Abstract
The aroma types of cream cheese affect its commercial value and consumer acceptability. However, the types of volatile substances and sensory characteristics of cream cheese at different fermentation stages are still unclear. Therefore, in this study, headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC-MS) and headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) were used to analyze the volatile substances in cream cheese fermentation. Orthogonal partial least squares discriminant analysis (OPLS-DA), odor activity value (OAV), relative odor activity value (ROAV) and variable projection importance (VIP) were used to identify the characteristic flavor substances in cream cheese fermentation. Finally, the relationship between key flavor substances and sensory characteristics was determined by partial least squares (PLS) analysis. A total of 34 and 36 volatile organic compounds were identified by HS-SPME-GC-MS and HS-GC-MS, respectively, and 14 characteristic flavor substances were found, based on VIP, ROAV and OAV models. Combined with sensory analysis and flavor substance changes, it was found that the cream cheese fermented for 15 d had the best flavor and taste. This study reveals the characteristics and contribution of volatile substances in cream cheese at different fermentation stages, which provides new insights into improving flavor and quality control.
Keywords: Cream cheese, Gas chromatography-ion mobility spectrometry, Gas chromatography-mass spectrometry, Flavor substances, Multivariate statistics
Graphical abstract
Highlights
-
•
A total of 34 and 36 VOCs were identified by HS-SPME/GC-MS and HS-GC-IMS, respectively.
-
•
GC–IMS and GC-MS were used to evaluate the flavor profile of cream cheese.
-
•
OPLS-DA, VIP, and OAV screened a total of 11 key flavor substances.
-
•
Six flavor attributes have good correlations with key flavor substances.
-
•
The best flavor and quality of cream cheese fermented for 15 d.
1. Introduction
Cream cheese is a kind of fresh cheese made from gel precipitation at casein's isoelectric point, which is not mature (Tadeu et al., 2022). It is delicate and soft, with huge market potential. The global cheese production in 2020 was approximately 21.3 million tons, with an estimated market size of US$65–68 billion. Furthermore, this number is increasing year by year (Wolfschool et al., 2021).
So far, some research has been done in cream cheese. For example, Amaral et al. (2022) studied the physical, chemical, rheological and microbial characteristics of light cream cheese coated with goat milk rich in phytosterols, and found that the formula containing phytosterols exhibited strong elasticity and firmness, with great fluidity. Brighenti et al. (2021) used whey cream instead of sweet cream to explore its effect on the texture and rheological properties of cream cheese, and found that addition of whey cream affected the texture and function of cream cheese. Tadeu da Veiga Correia et al. (2022) added sorghum flour into cream cheese formula to study the nutritional and antioxidant effects of the formula, and found that the addition of sorghum flour provided higher concentration of protein and improved its nutritional and antioxidant properties. However, to our knowledge, these studies are mainly focused on the physicochemistry and texture of cream cheese, little has been known on the flavor compounds in cream cheese. In addition, compared to other fermented cheeses, the flavor of cream cheese is appreciably lacking (Uzkuç and Karagül Yüceer, 2023). It has been reported that the extension of cheese ripening time can affect the proliferation and metabolism of microorganisms, and then promote the release of flavor substances (Zheng et al., 2018). However, as a kind of immature cheese, little research has focused on the influence of fermentation time on flavor release. It is thus hypothesized that the fermentation time may affect its flavor.
Flavor substances are important factors affecting product quality and consumer acceptance. The production of flavor substances in cheese mainly involves three biochemical reactions, including glycolysis, proteolysis and lipolysis. The metabolism of these substances produces volatile organic compounds (VOCs), such as aldehydes, alcohols, ketones and esters, which endows cheese with unique flavor and is a symbol of cheese ripening. HS-SPME-GC-MS has been widely used in the detection of VOCs due to its advantages of simple operation, good repeatability and high sensitivity. It was reported that acetone, 3-methylbutyral, isobutyric acid and δ-caprolactone were the key flavor substances in cheese (Chen et al., 2022a, Chen et al., 2022b, Chen et al., 2022c). Similarly, Gao et al. (2022) found that acetic acid, butyric acid, caproic acid, heptan-2-one, undecan-2-one and benzaldehyde were the main flavoring substances in the cream cheese. At the same time, it was found that GC-MS had some disadvantages, such as complex pretreatment, long detection time and insufficient sensitivity to small molecule flavor substances, leading to inaccurate detection results (Xiao et al., 2022a, Xiao et al., 2022b, Xiao et al., 2022c).
GC-IMS has emerged in recent years, which combines the high separation ability of GC with the high sensitivity and fast response characteristics of IMS. The identification information of flavor compounds and sample quality can be obtained simultaneously. However, HS-GC-IMS lacks a complete database, so it has limitations in the qualitative aspect of flavor substances (Yu, et al., 2021). Therefore, the combination of HS-GC-IMS and HS-SPME-GC-MS can more comprehensively reveal the flavor changes of fermented foods. However, this method has not yet been used to evaluate the flavor characteristics of cream cheese during fermentation.
This study aimed to evaluate the alterations in the flavor components of cream cheese during natural fermentation using a combination of GC-MS, GC-IMS and sensory analysis. Multiple statistical analysis was used to identify the key flavor components and sensory contributions during the fermentation process of the cream cheese, and the optimal fermentation time was determined. The results not only laid a theoretical foundation for the scientific research of cream cheese, but also provided assurance for quality control.
2. Materials and methods
2.1. Materials and chemicals
Cream was obtained from Fonterra Commercial Trading Co., Ltd. (Shanghai, China). 2-octanol (internal standard) was purchased from Aladdin (Shanghai, China).
2.2. Preparation of cream cheese
Cream cheese was prepared according to the method of Zhang et al. (2022); Malik et al. (2022), with minor modifications. One liter of whipped cream (2.6 g/hg protein, 35 g/hg fat) was homogenized at 15–18 MPa, then sterilized at 90 °C for 10 min. After the cream was cooled to 60 °C, 45 mL citric acid (450 g/dl) was added and continuously stirred for 10 min, and then filtered with sterile gauze to remove the whey and obtain cream cheese. The cream cheese was vacuum packed (−0.8 bar) and stored in an incubator maintained at 12 °C and 50–55% humidity, allowing it to mature within 20 d. Cream cheese samples were collected every 0 d, 5 d, 10 d, 15 d and 20 d. Each sample was kept at −20 °C for further determinations. The non-fermented cream cheese for 0 d was used as the control group.
2.3. GC-MS analysis
The method of flavor substances extraction and GC-MSanalysis were described by Li. et al. (2021) with some modifications. Briefly, 4 g of cream cheese were put into a 20 mL headspace extraction bottle, 10 μL of 2-octanol (1.00 mg/mL) were added as an internal standard, sealed, put in a water bath at 85 °C, stirred by magnetic force at a speed of 500 r/min, balanced it for 20 min, and then an extraction needle (50/30 Divinylbenzene/Carboxen/Polydimethylsiloxane 2 cm) was inserted for 30 min. Before using the extraction needle, it was activated at the GC inlet for 20 min (250 °C).
The detection of volatile flavor components was carried out on the GC-MS system (Agilent Technologies Inc., CA, USA) equipped with DB-WAX capillary column (30 mm × 0.25 mm × 0.25 μm, Agilent J & W, CA, USA). GC conditions: The temperature of the injector was 250 °C, and the GC separation of volatile substances was carried out at a flow rate of 1.0 mL/min under helium carrier gas using programming temperature mode. The column temperature was initially 40 °C and maintained for 3 min, then the temperature was increased to 150 °C at a rate of 4 °C/min. After that, the temperature was increased to 200 °C at a rate of 5 °C/min. Finally, the temperature was raised to 230 °C at a rate of 20 °C/min for 5 min. MS conditions: The ion source temperature was 230 °C, and the mass scanning range was 40–450 m/z. The qualitative analysis of volatile substances was based on the matching of their mass spectrometric data with NIST14 and NIST14S databases, and only compounds with a similarity greater than 80% were selected. Volatiles were quantified by semi-quantitative method with internal standards, and the content was expressed as mg/kg. 2-octanol (Aladdin Co., St. Shanghai, China) at 1.00 mg/mL was used as an internal standard for the quantitative analysis of GC-MS. OAVs were determined by dividing the concentration of each aroma substance by its odor threshold in water. The threshold of flavor substances in water was obtained by a previous method (Alewijn, et al., 2007).
2.4. GC-IMS analysis of volatile composition
HS-GC-IMS analysis of cream cheese followed the method of Ling et al. (2022) with minor modifications. Briefly, 3.00 g cream cheese and 3.00 g sodium chloride were put into a mortar and ground evenly, then added to a 20 mL headspace bottle to seal, followed by GC-IMS analysis. Each sample was repeated three times. GC (Agilent Technologies, Palo Alto, CA, USA) conditions: MXT-5 inner diameter: 0.53 mm, thickness: 1 μm, column temperature: 60 °C, drift gas (N2, purity ≥99.99%), flow rate: 50 mL/min, IMS (FlavourSpec®, Gesellschaft für analytische Sensorsysteme mbH, Dortmund, Germany) temperature: 45 °C. The relative amount of volatile compounds was expressed as the average of the peak area (Guo, et al., 2021). The identity citation of the volatile compounds was based on RI and drift time compared to the GC-IMS library. The NIST database and IMS database were used in the GC-IMS Library search software to qualitatively analyze the components (Li et al., 2023).
2.5. Calculation of odor activity value and relative odor activity value
OAV and ROAV were analyzed to determine the contribution of each volatile compound to the overall aroma. The calculation formula of OAV refers to Xi et al. (2024):
Ci is the concentration of the compound, Ti is the threshold of the compound in water, and OAVi is the odor activity value of compound. The formula of ROAV (Xiao et al., 2022a, Xiao et al., 2022b, Xiao et al., 2022c) is as follows:
ROAVi is the relative odor activity value of compound, and Tmax/Cmax is the maximum of Ci/Ti among all the compounds in the sample.
2.6. Sensory evaluation of cream cheese
Sensory evaluation analysis of cream cheese followed the method of Zhang et al. (2022) with minor modifications. Briefly, the sensory evaluation team of cream cheese at different fermentation phases was composed by 5 women and 5 men who have been trained in flavor discrimination, description and distinction, with experience and no smoking history. The sensory test meets the ethical standards of the institution and/or the national research council, and all participants agree to participate in this survey. The tested product is safe. All cheese samples were randomly numbered and placed in a closed container with lids. The characteristic terms of cream cheese aroma were finally determined through group discussion. The method of sensory evaluation was a ten points system, 0 meant that the aroma was not detected, 10 meant that the aroma was the strongest, and 0–10 meant that the aroma was gradually enhanced. Evaluators were asked to rinse their mouths with tap water between each sample. Each sample was repeated three times.
2.7. Statistical analysis
The GC-MS data were analyzed by Excel for means and standard deviation. SPSS 26.0 software was used for one-way ANOVA and the differences were analyzed for significance using Duncan's multiple comparisons at p < 0.05. Origin 2021b software was used for drawing. PCA, OPLS-DA and VIP analyses of volatile compounds were carried out using SIMCA 14.1. Interpolation Software Reporter, Gallery Plot and Dynamic PCA were used to plot the data of GC-IMS.
3. Results and discussion
3.1. HS-SPME-GC-MS
3.1.1. GC-MS of cream cheese at different fermentation stages
An important factor affecting consumer selectivity is the unique flavor of cheese. Therefore, in order to comprehensively understand the change of VOCs in cream cheese during ripening, HS-SPME-GC-MS was utilized to analyze VOCs. As shown in Table 1, 34 VOCs were identified by HS-SPME-GC-MS, including acids (12), esters (7), alcohols (5), ketones (5), aldehydes (3) and others (2). As shown in Fig. 1, acids were dominant in fermentation at D15, and then significantly declined, with the lowest level at D20. In addition, the level of alcohols was gradually increased from D0 to D10, whereas at D20, the content of the substances decreased. The proportion of esters and ketones significantly increased from D0 to D15 (p < 0.05). The content of aldehydes first increased and then decreased during the fermentation process. The levels of other VOCs, including d-limonene and heptadecane, increased from D0 to D15, but rapidly decreased at D20. These results indicate that fermentation can lead to changes in the VOCs of cream cheese, which may be related to the proliferation and metabolism of microorganisms (Wang, et al., 2023).
Table 1.
The VOCs of cream cheese and their threshold (mg/kg) and corresponding odor characteristics.
| NO. | Compounds | Threshold (mg/kg) a | Odor b | Fermentation stages (d) c |
||||
|---|---|---|---|---|---|---|---|---|
| D0 | D5 | D10 | D15 | D20 | ||||
| 1 | Acetic acid | 22,000 | sour | 0.03 ± 0.01 d | 0.04 ± 0.00 d | 0.26 ± 0.06c | 0.62 ± 0.01 b | 0.79 ± 0.05a |
| 2 | Benzoic acid | 1185 | nutty | – | 0.02 ± 0.02 d | 0.05 ± 0.01c | 0.07 ± 0.01 b | 0.09 ± 0.01a |
| 3 | 2-Methylpropionic acid | – | – | – | – | 0.17 ± 0.01a | – | – |
| 4 | 3-methylbutanoic acid | 130 | cheesy | 0.06 ± 0.06 b | 0.07 ± 0.01 b | 0.09 ± 0.00 b | 0.18 ± 0.01a | 0.06 ± 0.05 b |
| sweety | ||||||||
| greasy | ||||||||
| 5 | Decanoic acid | 13736.77 | rancidity greasy |
0.57 ± 0.06 b | 0.44 ± 0.29c | 0.07 ± 0.00 d | 0.94 ± 0.02a | 0.06 ± 0.01 d |
| 6 | Hexanoic acid | 3000 | sweety | 0.26 ± 0.06a | 0.19 ± 0.07 b | 0.28 ± 0.01a | 0.32 ± 0.00a | 0.07 ± 0.01c |
| 7 | Octanoic acid | 2701.23 | sweat | 0.38 ± 0.06 b | 0.22 ± 0.18c | 0.46 ± 0.00a | 0.35 ± 0.00 b | 0.086 ± 0.00c |
| rancidity | ||||||||
| 8 | Nonanoic acid | 3559.23 | rancidity | 0.04 ± 0.01 d | 0.04 ± 0.00 d | 0.07 ± 0.00c | 0.67 ± 0.00a | 0.09 ± 0.01 b |
| 9 | (E)-hept-2-enoic acid | – | green | 0 ± 0 | 0 ± 0 | 0.09 ± 0.00 b | 0.09 ± 0.01 b | 0.13 ± 0.00a |
| 10 | butanedioic acid | – | – | – | – | 0.14 ± 0.01a | 0.07 ± 0.00 b | – |
| 11 | dodecanoic acid | – | – | 0.89 ± 0.01c | 0.73 ± 0.22 d | 1.14 ± 0.01 b | 3.25 ± 0.03a | 0.33 ± 0.01e |
| 12 | Icosanoic acid | – | – | 1.51 ± 0.06 b | 1.69 ± 0.21a | 0.89 ± 0.06c | 0.85 ± 0.05c | 0.35 ± 0.01 d |
| 13 | 2,3-Butanediol | 141 | fruity | 0.14 ± 0.06 d | 0.38 ± 0.03c | 0.51 ± 0.00 b | 0.62 ± 0.00 ab | 0.72 ± 0.00a |
| onion | ||||||||
| 14 | 3-methylsulfanylpropan-1-ol | 2110.41 | – | – | – | 0.03 ± 0.00a | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| 15 | 2-hexyldecan-1-ol | – | – | 0.43 ± 0.06c | 0.34 ± 0.07e | 0.57 ± 0.00 b | 0.95 ± 0.01a | 0.35 ± 0.00 d |
| 16 | 2-phenylethanol | 28922.73 | rosey | – | 0 ± 0 | 0.05 ± 0.00a | 0.03 ± 0.00 b | – |
| 17 | 3-methylbutan-1-ol | 250 | malty burnty |
0.49 ± 0.01 b | 0.24 ± 0.34c | 1.35 ± 0.06a | – | – |
| 18 | 2-phenylacetaldehyde | 4 | honey | – | – | 0.02 ± 0.00a | – | – |
| sweety | ||||||||
| 19 | (E)-dec-2-enal | 50 | 0.02 ± 0.01 b | 0.02 ± 0.00 b | – | 0.03 ± 0.01a | – | |
| 20 | Decanal | 0.1 | greasy citrus |
0.02 ± 0.00 b | 0 ± 0 | – | 0.05 ± 0.00a | – |
| 21 | Heptan-2-one | 140 | sweety musty | 0.29 ± 0.01 ab | 0.13 ± 0.11 d | 0.26 ± 0.05BCE | 0.23 ± 0.04c | 0.47 ± 0.01a |
| 22 | 3-hydroxybutan-2-one | 55 | buttery | 0.13 ± 0.00 d | 0.16 ± 0.05c | 0.68 ± 0.05a | 0.69 ± 0.05a | 0.49 ± 0.00 b |
| 23 | Nonan-2-one | 1087 | floral | 0.39 ± 0.00 d | 0.36 ± 0.06e | 0.67 ± 0.02 b | 0.59 ± 0.02c | 0.95 ± 0.02a |
| fruity | ||||||||
| 24 | Undecan-2-one | 100 | fruity | 0.07 ± 0.00 d | 0.05 ± 0.02e | 0.37 ± 0.00c | 0.44 ± 0.0025 b | 0.86 ± 0.00a |
| 25 | Decan-2-one | – | – | 0.15 ± 0.01c | 0.15 ± 0.00c | 0.18 ± 0.02 b | 0.09 ± 0.01 d | 0.30 ± 0.01a |
| 26 | Butanoic acid, 1-Methyl-1-ethyl ester | – | – | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.05 ± 0.00a | 0 ± 0 |
| 27 | Cis-3,7-dimethyl-2,6-Octadien- | – | – | 0.05 ± 0.01 b | 0.04 ± 0.02c | 0.04 ± 0.02BCE | 0.07 ± 0.00a | – |
| 28 | δ-decalactone | 66 | – | 0.02 ± 0.01e | 0.11 ± 0.11 d | 0.37 ± 0.00 b | 0.55 ± 0.00a | 0.64 ± 0.01c |
| 29 | δ-octanolactone | 200 | – | 0.036 ± 0.01 d | 0.09 ± 0.07a | 0.05 ± 0.00cd | 0.09 ± 0.0019 b | 0.047 ± 0.00c |
| 30 | Propyl benzoate | – | – | 0 ± 0 | 0.01 ± 0.02c | 0.18 ± 0.00 b | 2.36 ± 0.00a | – |
| 31 | 6-heptyloxan-2-one | 53 | buttery | 0 ± 0 | 0.38 ± 0.54a | 0.46 ± 0.00 b | 0.02 ± 0.00 d | 0.09 ± 0.00c |
| fruity | ||||||||
| 32 | δ-Nonalactone | – | citrus | 0.29 ± 0.06 b | 0.19 ± 0.09c | 0.18 ± 0.00c | 0.70 ± 0.01a | 0.26 ± 0.00 b |
| sweety | ||||||||
| 33 | D-Limonene | 1000 | lemon | 2.39 ± 0.15c | 2.43 ± 0.05c | 3.98 ± 0.01 b | 15.05 ± 0.011a | – |
| citrus | ||||||||
| 34 | Heptadecane | – | – | 0.06 ± 0.01c | 0.05 ± 0.02 d | 0.05 ± 0.00 d | 0.09 ± 0.08 b | 0.14 ± 0.00a |
Note:”-” means not detected.
The flavor and threshold of all flavor compounds are from relevant literature (Que. et al., 2023; Wang. et al., 2022).
Odor descriptions were from the literatures (Liu. et al., 2022; Xiao. et al., 2022) or from FEMA database.
The concentration data of volatile compounds are shown as the mean value of peak volume ± SD (n = 3). In the same line, the difference between different letters is significant (p < 0.05).
Fig. 1.
Relative contents of volatile compounds in cream cheese at different fermentation times.
3.1.2. Volatile profiling of cream cheese during fermentation
Esters and lactones were the major compounds in cheese, with 5.52%, 16.00%, 16.56%, 48.68% and 13.24% compounds detected at D0, D5, D10, D15 and D20, respectively. The widely detected compounds included butanoic acid, 1-methyl-1-ethyl ester, cis-3,7-dimethyl-2,6-octadien-, δ-decalactone, δ-octanolactone, propyl benzoate, 6-heptyloxan-2-one and δ-nonalactone. Esters in cheese are mainly produced through esterification of fatty acids and alcohols (Table 1). They have floral and fruity fragrance with a low threshold, which can neutralize the irritating smell caused by excessive acids and make the cheese softer. Lactones in cheese are released by esterification of hydroxy fatty acids in triglycerides (Alewijn et al., 2007). They also make a great contribution to the flavor of milk and dairy products, which can effectively impact cheese milk flavor, fruit flavor, nut flavor and other excellent flavors (Ye et al., 2022). For instance, 6-heptyloxan-2-one imparts buttery and ruity flavor. The levels of most of esters and lactones were gradually increased from D0 toD10. The contents of propyl benzoate, butanoic acid, 1-methyl-1-ethyl ester, cis-3,7-dimethyl-2,6-octadien- and δ-nonalactone were the highest at D15. δ-nonalactone generates citrus and fruity flavor. δ-decalactone was detected in all time points of cream cheese and impacted sweety and coconut flavor (Chen et al., 2022a, Chen et al., 2022b, Chen et al., 2022c). It is composed of free fatty acids produced by oxidation and dehydration, giving cheese a fruity flavor (Jung et al., 2013).
As shown in Table 1, 2-phenylacetaldehyde was found only at D10. The concentrations of (E)-dec-2-enal and decanal were increased slowly from D0 to D15, but were not detected at D20. Decanal (soap, tallow) and 2-phenylacetaldehyde (honey, sweety) were the dominant aldehydes during the whole ripening process of cream cheese (Zhao et al., 2022a, Zhao et al., 2022b). Phenylalanine is further degraded to phenylpyruvate through aromatic amino acid transaminase and then catalyzed by phenylpyruvate decarboxylase to form 2-phenylacetaldehyde (Yang et al., 2021a, Yang et al., 2021b). Some aldehydes, such as decanal, were formed via lipid oxidation initiated by endogenous enzymes or microorganism activity, and (E)-dec-2-enal was derived from the oxidative degradation of C18:3n3 (Zhao et al., 2022a, Zhao et al., 2022b).
The main alcohol compounds in the cream cheese were 2,3-butanediol, 3-methylsulfanylpropan-1-ol, 2-hexyldecan-1-ol, 2-phenylethanol and 3-methylbutan-1-ol, similar to the result reported by Xu et al. (2020). The content of 3-methylbutan-1-ol was significantly increased from D0 to D10 (Table 1). 2-phenylethanol was detected only at D10 and D15, similar to the result reported by Yang et al. (2022). 3-methylbutan-1-ol is derived from the amino acid metabolic decomposition pathway and the anabolic pathway affects malty flavor. The concentration of 2,3-butanediol formed by the degradation of amino acid initiated by P. pentosaceus increased significantly (p < 0.05), with a maximum at D20, similar to the finding by Hu et al. (2019). 3-methylsulfanylpropan-1-ol which provides sweety odor is produced by the degradation of methionine via pyruvate decarboxylase and alcohol dehydrogenase. However, the concentration of 3-methylsulfanylpropan-1-ol was the highest at D10 and decreased from D15 to D20. This may be due to the decreased activities of the key enzymes. 2,3-butanediol provides a fruity flavor to cream cheese. Leucine is degraded into α-ketoisocaproate by leucine transferase and branched chain amino acid transaminase, decarboxylated to form 3-methylbutyraldehyde, which is decarboxylated by keto acid decarboxylase, and then reduced to 3-methylbutanol under the action of ethanol dehydrogenase (Caron et al., 2021). 2-phenylethanol is derived from the degradation of phenylalanine infinite by yeast and contributes to a rose flavor in cream cheese. In addition, the content of most of the alcohols tended to decrease during ripening, which may be caused by the esterification reaction between alcohols and aldehydes.
Acids are the most important VOCs in the fermentation of cream cheese. In the present study, the concentrations of 11 acids were determined via GC-MS analysis, including acetic acid, benzoic acid, 2-methylpropionic acid, 3-methylbutanoic acid, decanoic acid, hexanoic acid, octanoic acid, nonanoic acid, (E)-hept-2-enoic acid, butanedioic acid, dodecanoic acid and icosanoic acid, and the contents of most acid compounds changed significantly during the ripening of cream cheese (p < 0.05) (Table 1). During the ripening of cream cheese, the acids are mainly produced from three biochemical pathways: lipid hydrolysis, protein degradation and glycolysis. The contents of acetic acid and (E)-hept-2-enoic acid were increased during fermentation, similar to a report by Wang et al., 2022a, Wang et al., 2022b, Wang et al., 2022c. However, the contents of benzoic acid, octanoic acid and butanedioic acid were significantly increased from D0 to D15 and gradually decreased from D10 to D20. The same trends were found in other compounds, such as 3-methylbutanoic acid, hexanoic acid and nonanoic acid. The concentrations of octanoic acid and decanoic acid were decreased at D20, and this may result from the formation of heptan-2-one and 2-nonone which are generated by the β-oxidation and decarboxylation of octanoic acid and decanoic acid. The concentration of 3-methylbutanoic acid was increased during fermentation, similar to the study by Al-Dalali et al. (2022). This is because leucine is degraded to α-ketoisohexanoic acid by aminotransferase and then catalyzed by α-ketoacid decarboxylase to form 3-methyl butyraldehyde, and 3-methyl butyraldehyde can be further degraded into 3-methylbutanoic acid via aldehyde dehydrogenase (Chen et al., 2022a, Chen et al., 2022b, Chen et al., 2022c). Two non-volatile acids, dodecanoic acid and icosanoic acid, were also found during ripening of cream cheese. Icosanoic acid is a non-volatile substance that may be further degraded into VOCs during fermentation. Acetic acid produced via glycolysis and proteolysis provides a vinegary in cream cheese (Wang et al., 2022b; Zang et al., 2022). Decanoic acid usually generates a bad flavor in cheese. (E)-hept-2-enoic acid provides a green flavor. Benzoic acid can be produced by phenylalanine under the interaction of transaminases, enterobacteriaceae and micrococcus (Li et al., 2022). Hexanoic acid, octanoic acid and nonanoic acid which are produced via lactose fermentation and lipid hydrolysis generate sweety, sweat and vinegary. 2-methylpropionic acid which adds sour in cream cheese, is formed by the metabolism of the valine (Delgado et al., 2010).
Ketones are usually important components of flavor substances in cheese due to their low threshold and unique flavor. In the study, five ketones, including heptan-2-one, 3-hydroxybutan-2-one, nonan-2-one, 2-undmecanone and decan-2-one, were detected during the ripening of cream cheese. The concentration of decan-2-one was increased during the fermentation of cheese and similar trends were observed for nonan-2-one and undecan-2-one. The content of nonan-2-one was the highest at D20, the compound is an important volatile substance in fermented dairy products and derived from β-oxidation of fatty acids with subsequent decarboxylation of β-ketoacids and also gives cheese a floral, fruity and peachy flavor (Dan et al., 2022). Heptan-2-one was produced by incomplete β-oxidation via staphylococci (Leroy et al., 2006). Ketone compounds are common flavor compounds in cheese and make a great contribution to cheesy and sweety odor. Heptan-2-one and 3-hydroxybutan-2-one usually affect a creamy and cheesy odor, and are formed by the automatic oxidation of lipids in dairy products (Xie et al., 2022). 2,3-butanedione and 3-hydroxy-2-butanone are typical flavor substances in fermented dairy products, but 2,3-butanedione was not detected in this experiment. This may be related to weak stability of diacetyl, which is easily reduced to 3-hydroxybutan-2-one under the action of reductase.
3.2. HS-GC-IMS
3.2.1. Volatile fingerprints of cream cheese
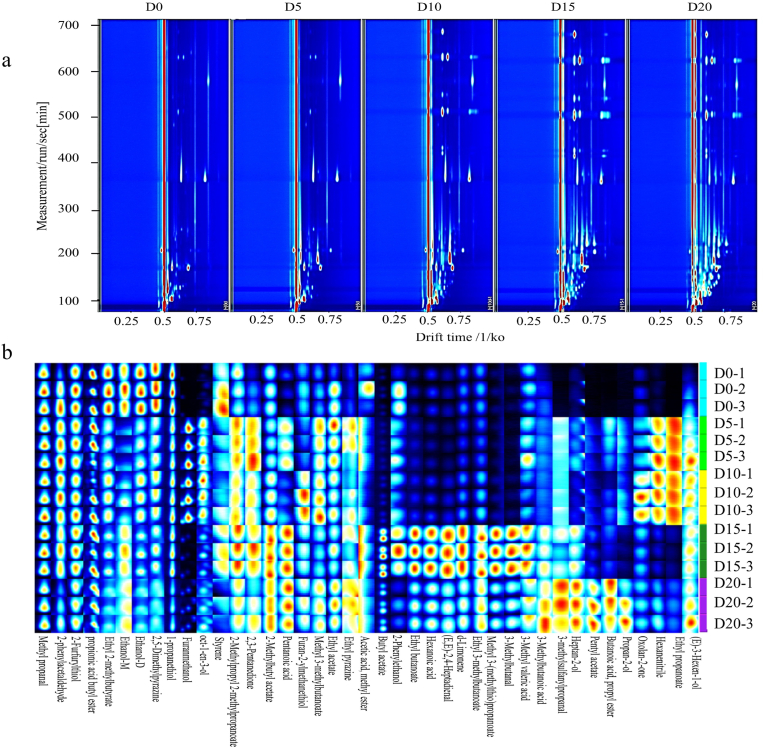
In order to comprehensively explore the changes in flavor substances before and after fermentation and determine the optimal fermentation stage, HS-GC-IMS was utilized to determine the VOCs in the cream cheese. A flavor analyzer was utilized to identify the VOCs of cream cheese during fermentation. The reporter plug-in was used to generate a two-dimensional top view of GC-IMS of volatile compounds in cream cheese at different fermentation stages (Fig. 2a). Most of the compounds were observed at the retention time of 100–400 s and the drift time of 0.5–1.5 s. However, the retention time of the cream cheese increased to 700 s at the later stage of fermentation. The VOCs at five different stages were basically the same, but the contents of some volatile compounds increased from D15 to D20. Therefore, fermentation can significantly affect the production of flavor substances.
Fig. 2.
Topographic plots (A) and gallery plots (B) of cream cheese for volatile compounds during different fermentation times via GC-IMS.
3.2.2. Differential flavors during ripening of cream cheese
Through HS-GC-IMS spectrum analysis, the drift time and retention index were compared, and the dynamic changes of VOCs in the ripening of cream cheese were detected by instrumental analysis. A total of 36 VOCs were found, including 14 esters, 8 alcohols, 5 aldehydes, 4 acids and 1 ketone (Table 2).
Table 2.
The VOCs identified by GC-IMS during fermentation.
| NO. | Volatile compounds | Odor a | CAS | Threshold (mg/kg) b | MW | RI c | Rt[s] d | Dt[ms] e | Relative content (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D5 | D10 | D15 | D20 | |||||||||
| Acid | |||||||||||||
| 1 | Hexanoic acid | sweety | C142621 | 1.8/1.84 | 116,2 | 1030,1 | 507,582 | 129,894 | 0.95 | 0.96 | 5.78 | 16.40 | 10.64 |
| 2 | Pentanoic acid | potato, cheese | C109524 | – | 102,1 | 934,2 | 224,507 | 151,836 | 0.77 | 0.81 | 0.87 | 18.81 | 16.57 |
| 3 | 3-Methyl valeric acid | – | C105431 | – | 116,2 | 938,7 | 232,955 | 125,478 | 1.33 | 2.90 | 5.06 | 21.13 | 15.49 |
| 4 | 3-methylbutanoic acid | Cheesy | C503742 | 0.13 | 102,1 | 850,0 | 114,974 | 120,083 | 3.54 | 5.02 | 18.31 | 18.71 | 36.05 |
| Sweety | |||||||||||||
| Alcohol | |||||||||||||
| 5 | 2-Phenylethanol | Malty burnty | C60128 | 390 | 122,2 | 1054,0 | 626,063 | 12,981 | 6.78 | 7.59 | 38.23 | 62.95 | 35.50 |
| 6 | oct-1-en-3-ol | Mushroom fragrance | C3391864 | 0.50/1.5 | 128,2 | 1002,6 | 371,268 | 162,876 | 31.91 | 57.07 | 41.60 | 28.01 | 24.31 |
| 7 | Furanmethanol | Burnt, sweet | C98000 | 1.9/2.0 | 98,1 | 904,6 | 169,176 | 137,158 | 29.28 | 42.49 | 38.52 | 51.25 | 44.65 |
| 8 | heptan-2-ol | soap | C543497 | 0.041/0.081 | 116,2 | 895,5 | 152,148 | 140,412 | 0.17 | 0.21 | 0.38 | 0.56 | 3.37 |
| 9 | Ethanol-D | Scent | C64175 | 200 | 46,1 | 906,6 | 172,861 | 112,278 | 21.34 | 23.08 | 16.74 | 7.55 | 5.45 |
| 10 | Ethanol-M | Scent | C64175 | 200 | 46.1 | 902,2 | 164,639 | 112,108 | 22.36 | 23.90 | 15.34 | 6.94 | 5.76 |
| 11 | (E)-3-Hexen-1-ol | green | C928972 | 0.07 | 100,2 | 863,0 | 124,493 | 124,297 | 5.06 | 9.22 | 11.36 | 5.20 | 3.23 |
| 12 | 1-propanethiol | – | C107039 | – | 76,2 | 861,2 | 123,175 | 1,18,003 | 0.36 | 0.25 | 0.21 | 0.17 | 0.13 |
| 13 | furan-2-ylmethanethiol | – | C98022 | – | 114,2 | 883,0 | 139,152 | 137,096 | 0.19 | 0.17 | 2.57 | 2.25 | 6.48 |
| 14 | Propan-2-ol | plastic, rubber | C67630 | 0.4 | 60,1 | 916,0 | 190,514 | 120,944 | 3.25 | 5.42 | 8.84 | 14.52 | 18.31 |
| Aldehydes | |||||||||||||
| 15 | (E,E)-2,4-Heptadienal | – | C4313035 | 0.015 | 110,2 | 1014,1 | 428,369 | 121,565 | 0.48 | 0.63 | 5.51 | 9.81 | 5.63 |
| 16 | 2-phenylacetaldehyde | Honey sweety | C122781 | 4/9 | 120,2 | 1004,4 | 380,18 | 126,345 | 40.92 | 50.41 | 40.75 | 30.00 | 20.08 |
| 17 | 3-Methylbutanal | cocoa, almond | C590863 | 1.2 | 86,1 | 934,7 | 225,513 | 140,676 | 1.07 | 2.56 | 5.22 | 34.27 | 22.09 |
| 18 | 3-methylsulfanylpropanal | Cooked vegetable | C3268493 | 0.04 | 104,2 | 920,6 | 199,025 | 140,068 | 0.53 | 1.41 | 5.64 | 5.52 | 12.92 |
| 19 | Methyl propanal | Wine | C78842 | – | 72,1 | 839,0 | 106,944 | 112,393 | 70.60 | 81.69 | 158.41 | 160.64 | 176.91 |
| Esters | |||||||||||||
| 20 | Ethyl butanoate | Fruity | C105544 | 1 | 116,2 | 1037,1 | 542,07 | 121,704 | 0.75 | 0.80 | 3.41 | 13.72 | 7.88 |
| 21 | methyl 3-methylbutanoate | – | C556241 | – | 116,2 | 1029,8 | 505,951 | 12,163 | 7.85 | 9.12 | 48.31 | 60.37 | 46.44 |
| 22 | Butyl acetate | Ethereal, solvent | C123864 | 0.01/0.1 | 116,2 | 1065,6 | 683,3 | 121,737 | 2.26 | 2.46 | 19.95 | 42.59 | 23.56 |
| 23 | ethyl 3-methylbutanoate | – | C108645 | – | 130,2 | 1053,9 | 625,302 | 165,323 | 2.05 | 2.04 | 3.84 | 16.18 | 9.36 |
| 24 | Methyl 3-(methylthio)propanoate | cooked potato, roasty | C13532188 | – | 134,2 | 1029,3 | 503,67 | 163,473 | 0.69 | 0.68 | 5.02 | 14.38 | 8.91 |
| 25 | Pentyl acetate | Fragrance | C628637 | – | 130,2 | 895,9 | 152,859 | 128,914 | 0.86 | 0.90 | 2.31 | 4.82 | 21.42 |
| 26 | Butanoic acid, propyl ester | – | C105668 | 0.5 | 130,2 | 885,1 | 140,668 | 125,374 | 0.58 | 0.59 | 6.57 | 4.39 | 7.99 |
| 27 | propionic acid butyl ester | – | C590012 | – | 130,2 | 895,9 | 152,858 | 1,28,747 | 2.45 | 2.34 | 2.62 | 2.23 | 1.45 |
| 28 | 2-Furfurylthiol | – | C98022 | – | 114,2 | 882,9 | 139,091 | 1,3698 | 1.67 | 1.23 | 1.69 | 1.32 | 0.56 |
| 29 | Ethyl acetate | pineapple | C141786 | 14 | 88,1 | 875,7 | 133,805 | 133,625 | 0.56 | 0.15 | 1.51 | 2.85 | 3.62 |
| 30 | Ethyl 2-methylbutyrate | – | C7452791 | – | 130,2 | 1044,8 | 580,573 | 167,534 | 23.43 | 28.93 | 24.15 | 23.56 | 30.33 |
| 31 | 2-methylpropyl 2-methylpropanoate | – | C97858 | – | 144,2 | 920,6 | 198,966 | 133,021 | 7.92 | 127.04 | 138.52 | 123.00 | 81.20 |
| 32 | ethyl propanoate | fruity, sweet | C105373 | – | 102,1 | 907,2 | 174,031 | 142,246 | 0.63 | 4.39 | 5.35 | 3.72 | 1.10 |
| 33 | oxolan-2-one | – | C96480 | – | 86,1 | 907,4 | 174,262 | 130,028 | 0.90 | 0.81 | 0.83 | 5.49 | 7.94 |
| 34 | Acetic acid, methyl ester | – | C79209 | – | 74,1 | 883,9 | 139,826 | 11,752 | 1.39 | 2.61 | 10.80 | 8.78 | 7.89 |
| 35 | 2-Methylbutyl acetate | sweat, acid | C624419 | 275 | 130,2 | 868,6 | 128,63 | 129,027 | 0.92 | 0.32 | 1.27 | 2.39 | 2.49 |
| Ketones | |||||||||||||
| 36 | 2,3-Pentanedione | – | C600146 | 0.02 | 100,1 | 1053,4 | 623,144 | 121,639 | 5.76 | 6.67 | 24.27 | 28.87 | 22.90 |
| Other | |||||||||||||
| 37 | d-Limonene | lemon, orange | C138863 | 0.2 | 136,2 | 1011,5 | 415,363 | 121,706 | 0.42 | 0.45 | 3.40 | 14.52 | 8.07 |
| 38 | Hexanenitrile | – | C628739 | – | 97,2 | 849,6 | 114,694 | 126,425 | 0.51 | 5.76 | 6.85 | 2.33 | 2.76 |
| 39 | Ethyl pyrazine | – | C13925003 | – | 108,1 | 897,9 | 156,654 | 118,218 | 2.67 | 4.21 | 6.61 | 4.84 | 4.93 |
| 40 | 2,5-Dimethylpyrazine | C123320 | 108,1 | 934,0 | 224,105 | 1,51,451 | 0.98 | 0.76 | 0.76 | 0.69 | 0.54 | ||
| 41 | styrene | Sweet, balsam, floral, plastic | C100425 | 3.6 | 104,2 | 924,1 | 205,511 | 152,612 | 1.52 | 8.90 | 7.97 | 12.93 | 4.27 |
Note: “-” means not detected.
The flavor and threshold of all flavor compounds are from relevant literature (Que. et al., 2023; Wang. et al., 2022).
Odor descriptions were from the literatures (Liu. et al., 2022; Xiao. et al., 2022) or from FEMA database.
RI: Represents the retention index calculated using n-ketones C4–C9 as external standard on FS-SE-54-CB-1 column.
Rt: Represents the retention time in the capillary GC column.
Dt: Represents the drift time in the drift tube.
The GC-IMS library plug-in was used to construct a fingerprint to compare the differences in VOCs before and after fermentation (Fig. 2b). Each row in the figure represents the signal peaks of different VOCs in the same sample, and each column represents the signal peaks of different VOCs in the sample. The color represents the relative content of VOCs, and the brighter the color, the higher the content. At D0, the concentrations of 2-phenylacetaldehyde and ethanol were higher than the other stages (Fig. 2b). The volatile substances at D5 were similar to D10. From D5 to D10, the contents of styrene, 2-methylpropyl 2-methylpropanoate, ethanol, 2-phenylacetaldehyde, oct-1-en-3-ol, furanmethanol, ethyl 2-methylbutyrate and methylpropanal were higher than the other stages. Oct-1-en-3-ol which provides a mushroom odor was produced by the oxidation of linoleic acid catalyzed by lipoxygenase (Yang et al., 2021a, Yang et al., 2021b). Styrene has an odd smell, such as resin and flowers odor (Ling et al., 2022). Ethanol generated by the degradation of pyruvate catalyzed by two key enzymes (pyruvate decarboxylase and alcohol dehydrogenase) plays an important role in fermentation (Huan et al., 2021). Furanmethanol generated by Maillard reaction and responsible for the warm “burnt” odor and cooked sugar taste, was mainly detected in smoked bacon (Du et al., 2021).
The concentrations of ethyl propanoate, 3-methyl valeric acid, 3-methylbutanal, methyl 3-(methylthio) propanoate, ethyl 3-methylbutanoate, limonene, (E,E)-2,4-heptadienal, hexanoic acid, ethyl butanoate, 2-phenylethanol, butyl acetate, pentanoic acid, 2-methylbutyl acetate, ethyl acetate, methyl 3-methylbutanoate, 2,3-pentanedione, acetic acid and methyl ester were the highest at D15 (Fig. 2b). The contents of 2-phenylethanol increased first, with maximum at D15, and then decreased. This result was similar to the results of GC-MS analysis. (E, E)-2,4-heptadienal is produced by the oxidation of phosphatidylcholine (Zhong et al., 2021). Ethyl butanoate, ethyl acetate and ethyl propanoate usually contribute to a fruit and cream odor (Zhong et al., 2021).
As shown in Fig. 3B, the content of VOCs at D20 was much higher, including propan-2-ol, (E)-hex-3-en-1-ol, 3-methylbutanoic acid, butanoic acid, propyl ester, hexanenitrile, oxolan-2-one, furan-2-ylmethanethiol, pentyl acetate, heptan-2-ol and 3-methylsulfanylpropanal. In summary, the contents of aldehydes and acids increased significantly from D0 to D15 (p < 0.05), which was characterized by the flavor of fatty and acidy, but slightly decreased at D20. The concentrations of alcohols and esters, which generate fruity and sour odor, were higher during the last fermentation phase of cream cheese. The results of HS-GC-IMS showed that there was a significant difference in the VOCs of cream cheese before and after fermentation. In addition, few dimers and trimers were detected in this study, which may be caused by the low proton affinity of the compounds and the internal problems of the instrument(Parastar and Weller, 2024; Natividad et al., 2018).
Fig. 3.
The PCA analysis of volatiles identified by GC–IMS(a) and GC-MS(b). The VIP ranking diagram of GC-IMS(c) and GC-MS(d).
3.3. Chemometrics analysis of cream cheese at different fermentation stages
3.3.1. Differential flavor substances in cream cheese
The PCA is a method of unsupervised multivariate statistical analysis that can be used to summarize the differences in volatile components and evaluate the consistency and variability between samples. Samples with similar aroma characteristics may approach or even overlap in the PCA graph. If the aroma characteristics of the samples are distinct, they will be separated from each other. As shown in Fig. 3a–b, the PCA results of GC-MS and GC-IMS were similar. The five samples were divided into four groups, and the samples at different fermentation stages were well separated, with two fermentation groups (D0, D5) arranged closer together in space. It indicates that fermentation time distinguishes flavor compounds in cream cheese effectively. To further determine the contribution of each of the VOCs to flavor, the importance of variables in the supervised OPLS-DA model projection (VIP) was determined. Volatility with VIP >1 is considered an important contribution to classification (Liu et al., 2023). After arrangement testing, the constructed OPLS-DA models all performed well (Fig. S1). According to the VIP values in the OPLS-DA model, 34 and 36 VOCs were selected in GC-MS and GC-IMS, respectively. For GC-MS, there were 11 flavor substances with a VIP greater than 1, which can be used as important substances to distinguish different fermentation stages, namely decanoic acid, benzoic acid terpene δ-caprolactone, decan-2-one, caproic acid, 2,3-butanediol, heptan-2-one, trans-9-octadecenol, limonene and 3-methylbutanoic acid (Fig. 3c–d). For GC-IMS, there were 12 flavor substances with a VIP >1, namely pentanoic acid, hexanitrile, pentyl acetate, furfuryl alcohol, 3-methylbutanoic acid, butyl acetate, isovaleraldehyde, oct-1-en-3-ol, phenylethanol, methyl isovalerate, isobutyraldehyde and 2-methylpropyl 2-methylpropanoate (Fig. 3c–d).
3.3.2. Comparison of detection results between HS-GC-IMS and HS-SPME-GC-MS
HS-GC-IMS and HS-SPME-GC-MS may have different detection abilities for flavor substances. It was found that more kinds of VOCs (41) were identified by HS-GC-IMS than HS-SPME-GC-MS(34). In addition, as shown in Fig. S1, acids accounted for the largest proportion (22–43%) in the compounds identified by GC-MS in cream cheese. In contrast, the main VOCs identified by HS-GC-IMS in cream cheese were esters (17–37%), alcohols (21–40%) and aldehydes (27–38%), similar to the report by Xi et al. (2024). Therefore, the integration of the two methods can be used to study VOCs in cream cheese more comprehensively.
3.3.3. Key flavor substances in cream cheese at different fermentation stages
The contribution of VOCs to the characteristic flavor composition of cream cheese is not only related to their concentration, but also is an important reference factor for its odor threshold. OAV is the ratio of aroma compound concentration to odor threshold, and a higher OAV indicates a greater contribution of flavor compounds to the entire flavor spectrum (Xiang et al., 2023).
In addition, GC-MS can determine key flavor compounds at different fermentation stages based on OAV, with 11 volatile substances having OAVs above one in five different stages, including 3-methylbutanoic acid, 2,3-butanediol, 3-methylbutan-1-ol, 2-phenylacetaldehyde, decanal, heptan-2-one, 3-hydroxybutan-2-one, undecan-2-one, 6-heptyloxan-2-one and d-limonene. 3-hydroxybutan-2-one is the key flavor contributor for odor and provides a buttery odor in cream cheese. 3-methylbutanoic acid is another important volatile and generates a cheesy odor. d-limonene was the only VOC with OAV greater than one in all alkenes, which contributed to the lemon odor of cream cheese. Decanal generates citrus odor for cheese. Its OAV was the highest among all the samples. The OAV of δ-decalactone was greater than 1 at D10-D20, but the OAV of 6-heptyloxan-2-one was higher than 1 only at D5, D10 and D20. Both compounds contributed to buttery and sweety odor.
Compared with OAV, ROAV analysis only needs relative concentration. Therefore, ROAV was used to analyze the data based on GC-IMS to determine the key active aroma compounds of cream cheese obtained from different stages (Table S1). As shown inTable S1, 17 VOCs were identified as key aromatic compounds in cream cheese, as their ROAVs exceeded 1, including hexanoic acid, 3-methylbutanoic acid, oct-1-en-3-ol, furanmethanol, heptan-2-ol and (e)-3-hexen, propan-2-ol, (E,E)-2,4-heptadienal, 2-phenylacetaldehyde, 3-methylbutanal, 3-methylsulfanylpropanal, ethyl butanoate, butyl acetate, butanoic acid, propyl ester, 2,3-Pentanedione d-limonene and styrene.
Finally, OAV and ROAV combined with VIP were used to screen the key flavor substances. Heptan-2-one, 3-hydroxybutan-2-one, undecan-2-one, and δ-caprolactone, 2,3-butanediol δ-dodecyl lactone, decanoic acid, benzoic acid, decan-2-one, limonene, 3-methylbutanoic acid and 2-phenylacetaldehyde, oct-1-en-3-ol and phenylethanol were found to be the substances that contribute significantly to the flavor during the fermentation process of cream cheese.
3.4. The correlation between flavor and sensory characteristics
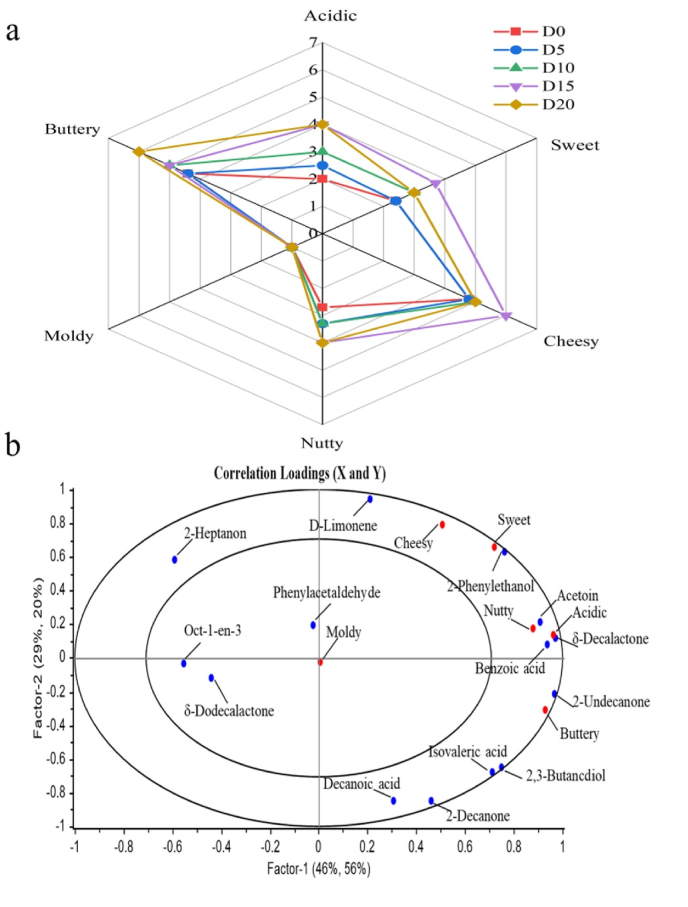
Ten assessors conducted sensory evaluation based on 6 indexes (acidic, sweet, cheesy, nutty, moldy, buttery) of cream cheese at different fermentation stages. As shown in Fig. 4a, the main differences in the sensory of cream cheese at different fermentation times were nutty, buttery and cheesy odor. The aroma of cheesy was significantly higher at D15 than the other stages, similar to the report by Zhang et al. (2022). This may be related to the change of 3-methylbutanoic acid content, which reached the maximum at D15 of fermentation and generated cheesy odor. The buttery is mainly produced by 3-hydroxybutan-2-one, with a high content during fermentation. It is a characteristic flavor substance of milk and dairy products. After 15–20 d of fermentation, the acidity reached the highest, and this may result from the high concentrations of acetic acid, 3-methylbutanoic acid, capric acid, caproic acid, caproic acid and azelaic acid. The sweetness of cheese is mainly related to ketones, such as 2-nonone and heptan-2-one, which make the cheese taste plump. PLS is a supervised classification technique that uses odor data matrices with FD values or OAV (X variable) and sensory contours (Y variable) (Liu et al., 2021). As shown in Fig. 4b, PLSR was used to establish the relationship between flavor characteristics and key flavor substances. It was found that 2-phenylethanol was highly correlated with sweetness, while 3-hydroxybutan-2-one, benzoic acid and δ-caprolactone were highly correlated with nut and sour taste, and undecan-2-one was strongly correlated with butter taste. These unique aromatic active compounds form the rich aroma of cream cheese. PLSR further validated the qualitative and quantitative analysis of flavor compounds, as well as the sensory analysis results of flavor substances. Finally, through analysing the changes in flavor components combined with sensory analysis, it was concluded that the optimal fermentation time for cream cheese was 15 d.
Fig. 4.
Sensory analysis (a) and PLSR analysis (b) of cream cheese during different fermentation periods.
4. Conclusions
In this study, the flavor of cream cheese samples at different stages was analyzed comprehensively. A total of 75 VOCs were identified, including 24 esters, 16 acids, 14 alcohols, 8 aldehydes, 6 ketones and 7 other substances. HS-GC-IMS detected more VOCs than GC-MS. Acids accounted for the largest proportion of the compounds identified by GC-MS in cream cheese. However, the main VOCs identified by HS-GC-IMS in cream cheese were esters, alcohols and aldehydes. Through the analysis of OAV and ROAV, 13 VOCs were identified as key aromatic compounds. PLSR analysis showed that 2-phenylethanol provided sweetness. 3-hydroxybutyl-2-ketone, benzoic acid and δ-caprolactone played an important role in the nutty and sour taste of cream cheese. Undecane-2-ketone provided butter flavor to cream cheese. Finally, different ripening stages had significant effects on the flavor of cream cheese, and the cream cheese fermented for 15 d showed the best flavor. The results help improve the understanding of the flavor changes of cream cheese at different maturity stages, and provide new insights into flavor evaluation and quality improvement of cream cheese.
Credit Author Statement
Chao-Kun Wei; Conceptualization, Validation, Writing – review & editing, Supervision, Project administration, Funding acquisition, Min Fan; Methodology, Formal analysis, Data curation, writing—original draft preparation, An-Ran Zheng; Methodology, Software, Investigation, Data curation, Meng-Song Wang; Formal analysis, Ning Jun; Resources. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Key research and development projects in Ningxia Province (2021BEB04049), National Natural Science Foundation of Ningxia Province (2021AAC03050).
Handling Editor: Professor Aiqian Ye
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100772.
Contributor Information
An-Ran Zheng, Email: zhenganran17@163.com.
Chao-Kun Wei, Email: weichaokun@nxu.edu.cn.
Meng-Song Wang, Email: wangmengsong11@163.com.
Ning Ju, Email: 708818908@qq.com.
Min Fan, Email: 2903876326@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Al-Dalali S., Zheng F., Sun B., Rahman T., Chen F. Tracking volatile flavor changes during two years of aging of Chinese vinegar by HS-SPME-GC-MS and GC-O. J. Food Compos. Anal. 2022;106 doi: 10.1016/j.jfca.2021.104295. [DOI] [Google Scholar]
- Alewijn M., Smit B.A., Sliwinski E.L., Wouters J.T.M. The formation mechanism of lactones in Gouda cheese. Int. Dairy J. 2007;17(1):59–66. doi: 10.1016/j.idairyj.2006.01.002. [DOI] [Google Scholar]
- Amaral J.B.S., Grisi C.V.B., Vieira E.A., Ferreira P.S., Rodrigues C.G., Diniz N.C.M., Cordeiro A.M. T.d.M. Light cream cheese spread of goat milk enriched with phytosterols: physicochemical, rheological, and microbiological characterization. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2022;157 doi: 10.1016/j.lwt.2022.113103. [DOI] [Google Scholar]
- Brighenti M., Govindasamy-Lucey S., Jaeggi J.J., Johnson M.E., Lucey J.A. Effect of substituting whey cream for sweet cream on the textural and rheological properties of cream cheese. J. Dairy Sci. 2021;104(10):10500–10512. doi: 10.3168/jds.2021-20338. [DOI] [PubMed] [Google Scholar]
- Caron T., Piver M.L., Peron A.C., Lieben P., Lavigne R., Brunel S., Chassard C. Strong effect of Penicillium roqueforti populations on volatile and metabolic compounds responsible for aromas, flavor and texture in blue cheeses. Int. J. Food Microbiol. 2021;354 doi: 10.1016/j.ijfoodmicro.2021.109174. [DOI] [PubMed] [Google Scholar]
- Chen C., Liu Z., Yu H., Xu Z., Tian H. Flavoromic determination of lactones in cheddar cheese by GC-MS-olfactometry, aroma extract dilution analysis, aroma recombination and omission analysis. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130736. [DOI] [PubMed] [Google Scholar]
- Chen C., Tian T., Yu H., Yuan H., Wang B., Xu Z., Tian H. Characterisation of the key volatile compounds of commercial Gouda cheeses and their contribution to aromas according to Chinese consumers' preferences. Food Chem. X. 2022;15 doi: 10.1016/j.fochx.2022.100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Yuan J., Yu H., Wang B., Huang J., Yuan H., Tian H. Characterization of metabolic pathways for biosynthesis of the flavor compound 3-methylbutanal by Lactococcus lactis. J. Dairy Sci. 2022;105(1):97–108. doi: 10.3168/jds.2021-20779. [DOI] [PubMed] [Google Scholar]
- Dan T., Hu H., Li T., Dai A., He B., Wang Y. Screening of mixed-species starter cultures for increasing flavour during fermentation of milk. Int. Dairy J. 2022;135 doi: 10.1016/j.idairyj.2022.105473. [DOI] [Google Scholar]
- Delgado F.J., González-Crespo J., Cava R., García-Parra J., Ramírez R. Characterisation by SPME–GC–MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010;118(1):182–189. doi: 10.1016/j.foodchem.2009.04.081. [DOI] [Google Scholar]
- Du H., Chen Q., Liu Q., Wang Y., Kong B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021;182 doi: 10.1016/j.meatsci.2021.108626. [DOI] [PubMed] [Google Scholar]
- Gao P., Zhang W., Wei M., Chen B., Zhu H., Xie N., Lv J. Analysis of the non-volatile components and volatile compounds of hydrolysates derived from unmatured cheese curd hydrolysis by different enzymes. LWT--Food Sci. Technol. 2022;168 doi: 10.1016/j.lwt.2022.113896. [DOI] [Google Scholar]
- Guo X., Schwab W., Ho C.T., Song C., Wan X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2021;376 doi: 10.1016/j.foodchem.2021.131933. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen Q., Wen R., Wang Y., Qin L., Kong B. Quality characteristics and flavor profile of Harbin dry sausages inoculated with lactic acid bacteria and Staphylococcus xylosus. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;114 doi: 10.1016/j.lwt.2019.108392. [DOI] [Google Scholar]
- Huan C., Du X., Wang L., Kebbeh M., Li H., Yang X., Zheng X. Transcriptome analysis reveals the metabolisms of starch degradation and ethanol fermentation involved in alcoholic off-flavor development in kiwifruit during ambient storage. Postharvest Biol. Technol. 2021;180 doi: 10.1016/j.postharvbio.2021.111621. [DOI] [Google Scholar]
- Jung H.J., Ganesan P., Lee S.J., Kwak H.S. Comparative study of flavor in cholesterol-removed Gouda cheese and Gouda cheese during ripening. J. Dairy Sci. 2013;96(4):1972–1983. doi: 10.3168/jds.2012-5644. [DOI] [PubMed] [Google Scholar]
- Leroy F., Verluyten J., De Vuyst L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006;106(3):270–285. doi: 10.1016/j.ijfoodmicro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Li X.M., Deng J.Y., Wu Y., Nie W., Wang Z.M., Zhou H., Xu B.C. Insight into the correlation between microbial diversity and flavor profiles of traditional dry-cured duck from the metabolomic perspective. Food Res. Int. 2022;156 doi: 10.1016/j.foodres.2022.111349. [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan L., Liu H., Liu H., Zhou Y., Li M., Gao R. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Sci. Hum. Wellness. 2023;12(1):173–182. doi: 10.1016/j.fshw.2022.07.035. [DOI] [Google Scholar]
- Ling H., Shi H., Chen X., Cheng K. Detection of the microbial diversity and flavor components of northeastern Chinese soybean paste during storage. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131686. [DOI] [PubMed] [Google Scholar]
- Liu H., Xu Y., Wu J., Wen J., Yu Y., An K., Zou B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021;150(Pt A) doi: 10.1016/j.foodres.2021.110784. [DOI] [PubMed] [Google Scholar]
- Liu A., Zhang H., Liu T., Gong P., Wang Y., Wang H., Yi H. Aroma classification and flavor characterization of Streptococcus thermophilus fermented milk by HS-GC-IMS and HS-SPME-GC-TOF/MS. Food Biosci. 2022;49 doi: 10.1016/j.fbio.2022.101832. [DOI] [Google Scholar]
- Liu N., Shen S., Huang L., Deng G., Wei Y., Ning J., Wang Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023;169 doi: 10.1016/j.foodres.2023.112845. [DOI] [PubMed] [Google Scholar]
- Malik S., De I., Singh M., Galanakis C.M., Alamri A.S., Yadav J.K. Isolation and characterisation of milk-derived amyloid-like protein aggregates (MAPA) from cottage cheese. Food Chem. 2022;373(Pt B) doi: 10.1016/j.foodchem.2021.131486. [DOI] [PubMed] [Google Scholar]
- Natividad J., Rocío G., Bruno M., Eiceman Gary A., Arce Lourdes. Stability of proton-bound clusters of alkyl alcohols, aldehydes and ketones in Ion Mobility Spectrometry. Talanta. 2018;185:299–308. doi: 10.1016/j.talanta.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Parastar H., Weller P. Benchtop volatilomics supercharged: How machine learning based design of experiment helps optimizing untargeted GC-IMS gas phase metabolomics. Talanta. 2024;2024 doi: 10.1016/j.talanta.2024.125788. [DOI] [PubMed] [Google Scholar]
- Que Z., Jin Y., Huang J., Zhou R., Wu C. Flavor compounds of traditional fermented bean condiments: Classes, synthesis, and factors involved in flavor formation. Trends Food Sci. Technol. 2023;133:160–175. doi: 10.1016/j.tifs.2023.01.010. [DOI] [Google Scholar]
- Tadeu da Veiga Correia V., D'Angelis D.F., Neris dos Santos A., Silva Roncheti E.F., Vieira Queiroz V.A., Fontes Figueiredo J.E., Fante C.A. Tannin-sorghum flours in cream cheese: physicochemical, antioxidant and sensory characterization. LWT- Food Sci. Technol. 2022;154 doi: 10.1016/j.lwt.2021.112672. [DOI] [Google Scholar]
- Uzkuç H., Karagül Yüceer Y. Effects of heat treatment, plant coagulant, and starter culture on sensory characteristics and volatile compounds of goat cheese. Int. Dairy J. 2023;140 doi: 10.1016/jidairyj.2023.105588. [DOI] [Google Scholar]
- Wang D., Wang M., Cao L., Wang X., Sun J., Yuan J., Gu S. Changes and correlation of microorganism and flavor substances during persimmon vinegar fermentation. Food Biosci. 2022;46 doi: 10.1016/j.fbio.2022.101565. [DOI] [Google Scholar]
- Wang H., Li Y., Xia X., Liu Q., Sun F., Kong B. Flavor formation from hydrolysis of pork meat protein extract by the protease from Staphylococcus carnosus isolated from Harbin dry sausage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2022;163 doi: 10.1016/j.lwt.2022.113525. [DOI] [Google Scholar]
- Wang D., Chen G., Tang Y., Li J., Huang R., Ye M., Zhang W. Correlation between autochthonous microbial communities and flavor profiles during the fermentation of mustard green paocai (Brassica juncea Coss.), a typical industrial-scaled salted fermented vegetable. LWT--Food Sci. Technol. 2022;172 doi: 10.1016/j.lwt.2022.114212. [DOI] [Google Scholar]
- Wang M., Fan M., Zheng A., Wei C., Liu D., Thaku K., Wei Z. Characterization of a fermented dairy, sour cream: Lipolysis and the release profile of flavor compounds. Food Chem. 2023;423 doi: 10.1016/j.foodchem.2023.136299. [DOI] [PubMed] [Google Scholar]
- Wolfschoon Pombo A.F. Cream cheese: Historical, manufacturing, and physico-chemical aspects. Int. Dairy J. 2021;117 doi: 10.1016/j.idairyj.2020.104948. [DOI] [Google Scholar]
- Xi B., Zhang J., Xu X., Li C., Shu Y., Zhang Y., Shi X., Shen Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2024;435 doi: 10.1016/j.foodchem.2023.137547. [DOI] [PubMed] [Google Scholar]
- Xiang L., Zhu W., Jiang B., Chen J., Zhou L., Zhong F. Volatile compounds analysis and biodegradation strategy of beany flavor in pea protein. Food Chem. 2023;402 doi: 10.1016/j.foodchem.2022.134275. [DOI] [PubMed] [Google Scholar]
- Xiao N., Xu H., Jiang X., Sun T., Luo Y., Shi W. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111584. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Zhu M., He C., Li Z., Liu Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT--Food Sci. Technol. 2022;169 doi: 10.1016/j.lwt.2022.113925. [DOI] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Xiao L., Zhang X., Yang C., Li Z., Zhu M., Liu Z., Wang Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS-SPME/GC-MS and HS-GC-IMS. Curr. Res. Food Sci. 2022;5:1788–1807. doi: 10.1016/j.crfs.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Xu B., Xu Y., Yao Z., Zhu B., Li X., Sun Y. Effects of different thermal treatment temperatures on volatile flavor compounds of water-boiled salted duck after packaging. LWT--Food Sci. Technol. 2022;154 doi: 10.1016/j.lwt.2021.112625. [DOI] [Google Scholar]
- Xu Z., Chen J., Shi X., Wang B., Zheng X., Zheng X. Characteristic physicochemical indexes and flavor compounds in Xinjiang Kazak cheese during ripening. Food Biosci. 2020;35 doi: 10.1016/j.fbio.2020.100586. [DOI] [Google Scholar]
- Yang Y., Wang B., Fu Y., Shi Y.G., Chen F.L., Guan H.N., Zhang N. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021;346 doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- Yang J., Wu S., Mai R., Lin L., Zhao W., Bai W. Formation of amino acid-derived volatile compounds in dry-cured mackerel (Scomberomorus niphonius): metabolic pathways involving microorganisms, precursors, and intermediates. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.130163. [DOI] [PubMed] [Google Scholar]
- Yang Y., Ai L., Mu Z., Liu H., Yan X., Ni L., Xia Y. Flavor compounds with high odor activity values (OAV > 1) dominate the aroma of aged Chinese rice wine (Huangjiu) by molecular association. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132370. [DOI] [PubMed] [Google Scholar]
- Ye T.-T., Liu J., Wan P., Liu S.-Y., Wang Q.-Z., Chen D.-W. Investigation of the effect of polar components in cream on the flavor of heated cream based on NMR and GC-MS methods. LWT--Food Sci. Technol. 2022;155 doi: 10.1016/j.lwt.2021.112940. [DOI] [Google Scholar]
- Yu H., Guo W., Xie T., Ai L., Tian H., Chen C. Aroma characteristics of traditional Huangjiu produced around Winter Solstice revealed by sensory evaluation, gas chromatography-mass spectrometry and gas chromatography-ion mobility spectrometry. Food Res. Int. 2021;145 doi: 10.1016/j.foodres.2021.110421. [DOI] [PubMed] [Google Scholar]
- Zhao D., Hu J., Chen W. Analysis of the relationship between microorganisms and flavor development in dry-cured grass carp by high-throughput sequencing, volatile flavor analysis and metabolomics. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130889. [DOI] [PubMed] [Google Scholar]
- Zhao D., Hu J., Zhou X., Chen W. Correlation between microbial community and flavor formation in dry-cured squid analysed by next-generation sequencing and molecular sensory analysis. Food Chem. X. 2022;15 doi: 10.1016/j.fochx.2022.100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Liu F., Shi X., Wang B., Li K., Li B., Zhuge B. Dynamic correlations between microbiota succession and flavor development involved in the ripening of Kazak artisanal cheese. Food Res. Int. 2018;105:733–742. doi: 10.1016/j.foodres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Zhong A., Chen W., Duan Y., Li K., Tang X., Tian X., Wang C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;149 doi: 10.1016/j.lwt.2021.111873. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.