph of an immunoblot of lysates of HeLa cells electrophoretically separated in denaturing gels and reacted with antibodies to cyclin B, cdc-25C, and wee-1. Replicate cultures were mock-infected or infected with HSV-1(F) and maintained in the presence of PAA (300 μg of PAA per ml). The antibody to US11 verified that PAA blocked viral DNA synthesis and precluded the synthesis of γ2 proteins dependent on the replication of viral DNA for their synthesis. Cells were incubated with or without PAA and then mock infected or infected with HSV-1(F). The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
The decrease in the levels of cyclins A and B and wee(hu)-1 and the changes in electrophoretic mobility of cdc-25C require the expression of α22/US1.5 and UL13 genes.
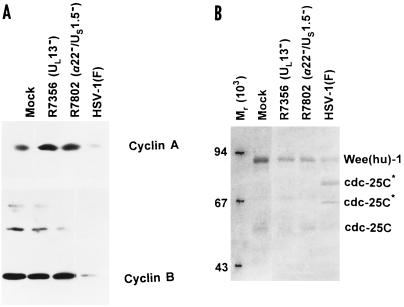
The UL13 protein kinase phosphorylates several viral and at least one cellular protein (EF-1δ) (19, 20, 36). Among the substrates of the UL13 protein kinase are the proteins encoded by α22/US1.5 genes. The functions of UL13 and of α22/US1.5 appear to be inter-related inasmuch as the phenotype of mutants lacking UL13 is similar to that lacking the α22/US1.5 genes. In this series of experiments we tested the hypothesis that the modification of cyclins A and B and of the associated proteins requires the expression of UL13 and of the α22/US1.5 genes. Replicate cultures of cells either mock-infected or infected with R7802 (α22−/US1.5−) or R7356 (UL3−) viruses were harvested at 16 h after infection and processed as described earlier in the text. Figure 6A shows that at 16 h after infection the levels of cyclins A and B present in mock-infected or R7802 or R7356 mutant-infected cells were similar, whereas the levels of cyclins A and B in wild-type-infected cells were reduced. Figure 6B shows that the reduction in the wee-1 kinase was less pronounced in mutant-infected cells then in wild-type virus-infected cells. In addition, the slow-migrating bands of cdc-25C characteristic of wild-type-infected cells were not detected in the lysates of mutant virus-infected cells (Fig. 6B).
FIG. 6.
Photograph of an immunoblot of lysates of HeLa cells mock infected or infected with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−), electrophoretically separated on polyacrylamide gels, and reacted with antibodies to cyclin A and B (A) or wee(hu)-1 and cdc-25C (B). The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
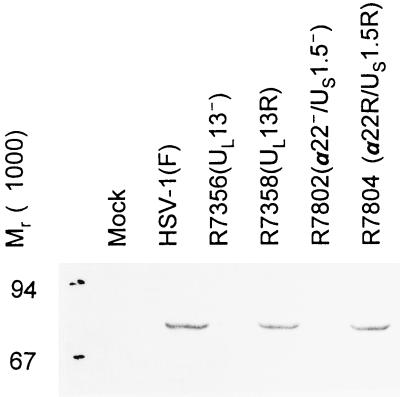
To verify that the phenotype of the R7356 and R7802 mutants observed in these studies was indeed due to the deletions in UL13 and α22/US1.5 genes, respectively, we also tested the UL13 repair virus (R7358) and the α22/US1.5 repair virus (R7804). The lysates of cells infected with either repaired virus exhibited the slow-migrating cdc-25C forms characteristic of wild-type-infected cells, indicating that the modification of cdc-25C in wild-type virus-infected cells was indeed mediated by UL13 and α22/US1.5 proteins (Fig. 7).
FIG. 7.
Photograph of an immunoblot of lysates of rabbit skin cells mock infected or infected with HSV-1(F), R7356 (UL13−), R7358 (UL13 repair), R7802 (α22−/US1.5−), or R7804 (repair of R7802), electrophoretically separated on a denaturing polyacrylamide gel, and reacted with antibodies to cdc-25C. The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
cdc2 remains active in HSV-1-infected cells.
The results of the experiments described above indicated that HSV-1 infection leads to decreased levels of cyclins A and B but also to a reduction in the amount of inhibitory phosphorylated cdc2. Effects on key regulators of cdc2 phosphorylation upon HSV-1 infection suggested that the inhibitory kinase wee-1 was reduced, whereas the activating phosphatase cdc-25C was phosphorylated and active. These observations suggested that cdc2 was active.
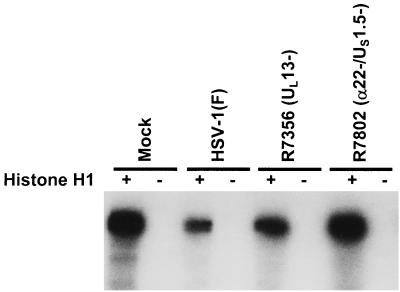
Most measurements of cdc2 activity described in the literature are based on assays of precipitates of cyclin B that should also contain cdc2. In these experiments immune precipitates of cyclin B from lysates of HeLa cells harvested 16 h after mock infection or infection with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−) were assayed for kinase activity by using histone H1 as a substrate as described in Materials and Methods. The results were as follows (Fig. 8): cyclin B-cdc2 activity was elevated in mock-infected cells and significantly decreased in HSV-1(F)-infected cells. Cyclin B-cdc2 kinase activity increased to mock-infected levels in R7356 (UL13−)- or R7802 (α22−/US1.5−)-infected cells.
FIG. 8.
Autoradiographic image of histone H1 phosphorylated in vitro by immune precipitates of cyclin B-cdc2 complex from replicate cultures of HeLa cells mock infected or infected with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−). The cyclin B-cdc2 complex was precipitated with cyclin B antibody from lysates of cells harvested 16 h after infection. The reaction mixtures consisted of the precipitate alone or precipitate plus histone H1. The mixtures were denatured and separated on denaturing polyacrylamide gels at the completion of the reaction and prior to autoradiography. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
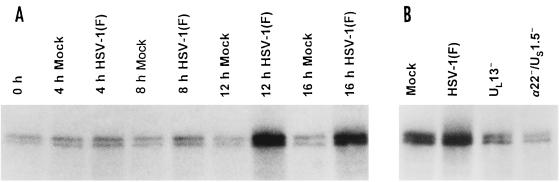
These results were not surprising since cyclin B levels were grossly reduced in cells infected with wild-type virus but not in cells infected with R7356 or R7802 mutants (Fig. 6) and therefore they measure the activity of the cyclin B-cdc2 complex but not the total activity of the residual cdc2 kinase. To measure the total residual activity of cdc2, we immunoprecipitated cdc2 directly and measured its capacity to phosphorylate histone H1 as described in Materials and Methods. The results were as follows (Fig. 9): cdc2 kinase activity in mock-infected cells peaked 4 h after seeding and to a lesser extent again at 16 h after seeding. In infected cells, the activity of cdc2 kinase began to increase between 4 and 8 h after infection and reached severalfold-higher levels at 12 and 16 h after infection (Fig. 9A). At these late times neither cyclin A nor cyclin B could be detected in infected cells.
FIG. 9.
Autoradiographic image of histone H1 phosphorylated in vitro by immune precipitates of cdc2 from lysates of HeLa cells. (A) cdc2 was immune precipitated from lysates of replicate cultures of HeLa cells harvested at the intervals shown after mock infection or infection with HSV-1(F). (B) cdc2 was immune precipitated from lysates of HeLa cells harvested 16 h after mock infection of infection with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−). The procedures were as described in the legend to Fig. 8 and in Materials and Methods.
Since cdc2 kinase activity peaked at 16 h after infection with HSV-1(F), we measured the effect of UL13 and α22/US1.5 gene products in lysates of cells harvested 16 h after infection. The results are shown in Fig. 9B. As seen in Fig. 9A, HSV-1(F) infection resulted in greater cdc2 histone H1 kinase activity compared to mock-infected cells. Infection with R7356 (UL13−) or R7802 (α22−/US1.5−) resulted in a decrease in kinase activity of cdc2 compared to extracts of wild-type-infected cells. These results are consistent with the observation that cdc-25C hyperphosphorylated bands were present in lysates of cells infected with HSV-1(F) but not in those infected with R7356 or R7802 (Fig. 6 and 7).
DISCUSSION
The progression of the cell through the cell cycle is tightly regulated at numerous checkpoints. As noted in the introduction, although HSV replicates efficiently in both dividing and nondividing cells, functions encoded by the virus appear to at least regulate, and possibly modify, cell cycle proteins. The observation that the wild-type virus stabilizes cyclin D3 involved in the G1/S interphase prompted us to examine the status of the cell cycle proteins involved in the G2/M interphase. The reasoning was that if the virus does activate or sustain cell cycle proteins involved in the G2/M interphase, the focus of viral activity would be bracketed to the G1/S/G2 phases. This appears not to be the case.
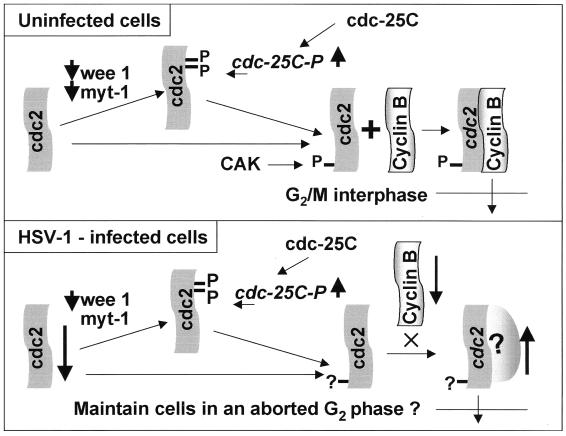
The top panel of Fig. 10 summarizes the events required for activation of cdc2. In brief, if the G2/M transition is blocked, it would be expected that the cdc2 would be phosphorylated near its N terminus by the inhibitory kinases wee-1 and myt-1, whereas cdc-25C would be inhibited (47). To overcome the G2/M arrest, the cdc-25C phosphatase would be activated by hyperphosphorylation. The activated cdc-25C phosphatase would remove the amino-terminal phosphates on cdc2, whereas the cyclin-activating kinase complex would phosphorylate the C-terminal phosphate of cdc2. The salient features of the results of HSV-1 infection are presented schematically in the bottom panel of Fig. 10. The significance of the results presented in this report is as follows.
FIG. 10.
Schematic diagram of the activation of cdc2 in uninfected cells (upper panel) and wild-type virus-infected cells (lower panel). The thick arrows indicate an increase (up) or a decrease (down) in activity. The key features shown in this report are decreased expression of the regulatory cyclins A and B and of cdc2 protein, the decrease in the levels of wee-1, the change in the phosphorylation of cdc2, and the hyperphosphorylation of cdc-25C. The cumulative effects of HSV-1 infection result in the upregulation of cdc2 activity. The experiment shown in Fig. 8 indicated that the kinase activity associated with cyclin B-cdc2 complex actually decreased in infected cells, whereas the kinase activity of precipitates obtained with antibody to cdc2 increased after infection (Fig. 9). The partner of cdc2 responsible for the higher cdc2 kinase activity in infected cells is unknown and marked with a question mark in the lower panel. CAK, cyclin-activating kinase complex.
(i) The levels of cyclins A and B were reduced to undetectable levels between 4 and 8 h after infection. This decrease was not observed in cells infected with mutants lacking the UL13 or the α22/US1.5 genes. Interpretation of these results by themselves is not straightforward. The decline in the levels of cyclins A and B in wild-type-virus-infected cells could be due to virus-directed degradation or to the relatively short half-life of these two proteins combined with total shutoff of protein synthesis. Conversely, the stabilization of cyclins A and B in cells lacking UL13 or α22/US1.5 gene products could be due to the absence of a signal for degradation of the proteins or a decrease in the expression of the virion host shutoff protein encoded by UL41 reported elsewhere (27).
(ii) The amount of cdc2 protein was reduced after infection and, in addition, the electrophoretic mobility of a fraction of cdc2 protein differed from that of the uninfected cell protein. The unexpected observation was that notwithstanding the reduction in total protein, the activity of cdc2 kinase as measured by phosphorylation of histone H1 was significantly higher than that present in mock-infected cells. The more significant observation was that the activity increased 4 to 8 h after cyclins A and B declined below detectable levels.
(iii) The increased activity of cdc2 kinase without an increase in detectable protein raised the possibility that cdc2 was specifically activated from an inactive state. Two series of experiments are concordant with this hypothesis. First, the studies on the inhibitory kinases revealed that, whereas the levels of myt-1 remained constant, the levels of wee-1 decreased. Of the known activating components, whereas the level of cdk7, a member of the cyclin-activating complex, remained unaltered, the cdc-25C phosphatase was hyperphosphorylated late in infection. The hyperphosphorylation of cdc-25C was particularly striking. Several proteins, including cdc-25C, share an epitope phosphorylated during the M phase. This epitope, in its phosphorylated state, reacts with monoclonal antibody MPM-2 (2, 22). In infected cells, one cdc-25C protein band reacted with the MPM-2 antibody, and both this reactivity and the slow-migrating cdc-25C protein bands were extinguished by treatment with phosphatase.
The second line of supporting evidence is based on the observation that the increase in cdc2 activity was mediated by both UL13 and α22/US1.5 gene products. We make a distinction between the disappearance of cyclins A and B and the high activity of cdc2 mediated by the viral gene products. While it could be argued that the disappearance of cyclins A and B is due to some nonspecific shutoff of protein synthesis, the higher levels of cdc2 activity must reflect a very specific viral function. Related to this line of evidence is the observation that cdc-25C was hyperphosphorylated and exhibited the MPM-2 phosphoepitope. The hyperphosphorylation of the cdc-25C protein was mediated by the products of the UL13 and α22/US1.5 genes. Since the α22/US1.5 and UL13 regulatory pathway affects the expression of many genes, the specific gene whose products mediate the phenotype observed with wild-type virus remains unknown.
Given the evidence that viral gene products alter the functions of cdc2 and of cdc-25C, the question arises whether viral gene products act on both cdc2 and cdc-25C or whether the changes reflect a single modification in a key regulatory protein. We should note that activated cdc2 hyperphosphorylates cdc-25C phosphatase that in turns keeps cdc2 active (15). The significance of such a positive feedback is that activation of only one of these two regulators may be sufficient to maintain both in an active state.
We are left with two experimentally unresolved questions. The first is how could infected cells exhibit a high level of cdc2 activity in the absence of detectable cyclin A or B, the cellular partner of cdc2? The second, equally intriguing question is why would HSV evolve a function that would maintain an active cdc2?
Several hypotheses may account for the observed activity of cdc2 in the absence of detectable levels of cyclin A or B. One hypothesis currently in favor is that cdc2 or one of its aberrantly modified forms has acquired a new partner, possibly a viral protein. Another, less-attractive but viable hypothesis is that the association of the aberrantly migrating forms with residual amounts of cyclin A or B exhibits a level of activity that is much higher than that of infected cell cdc2-cyclin A or B complexes. We do not know what the aberrantly migrating forms represent, and studies are in progress to define their function.
The question as to why HSV targets both cdc-25C and cdc2 remains for the moment unresolved. One hypothesis consistent with the biology of HSV infection centers on two key findings. The first is that cellular DNA synthesis is firmly shut off within a few hours after infection (38). The second concerns the functional life of cdc2. In uninfected cells, cdc2 kinase activity peaks at the G2/M interphase and is then shut off precipitously (21). This shutdown of cdc2 is the result of activation of cyclosome by cdc2 and ubiquitin-dependent degradation of cyclin B. The loss of cdc2 activity serves as a signal for mitosis to proceed. Except in the case of cells infected after deliberate mitotic arrest (37), infected cells do not proceed through mitosis and it could be argued that mitosis would in any event grossly interfere with HSV-1 replication. The compelling conclusion is that the infected cell does not complete its S phase and yet brings the cells to the brink but not across the G2/M interface since cdc2 is in an active form and the infected cell does not receive the signal for mitosis.
The preferred explanation for the entry of the infected cells into the S phase is to acquire or preserve proteins useful for viral nucleic acid metabolism (44). One explanation for the G2/M brinkmanship is to induce changes in cell morphology that would facilitate viral replication. cdc2 kinase is in part responsible for modifying the cellular architecture of the cell for mitosis and specifically for the phosphorylation of nuclear lamins to alter cell morphology (34). cdc2 may also be involved in modifying the structure of chromatin, and this would decrease its availability for transcription and ultimately result in its margination.
The studies reported here underscore the conclusions reported earlier that HSV encodes functions that scavenge the cell for cellular proteins that are subverted for use by the virus to attain specific goals (44). An understanding of the role of the activated cdc2 and cdc-25C proteins during infection may significantly enrich our understanding of the biology of the virus.
ACKNOWLEDGMENTS
We thank W. O. Ogle for invaluable discussions.
These studies were aided by grants from the National Cancer Institute (CA47451, CA71933, CA78766, and CA41068) of the U.S. Public Health Service.
REFERENCES
- 1.Atherton-Fessler S, Liu F, Gabrielli B, Lee M S, Cheng-Yuan P, Piwnica-Worms H. Cell cycle regulation of the p34cdc inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barratt R A, Kao G, McKenna W G, Kuang J, Muschel R J. The G2 block induced by DNA damage: a caffeine-resistant component independent of cdc25C, mpm-2 phosphorylation, and H1 kinase activity. Cancer Res. 1998;58:2639–2645. [PubMed] [Google Scholar]
- 3.Berns J I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2173–2197. [Google Scholar]
- 4.Booher R N, Hlman P S, Fattaey A. Human myt1 is a cell cycle-regulated kinase that inhibits cdc2 but not dck2 activity. J Biol Chem. 1997;272:22300–22306. doi: 10.1074/jbc.272.35.22300. [DOI] [PubMed] [Google Scholar]
- 5.Bruni R, Roizman B. Herpes simplex virus 1 regulatory protein ICP22 interacts with a new cell cycle-regulated factor and accumulates in a cell cycle-dependent fashion in infected cells. J Virol. 1998;72:8525–8531. doi: 10.1128/jvi.72.11.8525-8531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole C N. Polyomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1997–2025. [Google Scholar]
- 7.Ding M, Feng Y, Vandre D D. Partial characterization of the mpm-2 phosphoepitope. Exp Cell Res. 1997;231:3–13. doi: 10.1006/excr.1996.3439. [DOI] [PubMed] [Google Scholar]
- 8.Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit regulation. Cell. 1988;54:17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 9.Draetta G, Piwnica-Worms H, Morrison D, Druker B, Roberts T, Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988;336:738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- 10.Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta. 1997;1332:M53–M63. doi: 10.1016/s0304-419x(96)00049-2. [DOI] [PubMed] [Google Scholar]
- 11.Ejercito P, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 12.Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single point mutation in HCF causes temperature-sensitive cell cycle arrest and disrupts mutations VP16 function. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 13.Heichman K A, Roberts J M. Rules to replicate by. Cell. 1994;79:557–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 14.Hilton M J, Mounghane D, McLean T, Contractor N V, O'Neil J, Carpenter K, Bachenheimer S L. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1995;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann I, Clarke P R, Marcote M J, Karsenti E, Draetta G. Phosphorylation and activation of cdc25-C by cdc2-cyclin B and its involvement in the self amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997;78:3341–3348. doi: 10.1099/0022-1317-78-12-3341. [DOI] [PubMed] [Google Scholar]
- 17.Howley P M. Papillomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2045–2076. [Google Scholar]
- 18.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi Y, Van Sant C, Roizman B. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the Ul13 gene of herpes simplex virus 1. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Matsumura T, Roizman B, Hirai K. Cellular elongation factor 1δ is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J Virol. 1999;73:4456–4460. doi: 10.1128/jvi.73.5.4456-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King R W, Jackson P K, Kirschner M W. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 22.Kuang J, Ashorn C L, Gonzalez-Kuyvenhoven M, Penkala J E. Cdc25 is one of the mpm-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell. 1994;5:135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai A, Dunphy W G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- 24.McGowan C H, Russell P. Cell cycle regulation of human wee1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millar J B A, McGowan C H, Lenaers G, Jones R, Russell P. p80zcdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moria A O, Draetta G, Beach D, Wang J Y J. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry in mitosis. Cell. 1989;58:193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- 27.Ng T I, Chang Y E, Roizman B. Infected cell protein 22 of herpes simplex virus regulates the expression of virion host shutoff gene UL41. Virology. 1997;234:226–234. doi: 10.1006/viro.1997.8659. [DOI] [PubMed] [Google Scholar]
- 28.Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 29.Ogle W O, Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J Virol. 1999;73:4305–4315. doi: 10.1128/jvi.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Reilly D, Hanscombe O, O'Hare P. A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J. 1997;16:2420–2430. doi: 10.1093/emboj/16.9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker L L, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human wee1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 32.Parker L L, Sylvestre P J, Byrnes III M J, Liu F, Piwnica-Worms H. Identification of a 95-kDa wee1-like tyrosine kinase in HeLa cells. Proc Natl Acad Sci USA. 1995;92:9638–9642. doi: 10.1073/pnas.92.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 34.Peter M, Nakagawa J, Doree M, Labbe J C, Nigg E A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamin by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 35.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roizman B. Virus infection of cells in mitosis. I. Observations on the recruitment on cells in karyokinesis into giant cells induced by herpes simplex virus and bearing on the site of antigen formation. Virology. 1961;13:387–401. doi: 10.1016/0042-6822(61)90269-0. [DOI] [PubMed] [Google Scholar]
- 38.Roizman B, Roane P R., Jr Multiplication of herpes simplex virus. II. The relation between protein synthesis and the duplication of viral DNA in infected HEp-2 cells. Virology. 1964;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 39.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2296. [Google Scholar]
- 40.Roller R J, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schang L M, Rosenberg A, Schaffer P A. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 44.Van Sant C, Kawaguchi Y, Roizman B. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc Natl Acad Sci USA. 1999;96:8184–8189. doi: 10.1073/pnas.96.14.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilcock D, Lane D P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cell. Nature. 1991;349:429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Orlandi L, Wang P, Orr M S, Senderowicz A M, Sausville E A, Silvestrini R, Watanabe N, Piwnica-Worms H, O'Connor P M. UCN-01 abrogates G2 arrest through a cdc2-dependent pathway that is associated with inactivation of the wee1hu kinase and activation of the cdc25C phosphatase. J Biol Chem. 1998;273:33455–33464. doi: 10.1074/jbc.273.50.33455. [DOI] [PubMed] [Google Scholar]
- 47.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase cdc25C by cds or chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]