Abstract
In accordance with Article 43 of Regulation (EC) No 396/2005, EFSA received a mandate from the European Commission to perform a targeted risk assessment for residues of lambda‐cyhalothrin in poultry products (meat/muscle, fat, liver, kidney, edible offal) and bird's eggs. EFSA performed the acute (short‐term) and chronic (long‐term) dietary risk assessment considering the lambda‐cyhalothrin exposure via residues in food commodities from poultry and birds' eggs at the levels of the proposed temporary maximum residue level (MRL) of 0.03 mg/kg and 0.02 mg/kg, respectively. These temporary MRLs were derived by the European Commission from monitoring data provided by EU member States and compiled by EFSA in a central database. Based on the risk assessment results, EFSA concluded that the proposed temporary MRL is unlikely to pose a risk to consumer health.
Keywords: birds' eggs, consumer risk assessment, lambda‐cyhalothrin, pesticide, poultry, temporary MRL
BACKGROUND
Lambda‐cyhalothrin is an active substance used in plant protection products that is approved in the EU under Regulation (EC) No 1107/2009. 1 The maximum residue levels (MRLs) for lambda‐cyhalothrin are set in Annex II of Regulation (EC) No 396/2005. 2 The residue definition for MRL enforcement is established as lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S and S,R isomers).
Cyhalothrin is also a pharmacologically active substance that may be used in veterinary medical products (VMP). MRLs have been set by Commission Regulation (EC) 37/2010 3 for bovine fat, kidney and milk. MRLs have not been set for poultry and eggs. The residue definition under the VMP legislation covers cyhalothrin (sum of isomers). The use of cyhalothrin would lead to residues measured as lambda‐cyhalothrin.
Lastly, lambda‐cyhalothrin is also approved as biocidal active substance for use in biocidal products of product‐type 18 4 (insecticides), including the use as insecticide in animal housings.
The European Commission received information from Member States and by EFSA showing the presence of lambda‐cyhalothrin in commodities from poultry and birds' eggs leading to higher residues than the default MRL of 0.01 mg/kg laid down in Regulation (EC) No 396/2005.
The source of these residues in the commodities concerned has not been elucidated and may originate from its use as a biocide. MRLs for pesticides should take into account residues that may arise as a result of use of active substances, currently or formerly used in plant protection products, as biocides. Therefore, the European Commission considers that it may be appropriate to establish temporary MRLs for lambda‐cyhalothrin in commodities from poultry and birds' eggs in order to cover residues originating potentially from biocidal use.
The European Commission received recent monitoring data from EFSA, covering the period between 2018 and 2022. Based on these data, the setting of temporary MRLs for lambda‐cyhalothrin at the level of 0.03 mg/kg in commodities from poultry and 0.02 mg/kg in birds' eggs might be appropriate. Before proposing the amendment of existing MRLs for those products, the European Commission requests EFSA to assess the potential risk for EU consumers from these (higher) MRLs.
TERMS OF REFERENCE
EFSA is requested, according to Article 43 of Regulation (EC) No 396/2005:
to assess the acute (short‐term) exposure for European consumers related to lambda‐cyhalothrin residues in commodities from poultry (1016000) and in birds' eggs (1030000) at the level derived from the new monitoring data received (i.e. 0.03 mg/kg for commodities from poultry and 0.02 mg/kg for birds' eggs). In addition, the contribution of lambda‐cyhalothrin residues in commodities from poultry and birds' eggs at the level of 0.03 and 0.02 mg/kg, respectively, to the overall chronic (long‐term) exposure of European consumers should be assessed. The exposure/risk assessment shall be performed with the newest version of the PRIMo model (EFSA, 2018, 2019), based on the currently applicable residue definition which was derived by EFSA in 2014 (EFSA, 2014a)
to recommend, in case risks for consumers are identified for commodities from poultry and/or birds' eggs containing lambda‐cyhalothrin at the above‐mentioned levels, MRLs that do not pose unacceptable risks to consumers, and/or advise risk managers on alternative options
ASSESSMENT
Cyhalothrin 5 is the unresolved mixture of four cis‐isomers of (RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate; the molecular structure is presented in Appendix C. Cyhalothrin is not approved under Regulation (EC) No 1107/2009. In the EU, its use is permitted as an active ingredient in veterinary medicinal products (EMEA, 2001) for use in bovine species, authorised in accordance with Regulation (EU) 2019/6. 6 Although the Summary Report of the Committee for Veterinary Medicinal Products (CVMP) (EMEA, 2001) indicates that it is intended for external use against ectoparasites, the established MRLs would also apply if the substance were used by a route other than topical administration. 7
Lambda‐cyhalothrin is the ISO common name for the 1:1 mixture of (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropane carboxylate (IUPAC), representing two of the four isomers contained in cyhalothrin. The molecular structure can be found in Appendix C. The active substance is approved for the uses in plant protection products under Regulation (EC) No 1107/2009 and in biocidal products under Regulation (EU) No 528/2012. 8
Gamma‐cyhalothrin is the ISO common name for (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropane‐carboxylate (IUPAC), representing one of the two isomers contained in lambda‐cyhalothrin. The molecular structure can be found in Appendix C. Gamma‐cyhalothrin is also approved as active substance in plant protection products under Regulation (EC) no 1107/2009.
1. TOXICOLOGICAL REFERENCE VALUES
The toxicological profile of cyhalothrin was assessed by European Medicines Agency in the framework of the approval of cyhalothrin for uses in veterinary medicinal products (EMEA, 2001). The acceptable daily intake (ADI) value of 0.005 mg/kg body weight (bw) was derived. While no acute reference dose (ARfD) was established, the assessment did consider short‐term neurotoxicity studies as well as reports of effects in humans. The overall toxicological reference value (i.e. the ADI) was based on clinical signs of neurotoxicity seen in a 52‐week study in dogs, as this was the most sensitive endpoint reported (from both short‐term and long‐term studies).
The toxicological profile of lambda‐cyhalothrin was assessed by EFSA in the framework of the EU pesticides peer review (European Commission, 2020). The following toxicological reference values were derived:
Acceptable daily intake (ADI): 0.0025 mg/kg body weight (bw) per day.
Acute reference dose (ARfD): 0.005 mg/kg bw.
The toxicological profile of lambda‐cyhalothrin has also been assessed by the European Chemicals Agency (ECHA) upon the approval of the active substance for the use in biocidal products in the Assessment Report prepared by Sweden (Sweden, 2011). The following toxicological reference values were derived:
Acceptable daily intake (ADI): 0.005 mg/kg bw per day.
Acute reference dose (ARfD): 0.0075 mg/kg bw.
Toxicological reference values have been derived also for gamma‐cyhalothrin in the framework of the EU pesticides peer review (European Commission, 2014):
Acceptable daily intake: 0.0012 mg/kg bw per day.
Acute reference dose: 0.0025 mg/kg bw.
Cyhalothrin, lambda‐cyhalothrin and gamma‐cyhalothrin belong to the group of pyrethroid compounds, a class of synthetic insecticides. Pyrethroids are known to form common metabolites, i.e. 3‐phenoxybenzoic acid (PBA or 3‐PBA) and 3‐(4′‐hydroxyphenoxy)benzoic acid (PBA (OH) or 4‐OH‐PBA). These metabolites are frequently identified in commodities of plant origin, and therefore, its toxicity was assessed by EFSA Panel on Plant Protection Products and their Residues in a scientific opinion on toxicity of pyrethroid common metabolites (EFSA PPR Panel, 2022). For both metabolites, the following toxicological reference values were derived:
Acceptable daily intake: 0.1 mg/kg bw per day.
Acute reference dose: 1 mg/kg bw.
Compared to the parent pyrethroid compounds, the metabolites are less toxic. The assessment of consumer exposure to PBA and PBA(OH) from the uses of lambda‐cyhalothrin is not within the remit of the present mandate and was therefore not performed.
In the EU pesticides peer review, it was concluded that gamma‐cyhalothrin is the biologically most active isomer of cyhalothrin. Toxicokinetics and metabolism of gamma‐cyhalothrin, cyhalothrin and lambda‐cyhalothrin were considered unlikely to be different (EFSA, 2014b). On the basis of the assessment, a relative potency factor of 2 can be used for acute and chronic risk assessment to take into account the hazard contribution of gamma‐cyhalothrin to lambda‐cyhalothrin (EFSA, 2017).
The consumer risk assessment, according to the terms of reference of the present mandate is performed considering the toxicological reference values (TRVs) for lambda‐cyhalothrin as derived by EFSA in the EU pesticides peer review. It is noted that these values are more conservative that the TRVs derived for the active substance by ECHA.
2. RESIDUE LEVELS AND RESIDUE DEFINITIONS
2.1. Residue definitions
Under Regulation (EC) No 396/2005, the enforcement residue definition for lambda‐cyhalothrin in all plant and animal commodities is established as ‘lambda cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S and S,R isomers)’. For animal commodities, the residue is expected to accumulate in fat tissue. In Regulation (EC) No 396/2005, it is therefore flagged as fat‐soluble.
In the framework of the Article 12 MRL review, the residue definition for risk assessment for lambda‐cyhalothrin was derived on a tentative basis as ‘lambda‐cyhalothrin’ (alone) (EFSA, 2014a); it is applicable to all plant and animal commodities (raw and processed).
For processed commodities, the residue definition for risk assessment was set on a tentative basis, pending the assessment of the toxicological relevance of the degradation products formed under sterilisation conditions, i.e. compounds Ia, IV (PBAld) and gamma‐lactone (EFSA, 2014a).
For animal commodities, the residue definition for risk assessment was set on a tentative basis in the absence of metabolism studies in ruminants and poultry matrices with lambda cyhalothrin labelled on phenoxy moiety (EFSA, 2014a). The EU pesticides peer review considered the available metabolism studies with another pyrethroid active substance cypermethrin (labelled on phenoxy moiety) as acceptable to conclude that lambda‐cyhalothrin is the predominant compound in animal matrices, except in liver and kidney, where metabolites Ia, XI (cyclopropyl label metabolites), PBA, PBA (OH) and XIII (phenoxybenzyl moiety metabolites) were recovered predominantly (EFSA, 2014b). Although feeding studies were not triggered, the EU pesticides peer review noted, that in case of increase of EU livestock dietary burden, the magnitude of these compounds should be reconsidered and their toxicological properties may need to be addressed (EFSA, 2014b).
In 2021, the European Commission mandated EFSA to determine whether PBA and PBA (OH) (metabolites common to several pyrethroid substances) should be included in the residue definitions for risk assessment of pyrethroid active substances. EFSA concluded that to derive a final decision on the need to establish a residue definition which covers the common metabolites bearing the 3‐phenoxybenzoyl moiety resulting from the use of active substances belonging to the class of pyrethroids additional data are required, i.e. in vitro and/or in vivo micronucleus tests to address the aneugenicity of PBAld and reliable residue data on the expected residue concentration of PBA, PBA(OH) (including their conjugates) and PBAld in food resulting from the use of pyrethroids as active substances (a.s.) in plant protection products, biocides and veterinary medicinal products (EFSA, 2023a). Hence, a final decision on the establishment of a separate residue definition for common metabolites of pyrethroids is still pending.
2.2. Residue levels in poultry tissues and birds' eggs
Under Regulation (EC) No 396/2005, EU MRLs for ‘lambda cyhalothrin (includes gamma‐cyhalothrin) sum of R,S and S,R isomers’ in poultry tissues – muscle, fat, liver, kidney and edible offal – and in birds' eggs are set at the limit of quantification (LOQ) of 0.01 mg/kg. These MRLs are currently set on a tentative basis, pending the submission of information required to address the Article 12 confirmatory data gaps, related to:
information on toxicological relevance of degradation products (compounds Ia, IV (3‐phenoxybenzaldehyde; PBAld) and gamma lactone) formed under sterilisation conditions (data gap relevant for all poultry tissues and birds' eggs) and
toxicological properties of metabolites Ia and XI (data gap relevant for liver and kidney of poultry).
The deadline to submit Article 12 confirmatory data expired on 6 July 2020 and under this framework no new data have been submitted.
In the framework of the assessment of confirmatory data of the EU pesticides peer review for gamma‐cyhalothrin (EFSA, 2019c), the toxicological profile of compound Ia, 9 which is the primary metabolite resulting from the ester cleavage of cyhalothrin molecule has been addressed. It was concluded that the toxicity profile of this metabolite is covered by the toxicological reference values established for the parent (EFSA, 2019c).
For the remaining compounds identified in hydrolysis studies (PBAld and gamma lactone) and livestock metabolism studies (metabolite XI), the data gaps identified in the MRL review are not yet addressed.
Cyhalothrin may be used in veterinary medicines for cattle only. Its use in veterinary medicines for other food‐producing animal species is not allowed. Consequently, MRLs are not established for poultry tissues and birds' eggs under Commission Regulation (EC) 37/2010.
Lambda‐cyhalothrin is used in biocidal products of product‐type 18 (insecticides, acaricides and products to control other arthropods) for controlling of insects, ants, etc. When biocides are used in animal husbandry, the MRLs established in the Commission Regulation (EC) 37/2010 apply, in line with Art.10 10 of Regulation (EC) No 470/2009. 10 As mentioned above, no uses of cyhalothrin or lambda cyhalothrin are currently allowed in veterinary medicine for the treatment of poultry. Therefore, no residue limits have been established for poultry food commodities resulting from the authorised uses of lambda‐cyhalothrin in biocidal products.
The temporary MRLs proposed for commodities of poultry and birds' eggs under the present mandate have been derived from monitoring data submitted to EFSA in the framework of Art. 31 of Regulation (EC) No 396/2005 covering the period between 2018 and 2022.
Based on the monitoring data, the European Commission derived temporary MRL proposals of 0.03 mg/kg for poultry muscle, fat, liver, kidney and other edible offal, based on a statistical assessment considering the distribution of the residues measured. For eggs, a temporary MRL of 0.02 mg/kg was calculated. Under the present assessment, EFSA was not mandated to investigate either the source of lambda‐cyhalothrin residues leading to these MRL proposals or the validity of these data with respect to the nature and magnitude of lambda‐cyhalothrin residues in livestock.
3. CONSUMER RISK ASSESSMENT
In accordance with the terms of reference of the present mandate, EFSA performed the targeted short‐term and long‐term dietary risk assessment for the proposed temporary MRLs for lambda‐cyhalothrin of 0.03 mg/kg for poultry tissues (meat/muscle, fat, liver, kidney and other edible offal) (commodity code 1016000) and of 0.02 mg/kg for birds' eggs (commodity code 1030000).
As requested in the terms of reference, the consumer exposure assessment was performed for the risk assessment residue definition which was derived by the MRL review in 2014 as ‘lambda cyhalothrin’ (EFSA, 2014a). This residue definition was confirmed in 2017 (EFSA, 2017). For poultry products, the assumption was made that the proposed temporary MRLs refer to lambda‐cyhalothrin. It is noted that this residue definition was proposed on a tentative basis.
The consumer exposure calculation was performed applying the toxicological reference values derived for lambda‐cyhalothrin by the EU pesticides peer review: ADI of 0.0025 mg/kg bw per day and ARfD of 0.005 mg/kg bw (European Commission, 2020).
The assumptions and the input values for the chronic and the acute risk assessment are further explained in Sections 3.1 and 3.2.
Since the origin of the residues is not known, and the monitoring data do not allow to distinguish whether the residues measured refer to the more toxic gamma‐cyhalothrin, EFSA calculated also a worst‐case scenario, assuming that the residues in poultry matrices and birds' eggs consist of the more toxic gamma‐cyhalothrin (Section 3.3). In this scenario, the calculated exposure from poultry tissues and birds' eggs was compared with the toxicological reference values derived by the EU pesticides peer review for gamma‐cyhalothrin (see Section 1). To take into account the higher toxicity of gamma‐cyhalothrin, a relative potency factor of 2 was applied to the input values of poultry tissues (muscle, fat, liver, kidney, edible offals) and birds' eggs.
The dietary risk assessment was performed using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019). This exposure assessment model contains food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
3.1. Chronic (long‐term) dietary risk assessment
The chronic exposure calculation was performed with the temporary MRL proposals for poultry tissues and eggs; for other food commodities of plant and animal origin with MRLs set above the limit of quantification (LOQ), risk assessment values (supervised trials median residue (STMR) values) derived in the MRL review and in subsequent EFSA outputs and JMPR evaluations were used for the chronic risk assessment. If no STMR values could be retrieved, the calculations were performed with the MRL. In addition, the recent MRL proposal assessed by EFSA for avocados (EFSA, 2023b), although not legally implemented yet, was considered in the chronic exposure assessment. The commodities for which authorised uses of lambda cyhalothrin have not been reported in the framework of the Article 12 MRL review or in subsequent EFSA outputs were not considered in the chronic exposure assessment, assuming a no residue situation. The summary of input values is provided in Appendix A.
Assuming that all residues in plant and animal commodities are entirely composed of lambda‐cyhalothrin, no chronic consumer intake concern was identified, as the total calculated exposure to lambda‐cyhalothrin residues accounted for a maximum of 91% of the ADI (NL toddler diet).
The contribution of residues in poultry tissues to the total exposure was the highest for poultry muscle (1.74% ADI, GEMS/Food G10 diet); for the other poultry tissues, the contribution to the overall chronic exposure was below 0.05% of the ADI. Residues in chicken eggs accounted for up to 1.08% of the ADI (highest long‐term exposure was calculated for UK Infant). For other types of eggs, consumption data are not available. The lack of consumption data on these very minor commodities are not expected to have an impact on the total long‐term consumer exposure.
3.2. Acute (short‐term) dietary risk assessment
The acute exposure calculation was performed with the temporary MRL proposals for poultry tissues and eggs.
No acute intake concerns were identified for lambda‐cyhalothrin residues in commodities of poultry (muscle, fat, liver, kidney and edible offal) and birds' eggs containing lambda‐cyhalothrin residues at the proposed temporary MRLs.
The highest acute exposure was calculated for children for residues in poultry muscle (10.2% of the ARfD) and chicken eggs (5% of the ARfD) with lower exposure from the intake of poultry liver (0.7% of the ARfD) and poultry fat (0.1% of the ARfD); for other commodities and other eggs, no consumption data were available, and therefore, no exposure could be calculated. The lack of consumption data for these minor commodities is not expected to affect the conclusions on the acute exposure to lambda‐cyhalothrin residues.
For adults, the calculated acute exposure accounted for 7% of the ARfD from the intake of poultry muscle, 2.8% of the ARfD from poultry liver, 1.7% of the ARfD from chicken eggs, 0.8% of the ARfD from poultry kidney, 0.6% of the ARfD from quail eggs and 0.2% of the ARfD from poultry fat and goose eggs. For duck eggs and poultry edible offal, no consumption data are available to calculate the acute exposure. The lack of consumption data for these minor commodities is not expected to affect the conclusions on the acute exposure to lambda‐cyhalothrin residues.
EFSA notes that acute intake concerns are identified for other commodities not falling under the remit of the present mandate: pears, peaches, swine muscle and apples, boiled broccoli and boiled fennel. 11 These exceedances of the ARfD have been already noted in the previous EFSA outputs on lambda cyhalothrin (EFSA, 2020, 2023b). They are mainly related to the use of PRIMo 3.1, while for the MRL review in 2014, the previous version of PRIMo (rev. 2) was used. Due to changes in the large portion consumption data, for some commodities, the exposure calculated with PRIMo 3.1 is higher than the results derived with PRIMo 2. In the latest assessment of the import tolerance MRL application on avocados EFSA explored options to refine the acute exposure for these commodities (EFSA, 2023b). The conclusions derived therein will not be further reiterated under the present assessment as not affecting the conclusions on the commodities under consideration.
EFSA concludes that the temporary MRL proposals for lambda‐cyhalothrin at the level of 0.03 mg/kg in poultry muscle, fat, liver, kidney and edible offal and at the level of 0.02 mg/kg in birds' eggs are unlikely to pose an acute consumer risk.
The calculated acute and chronic consumer exposure is, however, affected by uncertainties related to the lack of toxicological assessment of metabolite XI potentially present in livestock kidney and liver and degradation products 3‐phenoxybenzaldehyde (PBald) and gamma lactone formed under sterilisation conditions. The relevance of these compounds for the products under consideration shall be investigated in the framework of the assessment of Article 12 confirmatory data.
The detailed results of the chronic and acute risk calculations derived with PRIMo rev. 3.1 are presented in Appendix B.
3.3. Consumer dietary risk assessment related to gamma‐cyhalothrin residues in poultry tissues and birds' eggs
EFSA performed additional consumer dietary risk assessment where the calculated chronic and acute exposure from poultry commodities and birds' eggs was compared with the toxicological reference values derived for gamma‐cyhalothrin. This was done by applying the relative potency factor of 2 to the temporary MRL proposals derived for poultry tissues (muscle, fat, liver, kidney and edible offal) and birds' eggs, noting that gamma‐cyhalothrin is two times more toxic than lambda‐cyhalothrin. For the remaining plant and animal commodities, the input values in the exposure calculation remained unchanged.
This exposure calculation reflects the worst‐case scenario assuming that all residues in poultry tissues and birds' eggs at the level of the proposed temporary MRLs are entirely composed of a more toxic gamma‐cyhalothrin.
Results of this exposure scenario indicated no chronic intake concerns. The calculated exposure accounted for a maximum of 92% of the ADI (NL toddler diet).
No acute intake concerns are associated with potential gamma‐cyhalothrin residues in poultry tissues and birds' eggs. The highest acute exposure for children was calculated from the intake of poultry muscle (20.4% of the ARfD) and chicken eggs (9.9% of the ARfD). The acute exposure from the intake of poultry liver and fat was estimated at 1.3% and 0.1% of the ARfD, respectively. For remaining minor commodities, no consumption data are available but that is not expected to affect the conclusions on the acute exposure to gamma‐cyhalothrin residues.
For adults, the acute exposure was the highest for poultry muscle (14.1% of the ARfD), poultry liver (5.6% of the ARfD) and chicken eggs (3.4% of the ARfD). For remaining commodities, the individual exposure was 0.4% of the ARfD for poultry fat, 1.5% of the ARfD for poultry kidney, 1.1% of the ARfD for quail eggs an 0.4% of the ARfD for goose eggs. For duck eggs and poultry edible offal, no consumption data are available to calculate the acute exposure.
EFSA concludes that residues of gamma‐cyhalothrin in poultry tissues at the level of 0.03 mg/kg and residues in eggs at a level of 0.02 mg/kg are unlikely to pose a chronic or acute consumer risk.
The detailed results of the chronic and acute risk calculations derived with PRIMo rev. 3.1 are presented in Appendix B.
CONCLUSIONS AND RECOMMENDATIONS
Overall, the exposure calculations performed by EFSA ascertained that the proposed temporary MRLs for lambda‐cyhalothrin at the level of 0.03 mg/kg in poultry muscle, fat, liver, kidney and edible offal and at the level of 0.02 mg/kg in birds' eggs are unlikely to pose an unacceptable risk to consumer health.
The calculated consumer exposure is affected by uncertainties related to the lack of information on the toxicological relevance of metabolite XI in livestock kidney and liver and of degradation products 3‐phenoxybenzaldehyde (PBald; (compound IV)) and gamma lactone in processed commodities undergoing sterilisation. The relevance of these compounds in the products under consideration shall be investigated in the framework of the assessment of Article 12 confirmatory data or in the upcoming renewal of the approval of lambda‐cyhalothrin.
Additionally, EFSA notes that the relevance of common pyrethroid metabolites (PBA, PBA(OH), including their conjugated forms, and PBAld) to the overall consumer dietary exposure from the uses of pyrethroid active substances remains not addressed thus adding to the uncertainty of the consumer exposure assessment.
There was no need to advise risk managers on alternative MRL options for commodities from poultry and birds' eggs, as outlined in the second bullet point of the terms of reference, since EFSA did not identify an unacceptable risk for consumers for the proposed temporary MRLs of 0.03 and 0.02 mg/kg for commodities from poultry and birds' eggs, respectively.
EFSA derived the following recommendations:
-
–
Since the origin of the residues in poultry is not clearly identified, the root cause for the occurrence of lambda‐cyhalothrin in the commodities concerned should be further investigated, and measures should be taken to limit/avoid residues in poultry products.
-
–
If the residues in poultry products are resulting from the use of biocidal products in accordance with the approved use, studies should be generated that would allow setting of definitive MRLs replacing the temporary MRLs.
-
–
As the residue definition covers a more toxic isomer (gamma‐cyhalothrin), analytical methods should be developed that would allow separation of lambda‐cyhalothrin from gamma‐cyhalothrin and other isomers of cyhalothrin in order to identify the nature of residues contained in poultry tissues and birds' eggs.
-
–
EFSA also recommends addressing the data gaps identified in the MRL review of lambda‐cyhalothrin and which are potentially relevant for the commodities from poultry and birds' eggs
The summary of the assessment and the recommendation are summarised below (Table 1).
TABLE 1.
Summary table.
| Code a | Commodity | Existing tMRLs (mg/kg) according to Reg. (EU) 2021/590 | Outcome of the assessment | |
|---|---|---|---|---|
| tMRL proposed (mg/kg) | Comment | |||
| Enforcement residue definition: lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers) | ||||
| (1016000) Poultry |
0.03 Risk management consideration required |
The proposed temporary MRL is unlikely to pose a consumer health concern neither for children nor for adults The consumer exposure assessment is affected by uncertainties related to the data gaps identified in the MRL review The risk management decision is required on whether the MRL is proposed on tentative basis, pending the assessment of Article 12 confirmatory data b |
||
| 1016010 | Muscle | 0.01* (ft.1) | ||
| 1016020 | Fat | 0.01* (ft.1) | ||
| 1016030 | Liver | 0.01* (ft.2) | ||
| 1016040 | Kidney | 0.01* (ft.2) | ||
| 1016050 | Edible offals (other than liver and kidney) | 0.01* (ft.1) | ||
| 1016990 | Others | 0.01* (ft.1) | ||
| 1030000 Birds' eggs |
0.02 Risk management consideration required |
The proposed temporary MRL is unlikely to pose a consumer health concern neither for children nor for adults The consumer exposure assessment is affected by uncertainties related to the data gaps identified in the MRL review The risk management decision is required on whether the MRL is proposed on tentative basis, pending the assessment of Article 12 confirmatory data b |
||
| 1030010 | Chicken | 0.01* (ft.1) | ||
| 1030020 | Duck | 0.01* (ft.1) | ||
| 1030030 | Geese | 0.01* (ft.1) | ||
| 1030040 | Quail | 0.01* (ft.1) | ||
| 1030990 | Others | 0.01* (ft.1) | ||
Abbreviation: tMRL, temporary maximum residue level.
Indicates that the MRL is set at the limit of quantification.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
It is noted that the data gap on the toxicity of compound Ia has been addressed in the framework of the assessment of confirmatory data of the EU pesticides peer review for the active substance gamma‐cyhalothrin where it was concluded that the toxicity profile of the metabolite CPCA (compound Ia) is covered by the toxicological reference values established for the parent (EFSA, 2019c).
The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take1 into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions and on the toxicological properties of some others (compounds Ia and XI) as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
ABBREVIATIONS
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- CXL
codex maximum residue limit
- ECHA
European Chemicals Agency
- EMA/EMEA
European Medicines Agency
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HR
highest residue
- IESTI
International estimated short‐term intake
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LOD
Limit of detection
- LOQ
limit of quantification
- LP
large portion
- MRL
maximum residue level
- PAFF
Standing Committee on Plants, Animals, Food and Feed
- PF
processing factor
- PPP
plant protection products
- PRIMo
(EFSA) Pesticide Residues Intake Model
- SCoPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- STMR
supervised trials median residue
- tMRL
temporary MRL
- TRV
Toxicological Reference Values
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2024‐00269
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
APPENDIX A. Input values for the exposure calculations
A.1. Consumer risk assessment
| Commodity | Existing/proposed MRL | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | ||
| Risk assessment residue definition: lambda cyhalothrin (tentative; EFSA, 2014a ) | |||||
| Citrus fruits | 0.2 | 0.003 | STMR × PeF (EFSA, 2015) | 0.0096 | HR × PeF (EFSA, 2015) |
| Tree nuts | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Apples | 0.08 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | STMR (EFSA, 2015, 2017) |
| Pears | 0.08 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | HR (EFSA, 2015, 2017) |
| Medlar | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Loquat | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Quinces | 0.2 | 0.08 | STMR (EFSA, 2015) | 0.1 | HR (EFSA, 2015) |
| Apricots | 0.15 | 0.03 | STMR (EFSA, 2015) | 0.07 | HR (EFSA, 2015) |
| Cherries | 0.3 | 0.13 | STMR (EFSA, 2015) | 0.18 | HR (EFSA, 2015) |
| Peaches | 0.15 | 0.03 | STMR (EFSA, 2015, 2017) | 0.07 | HR (EFSA, 2015) |
| Plums | 0.2 | 0.02 | STMR (EFSA, 2015, 2017) | 0.10 | HR (EFSA, 2015) |
| Table grapes | 0.08 | 0.01 | STMR (EFSA, 2015, 2017, a ) | 0.05 | STMR (EFSA, 2015, 2017, a ) |
| Wine grapes | 0.2 | 0.02 | STMR (EFSA, 2014a) | 0.09 | HR (EFSA, 2014a) |
| Strawberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Cane fruits | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Blueberries, Cranberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Currants | 0.2 | 0.06 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Gooseberries, Rose hips | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Mulberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Azaroles, Elderberries | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Table olives | 1 | 0.13 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Kaki/Japanese persimmons | 0.09 | 0.02 | STMR (EFSA, 2015) | 0.04 | HR (EFSA, 2015) |
| Kiwi | 0.05 | 0.0105 | STMR × PeF (EFSA, 2015) | 0.027 | HR × PeF (EFSA, 2015) |
| Avocados | 0.15 | 0.01 | STMR (pulp) (EFSA, 2023b) | 0.01 | HR (pulp) (EFSA, 2023b) |
| Bananas | 0.15 | 0.023 | STMR × PeF (EFSA, 2015) | 0.026 | HR × PeF (EFSA, 2015) |
| Mangoes | 0.2 | 0.014 | STMR × PeF (EFSA, 2015) | 0.0196 | HR × PeF (EFSA, 2015) |
| Potatoes | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Tropical roots and tuber veg | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Beetroot | 0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Carrots | 0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Celeriac | 0.07 | 0.03 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
|
Horseradish Jerusalem artichokes Parsnips Parsley root Salsify Swedes Turnips |
0.04 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Radishes | 0.15 | 0.02 | STMR (EFSA, 2015) | 0.05 | HR (EFSA, 2015) |
| Bulb vegetables | 0.2 | 0.05 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Tomatoes | 0.07 | 0.02 | STMR (EFSA, 2015, 2017) | 0.05 | HR (EFSA, 2015, 2017) |
| Peppers | 0.1 | 0.02 | STMR (EFSA, 2015, 2017) | 0.06 | HR (EFSA, 2015, 2017) |
| Aubergines | 0.3 | 0.03 | STMR (EFSA, 2015, 2017) | 0.18 | HR (EFSA, 2015, 2017) |
| Okra | 0.3 | 0.03 | STMR (EFSA, 2015) | 0.18 | HR (EFSA, 2015) |
| Cucumbers | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Gherkins | 0.15 | 0.04 | STMR (EFSA, 2015) | 0.06 | HR (EFSA, 2015) |
| Courgettes | 0.15 | 0.04 | STMR (EFSA, 2015, 2017) | 0.06 | HR (EFSA, 2015, 2017) |
| Cucurbits with inedible peel | 0.06 | 0.005 | STMR × PeF (EFSA, 2015, 2017) | 0.02 | STMR × PeF (EFSA, 2015, 2017) |
| Sweet corn | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Flowering brassica | 0.1 | 0.02 | STMR (EFSA, 2015, 2017) | 0.07 | HR (EFSA, 2015, 2017) |
| Brussels sprouts | 0.04 | 0.02 | STMR (EFSA, 2015) | 0.02 | HR (EFSA, 2015) |
| Head cabbages | 0.15 | 0.03 | STMR (EFSA, 2015, 2017) | 0.09 | HR (EFSA, 2015, 2017) |
| Chinese cabbages | 0.3 | 0.08 | STMR (EFSA, 2015, 2017) | 0.13 | HR (EFSA, 2015, 2017) |
| Kohlrabi | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Lamb's lettuces | 1.5 | 0.34 | STMR (EFSA, 2015) | 0.63 | HR (EFSA, 2015) |
| Lettuces | 0.15 | 0.03 | STMR (EFSA, 2015) | 0.06 | HR (EFSA, 2015) |
| Escarole | 0.07 | 0.02 | STMR (EFSA, 2015, 2017) | 0.04 | HR (EFSA, 2015, 2017) |
| Cresses, Land cresses | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Roman rocket | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Baby leaf crops | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Spinach | 0.6 | 0.20 | STMR (EFSA, 2015, 2017) | 0.22 | HR (EFSA, 2015, 2017) |
| Chards/Beet leaves | 0.2 | 0.05 | STMR (EFSA, 2015) | 0.08 | HR (EFSA, 2015) |
| Herbs and edible flowers | 0.7 | 0.23 | STMR (EFSA, 2015) | 0.42 | HR (EFSA, 2015) |
| Beans with pods | 0.4 | 0.11 | STMR (EFSA, 2015) | 0.17 | HR (EFSA, 2015) |
| Beans without pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Peas with pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Peas without pods | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Lentils | 0.2 | 0.02 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Asparagus | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Celeries | 0.2 | 0.05 | STMR (EFSA, 2019b) | 0.09 | HR (EFSA, 2019b) |
| Florence fennels | 0.3 | 0.11 | STMR (EFSA, 2019b) | 0.14 | HR (EFSA, 2019b) |
| Globe artichokes | 0.15 | 0.04 | STMR (EFSA, 2015) | 0.07 | HR (EFSA, 2015) |
| Leeks | 0.07 | 0.02 | STMR (EFSA, 2015) | 0.04 | HR (EFSA, 2015) |
| Wild fungi | 0.50 | 0.17 | STMR (EFSA, 2015, 2017) | 0.23 | HR (EFSA, 2015, 2017 |
| Pulses | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Oilseeds, except soyabeans | 0.2 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Soya beans | 0.05 | 0.05 | EU MRL (EFSA, 2015) b | 0.05 | EU MRL (EFSA, 2015) b |
| Olives for oil production | 0.5 | 0.11 | STMR (EFSA, 2015) | 0.11 | STMR (EFSA, 2015) |
| Barley | 0.5 | 0.09 | STMR (EFSA, 2015) | 0.09 | STMR (EFSA, 2015) |
| Maize/corn | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Oats | 0.3 | 0.09 | STMR (EFSA, 2015) | 0.09 | STMR (EFSA, 2015) |
| Rice | 0.2 | 0.04 | STMR (EFSA, 2019b) | 0.04 | STMR (EFSA, 2019b) |
| Sorghum | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Wheat, Rye | 0.05 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Coffee | 0.01* | 0.01 | STMR (FAO, 2015) | 0.01 | STMR (FAO, 2015) |
| Hops (dried) | 10 | 3.30 | STMR (EFSA, 2015) | 3.60 | HR (EFSA, 2015) |
| Seed spices | 0.3 | 0.02 | STMR (EFSA, 2020) | 0.13 | HR (EFSA, 2015) |
| Fruit spices, except cardamom | 0.3 | 0.02 | STMR (seed spices) (EFSA, 2020) | 0.13 | HR (EFSA, 2015) |
| Cardamom | 2 | 0.28 | STMR (FAO, 2015) | 3.06 | HR (EFSA, 2015) |
| Root and rhizome spices | 0.05 | 0.05 | STMR (EFSA, 2015) | 0.05 | HR (EFSA, 2015) |
| Sugar beet roots | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Sugar canes | 0.05 | 0.02 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Chicory roots | 0.01* | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Swine, meat c | 0.15 | 0.23 | STMR (EFSA, 2015; FAO, 2009) | 0.52 | HR (EFSA, 2015; FAO, 2009) |
| Swine, fat | 3 | 1.00 | STMR (EFSA, 2015; FAO, 2009) | 2.2 | HR (EFSA, 2015; FAO, 2009) |
| Swine, liver | 0.05 | 0.008 | STMR (EFSA, 2015; FAO, 2009) | 0.02 | HR (EFSA, 2015; FAO, 2009) |
| Swine, kidney | 0.2 | 0.03 | STMR (EFSA, 2015; FAO, 2009) | 0.09 | HR (EFSA, 2015; FAO, 2009) |
| Swine, edible offal | 3 | 0.03 | STMR (EFSA, 2020) d | 0.09 | HR (EFSA, 2020) d |
| Ruminant, meat c | 0.15 | 0.23 | STMR (EFSA, 2015; FAO, 2009) | 0.52 | HR (EFSA, 2015; FAO, 2009) |
| Ruminant, fat | 3 | 1.00 | STMR (EFSA, 2015; FAO, 2009) | 2.2 | HR (EFSA, 2015; FAO, 2009) |
| Ruminant, liver | 0.05 | 0.008 | STMR (EFSA, 2015; FAO, 2009) | 0.02 | HR (EFSA, 2015; FAO, 2009) |
| Ruminant, kidney | 0.2 | 0.03 | STMR (EFSA, 2015; FAO, 2009) | 0.09 | HR (EFSA, 2015; FAO, 2009) |
| Ruminant, edible offal | 3 | 0.03 | STMR (EFSA, 2020) d | 0.09 | HR (EFSA, 2020) d |
| Poultry meat c |
0.03 (proposed tMRL) |
0.03 | Proposed tMRL | 0.03 | Proposed tMRL |
| Poultry fat | 0.06 f | Proposed tMRL (0.03 mg/kg) × relative potency factor (2) g | 0.06 f | Proposed tMRL (0.03 mg/kg) × relative potency factor (2) g | |
| Poultry liver | |||||
| Poultry kidney | |||||
| Poultry, edible offal | |||||
| Equine, other farmed, meat c | 0.15 | 0.23 | STMR (EFSA, 2015; FAO, 2009) | 0.52 | HR (EFSA, 2015; FAO, 2009) |
| Equine, other farmed, fat | 3 | 1.00 | STMR (EFSA, 2015; FAO, 2009) | 2.2 | HR (EFSA, 2015; FAO, 2009) |
| Equine, other farmed, liver | 0.05 | 0.008 | STMR (EFSA, 2015; FAO, 2009) | 0.2 | HR (EFSA, 2015; FAO, 2009) |
| Equine, other farmed, kidney | 0.2 | 0.03 | STMR (FAO, 2009, 2015) | 0.09 | HR (EFSA, 2015; FAO, 2009) |
| Equine, other farmed edible offal | 3 | 0.03 | STMR (EFSA, 2020) d | 0.09 | HR (EFSA, 2020) d |
| Ruminant milk | 0.02 | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Birds' eggs |
0.02 (proposed tMRL) |
0.02 | Proposed tMRL | 0.02 | Proposed tMRL |
| 0.04 f | Proposed tMRL (0.02 mg/kg) × relative potency factor (2) g | 0.04 f | Proposed tMRL (0.02 mg/kg) × relative potency factor (2) g | ||
Abbreviations: HR, highest residue; PeF, peeling factor; STMR, supervised trials median residue.
STMR derived from the approved use of gamma‐cyhalothrin was multiplied by a potency factor of 2 to take into account the hazard contribution of gamma‐cyhalothrin to lambda‐cyhalothrin (EFSA, 2017). This was not considered relevant for lambda‐cyhalothrin, and therefore, this potency factor was not applied to the risk assessment of lambda‐cyhalothrin in line with the residue definition for risk assessment.
EU MRL implemented in MRL legislation and derived during the MRL review revision (EFSA, 2015)
Consumption figures in the EFSA PRIMo are expressed as meat. Since the a.s. is a fat‐soluble pesticides, STMR and HR residue values were calculated considering a 80%/90% muscle and 20%/10% fat content for mammal/poultry meat, respectively (FAO, 2016).
For edible offal of mammals, the input values derived for kidney were included in the calculation (EFSA, 2020). It is noted that the existing EU MRL for edible offal of mammals has been established on the basis of a CXL for fat (3 mg/kg). However, since it is unlikely that edible offal will consist purely of fat, the risk assessment values derived for kidney were used for the consumer exposure assessment.
MRL proposal derived by EFSA, 2023b; not implemented yet.
Value used only in the worst‐case exposure scenario (see Section 3.3), assuming that all residues at the level of proposed temporary MRL consist of gamma‐cyhalothrin.
Relative potency factor of 2 applied to take into account the hazard contribution of gamma‐cyhalothrin to lambda‐cyhalothrin.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
APPENDIX B. Pesticide residue intake model (PRIMo)
B.1.
-
1
Lambda‐cyhalothrin

-
2
Lambda‐cyhalothrin (using gamma‐cyhalothrin TRVs for poultry)

Additional consumer exposure assessment, assuming that all residues in poultry tissues and birds ‘eggs consist of gamma‐cyhalothrin. The toxicological potency factor of 2 was applied to the input value of 0.03 mg/kg for poultry tissues and of 0.02 mg/kg for bird's eggs to account for a higher toxicity of gamma‐cyhalothrin.
APPENDIX C. Used compound codes
C.1.
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
| Lambda‐cyhalothrin |
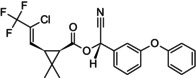
Reaction product comprising equal quantities of (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: reaction product comprising equal quantities of (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@@H]1[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl) = C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
|
| Gamma‐cyhalothrin |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F ZXQYGBMAQZUVMI‐GCMPRSNUSA‐N |

|
| Cyhalothrin |
(RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate Rothamsted‐style stereodescriptors: (RS)‐α‐cyano‐3‐phenoxybenzyl (1RS)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐enyl]‐2,2‐dimethylcyclopropanecarboxylate CC1(C)[C@H]([C@H]1\C=C(/Cl)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1.FC(F)(F)C(/Cl) = C/[C@H]1[C@@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C.Cl\C(=C/[C@@H]1[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl)=C/[C@H]1[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc3ccccc3)c2)C1(C)C OOAOVGPMANECPJ‐RWEUCVCFSA‐N |
|
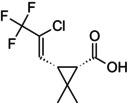
| Compound Ia |
(1R,3R)‐3‐[(1Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐en‐1‐yl]‐2,2‐dimethylcyclopropane‐1‐carboxylic acid—(1S,3S)‐3‐[(1Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐en‐1‐yl]‐2,2‐dimethylcyclopropane‐1‐carboxylic acid (1/1) Cl\C(=C/[C@H]1[C@@H](C(=O)O)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl) = C/[C@@H]1[C@H](C(=O)O)C1(C)C DPUIEEBDWOJPHB‐OBDQHKNMSA‐N |
|
|
Compound IV (PBAldehyde/PBAld) |
3‐phenoxybenzaldehyde O=Cc1cc(Oc2ccccc2)ccc1 MRLGCTNJRREZHZ‐UHFFFAOYSA‐N |

|
| Compound XI |
3‐[(1Z)‐2‐chloro‐3,3,3‐trifluoroprop‐1‐en‐1‐yl]‐2‐(hydroxymethyl)‐2‐methylcyclopropane‐1‐carboxylic acid Cl\C(=C/C1C(C(=O)O)C1(C)CO)C(F)(F)F DXNGARGFKRJLRK‐DJWKRKHSSA‐N |

|
| Gamma‐lactone (R947650) |
(1RS,4RS,5SR)‐4‐[(1RS)‐1‐chloro‐2,2,2‐trifluoroethyl]‐6,6‐dimethyl‐3‐oxabicyclo[3.1.0]hexan‐2‐one (Unstated stereochemistry) CC1(C)C2C(=O)OC(C(Cl)C(F)(F)F)C21 ZSSZFVGRINYCPY‐UHFFFAOYSA‐N |

|
|
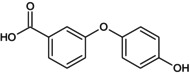
PBA(OH) 4‐OH‐3PBA |
3‐(4‐hydroxyphenoxy)benzoic acid O=C(O)c1cc(Oc2ccc(O)cc2)ccc1 OSGCDVKVZWMYBG‐UHFFFAOYSA‐N |
|
|
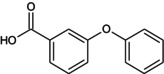
PBA 3‐PBA |
3‐phenoxybenzoic acid O=C(O)c1cc(Oc2ccccc2)ccc1 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |
|
| XIII (unstated stereochemistry) |
hydroxy(3‐phenoxyphenyl)acetic acid O=C(O)C(O)c1cc(Oc2ccccc2)ccc1 FPUCYPXKIFVDSD‐UHFFFAOYSA‐N |

|
Abbreviations: InChiKey, International Chemical Identifier Key; IUPAC, International Union of Pure and Applied Chemistry; SMILES, simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
It should be noted that Lambda‐cyhalothrin, Gamma‐cyhalothrin, Cyhalothrin, Compound Ia, Compound XI and Gamma‐lactone are identified as a pesticide active substance/metabolites that meet the definition of per‐ and polyfluoroalkyl substances (PFAS) based on its chemical structure (https://echa.europa.eu/hot‐topics/perfluoroalkyl‐chemicals‐pfas).
EFSA (European Food Safety Authority) , (2024). Targeted risk assessment of maximum residue levels for lambda‐cyhalothrin in commodities from poultry and birds' eggs. EFSA Journal, 22(6), e8816. 10.2903/j.efsa.2024.8816
Approved: 08 May 2024
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC (OJ L 309, 24.11.2009, p. 1). ELI: http://data.europa.eu/eli/reg/2009/1107/oj.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (OJ L 70, 16.3.2005, p. 1). ELI: http://data.europa.eu/eli/reg/2005/396/oj.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (OJ L 15, 20.1.2010, p. 1). ELI: http://data.europa.eu/eli/reg/2010/37(1)/oj.
Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market (OJ L 123, 24.4.1998, p. 1). https://eur‐lex.europa.eu/legal‐content/EN/TXT/?uri=CELEX%3A01998L0008‐20130820&qid=1712235837470.
It should be noted that Lambda‐cyhalothrin, Gamma‐cyhalothrin, Cyhalothrin, Compound Ia and Gamma‐lactone are identified as a pesticide active substance/metabolites that meet the definition of per‐ and polyfluoroalkyl substances (PFAS) based on its chemical structure (https://echa.europa.eu/hot‐topics/perfluoroalkyl‐chemicals‐pfas).
Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. PE/45/2018/REV/1. OJ L 4, 07/1/2019, p. 43–167.
It is noteworthy that a search of the Union Products Database of veterinary medicines suggests that there are currently no EU authorised veterinary medicinal products containing cyhalothrin.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 212 concerning the making available on the market and use of biocidal products. OJ L 167, 27.6.2012, p. 1–123.
The toxicity of CPCA (cyclopropyl carboxylic acid) has been addressed. Metabolite Ia is one of CPCA isomers.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OJ L 152, 16.6.2009, p. 11–22.
The MRLs for these commodities were established when previous versions of EFSA PRIMo were used for risk assessment. The higher exposure results derived with PRIMo 3.1 compared to PRIMo 3/PRIMo 2 can be explained by the higher consumption data and/or different unit weight data which trigger the IESTI case implemented in PRIMo 3.1.
REFERENCES
- EFSA (European Food Safety Authority) . (2014a). Reasoned opinion on the review of the existing maximum residue levels (MRLs) for lambda‐cyhalothrin according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 12(1), 3546. 10.2903/j.efsa.2014.3546 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014b). Conclusion on the peer review of the pesticide risk assessment of the active substance lambda‐cyhalothrin. EFSA Journal, 12(5), 3677. 10.2903/j.efsa.2014.3677 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015). Revision of the review of the existing maximum residue levels for the active substance lambda‐cyhalothrin. EFSA Journal, 13(12), 4324. 10.2903/j.efsa.2015.4324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , De Lentdecker, C. , Erdos, Z. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nougadere, A. , Pedersen, R. , Reich, H. , Sacchi, A. , Santos, M. , … Villamar‐Bouza, L. (2017). Reasoned opinion on the focused review of the existing maximum residue levels for lambda‐cyhalothrin in light of the unspecific residue definition and the existing good agricultural practices for the substance gamma‐cyhalothrin. EFSA Journal, 15(7), 4930. 10.2903/j.efsa.2017.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , Ferreira, L. , Greco, L. , Jarrah, S. , Leuschner, R. , Medina, P. , Miron, I. , Nougadere, A. , Pedersen, R. , Reich, H. , Santos, M. , Stanek, A. , Tarazona, J. , Theobald, A. , & Villamar‐Bouza, L. (2018). Guidance on use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA Journal, 16(1), 5147. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Pedersen, R. , Raczyk, M. , Reich, H. , Ruocco, S. , Sacchi, A. , Santos, M. , Stanek, A. , Tarazona, J. , … Verani, A. (2019a). Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication, 16(3), EN‐1605. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Abdourahime, H. , Anastassiadou, M. , Brancato, A. , Brocca, D. , Carrasco Cabrera, L. , De Lentdecker, C. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lostia, A. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nave, S. , Pedersen, R. , … Villamar‐Bouza, L. (2019b). Reasoned opinion on the modification of the existing maximum residue levels for lambda‐cyhalothrin in celeries, fennel and rice. EFSA Journal, 17(1), 5546. 10.2903/j.efsa.2019.5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019c). Technical report on the outcome of the consultation with member states, the applicant and EFSA on the pesticide risk assessment for gamma‐cyhalothrin in light of confirmatory data. EFSA supporting publication, 16(3), EN‐1599. 10.2903/sp.efsa.2019.EN-1599 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Bernasconi, G. , Brancato, A. , Carrasco Cabrera, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Rojas, A. , Sacchi, A. , Santos, M. , Stanek, A. , Theobald, A. , … Verani, A. (2020). Reasoned opinion on the modification of the existing maximum residue levels for lambda‐cyhalothrin in seed and fruit spices. EFSA Journal, 18(6), 6110. 10.2903/j.efsa.2020.6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023a). Statement on the review of the residue definitions for risk assessment of pyrethroids forming common metabolites. EFSA Journal, 21(5), 8022. 10.2903/j.efsa.223.8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Bellisai, G. , Bernasconi, G. , Cabrera, L. C. , Castellan, I. , del Aguila, M. , Ferreira, L. , Santonja, G. G. , Greco, L. , Jarrah, S. , Leuschner, R. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Ruocco, S. , Santos, M. , Scarlato, A. P. , Szot, M. , … Verani, A. (2023b). Setting of an import tolerance for lambda‐cyhalothrin in avocados. EFSA Journal, 21(12), e8464. 10.2903/j.efsa.2023.8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernandez‐Jerez, A. F. , Adriaanse, P. , Aldrich, A. , Berny, P. , Duquesne, S. , Focks, A. , Marinovich, M. , Millet, M. , Pelkonen, O. , Pieper, S. , Tiktak, A. , Topping, C. J. , Widenfalk, A. , Wilks, M. , Wolterink, G. , Binaglia, M. , Chiusolo, A. , Serafimova, R. , … Coja, T. (2022). Scientific opinion on toxicity of pyrethroid common metabolites. EFSA Journal, 20(10), 7582. 10.2903/j.efsa.2022.7582 [DOI] [Google Scholar]
- EMEA (European Medicines Agency) . (2001). Summary Report: cyhalothrin. Committee for veterinary medicinal products. EMEA/MRL/699/99‐Final, April, 2001. 1–6. https://www.ema.europa.eu/en/documents/mrl‐report/cyhalothrin‐summary‐report‐committee‐veterinary‐medicinal‐products_en.pdf
- European Commission . (2014). Final Review report for the active substance gamma‐cyhalothrin finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 10 October 2014 in view of the renewal of the approval of lambda‐cyhalothrin as active substance in accordance with Regulation (EC) No 1107/2009. SANCO/10606/2014 Rev 1, 10 October 2014.
- European Commission . (2020). Final Review report for the active substance lambda‐cyhalothrin finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 11 December 2015 in view of the renewal of the approval of lambda‐cyhalothrin as active substance in accordance with Regulation (EC) No 1107/2009. SANCO/12282/2014 Rev 5, 17 July 2020.
- FAO (Food and Agriculture Organization of the United Nations) . (2009). Lambda‐cyhalothrin in: Pesticide residues in food – 2008. Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 194.
- FAO (Food and Agriculture Organization of the United Nations) . (2015). Lambda‐cyhalothrin. In: Pesticide residuesin food–2015. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 226.
- FAO (Food and Agriculture Organization of the United Nations) . (2016). Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. Pesticide residues. 3rd Edition. FAO Plant Production and Protection Paper 225. 298 pp.
- Sweden . (2011). Assessment report for the Inclusion in Annex I or IA to Directive 98/8/EC concerning the placing biocidal products on the market of the active substance lambda‐cyhalothrin, Product‐type 18 (Insecticide), prepared by Sweden. May 2011. 101 pp. https://echa.europa.eu/documents/10162/6707c237‐87db‐9346‐5b81‐c72cc3c35d3f